Abstract
Background
Gastric cancer (GC) is one of the deadliest malignant tumors with unknown pathogenesis. Due to its treatment resistance, high recurrence rate, and lack of reliable early detection techniques, a majority of patients have a poor prognosis. Therefore, identifying new tumor biomarkers and therapeutic targets is essential. This review aims to provide fresh insights into enhancing the prognosis of patients with GC by summarizing the processes through which microRNAs (miRNAs) regulate the tumor microenvironment (TME) and highlighting their critical role in the TME.
Main text
A comprehensive literature review was conducted by focusing on the interactions among tumor cells, extracellular matrix, blood vessels, cancer-associated fibroblasts, and immune cells within the GC TME. The role of noncoding RNAs, known as miRNAs, in modulating the TME through various signaling pathways, cytokines, growth factors, and exosomes was specifically examined. Tumor formation, metastasis, and therapy in GC are significantly influenced by interactions within the TME. miRNAs regulate tumor progression by modulating these interactions through multiple signaling pathways, cytokines, growth factors, and exosomes. Dysregulation of miRNAs affects critical cellular processes such as cell proliferation, differentiation, angiogenesis, metastasis, and treatment resistance, contributing to the pathogenesis of GC.
Conclusions
miRNAs play a crucial role in the regulation of the GC TME, influencing tumor progression and patient prognosis. By understanding the mechanisms through which miRNAs control the TME, potential biomarkers and therapeutic targets can be identified to improve the prognosis of patients with GC.
Keywords: MicroRNA, Tumor microenvironment, Gastric cancer, Angiogenesis, Exosomes, Immune cells
Background
Gastric cancer (GC) is the fourth leading cause of cancer-related deaths worldwide and the fifth most prevalent disease globally [1]. This disease is often diagnosed at an advanced stage with metastases, as there are typically no early and accurate diagnostic methods or specific clinical symptoms. As a result, the 5-year survival rate for GC is only approximately 32% [2]. A noticeable process of connective tissue proliferation is present during solid tumor development, including that observed in GC. This process is intimately associated with immune cells, along with other types of mesenchymal stromal cells in the tumor microenvironment (TME) [3]. The TME includes fibroblasts, the extracellular matrix (ECM), blood vessels, endothelial cells, immune cells, and non-cellular elements such as cytokines and exosomes. Thus, the TME plays a significant role in cancer progression [4, 5] (Fig. 1). In this context, the external and internal environments in which the tumor cells are situated considerably affect the onset, development, and metastasis of the tumor, and they are both interdependent and competitive with each other [6]. Growth factors in the TME enhance the viability of tumor cells, reducing the uptake of chemotherapeutic agents or inactivating them. Additionally, the TME produces immunosuppressive factors, thereby promoting resistance to immunotherapy [7]. Therefore, understanding how the GC TME is regulated and applying these insights to clinical treatment is crucial to enhancing the poor prognosis of patients with GC.
Fig. 1.
TME composition in gastric cancer. TME: Tumor microenvironment
In 1993, Ambros et al. discovered the first microRNA (miRNA) in nematodes. This discovery revealed an important part of the noncoding genome that acts as a critical player in post-transcriptional gene regulation [8]. miRNA dysregulation has been identified in several human diseases, including heart disease, diabetes, cancer, and schizophrenia [9]. Dysregulation of miRNA expression levels in cancer has been associated with a range of biological features of human cancer development, including important roles in enhancing tumor cell proliferation, apoptosis, migration, epithelial-mesenchymal transition (EMT), metastasis, angiogenesis, autophagy, and interactions between malignant cells and the TME [10–12]. miRNA expression profiles in normal cells are very different from those in cancer tissues, and different tumor types and stages, including tumor development, progression, and metastasis, can be identified based on the expression of specific miRNAs [13, 14]. The regulation of miRNA expression has been associated with the suppression of oncogenic miRNAs and the replacement of tumor suppressor miRNAs [15]. Thus, miRNAs are a particularly important area of cancer research, with relevance to cancer prognosis, pathogenesis, diagnosis, and treatment, and are considered the perfect tool for improving cancer therapy [16].
Dysregulated miRNAs promote cancer-associated fibroblast (CAF) activation, inhibit myeloid-derived suppressor cells (MDSCs), inhibit T-cell differentiation, and facilitate angiogenesis, ultimately remodeling the TME [17]. Particularly, tumor cell-derived miRNAs are strongly associated with the production of an immunosuppressive TME and the loss of effector cells and reduced tumor immunogenicity; moreover, they are key determinants of cancer immune outcomes [18, 19]. Additionally, cancer cells secrete exosomes containing tumor suppressor miRNAs that propagate altered sets of miRNAs to different cellular compartments within the TME [20]. miRNAs may be key to immune-mediated tumor clearance, as miRNAs subtly repress genes and preferentially inhibit dose-sensitive targets [21].
Recently, miRNAs have been considered important potential biomarkers for gastric pathology, as they are frequently dysregulated in gastric tissues in preneoplastic lesions such as Helicobacter pylori infection, chronic gastritis, atrophic gastritis, and intestinal metaplasia, as well as in early-stage dysplasia and invasive cancers [22]. Meanwhile, increasing evidence indicates that miRNAs can be considered novel biomarkers; notably, many researchers have analyzed the miRNA profiles in serum and tissue samples from GC to assess their prognostic and diagnostic potential [23, 24] (Table 1). As previously described, miRNAs regulate mesenchymal interactions, immune invasion, and tumor angiogenesis, leading to malignant phenotypes of GC such as tumor growth, metastasis, angiogenesis, and drug resistance [25]. GC cells release extracellular vesicles (EVs) that are enriched in miR-1290. This miRNA enhances the inhibitory impact of GC cells on T-cell activation by targeting grainyhead-like 2 and activating the zinc finger E-box binding homeobox 1/programmed cell death ligand 1 (PD-L1) axis, facilitating GC cell immunological escape [26]. Drug resistance is one of the major challenges facing GC treatment, and manipulating miRNA expression has been shown to alleviate this therapeutic hurdle [27, 28]. Thus, miRNA-targeted GC therapies have great potential to enhance immunotherapy compared to existing therapies [29]. The investigation of microRNAs in GC have entered the clinical settings (Table 2).
Table 1.
miRNAs that potentially represent GC biomarkers
| Symbol | Materials | Function | Biomarker | sensitivity | specificity | Reference |
|---|---|---|---|---|---|---|
| miR-21 | Serum and PBMCs | Promote GC proliferation and invasion | Diagnostic and Prognostic |
88.4% (Serum) 79.6% (PBMCs) |
60.5% (Serum) 55.9% (PBMCs) |
[30] |
| miR-21 and miR-222 | Plasma | Regulate apoptosis, proliferation, and migration | Diagnostic |
86.7% (miR-21) 62.5% (miR-222) |
72.2% (miR-21) 56.2% (miR-222) |
[31] |
| miR-22 | Tissues | Suppress GC cell proliferation and invasion | Prognostic | - | - | [32] |
| miRNA-22-3p | Plasma | Inhibit GC growth and metastasis | Prognostic | - | - | [33] |
| miR-200c | Blood | Regulate invasiveness and migration | Diagnostic and prognostic | 65.4% | 100% | [34] |
| miR-28-5p | Cell lines | Inhibit GC migration and invasion | Prognostic | - | - | [35] |
| miR-29c | Tissues | Inhibit GC proliferation, adhesion, invasion, and migration | Diagnostic | - | - | [36] |
| miR-19b miR-106a | Serum Exosomal | Related to GC lymphatic metastasis | Diagnostic | 95% | 90% | [37] |
| miR-21 miR-106a | Gastric Juice | Increase GC proliferation, migration, and invasion | Diagnostic |
85.7% (miR-21) 73.8% (miR-106a) |
97.8% (miR-21) 89.3% (miR-106a) |
[38] |
| miR-24 and miR-101 | Tissues | Promote GC occurrence, development, infiltration and metastasis | Diagnostic and Prognostic | - | - | [39] |
| miR-124-3p | Tissues and cell lines | Suppressed GC proliferation and induce apoptosis | Prognostic | - | - | [40] |
| miR-129–1-3p and miR-129–2-3p | Gastric juice | GC suppressor activity | Diagnostic | 68.7% | 71.9% | [41] |
| miR-133a | Gastric juice | Inhibit GC proliferation, migration and invasion | Diagnostic | 85.9% | 84.8% | [42] |
| miR-140-5p | Tissues | Suppress GC proliferation and invasion | Prognostic | - | - | [43] |
| miR-181d | Tissues | Promote GC proliferation, migration and invasion | Prognostic | - | - | [44] |
| miR-187 | Tissues | Inhibit GC proliferation and induce cell cycle arrest at the G0/G1 phase | Prognostic | - | - | [45] |
| miR-196a/b | Plasma | OncomiRs | Monitoring, Diagnostic and Prognostic |
69.5% (miR-196a) 62.2% (miR-196b) |
97.6% (miR-196a) 96.1% (miR-196b) |
[46] |
| miR-196a | Plasma | Carcinogenesis | Diagnostic | 100.00% | 75.00% | [47] |
| miR-203 | Serum | Reduce GC EMT phenomena and tumor aggressiveness | Prognostic and Predict metastasis | - | - | [48] |
| miR-212 | Serum | Suppress GC proliferation and induce apoptosis | Prognostic | 95.1% | 78.7% | [49] |
| miR-302b | Tissues | Suppressed GC tumorigenesis and metastasis | Prognostic | - | - | [50] |
| miR-345 | Tissues and cell lines | Inhibit GC migration, stem-like cell phenotype, and EMT | Prognostic | - | - | [51] |
| miR-379 | Tissues and cell lines | Inhibit GC migration, invasion and EMT | Prognostic | - | - | [52] |
| miR-421 | Tissues | Promote GC metastasis, inhibit apoptosis, and induce cisplatin resistance | Prognostic | - | - | [53] |
| miR-421 | Plasma | Diagnostic | 66.29% | 95.56% | [54] | |
| miR-421 | Gastric juice | Carcinogenesis | Diagnostic | 71.4% | 71.7% | [55] |
| miR-484 | Tissues | Inhibit GC proliferation, migration, and invasion | Prognostic | - | - | [56] |
| miR-520a-3p | Tissues and cells | Inhibit GC proliferation, migration and invasion | Prognostic | - | - | [57] |
| miR-208a | Tissues | Promote GC proliferation and invasion | Prognostic | - | - | [58] |
| miR-552 | Tissues | Promote GC proliferation, migration, and invasion | Prognostic | - | - | [59] |
| miR-585 | Tissues and cell lines | Inhibit GC growth and migration | Monitoring | - | - | [60] |
| miR-601 | Tissues and cells | Promote GC proliferation, migration, and invasion | Prognostic | - | - | [61] |
| miR-1225-5p | Tissues | Inhibit GC proliferation, colony formation, migration and invasion | Diagnostic, Prognostic | - | - | [62] |
| miR-1236-3p | Tissues | Suppress GC migration and invasion |
Diagnostic, Prognostic, Monitoring, Recurrences |
73.68% | 60.53% | [63] |
| miR-718 | Tissues | Promote GC proliferation and invasion | Prognostic | - | - | [64] |
| miR-4257, miR-6785-5p, miR187-5p, and miR-5739 | Serum | Diagnostic |
98.3% (discovery set) 99.6% (validation set) |
97.7% (discovery set) 95.3% (validation set) |
[65] |
Abbreviations: miRNAs MicroRNAs, GC Gastric cancer
Table 2.
Summary of microRNAs in GC of clinical trials
| MicroRNA(s) | Source | Purpose | Enrolled | ClinicalTrials.gov identifier | Status | Organizing Location |
|---|---|---|---|---|---|---|
| miR-20a, miR-21, miR-106b, miR-199a, miR-223 | Blood | Diagnostic | 280 | NCT05901376 | Recruiting | Thailand |
| micro RNAs | Blood | Diagnostic | 6862 | NCT04329299 | Completed | Singapore |
| miR-215-5p | Tumor Tissues | Predictive | 35 | NCT01178944 | Completed | United States |
| micro RNAs | Serum | Diagnostic | 809 | NCT06342427 | Completed | United States, Japan |
| micro RNAs | Blood | Predictive | 150 | NCT06490055 | Recruiting | Japan |
| micro RNAs | Plasma | Diagnostic | 150 | NCT06277986 | Recruiting | China |
| micro RNAs | Blood | Predictive | 150 | NCT06490159 | Recruiting | Japan |
| Tissue and Blood | Predictive | 800 | NCT03253107 | Recruiting | Korea | |
| micro RNAs | Serum | - | 100 | NCT05544396 | Recruiting | Taiwan |
| micro RNAs | Blood | Diagnostic | 498 | NCT05224596 | - | China |
| micro RNAs | Blood | Diagnostic | 2430 | NCT05431621 | Completed | China |
| micro RNAs | Blood | Diagnostic | 15000 | NCT05633342 | Recruiting | Singapore |
Abbreviation: GC Gastric cancer
A comprehensive understanding of the biological mechanisms facilitated by miRNAs in the TME of GC may, therefore, offer valuable perspectives for the identification of antitumor drugs and the advancement of targeted cancer treatments in the future. This review emphasizes the pivotal role that miRNAs play in the TME and focuses on how control of the TME by miRNAs influences GC development. Increased understanding of these processes may assist in the development of new therapies for patients with GC and the identification of new biomarkers that can improve management and follow-up strategies for patients with GC.
Main text
miRNAs and H. pylori/Epstein–Barr virus in the GC TME
H. pylori infection is one of the most significant risk factors for GC [66]. Immune monitoring of the gastric mucosa may be impeded by H. pylori-induced activation of signal transducer and activator of transcription 1 (STAT1) and PD-L1 expression, allowing malignant lesions to develop into GC [67]. The H. pylori virulence factor CagA affects multiple types of miRNAs in GC cells [68]. CagA inhibits proliferative and antitumor effects of CD8 + T cells and increases PD-L1 levels in GC cell-derived exosomes via suppressing miRNA-34a and P53 [69]. Moreover, CagA promotes miR-543 overexpression, which inhibits autophagy by targeting sirtuin 1, subsequently inducing EMT and triggering cell invasion and migration [70]. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway is activated by H. pylori infection, which results in the TME secreting T regulatory cells (Tregs), suppressing tumor cell death, and enhancing the TME immunosuppressive state. This pathway activation aids in immune evasion, which in turn facilitates the development of tumors [71, 72]. On the contrary, H. pylori induces miR-223, which downregulates the expression of interleukin (IL)-6, IL-8, IL-1β, and tumor necrosis factor (TNF)-α and inhibits macrophage activation [73].
T helper 1 (Th1) and 17 (Th17) cell differentiation are influenced by miR-155 and contribute to immunity against H. pylori infection, along with infection-associated immunopathology [74]. However, Tsai et al. [75] noted that GC associated with H. pylori significantly increased miR-4286 and miR-18a-3p (5.73-fold and 6.02-fold, respectively). Moreover, invasion and miR-18a-3p, as well as lymph node metastases, tumor size, and tumor stage and miR-4286, have been shown to be significantly associated. Overexpression of miRNA-4286 and miR-18a-3p also inhibits benzodiazepine receptor-associated protein 1 expression while promoting the motility and proliferation of cancer cells. Furthermore, H. pylori infection induces IL-6, which affects STAT3 activity, inhibits miR-520d-5p expression, and activates the STAT3 and Janus kinase (JAK)/STAT pathway, leading to the proliferation of GC cells [76].
In H. pylori-infected T cells along with primary macrophages, miR-155 expression is dependent on forkhead box protein 3 (FOX3), indicating a potential functional relationship between the host immune response and miR-155 [77]. Huang et al. [78] indicated that miR-134 directly targets forkhead box protein M1 (FOXM1), and FOXM1 knockdown prevents the EMT induced by H. pylori CagA + /P + . Therefore, by targeting FOXM1, miR-134 suppresses invasion, proliferation, and EMT of SGC-7901 cells and may be protective against the GC process caused by H. pylori CagA + /P + (Fig. 2).
Fig. 2.
miRNAs and Helicobacter pylori in the gastric cancer TME. miRNAs: MicroRNAs, TME: Tumor microenvironment
Besides H. pylori, Epstein–Barr virus (EBV) is also a causative factor for GC [79]. EBV has been shown to be the first virus to encode its own miRNA. Immune escape is facilitated by EBV-encoded gene products, it-mediated epigenetic and structural variations, and miRNAs, which all assist in malignant transformation [80]. Furthermore, the EBV-miR-BART cluster, including miRBART-2, -4, -5, -18, and -22, is expressed in GC and linked to a poor prognosis [81]. Moreover, simultaneous infection with EBV also hinders the host response to H. pylori. Additionally, EBV synergism may strengthen the oncogenic potential of H. pylori CagA [82]. Notably, EMT-inducing transcription factors are induced in EBV-related GC upon downregulation of miR-200b and miR-200a [83]. In EBV-infected GC cells, miR-34a downregulation causes NADPH oxidase 2 upregulation, which promotes reactive oxygen species (ROS) generation and improves cell survival [84]. Notably, Choi et al. [85] determined that EBV-infected GC cells secrete miR-BART15-3p via exosomes that target the apoptosis inhibitor BRUCE. Subsequently, polybromo‐1 and FOXP1 separately suppress EBV-miR-BART17-3p along with EBV-miR-BART11 and increase PD-L1 transcription, thereby promoting tumor immune escape [86]. Low levels of viral antigen expression help EBV evade the host immune response. Additionally, viral miRNAs directly inhibit the release of the pro-inflammatory cytokine IL-12, thereby modulating the inflammatory response of T cells [87]. Moreover, viral miRNA-BART6-5p targets host cell Dicer and impairs host cell miRNA expression, thus helping EBV evade the host immune response and achieve chronic infection [88].
miRNAs regulate tumor angiogenesis in the GC TME
Angiogenesis plays a crucial role in cancer progression, as it is associated with immunosuppression and is essential for tumor growth, invasion, and metastasis [89, 90]. Based on the downstream targets of miRNAs, the expression of the most potent regulators of angiogenesis in different tumors has been extensively investigated, and a variety of miRNAs have been found to target angiogenic factors. Additionally to being a significant angiogenic agent, the vascular endothelial growth factor (VEGF) functions as an immunomodulator of the TME, promoting tumor-associated macrophages (TAMs) and Treg activation and preventing antigen presentation [91]. In addition to VEGF, phosphatase and tensin homolog (PTEN), mitogen-activated protein kinase (MAPK), and PI3K/AKT/mTOR are the major signaling pathways through which vascular-regulated miRNAs affect GC, and they are important mechanisms through which aberrant miRNAs regulate the development and progression of GC [92, 93].
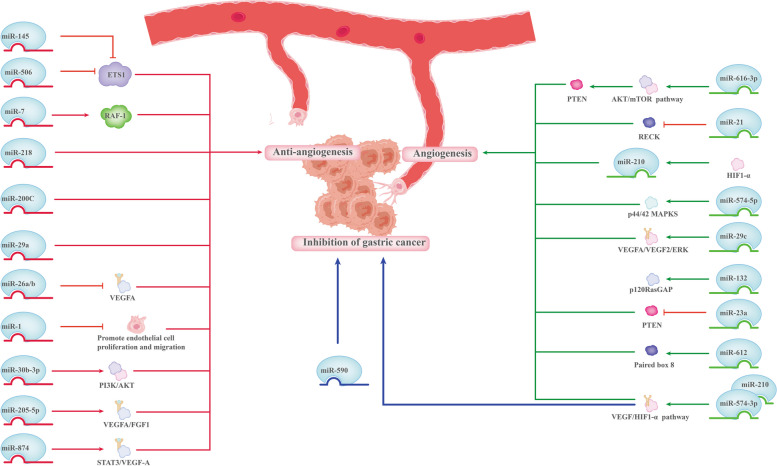
Wu et al. [94] observed that miR-616-3p overexpression in GC triggers the downstream AKT/mTOR signaling pathway, targets PTEN, and facilitates EMT and angiogenesis [95]. Furthermore, miR-21 targets the tumor suppressor gene RECK, which is linked to tumor metastasis and angiogenesis, to cause cancer [96]. MiR-132 has been demonstrated to activate endothelial cells and targets p120RasGAP to induce pathological angiogenesis [97]. Additionally, exosomes, generated from GC cells that carried miR-23a, induced angiogenesis in a co-culture system by suppressing PTEN [98]. When GC cells overexpressed miR-574-3p and miR-210, VEGF and hypoxia-inducible factor 1-alpha (HIF-1α) were upregulated, leading to increased GC cell proliferation, migration, and invasion along with angiogenesis [99, 100]. Subsequently, reduction of invasion, migration, angiogenesis, and EMT resulting from overexpression of paired box 8 on GC cells is replicated by ectopic expression of miR-612 [101].
Meanwhile, miR-574-5p inhibits the expression of protein tyrosine phosphatase non-receptor type 3 and increases phosphorylation of p44/42 MAPKs in GC cells, which promotes angiogenesis [102]. Through its modulation of cancer stem cells (CSCs) and the EMT, the miR-29c-VEGFA/VEGFR2/extracellular signal-regulated kinases (ERK) signaling axis serves as a significant player in the course of GC metastatic disease, making it a prospective acts for GC clinical interventions [103].
With the rapid development of research on miRNAs, their function in tumor suppression through their anti-angiogenic function offers multifaceted therapeutic potential for these molecules. For example, miR-26a/b can directly act on VEGFA in GC, and its overexpression can directly suppress VEGF expression and reduce cell proliferation and angiogenesis, thereby inhibiting GC growth in mice [104]. Besides facilitating GC cell proliferation and migration, a reduction in miR-1 may also trigger pro-angiogenic signaling and encourage endothelial cell migration and proliferation [95]. Moreover, through suppression of VEGFA and fibroblast growth factor 1 expression, miR-205-5p inhibits angiogenesis in GC [105].
Furthermore, the PI3K/AKT signaling pathway mediates invasion, metastasis, angiogenesis, and lymphangiogenesis in GC after the downregulation of miR-30b-3p [106]. By suppressing ETS1 expression through a binding site in the 3′-UTR, miR-145 and miR-506 inhibit GC cell invasion, metastasis, and angiogenesis [107, 108]. Through the STAT3/VEGFA pathway, downregulation of miR-874 facilitates tumor angiogenesis in GC tissues [109]. In GC, miR-590 can concurrently modulate neuropilin 1 and VEGFR1/2. Furthermore, miR-590 overexpression can suppress GC cell migration, invasion, proliferation, and migration, as well as the release of D-MVA both in vivo and in vitro [110]. Similarly, miR-7 targets Raf-1 to suppress angiogenesis and tumorigenesis in GC cells [111].
Zhang et al. [112] confirmed the anti-angiogenic action of miR-218 in GC and demonstrated that tumor angiogenesis inhibition might be achieved therapeutically by administering miR-218. GC with low expression of miR-200C was markedly enriched for angiogenesis, hypoxia, TGF-β signaling genomes, and EMT, all of which contribute to tumor development and metastasis [113]. Interestingly, miR-29a-low GC is enriched for genes correlated with cell apoptosis, proliferation, angiogenesis, and metastasis; it is linked to less anticancer immune cell infiltration and immune-related scores [114] (Fig. 3).
Fig. 3.
miRNAs regulate angiogenesis in the development of gastric cancer. miRNAs: MicroRNAs
Peritoneal dissemination is the main cause of patient mortality and the most frequent reason for tumor progression following GC surgery. Notably, GC development and peritoneal dissemination are significantly influenced by angiogenesis [115], and increased expression of VEGF has been found to promote the production of malignant ascites [116]. Additionally, the expression pattern of miRNAs in peritoneal exosomes serves as a valuable diagnostic tool for peritoneal metastasis treatment, reflecting the tumor load within the abdominal cavity [117]. Transitioning from these observations, research into the regulatory mechanisms of miRNAs in tumor angiogenesis has made significant strides. Despite remaining obstacles, these rapidly evolving findings will make way for the future application of miRNAs as predictive biomarkers for anti-angiogenic therapy and miRNA-based antitumor angiogenesis strategies.
miRNAs regulate CAFs in the GC TME
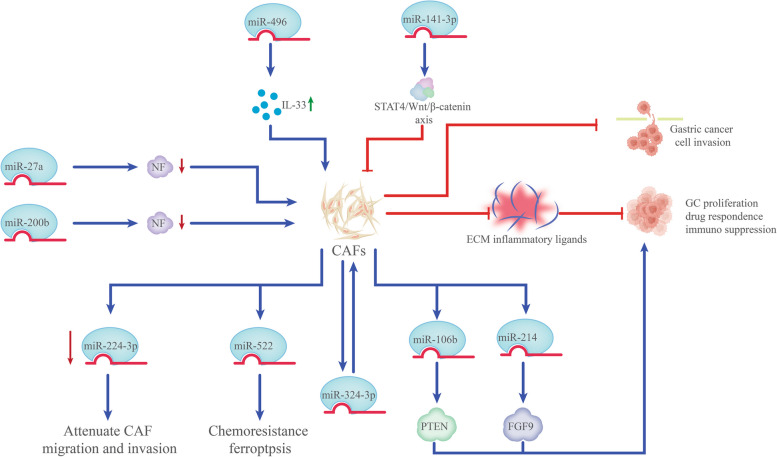
As the major cells in the TME of solid tumors, fibroblasts are controlled by a multitude of factors released by immune or tumor cells [118]. CAFs express a wide range of pro-inflammatory molecules, including chemokines, interleukins, and components of the ECM, which eventually stimulate the growth of tumors by regulating the inflammation associated with the tumor or directing intercellular communication [119]. In the TME, miRNAs are implicated in the whole process of CAF generation and their functional execution, promoting cancer cell proliferation, drug resistance, and immunosuppression via secreting ECM proteins, inflammatory ligands, and growth factors [120].
The high levels of miR-27a observed in GC cell exosomes stimulate the metastasis, motility, and proliferation of cancer cells both in vitro and in vivo, as well as the reprogramming of fibroblasts into CAFs [121]. Meanwhile, another study reported that transformation of CAFs in GC was linked to miR-200b downregulation. Particularly, methylation of the miR-200b promoter was detected in GC cases exhibiting elevated expression of the CAF-specific marker α-smooth muscle actin [122]. However, in contrast to normal fibroblasts, the expression of miR-224-3p was lower in CAFs from patients with squamous GC, and miR-224-3p mimics were found to attenuate CAF migration and invasion [123]. miR-214 in CAFs directly modulates fibroblast growth factor 9 expression, which facilitates cell invasion and GC migration in vitro [124]. Likewise, miR-496 upregulates IL-33, which amplifies CAFs’ tumor-promoting properties by improving GC cell proliferation, EMT, migration, and invasion [125].
It has been confirmed that CAFs elevate miR-106b levels, targeting PTEN to facilitate cell invasion and migration [126]. Zhang et al. [127] demonstrated that the heterogeneous nuclear ribonucleoprotein A1 axis and ubiquitin-specific protease 7 are activated by paclitaxel and cisplatin, which makes it easier for miR-522 to be secreted from CAFs through the de-ubiquitination pathway. Furthermore, miR-522 targets arachidonic acid lipoxygenase 15, which also prevents ROS accumulation. This suppresses ferroptosis in GC cells, causing GC cells to become resistant to chemotherapy [127]. Moreover, by targeting the STAT4/Wnt/β-catenin axis, miR-141-3p suppresses normal fibroblasts from transforming into CAFs, which in turn inhibits GC invasion and migration [128] (Fig. 4). Overall, the activation and creation of CAFs are intimately linked to miRNA dysregulation, which plays a role in both executive function and CAF generation. These results offer fresh perspectives on the relationship between GC cells and CAFs.
Fig. 4.
miRNAs regulate CAFs in the development of gastric cancer. miRNAs: MicroRNAs
miRNAs regulate immunosuppressive cells in the GC TME
The TME consists of various stromal cells such as macrophages, T cells, MDSCs, Tregs, and the ECM; furthermore, blood vessels, lymphatic vessels, cytokines, mediators, and other non-cellular components are vital in defending the human body against pathogen invasion. These cells also impact GC through the modulation of immune responses and the elimination of mutated or damaged cells [129, 130]. miRNAs are implicated in the function and maintenance of Tregs and macrophage polarization, maintaining homeostasis in vivo under physiological conditions and driving immune tolerance or immunosuppression under pathological conditions [131].
Macrophages are a crucial component of both the innate and adaptive immune systems, playing key roles in pathogen defense and the regulation of body homeostasis [132]. The polarization of macrophages is influenced by the PI3K/AKT and JAK/STAT pathways, along with critical regulators such as the STAT family, peroxisome proliferation-activated receptor-g (PPARg), and interferon modulator [133, 134]. This process can lead to the development of TAMs in response to chemokines, cytokines, and other growth factors secreted by tumor cells, as well as tumor-associated conditions. TAMs may adopt the M1 phenotype, which exhibits antitumor activity, or the M2 phenotype, which supports tumor growth [135]. Predominantly, TAMs align with the M2 phenotype and are more likely to promote tumor progression [136]. In GC tissues and ascites, TAMs are abundant and may enhance GC cell migration and invasion through the secretion of EVs [137]. Moreover, dysregulation of miRNAs in tumors facilitates the shift of macrophage polarization from M1 to M2, adversely impacting TAM phenotypes and suppressing the immune response [138]. The involvement of TAMs in GC underscores their complex role, suggesting that miRNA-based reprogramming of TAM polarization could advance tumor immunotherapy.
According to Yun et al. [139], downregulating miR-30c under hypoxic environments decreased mTOR and glycolysis activity in TAMs in GC and further suppressed M1 macrophage differentiation and antitumor effects. With PTEN and IFN-γ/STAT1, miR-21 modulates TAMs, enhancing tumor cell motility and M2 polarization while lowering the expression of PD-L1 and M1 polarization to promote cancer progression [140]. Interestingly, TAMs deficient in miR-21 had an inflammatory gene signature, and antagonism of miR-21 increased the level of granzyme B, which enhanced the cytotoxicity of CD8 + T cells in immune TME [141].
Additionally, exosomes derived from M2 macrophages may transfer miR-487a into GC cells, possibly facilitating GC progression through the downregulation of T-cell intracellular antigen [142]. Exosomes derived from M2 macrophages produce miR-588, which targets cylindromatosis and enhances resistance to cisplatin in GC cells [143]. Moreover, exosome miR-21 translocates directly from TAMs to GC cells and modulates GC resistance to cisplatin by targeting PTEN, suppressing apoptosis, and activating the PI3K/AKT signaling pathway [144]. These studies highlight the importance of miRNA regulation of macrophages through key signaling pathways.
Compared with other intra-abdominal tumors, GC is more prone to peritoneal metastases, and the peritoneal immune microenvironment is critical for GC progression [145, 146]. Notably, TAM in malignant ascites of GC showed a significant M2-like phenotype, which promotes peritoneal metastasis of GC [147, 148]. Microarray analysis revealed a significant connection in GC tissue between the expression of miR-210 and CD204 + M2-like TAM infiltration. TNF-α, released by CD204 + M2-like TAMs, upregulates miR-210 through NF-κB/HIF-1α signaling to facilitate GC progression [149]. In summary, these candidate preclinical and clinical miRNAs underscore their roles as TME immune modulators and their therapeutic potential. A deeper understanding of how different miRNAs influence the M1/M2 balance could aid in developing targeted therapies to re-educate macrophages toward the M1 phenotype.
After antigenic stimulation, naive CD4 + T cells differentiate into multiple effector Th subpopulations with distinct phenotypes, such as Th1, Th2, Treg, and IL-17-producing Th17 [150]. Certain miRNAs have been shown to regulate T-cell differentiation. For example, the differentiation of Treg/Th17 and Th1 cells is inhibited by miR-23 and -27, whereas miR-24 facilitates their differentiation, creating an immunosuppressive microenvironment conducive to GC progression and metastasis [151]. Furthermore, the miR-192-5p/Rb1/NF-κBp65 signaling axis stimulates Treg differentiation by modulating IL-10 production in GC while also facilitating EMT in tumor cells [152]. Importantly, exosomes promote the differentiation of primary neoplastic Treg cells at the expense of antitumor Th1/Th17 differentiation, suggesting that tumor miRNAs can orchestrate immune evasion through multiple simultaneous mechanisms [153, 154]. Furthermore, the secretion of exosomal miR-451, which escalates under low-glycemic conditions and is subsequently transferred to T cells, supports the differentiation of T cells into Th17 cells by diminishing AMP-activated protein kinase and enhancing mTOR activity, marking a potential indicator of poor prognosis [155]. Additionally, a hypoxic TME reduces miR-34a expression, resulting in elevated lactate levels in GC tumor-infiltrating lymphocytes and a reduction in Th1 cells and cytotoxic T lymphocytes (CTLs), thereby compromising the immune efficacy of GCs [156].
Moreover, MDSCs, a heterogeneous group of myeloid-derived cells, facilitate tumor invasion and metastasis through diverse mechanisms, with tumor miRNAs directly governing the recruitment and functionality of MDSCs [157]. Notably, MDSCs characterized by the expression of the myeloid differentiation factor schlafen4 + , a regulator of myeloid differentiation, have been identified in GC, particularly in preneoplastic lesions infected with H. pylori [158]. miR-130b is increased in Schlafen4 + GC cells and promotes gastric epithelial cell proliferation, which is essential for MDSCs to suppress T-cell functions [159]. Exosomes secreted by GC deliver miR-107 to host MDSCs and induce their amplification and activation by targeting DICER1 and PTEN genes, thus providing new cancer therapeutic targets for GC [160]. Furthermore, miR-200C reduces PTEN and friend of Gata 2 expression, induces the PI3K/Akt cascade, promotes MDSCs amplification, and suppresses immune response in TME [161] (Fig. 5).
Fig. 5.
miRNAs regulate immunosuppressive cells in the development of gastric cancer. miRNAs: MicroRNAs, CAFs: Cancer-associated fibroblasts
The miRNAs can also directly affect immunosuppressive signaling, thereby altering the TME. Meanwhile, miR-4510 inhibits GC cell metastasis by altering immunosuppressive signals in the TME through the downregulation of glypican-3 [162]. miR-148b-5p deficiency results in immunological tolerance and GC development via the CSF1 and miR-148b-5p/ATPIF1/TNFa + IL6 axis [163].
These studies suggest that many miRNAs play essential roles in regulating TME-mediated immunosuppressive mechanisms. However, this area of research still needs to be further explored.
miRNAs modulate immunoreactive cells in the TME of GC
T cells are vital to maintaining health and preventing disease and are divided into two main subpopulations: CD4 + and CD8 + T-cell subpopulations [164]. Longer survival from cancer is linked to infiltration of CD8 + T cells; however, low immunogenicity of tumor cells in the TME inhibits T lymphocyte immunological activity, which reduces their antitumor capacity [165]. Post-transcriptional gene regulation via miRNAs has emerged as a major control mechanism for a variety of biological processes, including T-cell development and function [166]. Given that T cells can perform both pro-inflammatory and pro-absorptive tasks, identification and characterization of miRNAs associated with T-cell function will reveal miRNA-mediated mechanisms as therapeutic targets for immunotherapy against a wide range of diseases with inflammatory and immunosuppressive environments [167].
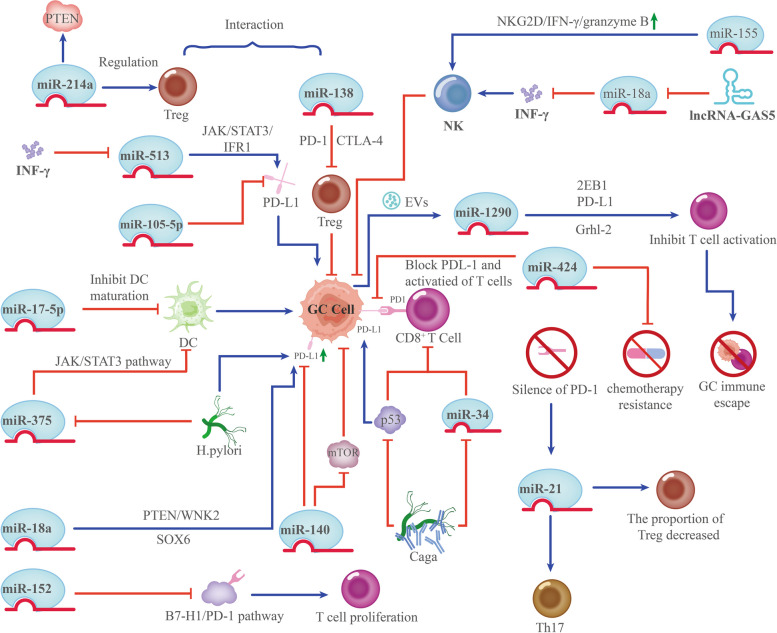
miRNAs regulate the expression of immune checkpoint ligands and protect tumors from T-cell-mediated lysis [168]. For instance, miR-105-5p, serving as a key player in the post-transcriptional suppression of PD-L1 in GC, prevents immunological escape resulting from upregulation of PD-L1 in cancer cells [169]. Furthermore, miR-424 has been identified as a potential inhibitor of the PD-L1/PD-1 pathway, and restoration of miR-424 expression reverses chemotherapy resistance [170].
miR-138 mainly modulates the immune system by interacting with CTLA-4 and PD-1 to repress tumor-infiltrating Tregs, thereby mitigating damage to immune-disordered cells in the TME [171]. Notably, H. pylori-positive GC has considerably higher PD-L1 expression levels, and miR-140 overexpression suppresses the proliferation and tumor growth of GC cells by blocking PD-L1 and mTOR activity [172]. Additionally, by repressing the expression of miR-513, reducing the translational repression of PD-L1, activating the pathway of JAK2/STAT1/IFR-1, and augmenting PD-L1 expression, INF-γ induces GC immune escape [173]. Notably, in vitro silencing of PD-1 enhances miR-21 expression, increases the proportion of Th17 cells, and decreases that of Treg cells [174]. miRNAs play an essential role in regulating the immune response, and miRNAs can interact with immune checkpoint inhibitors.
Additionally, elevated miR-152 levels improve immune responses by facilitating effector cytokine production and T-cell proliferation through the suppression of the B7-H1/PD-1 pathway. MiR-152 may be a potential therapeutic approach for GC [175]. Notably, manipulation of immune checkpoint protein expression by miRNA-based therapies combined with anti-immune checkpoint drugs may be an improved approach to GC treatment.
Dendritic cells (DCs) are the most potent antigen-presenting cells, capable of efficiently cross-presenting antigens. DCs contribute significantly to antitumor immunity by modulating the TME and attracting and activating anticancer T cells [176]. Thus, by impairing DC activation, antigen presentation, maturation, recruitment, and differentiation, TME and GC cells evade immune control [177]. Many miRNAs are implicated in the development and differentiation of DCs and in the regulation of inflammatory responses in DCs. Tumor miRNAs can directly or indirectly control DCs maturation and induce a tolerant state [178]. miR-17-5p decreased the secretion of TNF-α and IL-12 while increasing the production of IL-10. This shift inhibits the stimulation of T cells by DCs and promotes the expansion of Tregs. Furthermore, it can be utilized as a biomarker for GC originating from GC cells [179]. Additionally, in gastric TME, H. pylori can suppress miRNA-375 expression. This triggers the JAK2-STAT3 pathway, consequently promoting the release of VEGF, IL-10, and IL-6. These released factors promote DCs to differentiate immaturely and contribute to the induction of GC [180] (Fig. 6). These studies have demonstrated that miRNAs regulate the development, differentiation, and function of DCs, establishing them as pivotal regulators of the immune response. Another critical cellular component of innate immunity is the natural killer (NK) cells, which are essential in the immune response against cancer by killing tumor cells and secreting immunostimulatory cytokines [181]. Variations in miRNA expression influence the progression of NK and invariant NKT cells differently. For example, invariant NKT cells in the peripheral and thymus lymphoid organs are negatively regulated by miR-150 [182]. Conversely, miR-155 enhances NK cell function by increasing NKG2D, IFN-γ, and granzyme B production [183]. Furthermore, lncRNA-GAS5 enhances IFN-γ secretion by targeting miR-18a, thus promoting NK cell responses against GC cells [184]. In addition to modulating receptor signaling, miRNAs directly affect the production of effector molecules that determine NK cell activity.
Fig. 6.
miRNAs modulate immunoreactive cells in gastric cancer. miRNAs: MicroRNAs
Exosome-derived miRNAs regulate the GC TME
The discovery of exosomes and their multiple functions in cancer biology is undoubtedly one of the most exciting discoveries in recent years. Exosomes are nanoscale (30–150 nm in diameter) EVs that can transport a broad range of substances, including metabolites, proteins, lipids, and nucleic acids [185]. miRNAs in cancer-derived exosomes promote intercellular communication, targeting themselves and contributing to the regulation of multiple components of the immune system, ultimately modulating the TME to regulate GC development, metastasis, invasion, drug resistance, and angiogenesis [186, 187]. They are very valuable for the prognosis and early GC diagnosis and, to some extent, reflect the malignant characteristics of the tumor [188, 189]. Meanwhile, miRNAs play a role in the communication between tumor cells and TME through exosomal secretion and transport [190].
GC cells release exosomes containing miR-582-3p, which targets VEGF to stimulate cell invasion and proliferation [191]. Exosomes produced from GC cells carrying miR-135b have been found by Bai et al. [192] to lower FOXO1 protein levels and stimulate angiogenesis. GC cells can give rise to exosomes enriched in miR-301a-3p in hypoxic TMEs, which contribute to EMT, GC proliferation, invasion, and migration, along with HIF-1α accumulation [193]. Additionally, individuals with GC hepatic metastases demonstrate serum exosomes exhibiting considerably higher miR-519a-3p levels compared to individuals without liver metastases. Moreover, by targeting DUSP2, exosomal miR-519a-3p promotes the MAPK/ERK pathway, leading to M2-like polarization of macrophages, resulting in angiogenesis, facilitating the development of pre-metastatic niches in the liver, and accelerating the process of liver metastasis [194].
Exosomal miR-106a and miR-21-5p activates the TGF-β pathway by targeting TIMP2 and SMAD7, disrupts the mesothelial barrier, and promotes the peritoneal spread of GC by integrating into peritoneal mesothelial cells [195, 196]. Moreover, serum exosomes from patients with GC were enriched in miR-423-5p, and a significant correlation existed between lymph node metastasis and extracellular miR-423-5p levels, which facilitated cancer growth and metastasis [197].
Macrophages produce exosomes containing miR-16-5p that translocate to GC cells and target PD-L1 to activate T cells, thereby suppressing GC development [198]. Exosomes containing miR-21 are produced in tumors when the EMT transcription factor Snail activates miR-21. These exosomes are taken up by CD14 + human monocytes, which then cause a rise in M2 marker expression and ultimately accelerate tumor progression [199]. Furthermore, exosomal miR-15b-3p suppresses apoptosis in vivo and in vitro by inhibiting the expression of DYNLT1, cleaved caspase-3, and caspase-9. This promotes the proliferation, invasion, and migration of GC cells [200]. Additionally, GC cells secrete exosomes capable of delivering miR-107 to MDSCs, which causes the activation and amplification in MDSCs by targeting PTEN and DICER1 [160].
Notably, exosomal miR-122-5p inhibits both tumor development in vivo and GC cell migration and proliferation in vitro [201]. Furthermore, exosomal miR-139 produced by CAFs suppresses GC cell metastasis and tumor growth by decreasing the expression of matrix metalloproteinase 11 both in vitro and in vivo [202]. Moreover, exosomal miR-29b-1-5p generated from CAFs inhibits GC cell survival, invasion, and migration, as well as vascular mimicry development; however, it also stimulates apoptosis [203]. Additionally, CAF-derived EVs containing miR-199a-5p downregulate FKBP5, resulting in elevated AKT1 phosphorylation and mammalian target of rapamycin complex 1 activation, thereby promoting GC [204].
Chemotherapy is the cornerstone of cancer treatment; however, some individuals develop resistance to the drugs administered. GC has the highest rate of drug-resistant recurrence among all cancer types; this phenomenon considerably restricts the long-term prospects of patients with cancer, with 5-year survival rates dropping as low as 30% [205]. An increasing number of miRNAs have been found to be aberrantly expressed in drug-resistant GC tissues and are involved in the process of chemoresistance. These miRNAs function through complex mechanisms, including inactivation of apoptotic signaling pathways, loss of cell cycle checkpoint control, accelerated cell proliferation and autophagy flux, enhanced DNA damage repair, and drug transport and regulation. Furthermore, they activate CSCs and EMT [206–208] (Fig. 7). These correlations suggest that miRNA analysis will be a valuable tool for accurately assessing cellular sensitivity to chemotherapy and can be used to develop novel therapeutic approaches capable of overcoming resistance to GC chemotherapy [209] (Table 3).
Fig. 7.
miRNAs modulate GC chemoresistance through several mechanisms. miRNAs: MicroRNAs, GC: Gastric cancer
Table 3.
miRNAs that play roles in GC chemoresistance
| Symbol | Status | Signaling Pathway/ Targets | Function | Effects on chemosensitivity | Resistance | Reference |
|---|---|---|---|---|---|---|
| miR-1 | Downregulated | Sorcin | Promote the accumulation of intracellular drugs and enhance apoptosis | Increasing | Adriamycin, Vincristine | [210] |
| miR-7 | Downregulated | LDH-A | Increase apoptosis and caspase-3 activation | Increasing | Cisplatin | [211] |
| miR-16–1 | Downregulated | FUBP1 | Inhibit GC proliferation and invasion, and advanced apoptosis | Increasing | Adriamycin | [212] |
| miR-17 | Upregulated | EMT, DEDD | Inhibit apoptosis | Decreasing | Cisplatin, 5-Fluorouracil | [213] |
| miR-17-5p | Downregulated | P21 | Inhibit apoptosis | Decreasing | Cisplatin | [214] |
| mir-15b or miR-16 | Downregulated | Bcl-2 | Induce apoptosis | Increasing | Doxorubicin, Etoposide, Vincristine, Cisplatin | [215] |
| miR-19a/b | Upregulated | PI3K-Akt/ PTEN | Accelerate drug efflux and inhibit apoptosis | Decreasing | Cisplatin, 5-Fluorouracil, Adriamycin | [216] |
| miR-20a | Upregulated | NFκB/CYLD | Inhibit apoptosis | Decreasing | Cisplatin | [217] |
| miR-20a | - | PI3K-AKT and MAPK-ERK/ LRIG1 | Reduce apoptosis | Decreasing | Adriamycin, Vincristine | [218] |
| miR-21 | Upregulated | PTEN-PI3K-Akt/PTEN | Reduce antiproliferative effects and apoptosis | Decreasing | Cisplatin | [219] |
| miR-23b-3p | Downregulated | ATG12 and HMGB2 | Inhibit autophagy | Increasing | Vincristine, 5-Fluorouracil and Cisplatin | [220] |
| miR-25 | Upregulated | FOXO3a | Promote GC cycle progression | Decreasing | Cisplatin | [221] |
| miR-27a and miR-155 | Upregulated | RKIP | Inhibit apoptosis | Decreasing | 5-Fluorouracil and Oxaliplatin | [209] |
| miR-30a | Upregulated | beclin 1 | Suppress autophagy, induce apoptosis and G2/M cell cycle arrest | Increasing | Cisplatin | [222] |
| miR-31 | Downregulated | RhoA | Enhance apoptosis, inhibit cell cycle | Increasing | 5-Fluorouracil | [223] |
| miR-34 | - | Bcl-2, Notch, and HMGA2 | Induce GC apoptosis, Caspase-3 activation, and accumulate in G1 phase | Increasing | Docetaxel, Gemcitabine, Cisplatin, Doxorubicin | [224] |
| miR-34a | Upregulated | MET | Inhibit GC proliferation and induct apoptosis | Increasing | Cisplatin | [225] |
| miR-34c | Downregulated | Promote GC apoptosis and inhibit proliferation | Increasing | Paclitaxel, Cisplatin | [226] | |
| miR-34c-5p | Downregulated | MAPT | Regulate DNA methylation, inhibit GC proliferation and promote apoptosis | Increasing | Paclitaxel | [227] |
| miR-96 | Upregulated | FOXO1 | Promote GC proliferation | Decreasing | Cisplatin, Doxorubicin | [228] |
| miR-99a and miR-491 | Upregulated | AKT-FOX3A/ CAPNS1 | Induced GC apoptosis | Increasing | Cisplatin | [229] |
| miR-101 | Downregulated | p38MAPK and AKT / ANXA2 | Promote GC apoptosis | Increasing | Cisplatin, Vincristine | [230] |
| miR-106a | Upregulated | RUNX3 | Accelerate ADR efflux, and suppress apoptosis | Decreasing | Adriamycin, Vincristine | [231] |
| miR-106a | Upregulated | PI3K-AKT/ PTEN | Regulate GC apoptosis | Decreasing | Cisplatin | [232] |
| miR-126 | Downregulated | EZH2 | Promote GC proliferation and migration | Increasing | Vincristine, Adriamycin | [233] |
| miR-128 | Downregulated | HMGA2 | Increase GC apoptosis | Decreasing | Cisplatin | [234] |
| miR-129 | Downregulated | P-gp | Activate apoptotic pathway via upregulating caspase-9 and caspase-3 | Increasing | Cisplatin | [235] |
| miR-130b | Upregulated | CMPK1 | Reduce sensitivity and DNA damage | Increasing | 5-Fluorouracil | [236] |
| miR-132 | Upregulated | SIRT1-CREB-ABCG2/ SIRT1 | Regulate CSC | Decreasing | Cisplatin | [237] |
| miR-135a-5p | Upregulated | AP-2α/ BCL-2 | Enhance cell resistance to apoptosis | Increasing | Adriamycin | [238] |
| miR-135b | Upregulated | MAPK/ MST1 | Inhibit apoptosis, and induce proliferation | Decreasing | Cisplatin | [239] |
| miR-145 | Downregulated | CD44 | Regulate CSC | Decreasing | 5-Fluorouracil, Cisplatin | [240] |
| miR-148a-3p | Downregulated | AKAP1, RAB12 | Activate mitochondrial fission and apoptosis | Increasing | Cisplatin | [241] |
| miR‑138‑5p | Downregulated | ERCC | Regulate DNA damage repair | Increasing | Cisplatin | [242] |
| miR-155 | Upregulated | STAT3 and NF-κB | Inhibit GC apoptosis, promote proliferation | Decreasing | Cisplatin and 5-Fluorouracil | [243] |
| miR-155-5p | Upregulated |
GATA3 TP53INP1 |
Regulate EMT | Decreasing | Paclitaxel | [244] |
| miR-181a | Upregulated | MTMR3 | Attenuate GC apoptosis and autophagy | Decreasing | Cisplatin | [245] |
| miR-181a-2-3p | Upregulated | Inhibit GC apoptosis | Increasing | Cisplatin | [246] | |
| miR-181b | Downregulated | BCL2 | Induce apoptosis | Increasing | Vincristine, Cisplatin, Adriamycin, Etoposide, 5-Fluorouracil | [247] |
| miR-185 | Upregulated | ARC | Induce apoptosis | Increasing | Cisplatin, Doxorubicin | [248] |
| miR-193a-3p | Upregulated | Mitochondrial apoptosis/ SRSF2 | Inhibit apoptosis | Decreasing | Cisplatin | [249] |
| miR‑195‑5p | Downregulated | ZNF139 | Regulate MDR | Increasing | 5-Fluorouracil, Oxaliplatin | [250] |
| miR-200bc/429 | Downregulated | BCL2, XIAP | Induce apoptosis | Increasing | Vincristine, Cisplatin, Adriamycin, Etoposide, 5-Fluorouracil | [251] |
| miR-200c | Downregulated | Zinc finger E-box binding homeobox 2 | Induce apoptosis | Increasing | Cisplatin | [252] |
| miR-204 | Downregulated | Bcl-2 | Promote GC apoptosis | Increasing | 5-Fluorouracil; Oxaliplatin | [253] |
| miR-204 | Downregulated | TGFBR2 | Regulate EMT | Increasing | 5-Fluorouracil | [254] |
| miR-218 | Upregulated | mTOR | Induce apoptosis | Increasing | Cisplatin | [255] |
| miR-223 | Upregulated | FBXW7 | Regulate cell cycle and apoptosis | Decreasing | Cisplatin | [256] |
| miR-223 | Upregulated | FBXW7 | Regulate EMT | Decreasing | Doxorubicin | [257] |
| miR-193-3p | Upregulated | PTEN | Promote GC proliferation migration | Decreasing | 5Ffluorouracil | [258] |
| miR-301b-3p | Upregulated | TXNIP | Promote MDR | Decreasing | Cisplatin, Vincristine | [259] |
| miR-361-5p | - | PI3K-AKT-mTOR/ FOXM1 | Inhibit autophagy | Increasing | Docetaxel | [260] |
| miR-363 | Upregulated | FBW7 | Promote GC proliferation | Decreasing | docetaxel + cisplatin + 5-FU | [261] |
| miR-375 | Upregulated | PI3K-AKT/ ERBB2 | Anti-proliferative and apoptosis-inducing | Increasing | Cisplatin | [262] |
| miR-421 | Upregulated | E-cadherin and caspase-3 | Promote metastasis, inhibit apoptosis | Decreasing | Cisplatin | [53] |
| miR-424-3p | Downregulated | ABCC2 | Promote GC proliferation | Decreasing | Cisplatin | [263] |
| miR-429 | Downregulated | PI3K-AKT-mTOR/ SOX2 | Inhibit apoptosis | Decreasing | Cisplatin | [264] |
| miR-492 | Upregulated | DNMT3B | Induce GC proliferation | Decreasing | Cisplatin | [265] |
| miR-493 | MAD2L1 | Regulate chemosensitivity | Decreasing | Paclitaxel | [266] | |
| miR-495-3p | Downregulated | GRP78-mTOR/ GRP78 | Inhibit autophagy | Increasing | Vincristine, Adriamycin | [267] |
| miR-497 | Upregulated | Bcl-2 | Induce apoptosis | Increasing | Vincristine; Cisplatin; Etoposide; Adriamycin | [268] |
| miR-500a-3p | Upregulated | FBXW7 | Induce CSCs properties | Decreasing | Cisplatin | [269] |
| miR-503 | Downregulated | IGF1R, BCL2 | Inhibit GC proliferation, induce apoptosis | Increasing | Cisplatin | [270] |
| miR-508-5p | Downregulated | ZNRD1,ABCB1 | Induce apoptosis | Increasing | Vincristine; Adriamycin; 5-Fluorouracil; Cisplatin | [271] |
| miR-524-5p | Upregulated | SOX9 | Inhibit GC proliferation and invasion | Increasing | Cisplatin | [272] |
| miR-590-5p | Upregulated | AKT-ERK and STAT3/ RECK | Promote GC proliferation and invasion | Decreasing | Cisplatin and Paclitaxel | [273] |
| miR-623 | Downregulated | Cyclin D1 | Inhibit GC proliferation | Increasing | 5-Fluorouracil | [274] |
| miR-647 | Downregulated | ANK2-CD44-SNAIL1/ Ankyrins | Induce GC apoptosis and prevent cells from entering S phase of the cell cycle | Increasing | Vincristine | [275] |
| miR-648 | Downregulated | ET-1 | Induct apoptosis | Increasing | 5-Fluorouracil | [276] |
| miR-708-3p | Upregulated | ETNK1 | Promote GC proliferation and migration, inhibit apoptosis, and facilitate the transition from the G0/G1 to the G2/M phase | Decreasing | [277] | |
| miR-873-5p | Downregulated | THUMPD1 | Regulate migration, invasion, and chemoresistance | Increasing | Doxorubicin, 5-Fluorouracil, Cisplatin | [278] |
| miR-874 | Downregulated | ATG16L1 | Inhibit autophagy | Increasing | Cisplatin | [279] |
| miR-1229-3p | Upregulated | SLC22A7 | Induce chemoresistance | Decreasing | 5-Fluorouracil | [280] |
| miR-1284 | Downregulated | EIF4A1-JUN-MYC/ EIF4F | Promote cell cycle arrested at the G0/G1 phase, induce apoptosis | Increasing | Vincristine | [281] |
| miR-4295 | Upregulated | EGFR-PI3K-AKT/ LRIG1 | Induce apoptosis | Decreasing | Cisplatin | [282] |
Abbreviations: miRNAs MicroRNAs, GC Gastric cancer
This phenomenon of chemoresistance is also linked to miRNAs in exosomes. For example, patients with GC who have elevated miR-500a-3p levels in their plasma exosomes are more likely to be resistant to cisplatin, which lowers their progression-free survival rate [269]. Additionally, exosomes allow miR-21 to be transported from macrophages to GC cells, which significantly lowers the sensitivity of GC cells to cisplatin treatment both in vitro and in vivo, partly through modulation of the PTEN/PI3K/AKT signaling pathway [144].
Clinically, GC tissues display markedly elevated levels of miR-223 expression. Moreover, a strong correlation between high expression levels of plasma exosomal miR-223 and doxorubicin resistance is observed in patients with GC [257]. For example, biologically active miR-769-5p spreads cisplatin resistance by integrating into exosomes and infiltrating sensitive cells. Furthermore, by targeting CASP9, miR-769-5p enables the ubiquitin–proteasome pathway to degrade p53, an apoptosis-associated protein, while suppressing the downstream caspase pathway [283]. Furthermore, by modulating the high mobility group A2/mTOR/P-GP axis, exosome-secreted miR-107 dramatically increases the sensitivity of drug-resistant GC cells to chemotherapeutic drugs [284]. Finally, in paclitaxel-resistant GC cells, exosomal administration of miR-155-5p promotes chemoresistant phenotypes and EMT, which may be mediated by suppression of TP53INP1 and GATA3 [244] (Fig. 8). Overall, these findings imply that exosomal-derived miRNAs are essential for the development of medication resistance.
Fig. 8.
Exosome-derived miRNAs regulate TME and participate in the development of gastric cancer. miRNAs: MicroRNAs, TME: Tumor microenvironment
Conclusions
Despite treatment efforts, GC remains one of the deadliest tumors. Over the past years, growing research has indicated the significant role of the TME in the development, advancement, invasion, and metastasis of GC. Recent studies have shown a strong correlation between GC and miRNA dysregulation, which has a significant impact on TME-related activities and provides new insights into the relationship between immune cells, mesenchymal stromal cells, malignant cells, and non-cellular components of the TME, promoting tumor proliferation, angiogenesis, and metastasis.
Particularly, malignant and drug-resistant tumor cells secrete exosomes containing specific miRNAs. Therefore, exosomes are crucial for material exchange, energy flow, and signaling between the different cellular components of the TME. An in-depth study of the effect of miRNAs on TME is of great significance in furthering our understanding of the biology of GC. Based on the role of miRNAs in TME, the development of miRNAs as synergistic tumor immunotherapeutics is of great significance to improve the efficacy of monotherapy and reduce tumor survival.
Notably, several challenges remain to be addressed before these studies can be translated into clinical applications. Firstly, due to the complexity of the TME, the exact mechanisms of different miRNAs in different cell types in the TME remain largely unknown [285]. To select the optimal targets, a deeper understanding of the role of each specific miRNA in all immune cell subpopulations and their complete regulatory networks is essential. Additionally, given that naked miRNAs have a short half-life in vivo and are easily degraded, there is an urgent need to identify a safe, effective, and targeted vector to protect the miRNAs and ensure their delivery to the intended sites [286].
In conclusion, this review describes the communication mechanisms of miRNAs between the TME and GC tumor cells. Dysregulated miRNAs are found in both non-tumor and tumor cells within the TME, emphasizing the key role played by the TME and miRNAs in the development and metastasis of cancer. While their exact mechanism of action is still being investigated, several miRNAs have emerged as potential therapeutic targets and GC biomarkers. Exploring and studying the regulatory effects of naturally derived drugs on the TME at the miRNA level holds promise, especially considering the polygenic targeting of miRNAs and the anticancer effects of natural drugs on various types of mesenchymal stromal cells within the TME.
Acknowledgements
Not applicable.
Abbreviations
- GC
Gastric cancer
- TME
Tumor microenvironment
- CAFs
Cancer-associated fibroblasts
- miRNAs
MicroRNAs
- ECM
Extracellular matrix
- DC
Dendritic cells
- Tregs
Regulatory T cells
- MDSCs
Myeloid-derived suppressor cells
- EVs
Extracellular vesicles
- VEGF
Vascular endothelial growth factor
- IL
Interleukin
- JAK2-STAT3
Janus kinase 2
- MAPK/ERK
Mitogen-activated protein kinase/extracellular signal-regulated kinases
- EMT
Epithelial-mesenchymal transition
- PI3K/AKT/mTOR
Phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin
- FOXM1
Forkhead box M1
- PD-L1
Programmed death ligand 1
- HIF-1α
Hypoxia-inducible factor 1-alpha
- TAMs
Tumor-associated macrophages
- PTEN
Phosphatase and tensin homolog
- Th1
T helper 1 cells
- Th17
T helper 17 cells
- CTLs
Cytotoxic T lymphocytes
- NKs
Natural killer cells
- ROS
Reactive oxygen species
- EBV
Epstein–Barr virus
- ZEB1
Zinc finger E-box-binding homeobox 1
- TNF
Tumor necrosis factor
- CSCs
Cancer stem cells
Authors’ contributions
XZ Y and Y Z conceptualized the manuscript. XZ Y wrote the first draft. QH Z, FM L and LL Z contributed substantially by revising the manuscript. All authors approved the submitted version and are fully accountable for every aspect of the work.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82202989); the China Postdoctoral Science Foundation (No. 2022M722279, China); the Sichuan Science and Technology Program (No. 2023YFS0163, China); Postdoctoral Research Project of West China Hospital, Sichuan University, Chengdu, China (No. 2021HXBH045); Fundamental Research Funds for the Central Universities (No. 2022SCU1206); and Sichuan University Postdoctoral Interdisciplinary Innovation Fund (awarded to LZ, China).
Availability of data and materials
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xianzhe Yu and Yin Zhang contributed equally to this work.
Contributor Information
Fengming Luo, Email: fengmingluo@outlook.com.
Qinghua Zhou, Email: prof_qh_zhou@126.com.
Lingling Zhu, Email: zhulingling826@163.com.
References
- 1.Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. 10.1186/s13045-023-01451-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53. 10.1002/ijc.31937 [DOI] [PubMed] [Google Scholar]
- 3.Wei J, Yang Y, Wang G, Liu M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. 2022;13:1035276. 10.3389/fimmu.2022.1035276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Teixeira AF, Zhu HJ, Ten Dijke P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol Cancer. 2021;20:154. 10.1186/s12943-021-01463-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang E, Wang X, Gong Z, Yu M, Wu H, Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal Transduct Target Ther. 2020;5:242. 10.1038/s41392-020-00359-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C, Wei S, Dai S, Li X, Wang H, Zhang H, Sun G, Shan B, Zhao L. The NR_109/FUBP1/c-Myc axis regulates TAM polarization and remodels the tumor microenvironment to promote cancer development. J Immunother Cancer. 2023;11:e006230. 10.1136/jitc-2022-006230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd LNC, Andini KD, Peters GJ, Kazemier G, Giovannetti E. Heterogeneity and plasticity of cancer-associated fibroblasts in the pancreatic tumor microenvironment. Semin Cancer Biol. 2022;82:184–96. 10.1016/j.semcancer.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 8.Rawat M, Kadian K, Gupta Y, Kumar A, Chain PSG, Kovbasnjuk O, Kumar S, Parasher G. MicroRNA in Pancreatic Cancer: From Biology to Therapeutic Potential. Genes (Basel). 2019;10:752. 10.3390/genes10100752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berindan-Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64:311–36. 10.3322/caac.21244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holjencin C, Jakymiw A. MicroRNAs and their big therapeutic impacts: delivery strategies for cancer intervention. Cells. 2022;11(15):2332. [DOI] [PMC free article] [PubMed]
- 11.Xu X, Tao Y, Shan L, Chen R, Jiang H, Qian Z, Cai F, Ma L, Yu Y. The Role of MicroRNAs in Hepatocellular Carcinoma. J Cancer. 2018;9:3557–69. 10.7150/jca.26350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussen BM, Abdullah SR, Rasul MF, Jawhar ZH, Faraj GSH, Kiani A, Taheri M. MiRNA-93: a novel signature in human disorders and drug resistance. Cell Commun Signal. 2023;21:79. 10.1186/s12964-023-01106-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabortty A, Patton DJ, Smith BF, Agarwal P. miRNAs: Potential as biomarkers and therapeutic targets for cancer. Genes (Basel). 2023;14(7):1375. [DOI] [PMC free article] [PubMed]
- 14.Xuan J, Liu Y, Zeng X, Wang H. Sequence requirements for miR-424–5p regulating and function in cancers. Int J Mol Sci. 2022;23(7):4037. [DOI] [PMC free article] [PubMed]
- 15.Rhim J, Baek W, Seo Y, Kim JH. From molecular mechanisms to therapeutics: understanding MicroRNA-21 in cancer. Cells. 2022;11(18):2791. [DOI] [PMC free article] [PubMed]
- 16.Zhang C, Sun C, Zhao Y, Wang Q, Guo J, Ye B, Yu G. Overview of MicroRNAs as diagnostic and prognostic biomarkers for high-incidence cancers in 2021. Int J Mol Sci. 2022;23(19):11389. [DOI] [PMC free article] [PubMed]
- 17.Fanini F, Fabbri M. Cancer-derived exosomic microRNAs shape the immune system within the tumor microenvironment: State of the art. Semin Cell Dev Biol. 2017;67:23–8. 10.1016/j.semcdb.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzagalli M, Ebelt ND, Manuel ER. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin Cancer Biol. 2019;59:236–50. 10.1016/j.semcancer.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 19.Yi M, Xu L, Jiao Y, Luo S, Li A, Wu K. The role of cancer-derived microRNAs in cancer immune escape. J Hematol Oncol. 2020;13:25. 10.1186/s13045-020-00848-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia Z, Jia J, Yao L, Li Z. Crosstalk of Exosomal Non-Coding RNAs in The Tumor Microenvironment: Novel Frontiers. Front Immunol. 2022;13: 900155. 10.3389/fimmu.2022.900155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. 10.1038/nature03315 [DOI] [PubMed] [Google Scholar]
- 22.Link A, Kupcinskas J. MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: Current insights and future perspectives. World J Gastroenterol. 2018;24:3313–29. 10.3748/wjg.v24.i30.3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chadda KR, Blakey EE, Coleman N, Murray MJ. The clinical utility of dysregulated microRNA expression in paediatric solid tumours. Eur J Cancer. 2022;176:133–54. 10.1016/j.ejca.2022.09.010 [DOI] [PubMed] [Google Scholar]
- 24.Azari H, Nazari E, Mohit R, Asadnia A, Maftooh M, Nassiri M, Hassanian SM, Ghayour-Mobarhan M, Shahidsales S, Khazaei M, et al. Machine learning algorithms reveal potential miRNAs biomarkers in gastric cancer. Sci Rep. 2023;13:6147. 10.1038/s41598-023-32332-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, Zhang CD, Liang Y, Wu KZ, Pei JP, Dai DQ. The comprehensive upstream transcription and downstream targeting regulation network of miRNAs reveal potential diagnostic roles in gastric cancer. Life Sci. 2020;253: 117741. 10.1016/j.lfs.2020.117741 [DOI] [PubMed] [Google Scholar]
- 26.Liang Y, Liu Y, Zhang Q, Zhang H, Du J. Tumor-derived extracellular vesicles containing microRNA-1290 promote immune escape of cancer cells through the Grhl2/ZEB1/PD-L1 axis in gastric cancer. Transl Res. 2021;231:102–12. 10.1016/j.trsl.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 27.Zhu F, Wu Q, Ni Z, Lei C, Li T, Shi Y. miR-19a/b and MeCP2 repress reciprocally to regulate multidrug resistance in gastric cancer cells. Int J Mol Med. 2018;42:228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirzaei S, Zarrabi A, Hashemi F, Zabolian A, Saleki H, Ranjbar A, Seyed Saleh SH, Bagherian M, Sharifzadeh SO, Hushmandi K, et al. Regulation of Nuclear Factor-KappaB (NF-κB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021;509:63–80. 10.1016/j.canlet.2021.03.025 [DOI] [PubMed] [Google Scholar]
- 29.He S, Song W, Cui S, Li J, Jiang Y, Chen X, Peng L. Modulation of miR-146b by N6-methyladenosine modification remodels tumor-associated macrophages and enhances anti-PD-1 therapy in colorectal cancer. Cell Oncol (Dordr). 2023;46:1731–46. 10.1007/s13402-023-00839-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Li G, Wang Z, Yao Y, Chen R, Pu X, Wang J. Circulating MicroRNA-21 Is a Potential Diagnostic Biomarker in Gastric Cancer. Dis Markers. 2015;2015: 435656. 10.1155/2015/435656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emami SS, Nekouian R, Akbari A, Faraji A, Abbasi V, Agah S. Evaluation of circulating miR-21 and miR-222 as diagnostic biomarkers for gastric cancer. J Cancer Res Ther. 2019;15:115–9. 10.4103/jcrt.JCRT_592_17 [DOI] [PubMed] [Google Scholar]
- 32.Tang Y, Liu X, Su B, Zhang Z, Zeng X, Lei Y, Shan J, Wu Y, Tang H, Su Q. microRNA-22 acts as a metastasis suppressor by targeting metadherin in gastric cancer. Mol Med Rep. 2015;11:454–60. 10.3892/mmr.2014.2682 [DOI] [PubMed] [Google Scholar]
- 33.Chen TH, Chiu CT, Lee C, Chu YY, Cheng HT, Hsu JT, Wu RC, Yeh TS, Lin KH. Circulating microRNA-22-3p Predicts the Malignant Progression of Precancerous Gastric Lesions from Intestinal Metaplasia to Early Adenocarcinoma. Dig Dis Sci. 2018;63:2301–8. 10.1007/s10620-018-5106-4 [DOI] [PubMed] [Google Scholar]
- 34.Valladares-Ayerbes M, Reboredo M, Medina-Villaamil V, Iglesias-Díaz P, Lorenzo-Patiño MJ, Haz M, Santamarina I, Blanco M, Fernández-Tajes J, Quindós M, et al. Circulating miR-200c as a diagnostic and prognostic biomarker for gastric cancer. J Transl Med. 2012;10:186. 10.1186/1479-5876-10-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao F, Cheng Z, Wang P, Gong B, Huang H, Xing Y, Liu F. MicroRNA-28-5p inhibits the migration and invasion of gastric cancer cells by suppressing AKT phosphorylation. Oncol Lett. 2018;15:9777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han TS, Hur K, Xu G, Choi B, Okugawa Y, Toiyama Y, Oshima H, Oshima M, Lee HJ, Kim VN, et al. MicroRNA-29c mediates initiation of gastric carcinogenesis by directly targeting ITGB1. Gut. 2015;64:203–14. 10.1136/gutjnl-2013-306640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang N, Wang L, Yang Y, Gong L, Xiao B, Liu X. A serum exosomal microRNA panel as a potential biomarker test for gastric cancer. Biochem Biophys Res Commun. 2017;493:1322–8. 10.1016/j.bbrc.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 38.Cui L, Zhang X, Ye G, Zheng T, Song H, Deng H, Xiao B, Xia T, Yu X, Le Y, Guo J. Gastric juice MicroRNAs as potential biomarkers for the screening of gastric cancer. Cancer. 2013;119:1618–26. 10.1002/cncr.27903 [DOI] [PubMed] [Google Scholar]
- 39.Dong X, Liu Y. Expression and significance of miR-24 and miR-101 in patients with advanced gastric cancer. Oncol Lett. 2018;16:5769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F, Hu H, Zhao J, Zhang Z, Ai X, Tang L, Xie L. miR-124-3p acts as a potential marker and suppresses tumor growth in gastric cancer. Biomed Rep. 2018;9:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu X, Luo L, Wu Y, Yu X, Liu Y, Yu X, Zhao X, Zhang X, Cui L, Ye G, et al. Gastric juice miR-129 as a potential biomarker for screening gastric cancer. Med Oncol. 2013;30:365. 10.1007/s12032-012-0365-y [DOI] [PubMed] [Google Scholar]
- 42.Shao J, Fang PH, He B, Guo LL, Shi MY, Zhu Y, Bo P, Zhen-Wen ZW. Downregulated MicroRNA-133a in Gastric Juice as a Clinicopathological Biomarker for Gastric Cancer Screening. Asian Pac J Cancer Prev. 2016;17:2719–22. [PubMed] [Google Scholar]
- 43.Cha Y, He Y, Ouyang K, Xiong H, Li J, Yuan X. MicroRNA-140-5p suppresses cell proliferation and invasion in gastric cancer by targeting WNT1 in the WNT/β-catenin signaling pathway. Oncol Lett. 2018;16:6369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Guo Q, Lu Y, Tian T. Increased expression of miR-181d is associated with poor prognosis and tumor progression of gastric cancer. Cancer Biomark. 2019;26:353–60. 10.3233/CBM-190091 [DOI] [PubMed] [Google Scholar]
- 45.Chen W, Cui Y, Wang J, Yuan Y, Sun X, Zhang L, Shen S, Cheng J. Effects of downregulated expression of microRNA-187 in gastric cancer. Exp Ther Med. 2018;16:1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai MM, Wang CS, Tsai CY, Huang CG, Lee KF, Huang HW, Lin YH, Chi HC, Kuo LM, Lu PH, Lin KH. Circulating microRNA-196a/b are novel biomarkers associated with metastatic gastric cancer. Eur J Cancer. 2016;64:137–48. 10.1016/j.ejca.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 47.Chen TH, Lee C, Chiu CT, Chu YY, Cheng HT, Hsu JT, Tsou YK, Wu RC, Chen TC, Chang NC, et al. Circulating microRNA-196a is an early gastric cancer biomarker. Oncotarget. 2018;9:10317–23. 10.18632/oncotarget.23126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imaoka H, Toiyama Y, Okigami M, Yasuda H, Saigusa S, Ohi M, Tanaka K, Inoue Y, Mohri Y, Kusunoki M. Circulating microRNA-203 predicts metastases, early recurrence, and poor prognosis in human gastric cancer. Gastric Cancer. 2016;19:744–53. 10.1007/s10120-015-0521-0 [DOI] [PubMed] [Google Scholar]
- 49.Shao JP, Su F, Zhang SP, Chen HK, Li ZJ, Xing GQ, Liu HJ, Li YY. miR-212 as potential biomarker suppresses the proliferation of gastric cancer via targeting SOX4. J Clin Lab Anal. 2020;34: e23511. 10.1002/jcla.23511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang L, Hu H, He Y, McLeod HL, Xiao D, Chen P, Shen L, Zeng S, Yin X, Ge J, et al. The relationship between miR-302b and EphA2 and their clinical significance in gastric cancer. J Cancer. 2018;9:3109–16. 10.7150/jca.25235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Wang C, Yan S, Yang Y, Zhang X, Guo W. miR-345 inhibits migration and stem-like cell phenotype in gastric cancer via inactivation of Rac1 by targeting EPS8. Acta Biochim Biophys Sin (Shanghai). 2020;52:259–67. 10.1093/abbs/gmz166 [DOI] [PubMed] [Google Scholar]
- 52.Xu M, Qin S, Cao F, Ding S, Li M. MicroRNA-379 inhibits metastasis and epithelial-mesenchymal transition via targeting FAK/AKT signaling in gastric cancer. Int J Oncol. 2017;51:867–76. 10.3892/ijo.2017.4072 [DOI] [PubMed] [Google Scholar]
- 53.Ge X, Liu X, Lin F, Li P, Liu K, Geng R, Dai C, Lin Y, Tang W, Wu Z, et al. MicroRNA-421 regulated by HIF-1α promotes metastasis, inhibits apoptosis, and induces cisplatin resistance by targeting E-cadherin and caspase-3 in gastric cancer. Oncotarget. 2016;7:24466–82. 10.18632/oncotarget.8228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Wu L, Sun Y, Yin Q, Chen X, Liang S, Meng Q, Long H, Li F, Luo C, Xiao X. Mir-421 in plasma as a potential diagnostic biomarker for precancerous gastric lesions and early gastric cancer. PeerJ. 2019;7: e7002. 10.7717/peerj.7002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Cui L, Ye G, Zheng T, Song H, Xia T, Yu X, Xiao B, Le Y, Guo J. Gastric juice microRNA-421 is a new biomarker for screening gastric cancer. Tumour Biol. 2012;33:2349–55. 10.1007/s13277-012-0497-x [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Liu Y, Yao J, Li R, Fan X. Downregulation of miR-484 is associated with poor prognosis and tumor progression of gastric cancer. Diagn Pathol. 2020;15:25. 10.1186/s13000-020-00946-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su H, Ren F, Jiang H, Chen Y, Fan X. Upregulation of microRNA-520a-3p inhibits the proliferation, migration and invasion via spindle and kinetochore associated 2 in gastric cancer. Oncol Lett. 2019;18:3323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui HB, Ge HE, Wang YS, Bai XY. MiR-208a enhances cell proliferation and invasion of gastric cancer by targeting SFRP1 and negatively regulating MEG3. Int J Biochem Cell Biol. 2018;102:31–9. 10.1016/j.biocel.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 59.Feng X, Zhu M, Liao B, Tian T, Li M, Wang Z, Chen G. Upregulation of miR-552 Predicts Unfavorable Prognosis of Gastric Cancer and Promotes the Proliferation, Migration, and Invasion of Gastric Cancer Cells. Oncol Res Treat. 2020;43:103–11. 10.1159/000505377 [DOI] [PubMed] [Google Scholar]
- 60.Hu L, Wu H, Wan X, Liu L, He Y, Zhu L, Liu S, Yao H, Zhu Z. MicroRNA-585 suppresses tumor proliferation and migration in gastric cancer by directly targeting MAPK1. Biochem Biophys Res Commun. 2018;499:52–8. 10.1016/j.bbrc.2018.03.116 [DOI] [PubMed] [Google Scholar]
- 61.Min C, Zhang A, Qin J. Increased expression of miR-601 is associated with poor prognosis and tumor progression of gastric cancer. Diagn Pathol. 2019;14:107. 10.1186/s13000-019-0882-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng H, Zhang F, Lin X, Huang C, Zhang Y, Li Y, Lin J, Chen W, Lin X. MicroRNA-1225-5p inhibits proliferation and metastasis of gastric carcinoma through repressing insulin receptor substrate-1 and activation of β-catenin signaling. Oncotarget. 2016;7:4647–63. 10.18632/oncotarget.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.An JX, Ma ZS, Ma MH, Shao S, Cao FL, Dai DQ. MiR-1236-3p serves as a new diagnostic and prognostic biomarker for gastric cancer. Cancer Biomark. 2019;25:127–32. 10.3233/CBM-171026 [DOI] [PubMed] [Google Scholar]
- 64.Liu S, Tian Y, Zhu C, Yang X, Sun Q. High miR-718 Suppresses Phosphatase and Tensin Homolog (PTEN) Expression and Correlates to Unfavorable Prognosis in Gastric Cancer. Med Sci Monit. 2018;24:5840–50. 10.12659/MSM.909527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abe S, Matsuzaki J, Sudo K, Oda I, Katai H, Kato K, Takizawa S, Sakamoto H, Takeshita F, Niida S, et al. A novel combination of serum microRNAs for the detection of early gastric cancer. Gastric Cancer. 2021;24:835–43. 10.1007/s10120-021-01161-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi JM, Kim SG, Yang HJ, Lim JH, Cho NY, Kim WH, Kim JS, Jung HC. Helicobacter pylori Eradication Can Reverse the Methylation-Associated Regulation of miR-200a/b in Gastric Carcinogenesis. Gut Liver. 2020;14:571–80. 10.5009/gnl19299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Pan K, Vieth M, Gerhard M, Li W, Mejías-Luque R. JAK-STAT1 signaling pathway is an early response to helicobacter pylori infection and contributes to immune escape and gastric carcinogenesis. Int J Mol Sci. 2022;23(8):4147. [DOI] [PMC free article] [PubMed]
- 68.Yang F, Xu Y, Liu C, Ma C, Zou S, Xu X, Jia J, Liu Z. NF-κB/miR-223-3p/ARID1A axis is involved in Helicobacter pylori CagA-induced gastric carcinogenesis and progression. Cell Death Dis. 2018;9:12. 10.1038/s41419-017-0020-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Deng R, Chen S, Deng S, Hu Q, Xu B, Li J, He Z, Peng M, Lei S, et al. Helicobacter pylori CagA promotes immune evasion of gastric cancer by upregulating PD-L1 level in exosomes. iScience. 2023;26:108414. 10.1016/j.isci.2023.108414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi Y, Yang Z, Zhang T, Shen L, Li Y, Ding S. SIRT1-targeted miR-543 autophagy inhibition and epithelial-mesenchymal transition promotion in Helicobacter pylori CagA-associated gastric cancer. Cell Death Dis. 2019;10:625. 10.1038/s41419-019-1859-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koh V, Chakrabarti J, Torvund M, Steele N, Hawkins JA, Ito Y, Wang J, Helmrath MA, Merchant JL, Ahmed SA, et al. Hedgehog transcriptional effector GLI mediates mTOR-Induced PD-L1 expression in gastric cancer organoids. Cancer Lett. 2021;518:59–71. 10.1016/j.canlet.2021.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shao W, Yang Z, Fu Y, Zheng L, Liu F, Chai L, Jia J. The Pyroptosis-Related Signature Predicts Prognosis and Indicates Immune Microenvironment Infiltration in Gastric Cancer. Front Cell Dev Biol. 2021;9: 676485. 10.3389/fcell.2021.676485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsushima K, Isomoto H, Inoue N, Nakayama T, Hayashi T, Nakayama M, Nakao K, Hirayama T, Kohno S. MicroRNA signatures in Helicobacter pylori-infected gastric mucosa. Int J Cancer. 2011;128:361–70. 10.1002/ijc.25348 [DOI] [PubMed] [Google Scholar]
- 74.Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Müller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic Gastritis and Colitis. J Immunol. 2011;187:3578–86. 10.4049/jimmunol.1101772 [DOI] [PubMed] [Google Scholar]
- 75.Tsai CC, Chen TY, Tsai KJ, Lin MW, Hsu CY, Wu DC, Tsai EM, Hsieh TH. NF-κB/miR-18a-3p and miR-4286/BZRAP1 axis may mediate carcinogenesis in Helicobacter pylori-Associated gastric cancer. Biomed Pharmacother. 2020;132: 110869. 10.1016/j.biopha.2020.110869 [DOI] [PubMed] [Google Scholar]
- 76.Li T, Guo H, Zhao X, Jin J, Zhang L, Li H, Lu Y, Nie Y, Wu K, Shi Y, Fan D. Gastric Cancer Cell Proliferation and Survival Is Enabled by a Cyclophilin B/STAT3/miR-520d-5p Signaling Feedback Loop. Cancer Res. 2017;77:1227–40. 10.1158/0008-5472.CAN-16-0357 [DOI] [PubMed] [Google Scholar]
- 77.Fassi Fehri L, Koch M, Belogolova E, Khalil H, Bolz C, Kalali B, Mollenkopf HJ, Beigier-Bompadre M, Karlas A, Schneider T, et al. Helicobacter pylori induces miR-155 in T cells in a cAMP-Foxp3-dependent manner. PLoS ONE. 2010;5: e9500. 10.1371/journal.pone.0009500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang L, Wang ZY, Pan DD. Penicillin-binding protein 1A mutation-positive Helicobacter pylori promotes epithelial-mesenchymal transition in gastric cancer via the suppression of microRNA-134. Int J Oncol. 2019;54:916–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bai Y, Xie T, Wang Z, Tong S, Zhao X, Zhao F, Cai J, Wei X, Peng Z, Shen L. Efficacy and predictive biomarkers of immunotherapy in Epstein-Barr virus-associated gastric cancer. J Immunother Cancer. 2022;10(3):e004080. [DOI] [PMC free article] [PubMed]
- 80.Bauer M, Jasinski-Bergner S, Mandelboim O, Wickenhauser C, Seliger B. Epstein-Barr virus-associated malignancies and immune escape: the role of the tumor microenvironment and tumor cell evasion strategies. Cancers (Basel). 2021;13(20):5189. [DOI] [PMC free article] [PubMed]
- 81.Pandya D, Mariani M, He S, Andreoli M, Spennato M, Dowell-Martino C, Fiedler P, Ferlini C. Epstein-Barr Virus MicroRNA Expression Increases Aggressiveness of Solid Malignancies. PLoS ONE. 2015;10: e0136058. 10.1371/journal.pone.0136058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saju P, Murata-Kamiya N, Hayashi T, Senda Y, Nagase L, Noda S, Matsusaka K, Funata S, Kunita A, Urabe M, et al. Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein-Barr virus. Nat Microbiol. 2016;1:16026. 10.1038/nmicrobiol.2016.26 [DOI] [PubMed] [Google Scholar]
- 83.Shinozaki A, Sakatani T, Ushiku T, Hino R, Isogai M, Ishikawa S, Uozaki H, Takada K, Fukayama M. Downregulation of microRNA-200 in EBV-associated gastric carcinoma. Cancer Res. 2010;70:4719–27. 10.1158/0008-5472.CAN-09-4620 [DOI] [PubMed] [Google Scholar]
- 84.Kim SM, Hur DY, Hong SW, Kim JH. EBV-encoded EBNA1 regulates cell viability by modulating miR34a-NOX2-ROS signaling in gastric cancer cells. Biochem Biophys Res Commun. 2017;494:550–5. 10.1016/j.bbrc.2017.10.095 [DOI] [PubMed] [Google Scholar]
- 85.Choi H, Lee H, Kim SR, Gho YS, Lee SK. Epstein-Barr virus-encoded microRNA BART15-3p promotes cell apoptosis partially by targeting BRUCE. J Virol. 2013;87:8135–44. 10.1128/JVI.03159-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J, Ge J, Wang Y, Xiong F, Guo J, Jiang X, Zhang L, Deng X, Gong Z, Zhang S, et al. EBV miRNAs BART11 and BART17-3p promote immune escape through the enhancer-mediated transcription of PD-L1. Nat Commun. 2022;13:866. 10.1038/s41467-022-28479-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Albanese M, Tagawa T, Bouvet M, Maliqi L, Lutter D, Hoser J, Hastreiter M, Hayes M, Sugden B, Martin L, et al. Epstein-Barr virus microRNAs reduce immune surveillance by virus-specific CD8+ T cells. Proc Natl Acad Sci U S A. 2016;113:E6467-e6475. 10.1073/pnas.1605884113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iizasa H, Wulff BE, Alla NR, Maragkakis M, Megraw M, Hatzigeorgiou A, Iwakiri D, Takada K, Wiedmer A, Showe L, et al. Editing of Epstein-Barr virus-encoded BART6 microRNAs controls their dicer targeting and consequently affects viral latency. J Biol Chem. 2010;285:33358–70. 10.1074/jbc.M110.138362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shafqat A, Omer MH, Ahmed EN, Mushtaq A, Ijaz E, Ahmed Z, Alkattan K, Yaqinuddin A. Reprogramming the immunosuppressive tumor microenvironment: exploiting angiogenesis and thrombosis to enhance immunotherapy. Front Immunol. 2023;14:1200941. 10.3389/fimmu.2023.1200941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023;8:198. 10.1038/s41392-023-01460-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. 2019;25:5449–57. [DOI] [PubMed]
- 92.Kim S, Bae WJ, Ahn JM, Heo JH, Kim KM, Choi KW, Sung CO, Lee D. MicroRNA signatures associated with lymph node metastasis in intramucosal gastric cancer. Mod Pathol. 2021;34:672–83. 10.1038/s41379-020-00681-x [DOI] [PubMed] [Google Scholar]
- 93.Zhang H, Qu Y, Duan J, Deng T, Liu R, Zhang L, Bai M, Li J, Zhou L, Ning T, et al. Integrated analysis of the miRNA, gene and pathway regulatory network in gastric cancer. Oncol Rep. 2016;35:1135–46. 10.3892/or.2015.4451 [DOI] [PubMed] [Google Scholar]
- 94.Wu ZH, Lin C, Liu CC, Jiang WW, Huang MZ, Liu X, Guo WJ. MiR-616-3p promotes angiogenesis and EMT in gastric cancer via the PTEN/AKT/mTOR pathway. Biochem Biophys Res Commun. 2018;501:1068–73. 10.1016/j.bbrc.2018.05.109 [DOI] [PubMed] [Google Scholar]
- 95.Xie M, Dart DA, Guo T, Xing XF, Cheng XJ, Du H, Jiang WG, Wen XZ, Ji JF. MicroRNA-1 acts as a tumor suppressor microRNA by inhibiting angiogenesis-related growth factors in human gastric cancer. Gastric Cancer. 2018;21:41–54. 10.1007/s10120-017-0721-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gutiérrez J, Droppelmann CA, Salsoso R, Westermeier F, Toledo F, Salomon C, Sanhueza C, Pardo F, Leiva A, Sobrevia L. A Hypothesis for the Role of RECK in Angiogenesis. Curr Vasc Pharmacol. 2016;14:106–15. 10.2174/1570161113666151014130746 [DOI] [PubMed] [Google Scholar]
- 97.Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16:909–14. 10.1038/nm.2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Du J, Liang Y, Li J, Zhao JM, Lin XY. Gastric Cancer Cell-Derived Exosomal microRNA-23a Promotes Angiogenesis by Targeting PTEN. Front Oncol. 2020;10:326. 10.3389/fonc.2020.00326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang R, Miao Z, Liu Y, Zhang X, Yang Q. A positive feedback loop between miR-574-3p and HIF-1α in promoting angiogenesis under hypoxia. Microvasc Res. 2023;150: 104589. 10.1016/j.mvr.2023.104589 [DOI] [PubMed] [Google Scholar]
- 100.Bavelloni A, Ramazzotti G, Poli A, Piazzi M, Focaccia E, Blalock W, Faenza I. MiRNA-210: A Current Overview. Anticancer Res. 2017;37:6511–21. [DOI] [PubMed] [Google Scholar]
- 101.Wang L, Bo X, Zheng Q, Ge W, Liu Y, Li B. Paired box 8 suppresses tumor angiogenesis and metastasis in gastric cancer through repression of FOXM1 via induction of microRNA-612. J Exp Clin Cancer Res. 2018;37:159. 10.1186/s13046-018-0830-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang S, Zhang R, Xu R, Shang J, He H, Yang Q. MicroRNA-574-5p in gastric cancer cells promotes angiogenesis by targeting protein tyrosine phosphatase non-receptor type 3 (PTPN3). Gene. 2020;733: 144383. 10.1016/j.gene.2020.144383 [DOI] [PubMed] [Google Scholar]
- 103.Yu B, Zhu N, Fan Z, Li J, Kang Y, Liu B. miR-29c inhibits metastasis of gastric cancer cells by targeting VEGFA. J Cancer. 2022;13:3566–74. 10.7150/jca.77727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Si Y, Zhang H, Ning T, Bai M, Wang Y, Yang H, Wang X, Li J, Ying G, Ba Y. miR-26a/b Inhibit Tumor Growth and Angiogenesis by Targeting the HGF-VEGF Axis in Gastric Carcinoma. Cell Physiol Biochem. 2017;42:1670–83. 10.1159/000479412 [DOI] [PubMed] [Google Scholar]
- 105.Zhang J, Zhang J, Pang X, Chen Z, Zhang Z, Lei L, Xu H, Wen L, Zhu J, Jiang Y, et al. MiR-205-5p suppresses angiogenesis in gastric cancer by downregulating the expression of VEGFA and FGF1. Exp Cell Res. 2021;404: 112579. 10.1016/j.yexcr.2021.112579 [DOI] [PubMed] [Google Scholar]
- 106.Liu HT, Ma RR, Lv BB, Zhang H, Shi DB, Guo XY, Zhang GH, Gao P. LncRNA-HNF1A-AS1 functions as a competing endogenous RNA to activate PI3K/AKT signalling pathway by sponging miR-30b-3p in gastric cancer. Br J Cancer. 2020;122:1825–36. 10.1038/s41416-020-0836-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng L, Pu J, Qi T, Qi M, Li D, Xiang X, Huang K, Tong Q. miRNA-145 targets v-ets erythroblastosis virus E26 oncogene homolog 1 to suppress the invasion, metastasis, and angiogenesis of gastric cancer cells. Mol Cancer Res. 2013;11:182–93. 10.1158/1541-7786.MCR-12-0534 [DOI] [PubMed] [Google Scholar]
- 108.Li Z, Liu Z, Dong S, Zhang J, Tan J, Wang Y, Ge C, Li R, Xue Y, Li M, et al. miR-506 Inhibits Epithelial-to-Mesenchymal Transition and Angiogenesis in Gastric Cancer. Am J Pathol. 2015;185:2412–20. 10.1016/j.ajpath.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 109.Zhang X, Tang J, Zhi X, Xie K, Wang W, Li Z, Zhu Y, Yang L, Xu H, Xu Z. miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget. 2015;6:1605–17. 10.18632/oncotarget.2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mei B, Chen J, Yang N, Peng Y. The regulatory mechanism and biological significance of the Snail-miR590-VEGFR-NRP1 axis in the angiogenesis, growth and metastasis of gastric cancer. Cell Death Dis. 2020;11:241. 10.1038/s41419-020-2428-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin J, Liu Z, Liao S, Li E, Wu X, Zeng W. Elevated microRNA-7 inhibits proliferation and tumor angiogenesis and promotes apoptosis of gastric cancer cells via repression of Raf-1. Cell Cycle. 2020;19:2496–508. 10.1080/15384101.2020.1807670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang X, Dong J, He Y, Zhao M, Liu Z, Wang N, Jiang M, Zhang Z, Liu G, Liu H, et al. miR-218 inhibited tumor angiogenesis by targeting ROBO1 in gastric cancer. Gene. 2017;615:42–9. 10.1016/j.gene.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 113.Shichiri K, Oshi M, Ziazadeh D, Endo I, Takabe K. High miR-200c expression is associated with suppressed epithelial-mesenchymal transition, TGF-β signaling and better survival despite enhanced cell proliferation in gastric cancer patients. Am J Cancer Res. 2023;13:3027–40. [PMC free article] [PubMed] [Google Scholar]
- 114.Tokumaru Y, Oshi M, Huyser MR, Yan L, Fukada M, Matsuhashi N, Futamura M, Akao Y, Yoshida K, Takabe K. Low expression of miR-29a is associated with aggressive biology and worse survival in gastric cancer. Sci Rep. 2021;11:14134. 10.1038/s41598-021-93681-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng W, Liao Y, Xie Y, Wang Q, Li L, Chen Y, Zhao Y, Zhou J. Helicobacter pylori-induced fibroblast-derived Serpin E1 promotes gastric cancer growth and peritoneal dissemination through p38 MAPK/VEGFA-mediated angiogenesis. Cancer Cell Int. 2023;23:326. 10.1186/s12935-023-03177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Marzouki L, Stavrakos VS, Pal S, Giannias B, Bourdeau F, Rayes R, Bertos N, Najmeh S, Spicer JD, Cools-Lartigue J, et al. Soluble factors in malignant ascites promote the metastatic adhesion of gastric adenocarcinoma cells. Gastric Cancer. 2023;26:55–68. 10.1007/s10120-022-01338-1 [DOI] [PubMed] [Google Scholar]
- 117.Ohzawa H, Kumagai Y, Yamaguchi H, Miyato H, Sakuma Y, Horie H, Hosoya Y, Kawarai Lefor A, Sata N, Kitayama J. Exosomal microRNA in peritoneal fluid as a biomarker of peritoneal metastases from gastric cancer. Ann Gastroenterol Surg. 2020;4:84–93. 10.1002/ags3.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y, Shen H. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Mol Cancer. 2017;16:148. 10.1186/s12943-017-0718-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kennel KB, Bozlar M, De Valk AF, Greten FR. Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity. Clin Cancer Res. 2023;29:1009–16. 10.1158/1078-0432.CCR-22-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neophytou CM, Panagi M, Stylianopoulos T, Papageorgis P. The role of tumor microenvironment in cancer metastasis: molecular mechanisms and therapeutic opportunities. Cancers (Basel). 2021;13(9):2053. [DOI] [PMC free article] [PubMed]
- 121.Wang J, Guan X, Zhang Y, Ge S, Zhang L, Li H, Wang X, Liu R, Ning T, Deng T, et al. Exosomal miR-27a Derived from Gastric Cancer Cells Regulates the Transformation of Fibroblasts into Cancer-Associated Fibroblasts. Cell Physiol Biochem. 2018;49:869–83. 10.1159/000493218 [DOI] [PubMed] [Google Scholar]
- 122.Kurashige J, Mima K, Sawada G, Takahashi Y, Eguchi H, Sugimachi K, Mori M, Yanagihara K, Yashiro M, Hirakawa K, et al. Epigenetic modulation and repression of miR-200b by cancer-associated fibroblasts contribute to cancer invasion and peritoneal dissemination in gastric cancer. Carcinogenesis. 2015;36:133–41. 10.1093/carcin/bgu232 [DOI] [PubMed] [Google Scholar]
- 123.Takahashi S, Takagane K, Itoh G, Kuriyama S, Umakoshi M, Goto A, Yanagihara K, Yashiro M, Iijima K, Tanaka M. CCDC85A is regulated by miR-224-3p and augments cancer cell resistance to endoplasmic reticulum stress. Front Oncol. 2023;13:1196546. 10.3389/fonc.2023.1196546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang R, Sun Y, Yu W, Yan Y, Qiao M, Jiang R, Guan W, Wang L. Downregulation of miRNA-214 in cancer-associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J Exp Clin Cancer Res. 2019;38:20. 10.1186/s13046-018-0995-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang C, Liu J, He L, Wang F, Xiong B, Li Y, Yang X. The long noncoding RNA noncoding RNA activated by DNA damage (NORAD)-microRNA-496-Interleukin-33 axis affects carcinoma-associated fibroblasts-mediated gastric cancer development. Bioengineered. 2021;12:11738–55. 10.1080/21655979.2021.2009412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang TS, Yang XH, Chen X, Wang XD, Hua J, Zhou DL, Zhou B, Song ZS. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. FEBS Lett. 2014;588:2162–9. 10.1016/j.febslet.2014.04.050 [DOI] [PubMed] [Google Scholar]
- 127.Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, Zhang Q, Lin D, Ge S, Bai M, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19:43. 10.1186/s12943-020-01168-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou Y, Zhong JH, Gong FS, Xiao J. MiR-141-3p suppresses gastric cancer induced transition of normal fibroblast and BMSC to cancer-associated fibroblasts via targeting STAT4. Exp Mol Pathol. 2019;107:85–94. 10.1016/j.yexmp.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 129.Sammarco G, Varricchi G, Ferraro V, Ammendola M, De Fazio M, Altomare DF, Luposella M, Maltese L, Currò G, Marone G, et al. Mast cells, angiogenesis and lymphangiogenesis in human gastric cancer. Int J Mol Sci. 2019;20(9):2106. [DOI] [PMC free article] [PubMed]
- 130.Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696–707. 10.1111/cas.14521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bahmani L, Baghi M, Peymani M, Javeri A, Ghaedi K. MiR-141-3p and miR-200a-3p are involved in Th17 cell differentiation by negatively regulating RARB expression. Hum Cell. 2021;34:1375–87. 10.1007/s13577-021-00558-4 [DOI] [PubMed] [Google Scholar]
- 132.Li M, Yang Y, Xiong L, Jiang P, Wang J, Li C. Metabolism, metabolites, and macrophages in cancer. J Hematol Oncol. 2023;16:80. 10.1186/s13045-023-01478-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daniel B, Nagy G, Horvath A, Czimmerer Z, Cuaranta-Monroy I, Poliska S, Hays TT, Sauer S, Francois-Deleuze J, Nagy L. The IL-4/STAT6/PPARγ signaling axis is driving the expansion of the RXR heterodimer cistrome, providing complex ligand responsiveness in macrophages. Nucleic Acids Res. 2018;46:4425–39. 10.1093/nar/gky157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun X, Gao J, Meng X, Lu X, Zhang L, Chen R. Polarized Macrophages in Periodontitis: Characteristics, Function, and Molecular Signaling. Front Immunol. 2021;12: 763334. 10.3389/fimmu.2021.763334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022;23:1148–56. 10.1038/s41590-022-01267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nascimento C, Ferreira F. Tumor microenvironment of human breast cancer, and feline mammary carcinoma as a potential study model. Biochim Biophys Acta Rev Cancer. 2021;1876: 188587. 10.1016/j.bbcan.2021.188587 [DOI] [PubMed] [Google Scholar]
- 137.Zheng P, Luo Q, Wang W, Li J, Wang T, Wang P, Chen L, Zhang P, Chen H, Liu Y, et al. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018;9:434. 10.1038/s41419-018-0465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen C, Liu JM, Luo YP. MicroRNAs in tumor immunity: functional regulation in tumor-associated macrophages. J Zhejiang Univ Sci B. 2020;21:12–28. 10.1631/jzus.B1900452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhihua Y, Yulin T, Yibo W, Wei D, Yin C, Jiahao X, Runqiu J, Xuezhong X. Hypoxia decreases macrophage glycolysis and M1 percentage by targeting microRNA-30c and mTOR in human gastric cancer. Cancer Sci. 2019;110:2368–77. 10.1111/cas.14110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xi J, Huang Q, Wang L, Ma X, Deng Q, Kumar M, Zhou Z, Li L, Zeng Z, Young KH, et al. miR-21 depletion in macrophages promotes tumoricidal polarization and enhances PD-1 immunotherapy. Oncogene. 2018;37:3151–65. 10.1038/s41388-018-0178-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sahraei M, Chaube B, Liu Y, Sun J, Kaplan A, Price NL, Ding W, Oyaghire S, García-Milian R, Mehta S, et al. Suppressing miR-21 activity in tumor-associated macrophages promotes an antitumor immune response. J Clin Invest. 2019;129:5518–36. 10.1172/JCI127125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang X, Cai S, Shu Y, Deng X, Zhang Y, He N, Wan L, Chen X, Qu Y, Yu S. Exosomal miR-487a derived from m2 macrophage promotes the progression of gastric cancer. Cell Cycle. 2021;20:434–44. 10.1080/15384101.2021.1878326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cui HY, Rong JS, Chen J, Guo J, Zhu JQ, Ruan M, Zuo RR, Zhang SS, Qi JM, Zhang BH. Exosomal microRNA-588 from M2 polarized macrophages contributes to cisplatin resistance of gastric cancer cells. World J Gastroenterol. 2021;27:6079–92. 10.3748/wjg.v27.i36.6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. 10.1186/s13046-017-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gwee YX, Chia DKA, So J, Ceelen W, Yong WP, Tan P, Ong CJ, Sundar R. Integration of Genomic Biology Into Therapeutic Strategies of Gastric Cancer Peritoneal Metastasis. J Clin Oncol. 2022;40:2830. 10.1200/JCO.21.02745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takahashi K, Kurashina K, Yamaguchi H, Kanamaru R, Ohzawa H, Miyato H, Saito S, Hosoya Y, Lefor AK, Sata N, Kitayama J. Altered intraperitoneal immune microenvironment in patients with peritoneal metastases from gastric cancer. Front Immunol. 2022;13: 969468. 10.3389/fimmu.2022.969468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yamaguchi T, Fushida S, Yamamoto Y, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I, Munesue S, et al. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016;19:1052–65. 10.1007/s10120-015-0579-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eum HH, Kwon M, Ryu D, Jo A, Chung W, Kim N, Hong Y, Son DS, Kim ST, Lee J, et al. Tumor-promoting macrophages prevail in malignant ascites of advanced gastric cancer. Exp Mol Med. 2020;52:1976–88. 10.1038/s12276-020-00538-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen CW, Wang HC, Tsai IM, Chen IS, Chen CJ, Hou YC, Shan YS. CD204-positive M2-like tumor-associated macrophages increase migration of gastric cancer cells by upregulating miR-210 to reduce NTN4 expression. Cancer Immunol Immunother. 2024;73:1. 10.1007/s00262-023-03601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ruterbusch M, Pruner KB, Shehata L, Pepper M. In Vivo CD4(+) T Cell Differentiation and Function: Revisiting the Th1/Th2 Paradigm. Annu Rev Immunol. 2020;38:705–25. 10.1146/annurev-immunol-103019-085803 [DOI] [PubMed] [Google Scholar]
- 151.Cho S, Wu CJ, Yasuda T, Cruz LO, Khan AA, Lin LL, Nguyen DT, Miller M, Lee HM, Kuo ML, et al. miR-23∼27∼24 clusters control effector T cell differentiation and function. J Exp Med. 2016;213:235–49. 10.1084/jem.20150990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Song J, Lin Z, Liu Q, Huang S, Han L, Fang Y, Zhong P, Dou R, Xiang Z, Zheng J, et al. MiR-192-5p/RB1/NF-κBp65 signaling axis promotes IL-10 secretion during gastric cancer EMT to induce Treg cell differentiation in the tumour microenvironment. Clin Transl Med. 2022;12: e992. 10.1002/ctm2.992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yin Y, Cai X, Chen X, Liang H, Zhang Y, Li J, Wang Z, Chen X, Zhang W, Yokoyama S, et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res. 2014;24:1164–80. 10.1038/cr.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhuansun Y, Du Y, Huang F, Lin L, Chen R, Jiang S, Li J. MSCs exosomal miR-1470 promotes the differentiation of CD4(+)CD25(+)FOXP3(+) Tregs in asthmatic patients by inducing the expression of P27KIP1. Int Immunopharmacol. 2019;77: 105981. 10.1016/j.intimp.2019.105981 [DOI] [PubMed] [Google Scholar]
- 155.Liu F, Bu Z, Zhao F, Xiao D. Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci. 2018;109:65–73. 10.1111/cas.13429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ping W, Senyan H, Li G, Yan C, Long L. Increased Lactate in Gastric Cancer Tumor-Infiltrating Lymphocytes Is Related to Impaired T Cell Function Due to miR-34a Deregulated Lactate Dehydrogenase A. Cell Physiol Biochem. 2018;49:828–36. 10.1159/000493110 [DOI] [PubMed] [Google Scholar]
- 157.Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Myeloid-derived suppressor cells: Important contributors to tumor progression and metastasis. J Cell Physiol. 2018;233:3024–36. 10.1002/jcp.26075 [DOI] [PubMed] [Google Scholar]
- 158.Xiang X, Wu Y, Li H, Li C, Yan L, Li Q. Plasmacytoid Dendritic Cell-Derived Type I Interferon Is Involved in Helicobacter pylori Infection-Induced Differentiation of Schlafen 4-Expressing Myeloid-Derived Suppressor Cells. Infect Immun. 2021;89: e0040721. 10.1128/IAI.00407-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ding L, Li Q, Chakrabarti J, Munoz A, Faure-Kumar E, Ocadiz-Ruiz R, Razumilava N, Zhang G, Hayes MH, Sontz RA, et al. MiR130b from Schlafen4(+) MDSCs stimulates epithelial proliferation and correlates with preneoplastic changes prior to gastric cancer. Gut. 2020;69:1750–61. 10.1136/gutjnl-2019-318817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ren W, Zhang X, Li W, Feng Q, Feng H, Tong Y, Rong H, Wang W, Zhang D, Zhang Z, et al. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer Manag Res. 2019;11:4023–40. 10.2147/CMAR.S198886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mei S, Xin J, Liu Y, Zhang Y, Liang X, Su X, Yan H, Huang Y, Yang R. MicroRNA-200c Promotes Suppressive Potential of Myeloid-Derived Suppressor Cells by Modulating PTEN and FOG2 Expression. PLoS ONE. 2015;10: e0135867. 10.1371/journal.pone.0135867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ma HF, Shu P, Shi XH, Wang M, Jiang MF. Identification of miR-4510 as a metastasis suppressor of gastric cancer through regulation of tumor microenvironment via targeting GPC3. Clin Exp Metastasis. 2022;39:363–74. 10.1007/s10585-021-10143-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Y, Huo W, Sun L, Wu J, Zhang C, Wang H, Wang B, Wei J, Qu C, Cao H, Jiang X. Targeting miR-148b-5p Inhibits Immunity Microenvironment and Gastric Cancer Progression. Front Immunol. 2021;12: 590447. 10.3389/fimmu.2021.590447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sun L, Su Y, Jiao A, Wang X, Zhang B. T cells in health and disease. Signal Transduct Target Ther. 2023;8:235. 10.1038/s41392-023-01471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park J, Hsueh PC, Li Z, Ho PC. Microenvironment-driven metabolic adaptations guiding CD8(+) T cell anti-tumor immunity. Immunity. 2023;56:32–42. 10.1016/j.immuni.2022.12.008 [DOI] [PubMed] [Google Scholar]
- 166.Giri PS, Dwivedi M, Begum R. Decreased suppression of CD8(+) and CD4(+) T cells by peripheral regulatory T cells in generalized vitiligo due to reduced NFATC1 and FOXP3 proteins. Exp Dermatol. 2020;29:759–75. 10.1111/exd.14157 [DOI] [PubMed] [Google Scholar]
- 167.Naqvi RA, Gupta M, George A, Naqvi AR. MicroRNAs in shaping the resolution phase of inflammation. Semin Cell Dev Biol. 2022;124:48–62. 10.1016/j.semcdb.2021.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zabeti Touchaei A, Vahidi S. MicroRNAs as regulators of immune checkpoints in cancer immunotherapy: targeting PD-1/PD-L1 and CTLA-4 pathways. Cancer Cell Int. 2024;24:102. 10.1186/s12935-024-03293-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miliotis C, Slack FJ. miR-105-5p regulates PD-L1 expression and tumor immunogenicity in gastric cancer. Cancer Lett. 2021;518:115–26. 10.1016/j.canlet.2021.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu S, Tao Z, Hai B, Liang H, Shi Y, Wang T, Song W, Chen Y, OuYang J, Chen J, et al. miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat Commun. 2016;7:11406. 10.1038/ncomms11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wei J, Nduom EK, Kong LY, Hashimoto Y, Xu S, Gabrusiewicz K, Ling X, Huang N, Qiao W, Zhou S, et al. MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro Oncol. 2016;18:639–48. 10.1093/neuonc/nov292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhao M, Liu Q, Liu W, Zhou H, Zang X, Lu J. MicroRNA-140 suppresses Helicobacter pylori-positive gastric cancer growth by enhancing the antitumor immune response. Mol Med Rep. 2019;20:2484–92. [DOI] [PubMed] [Google Scholar]
- 173.Moon JW, Kong SK, Kim BS, Kim HJ, Lim H, Noh K, Kim Y, Choi JW, Lee JH, Kim YS. IFNγ induces PD-L1 overexpression by JAK2/STAT1/IRF-1 signaling in EBV-positive gastric carcinoma. Sci Rep. 2017;7:17810. 10.1038/s41598-017-18132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zheng X, Dong L, Wang K, Zou H, Zhao S, Wang Y, Wang G. MiR-21 Participates in the PD-1/PD-L1 Pathway-Mediated Imbalance of Th17/Treg Cells in Patients After Gastric Cancer Resection. Ann Surg Oncol. 2019;26:884–93. 10.1245/s10434-018-07117-6 [DOI] [PubMed] [Google Scholar]
- 175.Wang Y, Wang D, Xie G, Yin Y, Zhao E, Tao K, Li R. MicroRNA-152 regulates immune response via targeting B7–H1 in gastric carcinoma. Oncotarget. 2017;8:28125–34. 10.18632/oncotarget.15924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Friedrich M, Hahn M, Michel J, Sankowski R, Kilian M, Kehl N, Günter M, Bunse T, Pusch S, von Deimling A, et al. Dysfunctional dendritic cells limit antigen-specific T cell response in glioma. Neuro Oncol. 2023;25:263–76. 10.1093/neuonc/noac138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sathe A, Ayala C, Bai X, Grimes SM, Lee B, Kin C, Shelton A, Poultsides G, Ji HP. GITR and TIGIT immunotherapy provokes divergent multicellular responses in the tumor microenvironment of gastrointestinal cancers. Genome Med. 2023;15:100. 10.1186/s13073-023-01259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Scalavino V, Liso M, Serino G. Role of microRNAs in the regulation of dendritic cell generation and function. Int J Mol Sci. 2020;21(4):1319. [DOI] [PMC free article] [PubMed]
- 179.Cui ZJ, Xie XL, Qi W, Yang YC, Bai Y, Han J, Ding Q, Jiang HQ. Cell-free miR-17-5p as a diagnostic biomarker for gastric cancer inhibits dendritic cell maturation. Onco Targets Ther. 2019;12:2661–75. 10.2147/OTT.S197682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang Z, Chen S, Fan M, Ruan G, Xi T, Zheng L, Guo L, Ye F, Xing Y. Helicobacter pylori induces gastric cancer via down-regulating miR-375 to inhibit dendritic cell maturation. Helicobacter. 2021;26: e12813. 10.1111/hel.12813 [DOI] [PubMed] [Google Scholar]
- 181.Saultz JN, Freud AG, Mundy-Bosse BL. MicroRNA regulation of natural killer cell development and function in leukemia. Mol Immunol. 2019;115:12–20. 10.1016/j.molimm.2018.07.022 [DOI] [PubMed] [Google Scholar]
- 182.Bezman NA, Chakraborty T, Bender T, Lanier LL. miR-150 regulates the development of NK and iNKT cells. J Exp Med. 2011;208:2717–31. 10.1084/jem.20111386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. miR-155 regulates IFN-γ production in natural killer cells. Blood. 2012;119:3478–85. 10.1182/blood-2011-12-398099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou C, Li X, Zhang X, Liu X, Tan Z, Yang C, Zhang J. microRNA-372 maintains oncogene characteristics by targeting TNFAIP1 and affects NFκB signaling in human gastric carcinoma cells. Int J Oncol. 2013;42:635–42. 10.3892/ijo.2012.1737 [DOI] [PubMed] [Google Scholar]
- 185.Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–707. 10.7150/thno.41580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Eichmüller SB, Osen W, Mandelboim O, Seliger B. Immune modulatory microRNAs involved in tumor attack and tumor immune escape. J Natl Cancer Inst. 2017;109(10). [DOI] [PubMed]
- 187.Duan SL, Fu WJ, Jiang YK, Peng LS, Ousmane D, Zhang ZJ, Wang JP. Emerging role of exosome-derived non-coding RNAs in tumor-associated angiogenesis of tumor microenvironment. Front Mol Biosci. 2023;10:1220193. 10.3389/fmolb.2023.1220193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu S, Tang C, Zhang QW, Zhuang Q, Ye X, Xia J, Shi Y, Ning M, Dong ZX, Wan XJ. Overexpression of RAB31 in gastric cancer is associated with released exosomes and increased tumor cell invasion and metastasis. Cancer Med. 2023;12:13497–510. 10.1002/cam4.6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lu X, Lu J, Wang S, Zhang Y, Ding Y, Shen X, Jing R, Ju S, Chen H, Cong H. Circulating serum exosomal miR-92a-3p as a novel biomarker for early diagnosis of gastric cancer. Future Oncol. 2021;17:907–19. 10.2217/fon-2020-0792 [DOI] [PubMed] [Google Scholar]
- 190.Nail HM, Chiu CC, Leung CH, Ahmed MMM, Wang HD. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J Biomed Sci. 2023;30(1):69. [DOI] [PMC free article] [PubMed]
- 191.Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F, Ma P, Jiang H, Wu X, Shu Y, Xu T. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer. 2020;19:112. 10.1186/s12943-020-01208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bai M, Li J, Yang H, Zhang H, Zhou Z, Deng T, Zhu K, Ning T, Fan Q, Ying G, Ba Y. Retraction Notice to: miR-135b Delivered by Gastric Tumor Exosomes Inhibits FOXO1 Expression in Endothelial Cells and Promotes Angiogenesis. Mol Ther. 2022;30:2636. 10.1016/j.ymthe.2022.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xia X, Wang S, Ni B, Xing S, Cao H, Zhang Z, Yu F, Zhao E, Zhao G. Hypoxic gastric cancer-derived exosomes promote progression and metastasis via MiR-301a-3p/PHD3/HIF-1α positive feedback loop. Oncogene. 2020;39:6231–44. 10.1038/s41388-020-01425-6 [DOI] [PubMed] [Google Scholar]
- 194.Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, Xuan Z, Fang L, Yang J, Zhang L, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res. 2022;41:296. 10.1186/s13046-022-02499-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhu M, Zhang N, Ma J, He S. Integration of exosomal miR-106a and mesothelial cells facilitates gastric cancer peritoneal dissemination. Cell Signal. 2022;91: 110230. 10.1016/j.cellsig.2021.110230 [DOI] [PubMed] [Google Scholar]
- 196.Li Q, Li B, Li Q, Wei S, He Z, Huang X, Wang L, Xia Y, Xu Z, Li Z, et al. Exosomal miR-21-5p derived from gastric cancer promotes peritoneal metastasis via mesothelial-to-mesenchymal transition. Cell Death Dis. 2018;9:854. 10.1038/s41419-018-0928-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang H, Fu H, Wang B, Zhang X, Mao J, Li X, Wang M, Sun Z, Qian H, Xu W. Exosomal miR-423-5p targets SUFU to promote cancer growth and metastasis and serves as a novel marker for gastric cancer. Mol Carcinog. 2018;57:1223–36. 10.1002/mc.22838 [DOI] [PubMed] [Google Scholar]
- 198.Li Z, Suo B, Long G, Gao Y, Song J, Zhang M, Feng B, Shang C, Wang D. Exosomal miRNA-16-5p Derived From M1 Macrophages Enhances T Cell-Dependent Immune Response by Regulating PD-L1 in Gastric Cancer. Front Cell Dev Biol. 2020;8: 572689. 10.3389/fcell.2020.572689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hsieh CH, Tai SK, Yang MH. Snail-overexpressing Cancer Cells Promote M2-Like Polarization of Tumor-Associated Macrophages by Delivering MiR-21-Abundant Exosomes. Neoplasia. 2018;20:775–88. 10.1016/j.neo.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wei S, Peng L, Yang J, Sang H, Jin D, Li X, Chen M, Zhang W, Dang Y, Zhang G. Exosomal transfer of miR-15b-3p enhances tumorigenesis and malignant transformation through the DYNLT1/Caspase-3/Caspase-9 signaling pathway in gastric cancer. J Exp Clin Cancer Res. 2020;39:32. 10.1186/s13046-019-1511-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jiao Y, Zhang L, Li J, He Y, Zhang X, Li J. Exosomal miR-122-5p inhibits tumorigenicity of gastric cancer by downregulating GIT1. Int J Biol Markers. 2021;36:36–46. 10.1177/1724600821990677 [DOI] [PubMed] [Google Scholar]
- 202.Xu G, Zhang B, Ye J, Cao S, Shi J, Zhao Y, Wang Y, Sang J, Yao Y, Guan W, et al. Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing MMP11 expression. Int J Biol Sci. 2019;15:2320–9. 10.7150/ijbs.33750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wu C, Li D, Cheng X, Gu H, Qian Y, Feng L. Downregulation of cancer-associated fibroblast exosome-derived miR-29b-1-5p restrains vasculogenic mimicry and apoptosis while accelerating migration and invasion of gastric cancer cells via immunoglobulin domain-containing 1/zonula occluden-1 axis. Cell Cycle. 2023;22:1807–26. 10.1080/15384101.2023.2231740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang Y, Li T, Yang L, Zhang X, Wang X, Su X, Ji C, Wang Z. Cancer-associated fibroblast-released extracellular vesicles carrying miR-199a-5p induces the progression of gastric cancer through regulation of FKBP5-mediated AKT1/mTORC1 signaling pathway. Cell Cycle. 2022;21:2590–601. 10.1080/15384101.2022.2105092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534–42. 10.1016/j.cgh.2019.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang L, Lu Z, Zhao X. Targeting Bcl-2 for cancer therapy. Biochim Biophys Acta Rev Cancer. 2021;1876: 188569. 10.1016/j.bbcan.2021.188569 [DOI] [PubMed] [Google Scholar]
- 207.Osterlund P, Kinos S, Pfeiffer P, Salminen T, Kwakman JJM, Frödin JE, Shah CH, Sorbye H, Ristamäki R, Halonen P, et al. Continuation of fluoropyrimidine treatment with S-1 after cardiotoxicity on capecitabine- or 5-fluorouracil-based therapy in patients with solid tumours: a multicentre retrospective observational cohort study. ESMO Open. 2022;7: 100427. 10.1016/j.esmoop.2022.100427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021;18:473–87. 10.1038/s41571-021-00492-2 [DOI] [PubMed] [Google Scholar]
- 209.Li Y, Tian Z, Tan Y, Lian G, Chen S, Chen S, Li J, Li X, Huang K, Chen Y. Bmi-1-induced miR-27a and miR-155 promote tumor metastasis and chemoresistance by targeting RKIP in gastric cancer. Mol Cancer. 2020;19:109. 10.1186/s12943-020-01229-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Deng LM, Tan T, Zhang TY, Xiao XF, Gu H. miR-1 reverses multidrug resistance in gastric cancer cells via downregulation of sorcin through promoting the accumulation of intracellular drugs and apoptosis of cells. Int J Oncol. 2019;55:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jin HF, Wang JF, Shao M, Zhou K, Ma X, Lv XP. Down-Regulation of miR-7 in Gastric Cancer Is Associated With Elevated LDH-A Expression and Chemoresistance to Cisplatin. Front Cell Dev Biol. 2020;8: 555937. 10.3389/fcell.2020.555937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhao D, Zhang Y, Song L. MiR-16-1 Targeted Silences Far Upstream Element Binding Protein 1 to Advance the Chemosensitivity to Adriamycin in Gastric Cancer. Pathol Oncol Res. 2018;24:483–8. 10.1007/s12253-017-0263-x [DOI] [PubMed] [Google Scholar]
- 213.Wu DM, Hong XW, Wang LL, Cui XF, Lu J, Chen GQ, Zheng YL. MicroRNA-17 inhibition overcomes chemoresistance and suppresses epithelial-mesenchymal transition through a DEDD-dependent mechanism in gastric cancer. Int J Biochem Cell Biol. 2018;102:59–70. 10.1016/j.biocel.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 214.Wang Z, Ji F. Downregulation of microRNA-17-5p inhibits drug resistance of gastric cancer cells partially through targeting p21. Oncol Lett. 2018;15:4585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–9. 10.1002/ijc.23501 [DOI] [PubMed] [Google Scholar]
- 216.Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, Nie Y, Wu K, Shi Y, Fan D. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun. 2013;434:688–94. 10.1016/j.bbrc.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 217.Zhu M, Zhou X, Du Y, Huang Z, Zhu J, Xu J, Cheng G, Shu Y, Liu P, Zhu W, Wang T. miR-20a induces cisplatin resistance of a human gastric cancer cell line via targeting CYLD. Mol Med Rep. 2016;14:1742–50. 10.3892/mmr.2016.5413 [DOI] [PubMed] [Google Scholar]
- 218.Zhou L, Li X, Zhou F, Jin Z, Chen D, Wang P, Zhang S, Zhuge Y, Shang Y, Zou X. Downregulation of leucine-rich repeats and immunoglobulin-like domains 1 by microRNA-20a modulates gastric cancer multidrug resistance. Cancer Sci. 2018;109:1044–54. 10.1111/cas.13538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yang SM, Huang C, Li XF, Yu MZ, He Y, Li J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162–8. 10.1016/j.tox.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 220.An Y, Zhang Z, Shang Y, Jiang X, Dong J, Yu P, Nie Y, Zhao Q. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis. 2015;6: e1766. 10.1038/cddis.2015.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.He J, Qi H, Chen F, Cao C. MicroRNA-25 contributes to cisplatin resistance in gastric cancer cells by inhibiting forkhead box O3a. Oncol Lett. 2017;14:6097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Abbasi A, Hosseinpourfeizi M, Safaralizadeh R. All-trans retinoic acid-mediated miR-30a up-regulation suppresses autophagy and sensitizes gastric cancer cells to cisplatin. Life Sci. 2022;307: 120884. 10.1016/j.lfs.2022.120884 [DOI] [PubMed] [Google Scholar]
- 223.Korourian A, Roudi R, Shariftabrizi A, Madjd Z. MicroRNA-31 inhibits RhoA-mediated tumor invasion and chemotherapy resistance in MKN-45 gastric adenocarcinoma cells. Exp Biol Med (Maywood). 2017;242:1842–7. 10.1177/1535370217728460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ji Q, Hao X, Meng Y, Zhang M, Desano J, Fan D, Xu L. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. 10.1186/1471-2407-8-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang Z, Kong Y, Yang W, Ma F, Zhang Y, Ji S, Ma EM, Liu H, Chen Y, Hua Y. Upregulation of microRNA-34a enhances the DDP sensitivity of gastric cancer cells by modulating proliferation and apoptosis via targeting MET. Oncol Rep. 2016;36:2391–7. 10.3892/or.2016.5016 [DOI] [PubMed] [Google Scholar]
- 226.Zheng H, Wang JJ, Yang XR, Yu YL. Upregulation of miR-34c after silencing E2F transcription factor 1 inhibits paclitaxel combined with cisplatin resistance in gastric cancer cells. World J Gastroenterol. 2020;26:499–513. 10.3748/wjg.v26.i5.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wu H, Huang M, Lu M, Zhu W, Shu Y, Cao P, Liu P. Regulation of microtubule-associated protein tau (MAPT) by miR-34c-5p determines the chemosensitivity of gastric cancer to paclitaxel. Cancer Chemother Pharmacol. 2013;71:1159–71. 10.1007/s00280-013-2108-y [DOI] [PubMed] [Google Scholar]
- 228.Lang C, Xu M, Zhao Z, Chen J, Zhang L. MicroRNA-96 expression induced by low-dose cisplatin or doxorubicin regulates chemosensitivity, cell death and proliferation in gastric cancer SGC7901 cells by targeting FOXO1. Oncol Lett. 2018;16:4020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang Y, Xu W, Ni P, Li A, Zhou J, Xu S. MiR-99a and MiR-491 Regulate Cisplatin Resistance in Human Gastric Cancer Cells by Targeting CAPNS1. Int J Biol Sci. 2016;12:1437–47. 10.7150/ijbs.16529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bao J, Xu Y, Wang Q, Zhang J, Li Z, Li D, Li J. miR-101 alleviates chemoresistance of gastric cancer cells by targeting ANXA2. Biomed Pharmacother. 2017;92:1030–7. 10.1016/j.biopha.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 231.Zhang Y, Lu Q, Cai X. MicroRNA-106a induces multidrug resistance in gastric cancer by targeting RUNX3. FEBS Lett. 2013;587:3069–75. 10.1016/j.febslet.2013.06.058 [DOI] [PubMed] [Google Scholar]
- 232.Fang Y, Shen H, Li H, Cao Y, Qin R, Long L, Zhu X, Xie C, Xu W. miR-106a confers cisplatin resistance by regulating PTEN/Akt pathway in gastric cancer cells. Acta Biochim Biophys Sin (Shanghai). 2013;45:963–72. 10.1093/abbs/gmt106 [DOI] [PubMed] [Google Scholar]
- 233.Wang P, Li Z, Liu H, Zhou D, Fu A, Zhang E. MicroRNA-126 increases chemosensitivity in drug-resistant gastric cancer cells by targeting EZH2. Biochem Biophys Res Commun. 2016;479:91–6. 10.1016/j.bbrc.2016.09.040 [DOI] [PubMed] [Google Scholar]
- 234.Liang L, Kang H, Jia J. HCP5 contributes to cisplatin resistance in gastric cancer through miR-128/HMGA2 axis. Cell Cycle. 2021;20:1080–90. 10.1080/15384101.2021.1924948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lu C, Shan Z, Li C, Yang L. MiR-129 regulates cisplatin-resistance in human gastric cancer cells by targeting P-gp. Biomed Pharmacother. 2017;86:450–6. 10.1016/j.biopha.2016.11.139 [DOI] [PubMed] [Google Scholar]
- 236.Chu H, Han N, Xu J. CMPK1 Regulated by miR-130b Attenuates Response to 5-FU Treatment in Gastric Cancer. Front Oncol. 2021;11: 637470. 10.3389/fonc.2021.637470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang L, Guo X, Zhang D, Fan Y, Qin L, Dong S, Zhang L. Upregulated miR-132 in Lgr5(+) gastric cancer stem cell-like cells contributes to cisplatin-resistance via SIRT1/CREB/ABCG2 signaling pathway. Mol Carcinog. 2017;56:2022–34. 10.1002/mc.22656 [DOI] [PubMed] [Google Scholar]
- 238.Pan Y, Ren F, Zhang W, Liu G, Yang D, Hu J, Feng K, Feng Y. Regulation of BGC-823 cell sensitivity to adriamycin via miRNA-135a-5p. Oncol Rep. 2014;32:2549–56. 10.3892/or.2014.3546 [DOI] [PubMed] [Google Scholar]
- 239.Zhou J, Chen Q. Poor expression of microRNA-135b results in the inhibition of cisplatin resistance and proliferation and induces the apoptosis of gastric cancer cells through MST1-mediated MAPK signaling pathway. Faseb j. 2019;33:3420–36. 10.1096/fj.201800618RRR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zeng JF, Ma XQ, Wang LP, Wang W. MicroRNA-145 exerts tumor-suppressive and chemo-resistance lowering effects by targeting CD44 in gastric cancer. World J Gastroenterol. 2017;23:2337–45. 10.3748/wjg.v23.i13.2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li B, Wang W, Li Z, Chen Z, Zhi X, Xu J, Li Q, Wang L, Huang X, Wang L, et al. MicroRNA-148a-3p enhances cisplatin cytotoxicity in gastric cancer through mitochondrial fission induction and cyto-protective autophagy suppression. Cancer Lett. 2017;410:212–27. 10.1016/j.canlet.2017.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ning J, Jiao Y, Xie X, Deng X, Zhang Y, Yang Y, Zhao C, Wang H, Gu K. miR-138-5p modulates the expression of excision repair cross-complementing proteins ERCC1 and ERCC4, and regulates the sensitivity of gastric cancer cells to cisplatin. Oncol Rep. 2019;41:1131–9. [DOI] [PubMed] [Google Scholar]
- 243.Lin H, Ni R, Li D, Zhao M, Li Y, Li K, Zhang Q, Huang C, Huang S. LncRNA MIR155HG Overexpression Promotes Proliferation, Migration, and Chemoresistance in Gastric Cancer Cells. Int J Med Sci. 2023;20:933–42. 10.7150/ijms.82216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang M, Qiu R, Yu S, Xu X, Li G, Gu R, Tan C, Zhu W, Shen B. Paclitaxel-resistant gastric cancer MGC-803 cells promote epithelial-to-mesenchymal transition and chemoresistance in paclitaxel-sensitive cells via exosomal delivery of miR-155-5p. Int J Oncol. 2019;54:326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lin Y, Zhao J, Wang H, Cao J, Nie Y. miR-181a modulates proliferation, migration and autophagy in AGS gastric cancer cells and downregulates MTMR3. Mol Med Rep. 2017;15:2451–6. 10.3892/mmr.2017.6289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jin L, Ma X, Zhang N, Zhang Q, Chen X, Zhang Z, Ding G, Yu H. Targeting Oncogenic miR-181a-2-3p Inhibits Growth and Suppresses Cisplatin Resistance of Gastric Cancer. Cancer Manag Res. 2021;13:8599–609. 10.2147/CMAR.S332713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhu W, Shan X, Wang T, Shu Y, Liu P. miR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int J Cancer. 2010;127:2520–9. 10.1002/ijc.25260 [DOI] [PubMed] [Google Scholar]
- 248.Li Q, Wang JX, He YQ, Feng C, Zhang XJ, Sheng JQ, Li PF. MicroRNA-185 regulates chemotherapeutic sensitivity in gastric cancer by targeting apoptosis repressor with caspase recruitment domain. Cell Death Dis. 2014;5: e1197. 10.1038/cddis.2014.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee SD, Yu D, Lee DY, Shin HS, Jo JH, Lee YC. Upregulated microRNA-193a-3p is responsible for cisplatin resistance in CD44(+) gastric cancer cells. Cancer Sci. 2019;110:662–73. 10.1111/cas.13894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nie H, Mu J, Wang J, Li Y. miR-195-5p regulates multi-drug resistance of gastric cancer cells via targeting ZNF139. Oncol Rep. 2018;40:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol. 2012;69:723–31. 10.1007/s00280-011-1752-3 [DOI] [PubMed] [Google Scholar]
- 252.Jiang T, Dong P, Li L, Ma X, Xu P, Zhu H, Wang Y, Yang B, Liu K, Liu J, et al. MicroRNA-200c regulates cisplatin resistance by targeting ZEB2 in human gastric cancer cells. Oncol Rep. 2017;38:151–8. 10.3892/or.2017.5659 [DOI] [PubMed] [Google Scholar]
- 253.Sacconi A, Biagioni F, Canu V, Mori F, Di Benedetto A, Lorenzon L, Ercolani C, Di Agostino S, Cambria AM, Germoni S, et al. miR-204 targets Bcl-2 expression and enhances responsiveness of gastric cancer. Cell Death Dis. 2012;3: e423. 10.1038/cddis.2012.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Li LQ, Pan D, Chen Q, Zhang SW, Xie DY, Zheng XL, Chen H. Sensitization of Gastric Cancer Cells to 5-FU by MicroRNA-204 Through Targeting the TGFBR2-Mediated Epithelial to Mesenchymal Transition. Cell Physiol Biochem. 2018;47:1533–45. 10.1159/000490871 [DOI] [PubMed] [Google Scholar]
- 255.Zhang XL, Shi HJ, Wang JP, Tang HS, Wu YB, Fang ZY, Cui SZ, Wang LT. MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin. World J Gastroenterol. 2014;20:11347–55. 10.3748/wjg.v20.i32.11347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhou X, Jin W, Jia H, Yan J, Zhang G. MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J Exp Clin Cancer Res. 2015;34:28. 10.1186/s13046-015-0145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gao H, Ma J, Cheng Y, Zheng P. Exosomal Transfer of Macrophage-Derived miR-223 Confers Doxorubicin Resistance in Gastric Cancer. Onco Targets Ther. 2020;13:12169–79. 10.2147/OTT.S283542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jian B, Li Z, Xiao D, He G, Bai L, Yang Q. Downregulation of microRNA-193-3p inhibits tumor proliferation migration and chemoresistance in human gastric cancer by regulating PTEN gene. Tumour Biol. 2016;37:8941–9. 10.1007/s13277-015-4727-x [DOI] [PubMed] [Google Scholar]
- 259.Zhu T, Hu Z, Wang Z, Ding H, Li R, Wang J, Wang G. microRNA-301b-3p from mesenchymal stem cells-derived extracellular vesicles inhibits TXNIP to promote multidrug resistance of gastric cancer cells. Cell Biol Toxicol. 2023;39:1923–37. 10.1007/s10565-021-09675-0 [DOI] [PubMed] [Google Scholar]
- 260.Tian L, Zhao Z, Xie L, Zhu J. MiR-361-5p suppresses chemoresistance of gastric cancer cells by targeting FOXM1 via the PI3K/Akt/mTOR pathway. Oncotarget. 2018;9:4886–96. 10.18632/oncotarget.23513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhang PF, Sheng LL, Wang G, Tian M, Zhu LY, Zhang R, Zhang J, Zhu JS. miR-363 promotes proliferation and chemo-resistance of human gastric cancer via targeting of FBW7 ubiquitin ligase expression. Oncotarget. 2016;7:35284–92. 10.18632/oncotarget.9169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhou N, Qu Y, Xu C, Tang Y. Upregulation of microRNA-375 increases the cisplatin-sensitivity of human gastric cancer cells by regulating ERBB2. Exp Ther Med. 2016;11:625–30. 10.3892/etm.2015.2920 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 263.Li Y, Liu H, Cui Y, Chen H, Cui X, Shao J, Su F, He X. miR-424-3p Contributes to the Malignant Progression and Chemoresistance of Gastric Cancer. Onco Targets Ther. 2020;13:12201–11. 10.2147/OTT.S280717 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 264.Wang X, He R, Geng L, Yuan J, Fan H. Ginsenoside Rg3 Alleviates Cisplatin Resistance of Gastric Cancer Cells Through Inhibiting SOX2 and the PI3K/Akt/mTOR Signaling Axis by Up-Regulating miR-429. Front Genet. 2022;13: 823182. 10.3389/fgene.2022.823182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wu S, Xie J, Shi H, Wang ZW. miR-492 promotes chemoresistance to CDDP and metastasis by targeting inhibiting DNMT3B and induces stemness in gastric cancer. Biosci Rep. 2020;40(3):BSR20194342. [DOI] [PMC free article] [PubMed]
- 266.Makinoya M, Miyatani K, Matsumi Y, Sakano Y, Shimizu S, Shishido Y, Hanaki T, Kihara K, Matsunaga T, Yamamoto M, et al. Exosomal miR-493 suppresses MAD2L1 and induces chemoresistance to intraperitoneal paclitaxel therapy in gastric cancer patients with peritoneal metastasis. Sci Rep. 2024;14:10075. 10.1038/s41598-024-60967-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chen S, Wu J, Jiao K, Wu Q, Ma J, Chen D, Kang J, Zhao G, Shi Y, Fan D, Zhao G. MicroRNA-495-3p inhibits multidrug resistance by modulating autophagy through GRP78/mTOR axis in gastric cancer. Cell Death Dis. 2018;9:1070. 10.1038/s41419-018-0950-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-497 modulates multidrug resistance of human cancer cell lines by targeting BCL2. Med Oncol. 2012;29:384–91. 10.1007/s12032-010-9797-4 [DOI] [PubMed] [Google Scholar]
- 269.Lin H, Zhang L, Zhang C, Liu P. Exosomal MiR-500a-3p promotes cisplatin resistance and stemness via negatively regulating FBXW7 in gastric cancer. J Cell Mol Med. 2020;24:8930–41. 10.1111/jcmm.15524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wang T, Ge G, Ding Y, Zhou X, Huang Z, Zhu W, Shu Y, Liu P. MiR-503 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R and BCL2. Chin Med J (Engl). 2014;127:2357–62. 10.3760/cma.j.issn.0366-6999.20140318 [DOI] [PubMed] [Google Scholar]
- 271.Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li K, Zhou L, Sun Y, Li M, Zhou J, et al. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene. 2014;33:3267–76. 10.1038/onc.2013.297 [DOI] [PubMed] [Google Scholar]
- 272.Wang J, Xue X, Hong H, Qin M, Zhou J, Sun Q, Liang H, Gao L. Upregulation of microRNA-524-5p enhances the cisplatin sensitivity of gastric cancer cells by modulating proliferation and metastasis via targeting SOX9. Oncotarget. 2017;8:574–82. 10.18632/oncotarget.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Shen B, Yu S, Zhang Y, Yuan Y, Li X, Zhong J, Feng J. miR-590-5p regulates gastric cancer cell growth and chemosensitivity through RECK and the AKT/ERK pathway. Onco Targets Ther. 2016;9:6009–19. 10.2147/OTT.S110923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jiang L, Yang W, Bian W, Yang H, Wu X, Li Y, Feng W, Liu X. MicroRNA-623 Targets Cyclin D1 to Inhibit Cell Proliferation and Enhance the Chemosensitivity of Cells to 5-Fluorouracil in Gastric Cancer. Oncol Res. 2018;27:19–27. 10.3727/096504018X15193469240508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Cao W, Wei W, Zhan Z, Xie D, Xie Y, Xiao Q. Regulation of drug resistance and metastasis of gastric cancer cells via the microRNA647-ANK2 axis. Int J Mol Med. 2018;41:1958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Aliabadi P, Sadri M, Siri G, Ebrahimzadeh F, Yazdani Y, Gusarov AM, Kharkouei SA, Asadi F, Adili A, Mardi A, Mohammadi H. Restoration of miR-648 overcomes 5-FU-resistance through targeting ET-1 in gastric cancer cells in-vitro. Pathol Res Pract. 2022;239: 154139. 10.1016/j.prp.2022.154139 [DOI] [PubMed] [Google Scholar]
- 277.Shang J, Wang Q, Wang J, Xu B, Liu S. miR-708-3p promotes gastric cancer progression through downregulating ETNK1. Heliyon. 2023;9: e19544. 10.1016/j.heliyon.2023.e19544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chen Q, Lin L, Xiong B, Yang W, Huang J, Shi H, Wang Z. MiR-873-5p targets THUMPD1 to inhibit gastric cancer cell behavior and chemoresistance. J Gastrointest Oncol. 2021;12:2061–72. 10.21037/jgo-21-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Huang H, Tang J, Zhang L, Bu Y, Zhang X. miR-874 regulates multiple-drug resistance in gastric cancer by targeting ATG16L1. Int J Oncol. 2018;53:2769–79. [DOI] [PubMed] [Google Scholar]
- 280.Nishibeppu K, Komatsu S, Imamura T, Kiuchi J, Kishimoto T, Arita T, Kosuga T, Konishi H, Kubota T, Shiozaki A, et al. Plasma microRNA profiles: identification of miR-1229-3p as a novel chemoresistant and prognostic biomarker in gastric cancer. Sci Rep. 2020;10:3161. 10.1038/s41598-020-59939-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Cao W, Wei W, Zhan Z, Xie Y, Xiao Q. MiR-1284 modulates multidrug resistance of gastric cancer cells by targeting EIF4A1. Oncol Rep. 2016;35:2583–91. 10.3892/or.2016.4643 [DOI] [PubMed] [Google Scholar]
- 282.Yan R, Li K, Yuan DW, Wang HN, Zhang Y, Dang CX, Zhu K. Downregulation of microRNA-4295 enhances cisplatin-induced gastric cancer cell apoptosis through the EGFR/PI3K/Akt signaling pathway by targeting LRIG1. Int J Oncol. 2018;53:2566–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jing X, Xie M, Ding K, Xu T, Fang Y, Ma P, Shu Y. Exosome-transmitted miR-769-5p confers cisplatin resistance and progression in gastric cancer by targeting CASP9 and promoting the ubiquitination degradation of p53. Clin Transl Med. 2022;12: e780. 10.1002/ctm2.780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Jiang L, Zhang Y, Guo L, Liu C, Wang P, Ren W. Exosomal microRNA-107 reverses chemotherapeutic drug resistance of gastric cancer cells through HMGA2/mTOR/P-gp pathway. BMC Cancer. 2021;21:1290. 10.1186/s12885-021-09020-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Hao NB, He YF, Li XQ, Wang K, Wang RL. The role of miRNA and lncRNA in gastric cancer. Oncotarget. 2017;8:81572–82. 10.18632/oncotarget.19197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cui M, Wang H, Yao X, Zhang D, Xie Y, Cui R, Zhang X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front Genet. 2019;10:626. 10.3389/fgene.2019.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
No datasets were generated or analysed during the current study.