Abstract
Objective
To assess rates of traversing barriers to care to access optimal clinical outcomes in people with migraine internationally.
Background
People in need of medical care for migraine should consult a health care professional knowledgeable in migraine management, obtain an accurate diagnosis, and receive an individualized treatment plan, which includes scientific society guideline-recommended treatments where appropriate.
Methods
The Chronic Migraine Epidemiology and Outcomes-International (CaMEO-I) Study was a cross-sectional, web-based survey conducted from July 2021 through March 2022 in Canada, France, Germany, Japan, the United Kingdom, and the United States (US). Respondents who met modified International Classification of Headache Disorders, 3rd edition, criteria for migraine and had Migraine Disability Assessment Scale (MIDAS) scores of ≥ 6 (i.e., mild, moderate, or severe disability) were deemed to need medical care and were included in this analysis. Minimally effective treatment required that participants were currently consulting a health care professional for headache (barrier 1), reported an accurate diagnosis (barrier 2), and reported use of minimally appropriate pharmacologic treatment (barrier 3; based on American Headache Society 2021 Consensus Statement recommendations). Proportions of respondents who successfully traversed each barrier were calculated, and chi-square tests were used to assess overall difference among countries.
Results
Among 14,492 respondents with migraine, 8,330 had MIDAS scores of ≥ 6, were deemed in need of medical care, and were included in this analysis. Current headache consultation was reported by 35.1% (2926/8330) of respondents. Compared with the US, consultation rates and diagnosis rates were statistically significantly lower in all other countries except France where they were statistically significantly higher. Total appropriate treatment rates were also statistically significantly lower in all other countries compared with the US except France, which did not differ from the US. All 3 barriers were traversed by only 11.5% (955/8330) of respondents, with differences among countries (P < 0.001).
Conclusions
Of people with migraine in need of medical care for migraine, less than 15% traverse all 3 barriers to care. Although rates of consultation, diagnosis, and treatment differed among countries, improvements are needed in all countries studied to reduce the global burden of migraine.
Trial registration
NA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-024-01834-y.
Keywords: Migraine, Headache, Consultation, Unmet needs, Barriers to care, Headache-related disability
Introduction
Migraine is a debilitating neurological disease affecting over 1 billion people worldwide [1, 2]. People with migraine in need of medical care should consult a health care professional knowledgeable in the management of migraine, obtain an accurate diagnosis, and receive an individualized treatment plan that includes guideline-recommended treatments when appropriate. Despite advances in available treatment, migraine is underdiagnosed and undertreated and is associated with substantial disability [3, 4]. Additionally, access to health care, treatment, and medication availability vary worldwide [5]. To allow for equitable treatment of patients with migraine, health-related disparities must be reduced across countries.
Multiple large-scale epidemiologic studies have shown that most people with migraine do not seek or receive medical care and even fewer receive accurate diagnosis and guideline-recommended therapies in many countries [6–8]. Results from the OVERCOME study, which was a prospective, longitudinal, web-based survey of adults with migraine in the United States (US), found that only half (51.0%) of participants had consulted a health care professional for migraine care in the past 12 months. Only 58.6% of those who met International Classification of Headache Disorders, 3rd edition (ICHD-3), criteria for migraine reported having ever received a medical diagnosis [6]. Moreover, although acute treatment is recommended for all people with migraine, most individuals do not use an acute prescription or a migraine-specific acute treatment [7, 9]. Although not recommended, the use of opioids and barbiturates remains high [7]. Furthermore, low rates of preventive medication use among eligible candidates with migraine have been observed in real-world studies for several decades [9, 10].
The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study, conducted in 2012–2013, provided insights into barriers to care for migraine in the US, including consulting, diagnosis, and treatment. Of respondents with migraine, less than 10% traversed the barriers of consultation, diagnosis, minimally appropriate pharmacologic treatment, and avoidance of acute medication overuse [11]. The Chronic Migraine Epidemiology and Outcomes – International (CaMEO-I) Study was conducted a decade after the CaMEO Study. This analysis of the CaMEO-I Study sought to assess rates of traversing barriers to optimal clinical care in people with migraine internationally to better understand the current scope of this problem.
Methods
Study design
CaMEO-I was a cross-sectional, web-based survey conducted from July 2021 through March 2022 in Canada, France, Germany, Japan, the United Kingdom (UK), and the US [8]. Respondents with migraine who met screening and eligibility criteria were identified by the American Migraine Study/American Migraine Prevalence and Prevention (AMS/AMPP) migraine diagnostic questionnaire and invited to complete the survey module. In the survey module, barriers to care were assessed by collecting data on consultation patterns, self-reported medical diagnoses of migraine and related conditions, and prescription pharmacologic treatment patterns. The American Headache Society (AHS) 2021 Consensus Statement [12] recommendations were applied to data in all 6 countries to provide uniform criteria for who needs preventive therapy to facilitate comparisons across the studied countries. Available evidence-based treatments were identified for each country using country-specific lists from the published literature as reviewed by a headache expert from each country and the study advisory committee (Supplemental Table 1). When available, previously validated survey components in each language were used. When necessary, surveys were translated from English into the respective language of each country, reviewed by local speakers and the investigator in each country for both language and cultural accuracy. Wording and survey content were adjusted based on cognitive debriefing with native speakers who met criteria for migraine. After survey completion, responses from the surveys conducted in languages other than English were back-translated into English. Prior to participant recruitment, the study design was reviewed and approved by a central institutional review board or ethics committee for all countries included in the study.
Study population
Respondents were drawn from survey research panels established in each of the 6 countries by Dynata™. Eligible respondents were adults aged ≥ 18 years who could read and understand the national language of their country of residence, provided informed consent, and met modified criteria for migraine consistent with the ICHD-3 (mICHD-3). Respondents who were judged to be in need of medical care based on a Migraine Disability Assessment Scale (MIDAS) grade of at least II (i.e., mild [grade II], moderate [grade III], or severe [grade IV] disability), defined by a total MIDAS score ≥ 6, were included in this analysis.
Assessments
Barrier 1: appropriate current consulter
Consultation with a health care professional was assessed based on responses to the following question: “What type of doctor is currently managing or treating your headaches?” To successfully traverse this barrier, respondents were required to endorse a health care professional deemed suitable to provide ongoing care to treat their migraine/headache. A complete list of health care professionals by country who were identified as well-suited for headache management, given it is a chronic disease, are included in Table 1. This list varies slightly from country to country based on local consultation patterns.
Table 1.
Criteria for traversing barriers to care by country
| Barrier | Criteria | |||||
|---|---|---|---|---|---|---|
| US | Canada | Germany | France | UK | Japan | |
| Appropriate current consultera | GP, family physician, or IM doctor | GP, family physician, or IM doctor | GP, family physician, or IM doctor | GP, family physician, or IM doctor | GP, family physician, or IM doctor | GP, family physician, or IM doctor |
| NP or PA | NP or PA | NP or PA | - | Clinical NP | - | |
| Allergist | Allergist | Allergist | Allergist | Allergist | Allergist | |
| ENT | ENT | ENT | ENT | ENT | ENT | |
| Headache specialist | Headache specialist | Headache specialist | Headache specialist | Headache specialist | Headache specialist | |
| Neurologist | Neurologist | Neurologist | Neurologist | Neurologist | Neurologist | |
| Ob-Gyn | Ob-Gyn | Ob-Gyn | Ob-Gyn | Ob-Gyn | Ob-Gyn | |
| Pain specialist | Pain specialist | Pain specialist | Pain specialist | Pain specialist | Pain specialist | |
| - | Ophthalmologistb | - | Ophthalmologistb | Ophthalmologistb | Ophthalmologistb | |
| Psychiatrist | Psychiatrist | Psychiatrist | Psychiatrist | Psychiatrist | Psychiatrist | |
| - | PM&R physicianb | - | - | - | - | |
| - | - | Orthopedic physicianb | Orthopedic physicianb | - | - | |
| - | - | - | Rheumatologist | - | - | |
| Accurate diagnosis |
< 15 MHDs, diagnosis = migraine or menstrual migraine ≥ 15 MHDs, diagnosis = chronic migraine or transformed migraine |
|||||
| Appropriate treatmentc | Acute treatment (for any respondent with a diagnosis of migrained) | |||||
| NSAID | NSAID | NSAID | NSAID | NSAID | NSAID | |
| Triptan | Triptan | Triptan | Triptan | Triptan | Triptan | |
| Ergotamine | Ergotamine | Ergotamine | Ergotamine | Ergotamine | Ergotamine | |
| Isometheptene | - | - | - | Isometheptene | - | |
| Ditane | - | - | - | - | Ditane | |
| Gepantf | - | - | - | Gepantf | - | |
| - | - | - | Combo Rxg | - | - | |
| - | - | - | - | - | Other Rx analgesich | |
| Preventive treatment (AHS Consensus Statement used to define need for preventive treatmenti) | ||||||
| Anti-seizure | Anti-seizure | Anti-seizure | Anti-seizure | Anti-seizure | Anti-seizure | |
| Anti-depressant | Anti-depressant | Anti-depressant | Anti-depressant | Anti-depressant | Anti-depressant | |
| Calcium channel blockers and other anti-hypertensives | Calcium channel blockers and other anti-hypertensives | Calcium channel blockers and other anti-hypertensives | Calcium channel blockers and other anti-hypertensives | Calcium channel blockers and other anti-hypertensives | Calcium channel blockers and other anti-hypertensives | |
| Toxin injections | Toxin injections | Toxin injections | Toxin injections | Toxin injections | - | |
| Gepantf | Gepantf | Gepantf | - | Gepantf | Gepantf | |
| - | - | - | Other oral preventive | - | - | |
AHS, American Headache Society; CGRP, calcitonin gene–related peptide; ENT, ear, nose, and throat specialist; GP, general practitioner; HCP, health care professional; IM, internal medicine; MHD, monthly headache day; NP, nurse practitioner; NSAID, nonsteroidal anti-inflammatory drug; Ob-Gyn, obstetrician-gynecologist; PA, physician assistant; PM&R, physical medicine and rehabilitation physician; Rx, prescription; UK, United Kingdom; US, United States
aHealth care professionals varied by country based on input from headache experts in each respective country. bOnly a few patients received care from an ophthalmologist, orthopedic doctor, or PM&R. cAppropriate treatment as per AHS guidance. dRespondents were excluded if the only acute treatment received was an opioid. eDitans were considered appropriate acute treatment in the countries in which they were available at the time of the survey. fGepants were considered appropriate treatments in the countries in which they were available at the time of the survey. gIncludes acetaminophen + caffeine. hIncludes Rx aspirin and Rx acetaminophen. iPer the AHS Consensus Statement, preventive medication for migraine should be offered to people with ≥ 6 MHDs regardless of disability, those with ≥ 4 MHDs with at least mild disability, and those with ≥ 3 MHDs with at least severe disability
Barrier 2: accurate diagnosis
Diagnosis was assessed based on responses to the following question: “Have you ever been diagnosed by a doctor or other health professional with the following types of headaches?” To successfully traverse this barrier, respondents with episodic migraine (EM) needed to provide a response of migraine or menstrual migraine and respondents with chronic migraine (CM) needed to provide a response of CM or transformed migraine. Although some may consider a diagnosis of migraine as adequate for people with CM, the study team believes that recognition of CM is essential for adequate management.
Barrier 3: minimally appropriate treatment
Inclusion criteria for this analysis required that respondents report some level of disability associated with migraine, operationally defined as MIDAS scores of ≥ 6 corresponding to a grade of II or higher. MIDAS reliability and validity have been tested in multiple languages including American and British English, French, German, and Japanese [13–16], providing previously validated translations. The AHS 2021 consensus statement was used to define the need for acute and preventive treatment to apply a common definition across each country. There are other definitions that could have been applied. Although the AHS definition provided a common measure, we do not believe that this definition is empirically better than guidelines from other countries [17, 18]. Following the AHS 2021 Consensus Statement recommendations, minimally appropriate pharmacologic treatment was defined as the use of at least one recommended prescription acute treatment for migraine [19]. Minimally appropriate acute treatment was assessed based on responses to the following question: “Which of these medications (if any) are you currently using (or typically keep on hand) to relieve or treat your headaches when you have them?” To successfully traverse this barrier, respondents were required to select or write in their acute prescription treatment for migraine. Nonsteroidal anti-inflammatory drugs, triptans, and ergotamine were acceptable types of medications available in each country; however, acute prescription treatments varied by country as per treatment guidelines and are listed in Table 1. Gepants and ditans approved for acute treatment in the country in question were considered appropriate. Respondents were judged not to be receiving appropriate acute treatment if they received only a barbiturate (US and Canada) or only an opioid.
Minimally appropriate preventive treatment was assessed based on responses to the questions pertaining to treatments taken to prevent or reduce the frequency of headaches or migraine attacks. Respondents who met criteria to be offered preventive treatment based on the AHS 2021 Consensus Statement recommendation [12] were required to report receiving a preventive treatment for migraine to successfully traverse this barrier. The AHS Consensus Statement suggests that those with ≥ 3 monthly headache days (MHDs) and severe disability (MIDAS grade IV), ≥ 4 MHDs and some disability (MIDAS grade II or grade III), or ≥ 6 MHDs regardless of the level of disability should be offered a preventive treatment for migraine. Medications commonly used for or that are indicated for migraine or chronic migraine in the following classes were included: anti-seizure, anti-depressant, anti-hypertensive, toxin injections, and preventive calcitonin gene–related peptide medications (Table 1). Judgements about individual preventive treatments used were based on drug availability in each country.
Statistical analysis
The proportions of respondents who successfully traversed each barrier were calculated. Pooled estimates for the countries included in the study were generated by computing results for each individual country. Although data are presented separately for each country, for pooled estimates, countries were weighted uniformly since each country contributed data from roughly 2400 participants. Chi-square tests were used to determine whether an overall difference across the countries existed in the proportions of respondents who traversed each barrier: total sample consulting, total sample consulting and diagnosed, those consulting that are diagnosed, total sample consulting, diagnosed, and treating, and those consulting and diagnosed that are treating. We did not correct for multiple comparisons. If differences were observed across countries for a particular barrier (consultation, diagnosis and treatment), we conducted planned post hoc tests contrasting the US with each of the other countries. The significance level was set at < 0.05. Statistical analysis was performed using SPSS Statistics for Macintosh, version 29.0 (IBM, Armonk, NY).
Results
Analysis population
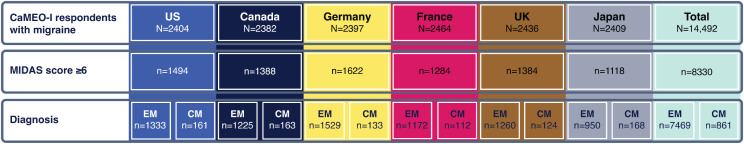
A total of 14,492 respondents were identified as having migraine based on mICHD-3 criteria (Fig. 1). The overall mean age of respondents with migraine was 41.7 years and the majority were female (71.2%) [8]. Of these respondents, 8330 (57.5%) were deemed in need of medical care for migraine and were included in this analysis. Of this subgroup, 7469 (89.7%) met criteria for EM and 861 (10.3%) met criteria for CM.
Fig. 1.
Analysis population. CaMEO-I, Chronic Migraine Epidemiology and Outcomes International; CM, chronic migraine; EM, episodic migraine; MIDAS, Migraine Disability Assessment; UK, United Kingdom; US, United States
Barrier 1: appropriate current consulter
A total of 2926 (35.1%) of 8330 respondents successfully traversed the consulting barrier (Table 2). Consultation rates differed to a statistically significant degree among countries (chi2 = 171.2; P < 0.001). In comparison with the US, rates of consultation were statistically significantly lower in Canada (chi2 = 21.0; P < 0.001), Germany (chi2 = 4.6; P = 0.033), the UK (chi2 = 21.3; P < 0.001), and Japan (chi2 = 42.2; P < 0.001) and significantly higher in France (chi2 = 27.2; P < 0.001). Rates of consultation were highest in France (48.5% [623/1284]) and lowest in Japan (26.6% [297/1118]). Current consultation was reported by a greater proportion of respondents with CM (43.2% [372/861]) than with EM (34.2% [2554/7469]).
Table 2.
Proportions of respondents traversing barriers to care among the eligible migraine sample
| US (N = 1494) |
Canada (N = 1388) |
Germany (N = 1662) |
France (N = 1284) |
UK (N = 1384) |
Japan (N = 1118) |
Totala (N = 8330) |
|
|---|---|---|---|---|---|---|---|
| Barrier 1: Current consulter b | |||||||
| Total sample consulting, % (95% CI) |
n = 578 38.7 (36.2, 41.2) |
n = 424 30.5 (28.1, 33.0) |
n = 582 35.0 (32.7, 37.3) |
n = 623 48.5 (45.8, 51.3) |
n = 422 30.5 (28.1, 32.9) |
n = 297 26.6 (24.0, 29.2) |
n = 2926 35.1 (34.1, 36.2) |
| Chi2 (P value) | - |
21.0 (< 0.001) |
4.6 (0.033) |
27.2 (< 0.001) |
21.3 (< 0.001) |
42.2 (< 0.001) |
171.2 (< 0.001) |
| Barrier 2: Accurate diagnosis (EM or CM) | |||||||
| Total sample consulting and diagnosed, % (95% CI) |
n = 404 27.0 (24.8, 29.3) |
n = 280 20.2 (18.1, 22.3) |
n = 385 23.2 (21.1, 25.2) |
n = 392 30.5 (28.0, 33.1) |
n = 279 20.2 (18.0, 22.3) |
n = 178 15.9 (13.8, 18.1) |
n = 1918 23.0 (22.1, 23.9) |
| Chi2 (P value) | - |
18.8 (< 0.001) |
6.3 (0.012) |
4.1 (0.043) |
18.8 (< 0.001) |
45.7 (< 0.001) |
99.0 (< 0.001) |
| Those consulting that are diagnosed, % (95% CI) |
69.9 (66.2, 73.7) |
66.0 (61.5, 70.6) |
66.2 (62.3, 70.0) |
62.9 (59.1, 66.7) |
66.1 (61.6, 70.7) |
59.9 (54.3, 65.5) |
65.6 (63.8, 67.3) |
| Chi2 (P value) | - |
1.7 (0.195) |
1.9 (0.172) |
6.5 (0.011) |
1.6 (0.204) |
8.7 (0.003) |
11.1 (0.050) |
| Barrier 3: Appropriate treatment | |||||||
| Those consulting and diagnosed that are treating, % (95% CI) |
n = 215 53.2 (48.3, 58.1) |
n = 127 45.4 (39.5, 51.2) |
n = 180 46.8 (41.8, 51.8) |
n = 184 46.9 (42.0, 51.9) |
n = 164 58.8 (53.0, 64.6) |
n = 85 47.8 (40.3, 55.2) |
n = 955 49.8 (47.6, 52.0) |
| Chi2 (P value) | - |
4.1 (0.043) |
3.3 (0.069) |
3.1 (0.077) |
2.1 (0.150) |
1.5 (0.224) |
16.1 (0.007) |
| Traversing all 3 barriers | |||||||
| Total sample consulting, diagnosed, and treating, % (95% CI) |
14.4 (12.6, 16.2) |
9.1 (7.6, 10.7) |
10.8 (9.3, 12.3) |
14.3 (12.4, 16.3) |
11.8 (10.1, 13.6) |
7.6 (6.1, 9.2) |
11.5 (10.8, 12.2) |
| Chi2 (P value) | - |
18.9 (< 0.001) |
9.1 (0.003) |
0.002 (0.964) |
4.1 (0.044) |
29.0 (< 0.001) |
47.6 (< 0.001) |
CM, chronic migraine; EM, episodic migraine; UK, United Kingdom; US, United States
Unless otherwise specified, Chi2 P values represent a comparison between the US and other countries
aChi2 P values are across all 6 countries
bCurrent consulters included respondents within the migraine population who had their migraine managed by a health care professional other than an emergency department or urgent care professional
Barrier 2: accurate diagnosis
Of respondents who successfully traversed the consulting barrier, 65.6% (1918/2926) reported an accurate diagnosis based on self-reported medical diagnosis (Table 2). An overall difference in the proportions of respondents who received a diagnosis of migraine was detected across all 6 countries among current consulters (chi2 = 11.1; P = 0.050). In comparison with the US, the proportion of respondents who received a diagnosis of migraine was statistically significantly lower in France (chi2 = 6.5; P = 0.011) and Japan (chi2 = 8.7; P = 0.003) among current consulters; differences between the US and other countries were not significant. The highest proportion of respondents who traversed the first barrier and reported an accurate diagnosis was observed in the US (69.9% [404/578]), while the lowest proportion was observed in Japan (59.9% [178/297]). Those with EM who traversed the first barrier were much more likely to receive an accurate diagnosis than those with CM (72.6% [1855/2544] vs. 16.9% [63/372]).
Among all 8330 respondents with migraine, 1918 (23.0%) successfully traversed the first 2 barriers. Of the 7469 respondents with EM, 1855 (24.8%) traversed both barriers and of the 861 respondents with CM, 63 (7.3%) traversed both barriers. An overall difference in the proportions of respondents who received a diagnosis of migraine was detected across all 6 countries among respondents who traversed barriers 1 and 2 (chi2 = 99.0; P < 0.001). Proportions of respondents who received a diagnosis of migraine differed between the US and all other countries; diagnostic rates were statistically significantly lower in Canada (chi2 = 18.8; P < 0.001), UK (chi2 = 18.8; P < 0.001), Germany (chi2 = 6.3; P = 0.012) and Japan (chi2 = 45.7; P < 0.001), and statistically significantly higher in France (chi2 = 4.1; P = 0.043; Table 2).
Barrier 3: minimally appropriate pharmacologic treatment
Of respondents who successfully traversed both the consulting and accurate diagnosis barriers, 49.8% (955/1918) reported receiving minimally appropriate pharmacologic treatment (Table 2). Minimally appropriate pharmacologic treatment was reported by a greater proportion of respondents with EM (50.2% [932/1855]) than with CM (36.5% [23/63]).
Among diagnosed consulters, an overall difference in the proportions of respondents who received minimally appropriate treatment was detected across all 6 countries (chi2 = 16.1; P = 0.007). The highest proportion of respondents who traversed the third barrier after having traversed the first and second was observed in the UK (58.8% [164/279]) and the lowest proportion was observed in Canada (45.4% [127/280]). Compared with the US, the proportion of respondents who received minimally appropriate treatment was statistically significantly lower in Canada (chi2 = 4.1; P = 0.043) among diagnosed consulters.
Among respondents who traversed both the consulting and accurate diagnosis barriers, rates of appropriate acute and preventive pharmacologic treatment were 79.8% (1530/1918) and 60.7% (1164/1918), respectively (Supplemental Table 2). The highest proportion of respondents who received appropriate acute pharmacologic treatment was observed in Japan (93.3% [166/178]) and the lowest proportion was observed in Canada (72.1% [202/280]). Compared with the US, the proportion of respondents who received appropriate acute pharmacologic treatment was statistically significantly higher in Japan (chi2 = 20.6; P < 0.001); the differences between the US and other countries were not statistically significant. The highest proportion of respondents who traversed the consulting and accurate diagnosis barriers and received appropriate preventive pharmacologic treatment was observed in the UK (68.1% [190/279]) and the lowest proportion was observed in Japan (50.0% [89/178]). Compared with the US, the proportion of respondents who received appropriate preventive pharmacologic treatment was statistically significantly lower in Germany (chi2 = 5.9; P = 0.015), France (chi2 = 6.4; P = 0.012), and Japan (chi2 = 13.5; P < 0.001).
If receiving minimally appropriate pharmacologic treatment was not contingent on receiving an accurate diagnosis, then minimally appropriate pharmacologic treatment was observed in 46.4% (1358/2926) of respondents (Supplemental Table 3). Among those who traversed barrier 1 but not necessarily barrier 2, rates of appropriate acute and preventive pharmacologic treatment were 75.4% (2205/2926) and 58.4% (1708/2926), respectively. The highest proportion of respondents who traversed barrier 1 but not necessarily barrier 2 and received appropriate acute pharmacologic treatment was observed in Japan (90.6% [269/297]) and the lowest proportion was observed in Canada (64.4% [273/424]). The proportion of respondents who received appropriate acute pharmacologic treatment was statistically significantly lower in Canada than in the US (chi2 = 9.7; P = 0.002) and statistically significantly higher in Japan than in the US (chi2 = 34.7; P < 0.001). The highest proportion of respondents who traversed barrier 1 but not necessarily barrier 2 and received appropriate preventive pharmacologic treatment was observed in the UK (65.6% [277/422]) and the lowest proportion was observed in Japan (46.8% [139/297]). Compared with the US, the proportion of respondents who received appropriate preventive pharmacologic treatment was statistically significantly lower in Germany (chi2 = 7.1; P = 0.008), France (chi2 = 5.8; P = 0.016), and Japan (chi2 = 22.4; P < 0.001).
Traversing all 3 barriers to care
Overall, 955 (11.5%) of 8330 respondents successfully traversed all 3 barriers. A total of 932 (12.5%) of 7469 respondents with EM and 23 (2.7%) of 861 respondents with CM traversed all 3 barriers. An overall difference in the proportions of respondents who traversed all 3 barriers was detected across all 6 countries (chi2 = 47.6; P < 0.001). Proportions of respondents who traversed all 3 barriers were statistically significantly lower in Canada (chi2 = 18.9; P < 0.001), Germany (chi2 = 9.1; P = 0.003), UK (chi2 = 4.1; P = 0.044), and Japan (chi2 = 29.0; P < 0.001) than in the US and not statistically significantly different in France (Table 2).
Discussion
This analysis from the CaMEO-I Study identified people with migraine who had unmet needs based on a MIDAS grade of II or higher (i.e., mild, moderate, or severe migraine-related disability) and assessed 3 levels of barriers to care including current consultation, accurate diagnosis, and appropriate treatment. Of 8330 respondents who were deemed in need of medical care for migraine, only 11.5% traversed all 3 barriers. The greatest barrier was at the level of consultation: only 35% of respondents reported that they were currently under the care of a clinician for headache deemed appropriate for their country. Respondents with CM were more likely to consult a health care professional than those with EM (43.2% vs. 34.2%), but less likely to receive an accurate diagnosis (16.9% vs. 72.6%). Those with CM were also less likely to receive minimal appropriate treatment than those with EM (36.5% vs. 50.2%). Additionally, only 12.5% of respondents with EM and 2.7% of respondents with CM traversed all 3 barriers.
Prior studies have demonstrated low consultation rates among people with migraine in the US [11, 20–22]. Data are limited on consultation rates from other countries [23–25]. In Denmark, almost one quarter of people with migraine have never consulted a health care professional for headache despite access to universal health care [26]. The CaMEO Study, conducted in the US in 2012, showed that 29.4% of respondents traversed the consulting barrier [11]. Among those with at least 1 lifetime consultation for headache, the US-based OVERCOME study revealed consultation rates of 28.1% with a neurologist and 15.6% with a headache specialist [6]. The majority of consultations were with a primary care provider (70.3%) [6]. The FRAMIG-3 study demonstrated that consultation rates in France were also low [25].
Reasons for a low rate of consultation may depend not only on the accessibility of health care professionals in primary and secondary care, but also on the willingness and/or ability of the person with migraine to seek medical care. Stigma is a known barrier to seeking care, and migraine is a stigmatized condition [27–30]. There are likely many other barriers to access such as socioeconomic, geographic, time/convenience, and other issues [11, 20, 21]. Although consultation rates in the US have increased by almost 10% within the past decade, they remain very low, with not much greater than half of people with migraine currently being cared for, indicating that improvement is still needed [11]. Moreover, the accessibility of health care varies among countries. For example, some countries, such as France, have access to universal health care, which has the potential to increase the likelihood of consultation. However, in Poland, many residents are entitled to free-of-charge health care and are encouraged to consult with neurologists for migraine, but misdiagnosis remains a concern [31].
In a prior publication regarding barriers to care, an accurate diagnosis of migraine among people who consulted a health care professional was reported by 39.5% of participants [21]. In a separate study, an accurate diagnosis of CM was reported by 24.6% of participants who consulted a health care professional [20]. Our findings suggest that among those who consult a health care professional for their migraine, more than half are likely to receive an accurate diagnosis. Although cross study comparisons are hazardous, the previous studies were similar in methodology to our present study, and the increased rate of diagnosis among consulters may reflect improved diagnostic rates, improvements in communication, or improvement in migraine awareness leading to better recall of migraine diagnoses [32]. Similar to results from previous studies, those with migraine/EM were much more likely to receive an accurate diagnosis than those with CM. Given the severity of CM and that some products are approved or reimbursed only for CM, the authors considered a diagnosis of CM as an important step in optimizing patient care and reducing stigma. As most people who seek care for migraine consult with a primary care professional [33], primary care education on diagnosis is imperative.
In the EUROLIGHT study, conducted from 2008 to 2009 in 10 countries across Europe, between 3.4% and 68.2% of participants with migraine used a triptan and between 1.6% and 41.7% of those who were eligible for preventive treatment received it [24, 34]. Additionally, in the CaMEO Study, 68.3% of respondents who traversed the consultation and diagnosis barriers received minimally appropriate acute treatment and 55.8% of those who were eligible for preventive treatment received it. Our study demonstrates that approximately 50% of respondents who traversed the barriers of consultation and accurate diagnosis received minimally appropriate pharmacologic treatment. Moreover, respondents with CM were less likely to receive minimally appropriate treatment than those with EM, which may have been due to the requirement for all respondents with CM to report receiving a preventive treatment. Although there may be some improvements in the use of migraine-specific medications, this continues to be a major barrier to care. Reasons for a lack of appropriate prescription treatment may stem from lack of recognition of the migraine burden, educational gaps in migraine care, and the perceptions and preferences of patients who may have concerns about side effects, long-term risks, and efficacy of medications [35].
Across all six countries in this report, we note patterns of underconsultation, underdiagnosis, and undertreatment. There were also statistical differences among countries for each barrier and in the overall proportions that traversed all 3 barriers. Notably, proportions of respondents who traversed all 3 barriers in the US differed from those in Canada, Germany, the UK, and Japan (Table 2). In Japan, among consulters with an accurate diagnosis, a high percentage (93.3%) received appropriate acute pharmacologic treatment, but only 50.0% received appropriate preventive pharmacologic treatment. These differences may be partially due to the willingness of people with migraine in different countries to seek care, the differences in health care systems in each country, and the treatments available for migraine in each country. Further exploration of these barriers to care and how to appropriately traverse these barriers in each country is an area of future interest to optimize patient care.
Defining barriers to care at 3 levels, underconsultation, underdiagnosis, and undertreatment, provides a country-specific report card that can inform the allocation of resources within a country at various levels of intervention. Underconsultation can be addressed at the level of public education, primary care screening, or electronic health record-based case-finding [36–40]. Barriers to diagnosis can be addressed through public and clinician education, screening, and case-findings. Barriers to treatment are best addressed through public and clinician education, the provision of real-time, treatment-decision support in electronic health records, and the adaptation of quality indicators in primary and secondary care.
This analysis is limited by reliance on self-reported data and the potential influence of selection bias. We also made a series of choices when other options were available. This paper focused on pharmacotherapy and did not consider devices or behavioral or lifestyle interventions, in part because data on nonpharmacologic interventions are limited. Only people with MIDAS scores of ≥ 6 were considered in need of better care in this analysis; however, someone with infrequent disabling headache might benefit from a more effective acute treatment. Assessing and comparing barriers to care is challenging because the clinicians who treat migraine, guidance on who needs treatment, and the available medication varies among countries. For example, we made the judgement that emergency department and urgent care (ED/UC) health care professionals were not appropriate for delivering long-term migraine care. This is not to suggest that ED/UC professionals lack the skills to treat migraine. We were guided instead by the practical limitations for continuity of care and follow-up in that setting [41, 42]. Similarly, some of the best headache clinicians in the world are neuro-ophthalmologists. Based on the judgement of health care professionals from each country, ophthalmologists were considered appropriate health care professionals in four of the six countries. Ophthalmologic consultation was not common, so this choice was unlikely to have had a major influence on our findings. We also used the US as the reference country for post hoc comparisons; statistically significant findings could be different if a different a priori reference country is selected.
Uniform criteria for diagnosis of migraine were based on the ICHD-3. Criteria for needing acute or preventive treatment also were uniform and based on the AHS Consensus Statement [12]. Although we recognize that there are other guidance documents we could have used, having a uniform definition was judged to be most helpful for purposes of making comparisons across countries. We anticipate that the national principal investigators of each country may want to run country-specific analyses using national or regional guidance on treatment needs and using their country as the reference for making comparisons among countries.
Finally, the medications considered appropriate were based on medications available in each country. For example, onabotulinumtoxinA is not available for migraine prevention in Japan and so was not considered there. In France, acetaminophen + caffeine was included because it is available as a prescription drug; however, other countries have similar formulations available over-the-counter that were not included in this analysis, although there is a body of evidence demonstrating the efficacy of caffeine combinations [43, 44].
Notable strengths include the collection of data from six countries, each represented by a headache expert or experts from that country. The use of uniform measures across countries provided similar snapshots of barriers to care in each country. Moreover, each country was represented by a systematically recruited, demographically representative sample of that country with more than 2000 individuals with migraine from each country.
Conclusion
Although advancements have been made, less than 15% of people with migraine who have some associated disability (a MIDAS grade of II or higher) and need medical care traverse all 3 barriers to care. These findings highlight the significant public health issue that people with migraine do not seek medical care at acceptable rates. These findings suggest that there is a large unmet need across all 6 countries for improving awareness and care for people living with migraine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the contributions of Ryan Bostic of MIST Research, Wilmington, NC, for data analyses. The authors also thank the study participants, site collaborators, and survey translators. Medical writing support was provided to the authors by Anny Wu, PharmD, of Peloton Advantage, LLC, an OPEN Health company, and was funded by AbbVie.
Abbreviations
- AHS
American Headache Society
- AMPP
American Migraine Prevalence and Prevention
- AMS
American Migraine Study
- CaMEO
Chronic Migraine Epidemiology and Outcomes
- CaMEO-I
Chronic Migraine Epidemiology and Outcomes – International
- CM
Chronic migraine
- ED
Emergency department
- EM
Episodic migraine
- ICHD-3
International Classification of Headache Disorders, 3rd edition
- MHD
Monthly headache day
- MIDAS
Migraine Disability Assessment Scale
- UC
Urgent care
- UK
United Kingdom
- US
United States
Author contributions
Study design: Dawn Buse, Richard Lipton. Collection and assembly of data: Kristina Fanning, Aubrey Manack Adams, Michael Seminerio. Data analysis: Richard Lipton. Data interpretation: All authors. Manuscript preparation: Aubrey Manack Adams. Manuscript review and revisions: All authors. Final approval of manuscript: All authors.
Funding
AbbVie funded this trial and contributed to the study design, the collection, analysis, and interpretation of data, and the review and approval of the final manuscript for publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Data availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select “Home.”
Declarations
Ethics approval and consent to participate
Prior to participant recruitment, the study design was reviewed and approved by a central institutional review board or ethics committee for all countries included in the study.
Consent for publication
Not applicable.
Competing interests
ML-M, reports personal fees for advisory boards, speaker panels, or investigation studies from Allergan, Amgen, Astellas, ATI, BMS, Boehringer, Boston Scientific, CoLucid, Convergence, GlaxoSmithKline, Grunenthal, IPSEN, Lundbeck, Lilly, Medtronic, Menarini, MSD, Novartis, Orion, Pfizer, Reckitt Benckiser, Saint-Jude, Sanofi-Aventis, Teva, UCB, UPSA, and Zambon. EL has received speaker fees and consulting fees from Allergan, Eli Lilly, and Teva Neuroscience; consulting fees from Aralez Pharmaceuticals, McKesson Canada, and Medscape; speaking fees, consulting, and reimbursement for travel from Novartis; and reimbursement for travel from I-GPAC. ZK has been a speaker and/or consultant and/or has received research support from Allergan, Amgen/Novartis, Eli Lilly, Merck, and Teva. RBL reports support for the present study from AbbVie, research support paid to his institution from the Czap Foundation, the National Headache Foundation, the National Institutes of Health, the S&L Marx Foundation, and the US Food and Drug Administration, and personal fees from AbbVie/Allergan, American Academy of Neurology, American Headache Society, Amgen, Biohaven, BioVision, Boston, Dr. Reddy’s (Promius), electroCore, Eli Lilly, GlaxoSmithKline, Grifols, Lundbeck (Alder), Merck, Pernix, Pfizer, Teva, Vector, and Vedanta Research. He holds stock/options in Axon, Biohaven, and Manistee. He is the overall principal investigator for the CaMEO-I Study. FS is a consultant, speaker, or scientific advisor for Amgen, Eli Lilly, Otsuka, and Teva, and the national principal investigator in Japan for the CaMEO-I Study. MM serves on the advisory board for Abbott, AbbVie, Eli Lilly, Lundbeck, Pfizer, Salvia, and Teva; has received payment for the development of educational presentations from AbbVie, Eli Lilly, Lundbeck, Pfizer, and Teva; and is the national principal investigator in the United Kingdom for the CaMEO-I Study. KF is managing director of MIST Research which has received research funding from AbbVie, Allay Lamp, Juva Health, NYC Langone Health and GlaxoSmithKline via grants to the National Headache Foundation. AMA and MS are employees of AbbVie and may hold AbbVie stock. KS was an employee of AbbVie at the time this study was conducted and may hold AbbVie stock. DCB has received grant support and honoraria from AbbVie, Amgen, Biohaven, Collegium, Eli Lilly and Company, Lundbeck, and Teva and for work on the editorial board of Current Pain and Headache Reports and is the national principal investigator in the United States for the CaMEO-I Study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Employee at the time of study conduct.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet 390:1211–1259 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of Disease Study 2015. Lancet 388:1545–1602 [DOI] [PMC free article] [PubMed]
- 3.Miller S, Matharu MS (2014) Migraine is underdiagnosed and undertreated. Practitioner 258:19–24 [PubMed] [Google Scholar]
- 4.Cohen F, Lipton RB (2024) Prevalence and burden of migraine in the United States: a systematic review. Headache 64:516–532 10.1111/head.14709 [DOI] [PubMed] [Google Scholar]
- 5.Raffaelli B, Rubio-Beltrán E, Cho SJ, De Icco R, Labastida-Ramirez A, Onan D et al (2023) Health equity, care access and quality in headache—part 2. J Headache Pain 24:167 10.1186/s10194-023-01699-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipton RB, Nicholson RA, Reed ML, Araujo AB, Jaffe DH, Faries DE et al (2022) Diagnosis, consultation, treatment, and impact of migraine in the US: results of the OVERCOME (US) study. Headache 62:122–140 10.1111/head.14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson S, Lipton RB, Ailani J, Reed ML, Fanning KM, Manack Adams A et al (2020) Characterization of acute prescription migraine medication use: results from the CaMEO study. Mayo Clin Proc 95:709–718 10.1016/j.mayocp.2019.11.025 [DOI] [PubMed] [Google Scholar]
- 8.Manack Adams A, Buse DC, Leroux E, Lanteri-Minet M, Sakai F, Matharu M et al (2023) Chronic migraine epidemiology and outcomes–international (CaMEO-I) study: methods and multi-country baseline findings for diagnosis rates and care. Cephalalgia 43:1–13 [DOI] [PubMed] [Google Scholar]
- 9.Lipton RB, Munjal S, Alam A, Buse DC, Fanning KM, Reed ML et al (2018) Migraine in America symptoms and treatment (MAST) study: baseline study methods, treatment patterns, and gender differences. Headache 58:1408–1426 10.1111/head.13407 [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68:343–349 10.1212/01.wnl.0000252808.97649.21 [DOI] [PubMed] [Google Scholar]
- 11.Buse DC, Armand CE, Charleston Lt, Reed ML, Fanning KM, Adams AM et al (2021) Barriers to care in episodic and chronic migraine: results from the chronic migraine epidemiology and outcomes study. Headache 61:628–641 10.1111/head.14103 [DOI] [PubMed] [Google Scholar]
- 12.Ailani J, Lipton RB, Goadsby PJ, Guo H, Miceli R, Severt L et al (2021) Atogepant for the preventive treatment of migraine. N Engl J Med 385:695–706 10.1056/NEJMoa2035908 [DOI] [PubMed] [Google Scholar]
- 13.Stewart WF, Lipton RB, Whyte J, Dowson A, Kolodner K, Liberman JN et al (1999) An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology 53:988–994 10.1212/WNL.53.5.988 [DOI] [PubMed] [Google Scholar]
- 14.Iigaya M, Sakai F, Kolodner KB, Lipton RB, Stewart WF (2003) Reliability and validity of the Japanese Migraine Disability Assessment (MIDAS) Questionnaire. Headache 43:343–352 10.1046/j.1526-4610.2003.03069.x [DOI] [PubMed] [Google Scholar]
- 15.Magnoux E, Freeman MA, Zlotnik G (2008) MIDAS and HIT-6 French translation: reliability and correlation between tests. Cephalalgia 28:26–34 10.1111/j.1468-2982.2007.01461.x [DOI] [PubMed] [Google Scholar]
- 16.Benz T, Lehmann S, Gantenbein AR, Sandor PS, Stewart WF, Elfering A et al (2018) Translation, cross-cultural adaptation and reliability of the German version of the migraine disability assessment (MIDAS) questionnaire. Health Qual Life Outcomes 16:42 10.1186/s12955-018-0871-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eigenbrodt AK, Ashina H, Khan S, Diener HC, Mitsikostas DD, Sinclair AJ et al (2021) Diagnosis and management of migraine in ten steps. Nat Rev Neurol 17:501–514 10.1038/s41582-021-00509-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pringsheim T, Davenport W, Mackie G, Worthington I, Aube M, Christie SN et al (2012) Canadian Headache Society guideline for migraine prophylaxis. Can J Neurol Sci 39:S1–59 [PubMed] [Google Scholar]
- 19.Ailani J, Burch RC, Robbins MS (2021) The American Headache Society consensus statement: update on integrating new migraine treatments into clinical practice. Headache 61:1021–1039 10.1111/head.14153 [DOI] [PubMed] [Google Scholar]
- 20.Dodick DW, Loder EW, Manack Adams A, Buse DC, Fanning KM, Reed ML et al (2016) Assessing barriers to chronic migraine consultation, diagnosis, and treatment: results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) study. Headache 56:821–834 10.1111/head.12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipton RB, Serrano D, Holland S, Fanning KM, Reed ML, Buse DC (2013) Barriers to the diagnosis and treatment of migraine: effects of sex, income, and headache features. Headache 53:81–92 10.1111/j.1526-4610.2012.02265.x [DOI] [PubMed] [Google Scholar]
- 22.Lipton RB, Stewart WF, Simon D (1998) Medical consultation for migraine: results from the American Migraine Study. Headache 38:87–96 10.1046/j.1526-4610.1998.3802087.x [DOI] [PubMed] [Google Scholar]
- 23.Groth M, Katsarava Z, Ehrlich M (2022) Results of the gErman migraine PatIent survey on medical care and prOPhylactic treatment experience (EPISCOPE). Sci Rep 12:4589 10.1038/s41598-022-08716-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrée C, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, Lainez JM et al (2011) The Eurolight project: the impact of primary headache disorders in Europe. Description of methods. J Headache Pain 12:541–549 10.1007/s10194-011-0356-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas C, Géraud G, Valade D, Chautard MH, Lantéri-Minet M (2006) Recognition and therapeutic management of migraine in 2004, in France: results of FRAMIG 3, a French nationwide population-based survey. Headache 46:715–725 10.1111/j.1526-4610.2006.00430.x [DOI] [PubMed] [Google Scholar]
- 26.Do TP, Dømgaard M, Stefansen S, Steiner TJ, Ashina M (2023) Characterizing healthcare utilization patterns in a Danish population with headache: results from the nationwide headache in Denmark (HINDER) panel. J Headache Pain 24:18 10.1186/s10194-023-01553-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross E, de la Ruiz E, Martelletti P (2023) The migraine stigma kaleidoscope view. Neurol Ther 12:703–709 10.1007/s40120-023-00456-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corrigan P (2004) How stigma interferes with mental health care. Am Psychol 59:614–625 10.1037/0003-066X.59.7.614 [DOI] [PubMed] [Google Scholar]
- 29.Seng EK, Shapiro RE, Buse DC, Robbins MS, Lipton RB, Parker A (2022) The unique role of stigma in migraine-related disability and quality of life. Headache 62:1354–1364 10.1111/head.14401 [DOI] [PubMed] [Google Scholar]
- 30.Shapiro RE, Nicholson RA, Seng EK, Buse DC, Reed ML, Zagar AJ et al (2024) Migraine-related stigma and its relationship to disability, interictal burden, and quality of life: results of the OVERCOME (US) study. Neurology 102:e208074 10.1212/WNL.0000000000208074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waliszewska-Prosół M, Straburzyński M, Czapińska-Ciepiela EK, Nowaczewska M, Gryglas-Dworak A, Budrewicz S (2023) Migraine symptoms, healthcare resources utilization and disease burden in a large Polish migraine cohort: results from Migraine in Poland—a nationwide cross-sectional survey. J Headache Pain 24:40 10.1186/s10194-023-01575-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipton RB, Stewart WF, Celentano DD, Reed ML (1992) Undiagnosed migraine headaches. A comparison of symptom-based and reported physician diagnosis. Arch Intern Med 152:1273–1278 10.1001/archinte.1992.00400180125021 [DOI] [PubMed] [Google Scholar]
- 33.Bigal ME, Serrano D, Reed M, Lipton RB (2008) Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 71:559–566 10.1212/01.wnl.0000323925.29520.e7 [DOI] [PubMed] [Google Scholar]
- 34.Katsarava Z, Mania M, Lampl C, Herberhold J, Steiner TJ (2018) Poor medical care for people with migraine in Europe - evidence from the Eurolight study. J Headache Pain 19:10 10.1186/s10194-018-0839-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansfield C, Gebben DJ, Sutphin J, Tepper SJ, Schwedt TJ, Sapra S et al (2019) Patient preferences for preventive migraine treatments: a discrete-choice experiment. Headache 59:715–726 10.1111/head.13498 [DOI] [PubMed] [Google Scholar]
- 36.Lipton RB, Amatniek JC, Ferrari MD, Gross M (1994) Migraine. Identifying and removing barriers to care. Neurology 44:S63–68 [PubMed] [Google Scholar]
- 37.Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J et al (2003) A self-administered screener for migraine in primary care: the ID Migraine validation study. Neurology 61:375–382 10.1212/01.WNL.0000078940.53438.83 [DOI] [PubMed] [Google Scholar]
- 38.Lipton RB, Serrano D, Buse DC, Pavlovic JM, Blumenfeld AM, Dodick DW et al (2016) Improving the detection of chronic migraine: development and validation of Identify Chronic Migraine (ID-CM). Cephalalgia 36:203–215 10.1177/0333102415583982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavlovic JM, Yu JS, Silberstein SD, Reed ML, Kawahara SH, Cowan RP et al (2019) Development of a claims-based algorithm to identify potentially undiagnosed chronic migraine patients. Cephalalgia 39:465–476 10.1177/0333102418825373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipton RB, Sico J, Seng EK (2023) Migraine screening in English and Spanish. Headache 63:843–845 10.1111/head.14520 [DOI] [PubMed] [Google Scholar]
- 41.Lim JH, Karimi L, Wijeratne T (2021) An evaluation of medication prescribing patterns for acute migraine in the emergency department: a scoping review. J Clin Med 10:1191 10.3390/jcm10061191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minen MT, Zhou K, Miller L (2020) A brief look at urgent care visits for migraine: the care received and ideas to guide migraine care in this proliferating medical setting. Headache 60:542–552 10.1111/head.13717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipton RB, Stewart WF, Ryan RE Jr., Saper J, Silberstein S, Sheftell F (1998) Efficacy and safety of acetaminophen, aspirin, and caffeine in alleviating migraine headache pain: three double-blind, randomized, placebo-controlled trials. Arch Neurol 55:210–217 10.1001/archneur.55.2.210 [DOI] [PubMed] [Google Scholar]
- 44.Charles A (2024) The role of caffeine in headache disorders. Curr Opin Neurol 37:289–294 10.1097/WCO.0000000000001249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select “Home.”