Abstract
Purpose
Chimeric antigen receptor (CAR) T-cell therapy is a new revolutionary method for treating refractory or relapsed hematologic malignancies, CAR T-cell therapy has been associated with cytokine release syndrome (CRS) and cardiotoxicity. We directed a systematic review and meta-analysis to determine the incidence and predictors of cardiovascular events (CVE) with CAR T-cell therapy.
Methods
We investigated PubMed, Embase, Cochrane Library, and ClinicalTrials.gov for studies reporting cardiovascular outcomes in CAR-T cell recipients. The study protocol was listed in the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42023478602). Twenty-three studies were included in this study.
Results
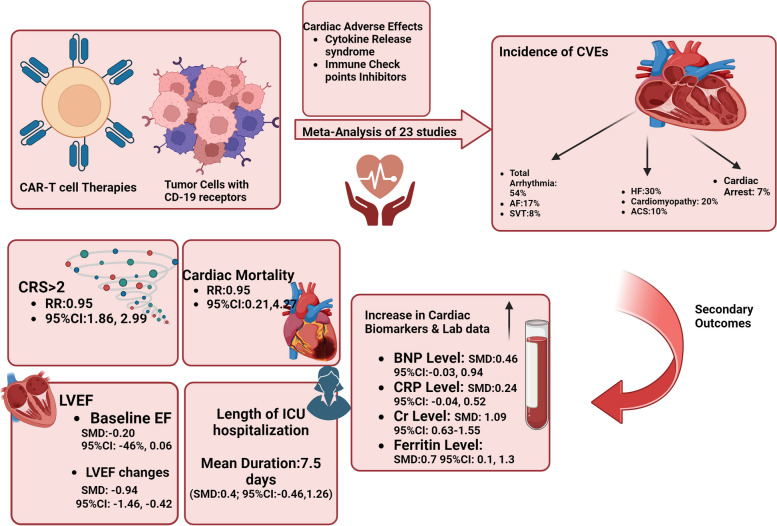
The pooled incidence of CVE was 54% for arrhythmias, 30% for heart failure, 20% for cardiomyopathy, 10% for acute coronary syndrome, and 7% for cardiac arrest. Patients with CVE had a higher incidence of cytokine release syndrome grade ≥ 2 (RR 2.36, 95% CI 1.86–2.99). The incidence of cardiac mortality in our meta-analysis was 2% (95% CI: 1%–3%). Left ventricular ejection fraction decline was greater in the CVE group (-9.4% versus -1.5%, p < 0.001). Cardiac biomarkers like BNP, CRP, creatinine, and ferritin were also elevated.
Conclusions
CAR T-cell therapy commonly leads to cardiotoxicity, mediated by cytokine release syndrome. Vigilant monitoring and tailored treatments are crucial to mitigate these effects. Importantly, there's no significant difference in cardiac mortality between groups, suggesting insights for optimizing preventive interventions and reducing risks after CAR T-cell therapy.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40959-024-00252-y.
Keywords: Chimeric antigen receptor cardiotoxicity, CAR T-cell cardiotoxicity, Chimeric antigen receptor t-cells, Cardiovascular events, Cardiotoxicity, Cardiac biomarkers, Cancer immunotherapy, Cardio-oncology, Cytokine release syndrome
Introduction
Chimeric antigen receptor (CAR) T-cell therapy is a revolutionary method in treating refractory or relapsed hematologic malignancies.
Despite its promising efficacy, CAR T-cell therapy has been associated with cytokine release syndrome (CRS) and cardiotoxicity. Cardiotoxicity related to CAR T-cell therapy has important clinical ramifications [1, 2] including tachycardia-induced LV dysfunction, myocardial injury, arrhythmias, hypotension, ST-segment changes on the electrocardiogram (ECG), and in some cases cardiac death [3–5]. While multiple studies have captured cardiovascular endpoints in patients receiving CAR T-cell therapy, a systematic appraisal of the available evidence to inform patients' and clinicians' expectations of cardiovascular risk related to this therapy has not yet been carried out. Thus, we performed a systematic review and meta-analysis to investigate the incidence of cardiotoxicity in patients receiving CAR T-cell therapy and its related predictors.
Methods
This systematic review and meta-analysis adhered to the procedures outlined in the Cochrane Handbook of Systematic Reviews and followed the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020) [6]. The study protocol was listed in the International Prospective Register of Systematic Reviews (PROSPERO) – PROSPERO ID: CRD42023478602 [7, 8].
Eligibility criteria
Inclusion criteria: Non-randomized clinical Trials and observational studies including patients with cardiotoxicity and cardiac adverse effects after all types of CAR T-cell therapies were considered. Studies that evaluated both the cardiovascular events (CVE) group and the non-cardiovascular events (non-CVE) group were included. Review articles, case reports, case series, conference abstracts, book chapters, studies on animal subjects, and non-English articles were excluded from this review.
Search strategy
PubMed, Embase, Cochrane Library, and Clinicaltrials.gov were searched up until September 2023 using the following keywords: “Cardiotoxicity”, “Cardiac-toxicity”, “Cardio-myopathic inflammatory process”, “Cardiac-biomarkers”, “CAR-T cell therapies”, “Chimeric antigen receptor immunotherapy”, “Chimeric antigen receptor T-cell”, and administrating a combination of subject words and free words. No publication date or publication status restrictions such as published or online first were considered. Reference lists of qualified studies and relevant reviews on this title were also screened.
Outcome measures
The primary outcome of our study was cardiotoxicity following CAR T-cell therapy, including cardiomyopathy, heart failure, arrhythmia, cardiac mortality, and acute coronary syndrome. Our secondary outcomes included laboratory cardiac and inflammatory biomarkers assessment, time to cardiotoxicity, days of intensive care unit (ICU) hospitalization, echocardiographic measurements, and hospitalization course and outcomes.
The included studies defined cardiomyopathy as an ejection fraction < 55%, diastolic dysfunction based on abnormal mitral inflow indices, shortening fraction < 28% [9], or as a reduction in LVEF > 10% from baseline to < 50% during the index hospitalization [10, 11]. Heart failure was identified with the same criteria, meeting three or more of the following criteria: (1) symptoms of heart failure, (2) clinical signs consistent with heart failure (such as pulmonary rales and lower extremity edema), (3) laboratory or imaging or radiographic findings (such as elevated brain natriuretic peptide (BNP) level, pleural effusion, Kerley B-lines or pulmonary edema, decreased left ventricular ejection fraction [LVEF]) and/or (4) initiation of new treatment for heart failure [12–14]. Also, myocardial infarction was defined with a rise and/or fall of hs-cTnT with at least one value above the 99th percentile upper reference limit, with the ischemia symptoms or development electrocardiogram [15]. arrhythmia was identified as an electrocardiogram showing a non-sinus rhythm with a rate greater than 120 beats per minute [12].
Data collection and management
Two independent reviewers reviewed all studies obtained from the systematic search of the title and abstract. After excluding articles meeting our exclusion criteria, the full texts of the remaining studies were retrieved, and screened by two reviewers, independently. Any discrepancies were discussed with a third reviewer. The following data were extracted from the selected studies: Author, year, study design, age, sex, sample size, baseline characteristics, type of cardiotoxicity and time to onset, laboratory and echocardiographic data, administered drugs, length of ICU hospitalization, and patient outcomes.
Risk of bias
The quality assessment of clinical trials included in our study was done using the ROBINS-I (Risk Of Bias In Non-randomized Studies—of Interventions) tool [16]. Observational studies were evaluated using the Newcastle–Ottawa Scale (NOS), which examines the studies in three main domains: the selection of the groups, the comparability of the groups, and assessing the outcome of interest [17]. Studies were considered good quality, fair quality, and poor quality based on their total scores of 7 or more, 4–6, and less than 4, respectively. Any conflicts were resolved through consultation.
Statistical analysis
A meta-analysis was conducted using a random-effects model to calculate pooled effect sizes and 95% confidence intervals (CI). The I2 statistic was utilized to assess heterogeneity among the studies. Potential publication bias was assessed using funnel plots and statistical tests, such as Egger’s test, to ensure the reliability of the findings for the outcomes with at least 10 effect sizes. All analyses were conducted using STATA 18 and R (meta-package). When the values reported in the manuscript were expressed as median and interquartile range (IQR) or median and range, and we were unable to obtain the mean and standard deviation (SD) from the authors, we employed the statistical techniques recommended by Luo et al. [18] and Wan et al. [19].
Results
Study selection
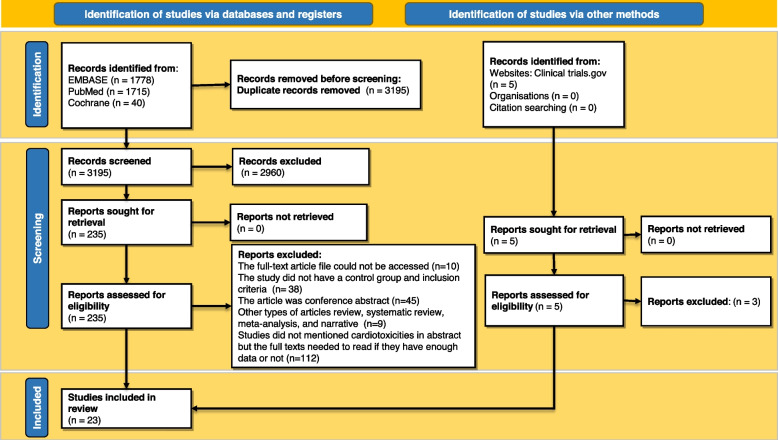
Figure 1 illustrates the study selection procedure. A total of 3538 records have been identified during the database search. A total of 3195 articles remained after duplicates were eliminated for the initial screening. Two independent reviewers evaluated the accuracy of 240 studies under the guidance of the leading member of the team before determining their definitive qualification. Publications that were not clinical trials or cohort studies were dismissed (Fig. 1). A comprehensive search identified a total of 23 studies meeting the inclusion criteria for this systematic review and meta-analysis. The selected studies encompassed a diverse range of chimeric antigen receptor T-cell (CAR-T) therapies. They included patients with a CVE group and a non-CVE group after CAR T-cell therapy.
Fig. 1.
Flow diagram of the study selection process based on PRISMA guidelines
Characteristics of the included studies
All included articles were published between 2015 and 2023. The mean ± SD age range is demonstrated in Table 1. The majority of patients were male. The toxicity onset time range was from 2 (0–9) to 371 (369–372) days. Table 1 summarizes the key characteristics of the included studies, highlighting the variety of CAR-T therapies, patient demographics, and follow-up durations.
Table 1.
Baseline characteristics of the included studies
| Trial/ Study Year, Country | Type of study | CAR-T Type/Trial Malignancy | Total | Case | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N: M/F |
Age | CRS≥2 total | ICU admission | Tocili-zumab | Milri-none | N | Age | CRS≥2 total | ICU admission | Tocili-zumab | Milri-none | CAR t-cell to cardiotoxicity (days) | |||
| Lee 2015 [20] | P | CTL019/ALL, CLL |
21 14/7 |
1-30 | 6 | 1 | 1 | 1 | 4 | ||||||
| Fitzgerald 2017 [21] | R | CTL019/B All |
39 20/19 |
11 (5-22) | 30 | 7.8 (2.9-14.9) | 13 | 1 | 14 | 13 | 1 | 5 (5-7) | |||
| Schuster 2017 [22] | R | CTL019/ Follicular Lymphoma |
14 7/7 |
59(43-72) | 16 | 1 | 8 | 4 | 1 | ||||||
| CTL019/Large B Cell lymphoma | 14 11/3 | 58(25-77) | |||||||||||||
| Neelapu 2017 [23] | P | axicabtagene ciloleucel/ refractory large B cell lymphoma | 101 68/33 | 58, range: 23-76 | 99 | ||||||||||
| Maude 2018 [24] | P | CTL019/ B-cell ALL | 75 43/32 | 11 (3-23) | 8 | ||||||||||
| Locke 2018 [25] | R | Axicabtagene ciloleucel/ B cell lymphoma |
108 73/35 |
58 (51–64) | 106 | 51 | |||||||||
| Burstein 2018 [9] | R | CART19/ B-cell ALL |
98 54/44 |
10 (2-27) | 21 | 6 | 24 | 9.75 (8-17) | 23 (13-28.5) | 21 | 6 | 4.6 (1-9) | |||
| Alvi 2019 [26] | R | Yescarta 68, Kymriah 1, Investigational CAR-T 66/ Diffuse large B-cell and Transformed Follicular Lymphoma, Multiple Myeloma |
137 93/44 |
62 (54-70) | 55 | 56 | 17 | 64 (55.3- 72.7) | 17 | 17 | 16 (6-31) | ||||
| Shalabi 2020 [27] | R | CD19-28ζ/ ALL, NHL |
52 41/11 |
13 (4-30) | 23 | 7 | 1 | 6 | 18 (10-30) | 6 | 4 | 1 | |||
| Ganatra 2020 [11] | R | axicabtagene ciloleucel and CTL019/NHL |
187 115-72 |
63 (range, 19–80) | 86 | 103 | 12 | 70 (63–80) | 11 | 22 (8-92) | 12 | 12.5 (2-24) | |||
| Lefebvre 2020 [14] | R | CTL019, axicabtagene ciloleucel /DLBCL, ALL, or CLL |
145 107/38 |
60 (50-66) | 36 | 31 | 50 (29–61) | 15 | 11 (6-151) | ||||||
| Grupp 2020 [28] | P |
62 34/28 |
|||||||||||||
| Qi 2021 [29] | R |
126 73/53 |
56 (range, 6-72) | 51 | 33 | 29 | |||||||||
| Goldman 2021 [10] | R | CTL019, axicabtagene ciloleucel /B-ALL |
2657 1321/815 |
60 (47-68) | 525 | 30 (3-182) | |||||||||
| Brammer 2021 [30] | R | CTL019, axicabtagene/ DLBCL, follicular lymphoma, mantle-cell lymphoma |
90 52/38 |
61 (50.1-71.9) | 36 | 17 | 17 | 2.5 (1-5) | |||||||
| Steiner 2022 [31] | R | CTL019, axicabtagene/ large B-cell | 165 | 60 (18-88) | 23 | 107 | 27 | 9 | 27 (10-54) | 24 | 2 (0-9) | ||||
| Ragoonanan 2022 [32] | R | CTL019/ ALL admitted to ICU |
205 127/78 |
39 | 13 (1.5-25) | 38 | 6 (2-52) | 6 (0-43) | |||||||
| Lee 2023 [33] | R | 78 | 16 | 58 | 11 | 6 | 2 (0-13) | 11 | |||||||
| Mahmood 2023 [13] | R | 202 | 79 | 87 | 33 | 23 | 12 (7-99) | 25 | |||||||
| Lefebvre 2023 [34] | P | Lymphoma, ALL |
44 34/10 |
58 (47-49) | 22 | 7 | 2 | 73 (52-94) | 371 (369-372) | 2 | 2 | ||||
| Lee 2023 [35] | P | CTL019/ DLBCL, TFL, B-ALL, MCL |
90 55/35 |
11 |
77.0 (69.5 -79.0) |
6 | 8 | ||||||||
| Patel 2023 [12] | R |
75 49/26 |
63.9 ± 13.1 | 9 | 70.4 ± 13.9 | 22 | |||||||||
| Noelle Frey 2023 [36] | P | CTL019/ CLL, ALL |
20 17/3 |
≥18 | 6 | 20 | ≥18 | 6 | |||||||
| Trial/ Study Year, Country | Type of study | CAR-T Type/Trial Malignancy | Control | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Age | CRS≥2 total | ICU admission | Tocili-zumab | Milri-none | ||||
| Lee 2015 [20] | P | CTL019/ALL, CLL | 18 | 3 | Of 21 patients, 1 developed cardiac arrest, complete response rate was 70%. | ||||
| Fitzgerald 2017 [21] | R | CTL019/B All | 25 | 0 | 0 | CRS happened in 36 patients, 5 of 7 patients with grade 3, 4 were treated in ICU. median time from infusion to ICU admission was 5.6 (3.7-6.2) | |||
| Schuster 2017 [22] | R | CTL019/ Follicular Lymphoma | 20 | 12 | 0 | Of 28 patients, 24 were responsive. | |||
| CTL019/Large B Cell lymphoma | |||||||||
| Neelapu 2017 [23] | P | axicabtagene ciloleucel/ refractory large B cell lymphoma | 2 | Of 101 patients, 75 were responsive and 48 had partial response | |||||
| Maude 2018 [24] | P | CTL019/ B-cell ALL | 69 | Of 75 patients, 61 were responsive and 45 had complete response. 19 patients died of whom 3 had cardiotoxicity | |||||
| Locke 2018 [25] | R | Axicabtagene ciloleucel/ B cell lymphoma | 57 | Of 108 patients, 89 were responsive and 69 had complete response. 56 patients died of whom, 2 had cardiotoxicity. | |||||
| Burstein 2018 [9] | R | CART19/ B-cell ALL | 74 | 11 (8-16) | 9 (4.5-17.5) | 0 | 0 | No cardiac related death was reported. 10 had new systolic dysfunction after treatment. Pretreatment factors were not associated with persistent dysfunction at discharge. | |
| Alvi 2019 [26] | R | Yescarta 68, Kymriah 1, Investigational CAR-T 66/ Diffuse large B-cell and Transformed Follicular Lymphoma, Multiple Myeloma | 120 | 58 (48.5-67.5) | 38 | 39 | 29 had positive troponin, of whom 8 had cardiotoxicity. Of 17 patients with cardiotoxicity, 6 died. 95% of CV events occurred after an elevated troponin. | ||
| Shalabi 2020 [27] | R | CD19-28ζ/ ALL, NHL | 46 | 13 (4-27) | 17 | 3 | 0 | All 6 patients with cardiac toxicity had CRS and transferred to ICU. 3 had steroid administration. Those who developed cardiac dysfunction had significantly lower baseline GLS. | |
| Ganatra 2020 [11] | R | axicabtagene ciloleucel and CTL019/NHL | 175 | 62 (19–78) | 75 | 16 (8-66) | 91 | incidence and impact of CAR T-cell therapy–associated cardiomyopathy was examined. Steroids were administered to 9 of 12 cardiomyopathy patients. 4 patients died, of whom 1 had cardiomyopathy. | |
| Lefebvre 2020 [14] | R | CTL019, axicabtagene ciloleucel /DLBCL, ALL, or CLL | 114 | 61 (54–67) | 21 | 41 CV events happened in 31 patients. median follow-up period was 456 days. 61 patients died. CRS occurred in median of 6 days. | |||
| Grupp 2020 [28] | P | ||||||||
| Qi 2021 [29] | R | 93 | 22 | Overall, 2 patients died, who had cardiotoxicity events. | |||||
| Goldman 2021 [10] | R | CTL019, axicabtagene ciloleucel /B-ALL | 2135 | cardiovascular and pulmonary adverse events was investigated following Car T cell therapy. CRS happened in 1457 patients. Of 525 cases, 162 died. Tachyarrhythmias and venous-thromboembolic events were reported more often in axicabtagene-ciloleucel than tisagenlecleucel. | |||||
| Brammer 2021 [30] | R | CTL019, axicabtagene/ DLBCL, follicular lymphoma, mantle-cell lymphoma | 73 | 19 | Among 90 patients, 17 developed cardiotoxicities. 80 patients developed CRS. | ||||
| Steiner 2022 [31] | R | CTL019, axicabtagene/ large B-cell | 132 | 14 | 16 (7-99) | 84 | Regarding 27 cardiotoxicities, 21 arrhythmias, 4 exacerbations of heart failure/cardiomyopathy, 4 cerebrovascular accidents, 3 myocardial infarctions, and 1 death was reported. | ||
| Ragoonanan 2022 [32] | R | CTL019/ ALL admitted to ICU | 166 | 11(0.3-25) | 7.5 (1-125) | Overall hospital length of stay and ICU admission were longer in case group. Death during ICU admission was 15.4% and 27.1% in case and control group. | |||
| Lee 2023 [33] | R | 67 | 10 | 47 | Of 11 cardiac events, 10 included new onset atrial fibrillation. BNP on day 5 was higher in patients with cardiac events. No mortality was reported in case group | ||||
| Mahmood 2023 [13] | R | 169 | 79 | 62 | Base line LEVF in case and control group was 56 ± 10 and 62 ± 6 respectively. 1 death was reported in case group. Overall, 51 patients received steroids of whom 15 were in case group. | ||||
| Lefebvre 2023 [34] | P | Lymphoma, ALL | 42 | 21 | Of 44 total patients, MACE developed in 2 patients. Mean LVEF in total patients and case subgroup was 62 ± 5 and 64 ± 6 respectively. 12 patients died. | ||||
| Lee 2023 [35] | P | CTL019/ DLBCL, TFL, B-ALL, MCL | 79 |
66.0 (56.5 - 73.0) |
25 | 42 | Of 90 patients, 11 developed adverse cardiac events. Ten patients developed atrial fibrillation, one of whom had a history of atrial fibrillation prior to CAR-T therapy | ||
| Patel 2023 [12] | R | 66 | 63.0 ± 12.8 | 22 |
Of 66 patients, 9 experienced cardiac events including cardiovascular death, new/worsening heart failure, and new/worsening arrhythmia within 1 year of treatment. |
||||
| Noelle Frey 2023 [36] | P | CTL019/ CLL, ALL | Overall response rate was 42.9% and 83.3% in CLL and ALL cases respectively. one patient died following myocardial infarction. | ||||||
Primary outcomes
Types of cardiovascular events
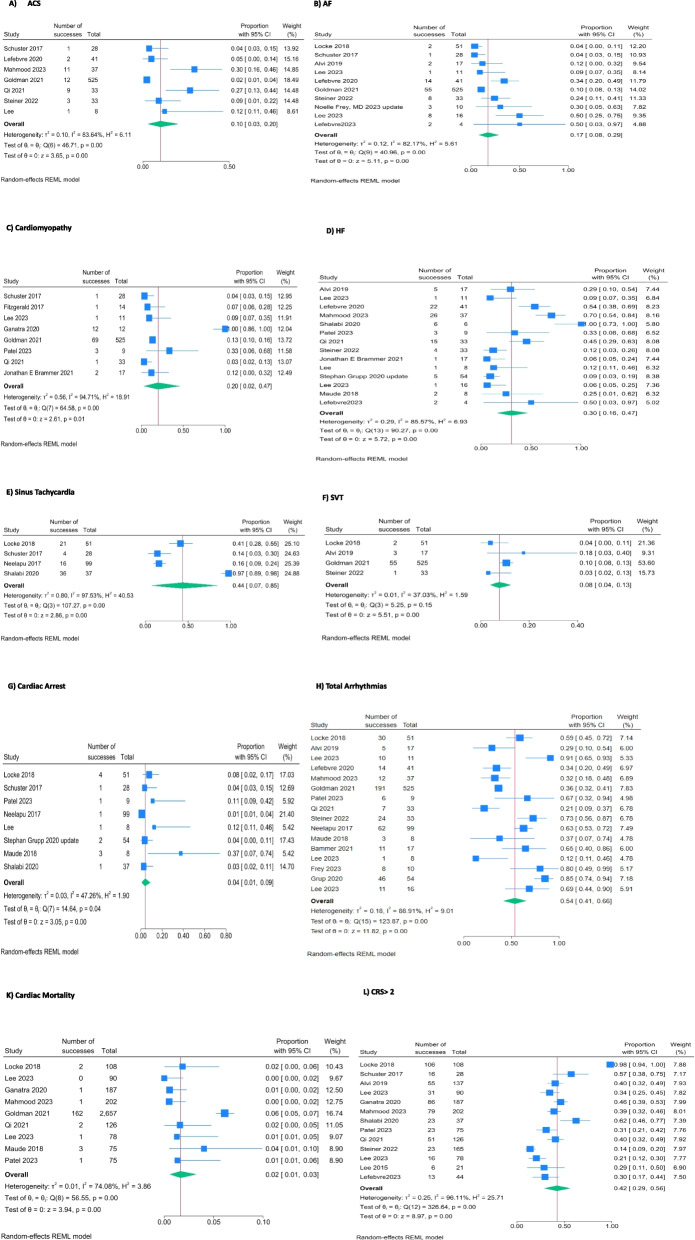
Among the identified CVEs, the most prevalent were arrhythmias (54%), heart failure (33%), and cardiomyopathy (20%). Figure 2 illustrates the distribution of CVE across the included studies [9–14, 20–36].
Fig. 2.
Forrest Plots for Incidence of Cardiac Adverse Effects
Incidence of other cardiovascular events
Our analysis revealed varying incidences of specific CVE in patients treated with CAR T-cell therapy. The analysis showed a prevalence rate of 17% for atrial fibrillation (95% CI: 8%–29%), 7% for cardiac arrest (95% CI: 2%–14%), 30% for heart failure (95% CI: 16%–47%), and 2% for cardiac mortality (95% CI: 1%–3%) in patients received CAR T-cell therapy. The pooled estimates for different outcomes are presented in Figs. 2 and 3 (completely in the Supplementary Table 1).
Fig. 3.
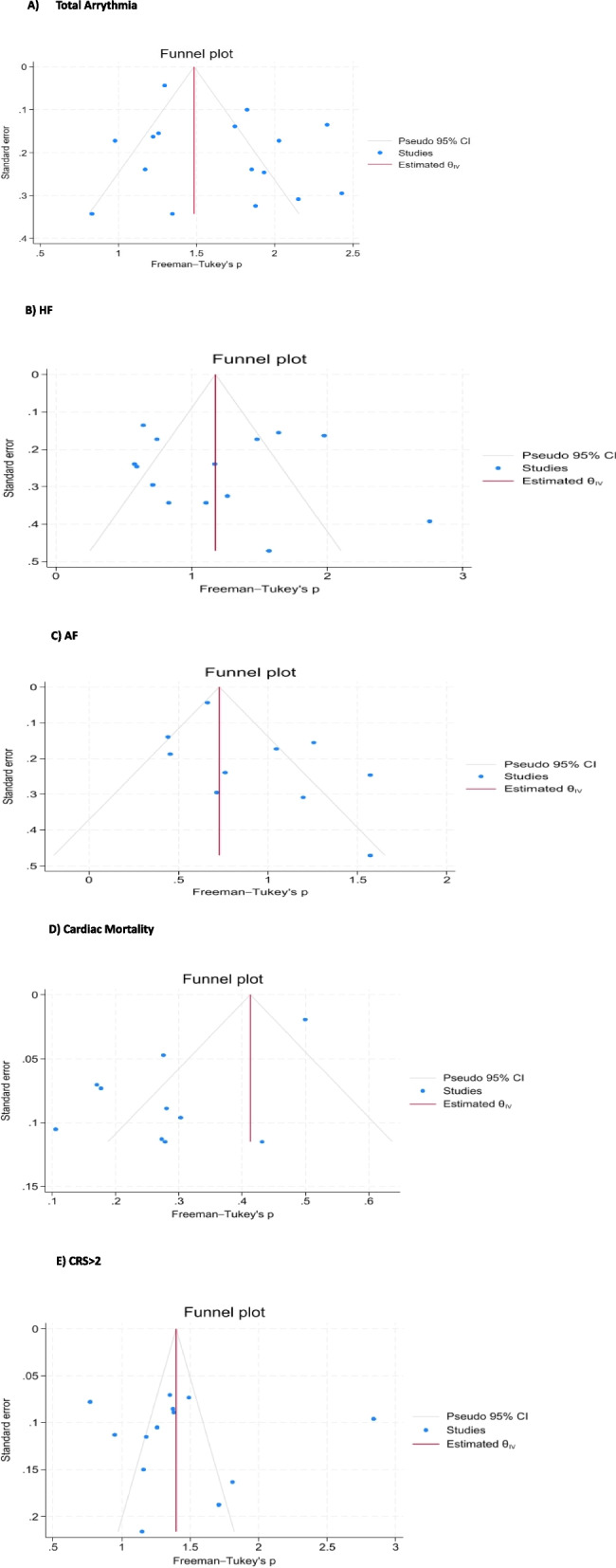
Funnel plot showing no evidence of publication bias
Secondary outcomes
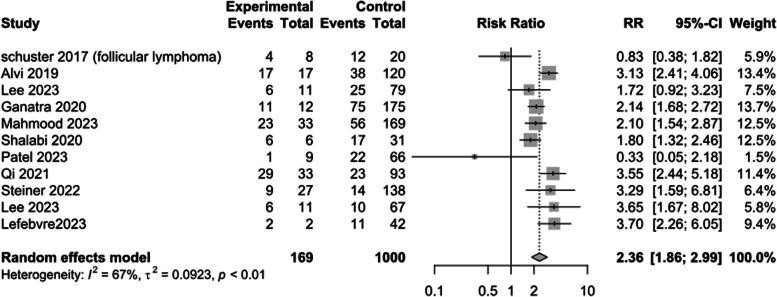
Risk of CRS > 2
Meta-analysis of 11 studies showed patients in the CVE group have a higher incidence of CRS > 2 during their CAR T-cell therapy (RR: 2.36; 95%CI: 1.86–2.99; I2 = 67%) [11–13, 20, 22, 25–27, 29, 31, 33–35] (Fig. 4).
Fig. 4.
Binary cytokine release syndrome (CRS)
Risk of cardiac mortality
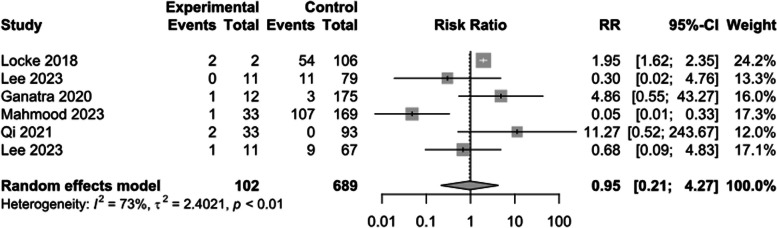
The risk of cardiac mortality following CAR T-cell therapy was compared between CVE and non-CVE groups. The results of our meta-analysis showed there was no significant difference between groups regarding the incidence of cardiac mortality (RR: 0.95; 95%CI: 0.21–4.27; I2 = 73%) [10–13, 24, 25, 29, 33, 35] (Fig. 5).
Fig. 5.
Binary cardiac mortality
Length of ICU hospitalization
Meta-analysis of 3 effect sizes showed no significant difference between CVE and non-CVE groups regarding the length of ICU hospitalization, with a mean duration of 7.5 days (SMD: 0.40; 95% CI: -0.46 to 1.26) [9, 11, 31] (Fig. 6).
Fig. 6.
Length of ICU stay
Left ventricular ejection fraction (LVEF)
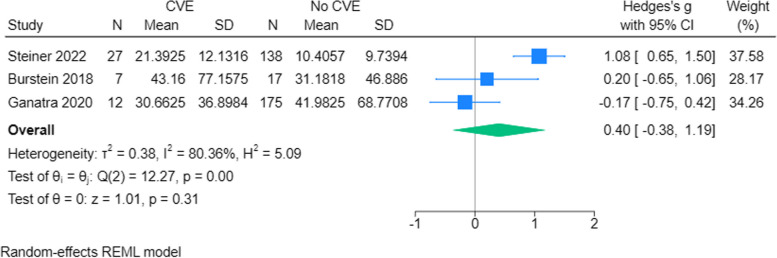
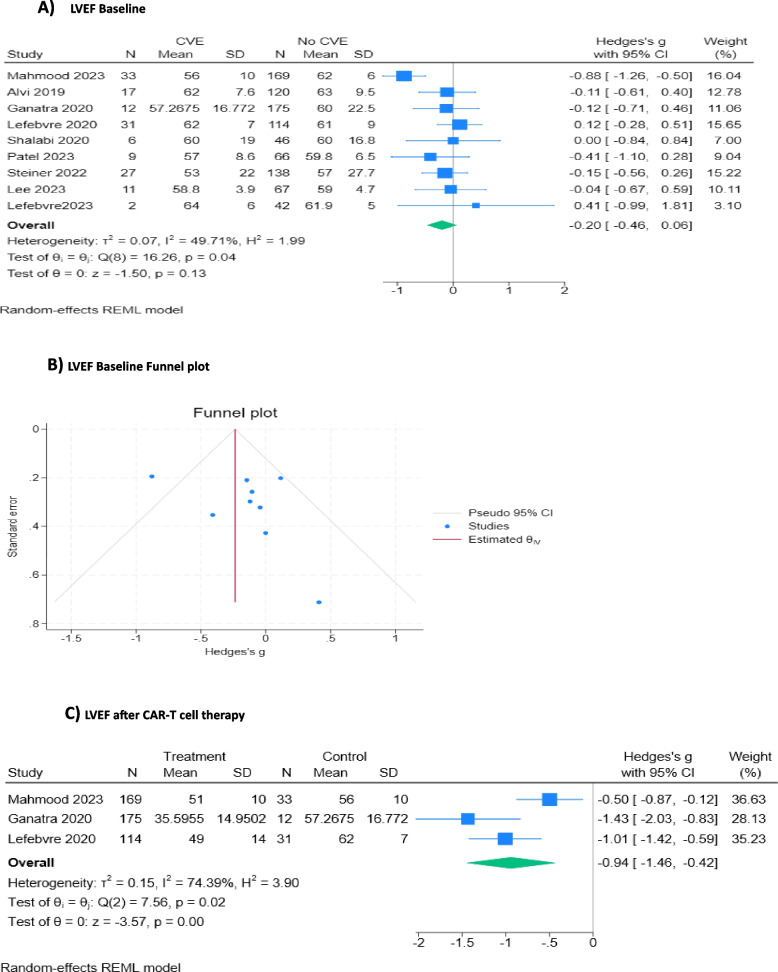
To explore the predictive value of Left Ventricular Ejection Fraction (LVEF) on CVE, we evaluated whether there was a difference between LVEF at baseline between those who experienced CVE vs those who did not. We found that patients who experienced CVE during the CAR T-cell regimen did not have statistically significantly lower LVEF at baseline than the non-CVE group (SMD: -0.20; 95% CI: -0.46 to 0.06) [11–14, 26, 27, 31, 33, 34] (Fig. 7A and B). A random effects model was applied to three studies that had LVEF assessment after CAR T-cell therapy. The CVE group had a statistically significant decrease in LVEF compared to the non-CVE group (SMD: -0.94; 95% CI: -1.46,—0.42) [11, 13, 14] (Fig. 7C).
Fig. 7.
LVEF at baseline and after CAR T-cell therapy
Cardiac biomarkers and laboratory data
In the meta-analysis of cardiotoxicity due to CAR T-cell therapy, elevated levels of BNP and CRP were observed in the CVE group, though the effects were modest (SMD_BNP: 0.46, 95% CI [-0.03, 0.94]; SMD_CRP: 0.24, 95% CI [-0.04, 0.52]) [9, 12, 13, 26, 31, 33, 35] (Fig. 8A and B). Creatinine levels showed a substantial increase in the CVE group (SMD: 1.09, 95% CI [0.63, 1.55]), suggesting potential renal involvement [12, 33, 34] (Fig. 8C). Ferritin levels also exhibited a notable rise (SMD: 0.7, 95% CI [0.1, 1.3]), indicating a possible association with inflammatory processes in cardiotoxicity [9, 12, 13, 31, 33] (Fig. 8D).
Fig. 8.
Cardiac Biomarkers and Other Laboratory Tests Level Changes
Publication bias
Funnel plots and the Egger test did not reveal significant publication bias, indicating that the included studies were distributed symmetrically around the pooled effect estimate. A funnel plot for each outcome with at least 10 effect sizes along with the Egger test is available in a supplementary file (Fig. 3).
Methodological risk of bias
The outcomes of quality assessments, applying the ROBINS-I tool for non-randomized Clinical Trials and NOS for observational cohort studies, are depicted in Tables 2 and 3.
Table 2.
Newcastle–Ottawa Scale (NOS) risk of bias assessment of the included cohort studies
| Author, Year | Selection (0–4) | Comparability (0–2) | Outcome (0–3) | Total score (0–9) |
|---|---|---|---|---|
| Fitzgerald 2017 [21] | 3 | 2 | 2 | 7 |
| Burstein 2018 [9] | 3 | 2 | 3 | 8 |
| Alvi 2019 [26] | 4 | 1 | 3 | 8 |
| Lee 2023 [35] | 4 | 1 | 3 | 8 |
| Ganatra 2020 [11] | 4 | 1 | 3 | 8 |
| Lefebvre 2020 [14] | 4 | 2 | 3 | 9 |
| Mahmood 2023 [13] | 3 | 2 | 3 | 8 |
| Shalabi 2020 [27] | 3 | 1 | 3 | 7 |
| Goldman 2021 [10] | 3 | 1 | 3 | 7 |
| Maude 2018 [24] | 3 | 2 | 3 | 8 |
| Patel 2023 [12] | 2 | 2 | 3 | 7 |
| Qi 2021 [29] | 3 | 2 | 3 | 8 |
| Ragoonanan 2022 [32] | 2 | 2 | 3 | 7 |
| Steiner 2022 [31] | 3 | 2 | 3 | 8 |
| Brammer 2021 [30] | 3 | 2 | 3 | 8 |
| Lee 2023 [33] | 3 | 2 | 3 | 8 |
| Lefebvre 2023 [34] | 4 | 2 | 2 | 8 |
Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome domain, Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome domain, Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome domain
Table 3.
ROBINS-I Tool, risk of bias assessments of the non-randomized clinical trials
| Study | D1: Bias due to confounding | D2: Bias in selection of participants into the study | D3: Bias in classification of interventions | D4: Bias due to deviations from intended interventions | D5: Bias due to missing data | D6: Bias in measurement of outcomes | D7: Bias in selection of the reported result | Overall |
|---|---|---|---|---|---|---|---|---|
| Lee 2015 [20] | Low | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Neelapu 2017 [23] | Low | Low | Low | Low | Low | Moderate | Low | Low |
| Schuster 2017 [22] | Low | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| Locke 2018 [25] | Low | Low | Low | Low | Low | Moderate | Low | Low |
| Stephan Grupp 2020 [28] | Low | Low | Low | Low | Moderate | Moderate | Low | Moderate |
| NOELLE Frey 2023 [36] | Low | Moderate | Low | Low | Low | Moderate | Low | Moderate |
Discussion
This systematic review and meta-analysis demonstrated a high incidence of cardiovascular toxicity, particularly arrhythmias (54%), heart failure (30%), and cardiomyopathy (20%), associated with CAR T-cell therapy. Our pooled analysis aligns with prior evidence that CRS provoked by CAR-T cell activation mediates downstream myocardial injury, with CRS grade ≥ 2 conferring over twice the risk of CVE. Additionally, notable post-treatment declines in LVEF among patients experiencing CVE substantiate the importance of cardiac monitoring. Overall, these data add to the growing literature on the pathophysiology, risk factors, predictive indicators, and management priorities for cardiovascular toxicities related to novel CAR-T cell regimens. Given the high prevalence of cardiotoxicity associated with CAR-T cells, our findings support baseline cardiac biomarker monitoring with BNP, troponin, ECG, and baseline echo as noted in the 2022 ESC cardio-oncology guidelines [37]; and these can be followed to evaluate for signs of cardiotoxicity.
Cardiotoxicity associated with CAR T-cell therapy is a multifaceted and intricate phenomenon that involves a variety of interrelated processes. Although CAR-T therapies have been remarkably effective in treating some hematological cancers, there is growing concern about how these treatments may affect the cardiovascular system. CRS, endothelial activation and dysfunction, direct myocardial inflammation and injury, and cardiotoxic cytokine release such as TNF-α and IFN-γ are some mechanisms of cardiotoxicity due to CAR T-cell therapy [2, 4, 38]. Current research points to immune checkpoint regulation modifications that could exacerbate these detrimental effects on the heart [39]. Molecular pathways called immune checkpoints regulate T-cell activation under physiological conditions to prevent uncontrollable autoreactivity and preserve self-tolerance [40]. Malignancies often exhibit dysregulation of checkpoint molecule expression, which facilitates immune evasion. Anti-PD1, anti-CTLA4, and other checkpoint inhibitors assist in changing this process to increase anti-cancer immunity [41]. Nevertheless, prior research in the context of CAR T-cell therapy demonstrates that the combination of checkpoint inhibition and increased immune stimulation can worsen both the severity of CRS and myocardial inflammation induced by CAR T cells [1, 42]. PD-1 aids in the regulation of cytotoxic activities, apoptosis, and cellular metabolism. Thus, PD-1 blockade may increase CAR T cell cytokine production and metabolic stressors, which could indirectly increase cardiotoxicity [43–45]. Furthermore, CTLA-4 and PD-1 typically protect T cell-mediated myocarditis [46]. Thus, inhibiting these checkpoint pathways in addition to CAR T-cell therapy may reduce the body's natural defenses against myocardial damage caused by dysregulated CAR T cells [1]. Investigations have linked the use of checkpoint inhibitors to a higher risk of life-threatening cardiac events, severe CRS, and early-onset cardiotoxicity in patients receiving CAR T-cell therapy [47, 48]. Guo et al. [49] showed cardiotoxicity occurred in 16.7% of patients across eight studies, which was in line with our results.
We noted the most common cardiovascular pathologies encountered were arrhythmias (6.5%), cardiomyopathy/heart failure (6.5%), and acute coronary syndrome (ACS)/ myocardial infarction (MI) (2.9%). Patients receiving CAR T-cell therapy had a high prevalence of arrhythmia (54%), heart failure (30%), sinus tachycardia (44%), and ACS (10%), which serve as surrogates for overall cardiotoxicity. Following CAR-T cell therapy, several CV problems have been documented, including high troponin arrhythmia, sudden cardiac death, and left ventricular (LV) dysfunction [50]. Sinus tachycardia and arterial hypotension can be primarily understood as consequences of CRS, while they can arise as independent CV toxicities. Tachycardia and low peripheral resistance are two systemic CRS consequences that strain the cardiovascular system and exacerbate toxicity manifestation [26]. Therefore, there seems to be some overlap in cardiovascular outcome trends but some discrepancies in the exact incidence values. In terms of potential risk factors, the article's results and our findings both identify cytokine release syndrome (CRS) as a risk factor for cardiovascular toxicity. Our data suggest that higher grades of CRS increase cardiotoxicity risk. The Guo et al. article noted a twofold higher risk for CVE with CRS, which is consistent with our results.
In the study by Chen et al. [51], most of the articles that were included were conference abstracts; however, in our study, we excluded conference abstracts. This meta-analysis reported an incidence of CVE of 25.6% with CAR T-cell therapy. Our results stated an overall cardiovascular event rate of 16%. So, there is a discrepancy between the two analyses, with the meta-analysis suggesting a higher overall CV event burden. For arrhythmias specifically, this meta-analysis noted an incidence of 19.2%, compared to the 59% rate in our results. Again, a large difference in the actual percentage between the two analyses. Heart failure rates were more closely aligned between the two analyses – the Chen et al. study reported a 5.3% HF incidence, while our results found a 30% incidence. Moreover, this article also looked at outcomes like CV deaths (1.8%), ACS (2.5%), cardiomyopathy (2.9%), and cardiac arrest (1.3%). We found a 7% cardiac arrest rate. The incidence of cardiac mortality in our meta-analysis was 2% (95% CI: 1%–3%) and there was no significant difference between groups regarding the incidence of cardiac mortality. This finding may provide insights into the optimal timing for administering preventive interventions, thereby reducing the risk of elevated cardiac mortality following CAR T-cell therapy. Moreover, we focused on cardiac mortality in CVE and non-CVE groups after CAR T-cell therapy specifically. The Chen et al. meta-analysis highlighted an increased risk of CV events with higher grade (≥ 2) CRS, aligning with our findings that CRS severity impacts cardiotoxicity risk, and also noted a very high prevalence (87.5%) of CRS among patients experiencing cardiovascular issues. Our results found that 42% of CV events were associated with CRS > 2.
Compared to the above meta-analysis, our results substantiate conclusions from earlier analyses regarding the role of severe CRS as an intermediary event predisposing patients to cardiotoxicity after CAR T-cell therapy. We did not find a statistically significant difference in cardiac mortality between patients with and without CVE. However, only a few studies included assessed this outcome, warranting further investigation. Regarding hospitalization, cardiovascular event status did not impact the duration of ICU stay following CAR-T cell infusion. Exploring potential predictors, we found that patients experiencing CVE had similar LVEF at baseline compared to event-free patients. However, the CVE group demonstrated a notable decline in LVEF following CAR T-cell therapy. Therefore, LVEF changes could serve as a meaningful dynamic indicator for cardiotoxicity risk stratification. Due to our results, monitoring LVEF changes may serve as a valuable predictor for identifying individuals at an elevated risk of cardiovascular complications following CAR T-cell therapy. Further research is warranted to establish the utility of LVEF as a routine monitoring tool in this context.
Moreover, our review highlights trends of elevation in BNP, CRP, creatinine, and ferritin levels among patients with CVE relative to others. These laboratory markers may have utility in the early detection of CAR T-cell therapy-associated cardiotoxicity. Mahmood et al. [13] and Lee et al. [35] focused specifically on cardiac and inflammatory biomarkers and CVE after CAR T-cell therapy in their recent studies. Nonetheless, additional studies are needed to define specific cut-offs for clinical application. Our results provide valuable insights into the association between cardiotoxicity in CAR T-cell therapy and the levels of BNP, CRP, creatinine, and ferritin and highlight specific biomarker trends in CAR T-cell therapy-related cardiotoxicity.
In the recent Lee et al. [33] study, multivariable analyses showed that cardiac events negatively impacted overall survival (OS) and progression-free survival (PFS), respectively. Their findings imply that cardiac events may result in worse PFS or OS; however, the small sample size limited the power to detect an association.
Many studies commonly employ tocilizumab and steroids to address cardiac events following CAR T-cell therapy. Lefebvre et al. [34] in their latest study that as patients were closely monitored and treated for CRS, suspected to be a major contributor to major adverse cardiovascular events (MACE), the incidence of MACE would also decrease. According to our findings, cardiac mortality did not significantly differ among the groups. This lack of statistical significance may be attributed to the effective management and control of cardiac adverse effects. Consequently, these results imply that the novel cancer therapy under consideration may be deemed safe, provided that practitioners possess a comprehensive understanding of the associated adverse effects and employ appropriate management strategies. However, the available data are insufficient for a comprehensive analysis of the correlation between these treatments and improvements in the prognosis of patients with cardiac adverse effects after CAR T-cell therapy. Consequently, we recommend future studies to delve deeper into this aspect. Additionally, there is a call for further research inquiries into the association between overall survival (OS) and progression-free survival (PFS) and the incidence of cardiac events following CAR-T cell therapies.
Limitations
This meta-analysis has certain limitations worth noting. In our analysis, we observed high incidence rates for arrhythmia (54%), heart failure (30%), and cardiomyopathy (20%) as cardiac adverse events post CAR T-cell therapy. These outcomes may be influenced by various confounding factors, such as patient age and pre-existing cardiovascular conditions. During our quality assessment, studies that adjusted for these confounding variables were rated higher in the quality assessment. This highlights the importance of considering patient demographics and baseline medical history when interpreting the incidence rates of cardiac adverse events. There was substantial heterogeneity among the included studies concerning aspects like CAR-T cell production techniques, conditioning regimens, completeness of cardiac imaging pre and post-CAR-T, follow-up approaches, and definitions of CVE. We applied random-effects models to account for between-study variability where appropriate. The majority of included studies were retrospectively designed, with inherent biases. Publication bias assessment was also restricted for some outcomes due to the small number of eligible studies. These factors could impact effect size estimations. Furthermore, data on the long-term trajectory of cardiovascular outcomes beyond the initial hospitalization period was scarce. Finally, the predictive accuracy of LVEF declines and biomarker trends requires further validation through higher-quality prospective research.
Conclusion
These results highlight the urgent need for careful observation, prompt diagnosis, and specialized treatment plans to mitigate the effects of cardiotoxicity in the CAR T-cell regimen. To further elucidate mechanisms, risk factors, and ideal management strategies, prospective studies with standardized methodologies should be given priority in future research. In the ever-changing field of cancer treatment, improving the safety and effectiveness of CAR-T cell therapies requires integrating biomarker evaluations, clinical parameters, and cardiovascular monitoring.
Supplementary Information
Additional file 1: Table 1. Types of cardiac adverse effects in patients received CAR T-cell therapy.
Acknowledgements
Not applicable.
Abbreviations
- CAR T cell
Chimeric antigen receptor T-cell
- CRS
Cytokine release syndrome
- ECG
Electrocardiogram
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- CVE
Cardiovascular events
- ROBINS-I
Risk of Bias In Non-randomized Studies - of Interventions
- NOS
Newcastle–Ottawa Scale
- LVEF
Left ventricular ejection fraction
- OS
Overall survival
- PFS
Progression-free survival
- MACE
Major adverse cardiovascular events
- ICU
Intensive care unit
- IQR
Interquartile range
- CI
Confidence interval
- SD
Standard deviation
- ACS
Acute coronary syndrome
- MI
Myocardial infarction
Authors’ contributions
SM: drafting of the manuscript, study conception and design, data acquisition/ ZE: drafting of the manuscript, data acquisition/ NS, AS: data analysis, drafting of the manuscript/ SM, MT, SS: data acquisition/ IM: drafting of the manuscript/ MN, AA, JK: critical revision/ KH: study conception and design, critical revision. All authors read and approved the final manuscript.
Authors’ Twitter handles
@SabaMalekiMD, @MichaelGNanna and @jennkwanMDPHD
Funding
This study did not receive any specific grant from funding agencies.
Availability of data and materials
The datasets used and analyzed in the current study are available upon reasonable request from the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stein-Merlob AF, Rothberg MV, Holman P, Yang EH. Immunotherapy-associated cardiotoxicity of immune checkpoint inhibitors and chimeric antigen receptor T cell therapy: diagnostic and management challenges and strategies. Curr Cardiol Rep. 2021;23(3):11. 10.1007/s11886-021-01440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burns EA, Gentille C, Trachtenberg B, Pingali SR, Anand K. Cardiotoxicity associated with anti-CD19 chimeric antigen receptor T-cell (CAR-T) Therapy: recognition, risk factors, and management. Diseases. 2021;9(1):20. 10.3390/diseases9010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilli M, Maggio L, Tinti L, Lamendola P, Lanza GA, Crea F, et al. Chimeric antigen receptor-T cell therapy-related cardiotoxicity in adults and children cancer patients: a clinical appraisal. Front Cardiovasc Med. 2023;10:1090103. [DOI] [PMC free article] [PubMed]
- 4.Patel NP, Doukas PG, Gordon LI, Akhter N. Cardiovascular toxicities of CAR T-cell therapy. Curr Oncol Rep. 2021;23(7):78. 10.1007/s11912-021-01068-0 [DOI] [PubMed] [Google Scholar]
- 5.Marar RI, Abbasi MA, Prathivadhi-Bhayankaram S, Daryanani A, Villarraga H, Anavekar N, et al. Cardiotoxicities of novel therapies in hematologic malignancies: chimeric antigen receptor T-cell therapy and bispecific t-cell engager therapy. JCO Oncol Pract. 2023;19(6):331–42. 10.1200/OP.22.00713 [DOI] [PubMed] [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. [DOI] [PMC free article] [PubMed]
- 7.International prospective register of systematic reviews. Available from: https://www.crd.york.ac.uk/prospero/#guidancenotes.
- 8.Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023443843.
- 9.Burstein DS, Maude S, Grupp S, Griffis H, Rossano J, Lin K. Cardiac profile of chimeric antigen receptor T cell therapy in children: a single-institution experience. Biol Blood Marrow Transplant. 2018;24(8):1590–5. 10.1016/j.bbmt.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 10.Goldman A, Maor E, Bomze D, Liu JE, Herrmann J, Fein J, et al. Adverse cardiovascular and pulmonary events associated with chimeric antigen receptor T-cell therapy. J Am Coll Cardiol. 2021;78(18):1800–13. 10.1016/j.jacc.2021.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganatra S, Redd R, Hayek SS, Parikh R, Azam T, Yanik GA, et al. Chimeric antigen receptor T-cell therapy-associated cardiomyopathy in patients with refractory or relapsed non-hodgkin lymphoma. Circulation. 2020;142(17):1687–90. 10.1161/CIRCULATIONAHA.120.048100 [DOI] [PubMed] [Google Scholar]
- 12.Patel N, Dalal P, Meng Z, Baldridge A, Cascino G, Sunderraj A, et al. Myocardial strain is associated with adverse cardiac events in patients treated with chimeric antigen receptor (CAR) T-cell therapy. Eur J Haematol. 2023;112(1):102–10. [DOI] [PubMed]
- 13.Mahmood SS, Riedell PA, Feldman S, George G, Sansoterra SA, Althaus T, et al. Biomarkers and cardiovascular outcomes in chimeric antigen receptor T-cell therapy recipients. Eur Heart J. 2023;44(22):2029–42. 10.1093/eurheartj/ehad117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefebvre B, Kang Y, Smith AM, Frey NV, Carver JR, Scherrer-Crosbie M. Cardiovascular effects of CAR T cell therapy: a retrospective study. JACC CardioOncol. 2020;2(2):193–203. 10.1016/j.jaccao.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–67. 10.1093/eurheartj/ehs184 [DOI] [PubMed] [Google Scholar]
- 16.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355. [DOI] [PMC free article] [PubMed]
- 17.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. 2000. [Google Scholar]
- 18.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- 19.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. [DOI] [PMC free article] [PubMed]
- 20.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45(2):e124–31. 10.1097/CCM.0000000000002053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–54. 10.1056/NEJMoa1708566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–44. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol. 2019;74(25):3099–108. 10.1016/j.jacc.2019.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalabi H, Sachdev V, Kulshreshtha A, Cohen JW, Yates B, Rosing DR, et al. Impact of cytokine release syndrome on cardiac function following CD19 CAR-T cell therapy in children and young adults with hematological malignancies. J Immunother Cancer. 2020;8(2):e001159. 10.1136/jitc-2020-001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grupp SA. Phase I/IIA Study of CART19 Cells for Patients With Chemotherapy Resistant or Refractory CD19+ Leukemia and Lymphoma (Pedi CART19). Available from: https://www.clinicaltrials.gov/study/NCT01626495. Updated 2020-03-23.
- 29.Qi K, Yan Z, Cheng H, Chen W, Wang Y, Wang X, et al. An analysis of cardiac disorders associated with chimeric antigen receptor T cell therapy in 126 patients: a single-centre retrospective study. Front Oncol. 2021;11:691064. [DOI] [PMC free article] [PubMed]
- 30.Brammer JE, Braunstein Z, Katapadi A, Porter K, Biersmith M, Guha A, et al. Early toxicity and clinical outcomes after chimeric antigen receptor T-cell (CAR-T) therapy for lymphoma. J Immunother Cancer. 2021;9(8):e002303. 10.1136/jitc-2020-002303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steiner RE, Banchs J, Koutroumpakis E, Becnel M, Gutierrez C, Strati P, et al. Cardiovascular events in patients treated with chimeric antigen receptor T-cell therapy for aggressive B-cell lymphoma. Haematologica. 2022;107(7):1555–66. 10.3324/haematol.2021.280009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragoonanan D, Bhar S, Mohan G, Beltramo F, Khazal SJ, Hurley C, et al. A multicenter study of ICU resource utilization in pediatric, adolescent and young adult patients post CAR-T therapy. Front Oncol. 2022;12:1022901. [DOI] [PMC free article] [PubMed]
- 33.Lee DH, Kumar A, Mohammed T, Peres LC, Alsina M, Bachmeier C, et al. Cardiac events after standard of care idecabtagene vicleucel for relapsed and refractory multiple myeloma. Blood Adv. 2023;7(16):4247–57. 10.1182/bloodadvances.2023009766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lefebvre B, Kang Y, Vakilpour A, Onoue T, Frey Noelle V, Brahmbhatt P, et al. Incidence of MACE in Patients Treated With CAR-T Cell Therapy. JACC: CardioOncology. 2023;5(6):747–54. [DOI] [PMC free article] [PubMed]
- 35.Lee DH, Chandrasekhar S, Jain MD, Mhaskar R, Reid K, Lee SB, et al. Cardiac and inflammatory biomarker differences in adverse cardiac events after chimeric antigen receptor T-cell therapy: an exploratory study. Cardiooncology. 2023;9(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frey N. CART19 to treat B-cell leukemia or lymphoma that are resistant or refractory to chemotherapy. Available from: https://clinicaltrials.gov/study/NCT01029366?tab=results&a=9. Updated 2023-06-22.
- 37.Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J. 2022;43(41):4229–361. 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 38.Rao A, Stewart A, Eljalby M, Ramakrishnan P, Anderson LD Jr, Awan FT, et al. Cardiovascular disease and chimeric antigen receptor cellular therapy. Front Cardiovasc Med. 2022;9:932347. 10.3389/fcvm.2022.932347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shalata W, Abu-Salman A, Steckbeck R, Mathew Jacob B, Massalha I, Yakobson A. Cardiac toxicity associated with immune checkpoint inhibitors: a systematic review. Cancers (Basel). 2021;13(20):5218. 10.3390/cancers13205218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee L, Gupta M, Sahasranaman S. Immune checkpoint inhibitors: an introduction to the next-generation cancer immunotherapy. J Clin Pharmacol. 2016;56(2):157–69. 10.1002/jcph.591 [DOI] [PubMed] [Google Scholar]
- 41.Sobhani N, Tardiel-Cyril DR, Davtyan A, Generali D, Roudi R, Li Y. CTLA-4 in regulatory T cells for cancer immunotherapy. Cancers. 2021;13(6):1440. [DOI] [PMC free article] [PubMed]
- 42.Khunger A, Battel L, Wadhawan A, More A, Kapoor A, Agrawal N. New insights into mechanisms of immune checkpoint inhibitor-induced cardiovascular toxicity. Curr Oncol Rep. 2020;22(7):65. 10.1007/s11912-020-00925-8 [DOI] [PubMed] [Google Scholar]
- 43.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–22. 10.1126/science.291.5502.319 [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Okazaki IM, Yoshida T, Chikuma S, Kato Y, Nakaki F, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–52. 10.1093/intimm/dxq026 [DOI] [PubMed] [Google Scholar]
- 45.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. 10.1016/1074-7613(95)90125-6 [DOI] [PubMed] [Google Scholar]
- 46.Lichtman AH. The heart of the matter: protection of the myocardium from T cells. J Autoimmun. 2013;45:90–6. 10.1016/j.jaut.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Totzeck M, Michel L, Lin Y, Herrmann J, Rassaf T. Cardiotoxicity from chimeric antigen receptor-T cell therapy for advanced malignancies. Eur Heart J. 2022;43(20):1928–40. 10.1093/eurheartj/ehac106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipe DN, Rajha E, Wechsler AH, Gaeta S, Palaskas NL, Alhajji Z, et al. Cardiotoxicity associated with immune checkpoint inhibitors and CAR T-cell therapy. Am J Emerg Med. 2021;50:51–8. 10.1016/j.ajem.2021.07.014 [DOI] [PubMed] [Google Scholar]
- 49.Guo M, Wang X, Xiao S, Liu A, Xu T, Huan C, et al. Preliminary assessment of cardiotoxicity in chimeric antigen receptor T cell therapy: a systematic review and meta-analysis. Clin Exp Med. 2023;23(6):2041–50. 10.1007/s10238-023-01042-z [DOI] [PubMed] [Google Scholar]
- 50.Ghosh AK, Chen DH, Guha A, Mackenzie S, Walker JM, Roddie C. CAR T cell therapy-related cardiovascular outcomes and management: systemic disease or direct cardiotoxicity?. JACC CardioOncol. 2020;2(1):97–109. 10.1016/j.jaccao.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen LR, Li YJ, Zhang Z, Wang P, Zhou T, Qian K, et al. Cardiovascular effects associated with chimeric antigen receptor T cell therapy in cancer patients: a meta-analysis. Front Oncol. 2022;12:924208. 10.3389/fonc.2022.924208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1. Types of cardiac adverse effects in patients received CAR T-cell therapy.
Data Availability Statement
The datasets used and analyzed in the current study are available upon reasonable request from the corresponding author.