Abstract
Ethnopharmacological relevance
Aster tataricus L.f., an extensively used herb in traditional Chinese medicine for more than 2000 years, is known as “Zi wan” or “Fan huncao”. Its dried root and rhizome hold great promise in the treatment of cough, asthma, tumor, inflammation, etc.
Aim of the study: This literature review summarizes the morphology characteristics, ethnopharmacological use, phytochemical properties, pharmacological effects, and potential applications of Aster tataricus. Furthermore, this review will discuss the future research trends and development prospects of this plant.
Materials and methods
Using “Aster tataricus L.f.”, “Traditional medicinal usage”, “Phytochemistry”, “Pharmacological effects” as the keywords and gathered relevant data on Aster tataricus L.f. using electronic databases (Elsevier, PubMed, ACS, CNKI, Google Scholar, Baidu Scholar, Web of Science), relevant books, and classic literature about Chinese herb.
Result
A total of 186 compounds have been isolated and identified from Aster tataricus, including terpenes, organic acids, peptides, and flavonoids. And Aster tataricus has been widely used as a natural cough suppressant and has anti-oxidative, anti-inflammatory, anti-depressive, and anti-tumor effects. In addition, Aster tataricus has also been reported to have damaging effects on the liver as well as other toxicities were discussed in this review.
Conclusions
Aster tataricus is an ancient herbal medicine with a broad spectrum of pharmaco logical activities that has been used for thousands of years in China, and has shown remarkable effectiveness in the treatment of various diseases, especially cough, asthma, inflammation. Although its rich chemical constituents have various pharmacological activities, the underlying mechanisms, as well as its toxicity and safety, remains unclear and warrant further investigation.
Keywords: Aster tataricus L.f., Morphology, Ethnopharmacology, Phytochemistry, Pharmacological activity
Highlights
-
•
This review summarized the botanical characteristics, chemical composition, and therapeutic effects of Aster tataricus based on traditional literature and modern evidence.
-
•
We reviewed domestic and international studies on the chemical composition of aster and summarized nearly 200 of its chemical constituents, and
-
•
provided a scientific basis for further research and exploitation of medicinal plants to develop more effective therapeutic drugs.
Abbreviations
- TCM
traditional Chinese medicine
- UHPLC-Q-TOF-MS
Ultra-high performance liquid chromatography coupled with triple quadrupole mass spectrometry
- HPLC-Q-TOF/MS
High-performance liquid chromatography quadrupole time-of-flight mass spectrometry
- HPLC-MS
High performance liquid chromatography-mass spectrometry
- Ovalbumin
OVA
- 1,1-Diphenyl-2-picrylhydrazyl radical
DPPH Median lethal dose: LD50
1. Introduction
Aster tataricus L. f. (Aster tataricus), also named Qing wan, Fan huncao, and Guan gongxu, is a perennial herb in the Asteraceae family, and it is widely cultivated in wetlands on shady slopes of low mountains, hilltops, low-mountain grasslands, and marshes at an altitude of 400–2000 m [1]. The medicinal use of its dried root and rhizome was first reported in Shennong Materia Medica [[2], [3], [4]]. Chinese Materia Medica states that Aster tataricus is widely distributed in Anhui, Hebei, Neimenggu, northeast China, Korea, and Japan.
Aster tataricus has been used in traditional Chinese medicine (TCM) for more than 2000 years. It is acrid and bitter in flavor, warm, and acts on the lung channels. It has remarkable therapeutic effects, especially cough relief and phlegm elimination [5]. According to folklore, Aster tataricus is usually brewed or decocted and administered orally to treat cough and asthma (Runfeizhike and Huatan) [6,7]. The active ingredients of medicinal plants are the material basis for the treatment and prevention of diseases and may guide the development of new and more effective therapeutic drugs. Phytochemical studies have shown that Aster tataricus is rich in various ingredients, encompassing terpenes, flavonoids, peptides, organic acids, and other compounds [[8], [9], [10], [11]]. With increasing interest in research on the pharmacological activities of Aster tataricus, researchers have found it have potent pharmacological activities, such as anti-tussive, anti-asthmatic, anti-tumor, anti-inflammatory, anti-bacterial, anti-oxidant, and anti-depressant activities [5,[12], [13], [14]]. To date, Aster tataricus has been widely used in various proprietary Chinese medications. This review summarizes the morphology characteristics, phytochemical composition, pharmacological effects, and medicinal applications of Aster tataricus, providing a reference for further research and development.
2. Botany
Aster tataricus, a member of the Asteraceae family, is distributed worldwide, especially in China, Korea, and Japan [15]. It was first reported in the Han Dynasty, and its name has been changed over time (Table 1). Aster tataricus is usually harvested in spring and autumn. Subsequently, the rhizomes (commonly called “Mugen”) and sediments are removed, and the roots are dried in the sun, either braided or unbraided.
Table 1.
Names of Aster tataricus in different literary works.
| Dynasty | Title | Name |
|---|---|---|
| Wei and Jin dynasties | Wupu Bencao | Qing wan |
| Records of Famous Doctors | Zi qian, Qing wan | |
| Southern Dynasty | Notes on the Materia Medica | Zi wan, Qing wan |
| Tang Dynasty | Xin Xiu Ben Cao | Zi qian, Qing wan |
| Qianjin Yifang | Zi qian, Qing wan | |
| Song Dynasty | Dou Men Fang | Fan huncao, Ye qianniu |
| Qing Dynasty | Ben Cao Shu | Zi wanrong |
| Compendium of Materia Medica | Qing wan, Zi wan, Fan huncao, Ye qianniu | |
| Textual Research on Reality and Titles of Plants | Guan gongxu |
According to Chinese Pharmacopoeia (2020 version), the rhizome of Aster tataricus exists as irregular lumps of varying sizes, with the stem and leaf stumps at the top, and its texture is slightly hard. According to Illustrations of Chinese Medicinal Plants and Colored Atlas of Chinese Folk Herbs, the stems of Aster tataricus (Fig. 1) grow erect to a height of approximately 1.5–2 m, are slightly branched at the upper end, and have sparsely distributed short bristles. Roots and foliage lush, withered at flowering time, and the leaves are long, elliptical, and obtuse-headed and sparsely setulose on both surfaces, with the base tapered or winged. The stalk is slender, and the margin is serrated. Capitula on branchlets with a dozen or more flowers are usually cephalic, and the stems are 2.5–3.3-cm long. The pedicels are long and densely setose. The involucre is hemispherical, with a length of 7 mm. The bracteolates are lanceolate, short, and slightly hairy and are arranged in whorls of three, with scarious margins. The corolla is purple, with a length of 1.6–1.7 cm and a width of 0.3–0.35 cm. The corolla tube is cylindrical with a yellow inner surface. The fruit is small and slightly compressed, with a length of 3 mm, and has white crown hair.
Fig. 1.
Plant morphology of Aster tataricus.
3. Ethnopharmacology
Since ancient times, scholars have been attempting to maximize the use of natural resources. The use of TCM in preventing and treating diseases has remarkably improved human health. Since the first mention of Aster tataricus in “Shen Nong Ben Cao Jing’’, its therapeutic effects on cough and asthma have been reported in various medical books. According to “Ming Yi Bie Lu”, Aster tataricus can be used to treat cough with blood in phlegm. Similar therapeutic effects of Aster tataricus have been mentioned in “Xin Xiu Ben Cao”, “Qian Jin Yi Fang”, “Zheng Lei Ben Cao”, and “Yao Jian”. Some traditional medical books, such as “Bei Ji Qian Jin Yao Fang”, “Ben Cao Cong Xin”, and “Zhong Hua Ben Cao”, have mentioned that Aster tataricus can be used to treat dyspepsia; however, this information is not found in modern books. In addition, “Dou Men Fang”, “Ben Cao Shu”, and “De Pei Ben Cao” have documented the use of Aster tataricus in the treatment of sore throats. The effects and contraindications of Aster tataricus reported in different literary works in different time periods are summarized in Table 2.
Table 2.
Ethnopharmacology of Aster tataricus throughout the Chinese dynasties.
| Dynasty | Efficacy | Contraindications | Title |
|---|---|---|---|
| Qin and Han dynasties | Relieves cough, increases Qi, regulates cold and heat in the chest, removes parasites and toxins, and tranquilizes the five viscera | Shen Nong Ben Cao Jing | |
| Northern and Southern dynasties | Treats pulmonary abscess, weakness caused by the “five Lao”, and pediatric convulsions | Not to be combined with Tian Xiong, Qu Mai, Lei Puan, Yuan Zhi, Fen Yin, and herba artemisias capillaris | Ming Yi Bie Lu |
| Tang dynasty | Relieves cough; increases Qi; regulates cold and heat in the chest; removes parasites and toxins; tranquilizes the five viscera; and treats pulmonary abscess, weakness caused by the “five Lao”, and pediatric convulsions | Not to be combined with Tian Xiong, Qu Mai, Lei Puan, Yuan Zhi, Gao ben, Fen Yin, and herba artemisias capillaris | Xin Xiu Ben Cao |
| Song dynasty | Shi Zhu and fever due to deficiency | Not to be combined with Tian Xiong, Qu Mai, Lei Puan, Yuan Zhi, Gao ben, Fen Yin, and Artemisia Chen | Jia You Ben Cao |
| Ming dynasty | Treats shortness of breath and cough | Not to be combined with Tian Xiong, Qu Mai, Lei Puan, Yuan Zhi, Gao ben, Fen Yin, and herba artemisias capillaris | Bencao Pinhui Jingyao |
| Qing dynasty | Protects the lungs and treats vomiting blood and cough with blood in phlegm | An appropriate amount to be used in patients with yin deficiency and lung-heat syndrome | Ben Cao Hai Li |
| Regulates cold and heat in the chest, relieves cough, increases Qi, and treats bloody sputum and pediatric convulsions | Not to be combined with Tian Xiong, Qu Mai, Yuan Zhi, Gao ben, and herba artemisias capillaris | Ben Cao Bei Yao | |
| Relieves cough due to exhaustion and treats hematochezia | Not to be combined with Tian Xiong, Qu Mai, Yuan Zhi, Gao ben, and herba artemisias capillaris | Ben Cao Qiu Zhen | |
| 1959 | Regulates cold and heat in the chest and relieves cough, vomiting blood, breathlessness, and pharyngitis | Not suitable for patients with heat in the lungs | Zhong Yao Zhi |
| 1999 | Reduces phlegm, suppresses cough, and treats bacterial infections | Saponins derived from Aster tataricus have strong hemolytic effects and their crude forms are not suitable for intravenous injection; the volatile oil of Aster tataricus is more toxic than that of Ligularia fischeri | Chinese Materia Medica |
| 2020 | Relieves cough with excessive phlegm and wheezing, chronic cough, and coughing up blood with exertion | Chinese pharmacopoeia |
To enhance its therapeutic efficacy and reduce its side effects, Aster tataricus was traditionally processed using refined honey, vinegar and wine, processing children's feces and ginger. However, modern processing methods mainly involve the use of honey and bran. The standard of pharmaceutical concoctions varies across regions. The main purpose of processing Aster tataricus is to reduce its coldness and enhance its therapeutic effects. Aster tataricus can clear heat and phlegm, thereby relieving cough. When consumed raw, it can Qingfei and has the effect of clearing heat and resolving phlegm, but it is only suitable for symptoms of obstructed lung qi and coughing up a lot of phlegm. Roasting Aster tataricus with honey increases its sweetness and enhances its effectiveness in moistening the lungs and relieving cough [2].
Aster tataricus can be used as not only a medication but also an ingredient in food products within a limited dose range. In particular, it has been used in tea, porridge, and soup. Ancient medical records show that Aster tataricus can be combined with other traditional Chinese herbs to treat asthma, cough, constipation, impotence, and carbuncles [16]. Shegan Mahuang Soup, which comprises 41.4-g Aster tataricus, Ephedra, and Belamcanda chinensis, is used to relieve phlegm, reduce cold, and treat lung and throat infections. Ze Qi Soup, which comprises 69-g Aster tataricus and Euphorbia helioscopia, is mainly used to treat occasional wheezing and coughing, body swelling, and restlessness. Bu Fei Soup, which comprises 3.1-g Aster tataricus and Sangbaipi, is used to treat cough and asthma due to lung deficiency. Zi Wan Soup, which comprises 13.8-g Aster tataricus, is effective in relieving upper airway obstruction [11].
With a long history of medicinal use, Aster tataricus has demonstrated good efficacy in the treatment of many complicated and recurrent diseases. It is a widely used source of natural bioactive components in TCM. In-depth research into the pharmacological effects of Aster tataricus is ongoing.
4. Phytochemistry
With the development of extraction and separation methods, scholars have identified several chemical constituents of Aster tataricus. To date, approximately 200 chemical compounds, including 73 terpenes, 34 flavonoids, 26 organic acids, 21 peptides, and 32 other compounds, have been isolated and identified from Aster tataricus, with terpenes being the main active components. The proportions of all chemical constituents are shown in Fig. 2, and their specific details are summarized in Table 3.
Fig. 2.
Proportion of each chemical constituent in Aster tataricus.
Table 3.
Specific details of the chemical constituents isolated from Aster tataricus.
| No. | Compounds | MF | Resource | Extraction methods | References |
|---|---|---|---|---|---|
| Terpenes (1–73) | |||||
| 1 | Shion-22-methoxy-20(21)-en-3-one | C31H52O2 | Roots and rhizomes | 95 % EtOH | [17] |
| 2 | Shion-22(30)-en-3,2,1-dione | C30H48O2 | Roots and rhizomes | 95 % EtOH | [17] |
| 3 | Shion-21-hydroxyl-22(30)-en-3-one | C30H50O2 | Roots and rhizomes | 95 % EtOH | [17] |
| 4 | Shion-22-methoxy-20(21)-en-3-ol | C31H54O2 | Roots and rhizomes | 95 % EtOH | [17] |
| 5 | Shione | C30H50O | Roots and rhizomes | 95 % EtOH | [17] |
| 6 | Epishionol | C30H52O | Roots and rhizomes | 95 % EtOH | [17] |
| 7 | Friedelane | C30H52 | Roots and rhizomes | 95 % EtOH | [17] |
| 8 | Epifriedelanol | C30H52O | Roots and rhizomes | 95 % EtOH | [17] |
| 9 | 24-Ethyl-5a-cholesta-7,22(E)-dien-3-one | C34H56O | Roots and rhizomes | 95 % EtOH | [17] |
| 10 | Xylonenone | C30H50O | Whole plant | MeOH | [18] |
| 11 | Friedelan-4-α-methyl-3β-OH | C30H52O | Whole plant | MeOH | [18] |
| 12 | β-Sitosterol | C29H50O | Whole plant | MeOH | [18] |
| 13 | Stigmasterol | C29H48O | Whole plant | MeOH | [18] |
| 14 | Campesterol | C28H48O | Whole plant | MeOH | [18] |
| 15 | Epifriedelanol | C29H50O | Roots and rhizomes | 95 % EtOH | [19] |
| 16 | α-Spinach sterols | C29H48O | Roots and rhizomes | 95 % EtOH | [19] |
| 17 | Betulin | C27H46O2 | Roots and rhizomes | 95 % EtOH | [19] |
| 18 | Oleanolic acid | C30H48O3 | Roots and rhizomes | 95 % EtOH | [19] |
| 19 | Shionoside A | C21H36O10 | Roots | MeOH | [20] |
| 20 | Shionoside B | C22H38O10 | Roots | MeOH | [20] |
| 21 | Epifriedelinol | C30H52O | Roots | MeOH | [20] |
| 22 | Astertarone A | C30H50O | Roots | MeOH | [21] |
| 23 | Shionone | C30H50O | Roots and rhizomes | MeOH | [21] |
| 24 | Friedelin | C30H50O | Roots and rhizomes | MeOH | [21] |
| 25 | Astertarone B | C31H52O2 | Roots | MeOH | [21] |
| 26 | Oleanic acid | C31H50O2 | Roots and rhizomes | Methanol | [22] |
| 27 | Taraxerol | C30H48O | Roots and rhizomes | Methanol | [22] |
| 28 | Betulin | C30H52O2 | Roots and rhizomes | Methanol | [22] |
| 29 | Taraxasterol | C30H50O | Roots and rhizomes | Methanol | [22] |
| 30 | Beta-amyrin | C30H50O | Roots and rhizomes | Methanol | [22] |
| 31 | Aster shionone A | C26H42O3 | Roots and rhizomes | Methanol | [23] |
| 32 | Aster shionone B | C29H46O2 | Roots and rhizomes | Methanol | [23] |
| 33 | Aster shionone C | C27H44O3 | Roots and rhizomes | Methanol | [23] |
| 34 | Aster shionone D | C27H46O | Roots and rhizomes | Methanol | [23] |
| 35 | Aster shionone E | C27H42O3 | Roots and rhizomes | Methanol | [23] |
| 36 | Aster shionone F | C27H42O2 | Roots and rhizomes | Methanol | [23] |
| 37 | Friedelan-3-ol | C30H52O2 | Roots | MeOH | [24] |
| 38 | Echinocystic acid | C30H48O4 | Roots and rhizomes | Methanol | [22] |
| 39 | Betulinic acid | C30H48O3 | Roots and rhizomes | Methanol | [22] |
| 40 | 2,3,24-Trihydroxyolean-12-en-28-oic acid | C30H48O5 | Roots and rhizomes | Methanol | [22] |
| 41 | 23-Hydroxybetulinic acid | C31H50O4 | Roots and rhizomes | Methanol | [22] |
| 42 | Shion-22-methoxy-20(21)-en-3-one | C31H52O2 | Rhizomes | Methanol | [23] |
| 43 | Shion-22-methoxy-20(21)-en-3β-ol | C31H54O2 | Rhizomes | Methanol | [23] |
| 44 | Shion-22(30)-en-3,21-dione | C29H46O2 | Rhizomes | Methanol | [23] |
| 45 | 15-Hydroxydehydroab ietic acid | C20H28O3 | Roots | MeOH | [25] |
| 46 | 7β-Hydroxydehydroabietie acid | C21H30O2 | Roots | MeOH | [25] |
| 47 | Junicedric acid | C21H34O4 | Roots | MeOH | [25] |
| 48 | (13S)-15-hydroxylubd-8(17)-en-19-oic acid | C20H34O3 | Roots | MeOH | [25] |
| 49 | (11S)-1β-hydroxyeudesm-4(14)-eno-13,6α-lactone | C15H22O3 | Roots | MeOH | [25] |
| 50 | Aster saponin G2 | C57H92O25 | Underground parts | Methanol | [26] |
| 51 | Aster saponin H | C46H74O18 | Underground parts | Methanol | [26] |
| 52 | 3-O-α-l-arabinopyranosyl-(1 → 6)-β-D-trihydroxyolean-12-en-28-oic acid | C41H66O14 | Underground parts | Methanol | [26] |
| 53 | Aster saponin G | C57H92O26 | Underground parts | Methanol | [26] |
| 54 | Aster saponin C2 | C73H118O37 | Underground parts | Methanol | [26] |
| 55 | Aster saponin A2 | C67H108O33 | Underground parts | Methanol | [26] |
| 56 | Aster lingulatoside D | C27H46O | Whole plant | – | [27] |
| 57 | Aster lingulatoside C | C27H44O3 | Whole plant | – | [27] |
| 58 | Aster lingulatoside B | C29H46O2 | Whole plant | 70 % EtOH | [28] |
| 59 | Aster lingulatoside A | C26H42O3 | Whole plant | 70 % EtOH | [28] |
| 60 | Aster saponin A | C68H110O33 | Roots | MeOH | [20] |
| 61 | Aster saponin B | C62H100O29 | Roots | MeOH | [20] |
| 62 | Aster saponin C | C74H120O37 | Roots | MeOH | [20] |
| 63 | Aster saponin D | C73H188O36 | Roots | MeOH | [20] |
| 64 | Aster batanoside B | C44H72O15 | Roots | 70 % EtOH | [29] |
| 65 | Aster batanoside C | C44H70O15 | Roots | 70 % EtOH | [29] |
| 66 | Aster saponin Hb | C42H66O13 | Aboveground parts | MeOH | [30] |
| 67 | Aster saponin E | C63H109O29 | Roots | MeOH | [20] |
| 68 | Aster saponin F | C63H108O28 | Roots | MeOH | [20] |
| 69 | Aster saponin Ha | C38H58O13 | Aboveground parts | MeOH | [20] |
| 70 | Aster saponin Hc | C58H92O25 | Aboveground parts | MeOH | [20] |
| 71 | Aster saponin Hd | C64H102O26 | Aboveground parts | MeOH | [20] |
| 72 | Aster batanoside F | C56H90O24 | Roots | MeOH | [20] |
| 73 | Foetidissimoside A | C56H90O18 | Aboveground parts | 70 % EtOH | [31] |
| Flavonoids (74–107) | |||||
| 74 | Kaempferol | C15H10O6 | Whole plant | MeOH | [18] |
| 75 | Apigenin-7-O-β-D-glucuronide | C22H20O10 | Whole plant | MeOH | [18] |
| 76 | Kaempferol-3-O-α-l-rhamnoside | C15H16O10 | Whole plant | MeOH | [18] |
| 77 | Kaempferol-7-O-α-l-rhamnopyranoside | C21H20O10 | Whole plant | MeOH | [18] |
| 78 | Quercetin | C15H12O7 | Roots and rhizomes | Acetone | [32] |
| 79 | Dihydromyricetin | C15H12O8 | Roots and rhizomes | Methanol | [22] |
| 80 | Myrictrin | C15H10O8 | Roots and rhizomes | Methanol | [22] |
| 81 | Myricetin | C15H10O8 | Roots and rhizomes | Methanol | [22] |
| 82 | Liquiritigenin | C15H12O4 | Roots and rhizomes | Methanol | [22] |
| 83 | Luteolin | C15H10O6 | Roots and rhizomes | Methanol | [22] |
| 84 | Naringenin | C15H12O5 | Roots and rhizomes | Methanol | [22] |
| 85 | Genistein | C15H10O5 | Roots and rhizomes | Methanol | [22] |
| 86 | Acacetin | C16H12O5 | Roots and rhizomes | Methanol | [22] |
| 87 | Genkwanin | C16H12O5 | Roots and rhizomes | Methanol | [22] |
| 88 | Apigenin | C15H10O5 | Roots and rhizomes | Methanol | [22] |
| 89 | Diosmetin | C16H14O6 | Roots and rhizomes | Methanol | [22] |
| 90 | Isorhamnetin | C16H14O7 | Roots and rhizomes | Methanol | [22] |
| 91 | Baicalein | C15H10O5 | Roots and rhizomes | Methanol | [22] |
| 92 | Wogonin | C16H12O5 | Roots and rhizomes | Methanol | [22] |
| 93 | Biorobin | C27H30O15 | Roots and rhizomes | Methanol | [22] |
| 94 | Baicalin | C21H18O11 | Roots and rhizomes | Methanol | [22] |
| 95 | Kaempferol-7-O-β-d-glucopyranoside | C21H20O11 | Roots and rhizomes | Methanol | [22] |
| 96 | Luteolin-7- galacturonide | C21H20O11 | Roots and rhizomes | Methanol | [22] |
| 97 | Genistin | C21H20O10 | Roots and rhizomes | Methanol | [22] |
| 98 | Hesperidin | C29H36O14 | Roots and rhizomes | Methanol | [22] |
| 99 | Isorhamnetin-3-O- neohespeidoside | C29H36O16 | Roots and rhizomes | Methanol | [22] |
| 100 | Isorhamnetin-3-O- glucoside | C22H22O12 | Roots and rhizomes | Methanol | [22] |
| 101 | Quercitrin | C21H20O11 | Roots and rhizomes | Methanol | [22] |
| 102 | Schaftoside | C26H28O14 | Roots and rhizomes | Methanol | [22] |
| 103 | Rutin | C28H32O15 | Roots and rhizomes | Methanol | [22] |
| 104 | Isoschaftoside | C26H28O14 | Roots and rhizomes | Methanol | [22] |
| 105 | Hyperoside | C21H20O12 | Roots and rhizomes | Methanol | [22] |
| 106 | Apigenin-5- rhamnoside | C21H20O9 | Roots and rhizomes | Methanol | [22] |
| 107 | Isoquercitrin | C21H20O12 | Roots and rhizomes | Methanol | [22] |
| Peptides (108–128) | |||||
| 108 | Astin A | C25H33Cl2N5O7 | Roots | – | [33] |
| 109 | Astin B | C25H33Cl2N5O7 | Roots | – | [34] |
| 110 | Astin C | C25H33Cl2N5O6 | Roots | – | [33] |
| 111 | Astin D | C25H32ClN5O6 | Roots | – | [33] |
| 112 | Astin E | C25H32ClN5O7 | Roots | – | [33] |
| 113 | Astin F | C25H34ClN5O6 | Roots | – | [33] |
| 114 | Astin G | C25H35N5O6 | Roots | – | [33] |
| 115 | Astin H | C25H32ClN5O7 | Roots | – | [33] |
| 116 | Astin I | C25H34ClN5O7 | Roots | – | [33] |
| 117 | Astin J | C25H33N5O9 | Roots | – | [33] |
| 118 | Astin K | C25H33Cl2N5O8 | Roots | Methanol | [35] |
| 119 | Astin L | C25H34ClN5O8 | Roots | Methanol | [22] |
| 120 | Astin M | C25H34ClN5O6 | Roots | Methanol | [35] |
| 121 | Astin N | C25H32ClN5O6 | Roots | Methanol | [35] |
| 122 | Astin O | C27H35Cl2N5O7 | Roots | Methanol | [35] |
| 123 | Astin P | C26H35Cl2N5O7 | Roots | Methanol | [35] |
| 124 | Asterinin A | C25H33N5O8 | Roots | Ethyl acetate | [36] |
| 125 | Asterinin B | C26H35N5O8 | Roots | Ethyl acetate | [36] |
| 126 | Asterinin C | C26H35N5O8 | Roots | Ethyl acetate | [36] |
| 127 | Asterinin D | C25H33N5O7 | Roots | – | [37] |
| 128 | Asterinin E | C26H35N5O9 | Roots | – | [37] |
| Organic acids (129–154) | |||||
| 129 | Nethyl caffeate | C10H10O4 | Roots and rhizomes | 95 % EtOH | [17] |
| 130 | O-hydroxybenzoic acid | C7H6O3 | Roots and rhizomes | 95 % EtOH | [17] |
| 131 | P-hydroxyacetophenone | C8H8O2 | Roots and rhizomes | 95 % EtOH | [17] |
| 132 | 4-Hydroxybenzoic acid | C7H6O3 | Roots and rhizomes | 95 % EtOH | [17] |
| 133 | 3-Hydroxy-4-methoxy benzoic acid | C8H8O4 | Roots and rhizomes | 95 % EtOH | [17] |
| 134 | 3,4-Dihydroxybenzoic acid | C7H6O4 | Roots and rhizomes | 95 % EtOH | [17] |
| 135 | Pyrogallic acid | C6H6O3 | Roots and rhizomes | Methanol | [22] |
| 136 | Benzoic acid | C7H6O2 | Roots and rhizomes | Methanol | [22] |
| 137 | Protocatechuate | C9H10O4 | Roots and rhizomes | Methanol | [22] |
| 138 | Caffeic acid | C9H8O4 | Roots and rhizomes | Methanol | [22] |
| 139 | Paeonol | C9H10O3 | Roots and rhizomes | Methanol | [22] |
| 140 | Ferulic acid | C10H10O4 | Roots and rhizomes | Methanol | [22] |
| 141 | Isoferulic acid | C10H10O4 | Roots and rhizomes | Methanol | [22] |
| 142 | Methyl caffeate | C10H12O4 | Roots and rhizomes | Methanol | [22] |
| 143 | Succinic acid | C4H6O4 | Roots and rhizomes | Methanol | [22] |
| 144 | 2,2-Dimethylsuccinic acid | C6H10O4 | Roots and rhizomes | Methanol | [22] |
| 145 | Chlorogenic acid | C16H18O9 | Roots and rhizomes | Methanol | [22] |
| 146 | Cryptochlorogenic acid | C16H18O9 | Roots and rhizomes | Methanol | [22] |
| 147 | 5-Caffeoylquinic acid | C16H18O9 | Whole plant | MeoH | [18] |
| 148 | 4-Caffeoylquinicacid | C16H18O9 | Whole plant | MeoH | [18] |
| 149 | 3-O-trans-feruloylquinicacid | C18H22O8 | Whole plant | MeoH | [18] |
| 150 | Cynarin | C25H24O12 | Roots and rhizomes | Methanol | [22] |
| 151 | 3,5-Dicaffeoylquinic acid | C25H24O12 | Roots and rhizomes | Methanol | [22] |
| 152 | 4,5-Dicaffeoylquinicacid | C25H26O12 | Whole plant | MeoH | [19] |
| 153 | 3,4-Dicaffeoylquinic acid | C25H24O12 | Roots and rhizomes | Methanol | [22] |
| 154 | 4,5-Dicaffeoylquinic acid | C25H24O12 | Roots and rhizomes | Methanol | [22] |
| Other compounds (155–186) | |||||
| 155 | 11β, 13-Dihydro-3-epizaluzanin C | C16H22O2 | Roots | MeoH | [25] |
| 156 | Dihydroestafiatol | C15H22O3 | Roots | MeoH | [25] |
| 157 | Dihydroestafiatone | C15H20O3 | Roots | MeoH | [25] |
| 158 | Dsoamberboin | C15H20O4 | Roots | MeoH | [25] |
| 159 | Daryolane-1,9β-diol | C14H24O2 | Roots | MeoH | [25] |
| 160 | 7-Hydroxycoumarin | C9H6O3 | Roots and rhizomes | 95 % EtOH | [17] |
| 161 | (−)-Clovane-2,9-diol | C15H26O2 | Roots | MeoH | [25] |
| 162 | 5-Hydroxymeth-yl-furfural | C6H6O3 | Roots and rhizomes | 95 % EtOH | [25] |
| 163 | P-hydroxybenzaldehyde | C7H6O2 | Roots and rhizomes | 95 % EtOH | [25] |
| 164 | Ferulic acid hexacosanyl ester | C37H64O4 | Roots and rhizomes | 95 % EtOH | [17] |
| 165 | Trans-hexacosane-1,2-dihydroxyethyl cinnamate vinegar | C37H64O4 | Roots and rhizomes | 95 % EtOH | [17] |
| 166 | Ethanone | C13H14O4 | Roots and rhizomes | 95 % EtOH | [17] |
| 167 | Viscidone | C13H14O4 | Roots and rhizomes | 95 % EtOH | [17] |
| 168 | Scopoletin | C10H8O4 | Roots and rhizomes | – | [32] |
| 169 | Emodin | C15H10O5 | Roots and rhizomes | – | [32] |
| 170 | Emodin anthrone | C15H10O5 | Roots and rhizomes | Methanol | [22] |
| 171 | Esculin | C15H16O9 | Roots and rhizomes | Methanol | [22] |
| 172 | 5-Hydroxymethyl-2- furaldehyde | C6H10O4 | Roots and rhizomes | Methanol | [22] |
| 173 | Benzaldehyde | C7H6O | Roots and rhizomes | Methanol | [22] |
| 174 | P-hydroxybenzaldehyde | C7H6O2 | Roots and rhizomes | Methanol | [22] |
| 175 | Esculetin | C9H6O4 | Roots and rhizomes | Methanol | [22] |
| 176 | Fraxetin | C10H8O5 | Roots and rhizomes | Methanol | [22] |
| 177 | Xanthotoxin | C12H8O4 | Roots and rhizomes | Methanol | [22] |
| 178 | Bergapten | C12H8O4 | Roots and rhizomes | Methanol | [22] |
| 179 | Isoscopoletin | C10H8O4 | Roots and rhizomes | Methanol | [22] |
| 180 | Psoralen | C11H6O3 | Roots and rhizomes | Methanol | [22] |
| 181 | Rhein | C15H8O6 | Roots and rhizomes | Methanol | [22] |
| 182 | 1-Acetoxy-2-ene€-4,6-decandiyne | C12H14O2 | Roots and rhizomes | 95 % EtOH | [17] |
| 183 | (E)-2-decend-4,6-diyn-1-ol | C10H12O | Roots and rhizomes | 95 % EtOH | [17] |
| 184 | Lachnophyllic acid | C10H10O2 | Roots and rhizomes | 95 % EtOH | [17] |
| 185 | N-octadecane | C18H38 | Whole plant | MeoH | [18] |
| 186 | N-triacontanol | C30H62O | Whole plant | MeoH | [18] |
4.1. Terpenes
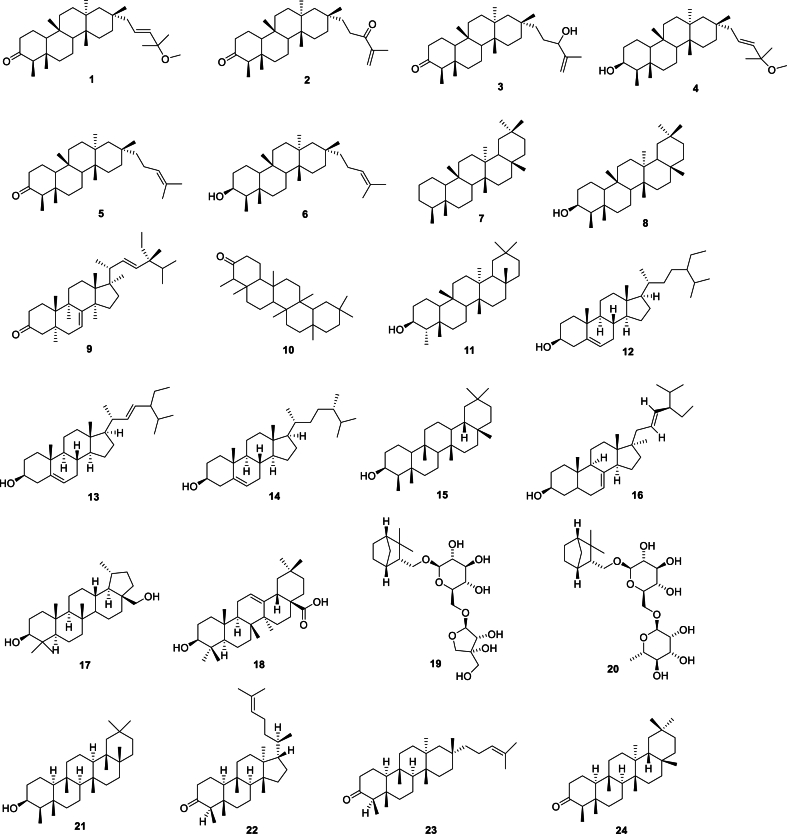
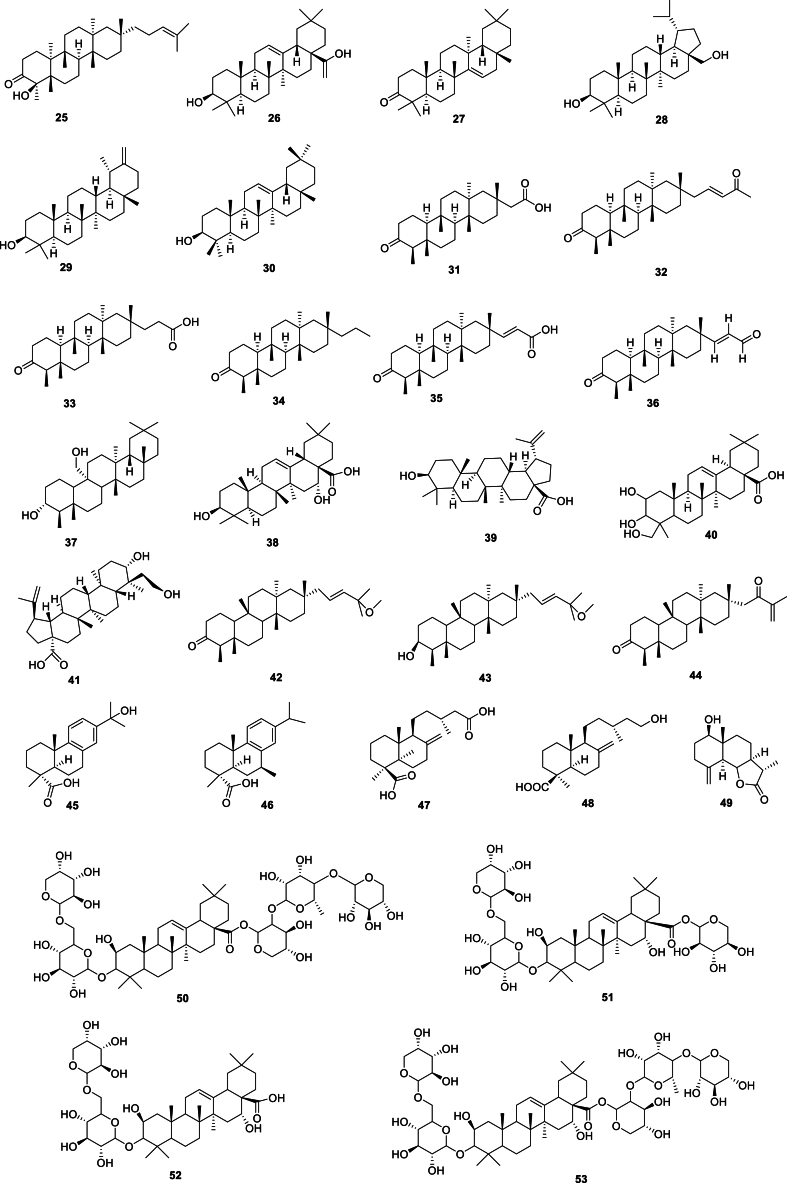
Terpenes are polymers of isoprene and its derivatives. They are synthesized from isoprene pyrophosphate, with most of them having a structure in which the isoprene residues are linked at the head and tail. Based on the number of isoprene units (5 carbon units), terpenes are classified as monoterpenes (2 units), sesquiterpenes (3 units), diterpenes (4 units), triterpenes (6 units), tetraterpenes (8 units), and polyterpenes (more than 8 units). Terpenes are the primary components of Aster tataricus. To date, 73 terpenes (Fig. 3) have been isolated from Aster tataricus, with triterpenes and triterpenoid saponins being the main types. In particular, shionane-type triterpenes have been identified as the main constituents of Aster tataricus and shown to have anti-tussive and expectorant activities [38]. According to the Chinese Pharmacopoeia (2020), shionone can be used as a maker for the quality control of Aster tataricus.
Fig. 3.
Structures of terpenes from Aster tataricus.
4.2. Flavonoids
Flavonoids are one of the most important natural compounds widely found in the in the plant kingdom [39]. They have a core 2-phenyl chromone nucleus without the substitution of an oxygen-containing group at the 3ꞌ end [40]. In a study, Aster tataricus was ultrasonicated with 25 mL of methanol for 30 min and subjected to UHPLC-Q-TOF-MS to yield different flavonoids [3]. To date, 34 flavonoids have been isolated from Aster tataricus, including flavonols, flavones, and their glycoside. The chemical structures of these molecules and sources are presented in Table 3 and Fig. 4.
Fig. 4.
Structures of flavonoids from Aster tataricus.
4.3. Peptides
Peptides are the characteristic components of Aster tataricus [41], mainly including oligopeptides, acyclic peptides, and chlorinated cyclic peptides [11]. They exhibit diverse biological activities, including anti-tumor and immune-regulatory activities [42]. However, the number of peptides isolated from Aster tataricus is limited. The two main types of peptide analogs identified in Aster tataricus are cyclic (117, 124–128) and chain (108–116, 118–123). The structures and sources of these peptides are presented in Table 3 and Fig. 5.
Fig. 5.
Structures of peptides from Aster tataricus.
4.4. Organic acids
Multiple organic acids are found in Aster tataricus, such as fatty acids, polyphenols, and carboxylic acids. These compounds are abundant in the leaves, roots, and especially fruits and are usually found in the form of salts or esters. To date, 26 organic acids have been isolated from Aster tataricus, all of which contain aromatic rings and are mostly classified as small molecules. The structures of these organic acids (129–154) are shown in Fig. 6.
Fig. 6.
Structures of organic acids from Aster tataricus.
4.5. Other compounds
In addition to the abovementioned common chemical compounds, more than 30 other compounds have been isolated from Aster tataricus. These compounds include 155–159, which are 7-membered cyclic compounds isolated from the methanolic extract of Aster tataricus [43], and 182–184, which are acetylenes isolated from the 95 % ethanolic extract of Aster tataricus [17]. Two chained alkanes named N-octadecane and N-triacontanol have been extracted from the methanolic extracts of whole plants of Aster tataricus [19]. In addition, coumarins and quinones have been identified in Aster tataricus (Fig. 7) .
Fig. 7.
Structures of other compounds from Aster tataricus.
5. Pharmacological activities
Aster tataricus has been used in TCM for the treatment of respiratory diseases for more than 2000 years. With the continuous progress of science and technology, numerous studies have investigated the pharmacological activities and mechanisms of action of the abovementioned bioactive compounds. Modern pharmacological studies have shown that Aster tataricus has a wide range of therapeutic effects (Fig. 8), including anti-cough and pro-expectoration, anti-asthmatic, anti-inflammatory, anti-tumor, anti-oxidant, anti-depressant, anti-bacterial, and anti-viral effects.
Fig. 8.
Pharmacological activities of Aster tataricus.
5.1. Anti-cough and pro-expectoration effects
In TCM, Zi wan is an important drug for suppressing coughs and promoting expectoration. The expectoration-inducing effects of Aster tataricus can be attributed to asterone and epimedanol, which have been isolated from petroleum ether and ethyl acetate extracts [44].
In a study, the composition of the volatile oil of Aster tataricus was analyzed via gas chromatography-mass spectrometry, and seven compounds were eventually identified. In vivo experiments and phenol red assay showed that 1-acetyl-trans-2-en-4,6-decadiyne, the main component of the volatile oil, had expectoration-inducing effects [45]. Aqueous extracts of Aster tataricus have been shown to reduce the frequency of cough induced by ammonia liquor in mice [46]. Yu et al. showed that Aster tataricus extracts (Fr-50) exerted remarkable pro-expectoration, anti-tussive, and anti-inflammatory effects at doses of 40 and 80 mg/kg. They used HPLC-Q-TOF/MS to investigate Fr-50 and found that chlorogenic acids (CGAs) eliminated or reduced tracheal inflammation, which is one of the main causes of cough and phlegm [47]. In addition, shionone and 1-acetoxy-2-ene (E)-4,6-decandiyne extracted from Aster tataricus have been identified as effective expectorants [48].
Triterpenoid saponins, which are one of the chemical constituents of Aster tataricus, are often considered expectorants [49]. Some studies have reported that shionone and epi-friedelanol isolated from Aster tataricus extracts can substantially decrease the frequency of ammonia-induced cough in mice [50].
Therefore, we speculate that triterpenoid saponins found in Aster tataricus may serve as primary expectorants that reduce airway inflammation and relieve cough, and its mechanism of action needs to be further investigated.
5.2. Anti-asthmatic effects
Asthma is a prime example of a “complex disease”. It is considered a syndrome instead of a disease because it is defined based on clinical characteristics rather than underlying mechanisms [51]. The principal clinical characteristics of asthma are reversible airflow obstruction, airway hyperresponsiveness, and airway inflammation [52].
Peng et al. [53] showed that the ethanolic extracts of Aster tataricus exhibited potent anti-asthmatic activity in guinea pigs. The mechanism of action was found to be related to the inhibition of tracheal smooth muscle M receptor, H1 receptor, and Ca2+ channels, which resulted in the inhibition of the inward flow of Ca2+. In addition, Chen et al. [54] showed that Aster tataricus extracts exerted anti-asthmatic effects by attenuating OVA-induced immune responses and inhibiting tracheal ring contraction.
5.3. Anti-inflammatory effects
According to folkloric and scientific literature, Aster tataricus has potential anti-inflammatory effects. In TCM, inflammation is called “Fa Yan”, which is a defense response to harmful stimuli and manifests as redness, swelling, heat, pain, and dysfunction. Inflammation is one of the common pathological conditions observed in clinical practice and is considered the first line of defense against invading pathogens [40]. However, unregulated inflammation can lead to allergies, cancer, and atherosclerosis [54].
Du et al. [55] reported that the ethanolic extract of Aster tataricus suppressed pro-inflammatory cytokines and activated the NF-κB signaling pathway, thereby exerting therapeutic effects against diabetes mellitus.
Zhang et al. [56] found that Aster tataricus exerted anti-neuroinflammatory effects by preventing the generation of free radicals, enhancing the activity of antioxidant enzymes, and suppressing the activity of pro-inflammatory cytokines.
Wang et al. [57] evaluated the protective effects of Aster tataricus extracts on CYP- or LPS + ATP-induced interstitial cystitis. The results showed that Aster tataricus extracts alleviated inflammation in rat bladder and urothelial cells by inhibiting the expression of pyroptosis-related proteins and downregulating the NLRP3/GSDMD-N signaling pathway.
Liu et al. [58] found that 4-hydroxyphenylacetic acid isolated from Aster tataricus alleviated inflammation by inhibiting the hypertonicity- and hypoxia-induced production of hypoxia-inducible factor 1-alpha in rats with seawater aspiration-induced lung injury.
Su et al. [59] analyzed the chemical composition of the methanolic extract of the rhizomes and roots of Aster tataricus and evaluated its anti-inflammatory activity. The results showed that lachnophyllol acetate was a candidate drug for the treatment of inflammatory diseases mediated by the NF-κB and MAPK signaling pathways.
5.4. Anti-tumor effects
Despite remarkable advancements in science and technology, malignant tumors remain a serious threat to human health worldwide [60]. The demand for anti-tumor drugs remains high as the global incidence of tumor increases. Natural active ingredients used in TCM have been reported to have therapeutic effects against tumors to some extent [61,62]. Pharmacological studies have shown that Aster tataricus has potential anti-tumor activity. For example, Zhou et al. [23] found that terpenes isolated from Aster tataricus induced tumor cell apoptosis.
Furthermore, plant-derived polysaccharides have been shown to possess anti-tumor properties [63]. Zhang et al. [64] reported that a water-soluble polysaccharide isolated from Aster tataricus induced apoptosis in SGC-7901 cells through calcium- and ΔΨm-dependent pathways, indicating that it may be used as a natural anti-cancer agent. Du et al. [65] isolated a homogeneous polysaccharide (ATP-II) from the 80 % ethanolic extract of Aster tataricus and assessed its anti-cancer effects and mechanism of action in glioma C6 cells. In vitro experiments showed that ATP-II effectively inhibited the proliferation of C6 cells by inducing DAN injury and apoptosis. In vivo experiments showed that ATP-II markedly inhibited the growth of C6-transplanted tumors and induced tumor cell apoptosis by increasing the Bax/Bcl-2 ratio and stimulating the activation of caspase-3, caspase-8, and caspase-9. These findings suggest that ATP-II is a safe and effective drug for the treatment of malignant glioma. Yao et al. [66] showed that the aqueous extract of Aster tataricus attenuated the proliferative and invasive abilities of human lung cancer A549 cells and inhibited the growth of transplanted tumors in nude mice by suppressing the Wnt/β-catenin signaling pathway.
5.5. Anti-oxidant effects
Anti-oxidants can prevent oxidation, both endogenously and exogenously, at low doses [67]. Phenolic acids and other classes of phenylpropanoids derived from medicinal plants are strong anti-oxidants and effective free radical scavengers [68]. Anti-oxidants are vital for human health, as they reduce the risk of free radical damage to cells [69].
Ng et al. [33] found that quercetin and kaempferol isolated from Aster tataricus exhibited the highest potency and possessed minimal pro-oxidant activity.
Zhang et al. [70] isolated active ingredients from the roots and flowers of Aster tataricus through solvent extraction and investigated the anti-oxidant activity of the extracts using DPPH assay. The results showed that both flower and roots extracts exerted strong anti-oxidant effects in a concentration- and solvent polarity-dependent manner.
Du et al. [55] found that the root extracts of Aster tataricus effectively alleviated diabetic retinopathy by controlling blood glucose levels and attenuating attenuating oxidative stress, and suppressing inflammatory mediators.
5.6. Anti-depressant effects
Depression is a common mental disorder. According to the World Health Organization, 5 % of adults have depression worldwide, with the incidence being higher among women than among men. Commercially available anti-depressants often have slow results and many side effects [71]. Wan et al. [72] identified the chemical constituents of Aster tataricus via HPLC-MS and evaluated their anti-depressant activity. The results revealed high levels of kaempferol, quercetin, chlorogenic acid, caffeic acid, and ferulic acid in Aster tataricus extracts. Among these compounds, quercetin, chlorogenic acid, and ferulic acid were found to have anti-depressant effects. However, the anti-depressant activity of Aster tataricus has been reported in limited studies and warrants further investigation.
5.7. Anti-bacterial and anti-viral effects
The anti-bacterial and anti-viral activities of Aster tataricus have been extensively investigated [73]. For instance, Zhou et al. [74] evaluated the anti-viral activities of six triterpenoids isolated from Aster tataricus. The results showed that Aster shionoes C remarkably inhibited the surface antigen of hepatitis B virus as well as its secretion and viral DNA replication.
Liu et al. [75] showed that the ethanolic extract of Aster tataricus exerted strong bacteriostatic effects against Varistaptyoccus aureas, Pasteurella maltocida, E.coli., Streptococci and Salmonella, with the lowest inhibitory concentrations of 0.80 g/mL, 0.05 g/mL, 0.50 g/mL, 0.20 g/mL, 0.20 g/mL, respectively.
5.8. Other pharmacological effects
In addition to the abovementioned effects, other pharmacological effects of Aster tataricus have been reported in previous studies. Astin C, one of the compounds isolated from the roots of Aster tataricus, can exert immunosuppressive effects by inducing T-cell apoptosis [76]. Li et al. [77] found that the cyclopeptide astin C exerted potent anti-cancer and immunosuppressive effects by binding to STING, a key cytosolic DNA sensor protein involved in natural immunity in humans.
Chen et al. [78] investigated the protective effects of Aster tataricus on acute lung injury caused by an endotoxin from Acanthopanax quinquefolia. Network pharmacology and experiments showed that the candidate compound in Aster tataricus extracts alleviated LPS-induced acute lung injury mainly by inhibiting the release of inflammatory factors and promoting the repair of the vascular endothelium.
Rho et al. [79] showed that Aster tataricus alleviated testosterone-induced benign prostatic hyperplasia in rats by promoting apoptosis and inhibiting inflammation. These results indicate that Aster tataricus can be used to treat inflammation associated with benign prostatic hyperplasia in clinical settings.
Recently, Li et al. [80] examined the bioactivities of five undescribed α-pyrone (neuropyrones A–E) derivatives from the endophytic fungus Neurospora dictyophora WZ-497 derived from the stems of Aster tataricus. The results showed that neuropyrones A–C exerted potent inhibitory effects on tyrosinase, with IC50 values of 0.38 ± 0.07, 0.49 ± 0.06, and 0.12 ± 0.01 mM, respectively.
Lee et al. [81] found that ethanolic extracts of Aster tataricus regulated osteoclast differentiation and alleviated osteoporosis as well as related metabolic changes after estrogen depletion. These results indicate that Aster tataricus can be used as an alternative treatment strategy for postmenopausal osteoporosis accompanied by metabolic imbalance.
5.9. Toxicity and safety
Modern pharmacological studies have primarily focused on the therapeutic effects of Aster tataricus against cough, tumors, and other diseases; however, studies investigating its toxic effects are limited.
To further improve the safety of Aster tataricus medication, toxicity and subtoxicity of it was evaluated by Peng et al. [5]. The study showed that Aster tataricus could produce toxic effects, mainly on the liver; much less on the heart. The acute oral toxicity experiment showed that Aster tataricus is capable of toxic effects and resulted in an LD50 of 15.74 g/kg BW in mice, The subchronic experiment, conducted at a dose of 0.34 g/kg/d.BW, demonstrated that the toxic components of Aster tataricus were mainly concentrated in the petroleum ether fraction, followed by the ethyl acetate fraction, the n-butyl alcohol fraction, the lower aqueous phase and the 75 % ethanol extract. In addition, terpenes can cause toxicity in tumor cells by inducing apoptosis and DNA mutations [23].
To provide an experimental basis for evaluating the safety of the utilization and development of Aster tataricus, in-depth studies on its potential toxicity should be carried out.
6. Conclusions and perspectives
The dried roots and rhizomes of Aster tataricus have been used in TCM for thousands of years [56]. Approximately 250 Aster species are found worldwide; of which, 100 species are found in China, mainly in Anhui and Hebei [5]. Ancient literary records indicate that Aster tataricus suppresses cough, alleviates asthma, improves eclampsia in children, urination, and relieves constipation. It is rich in active ingredients and has a wide range of pharmacological effects. This review summarized the morphology characteristics, chemical composition, and therapeutic effects of Aster tataricus based on traditional literature and modern evidence, providing a scientific basis for further research and exploitation of medicinal plants to develop more effective therapeutic drugs.
With the continuous development of research tools and instruments, numerous compounds have been isolated and identified from Aster tataricus, which has not only improved the understanding of its chemical constituents but also provided more comprehensive and accurate information for its medicinal and nutritional applications. Terpenes, flavonoids, organic acids, peptides, esters, coumarins, quinones, alkanes, and alkynes isolated from it exhibit a wide range of pharmacological activities. For example, terpenes, the main chemical constituents of Aster tataricus, can induce apoptosis and DNA mutations in tumor cells, thereby exerting anti-tumor effects. Caffeoylquinic acids and epifriedelinol possess strong anti-oxidant, anti-inflammatory, and anti-cancer activities. Scopoletin can effectively treat diabetes and alleviate inflammation and oxidative stress. In addition, astin C can suppresses the immune system by inducing T-cell apoptosis [33]. And its distinctive chlorinated pentacyclic structure and potential pharmacological activities have received substantial attention from researchers. Although several studies have reported the pharmacological effects of Aster tataricus, the translation of research findings into clinical practice is limited.
Remarkable progress has been made in research on the phytochemical composition and pharmacological effects of Aster tataricus. However, certain gaps in knowledge remain to be addressed. First, the pharmacological effects of terpenes, the main chemical constituents of Aster tataricus, should be evaluated comprehensively. Cyclic peptides have effective anti-tumor and immunosuppressive activities; however, few peptides have been extracted from Aster tataricus. Second, cancer is a leading cause of death worldwide, accounting for approximately 10 million deaths in 2020. The cyclopeptide astin C, isolated from the endophytic fungus Cyanodermella asteris derived from Aster tataricus, has been reported to have potent anti-cancer and immunosuppressive activities. It functions by binding to STING, a crucial cytosolic DNA sensor protein involved in innate immunity. Therefore, to facilitate the rational utilization of Aster tataricus resources and determine the optimal concentrations of its active compounds, the chemical composition and pharmacological effects of Cyanodermella asteris should be extensively investigated. Third, since ancient times, Aster tataricus has been used as an effective anti-cough agent and expectorant. Although modern pharmacological studies have validated these effects, the development of Aster tataricus into a drug remains an unaddressed concern. Last but not least, considering the pharmacological activities and potential health benefits of Aster tataricus, its toxicological profile should be analyzed intensively.
In addition to its medicinal value, the toxicity and safety of Aster tataricus warrants strict consideration, in order to provide experimental basis for the development and utilization of Aster tataricus. However, further research is warranted to validate these findings.
In conclusion, Aster tataricus is an important source of active components, with a wide range of pharmacological activity. However, the chemical compounds isolated from Aster tataricus are inadequate, and their mechanism of action warrants further investigation. Therefore, future research should be focused on the isolation and identification of chemical components from Aster tataricus, systematic analysis of their biological activities, in-depth exploration of pulmonary diseases, and strengthening drug development to expand the application of Aster tataricus.
Data availability
No data was used for the research described in the article.
CRediT authorship contribution statement
Xi-Ling Fan: Writing – review & editing, Writing – original draft. Zhong-Peng Qin: Writing – original draft, Formal analysis. Jian-Hui Wen: Writing – review & editing, Supervision. Zhen-Zhong Wang: Writing – review & editing, Supervision, Funding acquisition. Wei Xiao: Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing interest
No potential declaration of interest statement was reported by the authors.
Acknowledgements
This work was supported by the Jiangsu province transformation and upgrading plan of Industry and information industry-research on key technologies of multi-component traditional Chinese medicine.
Contributor Information
Zhen-Zhong Wang, Email: kyyywzz@163.com.
Wei Xiao, Email: xw_kanion@163.com.
References
- 1.Fan L.L., Wang X., Zhu X.J., Liu J., Yin H., Luo J.P., Xue Y., Jin Q.J., Yu W.D. Advances in research on chemical constituents and pharmacological effects of Aster. Jilin. J. Tradit. Chin. Med. 2019;39(2):269–273. [Google Scholar]
- 2.Zhai J.J., Duan M.Y., Li J.Y., Hu T.T., Meng X.S., Wang W.J. Textual research of aster. J. Liaoning. Univ. Tradit. Chin. Med. 2023;1–14 [Google Scholar]
- 3.Cai Y.J., Shi X.L., Liu H.Y., Wu L., Gu Y.T., Wang R. Effective components and pharmacological effects of Asteris Radix. J. Tradit. Chin. VeterMed. 2023;42(2):39–42. [Google Scholar]
- 4.Li H.Y., Li W.J., Ding X.Y., Zhang D., Xue Z.J., An Q., Zhan Z.L., Zheng Y.G. Herbal textual research on Asteris Radix et rhizoma in famous classical formulas. Chin. J. Exp. Tradit. Med. Form. 2023:1–13. [Google Scholar]
- 5.Peng W.J., Xin R.H., Luo Y.J., Liang G., Ren L.H., Liu Y., Wang G.B., Zheng J.F. Evaluation of the acute and subchronic toxicity of Aster tataricus L. F. Afr. J. Tradit. Complement. Altern. Med. 2016;13(6):38–53. doi: 10.21010/ajtcam.v13i6.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu P., Cheng S., Xiang J., Yu B., Zhang M., Zhang C., Xu X. Expectorant, antitussive, anti-inflammatory activities and compositional analysis of Aster tataricus. J. Ethnopharmacol. 2015;164:328–333. doi: 10.1016/j.jep.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Jiang K., Song Q., Wang L., Xie T.Z., Wu X., Wang P., Yin G., Ye W.C., Wang T.J. Antitussive, expectorant and anti-inflammatory activities of different extracts from Exocarpium Citri grandis. J. Ethnopharmacol. 2014;156:97–101. doi: 10.1016/j.jep.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Guo S.J., Zhao X.H., Cheng D.L. A new diterpenoid glucoside from Aster smithianus. Chin. Chem. Lett. 2004;15(12):1451–1453. [Google Scholar]
- 9.Sun Y.P., Li L., Liao M., Su M., Wan C.C., Zhang L.T., Zhang H.L. A systematic data acquisition and mining strategy for chemical profiling of Aster tataricus rhizoma (Ziwan) by UHPLC-Q-TOF-MS and the corresponding anti-depressive activity screening. J. Pharmaceut. Biomed. 2018;154:216–226. doi: 10.1016/j.jpba.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Dong X.V., Fu J., Yin X.B., Cao S.L., Li X.C., Lin L.F., Ni J. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother. Res. 2016;30:1207–1218. doi: 10.1002/ptr.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li K.J., Liu Y.Y., Wang D., Yan P.Z., Lu D.C., Zhao D.S. Radix Asteris: traditional Usage, phytochemistry and pharmacology of an important traditional Chinese medicine. Molecules. 2022;27(17):5388. doi: 10.3390/molecules27175388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton G.M. A calculated response: control of inflammation by the innate immune system. J. Clin. Invest. 2008;118(2):413–420. doi: 10.1172/JCI34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher J.L., Schwartzbaum J.A., Wrensch M., Wiemels J.L. Epidemiology of brain tumors. Neurol. Clin. 2007;25(4):867–890. doi: 10.1016/j.ncl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira M.R., Chenet A.L., Duarte A.R., Scaini G., Quevedo J. Molecular mechanisms underlying the anti-depressant effects of resveratrol: a review. Mol. Neurobiol. 2018;55(6):4543–4559. doi: 10.1007/s12035-017-0680-6. [DOI] [PubMed] [Google Scholar]
- 15.Han Y.Y., Gao F., Qin P.Y., Bai R., Tian J.Y., Liu X.C., Yuan C.C., Guo Q.H. Advances in studies on Asteris Radix et rhizoma and prediction of its quality markers. Modern. Chin. Med. 2023;25(3):655–664. [Google Scholar]
- 16.Tian H., Xu K.Y., Hang J.S., Piao C.L. On clinical application and dosage of Tatarian aster root. Jilin. J. Tradit. Chin. Med. 2021;41(1):99–102. [Google Scholar]
- 17.Zhou W.B. Studies on chemical constituents from Aster tataricus L.f., 2010. Hu. Bei. Chin. Med. 2010 [Google Scholar]
- 18.Shen J. Univ.; 2022. Study on the Chemical Constituents of Aster lingua. Southwest. Mizu. [Google Scholar]
- 19.Ye J. Tianjing. Univ.; 2007. Study on the Constituents and Their Primary Antitumor Effect in Aster tataricus & Semiaquilegia Adoxoides. [Google Scholar]
- 20.Nagao T., Okabe H., Yamaucchi T. Studies on the constituents of Aster tataricus L.f.I. structures of shionosides A and B, monoterpene glycosides isolated from the root. Chem. Pharm. Bull. 1988;36(2):571–577. [Google Scholar]
- 21.Akihisa T., Kimura Y., Koike K., Yasukawa K., Arai K., Suzuki Y., Nikaido T. Astertarone A: a triterpenoid ketone isolated from the roots of Aster tataricus L. Chem. Pharm. Bull. 1998;46:1824–1826. [Google Scholar]
- 22.Sun Y.P., Li L., Liao M., Su M., Wan C.C., Zhang L.T., Zhang H.L. A systematic data acquisition and mining strategy for chemical profiling of Aster tataricus rhizoma (Ziwan) by UHPLC-Q-TOF-MS and the corresponding anti-depressive activity screening. J. Pharmaceut. Biomed. 2018;154:216–226. doi: 10.1016/j.jpba.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Zhou W.B., Zeng G.Z., Xu H.M., He W.J., Zhang Y.M., Tan N.H. Astershionones A-F, six new anti-HBV shionane-type triterpenes from Aster tataricus. Fitoterapia. 2014;93:98–104. doi: 10.1016/j.fitote.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Virginia L. Bioactive saponins from Allium and aster plants. 2005;4(2–3):95–110. [Google Scholar]
- 25.Sun X.C. Nan. Kai. Univ.; 2017. Studies on the Chemical Constituents and Anti-inflammatory Activities from Callicarpa nudiflora and Ligularia fischeri. [Google Scholar]
- 26.Su X.D., Jang H.J., Wang C.Y., Lee S.W., Rho M.C., Kim Y.H., Yang S.Y. Anti-inflammatory potential of Saponins from Aster tataricus via NF-κB/MAPK activation. J. Nat. Prod. 2019;82(5):1139–1148. doi: 10.1021/acs.jnatprod.8b00856. [DOI] [PubMed] [Google Scholar]
- 27.Shao Y., Ho C.T., Chin C.K., Poobrasert O., Yang S.W., Cordell G.A. Asterlingulatosides C and D, cytotoxic triterpenoid saponins from Aster lingulatus. J. Nat. Prod. 1997;60(7):743–746. doi: 10.1021/np970080t. [DOI] [PubMed] [Google Scholar]
- 28.Shao Y., Ho C.T., Chin C.K., Rosen R.T., Hu B., Qin G.W. Triterpenoid saponins from Aster lingulatus. Phytochemistry. 1997;44(2):337–430. doi: 10.1016/s0031-9422(96)00551-1. [DOI] [PubMed] [Google Scholar]
- 29.Shao Y., Li Y.L., Zhou B.N. Phenolic and triterpenoid glycosides from Aster batangensis. Phytochemistry. 1996;41(6):1593–1598. doi: 10.1016/0031-9422(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka T., Nagao T., Okabe H., Yamauchi T. Studies on the constituents of Aster tataricus L.f.IV. structures of Aster saponins isolated from the herb. Chem Pharm Bull. 1990;38(5):1153–1157. [Google Scholar]
- 31.Shao Y., Zhou B.N., Lin L.Z., Cordell G.A. Triterpenoid saponins from Aster batangensis. Phytochemistry. 1995;38(4):927–933. doi: 10.1016/0031-9422(94)00744-e. [DOI] [PubMed] [Google Scholar]
- 32.Ng T.B., Liu F., Lu Y.H., Cheng C.H.K., Wang Z.T. Antioxidant activity of compounds from the medicinal herb Aster tataricus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003;136(2):109–115. doi: 10.1016/s1532-0456(03)00170-4. [DOI] [PubMed] [Google Scholar]
- 33.Morita H., Nagashima S., Uchiumi Y., Kuroki O., Takeya K., Itokawa H. Cyclic peptides from higher plants. XXVIII. Antitumor activity and hepatic microsomal biotransformation of cyclic pentapeptides, astins, from Aster tataricus. Chem. Pharm. Bull. 1996;44:1026–1032. doi: 10.1248/cpb.44.1026. [DOI] [PubMed] [Google Scholar]
- 34.Wang L., Li M.D., Cao P.P., Zhang C.F., Huang F., Xu X.H., Liu B.L., Zhang M. Astin B, a cyclic pentapeptide from Aster tataricus, induces apoptosis and autophagy in human hepatic L-02 cells. Chem. Biol. Interact. 2014;223:1–9. doi: 10.1016/j.cbi.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Xu H.M., Zeng G.Z., Zhou W.B., He W.J., Tan N.H. Astins K–P, six new chlorinated cyclopentapeptides from Aster tataricus. Tetrahedron. 2013;69:7964–7969. [Google Scholar]
- 36.Cheng D., Shao Y., Hartman R., Roder E., Zhao K. Oligopeptides from Aster tataricus. Phytochemistry. 1994;36(4):945–948. doi: 10.1016/s0031-9422(00)90468-0. [DOI] [PubMed] [Google Scholar]
- 37.Cheng D.L., Shao Y., Zhao K., Hartmann R., Roeder E. Pentapeptides from the roots of Aster tataricus. Pharmazie. 1996;51:185–186. [PubMed] [Google Scholar]
- 38.Sawai S., Uchiyama H., Mizuno S., Aoki T., Akashi T., Ayabe S.-I., Takahashi T. Molecular characterization of an oxidosqualene cyclase that yields shionone, a unique tetracyclic triterpene ketone of Aster tataricus. Febs. Lett. 2011;585:1031–1036. doi: 10.1016/j.febslet.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 39.Wang M., Bai Q.X., Zheng X.X., Hu W.J., Wang S., Tang H.P., Yu A.Q., Yang B.Y., Kuang H.X. Smilax China L.: a review of its botany, ethnopharmacology, phytochemistry, pharmacological activities, actual and potential applications. J. Ethnopharmacol. 2024;318(Pt B) doi: 10.1016/j.jep.2023.116992. [DOI] [PubMed] [Google Scholar]
- 40.Wang M., Tang H.P., Wang S., Hu W.J., Li J.Y., Yu A.Q., Bai Q.X., Yang B.Y., Kuang H.X. Acorus tatarinowii Schott: a Review of its botany, traditional uses, phytochemistry, and pharmacology. Molecules. 2023;28(11):4525. doi: 10.3390/molecules28114525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen S.L., Liu J., Feng M.H., Li Y.T., Jia Z.X., Yang S., Xiao H.B. Identification of peptides in Aster tataricus by the strategy of UPLC-Q-TOF-MS/MS combined with molecular network. J. Chin. Mass Spectrom. Soc. 2023;44(3):397–411. [Google Scholar]
- 42.Pernot M., Vanderesse R., Frochot C., Guillemin F., Barberi-Heyob M. Stability of peptides and therapeutic success in cancer. Expert. Opin. Drug. Metab. Toxicol. 2011;7(7):793–802. doi: 10.1517/17425255.2011.574126. [DOI] [PubMed] [Google Scholar]
- 43.Sun X.C. Nan. Kai. Univ.; 2017. Studies on the Chemical Constituents and Anti-inflammatory Activities from Callicarpa nudiflora and Ligularia fischeri. [Google Scholar]
- 44.Cai Y.J., Shi X.L., Liu H.Y., Wu L., Gu Y.T., Wang R. Effective components and pharmacological effects of Asteris Radix. J Tradit Chin Veteri Med. 2023;42(2):39–42. [Google Scholar]
- 45.Yang B., Xiao Y.Q., Liang R.X. 2008. Studies on Expectorant Compounds in Volatile Oil from Root and Rhizome of Aster tataricus; pp. 281–283. 03. [PubMed] [Google Scholar]
- 46.Zhang J.W., Dou C.G., Zhang M., Ma S.P., Huang F. Toxicity of Radix Asteris, flos farfarae and their combination. Chin. J. Clin. Pharmacol. Ther. 2007;(4):405–411. [Google Scholar]
- 47.Yu P., Cheng S., Xiang J., Yu B., Zhang M., Zhang C., Xu X. Expectorant, antitussive, anti-inflammatory activities and compositional analysis of Aster tataricus. J. Ethnopharmacol. 2015;164:328–333. doi: 10.1016/j.jep.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 48.Yang B., Xiao Y.Q., Liang R.X., Wang R.J., Li W., Zhang C., Cao Y., Wang Q.P., Wang L., Wang Y.Y. Studies on expectorant compounds in volatile oil from root and rhizome of Aster tataricus, China. J. Chin. Matera. Med. 2008;33:281–283. [PubMed] [Google Scholar]
- 49.Wang H.L., Gao J., Zhu D.N., Yu B.Y. Quality evaluation of Polygala japonica through simultaneous determination of six bioactive triterpenoid saponins by HPLC–ELSD. J. Pharm. Biomed. Anal. 2007;43(4):1552–1556. doi: 10.1016/j.jpba.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Liu K.Y., Zhang J.T., Gao W.Y., Zheng Y.N., Chen H.X. Triterpenes and steroids from Aster tataricus. Nat. Prod. Res. Dev. 2006;18:4–6. [Google Scholar]
- 51.Brusasco V., Pellegrino R. Complexity of factors modulating airway narrowing in vivo: relevance to assessment of airway hyperresponsiveness. J. Appl. Physiol. 2003;95:1305–1313. doi: 10.1152/japplphysiol.00001.2003. [DOI] [PubMed] [Google Scholar]
- 52.Bates J.H., Rincon M., Irvin C.G. Animal models of asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297(3):L401–L410. doi: 10.1152/ajplung.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng W.J., Xin R.H., Liu Y., Luo Y.J., Wang G.B., Luo C.Y., Xie J.S., Li J.Y., Zheng J.F. Effects of alcohol extract of Aster tataricus L.f. on the contraction of Guinea pig trzcheal smooth muscle in vitro. Chin. Animal. Husbandry. Veterinary. Med. 2016;43(6):1572–1578. [Google Scholar]
- 54.Chen Y.J., Wu H., Li Y.T., Liu J., Jia Z.X., Xu W.J., Xiao H.B., Wang W. Aster tataricus attenuates asthma efficiently by simultaneously inhibiting trcheal ring contraction and inflammation. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110616. [DOI] [PubMed] [Google Scholar]
- 55.Du H., Zhang M., Yao K.J., Hu Z.T. Protective effect of Aster tataricus extract on retinal damage on the virtue of its antioxidant and anti-inflammatory effect in diabetic rat. Biomed. Pharmacother. 2017;89:617–622. doi: 10.1016/j.biopha.2017.01.179. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H.T., Tian M., He Q.W., Chi N., Xiu C.M., Wang Y.B. Effect of Aster tataricus on production of inflammatory mediators in LPS stimulated rat astrocytoma cell line (C6) and THP-1 cells. Saudi. Pharm. J. 2017;25(3):370–375. doi: 10.1016/j.jsps.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X., Fan L., Yin H., Zhou Y.Q., Tang X.L., Fei X.J., Tang H.L., Peng J., Ren X.Q., Xue Y., Zhu C.L., Luo J.P., Jin Q.L., Jin Q.J. Protective effect of Aster tataricus extract on NLRP3-mediated pyroptosis of bladder urothelial cells. J. Cell Mol. Med. 2020;24(22):13336–13345. doi: 10.1111/jcmm.15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z.Y., Xi R.G., Zhang Z.R., Li W.P., Liu Y., Jin F.G., Wan g X.B. 4-hydroxyphenylacetic acid attenuated inflammation and edema via suppressing HIF-1α in seawater aspiration-induced lung injury in rats. Int. J. Mol. Sci. 2014;15(7):12861–12884. doi: 10.3390/ijms150712861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su X.D., Jang H.J., Li H.X., Kim Y.H., Yang S.Y. Identification of potential inflammatory inhibitors from Aster tataricus. Bioorg. Chem. 2019;92 doi: 10.1016/j.bioorg.2019.103208. [DOI] [PubMed] [Google Scholar]
- 60.Wang M., Hu W.J., Zhou X., Yu K., Wang Y., Yang B.Y., Kuang H.X. Ethnopharmacological use, pharmacology, toxicology, phytochemistry, and progress in Chinese crude drug processing of the lateral root of Aconitum carmichaelii Debeaux. (Fuzi): a review. J. Ethnopharmacol. 2023;301 doi: 10.1016/j.jep.2022.115838. [DOI] [PubMed] [Google Scholar]
- 61.Hu W.J., Yu A.Q., Wang S., Bai Q.X., Tang H.P., Yang B.Y., Wang M., Kuang H.X. Extraction, purification,structural characteristics, biological activities, and applications of the polysaccharides from Zingiber officinale Roscoe. (Ginger): a review. Molecules. 2023;28(9):3855. doi: 10.3390/molecules28093855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wan J., Jiang C.X., Tang Y., Ma G.L., Tong Y.P., Jin Z.X., Zang Y., Osman E.E.A., Li J., Xiong J., Hu J.F. Structurally diverse glycosides of secoiridoid, bisiridoid, and triterpene-bisiridoid conjugates from the flower buds of two Caprifoliaceae plants and their ATP-citrate lyase inhibitory activities. Bioorg. Chem. 2022;120 doi: 10.1016/j.bioorg.2022.105630. [DOI] [PubMed] [Google Scholar]
- 63.Xin T., Zhang F.B., Jiang Q.Y., Chen C.H., Huang D.Y., Li Y.J., Shen W.X., Jin Y.H., Sui G.J. The inhibitory effect of a polysaccharide from Codonopsis pilosula on tumor growth and metastasis in vitro. Int. J. Biol. Macromol. 2012;51(5):788–793. doi: 10.1016/j.ijbiomac.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y.X., Wang Q.S., Wang T., Zhang H.K., Tian Y., Luo H., Yang S., Wang Y., Huang X. Inhibition of human gastric carcinoma cell growth in vitro by a polysaccharide from Aster tataricus. Int. J. Biol. Macromol. 2012;51(4):509–513. doi: 10.1016/j.ijbiomac.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 65.Du L., Mei H.F., Yin X., Xing Y.Q. Delayed growth of glioma by a polysaccharide from Aster tataricus involve upregulation of Bax/Bcl-2 ratio, activation of caspase-3/8/9, and downregulation of the Akt. Tumour. Biol. 2014;35(3):1819–1825. doi: 10.1007/s13277-013-1243-8. [DOI] [PubMed] [Google Scholar]
- 66.Yao P.B., Liu Y.L., Zhu Z.G., Zhao H.H., Zhang J.X. Effects and aster water extract on proliferation and invasion on human lung cancer A549 cells, and tumorigenesis ability of nude mice. Chin. J. Pharm. Anal. 2022;42(3):380–386. [Google Scholar]
- 67.Liu Z.Q. Anti-oxidant in China: a thirty-year journey. Am. J. Chin. Med. 2019;47(5):1005–1024. doi: 10.1142/S0192415X19500514. [DOI] [PubMed] [Google Scholar]
- 68.Ma C.H., Dastmalchi K.V., Whitaker B.D., Kennelly E.J. Two new antioxidant malonated caffeoylquinic acid isomers in fruits of wild eggplant relatives. J. Agric. Food Chem. 2011;59(17):9645–9651. doi: 10.1021/jf202028y. [DOI] [PubMed] [Google Scholar]
- 69.Li K., Xia T.S., Jiang Y.P., Wang N.N., Lai L.Y., Xu S.Y., Yue X.Q., Xin H.L. A review on ethnopharmacology, phytochemistry, pharmacology and potential uses of Portulaca oleracea L. J. Ethnopharmacol. 2024;319(Pt 2) doi: 10.1016/j.jep.2023.117211. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y.P., Zhang H.L., Yang Y.S., Shi Z.C. In vitro antioxidant activity of different polar parts of Aster tataricus L. f. extracts. Lishizhen. Med. Mater. Med. Res. 2011;22(11):2799–2800. [Google Scholar]
- 71.de Oliveira M.R., Chenet A.L., Duarte A.R., Scaini G., Quevedo J. Molecular mechanisms underlying the anti-depressant effects of Resveratrol: a review. Mol. Neurobiol. 2018;55(6):4543–4559. doi: 10.1007/s12035-017-0680-6. [DOI] [PubMed] [Google Scholar]
- 72.Wan Y.C., Liu Y.Y., Yang H.T., Zhang Q.Y., Liao M., Zhang X., Zhang L.T. Simultaneous determination of nine constituents in Asteris Radix by HPLC-MS/MS. Chin. Tradit. Herb. Drugs. 2016;47(14):2534–2539. [Google Scholar]
- 73.Zhang Y.X., Wang Q.S., Wang T., Zhang H.K., Tian Y., Luo H., Yang S., Wang Y., Huang X. Inhibition of human gastric carcinoma cell growth in vitro by a polysaccharide from Aster tataricus. Int. J. Biol. Macromol. 2012;51(4):509–513. doi: 10.1016/j.ijbiomac.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 74.Zhou W.B., Zeng G.Z., Xu H.M., He W.J., Tan N.H. Astataricusones A-D and astataricusol A, five new anti-HBV shionane-type triterpenes from Aster tataricus L. f. Molecules. 2013;18(12):14585–14596. doi: 10.3390/molecules181214585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X.X., Tang Y.L., Liu Y.L., Xu K.H. Analysisi of effective constituents from Aster tataricus L.f. and extracting of alkaloid and its antibacterial test in vitro. J. Tradit. Chin. Vet. Med. 2006;(1):16–18. [Google Scholar]
- 76.Shen Y., Luo Q., Xu H.M., Gong F.Y., Zhou X.B., Sun Y., Wu X.F., Liu W., Zeng G.Z., Tan N.H., Xu Q. Mitochondria-dependent apoptosis of activated T lymphocytes induced by astin C, a plant cyclopeptide, for preventing murine experimental colitis. Biochem. Pharmacol. 2011;82(3):260–268. doi: 10.1016/j.bcp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 77.Li S.L., Hong Z., Wang Z., Li F., Mei J.H., Huang L.L., Lou X.W., Zhao S.M., Song L.H., Chen W., Wang Q., Liu H., Cai Y.N., Yu H.S., Xu H.M., Zeng G.Z., Wang Q.Y., Zhu J.J., Liu X., Tan N.H., Wang C. The cyclopeptide astin C specifically inhibits the innate immune CDN sensor STING. Cell Rep. 2018;25:3405–3421. doi: 10.1016/j.celrep.2018.11.097. [DOI] [PubMed] [Google Scholar]
- 78.Chen Y.J., Dong J.J., Liu J., Xu W.J., Wei Z.Y., Li Y.T., Wu H., Xiao H.B. Network pharmacology-based investigation of protective mechanism of Aster tataricus on lipopolysaccharide-induced acute lung injury. Int. J. Mol. Sci. 2019;20(3):543. doi: 10.3390/ijms20030543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rho J.H., Seo C.S., Park H.S., Jeong H.Y., Moon O.S., Seo Y.W., Son H.Y., Won Y.S., Kwun H.J. Asteris Radix et rhizoma suppresses testosterone-induced benign prostatic hyperplasia in rats by regulating apoptosis and inflammation. J. Ethnopharmacol. 2020;255 doi: 10.1016/j.jep.2020.112779. [DOI] [PubMed] [Google Scholar]
- 80.Li X., Gong Y.X., Feng L., Wang X.J., Wang J.W., Zhang A.X., Tan N.H., Wang Z. Neuropyrones A-E, five undescribed α-pyrone derivatives with tyrosinase inhibitory activity from the endophytic fungus Neurospora dictyophora WZ-497. Phytochemistry. 2023;207 doi: 10.1016/j.phytochem.2022.113579. [DOI] [PubMed] [Google Scholar]
- 81.Lee S.J., Yang H., Kim S.C., Gu D.R., Ryuk J.A., Jang S.A., Ha H. Ethanol extract of Radix Asteris suppresses osteoclast differentiation and alleviates osteoporosis. Int. J. Mol. Sci. 2023;24(22) doi: 10.3390/ijms242216526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.