Abstract
Interleukin-12 family cytokines have emerged as critical regulators of immunity with some members (IL-12, IL-23) associated with disease pathogenesis while others (IL-27, IL-35) mitigate autoimmune diseases. Each IL-12 family member is comprised of an α and a β chain, and chain-sharing is a key feature. Although four bona fide members have thus far been described, promiscuous chain-pairing between alpha (IL-23p19, IL-27p28, IL-12/IL-35p35) and beta (IL-12/IL-23p40, IL-27/IL-35Ebi3) subunits, predicts six possible heterodimeric IL-12 family cytokines. Here, we describe a new IL-12 member composed of IL-23p19 and Ebi3 heterodimer (IL-39) that is secreted by LPS-stimulated B cells and GL7+ activated B cells of lupus-like mice. We further show that IL-39 mediates inflammatory responses through activation of STAT1/STAT3 in lupus-like mice. Taken together, our results show that IL-39 might contribute to immunopathogenic mechanisms of systemic lupus erythematosus, and could be used as a possible target for its treatment.
Keywords: Autoimmunity, IL-12, IL-23p19, IL-27/IL-35Ebi3, IL-39, Inflammation, Systemic lupus erythematosus
Introduction
IL-12 family including IL-12, IL-23, IL-27, and IL-35, regulates both pro- and anti-inflammatory responses [1]. IL-12 and IL-23 play crucial roles in pathogenesis of autoimmune diseases by inducing the differentiation of Th1 and Th17 lymphocytes while IL-27 and IL-35 suppress inflammatory responses and limit tissue injury by promoting the expansion of regulatory B and T-cell subsets [2, 3].
Discovery of IL-23 in 2000 [4] led to the reevaluation of IL-12 and IL-23 in autoimmune diseases. For example, therapeutic targeting of IL-12p40 decreases pathology in many mouse models of autoimmune diseases [5], while disease is exacerbated in IL-12p35-deficient mice [6, 7]. Thus, IL-23 rather than IL-12 was found to be the critical cytokine for autoimmune inflammation including experimental immune-mediated disease [6–10]. Currently, at least ten therapeutic agents targeting IL-23 are being tested in the clinic for more than 17 human immune-mediated diseases [11]. Both IL-27 and IL-35 have immune-suppressive activities and are also cytokines with strikingly diverse influences on the immune response so that viable therapeutic targets may also be exploited for treatment of human inflammatory diseases [12, 13]. Thus, understanding immunobiology of IL-12 family cytokines would undoubtedly provide valuable knowledge that can be exploited therapeutically.
The IL-12 family cytokines are α/β heterodimers consisting of one α subunit (IL-23p19, IL-27p28, IL-12p35) and one β chain (IL-12p40, Ebi3) [14, 15]. Although there are currently four known members in the family, the predictable range of combinations is six and it is conceivable that additional pairings such as IL-23p19/Ebi3 are possible [12, 14–17]. In this study, we sought to discover additional IL-12 members that might exist in nature. By combining different alpha and beta IL-12 subunit proteins in vitro we detected a novel stable p19/Ebi3 heterodimeric complex by immunoprecipitation. We have characterized the p19/Ebi3 cytokine (IL-39) and demonstrated that it possesses biological activities in vitro and in vivo.
Results
IL-23p19 (p19) and Ebi3 form a composite factor (IL-39)
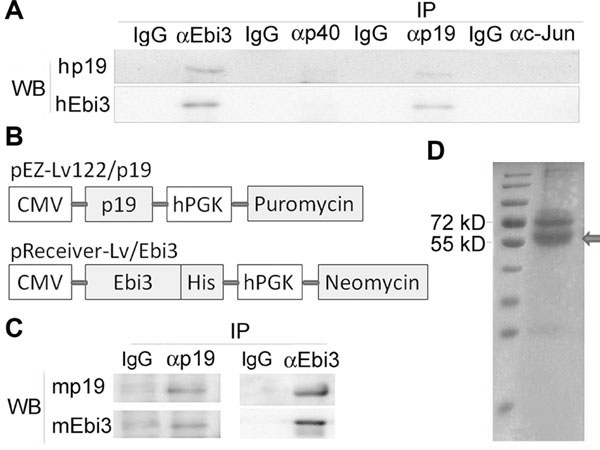
To examine whether p19 can form a stable complex with Ebi3, we mixed equal amounts of the two proteins and immunoprecipitation (IP)/Western blot analyses revealed formation of a stable human p19/Ebi3 complex (Fig. 1A). We could not detect the p19/Ebi3 following IP with isotype IgG or anti-c-Jun antibody, providing suggestive evidence for potential bona fide p19/Ebi3 cytokine. To confirm our finding in another animal species, we genetically engineered and expressed mouse p19 and Ebi3 subunits in CHO cells (Fig. 1B). IP of supernatants derived from transfectants with anti-p19 mAb and followed by Western blot analysis using anti-Ebi3 mAb confirmed coexpression p19 and Ebi3 and formation of a stable p19/Ebi3 heterodimer (Fig. 1C). We further confirmed this observation by reciprocal IP with anti-Ebi3 mAb and Western blotting with anti-p19 mAb and the p19/Ebi3 complex migrated on coomassie blue SDS-PAGE as a 54 KD protein (Fig. 1C, D). These results suggest that p19 and Ebi3 can form a stable p19/Ebi3 heterodimer referred here as IL-39.
Figure 1.
IL-23p19 and IL-27/IL-35Ebi3 can form a heterodimer, termed IL-39. (A) Detection and characterization of human IL-39 by immunoprecipitation (IP) and Western blot (WB) analyses under reducing conditions. (B) Schematic of the cDNA constructs used to genetically engineer mouse p19 and Ebi3 proteins. hPGK: human phosphoglycerate kinase 1 promoter. (C) Detection and characterization of mouse IL-39 recombinant proteins by IP and Western blot analyses under reducing conditions. (D) Coomassie Blue gel of the recombinant mouse IL-39 characterized on nonreducing polyacrylamide gels. Arrow indicates recombinant IL-39. (A, C, and D) Blots are representative of three independent experiments.
IL-39 is expressed in vitro and in vivo by activated B cells
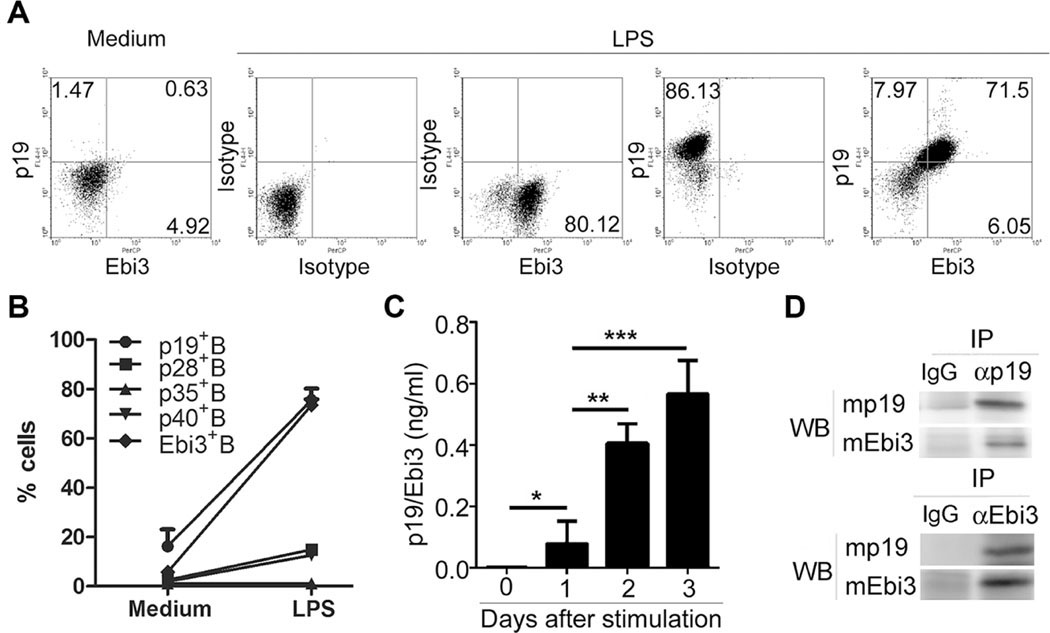
Ebi3 is expressed at a high levels in human B lymphoblast cell lines transformed in vitro by Epstein Barr Virus [18] and associates noncovalently with IL-12p35 to form IL-35, a heterodimeric cytokine secreted by B cells [2, 3]. We therefore examined whether activated B cells also produce IL-39. We show here that expression of both subunits of IL-39 is significantly upregulated in B cells activated with LPS (Fig. 2A, B) and time-dependent secretion of IL-39 was confirmed by Sandwich ELISA (Fig. 2C). IP/Western blot analysis of supernatants derived from LPS-stimulated B cells confirmed secretion of the IL-39 heterodimer (Fig. 2D). We also examined whether myeloid cell types, particularly antigen-presenting cells, express IL-39. We found that p19 and Ebi3 mRNA are expressed by DCs and macrophages (Supporting Information Fig. 1). However, additional studies are required to determine whether these cells can secrete the functional heterodimeric IL-39 cytokine.
Figure 2.
In vitro activated B cells expressed IL-39. (A, B) Primary B cells were sorted from 8-weeks-old female C57BL/6 mice by B220 microbeads, stimulated for 48 h with LPS, and analyzed by FACS. (A) The percentages of IL-39-expressing B cells and (B) statistical analysis of the percentage of IL-12 family cytokine subunits are shown. Isotype was used as the staining control. (C) The concentration of IL-39 in the cultured supernatant from LPS-stimulated B cells was measured by sandwich ELISA by using anti-p19 and Ebi3 antibody as coated and detected antibody, respectively. (B and C) Data are shown as mean + SEM (n = 8) from one experiment representative of three other similar experiments. (D) Detection of mouse natural IL-39 proteins in the cultured supernatant on days 3 after B cells were stimulated with LPS by IP and Western blot analyses under reducing conditions. Blots are representative of three independent experiments *p < 0.05, **p < 0.01, ***p < 0.001 (two tailed Student’s t-test).
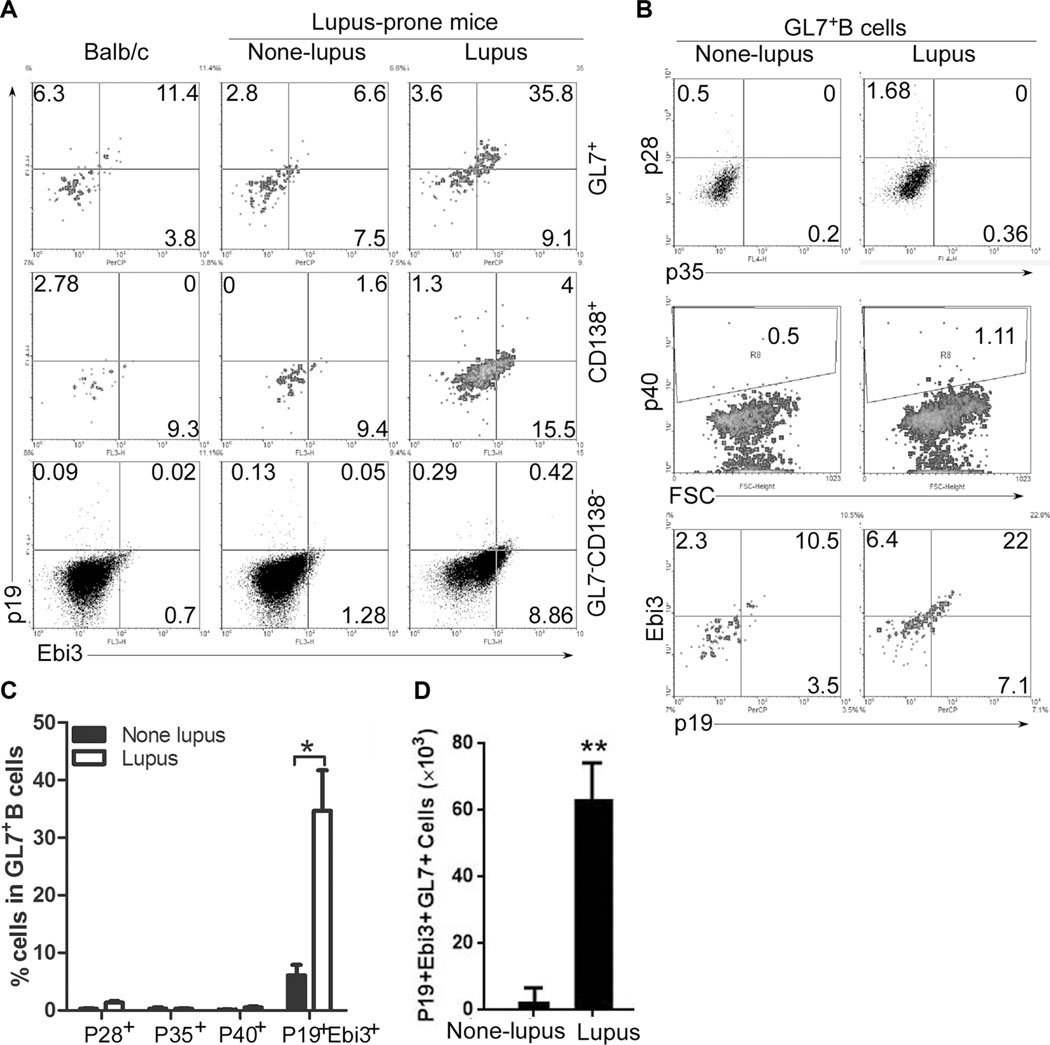
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the expansion of autoantibody-secreting B cells [19–21]. MRL/lpr mice bearing Fas/Fas ligand mutant genes develop autoimmunity and lymphoproliferation disease similar to human SLE and are considered a model of human SLE diseases [22, 23]. We therefore examined whether IL-39 is also expressed in vivo by activated B cells, the major cell type implicated in pathogenesis of SLE and the MRL/lpr model. We show here that in our lupus-like model, the MRL/lpr mice exhibit splenomegaly (Supporting Information Fig. 2A) and expansion of activated GL7+ B cells and CD138+ plasma cells (Supporting Information Fig. 2B, C). Although GL7 did not play a critical role in IL-39 expression in CD19-deficient B cells from CD19cre mice, IL-39 expressed mainly in GL7+ B cells activated in vitro by LPS (Supporting Information Fig. 2D). Furthermore, activated GL7+ B from lupus-like mice produced increased levels of IL-39 and the levels were significantly higher compared to CD138+ plasma cells (Fig. 3). In addition, compared to IL-39 subunits p19 and Ebi3, p28, p35, and p40 only slightly expressed in GL7+ B cells (Fig. 3B, C). Altogether, these results suggest that in vivo and in vitro activated B cells secrete IL-39.
Figure 3.
IL-39-expressing GL7+ B cells increased from lupus- like mice. (A) IL-39-expressing GL7+, CD138+, and CD138−GL7− B cells from 8-month-old female Balb/c, none lupus MRL/+ and lupus-like MRL/lpr mice were analyzed by FACS. (B, C) B cells from 8-month-old female none lupus MRL/+ and lupus-like MRL/lpr mice were analyzed by FACS. (B) The percentages of IL-39, p28, p35, and p40-expressing GL7+ B cells and (C) statistical analysis of the percentage are shown. (D) The absolute number of IL-39+GL7+ B cells per spleen from 8-month-old female nonlupus MRL/+ and lupus-like MRL/lpr mice are shown. (C and D) Data are shown as mean + SEM (n = 6) from one experiment representative of two other similar experiments. *p < 0.05, **p < 0.01 (two tailed Student’s t-test).
IL-39 induces inflammation in lupus-like mice
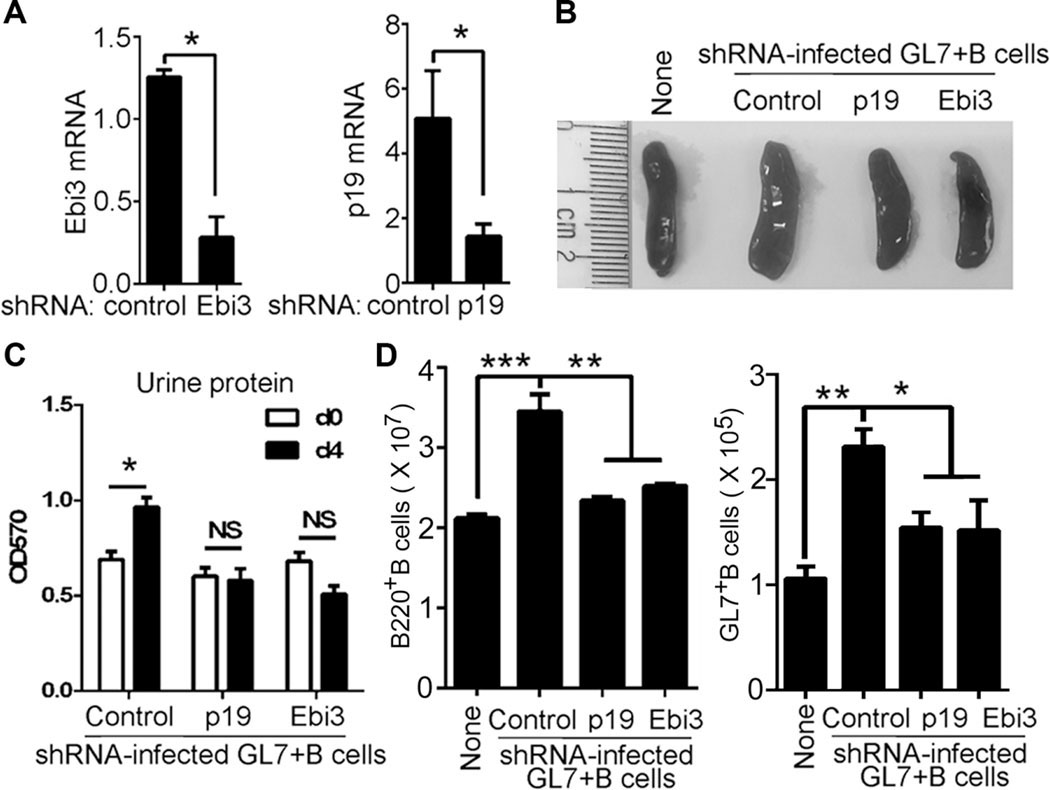
We next investigated potential role of IL-39 in lupus-like disease. We isolated and sorted GL7+ B cells from lupus-like mice and used shRNA to deplete p19, Ebi3, p19, p28, or p40 in the activated B cells (Fig. 4A, and Supporting Information Fig. 3A). We found that adoptive transfer of GL7+ B cells significantly enhanced the size of the spleen, the spleen weight, and proteinuria in lupus-like mice (Fig. 4B, and Supporting lnformation Fig. 3B–D), whereas p19 or Ebi3deficiency suppressed these effects mediated by GL7+ B cells (Fig. 4B, C, and Supporting Information Fig. 3B–D). Moreover, compared with mice that received GL7+ B cells depleted of p28, p35, or p40, mice that received p19- or Ebi3-deficient GL7+ B cells exhibited a phenotype characterized by much reduced spleen size and weight with less proteinuria (Supporting Information Fig. 3B–D).
Figure 4.
IL-39 induces inflammation in lupus-like Mice. (A) GL7+ B cells were sorted from 8-month-old female lupus-like MRL/lpr mice were sorted by FACS and infected with control, IL-39 subunits p19 or Ebi3-specific shRNA. On day 1 after infection, p19 and Ebi3 mRNA expression were analyzed by qPCR. (B–D) A 5 × 106 control, p19 or Ebi3-specific shRNA-infected GL7+ B220+ B cells per mouse were i.v. injected into 8-week-old female lupus-like MRL/lpr mice (six mice per group). Eight-week-old female lupus-like MRL/lpr mice into which either no cells, or transferred with control shRNA-infected GL7+ B cells, were used as no-cells transfer (None) and shRNA (Control) controls respectively. (B) On day 14 after cell transfer, spleens were harvested and photographed (representative of six spleens per experiments from three independent experiments). (C) Proteinuria was measured on day 0 and day 4 after cell transfer. (D) Absolute numbers of B220+ B and GL7+ B per spleen on days 7 after cell transfer. (A, C, and D) Data are shown as mean + SEM (n = 6) from one experiment representative of two other similar experiments. *p < 0.05, **p < 0.01 (two tailed Student’s t-test).
We also found that adoptive transfer of GL7+ B cells significantly enhanced GL7+ B-cell number in lupus-like mice (Fig. 4D, and Supporting Information Fig. 3E, F). On the other hand, we observed significant reductions in activated GL7+ B cells in mice that received Ebi3- or p19-depleted GL7+ B cells compared to controls or mice with adoptive transfer of p28-, p35-, or p40-deficient GL7+ B cells (Fig. 4D, and Supporting Information Fig. 3E, F). These results suggest that IL-39 might mediate inflammation in part by inducing the expansion of pathogenic B cells in lupus-like mice.
IL-39 signals through IL-23R/gp130 receptor and STAT1/STAT3 pathways
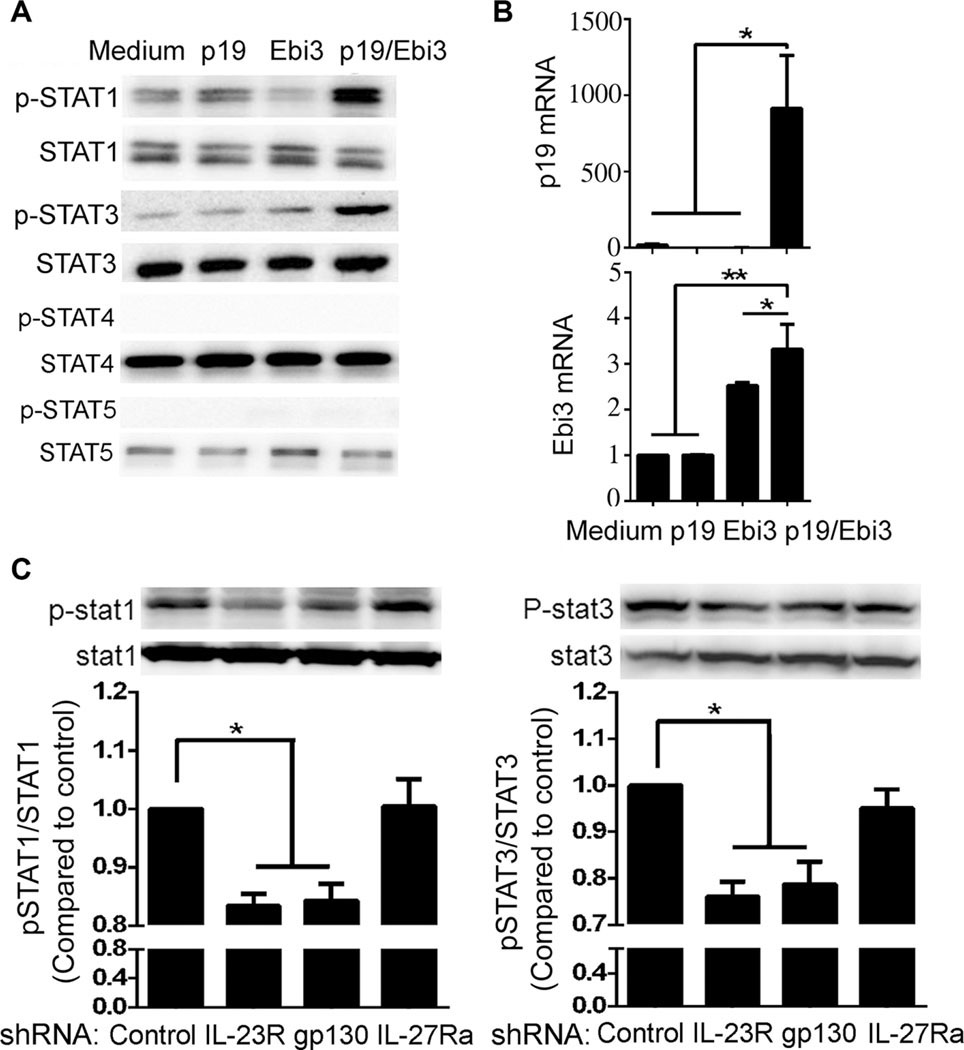
IL-12 family cytokines mediate their biological activities through activation of homodimeric or heterodimeric IL-12 cytokine receptor subunits (IL-12Rβ1, IL-12Rβ2, IL-23R, gp130, or IL-27Rα) and JAK/STAT signaling pathways [24]. Ebi3-related cytokines IL-27 and IL-35 activate STAT pathways in B cells [3, 25, 26] and so we examined whether IL-39 might mediate its effects through activation of STAT pathways. We first established that primary B cells express all IL-12 cytokine receptor subunits (Supporting Information Fig. 4A, B) and show here that IL-39 activated STAT1 and STAT3 but not STAT4 or STAT5(Fig. 5A). IL–35 has previously been shown to induce the expression of IL-35 subunits (p35 and Ebi3) in T cells [27] and B cells [3]. We therefore examined whether IL-39 could induce the expression of IL-39 subunits, p19 and Ebi3. It is therefore of note that IL-39 induced the expression of p19 and Ebi3 mRNA (Fig. 5B). Analysis of IL-23R-or gp130-deficient B cells further revealed that IL-39 mediates its biological activities through interactions with IL-23R/gp130 and activation of STAT1 and STAT3 pathways (Supporting Information Fig. 4C, Fig. 5C).
Figure 5.
IL-39 signals through IL-23R and gp130 receptor subunits and activates STAT1 and STAT3 pathways. (A) Primary B cells were sorted from 8-week-old female C57BL/6 mice by B220 microbeads, stimulated for 48 h with LPS, washed, and starved for 2 h in serum- free medium (0.5% BSA), followed by stimulation for 30 min with medium, p19, Ebi3, or IL-39. STAT activation was analyzed by Western blotting. Blots are representative of four independent experiments. (B) Primary B cells were sorted from 8-week-old female C57BL/6 mice by B220 microbeads, stimulated for 24 h with LPS in the presence of medium p19, Ebi3, or IL-39. IL-39 subunits (p19 and Ebi3) mRNA was determined using qPCR assay. Data are shown as mean + SEM (n = 8) from one experiment representative of two other similar experiments. (C) Control or IL-23R, IL-27Ra, gp130-specific shRNA-infected B cells described in Supporting Information Fig. 3C were stimulated for 2 days with LPS, washed, and starved for 2 h in serum-free medium (0.5% BSA), followed by stimulation for 30 min with IL-39. Cell lysates were separated by 8% SDS-PAGE and immunoblotted for phospho-Tyr701-STAT1 (pSTAT1) and total STAT1 (upper and left panel) or phosphor-Tyr705-STAT3(pSTAT3)andtotalSTAT3(upper and right panel). Band intensities of pSTAT1 and STAT1 or pSTAT3 and STAT3 were quantified by ImageProPlus 5.0 software. The density ratios of phosphorylated to total protein compared to control shRNA-infected group are shown as mean ± SEM (n = 3) of three independent experiments (lower panel). *p < 0.05, **p < 0.01 (two tailed Student’s t-test).
Discussion
A number of studies have indicated that the level of IL-12, IL-23, IL-27, and IL-35 is increased in the plasma of SLE patients compared with healthy controls [28–32]. Furthermore, IL-27 or IL-35 has also been shown to suppress lupus flare and nephritis in the MRL/lpr mice, suggesting that IL-27 and IL-35 may be therapeutically relevant in SLE [33, 34]. On the other hand, the role of other IL-12 members in pathogenesis of SLE is not well understood.
In this study, we have described a new IL-12 family heterodimeric cytokine (IL-39) composed of p19 and Ebi3. Identification of the IL-39 heterodimeric cytokine and demonstration that it is biologically active in vivo, suggest that additional IL-12 α- and β-subunit pairing may exist in nature [17]. Importantly, we show here that secretion of IL-39 (p19 and Ebi3) by activated B cells that mediate lupus-like diseases in MRL/lpr mice is significantly elevated compared to other IL-12 family cytokines, suggesting that that IL-39 may play important roles in the pathophysiology of SLE and other autoimmune diseases. Importantly, adoptive transfer of activated B cells depleted of p19 or Ebi3 into mice with lupus-like disease ameliorated hallmark features of SLE including the reduction of splenomegaly, pathogenic B cells and proteinuria (Fig. 4 and Supporting Information Fig. 3). In contrast, we observed a slight reduction in spleen weight of mice that received p28- or p35-deficient GL7+ B cells (Supporting Information Fig. 3C), underscoring the potential involvement of IL-39-producing B cells in the development of splenomegaly in lupus-like mice. However, in view of our data (Supporting Information Fig. 3D–F) showing that adoptive transfer of activated B cells depleted of p28, p35, or p40 also had minimal effects on pathogenic B cells and proteinuria, both of which are the hallmark features of SLE [21, 22, 35–38], it is still justifiable to conclude that the IL-39 subunits impact disease much more so than other cytokine components. Taken together, our data suggest that IL-39, secreted by activated B cells, may be an important proinflammatory cytokine and the potential therapeutic target in lupus diseases.
We found that other immunological cell types might also express IL-39. For example, BM-derived precursor dendritic cells (pDCs) and immature DCs (iDCs) express high levels of p19 and Ebi3 mRNAs and several factors of including SCF, IL-3, M-CSF, GM-CSF, FLT3 ligand, and IL-4 promoted their expression of p19, Ebi3 mRNA (Supporting Information Fig. 1). On the other hand, LPS appear to suppress p19 mRNA expression by mature DCs (mDCs), mature CD11c+ (cDC), or CD303+ plasmacytoid DCs (pDC) cells (Supporting Information Fig. 1). LPS also suppressed Ebi3 mRNA expression in a mouse macrophage line RAW264.7 cells (Supporting Information Fig. 1) consistent with the previous report that Ebi3 expression is decreased in LPS-induced M2 to M1 macrophage transformation [39]. These results suggest that the complex effects of LPS on p19 and Ebi3 expression in DCs, macrophages and B cells require additional studies.
In conclusion, we describe here a new IL-12 member IL-39 (IL-23p19/Ebi3) secreted by activated B cells that mediate inflammatory responses in lupus-like mice. Thus, IL-39 might contribute to immunopathogenic mechanisms of SLE.
Methods and materials
Mice
Seven-to-nine-week-old C57BL/6, Balb/c (Huafukang Corp., Beijing, China), Female lupus-like MRL/MpJ/lpr/lpr (MRL/lpr) mice and age-matched MRL/MpJ/+/+ (MRL/+) (Nanjing Biomedical Research Institute of Nanjing University, Nanjing, China) were bred in our animal facilities under specific pathogen-free conditions. Care, use, and treatment of mice in this study were in strict agreement with international guidelines for the care and use of laboratory animals. This study was approved by the Animal Ethics Committee of the Beijing Institute of Basic Medical Sciences.
Immunoprecipitation and Western blot analysis
A 5 ng/mL human IL-27/IL-35 Ebi3 subunit (Origene) and 5 ng/mL human IL-23 p19 subunit (Origene) were mixed in PBS. The human or mice Ebi3/p19 complex was immunoprecipitated with anti-human/mouse Ebi3, p40, p19, c-Jun (all from Santa Cruz Biotech) antibody that was precoupled to protein G–sepharose beads. Immunoprecipitates were resolved by SDS-PAGE, and blots were probed with anti-human/mouse Ebi3 and p19 antibody (Santa Cruz Biotech). In some experiments, anti-p-STAT1, p-STAT3, p-STAT4, p-STAT5, p-STAT6, STAT1, STAT3, STAT4, STAT5, STAT6, β-actin (Cell Signal Tech) were used. Preimmune serum (IgG) was used in parallel as a control, and signals were detected with horseradish peroxidase (HRP)-conjugated secondary F(ab’)2 (Zymed Labs) using the ECL system (Amersham).
Production and characterization of p19/Ebi3
The expression constructs pEZ-Lv122/mouse p19 and pReceiver-Lv18/mouse Ebi3 (GeneCopoeia) were transduced/cotransduced into CHO cells and stable transfectants were identified by drug selection (10 μg/mL Puromycin and Neomycin, Invitrogen). To ensure that the recombinant clone expressed p19/Ebi3, we isolated the expression vector and verified that no mutations were introduced during cloning or drug selection by DNA sequencing and that the construct was in the correct orientation. The recombinant protein(s) secreted by the CHO cells was sequentially purified by the Ni-NTA Purification system (Invitrogen), size-exclusion centricon filtration, and two consecutive cycles of FPLC gel filtration chromatography.
Cells were in vitro stimulated with LPS
Primary B cells from 8-weeks-old female C57BL/6 mice were sorted by B220 microbeads, and stimulated for 1, 2, 3 days in RPMI 1640 medium containing 10% FBS, 2 mM glutamine, penicillin (100 IU/mL), streptomycin (100 μg/mL), and 50 mM 2-mercapthoethanol with 1 μg/mL LPS (Sigma L2630 from Escherichia Coli 0111:B4) in the presence of 100 ng/mL p19, Ebi3, or p19/Ebi3.
Cytometric analysis and intracellular cytokine staining
Cells (1 × 106 cells/sample) were washed with fluorescence-activated cell sorting staining buffer (phosphate-buffered saline, 2% fetal bovine serum or 1% bovine serum albumin, 0.1% sodium azide). All samples were incubated with anti-Fc receptor Ab (BD Biosciences), prior to incubation with other Abs diluted in fluorescence-activated cell sorting buffer supplemented with 2% anti-Fc receptor Ab. For intracellular cytokine staining, 50 ng/mL PMA and 1 μg/mL ionomycin (Sigma-Aldrich) were added and then 10 μg/mL brefeldin A and 2 μM monensin were added 3 h later. After 3 h, cells were collected and fixed for 50 min with 1 mL fixation buffer (eBioscience). After washing, the fixed cells were stained. The samples were filtered immediately before analysis or cell sorting to remove any clumps. The following antibodies were purchased from eBioscience: fluorescence-conjugated anti-mouse p19,p28,p35,p40,Ebi3, IL-12Rβ1, IL-12Rβ2, IL-23R, IL-27Ra, gp130, B220, GL7 antibodies. Data collection and analyses were performed on a FACS Calibur flow cytometer using CellQuest software.
GL7+ B220+ cell sorting
B cells from the spleen of lupus-like mice were sorted using B220 microbeads and stimulated for 3 days with 1 μg/mL LPS. Multicolor flow cytometry was performed by gating on GL7 and B220 on the surface of activated B cells and used to directly sort GL7+ B220+ cells. All flow cytometry data were acquired with FACSCanto, FACSCantoII, or FACSAria (BD Biosciences), gated on live lymphocyte-sized cells on the basis of forward and side scatter, and analyzed using FlowJo software (Tree Star, Ashland, OR).
Control, p19, Ebi3, p28, p35, and p40-specific shRNA-infected GL7+ B220+ B cells were transferred into lupus-like mice
GL7+ B220+ cells described as above were infected with control, p19, Ebi3, p28, p35, and p40 (Santa Cruz Biotech)-specific shRNA. A total of 5 × 106 control, p19, Ebi3, p28, p35, and p40-specific shRNA-transfected GL7+ B220+ B cells per mouse were i.v. injected into 8-weeks-old female lupus-like MRL/lpr mice.
P19/Ebi3 concentration analysis by ELISA
The concentration of P19/Ebi3 was measured by ELISA. Briefly, for detection of p19/Ebi3, anti-p19, or anti-Ebi3 antibody (Santa Cruz Biotech) was coated in triplicate to the plate for overnight at 4°C and diluted supernatant were added to the plate for 1 h at 37°C; Then after washing, 4 μg/mL biotin rat anti-mouse Ebi3 or p19 antibody were added to the plate, and were incubated for another hour at 37°C. Thereafter, unbinding antibodies were washed off, followed by addition of avidin-HRP (1/1000 diluted) (eBiosciece). Plates were incubated for 1 h at 37°C. Finally, the color was developed by incubation with o-phenylenediamine. The OD was read at 492 nm with an ELISA reader (Bio-Rad). Standard curves were established to quantitate the amounts of the respective cytokines.
qPCR analysis
All RNA samples were DNA free. Each gene-specific primer pair used for qPCR analysis spanned at least an intron. Primers and probes (Supporting Information Table I) used for qPCR were purchased from Applied Biosystems and mRNA expression was normalized to the levels of β-Actin gene. Sequences of primer pairs are available upon request.
Assessment of proteinuria
Urine was manually expressed from each mouse on a weekly basis, collected into a sterile container and assayed for the presence of protein (specifically albumin) using a colorimetric method (Albustix Reagent Strips, Bayer Corporation, Elkhart, IN).
Statistics
Statistics were generated using t-test in GraphPad Prism (version 5.0, GraphPad Software Inc., USA) and values are represented as mean ± SEM. Results were considered statistically significant at p < 0.05.
Supplementary Material
Acknowledgments:
This study was supported by National Basic Research Program 973 Grants (2013CB530506, 2015CB553704), National Nature and Science Fund (81471529, 81401332, 81272320, 81471540, and 81472647), the Key Program of the Beijing Natural Science Foundation (7141007), and Service Industry Scientific Research of National Health and Family Planning Commission of China (2015SQ00192). Charles E. Egwuagu was supported by the Division of Intramural Research National Eye Institute, National Institutes of Health (EY000262-19 & EY000372-14).
Abbreviations:
- FACS
flow cytometry
- IP
mmunoprecipitation
- SLE
systemic lupus erythematosus
Footnotes
Conflict of interest: The authors declare no commercial or financial conflict of interest.
References
- 1.Sun L, He C, Nair L, Yeung J. and Egwuagu CE, Interleukin 12 (IL-12) family cytokines: role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015. 75: 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen P,Roch T,Lampropoulou V,O’Connor RA,Stervbo U,Hilgenberg E, Ries S. et al. , IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014. 507: 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT et al. , Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med 2014. 20: 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F. et al. , Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000. 13: 715–725. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W. et al. , Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest 2006. 116: 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD et al. , Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med 2003. 198: 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L. et al. , Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003. 421: 744–748. [DOI] [PubMed] [Google Scholar]
- 8.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP et al. , Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med 2008. 205: 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N. et al. , Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 2006. 25: 309–318. [DOI] [PubMed] [Google Scholar]
- 10.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA et al. , IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest 2006. 116: 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng MWL, Bowman EP, McElwee JJ, Smyth MJ, Casanova J, Cooper AM and Cua DJ, IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med 2015. 21: 719–729. [DOI] [PubMed] [Google Scholar]
- 12.Egwuagu CE, Yu CR, Sun L. and Wang R, Interleukin 35: critical regulator of immunity and lymphocyte-mediated diseases. Cytokine Growth F. R 2015. 26: 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida H. and Hunter CA, The immunobiology of interleukin-27. Annu. Rev. Immunol 2015. 33: 417–443. [DOI] [PubMed] [Google Scholar]
- 14.Vignali DA and Kuchroo VK, IL-12 family cytokines: immunological playmakers. Nat. Immunol 2012. 13: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kastelein RA,Hunter CA andCua,D.J.,DiscoveryandbiologyofIL-23 and IL-27: related but functionally distinct regulatorys of inflammation. Annu. Rev. Immunol 2007. 25: 242. [DOI] [PubMed] [Google Scholar]
- 16.Flores RR, Kim E, Zhou L, Yang C, Zhao J, Gambotto A. and Robbins PD, IL-Y, a synthetic member of the IL-12 cytokine family, suppresses the development of type 1 diabetes in NOD mice. Eur. J. Immunol 2015. 45: 3114–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang RX, Yu CR, Mahdi RM and Egwuagu CE, Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J. Biol. Chem 2012. 287: 36012–36021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devergne O, Birkenbach M. and Kieff E, Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc. Natl. Acad. Sci. USA 1997. 94: 12041–12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco P, Ueno H. and Schmitt N, T follicular helper (Tfh) cells in lupus: activation and involvement in SLE pathogenesis. Eur. J. Immunol 2016. 46: 281–290. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Liao J, Zhao M, Wu H, Yung S, Chan TM, Yoshimura A. et al. , Increased expression of TLR2 in CD4 (+) T cells from SLE patients enhances immune reactivity and promotes IL-17 expression through histone modifications. Eur. J. Immunol 2015. 45: 2683–2693. [DOI] [PubMed] [Google Scholar]
- 21.Vincent FB, Morand EF, Schneider P. and Mackay F, The BAFF/APRIL system in SLE pathogenesis. Nat. Rev. Rheumatol 2014. 10: 365–373. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa F, Animal models of cutaneous lupus erythematosus and lupus erythematosus photosensitivity. Lupus 1997. 6: 193–202. [DOI] [PubMed] [Google Scholar]
- 23.Cohen PL and Eisenberg RA, Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol 1991. 9: 243–269. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G, Pflanz S. and Kastelein RA, The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 2003. 19: 641–644. [DOI] [PubMed] [Google Scholar]
- 25.Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA and Devergne O, Differential effects of IL-27 on human B cell subsets. J. Immunol 2006. 176: 5890–5897. [DOI] [PubMed] [Google Scholar]
- 26.Charlot-Rabiega P, Bardel E, Dietrich C, Kastelein R. and Devergne O, Signaling events involved in interleukin 27 (IL-27)-induced proliferation of human naive CD4+ T cells and B cells. J. Biol. Chem 2011. 286: 27350–27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR et al. , The composition and signaling of the IL-35 receptor are unconventional. Nat. Immunol 2012. 13: 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du J, Li Z, Shi J. and Bi L, Associations between serum interleukin-23 levels and clinical characteristics in patients with systemic lupus erythematosus. J. Int. Med. Res 2014. 42: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 29.Xia LP, Li BF, Shen H. and Lu J, Interleukin-27 and interleukin-23 in patients with systemic lupus erythematosus: possible role in lupus nephritis. Scand. J. Rheumatol 2015. 44: 200–205. [DOI] [PubMed] [Google Scholar]
- 30.Mok MY., Wu HJ., Lo Y. and Lau CS., The relation of interleukin 17 (IL-17) and IL-23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus. J. Rheumatol 2010. 37: 2046–2052. [DOI] [PubMed] [Google Scholar]
- 31.Qiu F, Song L, Yang N. and Li X, Glucocorticoid downregulates expression of IL-12 family cytokines in systemic lupus erythematosus patients. Lupus 2013. 22: 1011–1016. [DOI] [PubMed] [Google Scholar]
- 32.Smith S, Gabhann JN, Higgs R, Stacey K, Wahren-Herlenius M, Espinosa A, Totaro MG et al. , Enhanced interferon regulatory factor 3 binding to the interleukin-23p19 promoter correlates with enhanced interleukin-23 expression in systemic lupus erythematosus. Arthritis Rheum. 2012. 64: 1601–1609. [DOI] [PubMed] [Google Scholar]
- 33.Pan HF, Tao JH and Ye DQ, Therapeutic potential of IL-27 in systemic lupus erythematosus. Expert. Opin. Ther. Targets 2010. 14: 479–484. [DOI] [PubMed] [Google Scholar]
- 34.Cai Z, Wong CK, Dong J, Chu M, Jiao D, Kam NW, Lam CW et al. , Remission of systemic lupus erythematosus disease activity with regulatory cytokine interleukin (IL)-35 in Murphy Roths Large (MRL)/lpr mice. Clin. Exp. Immunol 2015. 181: 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing C, Ma N, Xiao H, Wang X, Zheng M, Han G, Chen G. et al. , Critical role for thymic CD19+CD5+CD1dhiIL-10+ regulatory B cells in immune homeostasis. J. Leukocyte Biol 2015. 97: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma N, Liu X, Xing C, Wang X, Wei Y, Han G, Chen G. et al. , Ligation of metabotropic glutamate receptor 3 (Grm3) ameliorates lupus-like disease by reducing B cells. Clin. Immunol 2015. 160: 142–154. [DOI] [PubMed] [Google Scholar]
- 37.Ma N, Xing C, Xiao H, He Y, Han G, Chen G, Hou C. et al. , BAFF suppresses IL-15 expression in B cells. J. Immunol 2014. 192: 4192–4201. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Wei Y, Xiao H, Liu X, Zhang Yu, Han G, Chen G. et al. , Pre-existing CD19-independent GL7- Breg cells are expanded during inflammation and in mice with lupus-like disease. Mol. Immunol 2016. 71: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng XF, Hong YX, Feng GJ, Zhang GF, Rogers H, Lewis MA, Williams DW et al. ,Lipopolysaccharide-inducedM2toM1macrophage transformation for IL-12p70 production is blocked by Candida albicans mediated up-regulation of EBI3 expression. PLoS One 2013. 8: e63967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.