Abstract
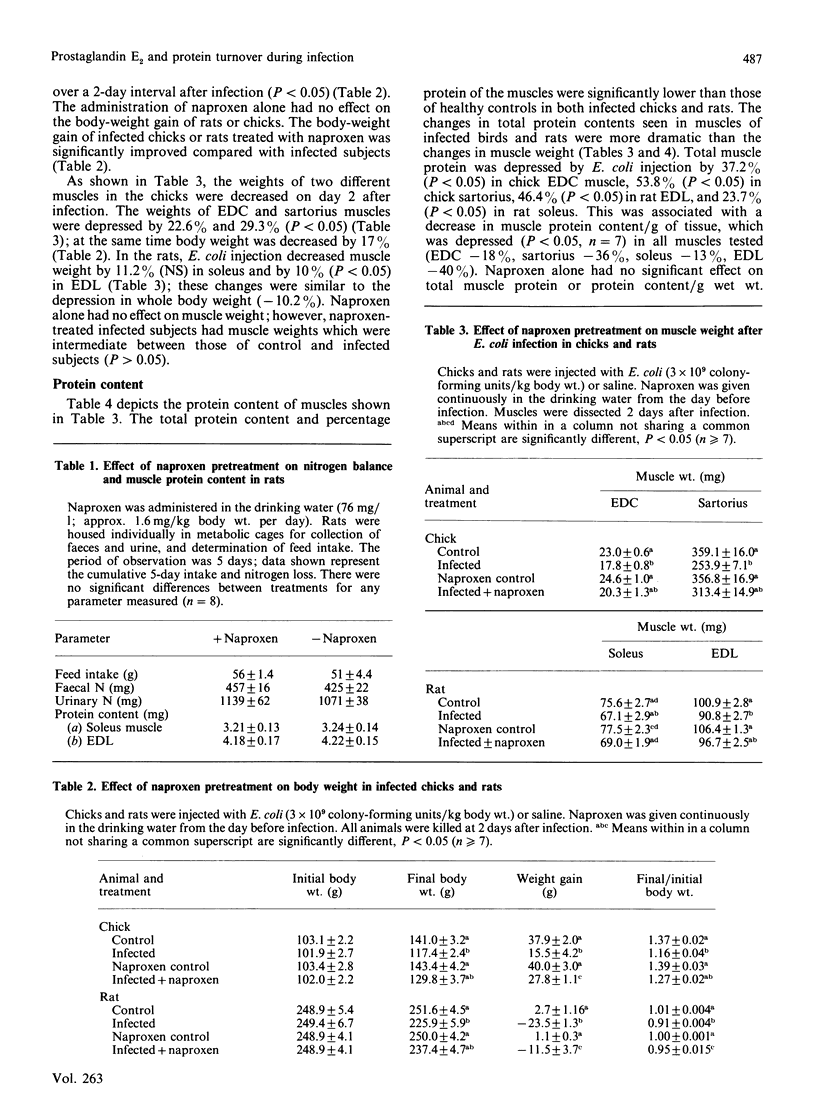
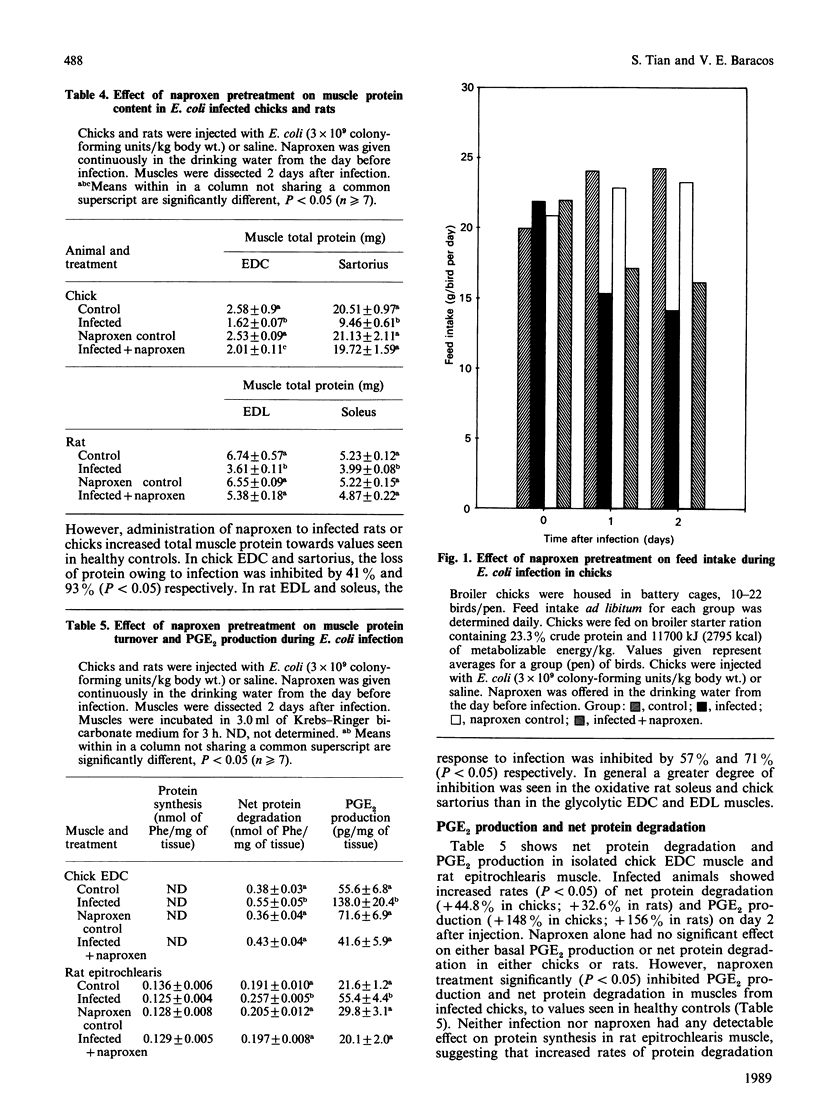
Systemic infection with Escherichia coli significantly decreased feed intake, slowed growth of the whole body and skeletal muscles, and severely inhibited muscle protein accumulation in both chicks and rats. Treatment with naproxen (6-methoxy-alpha-methyl-2-naphthaleneacetic acid), an inhibitor of prostaglandin production, decreased weight losses of body and muscle, and significantly inhibited muscle protein wasting in infected chicks and rats. E. coli infection increased net protein degradation by 44.8% (P less than 0.05) and prostaglandin E2 production by 148% (P less than 0.05) in isolated extensor digitorum communis muscle from chicks on day 2 after infection. Naproxen treatment significantly decreased net protein degradation and prostaglandin E2 production in infected chicks to values seen in muscles of healthy controls. Quantitatively and qualitatively similar results were seen in isolated rat epitrochlearis muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V. E., Langman M., Mak A. An in vitro preparation of the extensor digitorum communis muscle from the chick (Gallus domesticus) for studies of protein turnover. Comp Biochem Physiol A Comp Physiol. 1989;92(4):555–563. doi: 10.1016/0300-9629(89)90365-4. [DOI] [PubMed] [Google Scholar]
- Baracos V. E., Whitmore W. T., Gale R. The metabolic cost of fever. Can J Physiol Pharmacol. 1987 Jun;65(6):1248–1254. doi: 10.1139/y87-199. [DOI] [PubMed] [Google Scholar]
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Barnett J. G., Ellis S. Prostaglandin E2 and the regulation of protein degradation in skeletal muscle. Muscle Nerve. 1987 Jul-Aug;10(6):556–559. doi: 10.1002/mus.880100611. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Metabolic effects of infection. Prog Food Nutr Sci. 1984;8(1-2):43–75. [PubMed] [Google Scholar]
- Brogden R. N., Heel R. C., Speight T. M., Avery G. S. Naproxen up to date: a review of its pharmacological properties and therapeutic efficacy and use in rheumatic diseases and pain states. Drugs. 1979 Oct;18(4):241–277. doi: 10.2165/00003495-197918040-00001. [DOI] [PubMed] [Google Scholar]
- Fern E. B., McNurlan M. A., Garlick P. J. Effect of malaria on rate of protein synthesis in individual tissues of rats. Am J Physiol. 1985 Nov;249(5 Pt 1):E485–E493. doi: 10.1152/ajpendo.1985.249.5.E485. [DOI] [PubMed] [Google Scholar]
- Freund H. R., Muggia-Sullam M., LaFrance R., Gallon L. S., Barcelli U. O., Fischer J. E. Muscle prostaglandin production in the rat. Effect of abdominal sepsis and different amino acid formulations. Arch Surg. 1985 Sep;120(9):1037–1039. doi: 10.1001/archsurg.1985.01390330049009. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Fern E. B., Tomkins A. M., Waterlow J. C. Stimulation of protein synthesis and breakdown by vaccination. Br Med J. 1980 Jul 26;281(6235):263–265. doi: 10.1136/bmj.281.6235.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., Baracos V., Rodemann P., Waxman L., Dinarello C. Control of protein degradation in muscle by prostaglandins, Ca2+, and leukocytic pyrogen (interleukin 1). Fed Proc. 1984 Apr;43(5):1301–1306. [PubMed] [Google Scholar]
- Hasselgren P. O., Talamini M., LaFrance R., James J. H., Peters J. C., Fischer J. E. Effect of indomethacin on proteolysis in septic muscle. Ann Surg. 1985 Nov;202(5):557–562. doi: 10.1097/00000658-198511000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselgren P. O., Warner B. W., Hummel R. P., 3rd, James J. H., Ogle C. K., Fischer J. E. Further evidence that accelerated muscle protein breakdown during sepsis is not mediated by prostaglandin E2. Ann Surg. 1988 Apr;207(4):399–403. doi: 10.1097/00000658-198804000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J., Bohman S. O., Alster P., Berlin T., Cronestrand R., Sonnenfeld T. Biosynthesis of prostaglandins in microsomes of human skeletal muscle and kidney. Prostaglandins Leukot Med. 1983 Jul;11(3):269–279. doi: 10.1016/0262-1746(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Reeds P. J., Hay S. M., Glennie R. T., Mackie W. S., Garlick P. J. The effect of indomethacin on the stimulation of protein synthesis by insulin in young post-absorptive rats. Biochem J. 1985 Apr 1;227(1):255–261. doi: 10.1042/bj2270255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeds P. J., Palmer R. M. Changes in prostaglandin release associated with inhibition of muscle protein synthesis by dexamethasone. Biochem J. 1984 May 1;219(3):953–957. doi: 10.1042/bj2190953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie M. J. Muscle protein turnover and the wasting due to injury and disease. Br Med Bull. 1985 Jul;41(3):257–264. doi: 10.1093/oxfordjournals.bmb.a072060. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Ruff R. L., Secrist D. Inhibitors of prostaglandin synthesis or cathepsin B prevent muscle wasting due to sepsis in the rat. J Clin Invest. 1984 May;73(5):1483–1486. doi: 10.1172/JCI111352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]


