Abstract
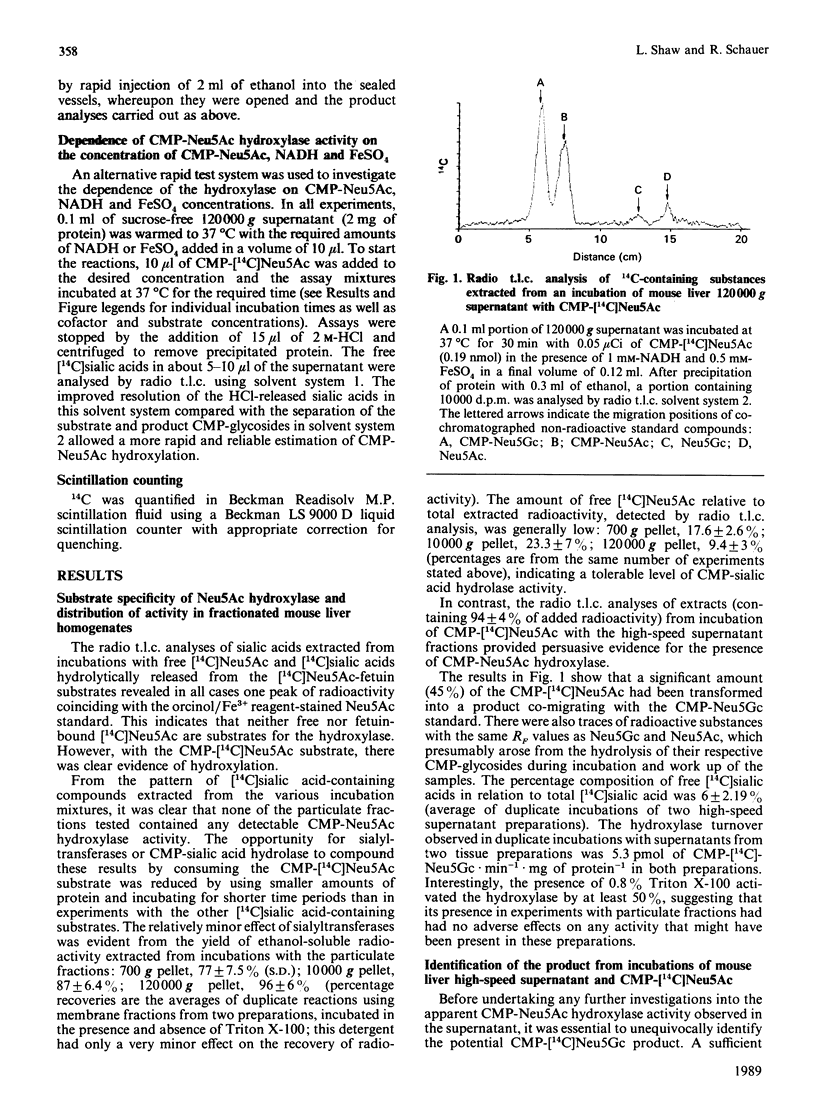
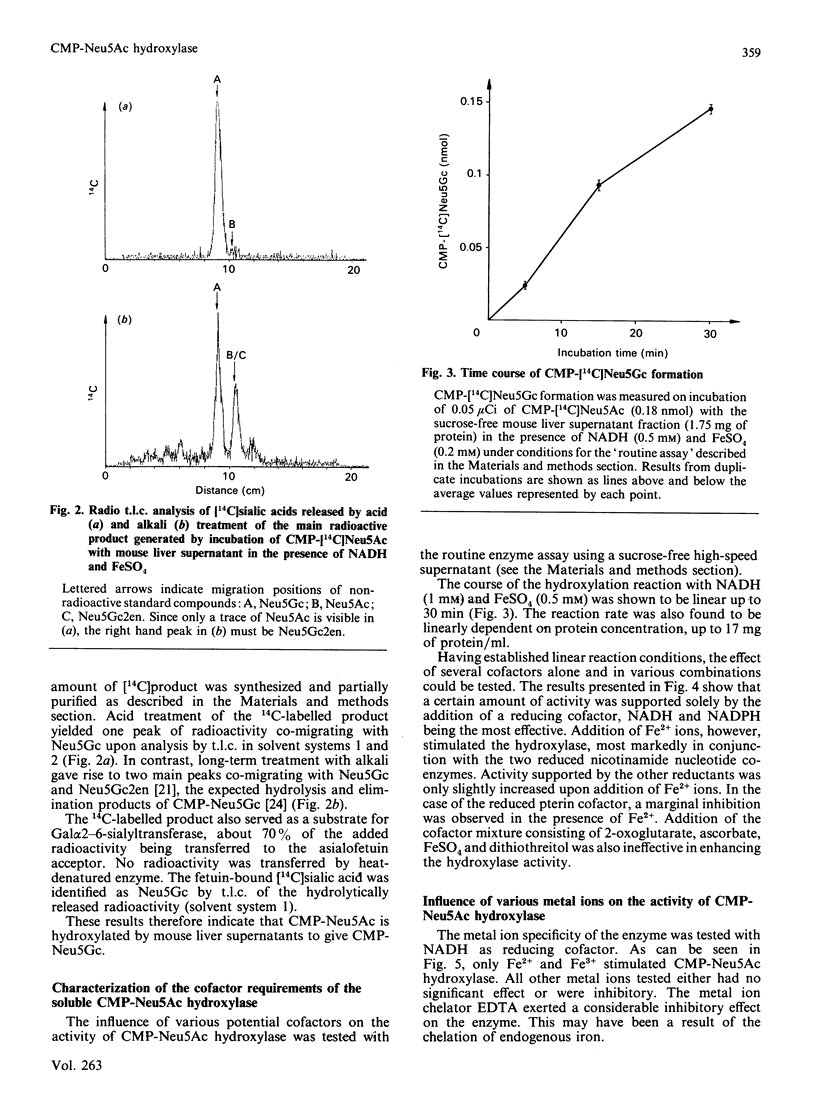
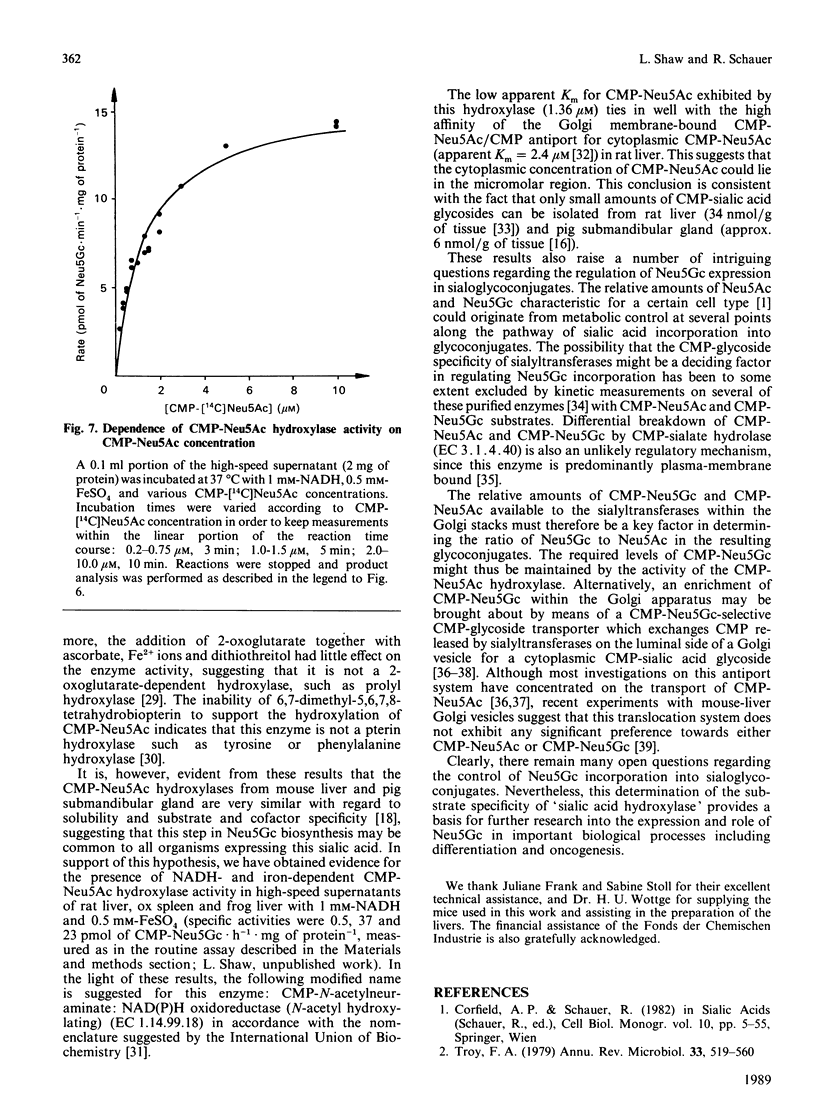
The finding that N-glycoloylneuraminic acid (Neu5Gc) in pig submandibular gland is synthesized by hydroxylation of the sugar nucleotide CMP-Neu5Ac [Shaw & Schauer (1988) Biol. Chem. Hoppe-Seyler 369, 477-486] prompted us to investigate further the biosynthesis of this sialic acid in mouse liver. Free [14C]Neu5Ac, CMP-[14C]Neu5Ac and [14C]Neu5Ac glycosidically bound by Gal alpha 2-3- and Gal alpha 2-6-GlcNAc beta 1-4 linkages to fetuin were employed as potential substrates in experiments with fractionated mouse liver homogenates. The only substrate to be hydroxylated was the CMP-Neu5Ac glycoside. The product of the reaction was identified by chemical and enzymic methods as CMP-Neu5Gc. All of the CMP-Neu5Ac hydroxylase activity was detected in the high-speed supernatant fraction. The hydroxylase required a reduced nicotinamide nucleotide [NAD(P)H] coenzyme and molecular oxygen for activity. Furthermore, the activity of this enzyme was enhanced by exogenously added Fe2+ or Fe3+ ions, all other metal salts tested having a negligible or inhibitory influence. This hydroxylase is therefore tentatively classified as a monooxygenase. The cofactor requirement and CMP-Neu5Ac substrate specificity are identical to those of the enzyme in high-speed supernatants of pig submandibular gland, suggesting that this is a common route of Neu5Gc biosynthesis. The relevance of these results to the regulation of Neu5Gc expression in sialoglycoconjugates is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOHM P., DAUBER S., BAUMEISTER L. Uber Neuraminsäure, ihr Vorkommen und ihre Bestimmung im Serum. Klin Wochenschr. 1954 Apr 1;32(13-14):289–292. doi: 10.1007/BF01467396. [DOI] [PubMed] [Google Scholar]
- Beau J. M., Schauer R., Haverkamp J., Kamerling J. P., Dorland L., Vliegenthart J. F. Chemical behaviour of cytidine 5'-monophospho-N-acetyl-beta-D-neuraminic acid under neutral and alkaline conditions. Eur J Biochem. 1984 Apr 2;140(1):203–208. doi: 10.1111/j.1432-1033.1984.tb08087.x. [DOI] [PubMed] [Google Scholar]
- Buscher H. P., Casals-Stenzel J., Schauer R., Mestres-Ventura P. Biosynthesis of N-glycolylneuraminic acid in porcine submandibular glands. Subcellular site of hydroxylation of N-acetylneuraminic acid in the course of glycoprotein biosynthesis. Eur J Biochem. 1977 Jul 15;77(2):297–310. doi: 10.1111/j.1432-1033.1977.tb11668.x. [DOI] [PubMed] [Google Scholar]
- Capasso J. M., Hirschberg C. B. Mechanisms of glycosylation and sulfation in the Golgi apparatus: evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7051–7055. doi: 10.1073/pnas.81.22.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale G. J., Udenfriend S. Prolyl hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):245–300. doi: 10.1002/9780470122860.ch6. [DOI] [PubMed] [Google Scholar]
- Carey D. J., Sommers L. W., Hirschberg C. B. CMP-N-acetylneuraminic acid: isolation from and penetration into mouse liver microsomes. Cell. 1980 Mar;19(3):597–605. doi: 10.1016/s0092-8674(80)80036-5. [DOI] [PubMed] [Google Scholar]
- Ferwerda W., Blok C. M., Van Rinsum J. Synthesis of N-acetylneuraminic acid and of CMP-N-acetylneuraminic acid in the rat liver cell. Biochem J. 1983 Oct 15;216(1):87–92. doi: 10.1042/bj2160087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S., Takemori Y., Yamaguchi M., Nakamura M., Ohkura Y. Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Anal Biochem. 1987 Jul;164(1):138–145. doi: 10.1016/0003-2697(87)90377-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Suzuki A., Yamakawa T., Wang C. H., Bonhomme F., Miyashita N., Moriwaki K. Expression of GM1 and GD1a in liver of wild mice. J Biochem. 1984 Jan;95(1):7–12. doi: 10.1093/oxfordjournals.jbchem.a134604. [DOI] [PubMed] [Google Scholar]
- Higashi H., Hirabayashi Y., Fukui Y., Naiki M., Matsumoto M., Ueda S., Kato S. Characterization of N-glycolylneuraminic acid-containing gangliosides as tumor-associated Hanganutziu-Deicher antigen in human colon cancer. Cancer Res. 1985 Aug;45(8):3796–3802. [PubMed] [Google Scholar]
- Higashi H., Ikuta K., Ueda S., Kato S., Hirabayashi Y., Matsumoto M., Naiki M. Characterization of N-glycolyneuraminic acid-containing glycosphingolipids from a Marek's disease lymphoma-derived chicken cell line, MSB1, as tumor-associated heterophile Hanganutziu-Deicher antigens. J Biochem. 1984 Mar;95(3):785–794. doi: 10.1093/oxfordjournals.jbchem.a134670. [DOI] [PubMed] [Google Scholar]
- Lepers A., Shaw L., Cacan R., Schauer R., Montreuil J., Verbert A. Transport of CMP-N-glycoloylneuraminic acid into mouse liver Golgi vesicles. FEBS Lett. 1989 Jul 3;250(2):245–250. doi: 10.1016/0014-5793(89)80731-8. [DOI] [PubMed] [Google Scholar]
- Muchmore E. A., Varki N. M., Fukuda M., Varki A. Developmental regulation of sialic acid modifications in rat and human colon. FASEB J. 1987 Sep;1(3):229–235. doi: 10.1096/fasebj.1.3.3623000. [DOI] [PubMed] [Google Scholar]
- Nöhle U., Shukla A. K., Schröder C., Reuter G., Schauer R., Kamerling J. P., Vliegenthart J. F. Structural parameters and natural occurrence of 2-deoxy-2,3-didehydro-N-glycoloylneuraminic acid. Eur J Biochem. 1985 Oct 15;152(2):459–463. doi: 10.1111/j.1432-1033.1985.tb09219.x. [DOI] [PubMed] [Google Scholar]
- Prince R. C., George G. N., Savas J. C., Cramer S. P., Patel R. N. Spectroscopic properties of the hydroxylase of methane monooxygenase. Biochim Biophys Acta. 1988 Jan 29;952(2):220–229. doi: 10.1016/0167-4838(88)90119-7. [DOI] [PubMed] [Google Scholar]
- Savage A. V., Koppen P. L., Schiphorst W. E., Trippelvitz L. A., Van Halbeek H., Vliegenthart J. F., Van den Eijnden D. H. Porcine submaxillary mucin contains alpha 2----3- and alpha 2----6-linked N-acetyl- and N-glycolylneuraminic acid. Eur J Biochem. 1986 Oct 1;160(1):123–129. doi: 10.1111/j.1432-1033.1986.tb09948.x. [DOI] [PubMed] [Google Scholar]
- Schauer R. Biosynthese der N-Glykoloylneuraminsäure durch eine von Ascorbinsäure bzw. NADPH abhängige N-Acetyl-hydroxylierende "N-Acetylneuraminat: O2-Oxidoreduktase" in Homogenaten der Unterkieferspeicheldrüse vom Schwein. Hoppe Seylers Z Physiol Chem. 1970 Jul;351(7):783–791. [PubMed] [Google Scholar]
- Schauer R. Characterization of sialic acids. Methods Enzymol. 1978;50:64–89. doi: 10.1016/0076-6879(78)50008-6. [DOI] [PubMed] [Google Scholar]
- Schauer R., Schoop H. J., Faillard H. Zur Biosynthese der Glykolyl-Gruppe der N-Glykolyl-neuraminsaure. Die oxydative Umwandlung der N-Acetyl-Gruppe zur Glykolyl-Gruppe. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):645–652. [PubMed] [Google Scholar]
- Schauer R., Wember M. Hydroxylation and O-acetylation of N-acetylneuraminic acid bound to glycoproteins of isolated subcellular membranes from porcine and bovine submaxillary glands. Hoppe Seylers Z Physiol Chem. 1971 Oct;352(10):1282–1290. doi: 10.1515/bchm2.1971.352.2.1282. [DOI] [PubMed] [Google Scholar]
- Schoop H. J., Schauer R., Faillard H. Zur Biosyntheses der N-Glykolyl-neuraminsäure. Die oxydative Enstehung von N-Glykolyl-neuraminsäure aus N-Acetyl-neuraminsäure. Hoppe Seylers Z Physiol Chem. 1969 Feb;350(2):155–162. [PubMed] [Google Scholar]
- Shaw L., Schauer R. The biosynthesis of N-glycoloylneuraminic acid occurs by hydroxylation of the CMP-glycoside of N-acetylneuraminic acid. Biol Chem Hoppe Seyler. 1988 Jun;369(6):477–486. doi: 10.1515/bchm3.1988.369.1.477. [DOI] [PubMed] [Google Scholar]
- Shiman R. Purification and assay of rat liver phenylalanine 4-monooxygenase. Methods Enzymol. 1987;142:17–27. doi: 10.1016/s0076-6879(87)42004-1. [DOI] [PubMed] [Google Scholar]
- Sommers L. W., Hirschberg C. B. Transport of sugar nucleotides into rat liver Golgi. A new Golgi marker activity. J Biol Chem. 1982 Sep 25;257(18):10811–10817. [PubMed] [Google Scholar]
- Troy F. A., 2nd The chemistry and biosynthesis of selected bacterial capsular polymers. Annu Rev Microbiol. 1979;33:519–560. doi: 10.1146/annurev.mi.33.100179.002511. [DOI] [PubMed] [Google Scholar]
- Van Dijk W., Maier H., Van den Eijnden D. H. CMP-N-acetylneuraminic acid hydrolase, an ectoenzyme distributed unevenly over the hepatocyte surface. Biochim Biophys Acta. 1977 Apr 1;466(1):187–197. doi: 10.1016/0005-2736(77)90218-8. [DOI] [PubMed] [Google Scholar]
- Verbert A., Cacan R., Cecchelli R. Membrane transport of sugar donors to the glycosylation sites. Biochimie. 1987 Feb;69(2):91–99. doi: 10.1016/0300-9084(87)90240-9. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Kwart L. D., Gibson D. T. Benzylic monooxygenation catalyzed by toluene dioxygenase from Pseudomonas putida. Biochemistry. 1988 Feb 23;27(4):1360–1367. doi: 10.1021/bi00404a041. [DOI] [PubMed] [Google Scholar]


