Abstract
Background
To investigate the survival outcome of “radical” GreenLight photoselective vaporization of bladder tumor (RPVBT) in conjunction with postoperative chemotherapy for patients with single, < 3 cm in diameter, T2 stage muscle invasive bladder cancer (MIBC).
Methods
Thirty-eight patients with single, < 3 cm, T2 stage bladder cancer were treated with RPVBT combined with chemotherapy and were included in the RPVBT group. To compare the differences in survival outcome, 80 patients with Ta/T1 bladder cancer and 30 patients with T2 bladder cancer were included as controls. The 80 patients with Ta/T1 bladder cancer underwent GreenLight photoselective vaporization of bladder tumors(PVBT), while 30 patients with T2 bladder cancer underwent radical cystectomy (RC) combined with pelvic lymph node dissection (PLND). Tumor recurrence and death were recorded, and recurrence-free survival (RFS) and overall survival (OS) curves were plotted to compare the survival difference between the RPVBT and control groups.
Results
No significant differences were observed in comorbidities or living habits between the RPVBT and control groups. Blood loss [RPVBT: 20 (IQR10, 20) vs. RC: 100 (IQR90, 150) mL] and postoperative hospital stay [RPVBT: 5.5 (IQR5, 6), vs. RC: 10 (IQR8, 12) days] in the RPVBT group were significantly lower than that in the RC group. Urinary tract infection [RPVBT: 6 (15.8%) vs. PVBT: 14 (17.5%)] and bladder irritation sign [RPVBT: 11 (28.9%) vs. PVBT: 23 (28.8%) ] were the most common short-term complications in the RPVBT group, with no statistical difference between the RPVBT and PVBT group. The median follow-up time for survival endpoints was 22 (16, 27) months for the included patients after surgery. The outcomes of tumor recurrence at 12, 24, and 36 months were 2 (5.3%), 3 (7.9%), and 5 (13.2%) patients in the RPVBT groups, 13 (16.3%) and 3 (10%) patients experienced recurrence in the PVBT and RC groups at 36 months. No significant differences were noted among the three groups (P = 0.778). Additionally, Kaplan–Meier survival analysis revealed no statistically significant differences in RFS (P = 0.791) and OS (P = 0.689) among the three groups.
Conclusions
Our findings indicate that RPVBT combined with chemotherapy is a simple and feasible treatment option with fewer complications and satisfactory survival outcomes in patients with single, < 3 cm, T2 stage bladder cancer.
Keywords: Bladder tumor, MIBC, PVBT, Greenlight laser
Background
The standard treatment for muscle invasive bladder cancer (MIBC) is widely acknowledged to be radical cystectomy (RC) combined with pelvic lymph node dissection (PLND). Several studies have proposed various organ-sparing strategies for patients with MIBC who cannot, or are unwilling to, undergo RC to preserve their quality of life without compromising tumor control.
Trimodal therapy (TMT) is recommended as the most beneficial organ-preserving strategy for MIBC [1, 2]. TMT typically involves a combination of surgery, radiation, and chemotherapy to retain bladder function and maintain tumor control without significantly compromising the quality of life. Complete transurethral resection of bladder tumor (TURBT) combined with chemotherapy and radiation is the most common combination. However, radiotherapy may lead to complications such as radiation enteritis and cystitis, which affect the quality of life [3, 4]. Moreover, salvageable RC becomes more challenging, and an organ-sparing strategy without radiotherapy lacks a definitive conclusion. Additionally, compared to TURBT, the GreenLight technique offers better hemostasis, a lower incidence of TUR syndrome, the absence of obturator nerve reflex, and a shorter learning curve. [5, 6]
Based on this research, we aimed to investigate the effect of “radical” Greenlight photoselective vaporization of bladder tumor (RPVBT) in conjunction with postoperative chemotherapy for patients with single, < 3 cm, T2 stage bladder cancer, providing reliable evidence for decision-making.
Methods
Patients and Population
Between January 2016 and December 2019, this retrospective observational study included 38 patients with single, < 3 cm, T2 stage bladder cancer who could not, or were unwilling to, undergo RC. These patients received RPVBT combined with chemotherapy. To compare differences in survival outcome, patients treated with PVBT and RC were included as controls. The PVBT group included 80 patients with Ta and T1 tumors, and the RC group included 30 patients with T2 tumors. All enrolled patients were confirmed to have urothelial carcinoma by cystoscopic biopsy, or no carcinoma in situ was observed. Additionally, no patient showed any signs of lymph node or distant metastases on imaging. Basic patient information (age, sex, body mass index [BMI], comorbidities, history of smoking and drinking) and tumor information (size, single/multiple, T stage and grade) were collected. This study was performed at the Tianjin Union Medical Center and was approved by the Institutional Ethics and Research Committee of Tianjin Union Medical Center. Four main investigators actively participated in the study, and informed consent was obtained from all participants.
Surgical Procedure
Patients in the PVBT and RPVBT groups were treated using a 180 W XPS GreenLight laser system (Boston Scientific, USA). The vaporization power was set to 60–120 W and the coagulation power was set to 20–30 W. PVBT was performed as previously described [7]. First, a cystoscopy was conducted to investigate tumor size, location, and number. Second, the bladder tumor was completely vaporized from the bottom if pedunculated. In contrast, wide-base tumors were vaporized and cut into small pieces to obtain pathological specimens. Vaporization reaches the bladder muscle layer. Furthermore, the surrounding 1–2 cm of the bladder mucosa was vaporized, and the disrupted vasculature was closed. Finally, cystoscopy was repeated to ensure complete tumor removal without damaging the ureteral orifice. An F-16 catheter was placed postoperatively. The catheters of patients in the PVBT group were removed 2–5 days after surgery, and immediate and regular bladder intravesical chemotherapy was administered. Regarding the RPVBT procedure, for tumors with a diameter of < 2 cm and a pedicle, the tumor pedicle should be fully exposed first; then, the tumor body should be vaporized and cut off along the root. For tumors with a diameter of 2–3 cm and a pedicle, the tumor surface can be vaporized to expose the tumor pedicle. For tumors with a wide base, the tumor body can be vaporized directly from the tumor surface, and intraoperative vaporization cutting can be used to obtain specimens. The depth of vaporization should be the fat layer outside the bladder muscle, and the tissue layers should be carefully identified intraoperatively to avoid bleeding caused by excessive local vaporization (Fig. 1). The bladder was filled moderately during the operation to avoid extravasation of the irrigation fluid. The duration of catheterization is 10–14 days, and postponing the first perfusion chemotherapy to approximately 2 weeks postoperatively is crucial for preventing fluid extravasation. Patients in the RC group underwent RC with standard PLND and urinary diversion of either the ileal conduit or the ureteral skin stoma. The chemotherapy regimen adopted is the GC scheme, which involves administering gemcitabine (1,000mg/m2) on the 1st and 8th days, and cisplatin (70mg/m2) on the 2nd day, and the chemotherapy cycle is repeated for 4 to 6 cycles.According to the EAU Guidelines and Chinese Urological Association (CUA) guidelines, the standards for adjuvant chemotherapy in the GC plan were as follows: multiple tumors, > 3 cm in diameter or high-grade tumors in the PVBT group, and high-grade tumors in the RC group. Patients in the RC group with a positive lymph node status received postoperative concurrent chemoradiation therapy.
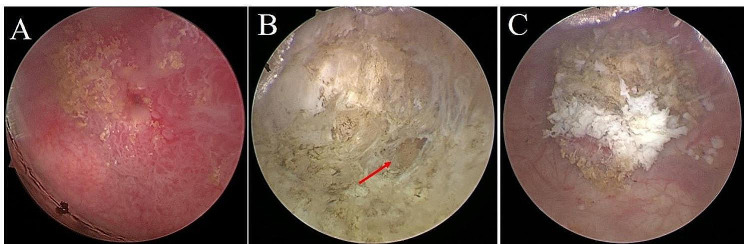
Fig. 1.
Vaporization depth of RPVBT. A: Bladder tumor. B: Fat layer outside the bladder muscle (red arrow). C: Specimens
Survival Outcome
Follow-up was regularly performed for the patients for up to 36 months after surgery. Tumor recurrence and death were considered the endpoints of observation, and the times of recurrence and death were recorded. Recurrence-free survival (RFS) and overall survival (OS) curves were plotted to compare survival among the three groups.
Statistical Analysis
The required sample size was calculated using the PASS 15 software (NCSS, Kaysville, Utah, USA), based on the pre-experimental data. The PVBT, RPVBT, and RC groups had values of 4.5, 5.5, and 12.5, respectively, with a standard deviation of 3.5, a two-sided α of 0.05, and a power of 0.90. Using One-Way Analysis of Variance F-Tests, it was determined that each group required at least six patients for enrollment. In this study, the final number of patients was 80, 38, and 30 in the PVBT, RPVBT, and RC groups, respectively, all meeting the minimum sample size requirement.
Continuous variables were first assessed for normality using the Shapiro–Wilk test. Continuous variables that followed a normal distribution were represented by the mean ± standard deviation and were compared using a one-way ANOVA. In contrast, continuous variables that did not follow a normal distribution were represented by the median (IQR) and were compared using non-parametric tests. Categorical variables were expressed as the number of patients (percentage), and a comparative analysis was performed using the chi-square test, continuous correction test, or Fisher’s exact test. RFS and OS were analyzed using Kaplan–Meier and log-rank tests. Post-hoc pairwise comparisons were conducted using the Bonferroni correction to adjust for multiple tests. Statistical significance was set at P < 0.05 and all statistical analyses were performed using SPSS 22.0.
Results
Thirty-eight patients with single, < 3 cm, T2 stage bladder cancer were included in the RPVBT group, comprising 28 male (73.7%) and 10 female patients (26.3%). The median age and mean BMI of patients were 61.5 (56.25, 75.75) and 24.51 ± 2.59, respectively. As controls, 80 patients with Ta/T1 bladder cancer and 30 patients with T2 bladder cancer were included in the PVBT and RC groups. Patient characteristics of the three groups are listed in Table 1.
Table 1.
The characteristics of patients between all groups
| Level | Total(n = 148) | PVBT(n = 80) | R-PVBT(n = 38) | RC(n = 30) | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age(median, IQR)(year) | 63 | 53, 73 | 63.5 | 58, 72 | 61.5 | 56.25, 75.75 | 59 | 57, 65.5 | 0.339 | |
| BMI(mean, SD)(kg/m²) | 24.62 | 3.09 | 24.8 | 3.25 | 24.51 | 2.59 | 24.28 | 3.3 | 0.714 | |
| gender(n/%) | 0.815 | |||||||||
| Male | 105 | 70.9% | 55 | 68.8% | 28 | 73.7% | 22 | 73.3% | ||
| Female | 43 | 29.1% | 25 | 31.3% | 10 | 26.3% | 8 | 26.7% | ||
| Tumor size(n/%) | < 0.001*# | |||||||||
| >3 cm | 38 | 25.7% | 21 | 26.3% | 0 | 0.0% | 17 | 56.7% | ||
| ≤ 3 cm | 110 | 74.3% | 59 | 73.8% | 38 | 100.0% | 13 | 43.3% | ||
| multiplicity(n/%) | 0.001*# | |||||||||
| Solitary | 125 | 84.5% | 67 | 83.8% | 38 | 100.0% | 20 | 66.7% | ||
| Multiple | 23 | 15.5% | 13 | 16.3% | 0 | 0.0% | 10 | 33.3% | ||
| T stage(n/%) | < 0.001*^ | |||||||||
| Ta | 49 | 33.1% | 49 | 61.3% | 0 | 0.0% | 0 | 0.0% | ||
| T1 | 31 | 20.9% | 31 | 38.8% | 0 | 0.0% | 0 | 0.0% | ||
| T2 | 68 | 45.9% | 0 | 0.0% | 38 | 100.0% | 30 | 100.0% | ||
| Grade(n/%) | 0.759 | |||||||||
| PUNLMP | 42 | 28.4% | 24 | 30.0% | 11 | 28.9% | 7 | 23.3% | ||
| Low | 73 | 49.3% | 38 | 47.5% | 19 | 50.0% | 16 | 53.3% | ||
| High | 33 | 22.3% | 18 | 22.5% | 8 | 21.1% | 7 | 23.3% | ||
RPVBT: “radical” GreenLight photoselective vaporization of bladder tumor; PVBT: GreenLight photoselective vaporization of bladder tumor; RC: radical cystectomy; PUNLMP: Papillary Urothelial Neoplasm of Low Malignant Potential
PUNLMP, RPVBT, PVBT, RC
Subgroup P<0.05 *RPVBT vs. PVBT, #RPVBT vs. RC, ^ PVBT vs. RC
one-way ANOVA: BMI; non-parametric tests: Age; chi-square test: gender, Tumor size, multiplicity, T stage, Grade
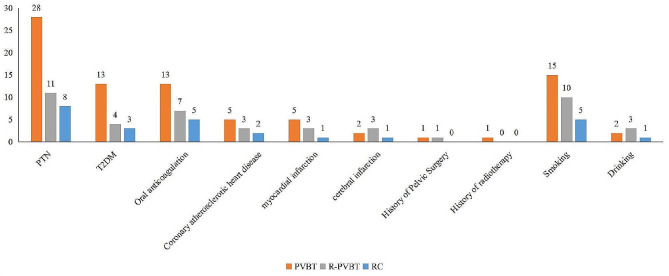
Regarding comorbidities in the RPVBT group, eleven (28.9%) and 4 (10.5%) patients had PTN and T2DM, respectively. Additionally, three (7.9%) suffered from myocardial infarction and cerebral infarction, respectively. Furthermore, seven patients (18.4%) were currently undergoing oral anticoagulant therapy, and 10 (26.3%) had a history of smoking. No obvious differences were observed in comorbidities or living habits among the three groups (P > 0.05), as shown in Table 2; Figs. 2 .
Table 2.
The comorbidities or living habits among the 3 groups
| Total(n = 148) | PVBT(n = 80) | R-PVBT(n = 38) | RC(n = 30) | P | |
|---|---|---|---|---|---|
| PTN(n/%) | 47 (31.8%) | 28 (35.0%) | 11 (29.0%) | 8 (26.7%) | 0.642 |
| T2DM(n/%) | 20 (13.5%) | 13 (16.3%) | 4 (10.5%) | 3 (10.0%) | 0.571 |
| Oral anticoagulation(n/%) | 25 (16.9%) | 13 (16.3%) | 7 (18.4%) | 5 (16.7%) | 0.877 |
| Coronary atherosclerotic heart disease(n/%) | 10 (6.8%) | 5 (6.3%) | 3 (7.9%) | 2 (6.7%) | 0.946 |
| myocardial infarction(n/%) | 9 (6.1%) | 5 (6.3%) | 3 (7.9%) | 1 (3.3%) | 0.734 |
| cerebral infarction(n/%) | 6 (4.1%) | 2 (2.5%) | 3 (7.9%) | 1 (3.3%) | 0.372 |
| History of Pelvic Surgery(n/%) | 2 (1.4%) | 1 (1.3%) | 1 (2.6%) | 0 (0.0%) | 0.643 |
| History of radiotherapy(n/%) | 1 (0.7%) | 1 (1.3%) | 0 (0.0%) | 0 (0.0%) | 0.652 |
| Smoking(n/%) | 30 (20.3%) | 15 (18.8%) | 10 (26.3%) | 5 (16.7%) | 0.545 |
| Drinking(n/%) | 6 (4.1%) | 2 (2.5%) | 3 (7.9%) | 1 (3.3%) | 0.372 |
Fig. 2.
Comorbidities and living habits among the three groups. No obvious differences were observed in comorbidities or living habits among the three groups
Blood loss and postoperative hospital stay in the RPVBT group were significantly lower than that in the RC group, and no significant differences were observed between the RPVBT and PVBT groups (Table 3). Urinary tract infection (UTI) and bladder irritation sign were the most common short-term complications in the RPVBT group, with no significantly difference between RPVBT and PVBT groups. Patients treated with RC experienced several postoperative adverse events, including intestinal obstruction, intestinal fistula, hydronephrosis, incision infection, and lymphatic leakage, which were not observed in the RPVBT group (Table 3). Moreover, there were 23 (28.8%), 38 (100%), and 7 (23.3%) patients treated with chemotherapy in PVBT, RPVBT, and RC, respectively. The toxicities related to chemotherapy included nausea, vomiting, leukopenia, thrombocytopenia, and anemia. No significant differences were observed among the three groups (Table 3).
Table 3.
The perioperative information and short-term complications
| Total(n = 148) | PVBT(n = 80) | R-PVBT(n = 38) | RC(n = 30) | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Operative time(median, IQR) (minutes) | 42.5 | 34, 62 | 40 | 33, 46 | 38 | 33.25, 46 | 290 | 260, 347.5 | < 0.001#^ | ||
| Blood loss(median, IQR) (ml) | 20 | 10, 40 | 10 | 10, 20 | 20 | 10, 20 | 100 | 90, 150 | < 0.001 | ||
| Hospital stay after surjery(median, IQR) (day) | 5 | 4, 8 | 4 | 4, 5 | 5.5 | 5,6 | 10 | 8, 12 | < 0.001#^ | ||
| Urinary diversion | |||||||||||
| Ureterocutaneous stoma(n/%) | 12 | 8.1% | 0 | 0.0% | 0 | 0.0% | 12 | 40.0% | < 0.001#^ | ||
| Bricker(n/%) | 18 | 12.2% | 0 | 0.0% | 0 | 0.0% | 18 | 60.0% | < 0.001#^ | ||
| Short-term complications | |||||||||||
| Hematuria(n/%) | 24 | 16.2% | 13 | 16.3% | 6 | 15.8% | 5 | 16.7% | 0.995 | ||
| UTI(n/%) | 25 | 16.9% | 14 | 17.5% | 6 | 15.8% | 5 | 16.7% | 0.973 | ||
| intestinal obstruction(n/%) | 3 | 2.0% | 0 | 0.0% | 0 | 0.0% | 3 | 10.0% | 0.002#^ | ||
| Intestinal fistula(n/%) | 1 | 0.7% | 0 | 0.0% | 0 | 0.0% | 1 | 3.3% | 0.138 | ||
| Hydronephrosis(n/%) | 4 | 2.7% | 0 | 0.0% | 0 | 0.0% | 4 | 13.3% | < 0.001^ | ||
| Bladder irritation sign(n/%) | 34 | 23.0% | 23 | 28.8% | 11 | 28.9% | 0 | 0.0% | 0.004#^ | ||
| Incision infection(n/%) | 2 | 1.4% | 0 | 0.0% | 0 | 0.0% | 2 | 6.7% | 0.019 | ||
| Lymphatic leakage(n/%) | 2 | 1.4% | 0 | 0.0% | 0 | 0.0% | 2 | 6.7% | 0.019 | ||
| Postoperative Treatment | |||||||||||
| Chemotherapy(n/%) | 68 | 45.9% | 23 | 28.8% | 38 | 100.0% | 7 | 23.3% | < 0.001*# | ||
| Toxicity related to chemotherapy | |||||||||||
| Nausea/vomiting(n/%) | 20 | 13.5% | 10 | 12.5% | 7 | 18.4% | 3 | 10.0% | 0.557 | ||
| Leukopenia(n/%) | 9 | 6.1% | 3 | 3.8% | 4 | 10.5% | 2 | 6.7% | 0.351 | ||
| Thrombocytopenia(n/%) | 3 | 2.0% | 1 | 1.3% | 0 | 0.0% | 2 | 6.7% | 0.118 | ||
| anemia(n/%) | 5 | 3.4% | 2 | 2.5% | 2 | 5.3% | 1 | 3.3% | 0.740 | ||
RPVBT: “radical” GreenLight photoselective vaporization of bladder tumor; PVBT: GreenLight photoselective vaporization of bladder tumor; RC: radical cystectomy; UTI: Urinary Tract Infection
Subgroup P<0.05 *RPVBT vs. PVBT, #RPVBT vs. RC, ^ PVBT vs. RC
non-parametric tests: Operative time, blood loss, Hospital stay after surjery; chi-square test: Urinary diversion, Short-term complications, Postoperative Treatment, Chemotherapy, Toxicity related to chemotherapy
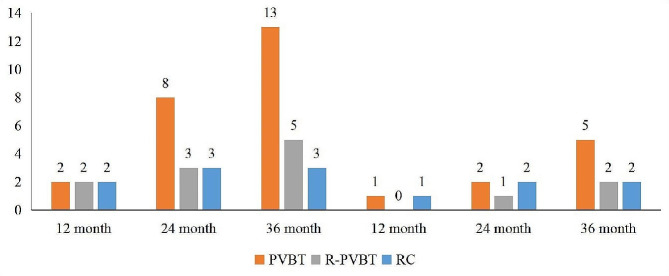
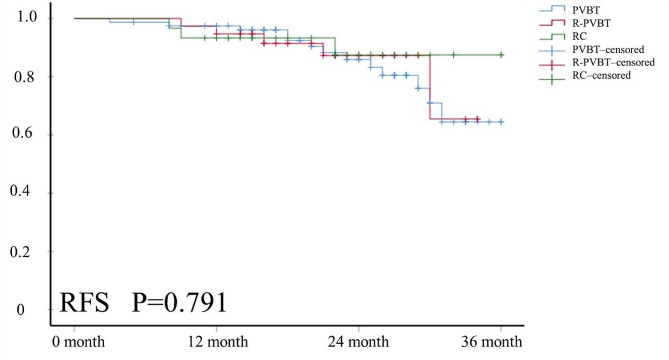
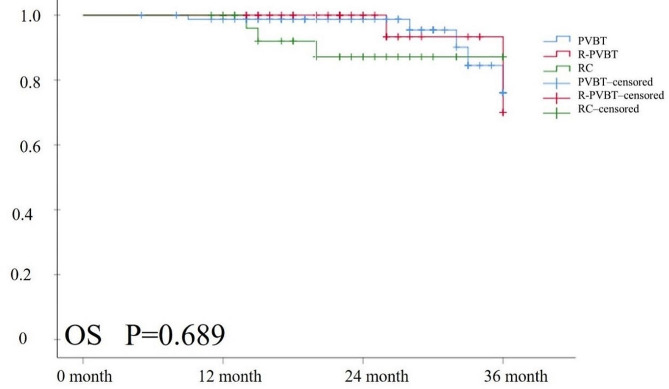
The median follow-up time for survival endpoints was 22 (16, 27) months for the included patients after surgery. The outcomes of tumor recurrence at 12, 24, and 36 months were 2 (5.3%), 3 (7.9%), and 5 (13.2%) patients in the RPVBT group (Fig. 3; Table 4). Four patients with bladder recurrence underwent secondary PVBT, and one patient with lymph node recurrence underwent salvage RC + PLND. There were 13 (16.3%) patients with tumor recurrence in the PVBT group; among them, 11 patients with bladder recurrence received secondary PVBT, two patients with bladder and lymph node recurrence underwent salvage RC + PLND. In RC group, Two patients experienced lymph node recurrence, and one patient had lung metastasis, while immunotherapy was administered to the three patients. No significant differences were noted among the three groups (P = 0.778). Kaplan–Meier survival analysis revealed no statistically significant differences in RFS (P = 0.791) or OS (P = 0.689) among the three groups (Figs. 4 and 5).
Fig. 3.
Outcomes of tumor recurrence and death among the three groups.The outcomes of tumor recurrence at 12, 24, and 36 months were 2 (5.3%), 3 (7.9%), 5 (13.2%) patients in the RPVBT group, 2 (5.3%), 8 (10.0%), 13 (16.3%) patients in the PVBT group, and 2 (6.7%), 3 (10.0%), 3 (10.0%) patients in the RC group. No significant differences were noted among the three groups (P = 0.778)
Table 4.
The outcomes of tumor recurrence and death among all groups
| Total(n = 148) | PVBT(n = 80) | R-PVBT(n = 38) | RC(n = 30) | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| tumor recurrence(n/%) | ||||||||||
| 12 month | 6 | 4.1% | 2 | 2.5% | 2 | 5.3% | 2 | 6.7% | 0.544 | |
| 24 month | 14 | 9.5% | 8 | 10.0% | 3 | 7.9% | 3 | 10.0% | 1.000 | |
| 36 month | 21 | 14.2% | 13 | 16.3% | 5 | 13.2% | 3 | 10.0% | 0.778 | |
| death(n/%) | ||||||||||
| 12 month | 2 | 1.4% | 1 | 1.3% | 0 | 0.0% | 1 | 3.3% | 0.430 | |
| 24 month | 5 | 3.4% | 2 | 2.5% | 1 | 2.6% | 2 | 6.7% | 0.497 | |
| 36 month | 9 | 6.1% | 5 | 6.3% | 2 | 5.3% | 2 | 6.7% | 1.000 | |
RPVBT: “radical” GreenLight photoselective vaporization of bladder tumor; PVBT: GreenLight photoselective vaporization of bladder tumor; RC: radical cystectomy;
Fig. 4.
The RFS curve outcome of the three groups. Kaplan-Meier survival analysis revealed no statistically significant differences in RFS (P = 0.791) among the three groups (Figs. 4 and 5)
Fig. 5.
The OS curve outcome of the three groups. Kaplan-Meier survival analysis revealed no statistically significant differences in OS (P = 0.689) among the three groups
Discussion
Our results are consistent with those of previous studies [8, 9], indicating an increase of old patients with high-risk bladder cancer. In this study, 47 (31.8%) and 20 (13.5%) patients had PTN and T2DM, and 9 (6.1%) and 6 (4.1%) patients suffered from myocardial infarction and cerebral infarction, respectively. Furthermore, 25 patients (16.9%) were currently undergoing oral anticoagulant therapy, and 30 (20.3%) had a history of smoking. For MIBC patients with multiple comorbidities, several studies have shown that RC had a high cure rate and a low recurrence rate in T2–T4a patients with MIBC [10, 11]. However, the high perioperative complication rate and impact on postoperative quality of life provided numerous patients with MIBC with the unwillingness to undergo RC [12, 13]. Reports show that the 1-month and 3-month major complication rates of RC were 14.4% and 21.7%, respectively [14]. Moreover, the mortality rate after RC gradually increased with age. Previous studies have shown that the mortality rate of patients aged 70–79 years after RC surgery was 5.4% at 3 months, whereas the rate increased to 9.2% in patients over 80 years [15–17]. Due to age, comorbidities, treatment toxicity, and subjective factors, increasing MIBC patients cannot undergo RC and remain hopeful for urgent adoption of organ-preserving treatment strategies.
TMT is a recognized treatment strategy for selected patients with MIBC [18, 19]. Fahmy et al. extracted data from 3402 patients treated with TMT and 26,891 patients with RC and observed no significant difference in the 10-year OS (30.9% vs. 35.1%) or DSS (50.9% vs. 57.8%) [20]. However, no large randomized controlled studies have been published on organ-preserving treatment strategies for MIBC. The difference in oncological outcomes between TMT and RC remains unclear, as reflected by the 5-year OS rates ranging from 36 to 74% [21]. Thus screening for the optimal patient population for TMT is challenging.
In this study, we used RPVBT surgery combined with adjuvant chemotherapy to treat patients with single, ≤ 3 cm, and T2 stage MIBC. Our data showed that patients with single, ≤ 3 cm T2 stage MIBC had similar RFS and OS with NMIBC treated with PVBT or patients with T2 stage MIBC who underwent RC. No reports exist on organ-conserving therapy with RPVBT + chemotherapy for patients with T2 stage, < 3 cm bladder cancer; however, a few research results are similar to our conclusions. Gofrit’s study showed that in cisplatin-eligible patients with a tumor diameter ≤ 3 cm, TMT provides an excellent disease-specific survival rate [22]. Giacalone et al. enrolled 475 patients with cT2-T4a MIBC and demonstrated that patients who underwent TMT had high rates of CR and confirmed DSS rates similar to those in RC, and patients with T2 tumor had a better outcome [2]. Kaushik’s comparative analysis showed that the median OS in MIBC patients treated with RC was longer than that in those who underwent TMT (36.2 vs. 24.2 months), and indicated that patients with T3 and T4 stagetumor in the TMT group had decreased OS compared to patients with T2 stage, which may be the selected patients [2]. Polineni’s study reported that selected T2 MIBC was an effective option with OS and DFS comparable to those of patients who underwent RC [23]. Ploussard et al. reported that the cancers best eligible for bladder preservation were those with low-volume T2 disease without in situ carcinoma [24]. Based on previous research and our findings, we believe that T2 stage, single, and tumor sizes of less than 3 cm in diameter are the populations for TMT treatment options.
The RPVBT for patients with MIBC had several technological advantages, as follows: (1) The GreenLight laser could vaporize tumors to the depth of the external fat of the bladder precisely, achieving local “radical.” (2) The fiber emits light laterally at 70°, which makes it easy to treat tumors on the anterior wall, top, and neck of the bladder. The GreenLight laser does not induce bladder perforation caused by the obturator nerve reflex, allowing the surgeon to completely vaporize the tumor. (4) Unlike TURBT, which directly contacts the tumor tissue, the GreenLight laser uses a non-contact method to vaporize tumors, which avoids tumor compression and potentially reduces the chance of tumor dissemination and metastasis. (5) Sealing vessels can prevent tumor cell implantation and transfer through the blood and lymphatic pathways. (6) The combination of postoperative adjuvant chemotherapy and bladder perfusion can reduce the rate of tumor recurrence. (7) RPVBT surgery can be performed in the supine position, which is suitable for patients who cannot undergo a lithotomy.
To address the potential challenge of irrigation fluid extravasation during the procedure, we perform vaporization resection of the tumor and close surrounding blood vessels prior to vaporizing to the extravesical fat, thereby shortening the surgical time after “bladder perforation”. Besides, when vaporizing the bladder muscle layer to the extravesical fat, we reduce the pressure of the irrigation fluid to prevent increased extravasation caused by excessive bladder pressure. To prevent tumor dissemination outside the bladder during RPVBT, we also tale several measures. Firstly, the risk of tumor metastasis is lower with “non-contact” Greenlight laser vaporization resection compared to TURBT. Secondly, by reducing irrigation fluid extravasation outside the bladder, we aim to minimize the risk of extravesical tumor spread. Lastly, after the vaporization resection is complete, we instill sterile water for injection into the bladder to further reduce the risk of tumor seeding.
Our study has certain limitations. First, retrospective studies on this topic are limited. Additionally, this study was a single-center effort, meaning that the enrolled patients were from a specific region. The number of patients enrolled in this study was relatively small, and the follow-up period was short. This highlights the necessity of substantiating our conclusions using larger samples and longer follow-up periods.
Conclusions
Our findings demonstrate that RPVBT combined with chemotherapy is a simple and feasible treatment option with fewer complications and satisfactory survival outcomes in patients with single, < 3 cm, T2 stage bladder cancer.
Acknowledgements
not applicable.
Author Contributions
Conceptualization, methodology, writing-original draft preparation by JL and YW Data curation, writing-reviewing and editing by FL and ZZ Software, validation and Supervision by YW and FL All authors approved the final version to be published.
Funding
This study was supported by the Tianjin Science and Technology Fund [No. 22JCYBJC01500] in collecting, analyzing, and interpreting data and in writing the manuscript.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethics Approval and Consent to Participate
This study was performed at the Tianjin Union Medical Center and was approved by the Institutional Ethics and Research Committee of Tianjin Union Medical Center.Informed consent was obtained from all the participants included in the study.
Consent for Publication
The authors declare their agreement for publication.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, et al. Propensity score analysis of Radical Cystectomy Versus bladder-sparing Trimodal Therapy in the setting of a multidisciplinary bladder Cancer Clinic. J Clin Oncol. 2017;35(20):2299–305. 10.1200/jco.2016.69.2327. 10.1200/jco.2016.69.2327 [DOI] [PubMed] [Google Scholar]
- 2.Giacalone NJ, Shipley WU, Clayman RH, Niemierko A, Drumm M, Heney NM, et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder Cancer: an updated analysis of the Massachusetts General Hospital Experience. Eur Urol. 2017;71(6):952–60. 10.1016/j.eururo.2016.12.020. 10.1016/j.eururo.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 3.Baumann BC, Sargos P, Efstathiou JA. Standard Versus Hypofractionated Radiation therapy for bladder Cancer: New insights, but questions remain. Int J Radiat Oncol Biol Phys. 2021;111(1):113–6. 10.1016/j.ijrobp.2021.04.048. 10.1016/j.ijrobp.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhury A, Porta N, Hall E, Song YP, Owen R, MacKay R, et al. Hypofractionated radiotherapy in locally advanced bladder cancer: an individual patient data meta-analysis of the BC2001 and BCON trials. Lancet Oncol. 2021;22(2):246–55. 10.1016/s1470-2045(20)30607-0. 10.1016/s1470-2045(20)30607-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan J, Wu K, Zhang N, Yang T, Liu N, Jiang Y, et al. Green-light laser en bloc resection versus conventional transurethral resection for initial non-muscle-invasive bladder cancer: a randomized controlled trial. Int J Urol. 2021;28(8):855–60. 10.1111/iju.14592. 10.1111/iju.14592 [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Gan G, Chen G, Wu GA, Systematic Review. Meta- analysis of Green-Light laser vaporization for superficial bladder. Urol J. 2020;17(6):578–86. 10.22037/uj.v16i7.5854. 10.22037/uj.v16i7.5854 [DOI] [PubMed] [Google Scholar]
- 7.Luo F, Ma C, Wu J, Li J. Prognostic value of the neutrophil-to-lymphocyte ratio in nonmuscle-invasive bladder Cancer treated with GreenLight Laser Vaporization. Photobiomodul Photomed Laser Surg. 2019;37(5):312–7. 10.1089/photob.2018.4592. 10.1089/photob.2018.4592 [DOI] [PubMed] [Google Scholar]
- 8.Gadzinski AJ, Psutka SP. Risk stratification metrics for bladder cancer: Comprehensive Geriatric assessments. Urol Oncol. 2020;38(9):725–33. 10.1016/j.urolonc.2020.01.003. 10.1016/j.urolonc.2020.01.003 [DOI] [PubMed] [Google Scholar]
- 9.Russell B, Häggström C, Holmberg L, Liedberg F, Gårdmark T, Bryan RT, et al. Systematic review of the association between socioeconomic status and bladder cancer survival with hospital type, comorbidities, and treatment delay as mediators. BJUI Compass. 2021;2(3):140–58. 10.1002/bco2.65. 10.1002/bco2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020;70(5):404–23. 10.3322/caac.21631. 10.3322/caac.21631 [DOI] [PubMed] [Google Scholar]
- 11.Zlotta AR, Ballas LK, Niemierko A, Lajkosz K, Kuk C, Miranda G, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023;24(6):669–81. 10.1016/s1470-2045(23)00170-5. 10.1016/s1470-2045(23)00170-5 [DOI] [PubMed] [Google Scholar]
- 12.Aminoltejari K, Black PC. Radical cystectomy: a review of techniques, developments and controversies. Transl Androl Urol. 2020;9(6):3073–81. 10.21037/tau.2020.03.23. 10.21037/tau.2020.03.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith ZL, Christodouleas JP, Keefe SM, Malkowicz SB, Guzzo TJ. Bladder preservation in the treatment of muscle-invasive bladder cancer (MIBC): a review of the literature and a practical approach to therapy. BJU Int. 2013;112(1):13–25. 10.1111/j.1464-410X.2012.11762.x. 10.1111/j.1464-410X.2012.11762.x [DOI] [PubMed] [Google Scholar]
- 14.Witjes JA, Feikema AAH. Organ-sparing strategies in muscle-invasive bladder Cancer. Cancer Manag Res. 2021;13:7833–9. 10.2147/cmar.S294099. 10.2147/cmar.S294099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Razdan S, Sljivich M, Pfail J, Wiklund PK, Sfakianos JP, Waingankar N. Predicting morbidity and mortality after radical cystectomy using risk calculators: a comprehensive review of the literature. Urol Oncol. 2021;39(2):109–20. 10.1016/j.urolonc.2020.09.032. 10.1016/j.urolonc.2020.09.032 [DOI] [PubMed] [Google Scholar]
- 16.Haque W, Verma V, Aghazadeh M, Darcourt J, Butler EB, Teh BS. Short-term Mortality Associated with definitive Chemoradiotherapy Versus Radical Cystectomy for muscle-invasive bladder Cancer. Clin Genitourin Cancer. 2019;17(5):e1069–79. 10.1016/j.clgc.2019.06.015. 10.1016/j.clgc.2019.06.015 [DOI] [PubMed] [Google Scholar]
- 17.Tran E, Souhami L, Tanguay S, Rajan R. Bladder conservation treatment in the elderly population: results and prognostic factors of muscle-invasive bladder cancer. Am J Clin Oncol. 2009;32(4):333–7. 10.1097/COC.0b013e31818b9486. 10.1097/COC.0b013e31818b9486 [DOI] [PubMed] [Google Scholar]
- 18.Polo-Alonso E, Kuk C, Guruli G, Paul AK, Thalmann G, Kamat A, et al. Trimodal therapy in muscle invasive bladder cancer management. Minerva Urol Nefrol. 2020;72(6):650–62. 10.23736/s0393-2249.20.04018-7. 10.23736/s0393-2249.20.04018-7 [DOI] [PubMed] [Google Scholar]
- 19.Hamad J, McCloskey H, Milowsky MI, Royce T, Smith A. Bladder preservation in muscle-invasive bladder cancer: a comprehensive review. Int Braz J Urol. 2020;46(2):169–84. 10.1590/s1677-5538.Ibju.2020.99.01. 10.1590/s1677-5538.Ibju.2020.99.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahmy O, Khairul-Asri MG, Schubert T, Renninger M, Malek R, Kübler H, et al. A systematic review and meta-analysis on the oncological long-term outcomes after trimodality therapy and radical cystectomy with or without neoadjuvant chemotherapy for muscle-invasive bladder cancer. Urol Oncol. 2018;36(2):43–53. 10.1016/j.urolonc.2017.10.002. 10.1016/j.urolonc.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Nagumo Y, Kojima T, Shiga M, Kandori S, Kimura T, Takaoka EI, et al. A single-institute experience of trimodal bladder-preserving therapy for histologic variants of urothelial carcinoma. Int J Clin Oncol. 2020;25(2):354–61. 10.1007/s10147-019-01553-4. 10.1007/s10147-019-01553-4 [DOI] [PubMed] [Google Scholar]
- 22.Gofrit ON, Meirovitz A, Frank S, Rabinovich I, Luwisch H, Yutkin V, et al. Trimodal therapy in T2-4aN0M0 bladder cancer–how to select the best candidate? Cancer Med. 2020;9(22):8491–7. 10.1002/cam4.3478. 10.1002/cam4.3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polineni P, Ashack L, Kalapurakal J, Morgans A, VanderWeele D, Kundu S, et al. Trimodality Treatment for muscle-invasive bladder Cancer: an institutional experience. Adv Radiat Oncol. 2021;6(5):100718. 10.1016/j.adro.2021.100718. 10.1016/j.adro.2021.100718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ploussard G, Daneshmand S, Efstathiou JA, Herr HW, James ND, Rödel CM, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014;66(1):120–37. 10.1016/j.eururo.2014.02.038. 10.1016/j.eururo.2014.02.038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.