Abstract
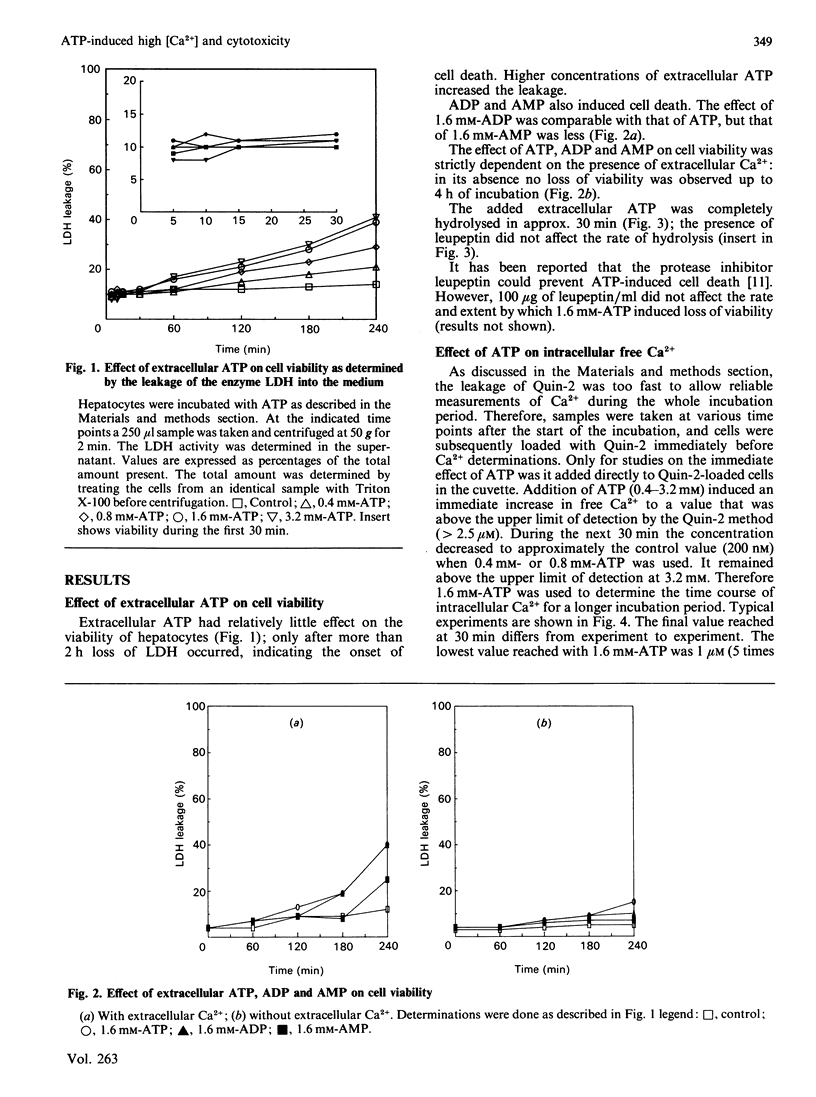
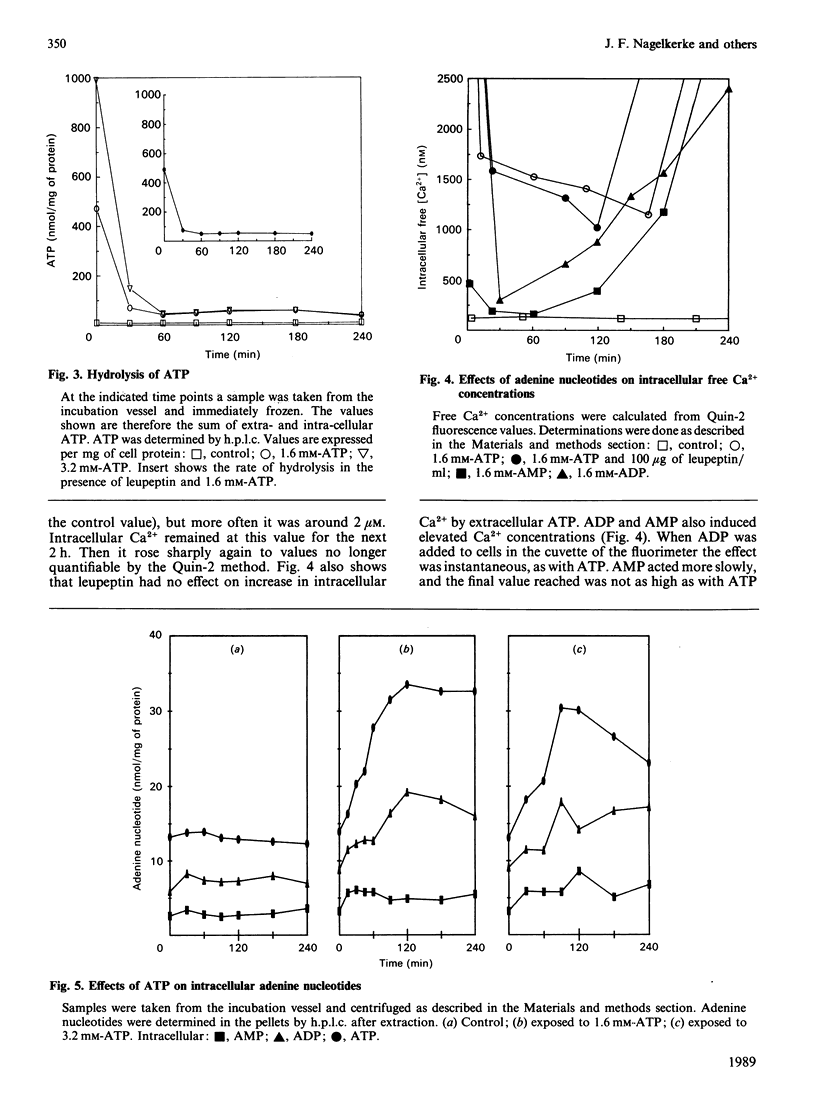
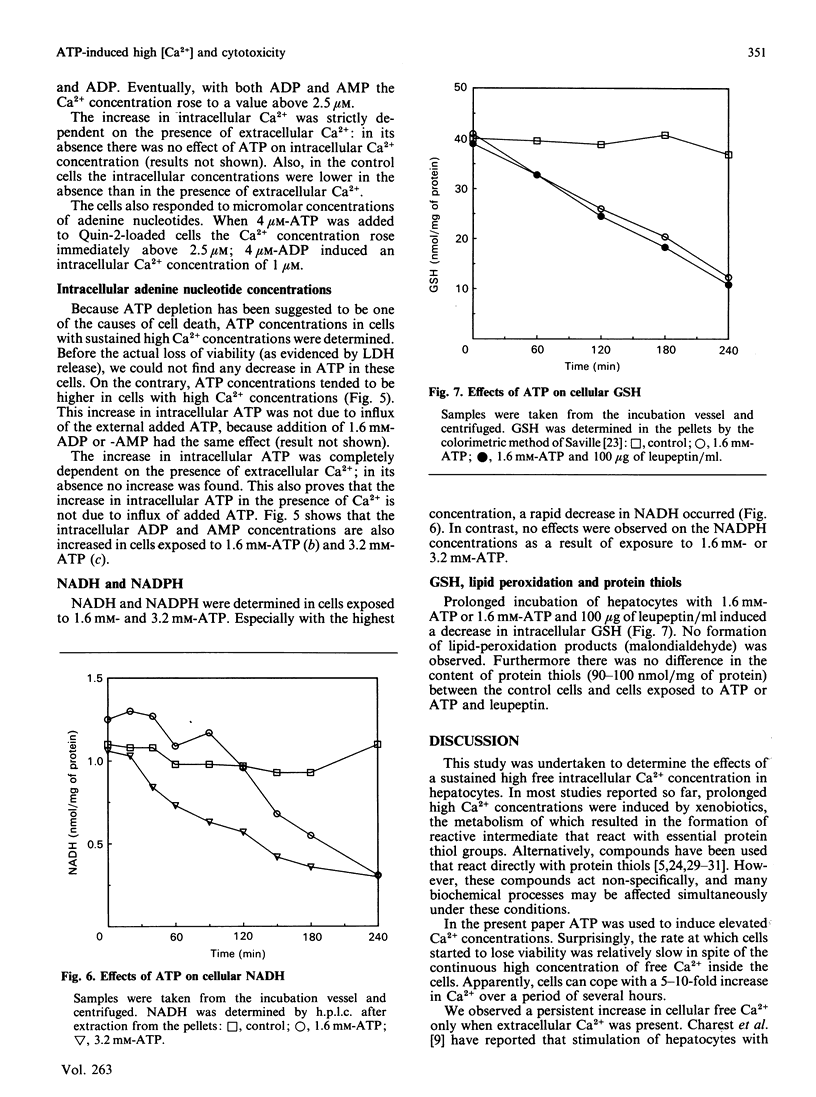
Isolated rat hepatocytes were incubated with ATP to induce high intracellular free Ca2+ concentrations as determined with the Quin-2 method. Immediately after addition of ATP, the intracellular concentration of Ca2+ rose from 200 nM to more than 2.5 microM. It stayed at this value during the first 1/2 h; the rise was absolutely dependent on extracellular Ca2+. After the first 1/2 h the Ca2+ concentration decreased to 1-2 microM (5-10 times the control value). These high intracellular free Ca2+ concentrations did not lead to an immediate loss of cell viability. Only after 2 h of incubation a substantial number of cells lost viability. This was preceded by a decrease in cellular NADH (greater than 40%) and accompanied by a sharp increase in the intracellular Ca2+ concentration. Under these conditions the NADPH concentration was not affected. Cellular GSH was decreased to 30% of the initial value, but no lipid peroxidation or protein-thiol depletion was observed. Intracellular ATP, ADP and AMP were increased in the presence of extracellular ATP. Ca2+-dependent proteases seemed not to be involved in cell death. These observations are consistent with a collapse of mitochondrial functions as a final trigger of cell death.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellomo G., Jewell S. A., Thor H., Orrenius S. Regulation of intracellular calcium compartmentation: studies with isolated hepatocytes and t-butyl hydroperoxide. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6842–6846. doi: 10.1073/pnas.79.22.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo G., Mirabelli F., Richelmi P., Orrenius S. Critical role of sulfhydryl group(s) in ATP-dependent Ca2+ sequestration by the plasma membrane fraction from rat liver. FEBS Lett. 1983 Oct 31;163(1):136–139. doi: 10.1016/0014-5793(83)81180-6. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Nicotera P., Orrenius S. Alterations in intracellular calcium compartmentation following inhibition of calcium efflux from isolated hepatocytes. Eur J Biochem. 1984 Oct 1;144(1):19–23. doi: 10.1111/j.1432-1033.1984.tb08425.x. [DOI] [PubMed] [Google Scholar]
- Berthon B., Binet A., Mauger J. P., Claret M. Cytosolic free Ca2+ in isolated rat hepatocytes as measured by quin2. Effects of noradrenaline and vasopressin. FEBS Lett. 1984 Feb 13;167(1):19–24. doi: 10.1016/0014-5793(84)80824-8. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Di Monte D., Ross D., Bellomo G., Eklöw L., Orrenius S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch Biochem Biophys. 1984 Dec;235(2):334–342. doi: 10.1016/0003-9861(84)90206-6. [DOI] [PubMed] [Google Scholar]
- Jones D. P. Determination of pyridine dinucleotides in cell extracts by high-performance liquid chromatography. J Chromatogr. 1981 Oct 9;225(2):446–449. doi: 10.1016/s0378-4347(00)80293-5. [DOI] [PubMed] [Google Scholar]
- Krell H., Baur H., Pfaff E. Transient 45Ca uptake and release in isolated rat-liver cells during recovery from deenergized states. Eur J Biochem. 1979 Nov;101(2):349–364. doi: 10.1111/j.1432-1033.1979.tb19727.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehninger A. L., Vercesi A., Bababunmi E. A. Regulation of Ca2+ release from mitochondria by the oxidation-reduction state of pyridine nucleotides. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1690–1694. doi: 10.1073/pnas.75.4.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. H. Novel ATP-dependent calcium transport component from rat liver plasma membranes. The transporter and the previously reported (Ca2+-Mg2+)-ATPase are different proteins. J Biol Chem. 1985 Jul 5;260(13):7850–7856. [PubMed] [Google Scholar]
- Lin S. H., Russell W. E. Two Ca2+-dependent ATPases in rat liver plasma membrane. The previously purified (Ca2+-Mg2+)-ATPase is not a Ca2+-pump but an ecto-ATPase. J Biol Chem. 1988 Sep 5;263(25):12253–12258. [PubMed] [Google Scholar]
- Lê Quôc K., Lê Quôc D. Involvement of the ADP/ATP carrier in calcium-induced perturbations of the mitochondrial inner membrane permeability: importance of the orientation of the nucleotide binding site. Arch Biochem Biophys. 1988 Sep;265(2):249–257. doi: 10.1016/0003-9861(88)90125-7. [DOI] [PubMed] [Google Scholar]
- Mirabelli F., Bellomo G., Nicotera P., Moore M., Orrenius S. Ca2+ homeostasis and cytotoxicity in isolated hepatocytes: studies with extracellular adenosine 5'-triphosphate. J Biochem Toxicol. 1986 Mar;1(1):29–39. doi: 10.1002/jbt.2570010105. [DOI] [PubMed] [Google Scholar]
- Mirabelli F., Salis A., Marinoni V., Finardi G., Bellomo G., Thor H., Orrenius S. Menadione-induced bleb formation in hepatocytes is associated with the oxidation of thiol groups in actin. Arch Biochem Biophys. 1988 Jul;264(1):261–269. doi: 10.1016/0003-9861(88)90593-0. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Högberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- Monck J. R., Reynolds E. E., Thomas A. P., Williamson J. R. Novel kinetics of single cell Ca2+ transients in stimulated hepatocytes and A10 cells measured using fura-2 and fluorescent videomicroscopy. J Biol Chem. 1988 Apr 5;263(10):4569–4575. [PubMed] [Google Scholar]
- Moore G. A., Rossi L., Nicotera P., Orrenius S., O'Brien P. J. Quinone toxicity in hepatocytes: studies on mitochondrial Ca2+ release induced by benzoquinone derivatives. Arch Biochem Biophys. 1987 Dec;259(2):283–295. doi: 10.1016/0003-9861(87)90495-4. [DOI] [PubMed] [Google Scholar]
- Moore M., Thor H., Moore G., Nelson S., Moldéus P., Orrenius S. The toxicity of acetaminophen and N-acetyl-p-benzoquinone imine in isolated hepatocytes is associated with thiol depletion and increased cytosolic Ca2+. J Biol Chem. 1985 Oct 25;260(24):13035–13040. [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Baldi C., Svensson S. A., Bellomo G., Orrenius S. Cystamine induces toxicity in hepatocytes through the elevation of cytosolic Ca2+ and the stimulation of a nonlysosomal proteolytic system. J Biol Chem. 1986 Nov 5;261(31):14628–14635. [PubMed] [Google Scholar]
- Nicotera P., Hartzell P., Davis G., Orrenius S. The formation of plasma membrane blebs in hepatocytes exposed to agents that increase cytosolic Ca2+ is mediated by the activation of a non-lysosomal proteolytic system. FEBS Lett. 1986 Dec 1;209(1):139–144. doi: 10.1016/0014-5793(86)81099-7. [DOI] [PubMed] [Google Scholar]
- Olafsdottir K., Pascoe G. A., Reed D. J. Mitochondrial glutathione status during Ca2+ ionophore-induced injury to isolated hepatocytes. Arch Biochem Biophys. 1988 May 15;263(1):226–235. doi: 10.1016/0003-9861(88)90631-5. [DOI] [PubMed] [Google Scholar]
- Pascoe G. A., Reed D. J. Relationship between cellular calcium and vitamin E metabolism during protection against cell injury. Arch Biochem Biophys. 1987 Mar;253(2):287–296. doi: 10.1016/0003-9861(87)90181-0. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Pozzan T. Using quin2 in cell suspensions. Cell Calcium. 1985 Apr;6(1-2):133–144. doi: 10.1016/0143-4160(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Schweinsberg P. D., Loo T. L. Simultaneous analysis of ATP, ADP, AMP, and other purines in human erythrocytes by high-performance liquid chromatography. J Chromatogr. 1980 Jan 11;181(1):103–107. doi: 10.1016/s0378-4347(00)81276-1. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sies H., Graf P., Estrela J. M. Hepatic calcium efflux during cytochrome P-450-dependent drug oxidations at the endoplasmic reticulum in intact liver. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3358–3362. doi: 10.1073/pnas.78.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke P. E., Hoek J. B., Farber J. L. Calcium-dependent and calcium-independent mechanisms of irreversible cell injury in cultured hepatocytes. J Biol Chem. 1986 Mar 5;261(7):3006–3012. [PubMed] [Google Scholar]
- Thor H., Hartzell P., Orrenius S. Potentiation of oxidative cell injury in hepatocytes which have accumulated Ca2+. J Biol Chem. 1984 May 25;259(10):6612–6615. [PubMed] [Google Scholar]
- van Berkel T. J., Kruijt J. K., Kempen H. J. Specific targeting of high density lipoproteins to liver hepatocytes by incorporation of a tris-galactoside-terminated cholesterol derivative. J Biol Chem. 1985 Oct 5;260(22):12203–12207. [PubMed] [Google Scholar]


