ABSTRACT
The human immunodeficiency virus type 1 (HIV-1) reservoir consists of latently infected cells which present a major obstacle to achieving a functional cure for HIV-1. The formation and maintenance of HIV-1 latency have been extensively studied, and latency-reversing agents (LRAs) that can reactivate latent HIV-1 by targeting the involved host factors are developed; however, their clinical efficacies remain unsatisfactory. Therefore, it is imperative to identify novel targets for more potential candidates or better combinations for LRAs. In this study, we utilized CRISPR affinity purification in situ of regulatory elements system to screen for host factors associated with the HIV-1 long terminal repeat region that could potentially be involved in HIV-1 latency. We successfully identified that origin recognition complex 1 (ORC1), the largest subunit of the origin recognition complex, contributes to HIV-1 latency in addition to its function in DNA replication initiation. Notably, ORC1 is enriched on the HIV-1 promoter and recruits a series of repressive epigenetic elements, including DNMT1 and HDAC1/2, and histone modifiers, such as H3K9me3 and H3K27me3, thereby facilitating the establishment and maintenance of HIV-1 latency. Moreover, the reactivation of latent HIV-1 through ORC1 depletion has been confirmed across various latency cell models and primary CD4+ T cells from people living with HIV-1. Additionally, we comprehensively validated the properties of liquid-liquid phase separation (LLPS) of ORC1 from multiple perspectives and identified the key regions that promote the formation of LLPS. This property is important for the recruitment of ORC1 to the HIV-1 promoter. Collectively, these findings highlight ORC1 as a potential novel target implicated in HIV-1 latency and position it as a promising candidate for the development of novel LRAs.
IMPORTANCE
Identifying host factors involved in maintaining human immunodeficiency virus type 1 (HIV-1) latency and understanding their mechanisms prepares the groundwork to discover novel targets for HIV-1 latent infection and provides further options for the selection of latency-reversing agents in the “shock” strategy. In this study, we identified a novel role of the DNA replication factor origin recognition complex 1 (ORC1) in maintaining repressive chromatin structures surrounding the HIV-1 promoter region, thereby contributing to HIV-1 latency. This discovery expands our understanding of the non-replicative functions of the ORC complex and provides a potential therapeutic strategy for HIV-1 cure.
KEYWORDS: HIV-1 latency, ORC1, LTR-associated factors, repressive epigenetic modifications, LLPS
INTRODUCTION
Due to the persistence of the human immunodeficiency virus type 1 (HIV-1) latent reservoir, HIV-1-infected individuals must receive combination anti-retroviral therapy (cART) for their entire lifetime (1–3). When cART is interrupted, the viruses harboring replication-competent proviruses within this latent reservoir will rebound rapidly, leading to viremia (4). The thorough eradication of HIV-1 reservoir is challenging. However, it is worth noting that most integrated proviruses are defective (5). Only 1–10 of every 106 resting CD4+ T cells contain intact and replication-competent proviruses (6, 7), some of which are also clonally expanded (8, 9). Although some cases of complete HIV-1 cure using stem cell transplantation have been reported in recent years, the stringent conditions required for this technique make it challenging to achieve universal accessibility (10–13). The concept of a functional cure rather than pursuing complete eradication of HIV-1 reservoir is gaining traction (14). The functional cure aims to minimize the size of the HIV-1 reservoir, with the “shock and kill” therapeutic strategy being the most extensively employed method; however, its clinical efficacy is not ideal (15). Therefore, it is imperative to elucidate the molecular mechanisms involved in the establishment and maintenance of HIV-1 latency in vivo, particularly from the perspective of epigenetic modulation.
Epigenetic modulation is extensively involved in the establishment and maintenance of HIV-1 latency. HIV-1 5′ long terminal repeat (LTR) is occupied by two precisely positioned nucleosomes, Nuc-0 and Nuc-1, whose remodeling and modifications directly impact HIV-1 latency (16). Histone deacetylase HDAC1 and HDAC2 can remove the active acetyl groups from histone 3 lysine 9 (H3K9) and H3K27, thus mediating hypoacetylation of the HIV-1 promoter (17). Repressive histone modifications of H3K9, H3K27, and H4K20, such as H3K9me2/3, H3K27me3, and H4K20me, which are catalyzed by G9a, Suv39h1, EZH2, and SMYD2, play a significant role in contributing to HIV-1 latency (18–21). Furthermore, a variety of proteins, including HP1, CBX4, and L3MBTL1, which recognize and maintain these repressive histone modifications, are also associated with HIV-1 latency (19, 20, 22, 23). HIV-1 promoter region also contains two conserved CpG islands that form DNA methylation sites in the latent state which are modified by DNMT1 and maintained by MBD2 (24–26).
In this study, we demonstrated that origin recognition complex 1 (ORC1), the largest subunit of the origin recognition complex, acts as a heterochromatin maintenance factor. ORC1 was found to recruit many repressive epigenetic proteins, including DNMT1, HDAC1/2, and modifiers of H3K9me3 and H3K27me3, leading to the enforcement of repressive epigenetic modifications on the HIV-1 LTR region, thereby promoting HIV-1 latency. Our findings suggest that ORC1 could act as a potential target for latency-reversing agents (LRAs) to achieve a functional cure for HIV-1.
RESULTS
ORC1 is identified as a potential host factor associated with the HIV-1 promoter
Utilizing RNA interference and single-guide (sgRNA) libraries to silence unidentified targets in HIV-1 latency models, combined with high-throughput sequencing techniques to identify differentially expressed genes, has become a widely adopted approach for identifying host factors associated with HIV-1 latency (27–29). Instead of large-scale library screening for intracellular proteins, our emphasis was directed toward a more targeted approach. Specifically, we focused on selective screening of host proteins that interact with the HIV-1 promoter in latently infected cells. This focus stems from the theory that these host proteins are strongly poised to directly affect the transcriptional regulation and silencing of HIV-1, ultimately influencing the formation and perpetuation of HIV-1 latency. Therefore, we screened and identified these host factors as potential candidates responsible for maintaining latent infection by utilizing CRISPR affinity purification in situ of regulatory elements (CAPTURE) system (30). This system comprises three components: HIV-1 LTR-specific sgRNA (sgLTR) or sgnon-target (sgNC), biotin acceptor site-tagged deactivated Cas9 (dCas9), and biotin ligase BirA. These components were co-transfected into an HIV-1 latency model based on HEK293T cells. The pseudotyped HIV-1 clone pNL4-3-ΔEnv/EGFP, derived from the infectious HIV-1 clone pNL4-3, was used to establish the HEK293T-based HIV-1 latency model. HEK293T cells were infected with the generated pseudotyped viruses, and GFP-positive cells were sorted and cultured until they became GFP negative. After several rounds of sorting, the HEK293T-based HIV-1 latency model was obtained. This cell model is convenient to manipulate and has been applied in research related to host factors involved in HIV-1 latency (31). After the transfection, the dCas9 was biotinylated in vivo , facilitated by the biotin ligase BirA. Subsequently, the biotinylated dCas9 was guided to specifically bind to the HIV-1 LTR by sgLTR (Fig. 1A). Then, we conducted streptavidin affinity purification on the biotinylated dCas9 complexes within both the sgLTR and sgNC groups. Following this, chromatin immunoprecipitation (ChIP)-qPCR assays and mass spectrometry (MS)-based proteomics analysis were then performed. In the purified dCas9 complex, we observed a significant enrichment of the HIV-1 promoter region in the sgLTR group compared to the sgNC group, establishing a basis for subsequent proteomic analysis (Fig. 1B). PEAKS Studio software with label-free quantification type was used to identify the enriched proteins in the sgLTR group compared to those in the sgNC group. Subsequently, non-nuclear proteins and proteins with low significance were excluded from the enriched proteomic analysis before searching for the host factors associated with HIV-1 latency. To further narrow down the selection, we conducted an additional prediction for host factors that could potentially bind to the HIV-1 promoter sequence using the hTFtarget database. This tool predicts candidate host factors based on specific sequences using the TRANSFAC/JASPAR/HOCOMOCO databases and ChIP-Seq data sets (32). Finally, we identified a series of host factors associated with HIV-1 latency within the CAPTURE screen, including RBBP4 (31), CPSF6 (33), HDAC1/2 (34), SMARCB1 (35), HMGA1 (36), SP1 (37), WDR82 (38), PCNA (31), SIN3A (39), KDM1A (40), SMC6 (41), CBX8 (16), TASOR (42), and BRD4 (43) (Fig. 1C). Interestingly, in the enriched protein results, we identified the presence of the origin recognition complex, with the largest subunit, ORC1, exhibiting notably high fold enrichment and significance in the overall enriched proteins (Table S1). Importantly, ORC1 was simultaneously identified in the hTF target database with a high prediction score (Fig. 1C; Table S1). Unlike the epigenetic and transcription regulators, ORC1 is primarily involved in DNA replication initiation. Its close association with the HIV-1 promoter has attracted our attention. This finding suggests its potential involvement in HIV-1 latency.
Fig 1.
ORC1 is identified as a potential host factor associated with the HIV-1 promoter. (A) Schematic of the CAPTURE system employed for screening host factors associated with the HIV-1 promoter. (B) The enrichment of the HIV-1 promoter region in purified chromatin DNA was evaluated by qPCR analysis. The primer set in the reference gene 36B4 served as the control region. Data are presented as means ± SEM in triplicates. P-values were calculated by unpaired Student’s t-test. **P < 0.01. (C) Host factors associated with HIV-1 latency were identified in CAPTURE screen. Conc.(fold enrichment) represents the concentration of proteins in sgLTR group as compared to that in sgNC group. (D–F) J-Lat 10.6 cells were infected with shLuc and shORC1 lentiviruses, respectively. Ninety-six after infection, LRAs including 500 nM SAHA, 1 µM JQ1, and 10 µM Bryostatin-1, were introduced to each group. After 24 hours, the percentage of GFP-positive cells as measured by flow cytometry, as shown in (D). The corresponding statistical results were presented in (E). The knockdown efficiency was validated by Western blot, as shown in (F). Data are presented as means ± SEM in triplicates. P-values were calculated by unpaired Student’s t-test. ***P < 0.001. (G) ChIP assay with antibody against ORC1 was performed in J-Lat 10.6 cells. The qPCR primer positions were presented in the upper panel. The lower panel showed the enrichment of ORC1 on the HIV-1 promoter through qPCR analysis. Data are presented as means ± SEM in triplicates. P-values were calculated by unpaired Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
Considering the potential role of all ORC subunits in HIV-1 latent infection, we designed specific short hairpin RNAs (shRNAs) targeting the six subunits of ORC (ORC1–6) and performed individual knockdown of each subunit in the J-Lat 10.6 model to assess the effect on HIV-1 reactivation. We found that the knockdown of each individual subunit of ORC resulted in an upregulation of HIV-1 expression. Notably, the depletion of ORC1 exhibited a more potent reactivation effect (Fig. S1A and B). Based on the knockdown of ORC1 in J-Lat 10.6 cells, we supplemented with well-established LRAs, including histone deacetylase inhibitors (SAHA), BET domain inhibitors (JQ1), and PKC pathway activator (Bryostatin-1). These LRAs are derived from different pathways and are widely used in clinical trials. The knockdown of ORC1 significantly enhanced HIV-1 reactivation upon stimulation with SAHA, JQ1, or Bryostatin-1 compared to the control (Fig. 1D through F). We also observed similar results in a heterogeneous latency model (23), J-Lat Mix, which consisted of a mixture of latently infected Jurkat cells (Fig. S1C through E). To validate the enrichment of ORC1 on the HIV-1 promoter, we performed chromatin immunoprecipitation assays targeting ORC1 in J-Lat 10.6 cells. The results demonstrated a significant enrichment of ORC1 throughout the entire regions of the HIV-1 LTR region compared to the host-virus junction region (Fig. 1G). These findings further support the results obtained from the previous analysis and provide additional confirmation of ORC1 involved in HIV-1 latency.
ORC1 is associated with repressive epigenetic elements
To further explore the molecular mechanism of ORC1 in maintaining HIV-1 latency, we next examined whether the knockdown of ORC1 had any impact on cell viability or cell cycle, as it is closely associated with HIV-1 expression and transcription regulation. The results showed that the knockdown of ORC1 did not suppress the viability and cell cycle progression of J-Lat 10.6 cells (Fig. S1F and G). Subsequently, we speculated whether the reactivation of latent HIV-1 could be attributed to changes in the chromatin structure of the integrated HIV-1 provirus, caused by the depletion of ORC1. By conducting assay for transposase-accessible chromatin with sequencing (ATAC-Seq) experiments, we observed alterations in the chromatin structure of the integrated HIV-1 provirus within J-Lat 10.6 when ORC1 was absent. Notably, there was a marked enhancement in the chromatin accessibility of the HIV-1 promoter region and gene-coding regions (Fig. 2A and B). Similarly, increased accessibility was observed across the entire chromatin (Fig. S2A). These results strongly suggest that ORC1 is likely involved in the regulation of chromatin structure of the HIV-1 provirus, thereby influencing HIV-1 latency, which is closely associated with epigenetic regulation. Therefore, to identify and explore ORC1-associated epigenetic regulatory factors involved in HIV-1 latency, we performed an affinity purification mass spectrometry (AP-MS) assay to identify ORC1-bound proteins. Flag-tagged ORC1 were transfected into Hela cells. After transfection, both ORC1 and ORC1-bound proteins were enriched using anti-Flag beads. Subsequently, the enriched proteins were separated by SDS-PAGE and analyzed by liquid chromatography-mass spectrometry (LC-MS/MS) (Fig. 2C). Through annotation using PEAKS Studio, we identified a series of candidate proteins enriched by ORC1. Additionally, we characterized several subgroups involved in chromatin binding, regulatory, and gene-specific transcription (Fig. 2D). Investigation of the interaction network of ORC1-enriched proteins using the STRING database revealed that, in addition to the DNA replication cluster, ORC1 also exhibited enrichment in protein cluster associated with chromatin remodeling and organization (Fig. 2E and F). This cluster included various repressive epigenetic elements. Among these heterochromatin-associated proteins, we found not only the previously well-reported ORC1/HP1α interaction but also several other representative repressive epigenetic proteins, including DNMT1, HDAC1/2, and polycomb repressive complex 2 (PRC2) subunits (EED and SUZ12) (16). Therefore, based on their respective functions, we classified these repressive epigenetic proteins into several representative epigenetic pathways, including (i) DNMT1-mediated DNA methylation, (ii) HDAC1/2-mediated histone deacetylation, and (iii) repressive histone methylation modifications H3K9me3 and H3K27me3, which are closely associated with HP1α and PRC2. These findings suggest that ORC1 may potentially participate in the regulation of chromatin structure and gene expression, facilitated by its interactions with proteins involved in epigenetic modifications. As a result, we hypothesized that ORC1 mediates HIV-1 latent infection by regulating these repressive epigenetic pathways. Subsequently, a series of experiments were performed to validate this hypothesis.
Fig 2.
ORC1 is associated with repressive epigenetic elements. (A and B) Chromatin accessibility within the HIV-1 proviral genome was assessed in J-Lat 10.6 cells treated with either shLuc or shORC1. Peaks, identified by MACS2, were normalized within each group and visualized using IGV tools. The knockdown efficiency was validated by Western blot, as shown in (B). (C) Hela cells were transfected with Flag-tagged ORC1. The transfection of empty vector was set as control group. Forty-eight hours after transfection, cells from each group were lysed and subsequently immunoprecipitated with anti-Flag beads, accompanied by treatment with nucleases. The enriched proteins were separated by SDS-PAGE and visualized through silver staining. (D) The enriched proteins identified by LC-MS/MS were proceeded to Gene Ontology analysis utilizing PANTHER classification system. (E and F) STRING network analysis was conducted on the enriched proteins with the interaction confidence threshold of 0.7. Clusters involving DNA replication, and chromatin remodeling and organization were identified.
ORC1 regulates the DNA methylation of the HIV-1 promoter by interacting with DNMT1
In contrast to the de novo DNA methyltransferases DNMT3A and DNMT3B, DNMT1 mainly mediates the inheritance of DNA methylation patterns during DNA replication (44). The role of ORC1 in DNA replication implies a potential connection between ORC1 and DNMT1. We conducted co-immunoprecipitation (co-IP) assays to verify the interaction between DNMT1 and ORC1 (Fig. 3A; Fig. S3A and B). Furthermore, we observed ORC1 obviously co-localized with DNMT1, reinforcing the evidence for their direct interaction (Fig. 3B). We postulated that this interaction could potentially influence DNA methylation of the HIV-1 promoter. Owing to the hypermethylated CpG island of the integrated provirus in the J-Lat 8.4 cell line compared to those of other monoclonal latency models, we utilized this cell line to investigate the relationship between DNA methylation and ORC1 knockdown. The knockdown of ORC1 in J-Lat 8.4 cells resulted in the upregulation of HIV-1 expression. This reactivation was significantly enhanced when supplemented with 5-aza-dC, a widely used inhibitor of DNMT1 methyltransferase (Fig. 3C and D). To determine if the enhanced reactivation could be a result of changes in the methylation levels of the HIV-1 promoter, we performed a bisulfite cytosine methylation assay to identify the CpG methylation profile of the HIV-1 promoter. We found the knockdown of ORC1 led to a mild decrease in CpG methylation within the HIV-1 promoter. Notably, when ORC1 knockdown was combined with 5-aza-dC supplementation, a more pronounced reduction in the CpG methylation of the HIV-1 promoter was observed (Fig. 3E). Specifically, the combination treatment resulted in a decrease in CpG methylation of approximately 25% compared to the group only treated with 5-aza-dC (Fig. 3E). To determine if the decrease in CpG methylation within the HIV-1 promoter was the result of a change in the binding of DNMT1 to the promoter region, we performed a ChIP assay targeting DNMT1 in J-Lat 8.4 cells following ORC1 knockdown. Our results demonstrated a notable reduction in the presence of DNMT1 on the HIV-1 promoter upon ORC1 depletion (Fig. 3F and G). Taken together, these results suggest that ORC1 recruits DNMT1 to the HIV-1 promoter and affects CpG methylation on the HIV-1 promoter, thereby contributing to HIV-1 latency.
Fig 3.
ORC1 regulates the DNA methylation of the HIV-1 promoter by interacting with DNMT1. (A) J-Lat 10.6 cells were lysed and immunoprecipitated with anti-DNMT1 antibody. The endogenous ORC1 enriched by DNMT1 was immunoblotted with anti-ORC1 antibody. (B) HEK293T cells transfected with GFP-tagged ORC1 were treated with AF568-tagged DNMT1 antibody and imaged using super-resolution structured illumination microscopy. (C and D) J-Lat 8.4 cells were infected with shLuc and shORC1 lentiviruses, respectively. Forty-eight hours after infection, cells were treated with 400 nM 5-aza-dC for 1 week. The percentage of GFP-positive cells was measured by flow cytometry, as shown in (C). The corresponding statistical results were presented in (D). Data are presented as means ± SEM in triplicates. P-values were calculated by unpaired Student’s t-test. ***P < 0.001. (E) The upper panel showed the two CpG islands of HIV-1 5′ LTR in J-Lat 8.4 cells. The lower panel presented the CpG methylation profile of HIV-1 5′ LTR in J-Lat 8.4 cells treated as described in (C). In the lower panel, the empty circles represent demethylated sites, while the filled circles represent methylated sites. Statistical significance between groups was determined by Mann-Whitney U test. *P < 0.05, **P < 0.01. (F) ChIP assay with antibody against DNMT1 was performed in shLuc- and shORC1-treated J-Lat 8.4 cells. Data are presented as means ± SEM in triplicates. P-values were calculated by unpaired Student’s t-test. **P < 0.01. (G) The knockdown efficiency of ORC1 in shRNA-infected J-Lat 8.4 cells was validated by Western blot.
HDAC1 and HDAC2 are recruited to the HIV-1 promoter by ORC1
Histone deacetylation, as a representative repressive epigenetic modification, has been extensively investigated in HIV-1 latent infection (34, 35). Among the proteins enriched by ORC1, we identified histone deacetylases HDAC1 and HDAC2. The interaction between ORC1 and HDAC1/2 was validated by co-IP assays (Fig. 4A and B; Fig. S3C through E). Considering the well-reported interaction between HDAC1/2 and DNMT1 (45, 46), we further confirmed that ORC1 could still interact with HDAC1/2 in the absence of DNMT1 and that the depletion of HDAC1/2 did not influence the interaction between ORC1 and DNMT1 (Fig. S3F and G). To explore whether ORC1 plays a role in recruiting HDAC1 and HDAC2 to the HIV-1 promoter, we conducted ORC1 knockdown in J-Lat 10.6 cells and observed a distinct reduction in the presence of HDAC1 and HDAC2 on the HIV-1 promoter (Fig. 4C and D). HDAC1 and HDAC2 are primarily responsible for the deacetylation of H3K9 and H3K27; therefore, we measured the levels of acetylation at H3K9 and H3K27 on the HIV-1 promoter (Fig. 4E through G). The results demonstrated that the depletion of ORC1 led to significant downregulation of H3K9ac and H3K27ac. Therefore, our findings strongly suggest that ORC1 recruits HDAC1 and HDAC2 to the HIV-1 promoter, thereby mediating hypoacetylation of the promoter region.
Fig 4.
HDAC1 and HDAC2 are recruited to the HIV-1 promoter by ORC1. (A) J-Lat 10.6 cells were lysed and immunoprecipitated with anti-HDAC1 antibody. The endogenous ORC1 enriched by HDAC1 was immunoblotted with anti-ORC1 antibody. (B) J-Lat 10.6 cells were lysed and immunoprecipitated with anti-HDAC2 antibody. The endogenous ORC1 enriched by HDAC2 was immunoblotted with anti-ORC1 antibody. (C–G) ChIP assay with antibodies against HDAC1, HDAC2, H3K9ac, and H3K27ac was performed in shLuc- and shORC1-treated J-Lat 10.6 cells. The knockdown efficiency was validated by Western blot, as shown in (G). Data are presented as means ± SEM in triplicates. P-values were calculated by unpaired Student’s t-test. *P < 0.05, **P < 0.01.
ORC1 maintains the repressive histone modifications enriched on the HIV-1 promoter
In the mass spectrometry results, we identified the presence of the heterochromatin protein HP1α. The interaction between ORC1 and HP1α was confirmed through the co-IP assay (Fig. 5A; Fig. S4A and B). As a heterochromatin-associated protein, HP1α can recruit various repressive epigenetic factors (47–49). However, in this study, the knockdown of endogenous HP1α did not affect the interaction between ORC1 and the mentioned repressive epigenetic proteins. This indicated that these interactions were independent of HP1α (Fig. S4C). Furthermore, a significant co-localization between ORC1 and HP1α was identified, and both proteins exhibited pronounced enrichment within the condensed chromatin regions marked by DAPI staining (Fig. 5B). These results implied a close association between ORC1/HP1α and heterochromatin. H3K9me3 serves as a hallmark of epigenetic modification for constitutive heterochromatin. HP1α plays a crucial role by acting as a “reader” protein that selectively recognizes the H3K9me3 modification, leading to its strong interaction with this specific epigenetic mark. Considering the interaction between ORC1 and HP1α, we hypothesized that ORC1 might be involved in the epigenetic pathway of H3K9me3 modification. Through co-IP assays, we verified the interactions between ORC1 and H3K9me3 modifiers, including SUV39H1, SUV39H2, and SETDB1 (Fig. 5C; Fig. S4D). Additionally, immunofluorescence assays also showed that the ORC1 significantly co-localized with these histone modifiers and H3K9me3, indicating that ORC1 was enriched in the heterochromatin regions marked by H3K9me3 (Fig. 5B). Based on these results, we postulated that ORC1 might affect H3K9me3 modification of the HIV-1 promoter. To investigate this, ChIP assays were performed, and a significant reduction in the enrichment of HP1α on the HIV-1 promoter was observed upon the knockdown of ORC1. This result was consistent with previous findings (50), suggesting that the depletion of ORC1 can influence the localization of HP1α in heterochromatin (Fig. 5D and E). However, the knockdown of HP1α did not influence the enrichment of ORC1 on the HIV-1 promoter, suggesting that this enrichment was independent of HP1α (Fig. S4E and F). Additionally, the accumulation of H3K9me3 on the HIV-1 promoter was significantly decreased upon the knockdown of ORC1 (Fig. 5D and E). These results suggest that ORC1 significantly influences the enrichment of H3K9me3 modifications on the HIV-1 promoter.
Fig 5.
ORC1 maintains the repressive histone modifications enriched on the HIV-1 promoter. (A) J-Lat 10.6 cells were lysed and immunoprecipitated with anti-HP1α antibody. The endogenous ORC1 enriched by HP1α was immunoblotted with anti-ORC1 antibody. (B) HEK293T cells transfected with GFP-tagged ORC1 were treated with AF568-tagged antibodies against HP1α, SUV39H1, and H3K9me3, and then imaged using super-resolution structured illumination microscopy (SR-SIM). (C) J-Lat 10.6 cells were lysed and immunoprecipitated with anti-SUV39H1 antibody. The endogenous ORC1 enriched by SUV39H1 was immunoblotted with anti-ORC1 antibody. (D and E) ChIP assay with antibodies against HP1α and H3K9me3 was performed in shLuc- and shORC1-treated J-Lat 10.6 cells. The knockdown efficiency was validated by Western blot, as shown in (E). (F) Flag-tagged ORC1 was transfected in J-Lat 10.6 cells. The endogenous EZH2 enriched by ORC1 was immunoblotted with anti-EZH2 antibody. (G) HEK293T cells transfected with GFP-tagged ORC1 were treated with AF568-tagged antibodies against EZH2 and H3K27me3, and then imaged using SR-SIM. (H and I) ChIP assay with antibody against H3K27me3 was performed in shLuc- and shORC1-treated J-Lat 10.6 cells. The knockdown efficiency was validated by Western blot, as shown in (I).
Mass spectrometry analysis of ORC1-enriched proteins identified the presence of core members of PRC2, including EED and SUZ12, which play crucial roles in mediating H3K27me3 modifications. The interaction between ORC1 and core components of PRC2 was validated using co-IP assays (Fig. 5F; Fig. S4G). Through immunofluorescence assays, we also observed significant co-localization of ORC1 with EZH2, the modifier of H3K27me3, and partial co-localization with H3K27me3 (Fig. 5G). Considering the association between ORC1 and PRC2, we knocked down ORC1 in J-Lat 10.6 cells and observed a noticeable reduction of H3K27me3 on the HIV-1 promoter, indicating that the depletion of ORC1 also influenced the H3K27me3 levels on the HIV-1 promoter (Fig. 5H and I). Taken together, these results show that ORC1 participates in maintaining the repressive histone modification of the HIV-1 promoter, thereby contributing to the establishment of HIV-1 latent infection.
ORC1 can form liquid-liquid phase separation condensates
In recent years, a growing number of proteins have been identified to form nuclear bodies and undergo liquid-liquid phase separation (LLPS), which are widely involved in various cellular organizations and functions (51). When the distribution of endogenous ORC1 was examined using super-resolution microscopy, the presence of hundreds of nuclear bodies was observed (Fig. 6A). Furthermore, protein structure prediction of ORC1 revealed the presence of extensive intrinsically disordered regions (IDRs), which are key structural features in the formation of the LLPS condensates (Fig. 6B). Therefore, we speculated that ORC1 bodies might also form LLPS. Owing to the liquid-like fluidity of LLPS condensates, they can constantly exchange substances with the surrounding environment. These protein bodies undergo internal diffusion, fusion, and fission, which serve as crucial evidences of the formation of LLPS condensates (52). To verify whether ORC1 bodies form LLPS, we performed fluorescence recovery after photobleaching (FRAP) assays on the GFP-tagged ORC1 bodies in live cells. The results showed that GFP-tagged ORC1 bodies bleached by a high-intensity 488 nm laser were able to gradually recover their fluorescence intensity to levels close to those before bleaching within 2–4 min (Fig. 6C and D). We also observed the morphology of GFP-tagged ORC1 protein bodies in live cells and discovered that they exhibited liquid-like fluidity, undergoing continuous fusion and fission (Fig. S5A and B). Additionally, 1,6-hexanediol has been reported to be widely used for disrupting the formation of phase-separated condensates. Treatment of GFP-tagged ORC1 protein bodies in live cells with 1,6-hexanediol resulted in a gradual disruption of ORC1 bodies, strongly suggesting the sensitivity of ORC1 bodies to 1,6-hexanediol and further supporting the theory that they are formed by LLPS (Fig. S5C). We attached a GFP tag to the IDRs of ORC1 and purified the fusion proteins in vitro (Fig. S5D). Induction of the formation of LLPS under different conditions in vitro demonstrated that GFP-tagged ORC1-IDR proteins exhibited the capacity to generate distinct droplets, particularly under conditions involving a gradual reduction in NaCl concentration (Fig. 6E). Meanwhile, as the protein concentration increased, the GFP-ORC1-IDR proteins exhibited an increase in both the quantity and size of the droplets formed (Fig. 6E). This phenomenon primarily arises owing to the influence of hypotonic solutions, and elevated protein concentrations promote the formation of LLPS. Taken together, these results from intracellular and in vitro assays suggest that ORC1 bodies are LLPS condensates.
Fig 6.
ORC1 bodies are phase-separated nuclear condensates. (A) Endogenous ORC1 in HEK293T cells was labeled by AF488-tagged antibody and imaged by super-resolution structured illumination microscopy (SR-SIM). (B) The predication of IDRs within ORC1 was performed using PONDR scores. IDRs were subdivided into sub-disordered regions: R1, R2, R3, and R4. The ATPase domain was segmented into four segments: C, D, E, and F. (C) FRAP assay was conducted in living HEK293T cells overexpressing GFP-tagged ORC1. Two ORC1 bodies were selected as regions of interest (ROI). One ROI served as the unbleached negative control, while another ROI was bleached using a high-intensity 488 nm laser. The images were captured every 5 seconds to record the recovery process and changes in fluorescence intensity of ROI. (D) The relative fluorescence intensities of both unbleached and bleached ORC1 bodies in each time point were measured at each time point. The fluorescence intensities at each time point were normalized to the pre-bleaching time point. (E) GFP-tagged ORC1 IDRs were purified in vitro. In the upper panel, 10 µg of proteins was added into Tris-HCl buffer with different NaCl gradients (ranging from 500 to 15.625 mM). In the lower panel, the protein concentration was gradient diluted from 10 µM in Tris-HCl buffer containing 50 mM NaCl. (F) GFP-tagged wild-type ORC1 and LLPS-deficient ORC1 in HEK293T cell were imaged by SR-SIM. (G) Flag-tagged ORC1 and LLPS-deficient ORC1 were transfected in HEK293T-based latency model, respectively. Forty-eight hours after transfection, ChIP assay with anti-Flag antibody was performed. Data are presented as means ± SEM in triplicates. P-values were calculated by unpaired Student’s t-test. **P < 0.01.
Subsequently, our attention was to identify the key regions of ORC1 that mediate the LLPS formation. Based on the prediction of the IDRs and the distribution of functional domains, ORC1 was divided into three distinct segments: the first region comprised the bromo-adjacent homology (BAH) domain located at the N terminus of ORC1 (amino acids 1–182), the second region consisted of the IDRs of ORC1 (amino acids 183–476), and the third region encompassed the ATPase domain at the C terminus of ORC1 (amino acids 477–861) (Fig. 6B). We found that the BAH domain of ORC1 lacked the regions associated with LLPS. In contrast, both the IDRs and ATPase domains of ORC1 contained LLPS-mediated regions (Fig. S6A). Through further structural analysis, we subdivided the IDRs of ORC1 into four sub-disordered regions: R1, R2, R3, and R4, and found that ORC1 (amino acids 1–476) failed to form nuclear bodies upon the deletion of both the R3 and R4 regions (Fig. S6A). This suggests that the regions responsible for LLPS within the IDRs are situated in R3 (amino acids 360–382) and R4 (amino acids 412–476). Subsequently, we conducted a screening to identify the regions within the ATPase domain that mediate the LLPS and subdivided the C-terminal region of ORC1 (amino acids 477–861) into four distinct segments: C, D, E, and F. The results showed that segments of D, E, and F all harbored LLPS-mediated regions (Fig. S6B). To further narrow down the functional regions, we performed additional structural predictions focusing on the C-terminus of ORC1. Our analysis revealed the presence of a coiled-coil structure within this region, a structural motif previously associated with the promotion of LLPS formation (31, 53, 54). Subsequently, we selectively deleted the coiled-coil structure within the D, E, and F segments (amino acids 577–636, amino acids 677–729, and amino acids 757–816, respectively). Upon implementing these deletions, we observed a notable absence of nuclear bodies, accompanied by a diffuse distribution of nuclear proteins throughout the nucleus (Fig. S6B). In summary, our findings indicated that the LLPS-mediated regions of ORC1 are located within the sub-disordered regions R3 and R4 as well as in the coiled-coil structure of the ATPase domain (Fig. 6F).
To investigate whether LLPS contributes to the function of ORC1, we conducted ChIP assays to assess the enrichment of both wild-type and LLPS-deficient ORC1 on the HIV-1 promoter. Remarkably, we observed that when ORC1 was unable to form LLPS condensates, its enrichment on the HIV-1 promoter was significantly decreased (Fig. 6G). This result strongly suggests that ORC1 interacts with the HIV-1 promoter in the form of LLPS, and the LLPS property of ORC1 plays a pivotal role in its effective localization and enrichment on the HIV-1 promoter.
ORC1 depletion reactivates latent HIV-1 in cells from people living with HIV-1
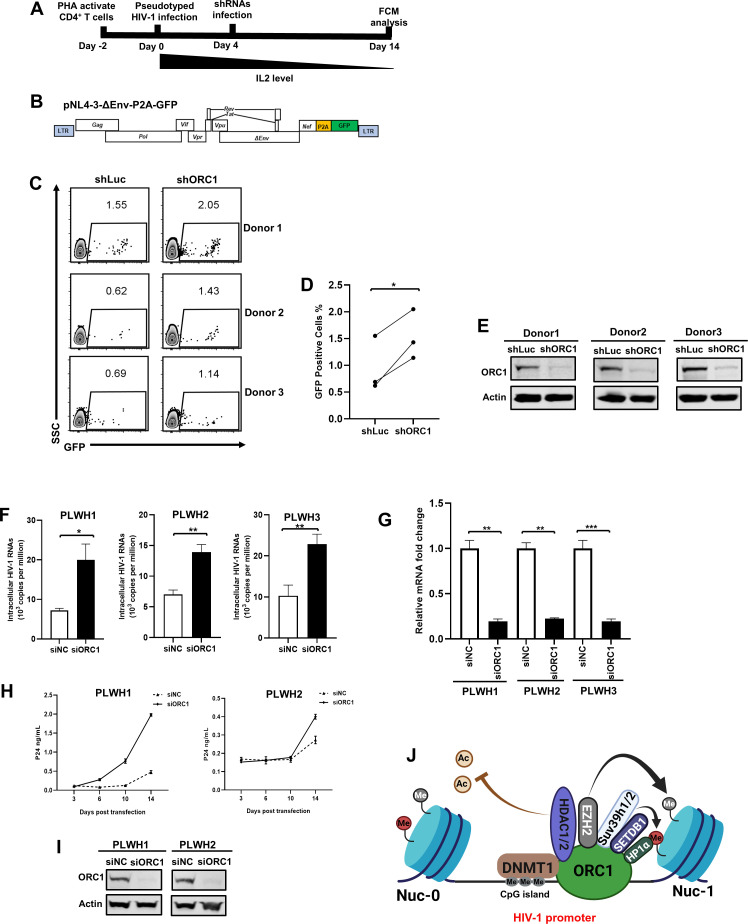
We have found that the downregulation of ORC1 was able to reactivate latent HIV-1 from Jurkat-derived latency cell lines. To strengthen our understanding of ORC1’s role in maintaining HIV-1 latency in primary CD4+ T cells, we established two different types of latency models using primary CD4+ T cells that were infected by pseudotyped HIV-1NL4-3-ΔEnv-P2A-GFP or HIV-1NL4-3-CM6. The former pseudotyped HIV-1 harbored a relatively intact HIV-1 genome, whereas the latter pseudotyped HIV-1 exhibited weaker virulence owing to the disruption of most HIV-1 genes (Fig. 7B; Fig. S7A) (55, 56). Phytohemagglutinin (PHA)-activated CD4+ T cells were initially infected with pseudotyped HIV-1, and the infected cells were subsequently divided into two groups. One group was transduced with lentiviral vector carrying control shLuc, whereas the other group was transduced with that carrying shORC1. After infection with the respective shRNAs, the cells were cultured in conditions with gradually decreasing IL-2 concentrations to induce latency. After a 2-week incubation period, the proportion of cells expressing GFP was assessed (Fig. 7A). The results showed that the depletion of ORC1 had a substantial effect on the upregulation of expression for both types of latently infected pseudotyped HIV-1 in primary CD4+ T cells (Fig. 7C through E; Fig. S7B through D).
Fig 7.
ORC1 depletion reactivates latent HIV-1 in cells from people living with HIV-1 (PLWH). (A) The procedure of ORC1 depletion-mediated HIV-1 reactivation in primary CD4+ T cells. (B) The schematic of pseudotyped HIV-1NL4-3-ΔEnv-P2A-GFP backbone. (C–E) The percentage of GFP-positive cells was measured by flow cytometry, as shown in (C). The corresponding statistical results were shown in (D). The knockdown efficiency of ORC1 in shRNA-infected primary CD4+ T cells was validated by Western blot, as shown in (E). Statistical significance between groups was determined by paired Student’s t-test with three replicates per group. *P < 0.05. (F and G) The measurement of HIV-1 cell-associated viral RNA of latently infected CD4+ T cells, which were isolated from PLWH and treated with either siNC or siORC1. The knockdown efficiency of ORC1 in PLWH treated with siRNAs was analyzed by RT-qPCR, as shown in (G). Data are presented as means ± SEM in triplicates. P-values were calculated by unpaired Student’s t-test. *P < 0.05, **P < 0.01, ***P < 0.001. (H–I) The replication-competent HIV-1 was detected by viral outgrowth assay. Primary CD4+ T cells from PLWH were treated with either siNC or siORC1. Irradiated healthy donor peripheral blood mononuclear cells and PHA-activated healthy donor CD4+ T cells were introduced to amplify the reactivated infectious HIV-1. The p24 levels of supernatants at each time point were collected and measured. The knockdown efficiency of ORC1 in PLWH treated with siRNAs was analyzed by Western blot, as shown in (I). (J) The schematic of ORC1-mediated HIV-1 latency.
Next, we determined whether the depletion of ORC1 can reactivate latent HIV-1 in resting CD4+ T cells from people living with HIV-1 (PLWH) receiving suppressive cART. We isolated CD4+ T cells from the peripheral blood of PLWH and knocked down ORC1 expression. Subsequently, the transcripts of the cell-associated viral RNA (CA-RNA) were measured. We found that ORC1 depletion was able to reactivate more CA-RNA compared to that of the negative control group (Fig. 7F and G). Because the HIV-1 latent reservoir comprises a substantial number of replication-defective proviruses, the elevation in HIV-1 transcripts does not necessarily indicate the reactivation of infectious HIV-1. Therefore, we conducted a viral outgrowth assay (VOA) to specifically detect the reactivation of replication-competent HIV-1. After the knockdown of ORC1 in CD4+ T cells isolated from PLWH, we found the depletion of ORC1 successfully induced more infectious HIV-1 than in the negative control group (Fig. 7H and I). Overall, these results suggest that ORC1 contributes to HIV-1 latency in primary CD4+ T cells, and the depletion of ORC1 can reactivate latent HIV-1 in cells from PLWH.
DISCUSSION
In the present study, we successfully identified ORC1 as a novel host factor that contributes to HIV-1 latency. This was confirmed in various HIV-1 latency cell models and CD4+ T cells isolated from PLWH. Subsequently, the molecular mechanisms by which ORC1 contributes to HIV-1 latency were elucidated detailly, including its effects on DNA methylation, histone deacetylation, and methylation. Furthermore, we identified the LLPS properties of ORC1 and located the key regions responsible for phase separation. The enrichment of LLPS-deficient ORC1 was significantly reduced on the HIV-1 promoter compared to the wild type. Based on these results, we proposed a model of ORC1 that contributes to HIV-1 latency (Fig. 7J). In latent status, ORC1 serves as a chromatin-binding scaffold protein, recruiting various repressive epigenetic proteins, such as DNMT1, HDAC1/2, HP1α, H3K9me3 modifiers, and H3K27me3 modifier, to the HIV-1 promoter region. This orchestrated recruitment facilitates the formation and maintenance of a condensed chromatin conformation around the HIV-1 promoter, contributing to the maintenance of HIV-1 latency.
The ORC complex was originally identified as a heterohexamer in budding yeast that mediates the initiation of DNA replication (57). ORC binds to chromosomal DNA, facilitating the recruitment of various replication factors, including Cdc6, Cdt1, and Mcm2-7, to form a pre-replicative complex and initiate DNA replication (58, 59). In addition to this essential role, ORC is involved in various other cellular functions, particularly in transcriptional silencing and heterochromatin formation (60, 61). In S. cerevisiae, ORC1 interacts with the heterochromatin protein SIR1 and plays a crucial role in maintaining heterochromatin and regulating gene expression (62). This phenomenon is conserved, and in mammals, the association between ORC1 and heterochromatin is predominantly related to its interaction with the heterochromatin protein HP1 (50, 63). Meanwhile, ORC also plays a significant role in maintaining telomere heterochromatin through its interactions with the TRF2 protein and telomere repeat-encoding RNA (64, 65). Recent studies have reported that ORC1 can also regulate the transcription of particular genes, such as CCEN1, which encodes the cyclin E protein (66). However, the specific mechanisms by which ORC1 participates in heterochromatin formation and gene expression have not been fully elucidated. In most previous studies, ORC appeared to act as an intermediary regulatory element in heterochromatin formation. In this study, we uncovered a dominant role of ORC1 in heterochromatin establishment and maintenance. ORC1 interacts with various repressive epigenetic modifying enzymes, such as DNA methyltransferase, histone deacetylases, and histone methyltransferases, which label nucleosomes with epigenetic marks and facilitate the formation of heterochromatin structures.
The recruitment of DNMT1 by ORC1, which notably affects DNA methylation levels on the HIV-1 LTR, is a novel finding in this study. During DNA replication, DNMT1 functions to replicate DNA methylation marks onto newly synthesized DNA strands. This mechanism is largely facilitated by its interaction with the replication factor PCNA (67). However, once replication initiation is completed and S phase has been entered, ORC1 is removed from the ORC complex and subsequently degraded (58). Therefore, based on our results, it is probable that prior to the initiation of replication, DNMT1 is recruited to the replication origins by ORC1, mediating the subsequent inheritance of DNA methylation. Similarly, during the G1 phase, in the absence of DNA replication activity, ORC1 binding to heterochromatin regions may also recruit DNMT1 through the same interaction, thereby contributing to the maintenance of DNA methylation levels in these regions. However, confirming the prevalence of this phenomenon still requires further relevant studies. The latently HIV-1-infected CD4+ T cells remain in a resting, non-dividing state, providing ORC1 with a greater opportunity to exert its function.
The colocalization of the ORC complex and HP1α in heterochromatin has been widely reported, and the depletion of individual ORC subunits disrupts HP1α localization (50, 68). In our results, we found that the recruitment of HP1α to heterochromatin was influenced by ORC1, whereas the recruitment of ORC1 to heterochromatin remained unaffected by HP1α. The depletion of HP1α did not influence the recruitment of repressive epigenetic elements by ORC1. These findings suggest a major role of ORC1 in heterochromatin formation, which is independent of the well-known ORC1/HP1α interaction. Based on the recruitment of HP1α by ORC1, we revealed that ORC1 was able to affect the enrichment of repressive histone modification H3K9me3, which is recognized by HP1α, on the HIV-1 promoter. We also found that ORC1 was involved in regulating the level of H3K27me3 of the HIV-1 promoter, although to a lesser extent compared to its effect on H3K9me3. Meanwhile, the co-localization of ORC1 and H3K27me3 was not as evident as that observed with H3K9me3. Therefore, it is probable that ORC1 preferentially localizes to constitutive heterochromatin regions enriched with H3K9me3 and serves as an important mediator in its regulation. Nevertheless, further functional and enzymatic assays are needed to validate this hypothesis. Taken together, it is likely that ORC1 acts as a scaffold protein that recruits various repressive epigenetic proteins to heterochromatin, thereby facilitating chromatin organization. This is supported by the increased chromatin accessibility at the HIV-1 proviral genome observed upon the knockdown of ORC1.
Our study offered a more comprehensive validation of the phase separation of ORC1 compared with previous studies (69). We demonstrated the internal diffusion of nuclear ORC1 bodies using FRAP and induced the formation of phase-separated droplets with purified ORC1 protein in vitro, thus confirming the LLPS phenotype of ORC1. ORC1 probably plays an important role in the phase separation of the ORC complex and the replication initiation factor CDC6 (69). The N-terminal IDRs of Drosophila ORC1 were required for Drosophila ORC phase separation and the association with chromatin (70). However, in human ORC1, apart from the IDRs, C-terminal coiled-coil structures, known to facilitate protein-protein interactions, also influence the phase separation of ORC1. The absence of both IDRs and C-terminal coiled-coil regions in human ORC1 results in impaired phase separation and substantially diminishes its capacity for chromatin recruitment. Recent studies have reported the association between LLPS proteins and HIV-1 latency, such as CAF-1 complex and CBX4 (23, 31). We explored the potential correlation between LLPS of ORC1 and HIV-1 latency and found that the LLPS of ORC1 influenced its enrichment on the HIV-1 promoter. Nevertheless, it remains to be determined whether the LLPS of ORC1 directly influences HIV-1 latency, heterochromatin formation, and DNA replication.
Because of the necessity of ORC1 in DNA replication, it is essential to identify distinct functional domains of ORC1 involved in DNA replication and epigenetic regulation. In Saccharomyces cerevisiae, the transcriptional silencing function is independent of the role of ORC in DNA replication and is mediated by separate protein domains (71, 72). It is highly probable that distinct domains contribute to heterochromatin maintenance and initiation of DNA replication within human ORC1. Therefore, screening for specific inhibitors targeting the functional domains responsible for the recruitment of ORC1 to heterochromatin and combining them with other LRAs could enhance the effectiveness of the “shock and kill” strategy and contribute to achieving a functional cure for HIV-1. We noticed that all the subunits of the ORC complex influenced HIV-1 latency in the J-Lat model. Apart from ORC1, other subunits may also be involved in HIV-1 latency, and further investigation is required to elucidate their specific roles in HIV-1 latency. It is possible that targeting all subunits or multiple subunits of the ORC complex could lead to more effective activation of latent HIV-1. Further studies should focus on identifying other subunits of the ORC complex that contribute to HIV-1 latency.
In conclusion, these findings of ORC1 have expanded our understanding of the non-replicative functions of the ORC complex and provided important insights into the molecular pathways governing HIV-1 latency. These insights may have implications for the development of potential therapeutic strategies targeting ORC1 and reactivating latent HIV-1 reservoirs, thereby contributing to the achievement of a functional cure for HIV-1.
MATERIALS AND METHODS
Study participants
PLWH were recruited for this study by the Department of Infectious Diseases in Guangzhou Eighth People’s Hospital, Guangzhou Medical University. All patients were recruited based on the criteria of prolonged continuous suppression of plasma HIV-1 viremia on cART, which means undetectable plasma HIV-1 RNAs (<50 copies/mL) and high CD4+ T-cell number (more than 350 cells/mm3) for at least 6 months.
Unidentified human peripheral blood mononuclear cells (PBMCs) of healthy blood donors were provided by Shenzhen Blood Center. We did not have any interaction with these human subjects or protected information, and therefore no informed consent was required.
Cell culture
HEK293T cells (ATCC, CRL-3216) and HeLa cells (ATCC, CCL-2) were obtained from ATCC. The adherent cell lines mentioned above were cultured in DMEM supplemented with 1% penicillin-streptomycin and 10% FBS. Jurkat cells were obtained from ATCC. J-Lat 8.4 (NIH AIDS Reagent Program, Cat#9847) and J-Lat 10.6 cells (NIH AIDS Reagent Program, Cat#9849), constructed by Dr. Eric Verdin (The Buck Institute for Research on Aging, Novato, CA) Laboratory, were obtained from Robert F. Siliciano (Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA). Suspension cells, including Jurkat, J-Lat 8.4, and J-Lat 10.6, were cultured in RPMI 1640 supplemented with 1% penicillin-streptomycin and 10% FBS. PBMCs and primary CD4+ T cells, which were isolated from healthy donors and PLWH, were cultured in RPMI 1640 supplemented with 1% penicillin-streptomycin and 10% FBS. All cells were cultured in a sterile incubator at 37°C and 5% CO2.
CRISPR affinity purification in situ of regulatory elements—proteomics
This method was based on CRISPR affinity purification in situ of regulatory elements system established by Liu et al., with some modifications (30, 73). HIV-1 LTR-targeted sgRNA (5′-GCCCGTCTGTTGTGTGACTC-3′) or sgnon-target (5′-ACGGAGGCTAAGCGTCGCAA-3′) was constructed into pSLQ1651 vector. The components of CAPTURE system, including pSLQ1651-sgLTR or pSLQ1651-sgNT, pEF1a-FB-dCas9, and pEF1a-BirA-V5 vectors, were co-transfected into established HEK293T latently infected cells. Forty-eight hours after transfection, 40 million cells from each group were harvested and cross-linked with 2% formaldehyde (Sigma-Aldrich) for 10 min at room temperature and quenched with 250 mM glycine for 5 min. After centrifugation to remove the supernatants, the cross-linked cells were lysed with 2 mL RIPA buffer (10 mM Tris-HCl, 1 mM EDTA, 0.1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, pH 8.0) for 15 min on ice. The nuclear pellets were collected by centrifugation at 12,000 g for 10 min and suspended in 1 mL 0.5% SDS lysis buffer (0.5% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.0). The suspension was then sonicated using VibraCell VCX-130 sonicator for 5 min (20 s on, 30 s off) at 50% amplitude to shear the chromatin fragments to a size of 200–500 bp. The samples were centrifuged at 14,000 g for 15 min at 4°C. Twenty microliters of sheared chromatin were reserved as an input sample for further ChIP-qPCR analysis. The residual supernatants were added to a final concentration of 150 mM NaCl and then incubated with 20 µL MyOne Streptavidin T1 Dynabeads (ThermoFisher) overnight at 4°C with rotation. The incubated beads were washed five times with 1 mL STN buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% NP-40, 0.5% Triton X-100) and washed an additional three times with 1 mL STN buffer without NP-40. For the downstream ChIP-qPCR, the beads containing chromatin DNA and input samples were treated with SDS elution buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.0) and proceeded to reverse cross-linking at 65°C overnight, followed by the treatment with RNase A at 37°C for 30 min and protease K at 65°C for 2 hours. Then, DNA fragments were purified with DNA purification spin columns (CST). Primers targeting the HIV-1 promoter region and human gene 36B4 as a control were used for qPCR analysis and listed in Table S3.
For the downstream proteomics, the beads were resuspended with 500 µL 0.5 M Tris (pH 8.5) solution and added to a final concentration of 20 mM TCEP [tris(2-carboxyethyl)phosphine, Sigma-Aldrich] at room temperature for 1 hour with rotation. Four microliters of MMTS (S-methyl methanethiosulfonate, ThermoFisher) were added into the sample and rotated at room temperature for 20 min. Ten micrograms of mass spectrometry-grade trypsin were added into the sample and rotated at 37°C overnight. Then, the beads were removed, and the treated protein solution was separated by centrifugation at 12,000 g for 3 min using Pierce cellulose membrane tubes (ThermoFisher). The sample was added to a final concentration of 3 M NaCl and incubated at 95°C for 1 hour. Subsequently, the solvent was removed under vacuum, and the residue was re-dissolved using 100 µL of 0.1% TFA (ThermoFisher). Following this, the sample was concentrated under vacuum to a final volume of 10–20 μL. The peptides in the concentrated sample were desalted and enriched using a C18 ZipTip (Millipore) and then re-dissolved in 50% ACN containing 0.1% TFA. The solvent was removed once again under vacuum. Finally, the peptides were reconstituted in 20 µL of 0.01% formic acid and subjected to LC-MS/MS analysis. The MS data of sgNC and sgLTR groups in the CAPTURE screen were analyzed by PEAKS Studio v.8 software against the Uniprot human databases. The search was performed with the following parameters: (i) quantification type: label-free quantification, (ii) mass error tolerance: 10.0 ppm, (iii) retention time shift tolerance: 6.0 min, (iv) peptide false discovery rate: 1%. The proteins with fold enrichment (sgLTR/sgNC ratio) >1 were considered to be enriched in sample with sgLTR relative to the sgNC control.
Gene knockdown mediated by shRNA lentivirus and siRNA
shRNAs targeting Luciferase and ORC1-6 were separately constructed into the pLKO.1-RFP vector, derived from pLKO.1-puro. The puro-tag of pLKO.1-puro was replaced with RFP-tag. To produce shRNA lentiviruses, 3 µg of VSV-G envelope plasmid, 6 µg of lentiviral packaging plasmid pCMVΔR8.2, and 6 µg of shRNA expression vectors were co-transfected into HEK293T cells using Lipo2000 reagent (ThermoFisher). Forty-eight hours after transfection, the virus supernatants were concentrated with PEG6000 and then used to infect target cells, including J-Lat cell lines and primary CD4+T cells. Ninety-six hours after infection, the infected cells were treated with 500 nM SAHA, 1 µM JQ-1, and 10 µM Bryostatin-1. Twenty-four hours later, each group of the GFP-positive-infected cells was detected using BD LSR Fortessa flow cytometer and analyzed with FlowJo V10 software. The percentage of RFP-positive cells indicated the efficiency of shRNA lentiviral infection. The knockdown efficiency was confirmed through qPCR and Western blot analysis.
siRNAs targeting human ORC1, HP1α, HDAC1/2, DNMT1, and the negative control siNC were purchased from RiboBio (Guangzhou, China). For the transfection of siRNA in adherent cells, a final concentration of 100 µM siRNAs was transfected into cells using RNAiMAX (ThermoFisher) when the cell confluence reached 60%–80%. For the transfection of siRNA in primary CD4+ T cells, 50 pmol of siRNAs was transfected into 1 million cells using 4D-Nucleofector (Lonza). The knockdown efficiency was confirmed through qPCR and Western blot analysis. The sequences of shRNAs and siRNAs were provided in Table S4.
RNA isolation and real-time RT-PCR
Total cellular RNA was extracted using TRIzol reagent (ThermoFisher), and cDNA was then synthesized with the PrimeScript RT Reagent Kit (Takara). Real-time PCR was performed using SYBR EX-taq premix (TaKaRa) on a CFX96 Real-Time PCR Detection Instrument (Bio-Rad). Human β-actin mRNA was measured as an internal control. Primers used for the detection of targeted genes were listed in Table S3. To quantify HIV-1 cell-associated RNA, a specific reverse primer was used for the reverse transcription of HIV-1 RNA: 5′-GCTTCAGCAAGCCGAGTCCTGCGTC-3′. Real-time PCR was then conducted to detect reverse-transcribed HIV-1 cDNA using the following primer pairs: HIVTotRNA Forward Primer: 5′-CTGGCTAACTAGGGAACCCACTGCT-3′ and HIVTotRNA Reverse Primer: 5′-GCTTCAGCAAGCCGAGTCCTGCGTC-3′. After quantification, in vitro transcribed HIV-1 RNA was used as the external control for measuring HIV-1 RNAs. The Ct value of each group was converted to mass and further transformed into copies. The final expression of HIV-1 cell-associated RNA was represented as 103 copies of viral RNA per million CD4+ T cells, as previously described (71, 72).
Co-immunoprecipitation and Western blot
Hela cells, transfected with Flag-tagged protein expression vectors, or J-Lat 10.6 cells were prepared for the co-IP assays. Cells were lysed with NP-40 lysis buffer [10 Mm Tris-HCl buffer, pH 7.5, 150 mM NaCl, 0.5% NP-40, 1% Triton X-100, 10% Glycerol, 2 mM EDTA, 1 mM NaF, 1 mM Na3VO4, and 1% protease inhibitor cocktail (Sigma-Aldrich)] for 30 min on ice with brief vertexing every 10 min. The lysates were centrifuged at 12,000 rpm for 10 min and then incubated with anti-Flag-tag beads (M8823, Sigma-Aldrich) or the specified immunoprecipitating antibodies while rotating overnight. After incubation, the immunoprecipitated products were washed five times with STN buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% NP-40, 0.5% Triton X-100) and eluted by boiling in SDS loading buffer at 95°C for 10 min. The eluted proteins were separated by SDS-PAGE and further immunoblotted with the indicated antibodies. Furthermore, 10% of the lysates were set as the input for co-IP assays. GAPDH (Proteintech, 10494–1-AP) or β-actin (Proteintech, 60008–1-Ig) was used as loading control. The secondary antibodies used were 680RD goat anti-mouse IgG antibody (LI-COR Biosciences, 926–68070) and 800CW goat anti-rabbit IgG antibody (LI-COR Biosciences, 926–32211). Images were acquired with an Odyssey CLX imager (LI-COR Biosciences) and analyzed by Image Studio Lite Ver 4.0 (LI-COR Biosciences).
The following specific antibodies were utilized for co-IP or immunoblotting: ORC1 antibody (A301-892A, Bethyl Laboratories, 1:1,000 for western blot), anti-DNMT1 antibody (ab188453, Abcam, 1:1,000 for western blot), anti-DNMT1 antibody [ab13537, Abcam, 1:50 for immunoprecipitation (IP)], anti-HDAC1 (10197–1-AP, Proteintech, 1:10,000 for western blot and 1:50 for IP), anti-HDAC2 (12922–3-AP, Proteintech, 1:10,000 for western blot and 1:50 for IP), anti-HP1α antibody (2616S, CST, 1:1,000 for western blot and 1:50 for IP), anti-SUV39H1 antibody (10574–1-AP, Proteintech, 1:1,000 for western blot), anti-SUV39H1 antibody (39785, Active Motif, 1:50 for IP), anti-EZH2 antibody (21800–1-AP, Proteintech, 1:1,000 for western blot), Anti-DDDDK Tag antibody (M185-3L, 1:1,000 for western blot).
ChIP-qPCR
Chromatin immunoprecipitation was performed following the experimental protocol provided by SimpleChIP Enzymatic Chromatin IP Kit (CST). Briefly, 4 × 106 cells were prepared for each immunoprecipitation. J-Lat 8.4 or J-Lat 10.6 cells infected with shORC1 were used to investigate the influence on the HIV-1 promoter in the absence of ORC1. HEK293T-based latently infected cells transfected with either ORC1 or LLPS-deficient ORC1 were utilized to investigate the enrichment of ORC1 on the HIV-1 promoter. Cells from each group were cross-linked with 1% formaldehyde (Sigma-Aldrich) for 10 min and quenched with 125 mM glycine for 5 min at room temperature. The supernatants were removed by centrifugation at 500 g for 5 min at 4°C, and then the cell pellets were lysed with provided buffer A (CST) for 10 min on ice. The nuclear pellets were collected by centrifugation at 500 g for 5 min at 4°C and then resuspended in 100 µL of buffer B (CST) containing 0.5 µL of micrococcal nuclease for chromatin digestion. After digestion for 20 min at 37°C, the supernatants were removed by centrifugation at 13,000 rpm for 1 min at 4°C. The nuclear pellets were resuspended in 500 µL of ChIP buffer (CST) and subjected to sonication for 1 min (20 s on, 30 s off) at 40% amplitude to disrupt the nuclear membrane. The supernatants containing chromatin sample were collected by centrifugation at 10,000 rpm for 10 min at 4°C. One-tenth of the chromatin sample was used to determine the concentration and fragment length of the sheared chromatin DNA by measuring OD260 and by electrophoresis on a 1% agarose gel, respectively. The fragment length of the sheared chromatin DNA should be between 150 and 900 bp in length.
For each IP preparation, 10 µg of chromatin DNA was resuspended in 500 µL of ChIP buffer, and 2% of chromatin DNA was used as the input sample to calculate the enrichment efficiency. The chromatin DNA was incubated with indicated antibodies at 4°C overnight with rotation. ChIP-Grade Protein G Magnetic Beads (CST) were added to each IP reaction and incubated with the IP samples for another 2 hours at 4°C while rotating. Each IP sample was washed three times with low-salt and one time with high-salt washing buffers. The input sample and immunoprecipitated products were eluted using ChIP elution buffer (CST) at 65°C for 2 hours while shaking at 1,200 rpm. The eluted DNA samples were reverse cross-linked with Proteinase K (CST) and purified using DNA purification spin columns (CST). ChIP primers used to detect the enrichment of the HIV-1 promoter were listed in Table S3.
The following specific antibodies were utilized for ChIP: ORC1 antibody (ab85830, Abcam, 1:25 for ChIP), anti-DNMT1 antibody (ab13537, Abcam, 1:25 for ChIP), anti-HDAC1 (10197–1-AP, Proteintech, 1:25 for ChIP), anti-HDAC2 (12922–3-AP, Proteintech, 1:25 for ChIP), anti-HP1α antibody (2616S, CST, 1:25 for ChIP), anti-Histone H3K9ac antibody (ab4441, Abcam, 5 µg for ChIP), anti-Histone H3K27ac antibody (ab4729, Abcam, 5 µg for ChIP), anti-Histone H3K9me3 antibody (ab8898, Abcam, 5 µg for ChIP), anti-Histone H3K27me3 antibody (ab192985, Abcam, 5 µg for ChIP).
Immunofluorescence
HEK293T cells were seeded on poly-lysine-treated coverslips. GFP-tagged ORC1 expression vectors were transfected into HEK293T cells when the cell confluence reached 60%–80%. Twenty-four hours after transfection, cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences) for 10 min at room temperature and then washed three times with PBS for 5 min each on a shaker. The fixed cells were permeabilized with 0.2% Triton X-100 (Sigma-Aldrich) for 15 min on a shaker and then washed three times with PBS for 5 min each on a shaker. For groups aiming to investigate the distribution of endogenous proteins or modifications, the fixed cells were blocked in 5% BSA/PBS for 1 hour on a shaker at room temperature and then washed three times with 0.1% Tween 20/PBS for 5 min each on a shaker. The blocked samples were proceeded to specific primary antibodies incubation for 1 hour on a shaker at room temperature. After washing with 0.1% Tween 20/PBS, cells were incubated with fluorescently labeled secondary antibodies for another 1 hour on a shaker at room temperature. Following incubation, cells were washed with 0.1% Tween 20/PBS and treated with DAPI (ThermoFisher) solution to stain chromatin DNA. After staining DNA with DAPI, the coverslips with cells were mounted on the glass slide using Antifade Mountant (ThermoFisher). The prepared samples were imaged using super-resolution structured illumination microscopy. The images were reconstructed and analyzed with the N-SIM module of the NIS-Elements AR software (Nikon).
Fluorescence recovery after photobleaching
HEK293T cells were seeded into eight-well optical culture plates and transfected with GFP-tagged ORC1 expression vectors. After 24 hours, cells were imaged under Nikon Confocal A1 microscope with a 488 nm laser. Within the field of view, two ORC1 bodies were selected as regions of interest (ROI). One ROI served as the unbleached negative control, while another ROI was bleached using a high-intensity 488 nm laser. Images were captured every 5 s to record the recovery process and changes in fluorescence intensity of the ROIs. The fluorescence intensities at each time point were normalized to the pre-bleaching time point.
Protein purification
The open reading frame (ORF) of GFP-tagged ORC1 IDRs was constructed into PET-28a vector. The constructed vectors were transformed into BL21 competent cells (Takara). A single clone was selected and amplified in LB medium containing kanamycin at 37°C with shaking at 220 rpm. When the OD600 value reached 0.6–0.8, IPTG (Takara) was added to the bacterial culture to induce protein production, and then the culture was transferred to a 16°C shaker. After 18 hours, the bacterial culture was centrifuged, washed with PBS, and then resuspended in Tris-HCl buffer (50 mM pH 7.5 Tris-HCl, 500 mM NaCl, 1% protease inhibitor cocktail). The resuspended bacteria were subjected to high-pressure homogenization, followed by centrifugation and filtration to remove residual bacteria. The solution containing proteins was incubated with Ni-NTA agarose (QIAGEN) to enrich His-tagged proteins. The enriched proteins on Ni-NTA agarose were washed with Tris-HCl buffer containing 50 mM imidazole and eluted with Tris-HCl buffer containing 500 mM imidazole. The eluted proteins were concentrated by using 30 kDa ultrafiltration units. Imidazole was removed, and the buffer was exchanged with Tris-HCl buffer. The purified proteins were stored at −80°C or used for downstream experiments.
In vitro droplet formation assay
The concentration of purified GFP-ORC1-IDR proteins was determined using the BCA protein quantification assay (ThermoFisher). Ten micrograms of proteins were added into Tris-HCl buffer with different NaCl gradients (ranging from 15.625 to 500 mM). In another condition for droplet formation, the protein concentration was gradient diluted from 10 µM in Tris-HCl buffer containing 50 mM NaCl. These protein solutions were placed on glass slides and imaged under Nikon Confocal A1 microscope with a 488 nm laser.
Bisulfite cytosine methylation assay
Genomic DNAs from J-Lat 8.4 cells treated with shRNA lentiviruses and 5-aza-dC were isolated using DNeasy Blood & Tissue Kit (QIAGEN). Sodium bisulfite conversion of the DNA was performed using the EpiTect Bisulfite Kit (QIAGEN). The bisulfite-converted DNA was then amplified in a nested PCR reaction with the following conditions: 1× (95°C—5 min), 40× (95°C—30 s, 55℃—30 s, 72°C—1 min), 1× (72°C—5 min). The primers used in the first round PCR were as follows: F1(5′-TTTATTGATTTTTGGATGGTGTTAT-3′) and R1(5′- CCATTTACCCCTAAATATTCTACAC-3′). The primers used in the second round PCR were F2(5′-ATATTTTGTGAGTTTGTATGGGATG-3′) and R2(5′- CCCAATATTTATCTACAA-3′). The amplification products were constructed into pMD-18T vector system (Takara). At least 20 clones were sequenced and quantified using the Quantification Tool for Methylation Analysis (QUMA) software. Only PCR clones with at least 95% conversion of cytosines outside CpGs were taken into account.
HIV-1 latency model construction
HIV-1 latency models established in Jurkat cells and HEK293T cells have been described in previous studies (23, 41). Briefly, the pNL4-3-ΔEnv/ΔNef-d2EGFP-pseudotyped HIV-1 construct, derived from pNL4-3, was used to establish the Jurkat latency model. A portion of env gene was deleted for a single round of infection of the virus, and an open reading frame containing destabilized, enhanced GFP was inserted into nef gene. A total of 3 µg of VSV-G envelope plasmid and 9 µg of pseudotyped virus construct was co-transfected into HEK293T cells. Forty-eight hours after transfection, the virus supernatants were concentrated with PEG6000 and then used to infect Jurkat cells. Forty-eight hours post-infection, GFP-negative cells were sorted using fluorescence-activated cell sorting (FACS) and then cultured for another week to facilitate recovery. The sorted cells were subsequently activated by TNFα. The reactivated GFP-positive cells were sorted and further cultured for 2 weeks to recover. If any GFP-positive cells persisted, GFP-negative cells were further sorted again. After several rounds of sorting, completely GFP-negative Jurkat cells infected with pseudotyped HIV-1 were obtained. These cells were sensitive to common LRAs. This Jurkat latency cell line was named as J-Lat Mix and was used to investigate the influence of ORC1 on HIV-1 latency maintenance.
The establishment of HEK293T latency model is similar to that of the J-Lat Mix model. HIV-1 pseudoviruses packaged with the NL4-3-ΔEnv/EGFP construct were used to infect HEK293T cells while ensuring that no more than 20% of cells were infected. GFP-positive HEK293T cells were sorted by FACS. After 4 weeks of culturing, almost all of GFP-positive cells would be GFP-negative cells. These obtained latent cells showed modest sensitivity to common LRAs. Genomic DNA PCR indicated that almost all the cells had been infected, suggesting that most of these cells were in deep latency.
The CD4+ T cell latency models were established based on the pseudotyped HIV-1 clone HIV-1NL4-3-ΔEnv-P2A-GFP or HIV-1NL4-3-CM6. The pseudotyped HIV-1NL4-3-ΔEnv-P2A-GFP was derived from the wild-typed HIV infectious clone pNL4-3. A portion of env gene was deleted for a single round of infection of the virus, and an ORF containing P2A and GFP was inserted downstream of the nef gene to indicate HIV expression. HIV-1NL4-3-ΔEnv-P2A-GFP-pseudotyped viruses were produced in the presence of VSV-G envelope plasmid. The pseudotyped HIV-1NL4-3-CM6 was obtained from Robert F. Siliciano (Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA). The ORF of GFP was inserted into the env gene, while other HIV-1 genes, including gag, pol, vif, vpr, vpu, and nef, were mutated to eliminate their expression. HIV-1NL4-3-CM6-pseudotyped viruses were produced in the presence of the VSV-G envelope plasmid and the lentiviral packaging plasmid pC-Help.
Viral outgrowth assay
VOA was used to detect the reactivation of replication-competent HIV-1. Primary CD4+ T cells isolated from PLWH were subsequently transfected with siNC and siORC1 using 4D-Nucleofector. Forty-eight hours after transfection, 10 million irradiated PBMCs from healthy donors and IL2 were added into each group. Twenty-four hours later, 4 million PHA-activated CD4+ T cells from healthy donors were introduced to each group, serving to amplify the reactivated infectious HIV-1. After culturing for 7 days, an equal number of PHA-activated CD4+ T cells were reintroduced to each group. The supernatants from each group were collected every 4 days and stored for further analysis. The monitoring process continued until day 14. Supernatants collected at different time points were used to detect the presence of HIV-1 antigen with p24 ELISA Kit (Abcam).
Affinity purification mass spectrometry
Empty vectors and Flag-tagged ORC1 expression vectors were transfected into Hela cells, respectively. Forty-eight hours after transfection, cells from each group were harvested and lysed with NP-40 lysis buffer, followed by immunoprecipitation with anti-Flag beads. Nucleases were added to the immunoprecipitation reaction to eliminate DNA/RNA contaminants. Enriched proteins were eluted by boiling in SDS loading buffer and separated by 4%–12% gradient SDS-PAGE. The separated proteins were visualized with the ProteoSilver Plus silver stain kit (Sigma-Aldrich). Then, the entire gel was cut into pieces and subjected to in-gel digestion with trypsin. All generated peptides were proceeded to LC-MS/MS analysis. The MS data of empty control and ORC1-IP groups in AP-MS were analyzed similarly to that in the CAPTURE screen. The enriched proteins were listed in Table S2.
ATAC-Seq
J-Lat 10.6 cells infected with shLuc and shORC1 were used to build ATAC-Seq library. The library was built with TruePrep DNA Library Prep Kit V2 (Vazyme). Briefly, approximately 30,000 cells from each group were lysed with 50 µL lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% Igepal CA-630) for 10 min on ice. After centrifuging to remove supernatants, the nuclear pellets were treated with Tn5 transposase and purified with VAHTS DNA Clean Beads (Vazyme), followed by PCR amplification. The amplified library was then purified again using VAHTS DNA Clean Beads (Vazyme) before proceeding to next-generation sequencing. The raw ATAC-Seq reads generated from shLuc and shORC1 groups were trimmed, filtered, and quality controlled by FastQC tool. The HIV-1 reference genome (version K03455.1) was inserted into the human reference genome Hg38 at the chr9:136468579 sites to construct the chimeric genome using samtools. Then, the reads were aligned to the chimeric genome by BWA software. Non-uniquely mapped reads or those with MAPQ scores below 20 were eliminated, and PCR duplicates were marked using Picard MarkDuplicates. ATAC peaks were called using the MACS2 callpeak function, and read counts were normalized by calculating reads per kilobase of bin per million of reads. Additionally, signals within blacklisted regions were filtered to allow downstream analysis. The generated bigWig files of each group were visualized in IGV tools. The integrated HIV-1 genome with specific sites was then amplified. Tag density from different groups was calculated by normalizing to the total mapped reads. Additionally, we use the computeMatrix and plotHeatmap tools from the deeptools software to analyze signal density around peak center across the entire genome (± 1 kb), based on the bigWig files.
Statistical analysis
Results of three experiments are presented as means ± standard errors of the means. Statistical analyses were conducted using GraphPad Prism 8. Value of P < 0.05 was considered statistically significant and denoted by an asterisk (*), while values of P < 0.01 and P < 0.001 were represented by two asterisks (**) or three asterisks (***), indicating more and most significant difference, respectively.
ACKNOWLEDGMENTS
We thank the clinicians at the Guangzhou Eighth People’s Hospital for their help with the collection of blood samples from HIV-1-infected patients.
This work was supported by the National Key R&D Program of Department of Science and Technology of China (2022YFC0870700), the Important Key Program of Natural Science Foundation of China (NSFC) (92369205, 92169201), the Guangdong Basic and Applied Research Foundation (2022B1111020004), the Emergency Key Program of Guangzhou National Laboratory (EKPG21-24) to H.Z., the Natural Science Foundation of China (82072265) to L.L., the National Key Research and Development Program (2021YFC2301904) and Basic and Applied Basic Research Fund Committee of Guangdong Province (SL2022A04J01923) to B.L., and Shenzhen Blood Center Scientific Research Project (SZBC202202) to L.W.
M.Z. designed the experiments, performed most of these experiments, analyzed the data, and manuscript writing; T.Y., M.Y., X.L., J.D., performed some of the experiments; S.W., Z.Z., Y.L., W.Z., B.X., Y.W., L.W., T.C., R.L., T.P., X.M. provided scientific expertise and the interpretation of data for the work; L.L., B.L., and H.Z. contributed to the idea generation, experimental design, and manuscript writing and conceived the project.
Contributor Information
Linghua Li, Email: llheliza@126.com.
Bingfeng Liu, Email: liubf5@mail.sysu.edu.cn.
Hui Zhang, Email: zhangh92@mail.sysu.edu.cn.
Frank Kirchhoff, Ulm University Medical Center, Ulm, Germany.
DATA AVAILABILITY
All data generated or analyzed during this study are included in the article and supplemental files.
ETHICS APPROVAL
The Ethics Review Boards of Sun Yat-Sen University and Guangzhou Eighth People’s Hospital approved this study.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jvi.00035-24.
Figures S1 to S7.
Host factors enriched in CAPTURE screen & prediction of host factors binding to the HIV-1 promoter using the hTFtarget database.
Protein list of ORC1 affinity purification mass spectrometry (AP-MS).
Primers used in qPCR analysis.
Sequences of shRNAs and siRNAs.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 9:727–728. doi: 10.1038/nm880 [DOI] [PubMed] [Google Scholar]
- 2. Strain MC, Günthard HF, Havlir DV, Ignacio CC, Smith DM, Leigh-Brown AJ, Macaranas TR, Lam RY, Daly OA, Fischer M, Opravil M, Levine H, Bacheler L, Spina CA, Richman DD, Wong JK. 2003. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci U S A 100:4819–4824. doi: 10.1073/pnas.0736332100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5:512–517. doi: 10.1038/8394 [DOI] [PubMed] [Google Scholar]
- 4. Davey RT, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, Kovacs JA, Polis MA, Walker RE, Falloon J, Masur H, Gee D, Baseler M, Dimitrov DS, Fauci AS, Lane HC. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 96:15109–15114. doi: 10.1073/pnas.96.26.15109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruner KM, Wang Z, Simonetti FR, Bender AM, Kwon KJ, Sengupta S, Fray EJ, Beg SA, Antar AAR, Jenike KM, et al. 2019. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 566:120–125. doi: 10.1038/s41586-019-0898-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ho Y-C, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DIS, Lai J, Blankson JN, Siliciano JD, Siliciano RF. 2013. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155:540–551. doi: 10.1016/j.cell.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hiener B, Horsburgh BA, Eden J-S, Barton K, Schlub TE, Lee E, von Stockenstrom S, Odevall L, Milush JM, Liegler T, Sinclair E, Hoh R, Boritz EA, Douek D, Fromentin R, Chomont N, Deeks SG, Hecht FM, Palmer S. 2017. Identification of genetically intact HIV-1 proviruses in specific CD4+ T cells from effectively treated participants. Cell Rep 21:813–822. doi: 10.1016/j.celrep.2017.09.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, Hughes SH. 2014. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345:179–183. doi: 10.1126/science.1254194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wagner TA, McLaughlin S, Garg K, Cheung CYK, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, Frenkel LM. 2014. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345:570–573. doi: 10.1126/science.1256304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jensen B-EO, Knops E, Cords L, Lübke N, Salgado M, Busman-Sahay K, Estes JD, Huyveneers LEP, Perdomo-Celis F, Wittner M, et al. 2023. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat Med 29:583–587. doi: 10.1038/s41591-023-02213-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsu J, Van Besien K, Glesby MJ, Pahwa S, Coletti A, Warshaw MG, Petz L, Moore TB, Chen YH, Pallikkuth S, Dhummakupt A, Cortado R, Golner A, Bone F, Baldo M, Riches M, Mellors JW, Tobin NH, Browning R, Persaud D, Bryson Y, International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1107 Team . 2023. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 186:1115–1126. doi: 10.1016/j.cell.2023.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, Martinez-Picado J, Nijhuis M, Wensing AMJ, Lee H, Grant P, Nastouli E, Lambert J, Pace M, Salasc F, Monit C, Innes AJ, Muir L, Waters L, Frater J, Lever AML, Edwards SG, Gabriel IH, Olavarria E. 2019. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 568:244–248. doi: 10.1038/s41586-019-1027-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, Schneider T. 2011. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood 117:2791–2799. doi: 10.1182/blood-2010-09-309591 [DOI] [PubMed] [Google Scholar]
- 14. Liu C, Ma X, Liu B, Chen C, Zhang H. 2015. HIV-1 functional cure: will the dream come true? BMC Med 13:284. doi: 10.1186/s12916-015-0517-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zerbato JM, Purves HV, Lewin SR, Rasmussen TA. 2019. Between a shock and a hard place: challenges and developments in HIV latency reversal. Curr Opin Virol 38:1–9. doi: 10.1016/j.coviro.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan S, Iqbal M, Tariq M, Baig SM, Abbas W. 2018. Epigenetic regulation of HIV-1 latency: focus on polycomb group (PcG) proteins. Clin Epigenetics 10:14. doi: 10.1186/s13148-018-0441-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hakre S, Chavez L, Shirakawa K, Verdin E. 2011. Epigenetic regulation of HIV latency. Curr Opin HIV AIDS 6:19–24. doi: 10.1097/COH.0b013e3283412384 [DOI] [PubMed] [Google Scholar]
- 18. Imai K, Togami H, Okamoto T. 2010. Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294. J Biol Chem 285:16538–16545. doi: 10.1074/jbc.M110.103531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. du Chéné I, Basyuk E, Lin Y-L, Triboulet R, Knezevich A, Chable-Bessia C, Mettling C, Baillat V, Reynes J, Corbeau P, Bertrand E, Marcello A, Emiliani S, Kiernan R, Benkirane M. 2007. Suv39H1 and HP1gamma are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. EMBO J 26:424–435. doi: 10.1038/sj.emboj.7601517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boehm D, Jeng M, Camus G, Gramatica A, Schwarzer R, Johnson JR, Hull PA, Montano M, Sakane N, Pagans S, Godin R, Deeks SG, Krogan NJ, Greene WC, Ott M. 2017. SMYD2-mediated histone methylation contributes to HIV-1 latency. Cell Host Microbe 21:569–579. doi: 10.1016/j.chom.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedman J, Cho WK, Chu CK, Keedy KS, Archin NM, Margolis DM, Karn J. 2011. Epigenetic silencing of HIV-1 by the histone H3 lysine 27 methyltransferase enhancer of Zeste 2. J Virol 85:9078–9089. doi: 10.1128/JVI.00836-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taura M, Song E, Ho YC, Iwasaki A. 2019. Apobec3A maintains HIV-1 latency through recruitment of epigenetic silencing machinery to the long terminal repeat. Proc Natl Acad Sci U S A 116:2282–2289. doi: 10.1073/pnas.1819386116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu L, Pan T, Zhou M, Chen T, Wu S, Lv X, Liu J, Yu F, Guan Y, Liu B, Zhang W, Deng X, Chen Q, Liang A, Lin Y, Wang L, Tang X, Cai W, Li L, He X, Zhang H, Ma X. 2022. CBX4 contributes to HIV-1 latency by forming phase-separated nuclear bodies and SUMOylating EZH2. EMBO Rep 23:e53855. doi: 10.15252/embr.202153855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kauder SE, Bosque A, Lindqvist A, Planelles V, Verdin E. 2009. Epigenetic regulation of HIV-1 latency by cytosine methylation. PLoS Pathog 5:e1000495. doi: 10.1371/journal.ppat.1000495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blazkova J, Trejbalova K, Gondois-Rey F, Halfon P, Philibert P, Guiguen A, Verdin E, Olive D, Van Lint C, Hejnar J, Hirsch I. 2009. CpG methylation controls reactivation of HIV from latency. PLoS Pathog 5:e1000554. doi: 10.1371/journal.ppat.1000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trejbalová K, Kovářová D, Blažková J, Machala L, Jilich D, Weber J, Kučerová D, Vencálek O, Hirsch I, Hejnar J. 2016. Development of 5' LTR DNA methylation of latent HIV-1 provirus in cell line models and in long-term-infected individuals. Clin Epigenetics 8:19. doi: 10.1186/s13148-016-0185-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang H, Kong W, Jean M, Fiches G, Zhou D, Hayashi T, Que J, Santoso N, Zhu J. 2019. A CRISPR/Cas9 screen identifies the histone demethylase MINA53 as a novel HIV-1 latency-promoting gene (LPG). Nucleic Acids Res 47:7333–7347. doi: 10.1093/nar/gkz493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das B, Dobrowolski C, Luttge B, Valadkhan S, Chomont N, Johnston R, Bacchetti P, Hoh R, Gandhi M, Deeks SG, Scully E, Karn J. 2018. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 115:E7795–E7804. doi: 10.1073/pnas.1803468115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Z, Hajian C, Greene WC. 2020. Identification of unrecognized host factors promoting HIV-1 latency. PLoS Pathog 16:e1009055. doi: 10.1371/journal.ppat.1009055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu X, Zhang Y, Chen Y, Li M, Zhou F, Li K, Cao H, Ni M, Liu Y, Gu Z, Dickerson KE, Xie S, Hon GC, Xuan Z, Zhang MQ, Shao Z, Xu J. 2017. In situ capture of chromatin interactions by biotinylated dCas9. Cell 170:1028–1043. doi: 10.1016/j.cell.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma X, Chen T, Peng Z, Wang Z, Liu J, Yang T, Wu L, Liu G, Zhou M, Tong M, Guan Y, Zhang X, Lin Y, Tang X, Li L, Tang Z, Pan T, Zhang H. 2021. Histone chaperone CAF-1 promotes HIV-1 latency by leading the formation of phase-separated suppressive nuclear bodies. EMBO J 40:e106632. doi: 10.15252/embj.2020106632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Q, Liu W, Zhang H-M, Xie G-Y, Miao Y-R, Xia M, Guo A-Y. 2020. hTFtarget: a comprehensive database for regulations of human transcription factors and their targets. Genomics Proteomics Bioinformatics 18:120–128. doi: 10.1016/j.gpb.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng Y, Schubert HL, Singh PK, Martins LJ, Engelman AN, D’Orso I, Hill CP, Planelles V. 2021. Cleavage and polyadenylation specificity factor 6 is required for efficient HIV-1 latency reversal. mBio 12:e0109821. doi: 10.1128/mBio.01098-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sheridan PL, Mayall TP, Verdin E, Jones KA. 1997. Histone acetyltransferases regulate HIV-1 enhancer activity in vitro. Genes Dev 11:3327–3340. doi: 10.1101/gad.11.24.3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boese A, Sommer P, Holzer D, Maier R, Nehrbass U. 2009. Integrase interactor 1 (Ini1/hSNF5) is a repressor of basal human immunodeficiency virus type 1 promoter activity. J Gen Virol 90:2503–2512. doi: 10.1099/vir.0.013656-0 [DOI] [PubMed] [Google Scholar]
- 36. Eilebrecht S, Le Douce V, Riclet R, Targat B, Hallay H, Van Driessche B, Schwartz C, Robette G, Van Lint C, Rohr O, Benecke AG. 2014. HMGA1 recruits CTIP2-repressed P-TEFb to the HIV-1 and cellular target promoters. Nucleic Acids Res 42:4962–4971. doi: 10.1093/nar/gku168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang G, Espeseth A, Hazuda DJ, Margolis DM. 2007. c-Myc and Sp1 contribute to proviral latency by recruiting histone deacetylase 1 to the human immunodeficiency virus type 1 promoter. J Virol 81:10914–10923. doi: 10.1128/JVI.01208-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ait Said M, Bejjani F, Abdouni A, Ségéral E, Emiliani S. 2023. Premature transcription termination complex proteins PCF11 and WDR82 silence HIV-1 expression in latently infected cells. Proc Natl Acad Sci U S A 120:e2313356120. doi: 10.1073/pnas.2313356120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jean MJ, Power D, Kong W, Huang H, Santoso N, Zhu J. 2017. Identification of HIV-1 Tat-associated proteins contributing to HIV-1 transcription and latency. Viruses 9:67. doi: 10.3390/v9040067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Le Douce V, Colin L, Redel L, Cherrier T, Herbein G, Aunis D, Rohr O, Van Lint C, Schwartz C. 2012. LSD1 cooperates with CTIP2 to promote HIV-1 transcriptional silencing. Nucleic Acids Res 40:1904–1915. doi: 10.1093/nar/gkr857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Irwan ID, Bogerd HP, Cullen BR. 2022. Epigenetic silencing by the SMC5/6 complex mediates HIV-1 latency. Nat Microbiol 7:2101–2113. doi: 10.1038/s41564-022-01264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matkovic R, Morel M, Lanciano S, Larrous P, Martin B, Bejjani F, Vauthier V, Hansen MMK, Emiliani S, Cristofari G, Gallois-Montbrun S, Margottin-Goguet F. 2022. TASOR epigenetic repressor cooperates with a CNOT1 RNA degradation pathway to repress HIV. Nat Commun 13:66. doi: 10.1038/s41467-021-27650-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conrad RJ, Fozouni P, Thomas S, Sy H, Zhang Q, Zhou MM, Ott M. 2017. The short isoform of BRD4 promotes HIV-1 latency by engaging repressive SWI/SNF chromatin-remodeling complexes. Mol Cell 67:1001–1012. doi: 10.1016/j.molcel.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones PA, Liang G. 2009. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet 10:805–811. doi: 10.1038/nrg2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fuks F, Burgers WA, Brehm A, Hughes-Davies L, Kouzarides T. 2000. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat Genet 24:88–91. doi: 10.1038/71750 [DOI] [PubMed] [Google Scholar]
- 46. Sarkar S, Abujamra AL, Loew JE, Forman LW, Perrine SP, Faller DV. 2011. Histone deacetylase inhibitors reverse CpG methylation by regulating DNMT1 through ERK signaling. Anticancer Res 31:2723–2732. [PubMed] [Google Scholar]
- 47. Fuks F, Hurd PJ, Deplus R, Kouzarides T. 2003. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic Acids Res 31:2305–2312. doi: 10.1093/nar/gkg332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oliviero G, Brien GL, Waston A, Streubel G, Jerman E, Andrews D, Doyle B, Munawar N, Wynne K, Crean J, Bracken AP, Cagney G. 2016. Dynamic protein interactions of the polycomb repressive complex 2 during differentiation of pluripotent cells. Mol Cell Proteomics 15:3450–3460. doi: 10.1074/mcp.M116.062240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Russo V, Bernabò N, Di Giacinto O, Martelli A, Mauro A, Berardinelli P, Curini V, Nardinocchi D, Mattioli M, Barboni B. 2013. H3K9 trimethylation precedes DNA methylation during sheep oogenesis: HDAC1, SUV39H1, G9a, HP1, and Dnmts are involved in these epigenetic events. J Histochem Cytochem 61:75–89. doi: 10.1369/0022155412463923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prasanth SG, Shen Z, Prasanth KV, Stillman B. 2010. Human origin recognition complex is essential for HP1 binding to chromatin and heterochromatin organization. Proc Natl Acad Sci U S A 107:15093–15098. doi: 10.1073/pnas.1009945107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peng PH, Hsu KW, Wu KJ. 2021. Liquid-liquid phase separation (LLPS) in cellular physiology and tumor biology. Am J Cancer Res 11:3766–3776. [PMC free article] [PubMed] [Google Scholar]
- 52. Alberti S, Gladfelter A, Mittag T. 2019. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176:419–434. doi: 10.1016/j.cell.2018.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fang X, Wang L, Ishikawa R, Li Y, Fiedler M, Liu F, Calder G, Rowan B, Weigel D, Li P, Dean C. 2019. Arabidopsis FLL2 promotes liquid-liquid phase separation of polyadenylation complexes. Nature 569:265–269. doi: 10.1038/s41586-019-1165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ludwiczak J, Winski A, Szczepaniak K, Alva V, Dunin-Horkawicz S. 2019. DeepCoil-a fast and accurate prediction of coiled-coil domains in protein sequences. Bioinformatics 35:2790–2795. doi: 10.1093/bioinformatics/bty1062 [DOI] [PubMed] [Google Scholar]
- 55. He Z, Jing S, Yang T, Chen J, Huang F, Zhang W, Peng Z, Liu B, Ma X, Wu L, Pan T, Zhang X, Li L, Cai W, Tang X, Zhang J, Zhang H. 2020. PIWIL4 maintains HIV-1 latency by enforcing epigenetically suppressive modifications on the 5’ long terminal repeat. J Virol 94:e01923-19. doi: 10.1128/JVI.01923-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim M, Hosmane NN, Bullen CK, Capoferri A, Yang HC, Siliciano JD, Siliciano RF. 2014. A primary CD4+ T cell model of HIV-1 latency established after activation through the T cell receptor and subsequent return to quiescence. Nat Protoc 9:2755–2770. doi: 10.1038/nprot.2014.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bell SP, Stillman B. 1992. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357:128–134. doi: 10.1038/357128a0 [DOI] [PubMed] [Google Scholar]
- 58. DePamphilis ML. 2005. Cell cycle dependent regulation of the origin recognition complex. Cell Cycle 4:70–79. doi: 10.4161/cc.4.1.1333 [DOI] [PubMed] [Google Scholar]
- 59. Bell SP, Dutta A. 2002. DNA replication in eukaryotic cells. Annu Rev Biochem 71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425 [DOI] [PubMed] [Google Scholar]
- 60. Chesnokov IN. 2007. Multiple functions of the origin recognition complex. Int Rev Cytol 256:69–109. doi: 10.1016/S0074-7696(07)56003-1 [DOI] [PubMed] [Google Scholar]
- 61. Sasaki T, Gilbert DM. 2007. The many faces of the origin recognition complex. Curr Opin Cell Biol 19:337–343. doi: 10.1016/j.ceb.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 62. Hou Z, Bernstein DA, Fox CA, Keck JL. 2005. Structural basis of the Sir1-origin recognition complex interaction in transcriptional silencing. Proc Natl Acad Sci U S A 102:8489–8494. doi: 10.1073/pnas.0503525102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lidonnici MR, Rossi R, Paixão S, Mendoza-Maldonado R, Paolinelli R, Arcangeli C, Giacca M, Biamonti G, Montecucco A. 2004. Subnuclear distribution of the largest subunit of the human origin recognition complex during the cell cycle. J Cell Sci 117:5221–5231. doi: 10.1242/jcs.01405 [DOI] [PubMed] [Google Scholar]
- 64. Tatsumi Y, Ezura K, Yoshida K, Yugawa T, Narisawa-Saito M, Kiyono T, Ohta S, Obuse C, Fujita M. 2008. Involvement of human ORC and TRF2 in pre-replication complex assembly at telomeres. Genes Cells 13:1045–1059. doi: 10.1111/j.1365-2443.2008.01224.x [DOI] [PubMed] [Google Scholar]
- 65. Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. 2009. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell 35:403–413. doi: 10.1016/j.molcel.2009.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hossain M, Stillman B. 2016. Opposing roles for DNA replication initiator proteins ORC1 and CDC6 in control of Cyclin E gene transcription. Elife 5:e12785. doi: 10.7554/eLife.12785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Iida T, Suetake I, Tajima S, Morioka H, Ohta S, Obuse C, Tsurimoto T. 2002. PCNA clamp facilitates action of DNA cytosine methyltransferase 1 on hemimethylated DNA. Genes Cells 7:997–1007. doi: 10.1046/j.1365-2443.2002.00584.x [DOI] [PubMed] [Google Scholar]
- 68. Prasanth SG, Prasanth KV, Siddiqui K, Spector DL, Stillman B. 2004. Human Orc2 localizes to centrosomes, centromeres and heterochromatin during chromosome inheritance. EMBO J 23:2651–2663. doi: 10.1038/sj.emboj.7600255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hossain M, Bhalla K, Stillman B. 2021. Multiple, short protein binding motifs in ORC1 and CDC6 control the initiation of DNA replication. Mol Cell 81:1951–1969. doi: 10.1016/j.molcel.2021.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Parker MW, Bell M, Mir M, Kao JA, Darzacq X, Botchan MR, Berger JM. 2019. A new class of disordered elements controls DNA replication through initiator self-assembly. Elife 8:e48562. doi: 10.7554/eLife.48562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li YC, Cheng TH, Gartenberg MR. 2001. Establishment of transcriptional silencing in the absence of DNA replication. Science 291:650–653. doi: 10.1126/science.291.5504.650 [DOI] [PubMed] [Google Scholar]
- 72. Dillin A, Rine J. 1997. Separable functions of ORC5 in replication initiation and silencing in Saccharomyces cerevisiae. Genetics 147:1053–1062. doi: 10.1093/genetics/147.3.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu X, Zhang Y, Chen Y, Li M, Shao Z, Zhang MQ, Xu J. 2018. CAPTURE: in situ analysis of chromatin composition of endogenous genomic loci by biotinylated dCas9. Curr Protoc Mol Biol 123:e64. doi: 10.1002/cpmb.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S7.
Host factors enriched in CAPTURE screen & prediction of host factors binding to the HIV-1 promoter using the hTFtarget database.
Protein list of ORC1 affinity purification mass spectrometry (AP-MS).
Primers used in qPCR analysis.
Sequences of shRNAs and siRNAs.
Data Availability Statement
All data generated or analyzed during this study are included in the article and supplemental files.