Abstract
Spontaneous regression of cancer is a rare biological phenomenon and the mechanisms underlying it are poorly understood. There have been few reports of temporal changes in morphology and metabolism associated with spontaneous regression. Here, we report an 80-year-old man who presented with right upper quadrant pain. He was diagnosed with stage IVA lung cancer, but without treatment, rib metastasis disappeared 4 months after the diagnosis. Although mediastinal lymph node metastasis regressed partially it began to grow 10 months after the diagnosis. In this case, complete and partial spontaneous tumor regressions were observed in the patient, allowing for a comparison of morphological and metabolic changes during each occurrence by serial computed tomography (CT) and 18F-fluodeoxyglucose positron emission tomography with computed tomography (FDG-PET/CT). We observed that the rib metastasis with high FDG uptake on initial PET/CT was composed of cancer cells as well as intratumoral immune cells, whereas recurrent mediastinal lymph node metastasis with high FDG uptake on follow-up PET/CT was composed of cancer cells with few immune cells. Our findings suggest that hypermetabolism within the rib metastasis on initial PET/CT reflected immune activation, whereas hypermetabolism within the mediastinal lymph node on follow-up PET/CT reflected tumor activation.
Keywords: Lung cancer, Spontaneous regression, Percutaneous biopsy, 18F-fluodeoxyglucose, Positron emission tomography, Computed tomography
Case report
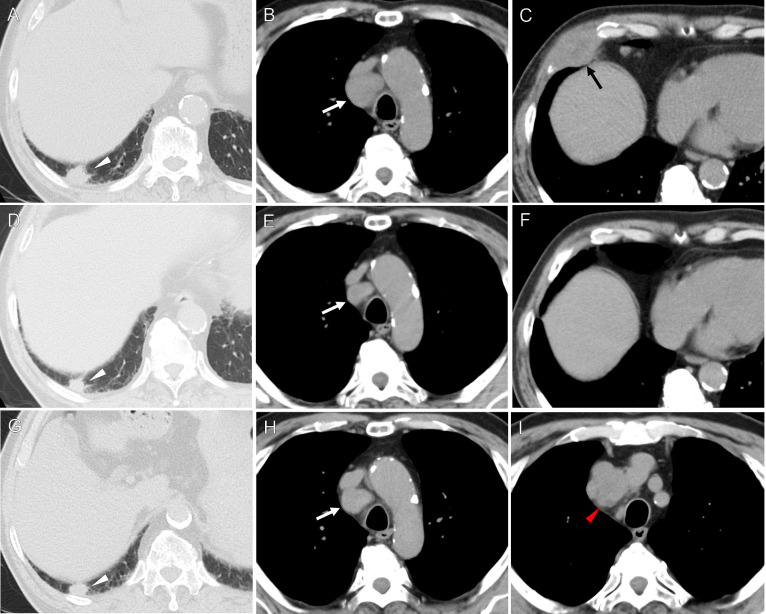
An 80-year-old man presented to our hospital with right upper quadrant pain that corresponded to the right lower ribs. He was an ex-smoker (40 cigarettes/day for 20 years, cessation for 40 years), and his medical history included dyslipidemia, hypertension, and diabetes mellitus. A laboratory examination revealed a white blood cell count of 8200 /µl, a C-reactive protein level of 1.64 mg/dL, and no elevation of serum tumor markers. A computed tomography (CT) scan revealed a 50×31 mm mass lesion in the seventh right rib with bone destruction. There was also a 22×14 mm lung mass in the right lower lobe and a 32×26 mm enlarged mediastinal lymph node in the right lower paratracheal region, with no other abnormalities (Fig. 1).
Fig. 1.
Temporal changes of chest CT findings.
(A-C): The initial CT scan showed a 22 × 14 mm lung nodule in the right lower lobe (white arrowhead), a 32 × 26 mm enlarged mediastinal lymph node in the right lower paratracheal region (white arrow), and a 50 × 31 mm mass in the seventh right rib (black arrow). (D-F): A CT scan 4 months after the biopsy showed the rib mass had disappeared and the size of the lung nodule and right lower paratracheal lymph node had decreased. (G-I): A CT scan 14 months after the biopsy showed the right lower paratracheal lymph node had regrown and a 36 × 24 mm upper paratracheal lymph node was present (red arrowhead). There was no apparent increase in the size of the lung nodule.
Two weeks after the initial CT, a percutaneous ultrasound-guided 18-gauge needle biopsy of the rib mass was performed twice without complication. The CT scan at the time of biopsy showed the size of the rib tumor was unchanged from the initial CT. Histologic examination of the biopsy indicated a poorly differentiated carcinoma with abundant inflammatory cell infiltration (Fig. 2). Immunohistochemical staining for cytokeratin MNF116, CD56, and P40 was positive, suggesting combined neuroendocrine and squamous cell carcinoma. Three weeks after the biopsy, 18F-fluodeoxyglucose positron emission tomography with computed tomography (FDG-PET/CT) (Discovery IQ, GE Healthcare, Milwaukee, WI, USA) was performed for initial staging. PET/CT images were obtained starting 50 min after an intravenous administration of 190 MBq 18F-FDG. The PET/CT showed highly increased uptake in biopsied rib metastasis with a maximum standardized uptake value (SUVmax) of 12.3, with mild FDG uptake in the lung lesion and mediastinal lymph metastasis with an SUVmax of 3.2 and 3.1, respectively, without other abnormalities (Fig. 3). Therefore, a tentative diagnosis of stage IVA lung cancer according to the eighth edition of the American Joint Committee on Cancer/Union for International Cancer Control tumor, node, metastasis staging classification was proposed. The patient did not want further therapy except for an analgesic, and he was followed up thereafter.
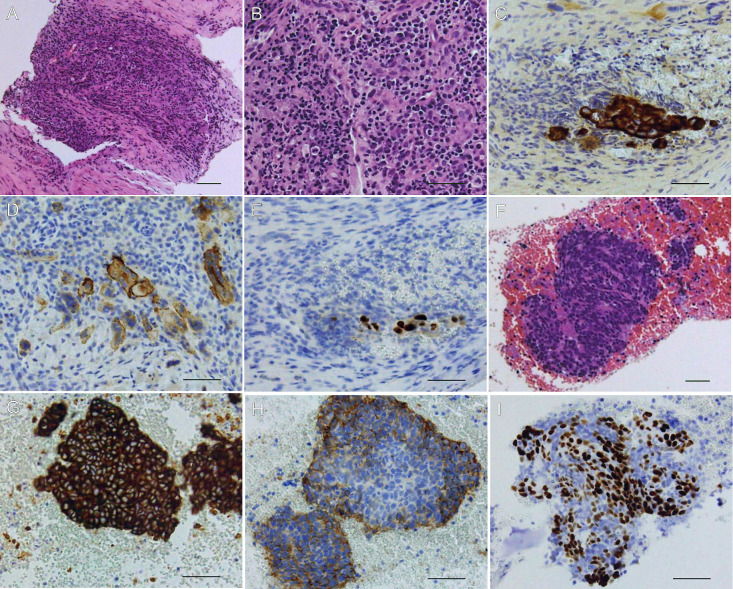
Fig. 2.
Histopathological findings of rib and mediastinal lymph node specimens.
(A, B): Hematoxylin and eosin staining of the rib biopsy tissue showing tumor cells with abundant inflammatory cell infiltration. Immunohistochemical staining of the rib specimen showed that the tumor cells were positive for (C) cytokeratin MNF 116, (D) CD56, and (E) p40. (F): Hematoxylin and eosin staining of mediastinal lymph node biopsy tissue showing tumor cells with scant inflammatory cell infiltration. Immunohistochemical staining showed that the tumor cells were positive for (G) cytokeratin MNF 116, (H) CD56, and (I) p40. Scale bars represent 50 µm.
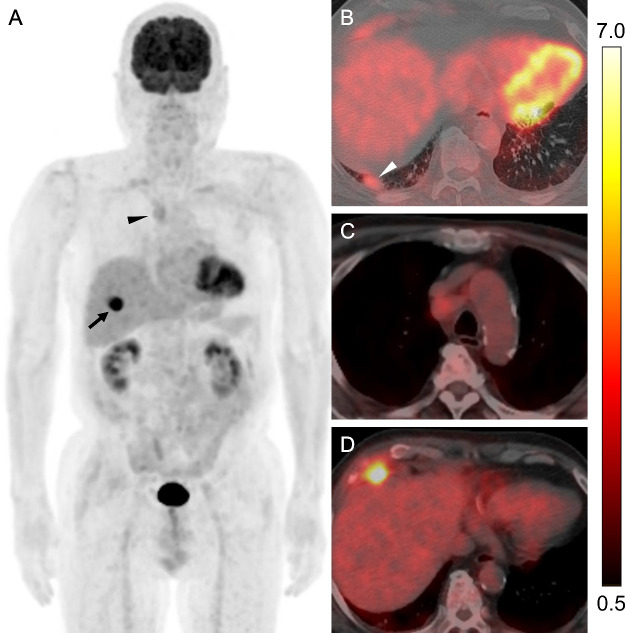
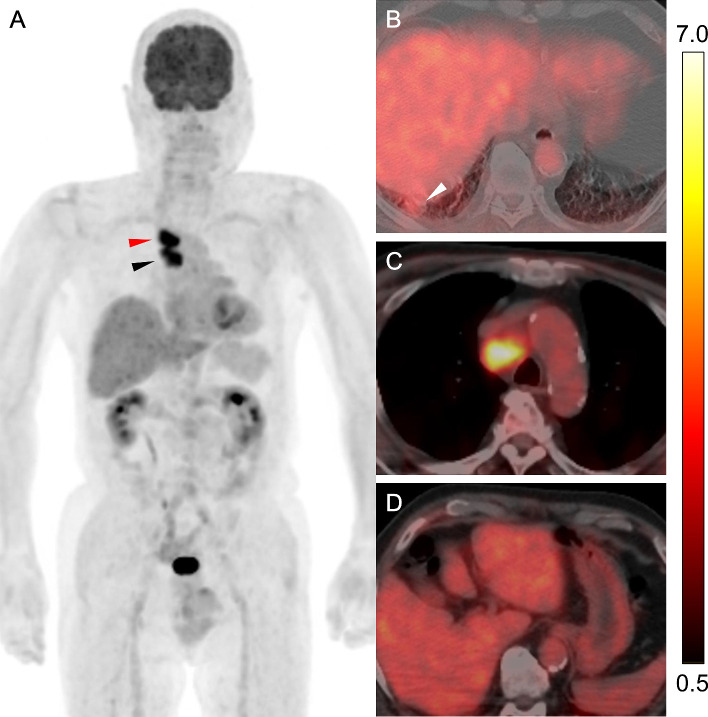
Fig. 3.
FDG-PET/CT scan for initial staging in lung cancer.
(A) Maximum intensity projection and (B-D) transaxial fused PET/CT for initial staging showed high FDG uptake in the rib tumor (SUVmax, 12.3) (black arrow) and mild FDG uptake in the lung nodule (SUVmax, 3.2) (white arrowhead) and right lower paratracheal lymph node (SUVmax, 3.1) (black arrowhead). Blood glucose level at the administration of the tracer was 146 mg/dL. The color scale in the fused PET/CT images shown on the right side of this figure ranged from SUV 0.5–7.0. SUV, Standardized uptake value; SUVmax, maximum SUV.
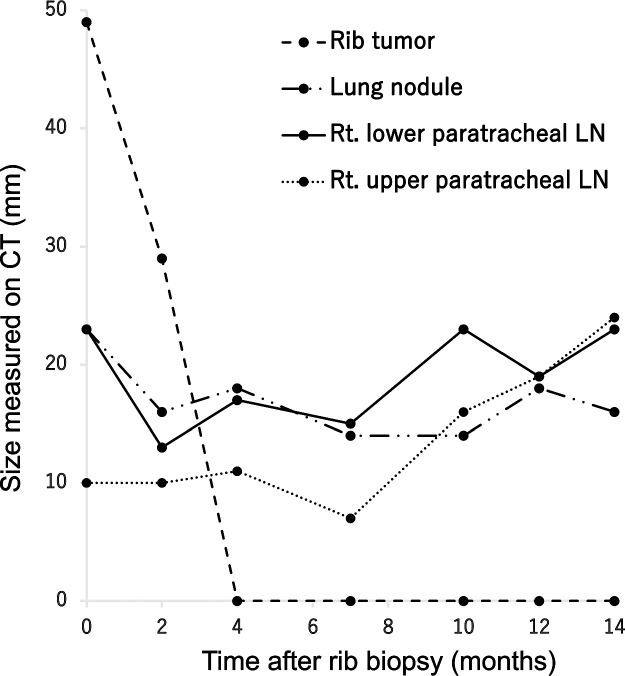
Figure 4 shows the tumor size on follow-up CT at different time points after the rib biopsy. Two months after the biopsy, the size of all tumor lesions had decreased, and 4 months after the biopsy, the biopsied rib metastasis had completely disappeared, but the right lower paratracheal lymph node continued to show slight growth and shrinkage, resulting in a large increase in the size with right upper paratracheal lymph node enlargement 14 months after the biopsy (Fig. 1). Therefore, endobronchial ultrasound-guided transbronchial needle aspiration of the mediastinal lymph node was performed. Histologic examination of the specimens revealed a carcinoma that was immunohistochemically similar to the specimen of the rib biopsy although inflammatory cell infiltration was scant (Fig. 2). Three weeks after endobronchial ultrasound-guided transbronchial needle aspiration, a follow-up PET/CT scan showed the complete disappearance of FDG uptake in rib metastasis, whereas FDG uptake in the lung lesion, right lower paratracheal lymph node, and right upper paratracheal lymph node metastases with SUVmax was 2.7, 8.7, and 9.8, respectively, without other abnormalities (Fig. 5).
Fig. 4.
Temporal changes in tumor size on follow-up CT after rib biopsy.
The rib tumor completely regressed 4 months after the biopsy. The lung nodule and right lower paratracheal lymph node showed slight growth and shrinkage after the biopsy, but the mediastinal lymph node was obviously enlarged 10 months after the biopsy. The sizes of the 4 lesions (rib tumor, lung nodule, right lower paratracheal lymph node, right upper paratracheal lymph node) are represented by a dashed line, dashed-dotted line, solid line, and dotted line, respectively. The sizes of the rib tumor and lung nodule were measured as the diameter of the long axis. The size of lymph nodes was measured as the diameter of the short axis.
Fig. 5.
FDG-PET/CT scan for restaging in recurrent lung cancer.
(A) Maximum intensity projection and (B-D) transaxial fused PET/CT at follow-up showed high FDG uptake in the right lower paratracheal and right upper paratracheal lymph nodes (SUVmax, 8.7 and 9.8) (black arrowhead, red arrowhead, respectively), whereas there was mild FDG uptake into the lung nodule (SUVmax, 2.7) (white arrowhead). A follow-up PET/CT showed the complete disappearance of FDG uptake by the rib tumor. The blood glucose level at the administration of the tracer was 175 mg/dL. The color scale in the fused PET/CT images shown on the right side of this figure ranged from SUV 0.5-7.0. SUV, Standardized uptake value; SUVmax, maximum SUV.
Discussion
Spontaneous regression (SR) is defined as “the partial or complete disappearance of a tumor in the absence of any treatment capable of regression” [1]. SR of cancer is estimated to occur in 1 in every 60,000 to 100,000 cancer patients according to the type of cancer [2]. The obvious putative mechanism of such spontaneous tumor regression involves immune rejection [3,4]. The clinical implication of SR is that there might be a rare, but extremely effective, mechanism that can eradicate cancer cells after the development of advanced malignancy.
FDG-PET/CT, a noninvasive whole-body imaging modality that can capture metabolic information, has been extensively used in clinical practice. The uptake of FDG reflects the glucose metabolism of cells within the tumor area, and can reflect both the state of tumor cells and immune cell activation [5,6].
To the best of our knowledge, this is the first known case of the complete and partial SR of metastases of lung cancer with a documented change in tumor size on close follow-up CT. We also observed changes in tumor metabolism, possibly associated with anti-cancer immunity, on serial PET/CT.
Although our patient's rib tumor exhibited a pre-existing immune infiltrate in the biopsy specimen, the percutaneous biopsy may have contributed to the further enhanced the anti-cancer effect, because the lung lesion and mediastinal lymph node metastasis without percutaneous biopsy did not undergo complete regression. Cole reported 176 cases of spontaneous cancer regression, of which 40% were associated with some type of operative trauma [7]. The healing process associated with trauma causes an increase in immunological resistance to tumor growth and may induce spontaneous remission [8]. Therefore, we speculate that in our case, the percutaneous biopsy caused tissue injury such as damage to the neovasculature, which may have induced necrosis that promoted locoregional inflammation. Indeed, only the biopsied rib metastasis showed considerably higher FDG uptake compared with the other lesions. Nevertheless, the rib lesion underwent complete remission.
In addition, the lung lesion and mediastinal metastasis partially regressed after the biopsy and continued to show slight growth and shrinkage without apparent enlargement for 10 months. The PET/CT for initial staging showed much less FDG uptake in the mediastinal metastasis and the lung lesion than in the rib metastasis. Previous studies showed that cytokines produced by activated immune cells limited tumor growth by inducing apoptosis [9,10]. Therefore, the occurrence of apoptosis that does not induce inflammation [11] may have led to the partial regression, following the rib tumor injury resulting in spillage of the contents of the cell into surrounding tissues. However, because we could not perform biomarker analysis of the host immune response by flow cytometry, the evaluation of local and systemic immune responses is inadequate.
We also observed changes in FDG uptake in the right lower paratracheal lymph node metastasis between the initial PET/CT and follow-up PET/CT. Values of SUVmax were lower in the initial PET/CT than in the follow-up PET/CT, but there were no differences in the tumor size. Although which host and tumor characteristics were involved in this phenomenon remain unclear, increased FDG uptake in the right lower paratracheal lymph node might be related to the tumor rather than immune cell infiltration because tumors in the late-stage of disease become gradually less immunogenic [12]. Indeed, the mediastinal lymph node was growing at the time of the follow-up PET/CT and mediastinal biopsy tissue showed scant immune cell infiltration. Even though the lung nodule maintained low FDG uptake in the follow-up PET/CT, it is not uncommon to observe variable growth rates of primary and metastatic lesions in clinical practice.
Our patient had no changes in medications except for analgesics. Although the patient had received vaccinations for coronavirus disease 2019 before and after the diagnosis, it would be reasonable to assume that a systemic antitumor effect caused by boosting the immune response was not the most influential factor in the regression of rib metastasis because only the rib tumor, which was the largest lesion in our patient, regressed dramatically over a short duration after the biopsy whereas other lesions only regressed slightly. However, as biopsy-induced tumor shrinkage is very rare, the abundant lymphocytes present in the tumor before biopsy probably also had an important role as did the immunotherapy [13].
This case report had some limitations. First, we were not able to prove malignancy of the primary tumor in the lower lobe of the right lung as in another reported case of SR [14,15]. However, histological examination of the rib and mediastinal lesions showed pan-cytokeratin positivity, so in theory, this might be a metastasis from epithelial cancer. There was a proven metastatic lymph node in an expected position for lung cancer. In addition, the staging procedures did not reveal any other lesions and no malignancy developed elsewhere during 14 months of follow-up. Therefore, the most reasonable explanation for the findings in our case is a primary lung cancer with lymph node and rib metastases. Second, the follow-up was still short in this case and long-term follow-up is needed to determine the status of the tumor.
In conclusion, we report the serial CT and PET/CT findings of a rare case of SR of lung cancer. Our findings may indicate that hypermetabolism within the rib metastasis on initial PET/CT reflected immune activation whereas hypermetabolism within the mediastinal lymph node on follow-up PET/CT reflected tumor activation. In this case, conventional CT and biopsy information was helpful for assessing tumor viability, because FDG cannot differentiate between proliferative tumor cells and tumor-infiltrating immune cells.
Data statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Patient consent
Written informed consent was obtained from the patient described in this manuscript.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments: The authors acknowledge the contribution of outside experienced pathologists from the Department of Diagnostic Pathology, Nara Medical University, Kashihara, Japan, regarding a second opinion to confirm pathological diagnosis. We thank J. Ludovic Croxford, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
- 1.Radha G, Lopus M. The spontaneous remission of cancer: current insights and therapeutic significance. Transl Oncol. 2021;14 doi: 10.1016/j.tranon.2021.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobosz P, Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front Immunol. 2019;10:2965. doi: 10.3389/fimmu.2019.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franses JW, Bhan I, Pankaj A, Ting DT, Deshpande V, Tanabe K. Spontaneous immune-mediated regression of hepatocellular carcinoma with high tumor mutational burden. JCO Precis Oncol. 2021;5:1040–1043. doi: 10.1200/PO.21.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sousa LG, McGrail DJ, Li K, Marques-Piubelli ML, Gonzalez C, Dai H, et al. Spontaneous tumor regression following COVID-19 vaccination. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2021-004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1970–1980. [PubMed] [Google Scholar]
- 6.Yamane T, Daimaru O, Ito S, Nagata T, Yoshiya K, Fukaya N, et al. Drug-induced pneumonitis detected earlier by 18F-FDG-PET than by high-resolution CT: a case report with non-Hodgkin's lymphoma. Ann Nucl Med. 2008;22:719–722. doi: 10.1007/s12149-008-0183-7. [DOI] [PubMed] [Google Scholar]
- 7.Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201–209. doi: 10.1002/jso.2930170302. [DOI] [PubMed] [Google Scholar]
- 8.Challis GB, Stam HJ. The spontaneous regression of cancer. A review of cases from 1900 to 1987. Acta Oncol. 1990;29:545–550. doi: 10.3109/02841869009090048. [DOI] [PubMed] [Google Scholar]
- 9.Jorgovanovic D, Song M, Wang L, Zhang Y. Roles of IFN-γ in tumor progression and regression: a review. Biomark Res. 2020;8:49. doi: 10.1186/s40364-020-00228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai X, Zhang J, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, et al. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med (Maywood) 2015;240:760–773. doi: 10.1177/1535370215579167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia Q, Wang A, Yuan Y, Zhu B, Long H. Heterogeneity of the tumor immune microenvironment and its clinical relevance. Exp Hematol Oncol. 2022;11:24. doi: 10.1186/s40164-022-00277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melssen MM, Sheybani ND, Leick KM, Jr Slingluff CL. Barriers to immune cell infiltration in tumors. J Immunother Canc. 2023;11 doi: 10.1136/jitc-2022-006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Pastorini A, Plönes T, Brockmann M, Ludwig C, Beckers F, Stoelben E. Spontaneous regression of non-small cell lung cancer after biopsy of a mediastinal lymph node metastasis: a case report. J Med Case Rep. 2015;9:217. doi: 10.1186/s13256-015-0702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otani M, Nishimori M, Iwasa H, Iwamura M, Izumi T, Nakaji K, et al. Spontaneous regression of small cell lung cancer associated with Lambert-Eaton myasthenic syndrome: case report. Radiol Case Rep. 2023;18:4036–4041. doi: 10.1016/j.radcr.2023.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]