Visual Abstract
Key Words: chronic kidney disease, clopidogrel, coronary artery disease, diabetes mellitus, pharmacodynamic, pharmacokinetic, platelets
Highlights
-
•
Patients with DM have impaired clopidogrel-mediated platelet P2Y12 inhibition, exacerbated if CKD is present.
-
•
Potential mechanism(s) include altered drug absorption and/or metabolism and/or platelet P2Y12 activity.
-
•
CKD was associated with increased maximal platelet aggregation, which was not reflected in differences in the PRI or PRUs.
-
•
These findings could be attributed partially to upregulation of the P2Y12 signaling pathway but not to differences in drug absorption or metabolism.
-
•
Further studies are needed to determine the mechanism(s) by which CKD can lead to upregulation of P2Y12 signaling activity in DM patients.
Summary
This prospective ex vivo and in vitro pharmacodynamic (PD)/pharmacokinetic investigation was conducted in patients with diabetes mellitus with (n = 31) and without chronic kidney disease (n = 30). PD assessments included platelet reactivity index, maximum platelet aggregation, and P2Y12 reaction units. Ex vivo pharmacokinetic assessments included plasma levels of clopidogrel and its active metabolite. In vitro PD assessments were conducted on baseline samples incubated with escalating concentrations of clopidogrel and its active metabolite. Among patients with diabetes mellitus treated with clopidogrel, impaired renal function was associated with increased maximum platelet aggregation. This finding could be attributed partially to upregulation of the P2Y12 activity without differences in drug absorption or metabolism. (Impact of Chronic Kidney Disease on Clopidogrel Effects in Diabetes Mellitus; NCT03774394)
Clopidogrel is the most widely used oral P2Y12 receptor inhibitor and is recommended to prevent ischemic events in patients with atherosclerotic disease, particularly in those undergoing percutaneous cardiac intervention.1 Importantly, patients with diabetes mellitus (DM) have consistently shown to have impaired platelet inhibitory response to clopidogrel, contributing to their increased risk of atherothrombotic recurrences compared with patients without DM.2, 3, 4, 5, 6, 7 Of note, DM is among the most important determinants for the development of chronic kidney disease (CKD), and also a risk factor for recurrent atherothrombotic events.8,9 This factor can explain why clinical outcome studies have shown a gradient of risk according to the presence or absence of DM and CKD, with patients having both risk factors at highest risk of recurrent atherothrombotic events.8,10 These observations could be in part explained by the enhanced magnitude of impaired clopidogrel-mediated platelet inhibition in patients with DM with coexisting CKD.11
Prior investigations have shown that the reduced level of platelet P2Y12 inhibition mediated by clopidogrel in patients with DM could be attributed to lower plasma levels of clopidogrel active metabolite (C-AM) compared with patients without DM.12 Moreover, among clopidogrel-treated patients with DM, those with CKD have increased platelet reactivity than those without CKD.11 These latter observations have been suggested to be attributed to increased activity of the platelet P2Y12 receptor signaling pathway.13 However, comprehensive pharmacokinetic (PK) and pharmacodynamic (PD) assessments that would allow a better understanding of the underlying mechanism(s) leading to the enhanced degree of impaired clopidogrel response resulting in increased platelet reactivity among patients with DM with CKD compared with those without are lacking, leading to the design of this prospective investigation.
Methods
Patient population
Patients were screened at the outpatient clinic of the Division of Cardiology–University of Florida College of Medicine Jacksonville. Details on study inclusion and exclusion criteria are provided in the Supplemental Appendix. In brief, patients were eligible for the study if they were ≥18 years of age, had stable ischemic heart disease (SIHD) on low-dose aspirin (81 mg/d) for ≥30 days as part of standard of care, and a diagnosis of type 2 DM. All patients needed to be on treatment with oral hypoglycemic agents and/or insulin for ≥2 months without any changes in their regimen. Key exclusion criteria included any active bleeding, high risk for bleeding, use of an oral P2Y12 receptor inhibitor or an oral anticoagulant in the prior 30 days, clinical indication (eg, recent acute ischemic event) to be on an oral P2Y12 receptor inhibitor, end-stage renal disease on hemodialysis, and known allergies to clopidogrel.
Patients were stratified according to CKD status into patients with CKD and patients without CKD groups. CKD was defined according to the functional definition of the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines (CKD: glomerular filtration rate [GFR] of <60 mL/min/1.73 m2; patients without CKD: GFR of ≥60 mL/min/1.73 m2).14,15 The rationale for considering the functional classification to initially stratify patients is in line with clinical studies showing the increased cardiovascular risk according to GFR strata.11,16,17 The GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation, as suggested by guidelines as the most accurate method to calculate GFR, especially for values in the normal range (>60 mL/min/1.73 m2). In addition to using the Chronic Kidney Disease Epidemiology Collaboration equation to enhance the sensitivity of our assessments evaluating the impact of CKD status on PK/PD profiles being tested, CKD was also classified according to markers of kidney damage, particularly albuminuria.14,15 Albuminuria was evaluated as the albumin-to-creatinine ratio (ACR) expressed in mg/g, which is approximately equivalent to the albumin excretion rate. According to KDIGO guidelines, CKD was defined as an ACR of >30 mg/g and patients without CKD as an ACR of ≤30 mg/g.14,15 The study complied with the Declaration of Helsinki, was approved by the Western Institutional Review Board, and all patients gave written informed consent. The study was registered in Clinicaltrials.gov (NCT03774394).
Study design
This prospective investigation included ex vivo and in vitro experiments in which comprehensive PK and PD assessments were carried out. Eligible patients were administered a 600-mg loading dose (LD) of clopidogrel followed by a single 75-mg maintenance dose (MD) administered after 24 hours. Ex vivo assessments, including PK and PD measurements, were conducted on blood samples collected at a total of 8-time points: baseline (before LD administration) and 30 minutes, 1, 2, 4, 6, and 24 hours after LD before administration of MD (trough), and 2 hours after administration of the 75-mg MD of clopidogrel (peak).
Blood samples collected at baseline (ie, before LD administration) were used for the in vitro experiments. PD testing was performed before and after incubation (for 30 minutes at 37°C) with escalating concentrations of clopidogrel’s active metabolite (C-AM) (1, 3, and 10 μmol/L) to explore the functional status of the P2Y12 signaling pathway.11,12 Daiichi Sankyo Co, Ltd provided C-AM. A flow diagram of the study design is presented in Figure 1.
Figure 1.
Study Design
C-AM = clopidogrel active metabolite; CKD = chronic kidney disease; DM = diabetes mellitus; LD = loading dose; MD = maintenance dose; PD = pharmacodynamic; PK = pharmacokinetic; SIHD = stable ischemic heart disease.
Blood sampling and laboratory assessments
A detailed description of PD and PK assessments is provided in the Supplemental Appendix. In brief, peripheral venous blood samples were drawn through a short venous catheter inserted into a forearm vein and collected in citrate, ethylenediamine tetraacetic acid, and serum tubes as appropriate for assessments. The first 2 to 4 mL of blood were discarded to avoid spontaneous platelet activation. PD assessments were conducted using 3 different assays: 1) whole blood vasodilator-stimulated phosphoprotein (Biocytex Inc.) with results reported as platelet reactivity index (PRI); 2) VerifyNow PRU system with results reported in P2Y12 reaction units (PRU); and 3) light transmission aggregometry (LTA, Chrono-Log Corp). After adenosine diphosphate (ADP) (5 and 20 μmol/L) stimuli with results reported as the maximum platelet aggregation (MPA).18, 19, 20 PK assessments included determination of plasma concentration of clopidogrel and its major active metabolite (R-130964). A commercial laboratory (Q Squared Solutions BioSciences LLC, Inc) blinded to the nature of the samples determined the plasma concentration of clopidogrel and C-AM using liquid chromatography with tandem mass spectrometry, according to standard protocols.12,21 For clopidogrel and its active metabolite (R-130964), the area under the plasma concentration vs time curve (AUC) from time 0 to the last measurable concentration (AUC0-last), maximum plasma concentration (Cmax), and time to Cmax (Tmax) were estimated. Cytochrome P450 2C19 (CYP2C19) genetic polymorphisms (∗1, ∗2, ∗3, and ∗17) allele status were assessed with the Genomadix Cube CYP2C19 system (Genomadix) as previously described.22 Albuminuria was measured on random untimed spot urine samples. Metabolic status and glycemic control were assessed at baseline by measuring fasting plasma glucose, hemoglobin A1c, and lipid profile.
Sample size calculation and study endpoints
The sample size was determined based on assumptions derived for the ex vivo PD component of the study, in particular, the comparison of PRI values at 6 hours after a 600-mg clopidogrel LD between patients with DM with and without CKD (primary endpoint). Assuming a common standard deviation of 10 PRI and an approximately 10% rate of invalid results owing to hemolysis or dropout, we hypothesized to detect an absolute difference of 10% in PRI with 60 patients (30 with CKD and 30 without CKD), with 95% power and a 2-tailed alpha value of 0.05. PRI was chosen in line with prior investigations because it is most specific to define the functional activity of the P2Y12 signaling pathway.12 A cutoff of a 10% absolute change in the PRI was chosen as this has been associated with a 44% relative decrease in thrombotic events in patients undergoing percutaneous cardiac intervention.23 Other exploratory endpoints included PD assessments (PRI, PRU, and MPA) at each time point as part of the ex vivo component of the experimental design; PK assessments (clopidogrel and C-AM plasma concentrations, Tmax, Cmax, and AUC[0-tlast]) as a part of the ex vivo component of the experimental design, and PD assessments (PRI, PRU, and MPA) as a part of the in vitro component of the experimental design. High platelet reactivity (HPR) on treatment, a marker of thrombotic risk, was defined as a PRU of >208, PRI of >50%, LTA-ADP 20 μmol/L of >59%, and 5 μmol/L of >46%, in line with consensus definitions.24 A sensitivity analysis was performed for the ex vivo PD component of the study using ACR to classify CKD.
Statistical analysis
Conformity to the normal distribution was evaluated for continuous variables with the Kolmogorov-Smirnov test. For baseline characteristics, continuous variables are expressed as mean ± SD or median with 25th-75th percentiles (Q1-Q3), unless otherwise specified, and categorical variables are expressed as frequency and percentage. The chi-square or Fisher exact tests (if the expected value in any cell was <5) were used to compare categorical variables between 2 groups, and the Student t-test or Mann-Whitney U test was used to compare continuous variables, where appropriate. A univariate analysis of covariance (ANCOVA) model using a general linear model using the corresponding baseline platelet function value and oral hypoglycemic agents use as covariates was used to calculate the difference between groups at each time point and C-AM dosing. A mixed between-within subjects ANCOVA with polynomial contrast, also adjusted for baseline platelet reactivity and oral hypoglycemic agents use, was conducted with a general linear model to evaluate the overall difference between groups across time points and across C-AM doses. A repeated-measures ANCOVA model, also adjusted by baseline platelet function value and oral hypoglycemic agents use, was used to evaluate the overall difference between groups. ANCOVA models were performed for the previously mentioned analyses in line with other PK/PD studies.12,25,26 In line with prior investigations, given the translational and exploratory nature of the analysis, there was no adjustment for multiple comparisons in the primary endpoint analysis.19,27,28 PD results are reported as least-squares means with 95% CIs. A 2-sided P value of <0.05 indicated a statistically significant difference for superiority for all the analyses performed. Statistical analysis was performed using SPSS version 29.0 software (SPSS Inc). Graphs were plotted with GraphPad Prism version 9.1.0 (Dotmatics).
The safety population included all randomized patients exposed to the study medication. The PD population included all patients with PD data without a major protocol deviation. The PD population was used to analyze all primary and exploratory PK/PD endpoints. The data, analytical methods, and study materials will not be made available to other researchers for the purposes of reproducing the results or replicating the procedure.
Results
Patient Population
Between August 21, 2019, and May 23, 2022, a total of 65 patients provided written informed consent to participate in the study. Of these, 1 screen failed and 3 withdrew informed consent. The remaining 61 patients received at least 1 dose of the medication representing the safety population. Of these, 2 patients withdrew consent after 1 dose of study medication in the CKD group; in patients without CKD, no patients were excluded. Ultimately, 59 patients (CKD, n = 29; non-CKD, n = 30) completed the study and had valid primary endpoint data representing the PD population (Supplemental Figure 1). Patient characteristics were similar between groups, except for hypoglycemic agent use, which was higher in the non-CKD group (P = 0.010), and insulin use, which was higher in the CKD group (P = 0.026) (Table 1). Hemoglobin A1c levels were similar between the CKD and non-CKD groups (7.7 ± 1.4 vs 7.7 ± 1.7; P = 0.91), and there were no differences in the distribution of the CYP2C19 genotype polymorphisms between groups (P = 0.77) (Table 1, Supplemental Table 1).
Table 1.
Baseline Characteristics
| CKD (n = 31) | Patients Without CKD (n = 30) | P Value | |
|---|---|---|---|
| Age, y | 68.4 ± 10.8 | 65.2 ± 6.8 | 0.17 |
| Female | 17 (54.8) | 17 (56.7) | |
| Body mass index, kg/m2 | 34.2 ± 5.8 | 34.1 ± 7.7 | |
| Race | 0.17 | ||
| Black | 9 (29.0) | 15 (50.0) | |
| White | 21 (67.7) | 15 (50.0) | |
| Hispanic | 1 (3.2) | 0 | |
| Current smoking | 3 (9.6) | 3 (10.0) | 1.00 |
| Hypertension | 31 (100.0) | 30 (100.0) | 1.00 |
| Diabetes mellitus | 31 (100.0) | 30 (100.0) | 1.00 |
| Hyperlipidemia | 29 (93.5) | 27 (90.0) | 0.61 |
| Family history of premature CAD | 11 (35.5) | 13 (43.3) | 0.53 |
| PAD | 4 (12.9) | 2 (6.7) | 0.41 |
| Stroke | 5 (16.1) | 2 (6.7) | 0.25 |
| Prior MI | 12 (38.7) | 9 (30.0) | 0.47 |
| Prior PCI | 12 (38.7) | 9 (30.0) | 0.25 |
| Prior CABG | 8 (25.8) | 10 (33.3) | 0.52 |
| Congestive heart failure | 11 (35.5) | 7 (23.3) | 0.30 |
| Left ventricular ejection fraction | 51.9 ± 12.2 | 52.6 ± 12.7 | 0.89 |
| Medications | |||
| ASA | 31 (100.0) | 30 (100.0) | 1.00 |
| Statins | 30 (96.8) | 30 (100.0) | 1.00 |
| Beta-blockers | 28 (90.3) | 24 (80.0) | 0.26 |
| ACE inhibitors or ARB | 22 (71.0) | 20 (69.0) | 0.87 |
| Nitrates | 12 (38.7) | 11 (36.7) | 0.87 |
| Proton pump inhibitors | 9 (29.0) | 11 (36.7) | 0.52 |
| Calcium channel blockers | 15 (48.4) | 15 (50.0) | 1.00 |
| Oral antidiabetic drug | 15 (48.4) | 24 (80.0) | 0.010 |
| Insulin | 18 (60.0) | 9 (31.0) | 0.026 |
| Hemoglobin, g/dL | 12.6 ± 1.9 | 13.1 ± 1.5 | 0.28 |
| Hematocrit, % | 38.5 ± 5.3 | 40.6 ± 4.3 | 0.09 |
| Platelet count, ×103/μL | 264.1 ± 119.4 | 254.1 ± 68.2 | 0.69 |
| Serum creatinine, mg/dL | 1.5 ± 0.4 | 0.9 ± 0.2 | <0.001 |
| eGFR (mL/min/1.73 m2) | 45.6 ± 8.5 | 83.7 ± 14.3 | <0.001 |
| ACR, mg/dL | 34.6 (16.9-69.7) | 18.4 (1.1-25.3) | 0.019 |
| Total cholesterol, mg/dL | 156.0 ± 88.3 | 144.5 ± 39.0 | 0.52 |
| LDL-C, mg/dL | 71.9 ± 34.4 | 71.6 ± 34.3 | 0.97 |
| HDL-C, mg/dL | 42.7 ± 16.8 | 46.8 ± 14.5 | 0.33 |
| Triglycerides | 133.5 (76.0-193.0) | 114.0 (70.0-175.0) | 0.52 |
| Fasting glucose | 142.0 (100.0-188.0) | 138.5 (114.0-167.0) | 0.42 |
| HbA1c, % | 7.7 ± 1.4 | 7.7 ±.17 | 0.91 |
| CYP2C19 genetics | |||
| No LOF | 20 (66.6) | 20 (66.6) | |
| Heterozygous LOF | 9 (30.0) | 9 (30.0) | |
| Homozygous LOF | 0 | 1 (3.33) |
Values are mean ± SD, n (%), or median (25th-75th percentiles).
ACE = angiotensin-converting enzyme; ACR = albumin-to-creatinine ratio; ARB = angiotensin receptor blocker; ASA = aspirin; CABG = coronary artery bypass graft; CAD = coronary artery disease; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; HbA1c = hemoglobin A1c; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; LOF = ; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PAD = peripheral artery disease; PCI = percutaneous coronary intervention; PPI = proton pump inhibitor.
Ex vivo PD assessments
PRI levels were overall very high across study time points. Although the PRI was numerically higher in patients with CKD vs patients without CKD at 1 and 2 hours after the LD, there were no significant differences in PRI levels during the 6 hours after a 600-mg LD of clopidogrel between patients with CKD and patients without CKD in the unadjusted analysis (P = 0.87) as well as after adjusting for baseline PRI values and oral hypoglycemic agents (P = 0.49) (Figure 2A). At 6 hours (primary endpoint), there were no significant differences in PRI levels between patients with CKD or without CKD, both in the unadjusted (P = 0.96) and adjusted (CKD: 69.7 [95% CI: 58.9-80.4] vs non-CKD: 64.9 [95% CI: 54.3-75.7]; P = 0.55) analyses (Figure 2A). No significant interaction was observed in the primary endpoint analysis based on CKD and CYP2C19 genetic polymorphism (Pinteraction = 0.56). There were no differences in the PRI between groups at 24 hours after the LD as assessed with trough levels in either the unadjusted and adjusted analyses (CKD: 71.8 [95% CI: 60.7-82.9] vs non-CKD: 69.4 [95% CI: 59.1-79.8]), as well as peak levels (2 hours after a 75-mg MD of clopidogrel; CKD: 70.8 [95% CI: 59.2-82.3] vs non-CKD: 66.9 [95% CI: 56.2-77.7]). HPR rates were overall very high and similar between groups (Supplemental Table 2).
Figure 2.
Ex Vivo Pharmacodynamic Assessment After Clopidogrel LD and MD of Clopidogrel
(A) Platelet reactivity index measured by the vasodilator-stimulated phosphoprotein assay. (B) Platelet aggregation measured by light transmission aggregometry after stimulation with 20 μmol/L ADP. (C) P2Y12 reaction units measured by the VerifyNow-P2Y12 assay. ANCOVA method was used to generate the curves. ∗Adjusted for baseline platelet reactivity and oral hypoglycemic agents and analyzed from 0 to 6 hours after 600-mg LD of clopidogrel. †P < 0.05; adjusted for baseline platelet reactivity and oral hypoglycemic agents. Values are expressed as least-squares means and error bars indicate 95% CIs. ADP = adenosine diphosphate; ANCOVA = analysis of covariance; other abbreviations as in Figure 1.
Platelet reactivity as reported by MPA using LTA with ADP 20 μmol/L was significantly higher in patients with CKD than patients without CKD during the 6 hours after the LD both in the unadjusted (P = 0.004) and adjusted (P = 0.007) analyses (Figure 2B). At 6 hours, there were no significant differences in MPA levels between patients with CKD or without CKD, either in the unadjusted (P = 0.37) or adjusted (CKD: 41.8 [95% CI: 34.1-49.6] vs non-CKD: 36.4 [95% CI: 28.8-44.0]; P = 0.34) analyses. There were no differences in MPA between groups at 24 hours after LD at trough (adjusted analysis, CKD: 47.5 [95% CI: 39.9-55.1] vs non-CKD: 43.9 [95% CI: 36.9-51.0]) and peak (adjusted analysis, CKD: 43.3 [95% CI: 35.9-50.7] vs non-CKD: 39.2 [95% CI: 32.4-46.1]). MPA assessed by LTA with 5 μmol/L ADP showed consistent findings with 20 μmol/L ADP (Supplemental Figure 2). There were significantly higher rates of HPR in the CKD group compared with the group without CKD at 1 hour with an LTA-ADP of 20 μmol/L (Supplemental Table 3) and at 30 minutes and 1 hour with an LTA-ADP of 5 μmol/L (Supplemental Table 4).
Platelet reactivity according to VerifyNow PRU was numerically increased in patients with CKD vs without CKD across the first 6 hours after the LD. However, there were no significant differences in PRU levels between patients with CKD and patients without CKD in the unadjusted analysis (P = 0.18) or after adjustment (P = 0.65) (Figure 2C). At 6 hours, there were no significant differences in PRU levels between patients with CKD or without CKD, both in the unadjusted (P = 0.76) and adjusted (CKD: 163.4 [95% CI: 129.4-197.3] vs non-CKD: 156.7 [95% CI: 122.8-190.7]; P = 0.79) analyses. There were no differences in PRU between groups at 24 hours after LD at trough (adjusted analysis, CKD: 140.2 [95% CI: 105.7-174.7] vs non-CKD: 136.2 [95% CI: 104.2-168.2]) and peak (adjusted analysis, CKD: 132.3 [95% CI: 99.3-165.4] vs non-CKD: 131.0 [95% CI: 100.4-161.6]). There was a significantly higher rate of HPR in the CKD group compared with the non-CKD group at 1 hour (Supplemental Table 5).
When CKD was defined according to ACR, there were no differences in platelet reactivity between patients with CKD and patients without CKD according to PRI (Supplemental Figure 3), MPA with LTA using ADP 20 μmol/L (Supplemental Figure 4) and 5 μmol/L (Supplemental Figure 5), and PRU (Supplemental Figure 6).
PK assessments
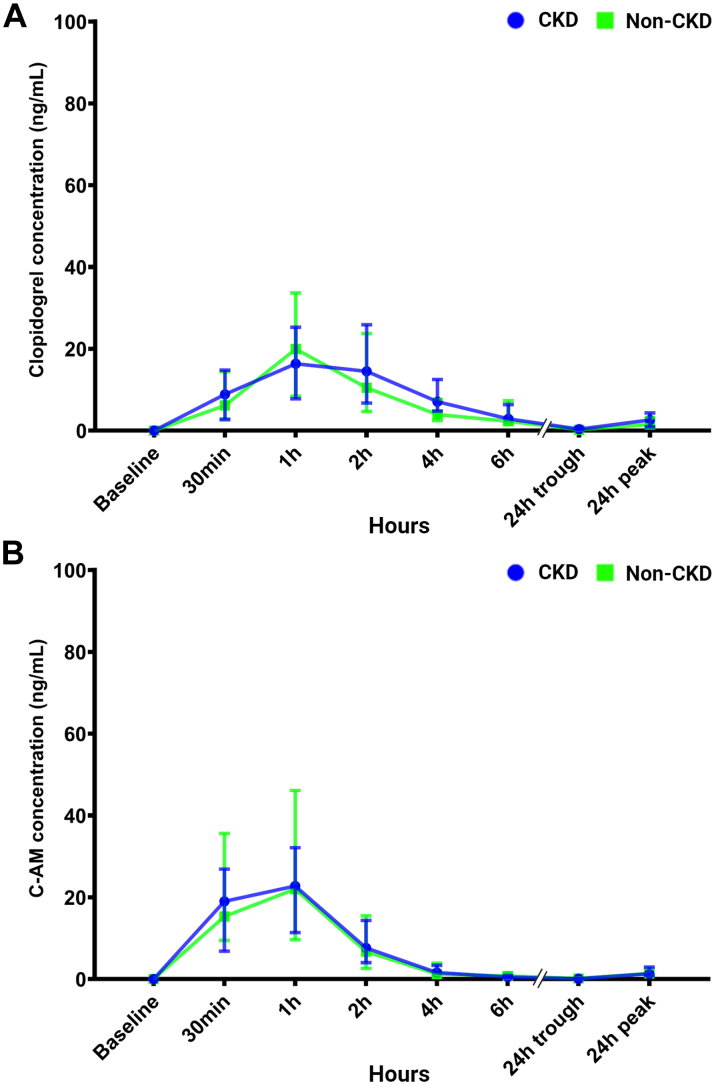
After the 600-mg LD and 75-mg MD of clopidogrel, plasma levels of clopidogrel and C-AM were similar between patients with CKD and patients without CKD throughout the 24-hour time course (Figures 3A and 3B). During the 6 hours after a 600-mg LD of clopidogrel, there were no differences in exposure to clopidogrel and C-AM between patients with CKD and patients without CKD (Table 2). There were no differences between the geometric mean (range) for clopidogrel AUC[0-tlast] (58.0 [95% CI: 40.3-100.8] ng·h/mL vs 45.1 [95% CI: 24.3-100.8] ng·h/mL; P = 0.25) and C-AM AUC[0-tlast] (47.1 [95% CI: 32.8-62.7] ng·h/mL vs 39.6 [95% CI: 19.8-85.8] ng·h/mL; P = 0.65) between CKD and patients without CKD. There were no significant differences when clopidogrel and C-AM levels analyses were adjusted according to CYP2C19 genotypes (P = 0.42 and P = 0.54, respectively). When CKD was defined according to ACR, clopidogrel and C-AM levels were similar between patients with CKD and patients without CKD (Supplemental Figure 7), and there were no significant differences in any of the assessed PK parameters (Supplemental Table 6).
Figure 3.
Plasma Concentrations of Clopidogrel and C-AM
(A) Plasma levels of clopidogrel and (B) C-AM during the 24 hours after a 600-mg LD and 75-mg MDs of clopidogrel. Values are expressed as medians and error bars indicate 25th and 75th percentiles. Abbreviations as in Figure 1.
Table 2.
Pharmacokinetic Profiles of Clopidogrel and C-AM According to CKD Status
| CKD | Patients Without CKD | P Value | |
|---|---|---|---|
| Clopidogrel | |||
| Tmax, h | 1.0 (1.0-2.0) | 1.0 (1.0-2.0) | 0.99 |
| Cmax, (ng/mL) | 21.3 (13.7-35.2) | 21.0 (11.1-33.8) | 0.88 |
| AUC0-last (ng·h/mL) | 58.0 (40.3-100.8) | 45.1 (24.7-93.7) | 0.25 |
| C-AM | |||
| Tmax, h | 1.0 (0.5-1.0) | 1.0 (0.5-1.0) | 0.49 |
| Cmax, (ng/mL) | 26.9 (19.1-31.4) | 22.6 (14.4-48.6) | 0.99 |
| AUC0-last (ng·h/mL) | 47.1 (32.8-62.7) | 39.6 (19.8-85.8) | 0.65 |
Values are median (25th-75th percentiles). Analyses done after a 600-mg loading dose from 0 to 6 hours. Tmax is reported as median (range). Cmax and AUC0-last are reported as geometric mean (range).
AUC0-last = area under the plasma concentration vs time curve from time 0 to the last measurable concentration; Cmax = maximum observed plasma concentration; Tmax = time to maximum observed plasma concentration; other abbreviations as in Table 1.
In vitro PD assessments
In vitro incubation of blood samples collected at baseline with escalating concentrations of C-AM showed a non-significant difference in PRI between CKD and patients without CKD in the unadjusted analysis (P = 0.69) but significantly higher PRI levels in CKD compared with patients without CKD in the adjusted analysis (P = 0.005) (Figure 4A). There were no differences in MPA according to LTA with 20 μmol/L ADP in either the unadjusted (P = 0.340) or adjusted (P = 0.922) analyses (Figure 4B). Patients with CKD had marginally higher MPA compared with patients without CKD according to LTA with 5 μmol/L ADP in the adjusted analysis (P = 0.038) but not in the unadjusted analysis (P = 0.11) (Supplemental Figure 8). There were no differences in PRU between patients with CKD and patients without CKD in both the unadjusted (P = 0.076) and the adjusted (P = 0.267) analyses (Figure 4C).
Figure 4.
In Vitro Pharmacodynamic Assessments After Incubation With Escalating Concentrations of C-AM
(A) Platelet reactivity index measured by the vasodilator-stimulated phosphoprotein assay. (B) Platelet aggregation measured by light transmission aggregometry after stimulation with 20 μmol/L ADP. (C) P2Y12 reaction units measured by the VerifyNow-P2Y12 assay. ∗Adjusted for baseline platelet reactivity and oral hypoglycemic agents, P values indicate the overall differences between groups assessed by repeated-measures ANCOVA. †P < 0.05, adjusted for baseline platelet reactivity and oral hypoglycemic agents. ††P < 0.01, adjusted for baseline platelet reactivity and oral hypoglycemic agents. Values are expressed as least-squares means and error bars indicate 95% CIs. Abbreviations as in Figures 1 and 2.
Discussion
This investigation was designed to identify potential mechanism(s) associated with impaired clopidogrel-mediated platelet P2Y12 inhibitory effects in patients with DM stratified according to the presence or absence of CKD. The study design included both ex vivo and in vitro experiments with comprehensive PD and PK assessments. The key observations from our study can be summarized as follows: 1) after clopidogrel LD administration, MPA during the first 6 hours, but not at 24 hours, was higher in patients with DM with CKD than those without, but no significant differences were observed in PRI and PRU levels, albeit there were numerical differences between groups following the same trend; 2) no differences were observed in plasma levels of clopidogrel (ie, indicative of drug absorption) and C-AM (ie, indicative of drug metabolism) between patients with and without CKD; 3) in vitro incubation with escalating concentrations of C-AM showed impaired inhibition of PRI, but not MPA and PRU, in patients with CKD compared with those without; and 4) finally, consistent results were found using alternative definitions of CKD, such as urine ACR.
Clopidogrel is the most broadly used oral platelet P2Y12 inhibitor and is recommended to prevent ischemic events in patients with atherosclerotic disease.1 Although most commonly used as an adjunct to low-dose aspirin (ie, dual antiplatelet therapy) in high-risk settings such as after an acute ischemic event or percutaneous cardiac intervention, clopidogrel monotherapy may also be used for long-term secondary prevention in patients with stable atherosclerotic disease.1,29,30 PD investigations have consistently shown that patients with DM have impaired platelet inhibitory response to clopidogrel resulting in higher rates of on-treatment HPR, a marker of thrombotic risk, compared with patient without DM.2, 3, 4, 5, 6, 7,12,24 Clinically, this is manifested as an increased risk for on-treatment atherothrombotic events (ie, myocardial infarction, stent thrombosis, stroke, or acute limb ischemia) in patients with DM compared with those without.4,10 Importantly, DM is a key determinant for deterioration of renal function, and patients with concomitant CKD have an enhanced risk of recurrent atherothrombotic events.8,31 PD studies have also shown patients with CKD to have impaired clopidogrel-mediated platelet inhibition, albeit with inconsistent findings and potentially dependent on whether patients also have DM.11,31, 32, 33, 34 These findings suggest that, when DM and CKD coexist, there is an interaction as reflected in both ex vivo PD experiments and clinical outcomes studies showing that patients with DM with CKD have an enhanced degree of impaired clopidogrel-mediated inhibition and a higher risk of recurrent atherothrombotic events, compared with those without CKD.8,10
The presence of lower levels of C-AM in DM compared with patients without DM has been identified as a key factor of their reduced clopidogrel-mediated platelet inhibitory effects and elevated rates of HPR.12 In vitro experiments suggest that patients with DM who also have CKD may also be affected by upregulation of the P2Y12 receptor signaling pathway.13 However, these previous preliminary findings have yet to be validated in a dedicated investigation specifically designed in patients with DM stratified according to CKD status in which detailed experiments, both ex vivo and in vitro, with comprehensive PK and PD assessments, are carried out. Hence, the design and conduct of the current investigation.
Our study showed that, during the first 6 hours, platelet reactivity assessed ex vivo by MPA using LTA after ADP (5 and 20 μmol/L) is increased in patients with CKD compared with those without. This finding is consistent with a prior observational PD investigation.13 However, PRI and PRU levels were not affected significantly by CKD status. Both whole blood vasodilator-stimulated phosphoprotein PRI and VerifyNow-PRU assays have in common that, in addition to ADP, they also use prostaglandin E1, which is a suppressor of intracellular free calcium levels for diminishing the nonspecific stimulation of the ADP-binding to P2Y1 receptors.35,36 This factor allows these assays to be more specific to P2Y12 signaling and less influenced by the contribution of the P2Y1 ADP receptor. LTA-MPA using ADP stimuli is more reflective of overall purinergic signaling, which can also be modulated by other determinants (eg, lipid plasma, hemolysis, platelet count) compared with other platelet function tests, particularly when used for ex vivo testing.34,35 It is, however, important to note that the differences in MPA were no longer present at 24 hours when patients are on maintenance clopidogrel 75 mg therapy, questioning the long-term clinical implications of our study observations. Our in vitro findings showed that incubation with escalating doses of C-AM was associated with impaired inhibition of PRI, but not MPA and PRU, levels. More specifically, compared with those without CKD, platelets from patients with CKD exhibit higher PRI despite being incubated with the same concentration of C-AM, suggesting some degree of resistance to P2Y12 receptor inhibition. Overall, these findings suggest that, among patients with DM, those with CKD exhibit higher platelet aggregation, which can only in part be explained by increased activity of the P2Y12 signaling pathway, possibly with other factors contributing to these observations. These observations align with a prior study suggesting an upregulation of the P2Y12 receptor signaling pathway in patients with concomitant DM and CKD.13 However, in that preliminary study, patients with DM and CKD had higher levels of PRU compared with those without CKD, similar to the unadjusted analysis of the present investigation.
Although a prior investigation showed that patients with DM have lower plasma levels of C-AM than those without DM, plasma levels of clopidogrel were not assessed, not allowing to fully ascertain if this was attributed to impaired absorption or metabolism.12 Moreover, the impact of CKD, known to affect the absorption and metabolism of various drugs, was not assessed in prior investigations specific to understanding the mechanism(s) of impaired clopidogrel-mediated antiplatelet effects in patients with DM.12 Our comprehensive PK assessments, including Tmax, Cmax, and AUC0-last, of clopidogrel and C-AM plasma levels at 8 time points before and after clopidogrel exposure showed no differences between patients with and without CKD, ruling out that the differences observed in platelet aggregation between these groups could be attributed to impaired drug absorption or metabolism. The absence of differences between patients with and without CKD in glycemic control, which can potentially increase platelet reactivity, as well as any imbalance in CYP2C19 genetic polymorphisms associated with impaired clopidogrel metabolism, suggest that other mechanisms may be associated with the increased platelet aggregation of clopidogrel-treated patients with concomitant DM and CKD.
Platelets from patients with DM exhibit upregulation of P2Y12 receptor signaling.37 Chronic hyperglycemia increases intracellular reactive oxygen species and nuclear factor-κβ pathway activation, which can upregulate P2Y12 expression, increase platelet reactivity, and promote constitutive activation (ie, activation of the receptor despite absence of the agonist).37 A prior study has suggested that constant exposure to higher levels of dinucleoside polyphosphates, which can act as agonists of purinergic signaling, are associated with an upregulation of P2Y12 pathway signaling.38 In patients with concomitant DM and CKD, these mechanisms can lead to an enhanced upregulation of P2Y12 pathway signaling compared with patients with DM without CKD. These considerations may explain the increased platelet aggregation in patients with concomitant DM and CKD in our study and prior assessments.11,13 These observations could also explain the enhanced ischemic benefit of potent P2Y12 inhibitors in patients with concomitant DM and CKD compared with patients with only 1 or none of these risk factors.10,39
Ultimately, the main results of the study were confirmed by means of an alternative definition of CKD. Urine ACR, a common endpoint in CKD clinical trials, was chosen as it is complementary to the GFR definition, as together with the presence and duration of DM, it can establish the diagnosis of kidney disease related to DM without needing a biopsy.14,15 Moreover, the American Diabetes Association and KDIGO propose albuminuria (ie, urine ACR ≥30 mg/g) as an indicator of disease control in patients with CKD and DM to reduce CKD progression and cardiovascular events.14,15 Overall, the PK/PD profile results were consistent when CKD was defined according to ACR, showing no significant differences in the primary or exploratory endpoints, underscoring the robustness of our study results.
Study limitations
The PK/PD nature of this investigation does not allow for drawing any definitive conclusions on the clinical implications of the observed findings. Our study was conducted in patients with SIHD, and whether results can be extrapolated to patients with an acute coronary event characterized by a hyperreactive platelet phenotype and who are more susceptible to absorption and metabolism abnormalities requires dedicated investigation.40 Although our investigation is the most comprehensive to date exploring the mechanisms associated with differences in clopidogrel response profiles in patients with DM with and without CKD, the complex nature of the experiments limited our study to a relatively small number of patients, which could have resulted in it being underpowered for some of the assessments, albeit powered in our study assumptions based on available data.12 Indeed, the inherent differences between patients with and without CKD indicate that additional confounders, other than those already accounted for in our statistical adjustments, may emerge in a larger study. Also, the potential mechanism(s) by which CKD status can lead to increased activity of the platelet P2Y12 signaling pathway in patients with DM (ie, either increased receptor expression or levels of dinucleoside polyphosphates) requires further research. Ultimately, although in vitro incubation with escalating concentrations of C-AM showed impaired inhibition of PRI in patients with DM with CKD compared with those without, suggesting the presence of some degree of upregulation of P2Y12 activity, it cannot be ruled out that these observations be attributed to the presence of alternative platelet ADP receptors (eg, P2Y13 or P2Y14) or weaker prostaglandin E1 stimulated Gs signaling without any changes in P2Y12 activity.
Conclusions
Among patients with DM and SIHD, after a 600-mg LD of clopidogrel, those with CKD had higher MPA, but not PRI or PRU, than patients without CKD. These findings can be attributed partly to increased activity of the platelet P2Y12 signaling pathway, but not by apparent differences in clopidogrel absorption or metabolism. Further research is warranted to define the mechanisms by which CKD status impacts the functional status of the platelet P2Y12 signaling pathway in patients with DM.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with DM exhibit impaired platelet inhibition in response to clopidogrel compared with those without DM, contributing to their increased risk of atherothrombotic events. In particular, clinical studies have shown a gradient of risk according to the presence or absence of DM and CKD, with patients having both risk factors at the highest risk. The underlying biological mechanism(s) explaining these clinical findings are poorly understood. Notably, a better understanding of these mechanisms could potentially lead to targeted antiplatelet therapy in patients with DM.
TRANSLATIONAL OUTLOOK: Our current investigation demonstrates that, among patients with DM and SIHD treated with a 600-mg LD of clopidogrel, those with CKD had higher maximal platelet aggregation than patients without CKD, without differences in the PRI and PRU levels. These findings could be attributed partly to increased activity of the platelet P2Y12 signaling pathway, but not differences in drug absorption or metabolism; there were no differences in the PK profiles of clopidogrel and its active metabolite according to the presence or absence of CKD. Future research should focus on directly determining the P2Y12 signaling pathway status to confirm if its upregulation can explain the overall differences in platelet aggregation. Ultimately, the clinical implications of these findings regarding the selection of optimal antiplatelet therapy for patients with DM remain to be determined.
Funding Support and Author Disclosures
This study was funded by an investigator-initiated grant from the University of Florida and the Scott R. MacKenzie Foundation. The Scott R. MacKenzie Foundation had no role in the study design conception, conduct of the study, or decision to publish these results. Work by Drs Cavallari, Angiolillo, and Franchi is supported by the National Institutes of Health National Heart, Lung, and Blood Institute (1R01HL149752). Dr Franchi has received payment as an individual for consulting fee or honoraria from AstraZeneca, Bayer and Sanofi; and institutional payments for grants from PLx Pharma and The Scott R. MacKenzie Foundation. Dr Galli has received consulting fees or honoraria from Terumo, outside the present work. Dr Cavallari has received research support from Accriva Diagnostics. Dr Angiolillo has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Daiichi-Sankyo, Eli Lilly, Faraday, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura; h and his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Faraday, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co. Merck, Novartis, and the Scott R. MacKenzie Foundation. Dr Jakubowski was an employee of Eli Lilly and Company at the time of the studies. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section and supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Angiolillo D.J., Galli M., Collet J.P., Kastrati A., O'Donoghue M.L. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention. 2022;17:e1371–e1396. doi: 10.4244/EIJ-D-21-00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angiolillo D.J., Fernandez-Ortiz A., Bernardo E., et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2435. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 3.Angiolillo D.J., Bernardo E., Sabate M., et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2007;50:1541–1547. doi: 10.1016/j.jacc.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 4.Ferreiro J.L., Angiolillo D.J. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123:798–813. doi: 10.1161/CIRCULATIONAHA.109.913376. [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo D.J., Capodanno D., Danchin N., et al. Derivation, validation, and prognostic utility of a prediction rule for nonresponse to clopidogrel: the ABCD-GENE score. JACC Cardiovasc Interv. 2020;13:606–617. doi: 10.1016/j.jcin.2020.01.226. [DOI] [PubMed] [Google Scholar]
- 6.Mangiacapra F., Bressi E., Colaiori I., et al. Interaction between diabetes mellitus and platelet reactivity in determining long-term outcomes following percutaneous coronary intervention. J Cardiovasc Transl Res. 2020;13:668–675. doi: 10.1007/s12265-019-09931-z. [DOI] [PubMed] [Google Scholar]
- 7.Shahim B., Redfors B., Stuckey T.D., et al. On-treatment platelet reactivity and ischemic outcomes in patients with diabetes mellitus: two-year results from ADAPT-DES. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.026482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales J., Handelsman Y. Cardiovascular outcomes in patients with diabetes and kidney disease: JACC review topic of the week. j Am Coll Cardiol. 2023;82:161–170. doi: 10.1016/j.jacc.2023.04.052. [DOI] [PubMed] [Google Scholar]
- 9.Ndumele C.E., Rangaswami J., Chow S.L., et al. Cardiovascular-kidney-metabolic health: a presidential advisory from the american heart association. Circulation. 2023;148:1606–1635. doi: 10.1161/CIR.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 10.Franchi F., James S.K., Ghukasyan Lakic T., et al. Impact of diabetes mellitus and chronic kidney disease on cardiovascular outcomes and platelet P2Y(12) receptor antagonist effects in patients with acute coronary syndromes: insights from the PLATO trial. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angiolillo D.J., Bernardo E., Capodanno D., et al. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. doi: 10.1016/j.jacc.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 12.Angiolillo D.J., Jakubowski J.A., Ferreiro J.L., et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64:1005–1014. doi: 10.1016/j.jacc.2014.06.1170. [DOI] [PubMed] [Google Scholar]
- 13.Engwenyu L.R., Franchi F., Rollini F., et al. Impact of chronic kidney disease on platelet P2Y(12) receptor signalling in patients with type 2 diabetes mellitus. Thromb Haemost. 2017;117:201–203. doi: 10.1160/TH16-08-0594. [DOI] [PubMed] [Google Scholar]
- 14.Inker L.A., Astor B.C., Fox C.H., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 15.Levey A.S., Becker C., Inker L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313:837–846. doi: 10.1001/jama.2015.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Best P.J., Steinhubl S.R., Berger P.B., et al. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2008;155:687–693. doi: 10.1016/j.ahj.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 17.Dasgupta A., Steinhubl S.R., Bhatt D.L., et al. Clinical outcomes of patients with diabetic nephropathy randomized to clopidogrel plus aspirin versus aspirin alone (a post hoc analysis of the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance [CHARISMA] trial) Am J Cardiol. 2009;103:1359–1363. doi: 10.1016/j.amjcard.2009.01.342. [DOI] [PubMed] [Google Scholar]
- 18.Franchi F., Rollini F., Rivas Rios J., et al. Pharmacodynamic effects of switching from ticagrelor to clopidogrel in patients with coronary artery disease: results of the SWAP-4 study. Circulation. 2018;137:2450–2462. doi: 10.1161/CIRCULATIONAHA.118.033983. [DOI] [PubMed] [Google Scholar]
- 19.Franchi F., Rollini F., Rivas A., et al. Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. 2019;139:1661–1670. doi: 10.1161/CIRCULATIONAHA.118.038317. [DOI] [PubMed] [Google Scholar]
- 20.Franchi F., Rollini F., Aggarwal N., et al. Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease: the OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus)-4 study. Circulation. 2016;134:780–792. doi: 10.1161/CIRCULATIONAHA.116.023402. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M., Pang H., Kawabata K., Farid N.A., Kurihara A. Quantitative determination of clopidogrel active metabolite in human plasma by LC-MS/MS. J Pharm Biomed Anal. 2008;48:1219–1224. doi: 10.1016/j.jpba.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Franchi F., Rollini F., Rivas J., et al. Prasugrel versus ticagrelor in patients with CYP2C19 loss-of-function genotypes: results of a randomized pharmacodynamic study in a feasibility investigation of rapid genetic testing. JACC Basic Transl Sci. 2020;5:419–428. doi: 10.1016/j.jacbts.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonello L., Mancini J., Pansieri M., et al. Relationship between post-treatment platelet reactivity and ischemic and bleeding events at 1-year follow-up in patients receiving prasugrel. J Thromb Haemost. 2012;10:1999–2005. doi: 10.1111/j.1538-7836.2012.04875.x. [DOI] [PubMed] [Google Scholar]
- 24.Sibbing D., Aradi D., Alexopoulos D., et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y(12) receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1521–1537. doi: 10.1016/j.jcin.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Wallentin L., Varenhorst C., James S., et al. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008;29:21–30. doi: 10.1093/eurheartj/ehm545. [DOI] [PubMed] [Google Scholar]
- 26.Wiviott S.D., Trenk D., Frelinger A.L., et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116:2923–2932. doi: 10.1161/CIRCULATIONAHA.107.740324. [DOI] [PubMed] [Google Scholar]
- 27.Lancaster G.A., Dodd S., Williamson P.R. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 28.Rollini F., Franchi F., Hu J., et al. Crushed prasugrel tablets in patients with STEMI undergoing primary percutaneous coronary intervention: the CRUSH study. J Am Coll Cardiol. 2016;67:1994–2004. doi: 10.1016/j.jacc.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 29.Lawton J.S., Tamis-Holland J.E., Bangalore S., et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(2):e21–e129. doi: 10.1016/j.jacc.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Knuuti J., Wijns W., Saraste A., et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 31.Bonello L., Angiolillo D.J., Aradi D., Sibbing D. P2Y(12)-ADP receptor blockade in chronic kidney disease patients with acute coronary syndromes. Circulation. 2018;138:1582–1596. doi: 10.1161/CIRCULATIONAHA.118.032078. [DOI] [PubMed] [Google Scholar]
- 32.Tello-Montoliu A., Ferreiro J.L., Kodali M.K., et al. Impact of renal function on clopidogrel-induced antiplatelet effects in coronary artery disease patients without diabetes mellitus. J Thromb Thrombolysis. 2013;36:14–17. doi: 10.1007/s11239-012-0828-1. [DOI] [PubMed] [Google Scholar]
- 33.Baber U., Mehran R., Kirtane A.J., et al. Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents registry. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.115.001683. [DOI] [PubMed] [Google Scholar]
- 34.Franchi F., Rollini F., Angiolillo D.J. Defining the link between chronic kidney disease, high platelet reactivity, and clinical outcomes in clopidogrel-treated patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.115.002760. [DOI] [PubMed] [Google Scholar]
- 35.Angiolillo D.J., Been L., Rubinstein M., Martin M., Rollini F., Franchi F. Use of the VerifyNow point of care assay to assess the pharmacodynamic effects of loading and maintenance dose regimens of prasugrel and ticagrelor. J Thromb Thrombolysis. 2021;51:741–747. doi: 10.1007/s11239-021-02386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franchi F., Rollini F., Cho J.R., Ferrante E., Angiolillo D.J. Platelet function testing in contemporary clinical and interventional practice. Curr Treat Options Cardiovasc Med. 2014;16:300. doi: 10.1007/s11936-014-0300-y. [DOI] [PubMed] [Google Scholar]
- 37.Hu L., Chang L., Zhang Y., et al. Platelets express activated P2Y(12) receptor in patients with diabetes mellitus. Circulation. 2017;136:817–833. doi: 10.1161/CIRCULATIONAHA.116.026995. [DOI] [PubMed] [Google Scholar]
- 38.Chang H., Yanachkov I.B., Michelson A.D., et al. Agonist and antagonist effects of diadenosine tetraphosphate, a platelet dense granule constituent, on platelet P2Y1, P2Y12 and P2X1 receptors. Thromb Res. 2010;125:159–165. doi: 10.1016/j.thromres.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franchi F., Rollini F., Been L., et al. Impact of chronic kidney disease on the pharmacodynamic and pharmacokinetic effects of ticagrelor in patients with diabetes mellitus and coronary artery disease. Eur Heart J Cardiovasc Pharmacother. 2022;8:452–461. doi: 10.1093/ehjcvp/pvab042. [DOI] [PubMed] [Google Scholar]
- 40.Heestermans A.A., van Werkum J.W., Taubert D., et al. Impaired bioavailability of clopidogrel in patients with a ST-segment elevation myocardial infarction. Thromb Res. 2008;122:776–781. doi: 10.1016/j.thromres.2008.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.