Abstract
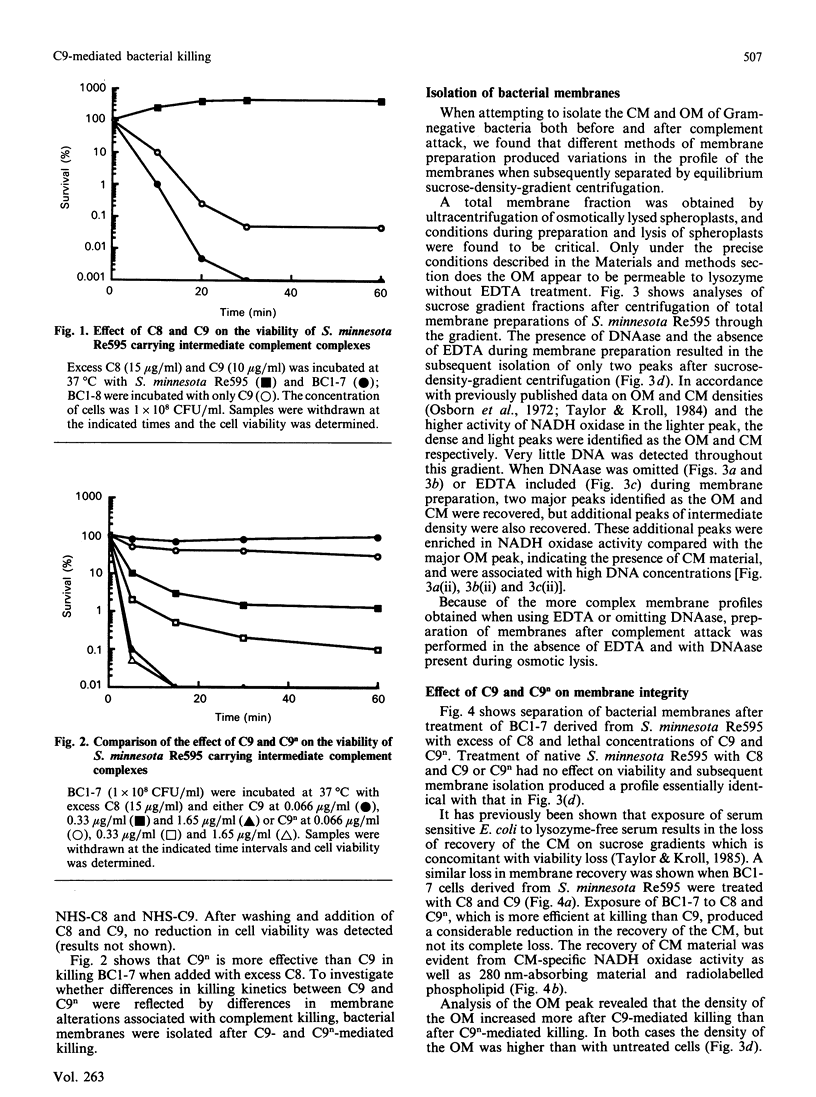
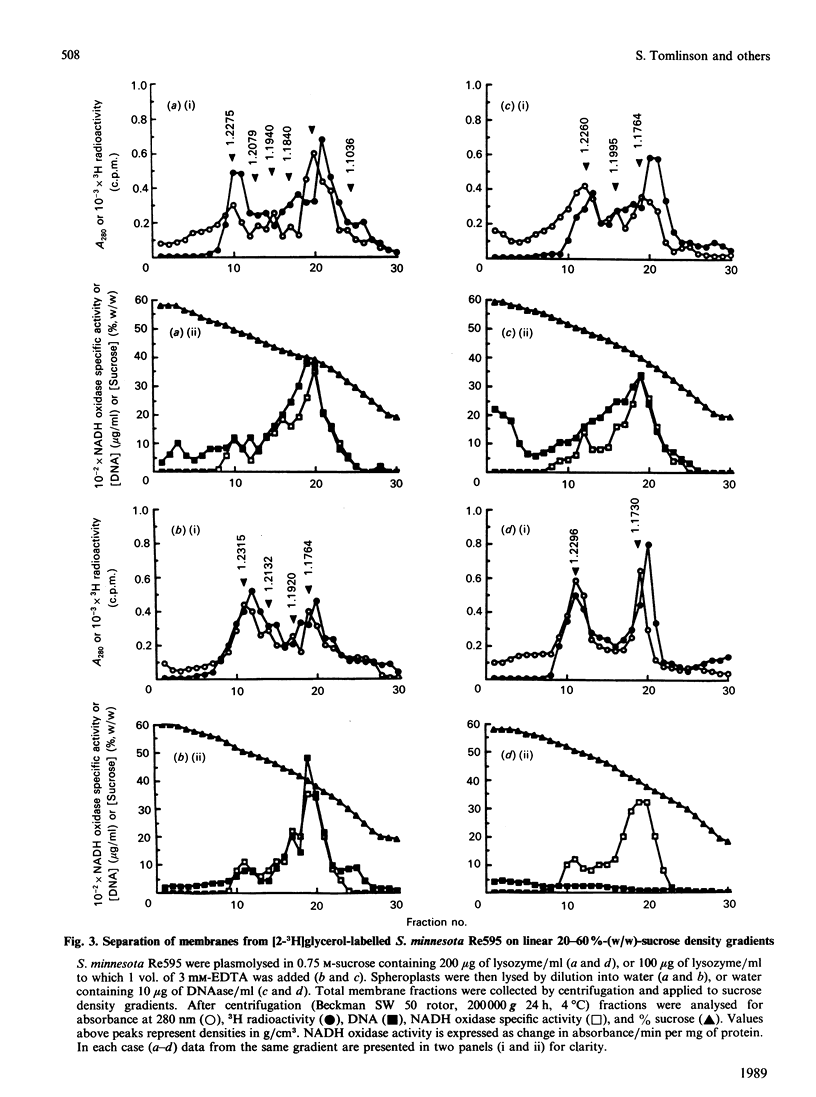
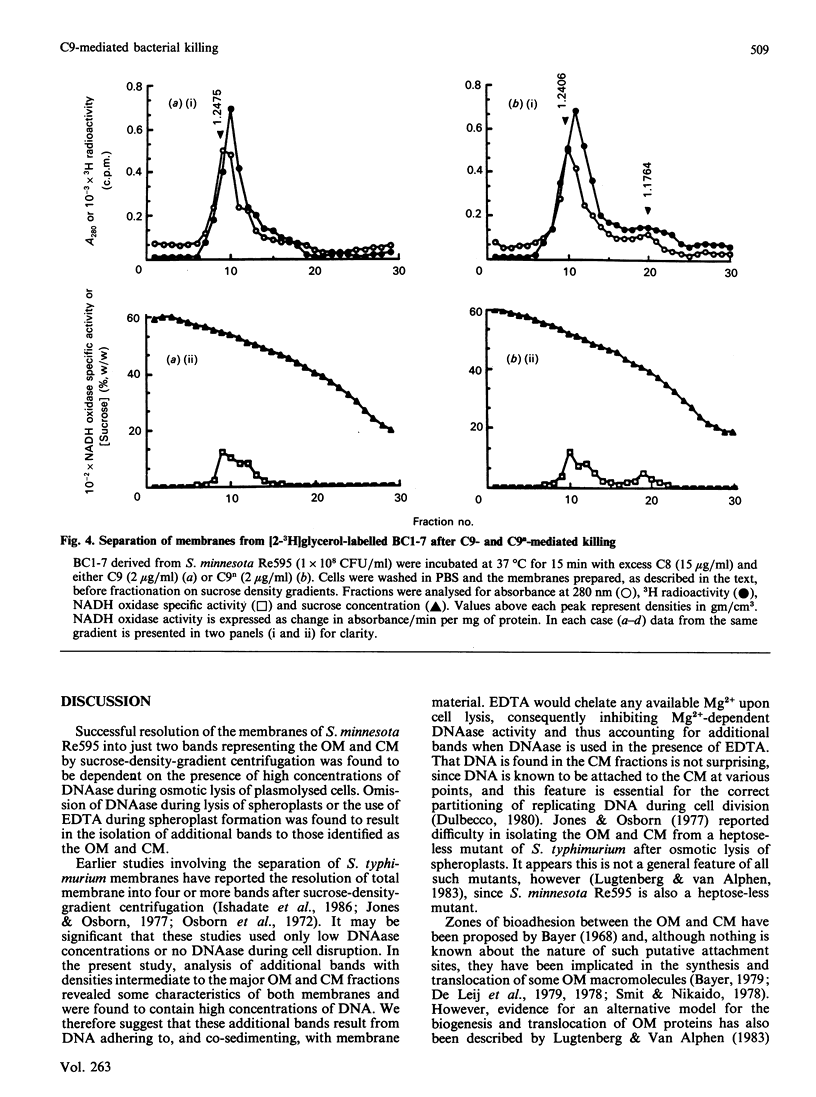
The effect of C5b-9 deposition on the envelope of target Gram-negative bacteria was studied. In order to understand the changes occurring after complement deposition on the bacterial surface, the preparation of Gram-negative bacterial membranes by different methods involving the osmotic lysis of spheroplasts was investigated. Subsequent fractionation of the outer membrane (OM) and cytoplasmic membrane (CM) by sucrose-density-gradient centrifugation showed differences in the membrane profiles obtained. The results indicate that optimum separation of OM and CM components requires effective digestion of DNA in the total membrane preparation before density-gradient fractionation. Salmonella minnesota Re595 carrying the intermediate complement complex C5b-7 (BC1-7) or C5b-8 (BC1-8) were efficiently killed upon incubation with purified C8 + C9 or C9 respectively. Human-alpha-thrombin-cleaved C9 (C9n), which is unable to form tubular poly(C9), was shown to be more effective at killing than native C9. By using an optimized system for the separation of OM and CM, it was found that, subsequent to lethal complement attack, the CM could not be recovered when C9 was used as the terminal complement component, but was recovered with reduced yield when C9n replaced C9. The results show that inability to recover the CM on sucrose density gradients after complement attack may not be a consequence of an essential membrane damage event required for complement-mediated killing of Gram-negative bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraha A., Morgan B. P., Luzio J. P. The preparation and characterization of monoclonal antibodies to human complement component C8 and their use in purification of C8 and C8 subunits. Biochem J. 1988 Apr 1;251(1):285–292. doi: 10.1042/bj2510285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Kuller G., Muhly M., Fromm S., Seibert G., Parrisius J. Formation of transmural complement pores in serum-sensitive Escherichia coli. Infect Immun. 1987 Jan;55(1):206–210. doi: 10.1128/iai.55.1.206-210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5655–5659. doi: 10.1073/pnas.75.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E. F., Schmetz M. A., Foulds J., Hammer C. H., Frank M. M., Joiner K. A. Multimeric C9 within C5b-9 is required for inner membrane damage to Escherichia coli J5 during complement killing. J Immunol. 1987 Feb 1;138(3):842–848. [PubMed] [Google Scholar]
- Dankert J. R., Esser A. F. Bacterial killing by complement. C9-mediated killing in the absence of C5b-8. Biochem J. 1987 Jun 1;244(2):393–399. doi: 10.1042/bj2440393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankert J. R., Esser A. F. Complement-mediated killing of Escherichia coli: dissipation of membrane potential by a C9-derived peptide. Biochemistry. 1986 Mar 11;25(5):1094–1100. doi: 10.1021/bi00353a023. [DOI] [PubMed] [Google Scholar]
- Dankert J. R., Esser A. F. Proteolytic modification of human complement protein C9: loss of poly(C9) and circular lesion formation without impairment of function. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2128–2132. doi: 10.1073/pnas.82.7.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi O., Nojima S. Lipase activity of detergent-resistant phospholipase A in Escherichia coli. Biochim Biophys Acta. 1974 Oct 16;369(1):64–69. [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the action of serum and the role of lysozyme in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2118–2126. doi: 10.1128/jb.96.6.2118-2126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopcroft D. W., Mason D. R., Scott R. S. Standardization of insulin secretion from pancreatic islets: validation of a DNA assay. Horm Metab Res. 1985 Nov;17(11):559–561. doi: 10.1055/s-2007-1013606. [DOI] [PubMed] [Google Scholar]
- Inoue K., Yonemasu K., Takamizawa A., Amano T. [Studies on the immune bacteriolysis. XIV. Requirement of all nine components of complement for immune bacteriolysis]. Biken J. 1968 Sep;11(3):203–206. [PubMed] [Google Scholar]
- Ishidate K., Creeger E. S., Zrike J., Deb S., Glauner B., MacAlister T. J., Rothfield L. I. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem. 1986 Jan 5;261(1):428–443. [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Schmetz M. A., Sanders M. E., Murray T. G., Hammer C. H., Dourmashkin R., Frank M. M. Multimeric complement component C9 is necessary for killing of Escherichia coli J5 by terminal attack complex C5b-9. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4808–4812. doi: 10.1073/pnas.82.14.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- Kroll H. P., Bhakdi S., Taylor P. W. Membrane changes induced by exposure of Escherichia coli to human serum. Infect Immun. 1983 Dec;42(3):1055–1066. doi: 10.1128/iai.42.3.1055-1066.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll H. P., Voigt W. H., Taylor P. W. Stable insertion of C5b-9 complement complexes into the outer membrane of serum treated, susceptible Escherichia coli cells as a prerequisite for killing. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Dec;258(2-3):316–326. doi: 10.1016/s0176-6724(84)80050-4. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Morgan B. P., Daw R. A., Siddle K., Luzio J. P., Campbell A. K. Immunoaffinity purification of human complement component C9 using monoclonal antibodies. J Immunol Methods. 1983 Nov 25;64(3):269–281. doi: 10.1016/0022-1759(83)90434-9. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Podack E. R., Tschoop J., Müller-Eberhard H. J. Molecular organization of C9 within the membrane attack complex of complement. Induction of circular C9 polymerization by the C5b-8 assembly. J Exp Med. 1982 Jul 1;156(1):268–282. doi: 10.1084/jem.156.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Nikaido H. Outer membrane of gram-negative bacteria. XVIII. Electron microscopic studies on porin insertion sites and growth of cell surface of Salmonella typhimurium. J Bacteriol. 1978 Aug;135(2):687–702. doi: 10.1128/jb.135.2.687-702.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Goldschneider I. The serum bactericidal system: ultrastructural changes in Neisseria meningitidis exposed to normal rat serum. J Exp Med. 1969 Jan 1;129(1):51–79. doi: 10.1084/jem.129.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Effect of lethal doses of complement on the functional integrity of target enterobacteria. Curr Top Microbiol Immunol. 1985;121:135–158. doi: 10.1007/978-3-642-45604-6_7. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Interaction of human complement proteins with serum-sensitive and serum-resistant strains of Escherichia coli. Mol Immunol. 1984 Jul;21(7):609–620. doi: 10.1016/0161-5890(84)90046-4. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Kroll H. P. Killing of an encapsulated strain of Escherichia coli by human serum. Infect Immun. 1983 Jan;39(1):122–131. doi: 10.1128/iai.39.1.122-131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLAW A. C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J Exp Med. 1962 Jun 1;115:1231–1249. doi: 10.1084/jem.115.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. I. Location of the lethal lesion. J Immunol. 1981 Sep;127(3):1146–1151. [PubMed] [Google Scholar]
- de Leij L., Kingma J., Witholt B. Insertion of newly synthesized proteins into the outer membrane of Escherichia coli. Biochim Biophys Acta. 1978 Sep 22;512(2):365–376. doi: 10.1016/0005-2736(78)90260-2. [DOI] [PubMed] [Google Scholar]
- de Leij L., Kingma J., Witholt B. Nature of the regions involved in the insertion of newly synthesized protein into the outer membrane of Escherichia coli. Biochim Biophys Acta. 1979 May 17;553(2):224–234. doi: 10.1016/0005-2736(79)90227-x. [DOI] [PubMed] [Google Scholar]


