Abstract
The increasing need for alternative and sustainable energy sources, prompted by the depletion of fossil fuels and the rise in greenhouse gas emissions, has generated attention towards exploring fast-growing filamentous fungi as a potential bioenergy source. This study aimed to optimize Ganoderma lucidum production for elevated biomass and lipid yields in submerged liquid fermentation. The optimization involved varying initial pH, glucose concentration, and agitation rate using response surface methodology (RSM) with central composite design (CCD). Glucose concentration and initial pH significantly influenced biomass production, while agitation rate had an insignificant effect. For lipid production, glucose concentration, initial medium pH, and agitation rate were identified as significant factors. The optimized conditions (initial pH 6, 50 g/L glucose concentration, and 113.42 rpm) were validated in 500 mL shake flasks and a 3 L Air-L-Shaped Bioreactor (ALSB). Shake flask results showed 8.33 g/L of biomass and 2.17 % of lipid, while the ALSB system produced 5.32 g/L of biomass and 2.35 % lipid. The obtained Ganoderma lucidum mycelial lipid underwent acid-catalysed transesterification to produce biodiesel, which was subjected to several tests to comply ASTM and EN standards. This study serves as a valuable reference for future biodiesel applications through the optimization of Ganoderma lucidum biomass and lipid production.
Keywords: Fungi, Optimization, Liquid fermentation, Lipid, Biodiesel
Graphical abstract
Highlights
-
•
The biomass and total lipid production were optimized using RSM.
-
•
Optimum level of initial pH, glucose and agitation are pH 6, 50 g/L and 113.42 rpm.
-
•
Lipid is extracted from G. lucidum mycelium biomass using solvent extraction.
-
•
The obtained Ganodiesel complied ASTM D6751 and EN 14214 standards.
1. Introduction
The rapid growth of the population coupled with the need for lifestyle improvement has greatly increased the demand for water, food, and energy resources. Fossil fuels are contributing as the primary energy for the world's global energy demand and economy, particularly transportation fuel, which has a substantial environmental impact. Furthermore, fossil fuel resources are non-renewable and depleted on a daily basis [1,2]. Thus, there are growing appeals for biodiesel as a feasible replacement to fossil fuels due to its advantages over other biofuels. Biodiesels are renewable, bio-degradable, low emission of pollutants, low toxicity, eco-friendly, high lubricity and suitable for diesel engines with minimal mechanical modifications [3].
Nevertheless, biodiesel production also faces some technical challenges. The critical issue is the requirement for high productivity of raw materials, which can influence market demand [3,4]. First-generation feedstocks involve in competition with food production and land use change due to large-scale cultivation of edible crops for biodiesel production. Second-generation biodiesel and third-generation biodiesel, which employs non-edible crops and algae feedstocks, respectively, confronts the challenges such as environmental concerns associated with intensive water and fertilizer use, as well as scale-up challenges and cost competitiveness [4]. Therefore, there is a need to explore promising new source of feedstocks to enable cost-effective, landless biodiesel production without contributing to food-energy conflicts. There has been less previous evidence for the use of fungi-based feedstock. Fungi can grow rapidly and are highly productive, often employed in biotechnology applications. It would be of special interest to study the effectiveness of fungi-based feedstocks as a new source of biodiesel [5]. Several fungi species, such as Mucor circinelloides, Mortierella isabelline and Mortierella alpina have been identified for their lipid ability to produce lipids [6]. Fazili et al. [7] reported that Mucor circinelloides produces relatively high lipid yields (15–52 %) but limited by a slow cell growth rate and low biomass yield.
Ganoderma lucidum has been extensively studied for its medicinal properties and bioactive compounds, and exploiting these diverse properties makes it as an attractive fungi for lipid production research [8]. Although some previous studies have focused on the lipid content of Ganoderma lucidum, primarily in the fruiting body or spores, there remains a gap in knowledge regarding its mycelium lipids. Ganoderma lucidum is a xylotrophic basidial fungi that capable of accumulating high level of lipids in the mycelium, the majority of which are triglycerides [9]. Several studies have shown that Ganoderma lucidum produces lipid with a fatty acid composition consisting mainly of oleic acid (C18:1), linoleic acid (C18:2) and palmitic acid (C16:0), which could be a potential source for biodiesel production [8,10]. Salvatore et al. [8] reported that lipid extraction from Ganoderma lucidum spores using n-hexane yielded 26.43 %, comprising 26.4 % saturated fatty acids, 70.3 % of monounsaturated fatty acids, and 3.19 % polyunsaturated fatty acids. Additionally, its simple, fast-growing nature and adaptability to different growth conditions enhances its potential for large-scale lipid production.
The cultivation of Ganoderma lucidum through submerged-liquid fermentation (SLF) generates a higher yield of mycelium biomass under controlled parameters and requires shorter fermentation times compared to solid fermentation. It also simplifies downstream processing as mycelium can be harvested directly from the liquid medium. Recent studies have introduced an innovative Air-L-Shaped Bioreactor (ALSB) for fungi liquid fermentation, addressing challenges encountered with other bioreactors. The ALSB design can eliminate issues such as fungi clumping and growth on the vessel walls, which can hinder optimal growth and biomass production [11]. In this study, the ALSB will be utilized to validate optimized biomass and lipid production models.
Furthermore, this research will apply response surface methodology (RSM) to optimize the fermentation conditions of Ganoderma lucidum, in terms of pH, agitation (rpm) and glucose concentration (g/L). Several researches have reported a correlation between the growth of Ganoderma lucidum mycelium and various factors, including medium initial pH, nutrient composition, and agitation. Glucose serves as an essential carbon source for Ganoderma lucidum mycelial growth, with concentrations typically ranging from 10 to 50 g/L. Moreover, acidic pH medium has been identified as more favourable for Ganoderma lucidum mycelial growth and synthesis of metabolites. The moderate agitation rate effectively promotes to enhance nutrient mixing and oxygen transfer, thereby facilitating mycelium growth and metabolites production [12,13]. According to Supramani et al. [12], RSM was utilized to determine the optimal fermentation conditions for mycelium biomass and polysaccharide production. RSM is an effective method to determine the interaction and relationship that exists between the set of experimental factors and response variables compared to the conventional “one-factor-at-a-time” (OFAAT) method. RSM allows to evaluate the independent variables and analyse the significant and insignificant factors [5].
This paper aims to optimize the biomass and lipid production of Ganoderma lucidum mycelium for Ganodiesel production. This study will apply solvent extraction (SVE) to extract the Ganoderma lucidum mycelium lipid. Solvent extraction was chosen as it offers comparable yield to other methods, while being simple, requiring less time, minimal biomass requirement and without the need for expensive tools for lipid extraction [10]. The obtained lipid will be converted into biodiesel and tested for compliance with biodiesel standards.
2. Materials and methods
2.1. Strain selection and media composition
Ganoderma lucidum strain QRS 5120 was cultivated in the Functional Omics and Bioprocess Development Laboratory, Institute of Biological Sciences, University Malaya. The Ganoderma lucidum mycelium was cut from the mother plate with the dimension of 1 cm × 1 cm, followed by subculturing on the potato dextrose agar (PDA) plate. The culture plates were incubated at room temperature for 7 days. The culture plates were kept for further use in batch fermentation. The medium composition for two-seed cultures used in all stages of fermentation are constant at (g/L): Yeast Extract (1), NH4Cl (4), MgSO4 (0.5), KH2PO4 (0.5) and K2HPO4 (0.5), unless otherwise stated [13].
2.2. Submerged-liquid fermentation
The submerged-liquid fermentation was conducted according to Hassan et al. [5] with slight modifications. The inoculum was prepared in a 500-mL Erlenmeyer flask accordingly to the media composition and fermentation conditions in Table 1. The media was fixed at the following conditions: 50–150 rpm agitation, 10 g/L-50 g/L glucose concentration, and initial pH 4–6. The first seed culture was prepared by cutting 3 mycelial agar squares and inoculated in a 500 mL Erlenmeyer flask containing 100 mL medium. The first seed culture was incubated for 10 days. Then, the first seed culture containing mycelium pellet was homogenised using a sterile Waring hand mixer. After that, 40 mL (20 % v/v) of the blended mycelium was transferred into another 500 mL Erlenmeyer flask containing 160 mL fresh medium (second seed culture) and incubated for another 10 days.
Table 1.
Experimental range and levels of the independent variables.
| Independent variables | Range and levels |
||
|---|---|---|---|
| −1 | 0 | 1 | |
| Initial pH | 4 | 5 | 6 |
| Glucose (g/L) | 10 | 30 | 50 |
| Agitation (rpm) | 50 | 100 | 150 |
2.3. Optimization of fermentation conditions using RSM
The fermentation conditions of Ganoderma lucidum including initial pH, glucose (g/L) and agitation (rpm) were optimized using Response Surface Methodology (RSM). RSM with central composite design (CCD) was selected to establish experimental set in Design Expert 13.0 software. The experimental range and levels of the variables for this research is shown in Table 1. The alpha value was set to 1.0. The lowest level for variables were initial pH, pH 4; glucose concentration, 10 g/L; and agitation rate, 50 rpm; and the highest level were initial pH 6; glucose concentration, 50 g/L; and agitation rate, 150 rpm. Then, 20 experiments were generated using CCD design with selected variables and responses, as indicated in Table 1. All experiments were carried out in triplicate.
2.4. Verification of optimized model in 500-mL shake flask and 3 L air-l-shaped bioreactor (ALSB)
The RSM model was applied to develop the optimized values for maximising biomass and lipid production of Ganoderma lucidum. The optimized values were verified in 500-mL shake flask fermentation in triplicate. The verification experiment for biomass and lipid production was carried out in a 3 L ALSB for the consideration of the feasibility to scale-up the Ganoderma lucidum cultivation for biodiesel production. ALSB design, featuring a vessel with an L-shaped part and devoid of sharp corners and baffles, minimizes the risk of mycelial wall growth. The inclusion of L-shaped stirrer with air sparging function ensures uniform mixing of the fermentation media and prevents mycelium anchoring. The 20 % of second seed culture was used as the inoculum for the ALSB with 2 L of total working volume (w/v). The verification assessments for both shake flasks and bioreactors were performed in dark for 7–10 days. The cultured mycelium was harvested when it reached at the stationary phase of growth for the calculation of total biomass and lipid production [11].
2.5. Pellet morphology
An optical microscope (CX23LEDFRS1, Hamburg, Germany) was used to analyse the morphology of sample pellets. A 10 mL culture sample was collected and transferred from the shake flask to a petri dish. The pellets from each culture were randomly selected from the petri dish and placed onto a slide for microscope observation at 4× magnification. The pellet morphology from each culture was observed in triplicate [14].
2.6. Lipid extraction
Lipid extraction for Ganoderma lucidum mycelial biomass was performed through solvent extraction method as described by Jahis et al. [10] with slight modification. The dried mycelium biomass was ground into a fine powder. The lipid was extracted using hexane (Grade AR, Sigma-Aldrich, Gillingham, Dorset, U.K) at a 1:100 (g/mL) of biomass to solvent ratio. The biomass powder was soaked in a 1 L beaker containing hexane and placed on a hot plate. Then, the sample was stirred for 2 h using magnetic stirrer at 60 °C. The sample was filtered using Whatman GF/C glass fibre filter, followed by hexane evaporation using a rotary evaporator at 40 °C.
2.7. Analytical methods
2.7.1. Determination of mycelium biomass
The pre-dried and pre-weighed filter paper was used to filter the fermentation broth. The fermentation broth was filtered through a Buchner funnel filter set and the mycelial biomass was washed repeatedly with distilled water. The mycelial biomass was dried in a food dehydrator at 35 °C to a constant weight. The dry weight of mycelial biomass was calculated by subtracting the pre-weighed filter paper [13].
2.7.2. Lipid yield determination
The extracted lipid will remain dissolved in the hexane solvent, thus rotary evaporator was used to remove the excess solvent. The mass of lipid remaining was weighed after the evaporation of hexane. The extracted lipid yield was calculated by applying the formula as follow [14]:
whereas the coefficient mi (g/L) is the weight of recovered lipid while ms (g/L) is the weight of pre-dried and pre-weighed biomass used for SVE extraction.
2.8. Statistical analysis
The internal statistical tool in Design Expert 13.0 software (Stat-Ease, Minneapolis, USA) was applied to conduct analysis of variance (ANOVA) for the CCD quadratic model. The statistical significance for each of the model coefficients will be presented by values of p < 0.05.
2.9. Biodiesel production through transesterification
Acid-catalysed transesterification as described by Redzwan et al. [15] with some modifications was performed to convert the extracted Ganoderma lucidum lipids to biodiesel. Acid-catalysed transesterification was conducted in a laboratory scale by using a 1000 mL round-bottomed glass flask. Sulphuric acid was used as the acidic catalyst while anhydrous methanol (99 %) was used as the reactant. The reaction product was evaporated by rotary evaporator at 70 °C for 20 min. Then, gravity settling using separating funnel was performed to separate the upper layer fatty acid methyl ester (FAME) from glycerol at lower layer.
2.10. Determination of biodiesel properties
To determine the compliance of Ganoderma lucidum biodiesel with the standard specifications, the biodiesel characteristics of biodiesel was examined by conducting various tests following the international standards [16,17]. Kinematic viscosity was assessed at 40 °C using the Automatic Kinematic Viscosity Measuring System AKV-201, following the guidelines outlined in ASTM D445. The cloud point, determined through ASTM D6749, was analyzed utilizing the Mini Cloud Point Tester MPC-102 within a temperature range spanning from −60 °C to 51 °C. Ignition point evaluation was performed using the Pensky-Martens Closed Cup Automated Flash Point Tester APM-7, in accordance with ASTM D93 specifications. The density was measured through a density meter, following the ASTM D4052 standard. Oxidation stability testing was conducted on Rancimat 743 (Methrom, Herisau, Switzerland), adhering to EN 14112 standards. All assessments were carried out using certified equipment supplied by TANAKA Scientific Ltd., based in Tokyo. For the measurement of acid number, ester content, total glycerol content, monoglyceride, diglyceride, triglyceride, all the tests were conducted according to the European Standard Methods with suitable modifications where applicable [16].
3. Results and discussion
3.1. Optimization
This study employed RSM to evaluate the effect of initial pH, glucose concentration (g/L) and agitation rate on the biomass, and lipid production. A total of 20 different runs were conducted on the production of biomass and lipid from Ganoderma lucidum, as outlined in Table 2. The significance of each coefficient was determined using ANOVA, where significance was denoted by a p-value less than 0.05.
Table 2.
Experimental sets designed by RSM and the results for the biomass and lipid yields.
| Run No. | Variables |
Responses |
|||||
|---|---|---|---|---|---|---|---|
| Initial |
Glucose |
Agitation |
Biomass |
Total Lipid (%) |
|||
| pH | (g/L) | (rpm) | (DCW g/L) |
||||
| Actual | Predicted | Actual | Predicted | ||||
| 1 | 4 | 30 | 100 | 5.28 | 6.03 | 2.90 | 2.77 |
| 2 | 4 | 50 | 150 | 2.30 | 2.46 | 2.19 | 2.26 |
| 3 | 6 | 10 | 50 | 1.03 | 0.81 | 4.00 | 3.98 |
| 4 | 4 | 50 | 50 | 3.95 | 3.64 | 2.94 | 3.02 |
| 5 | 5 | 30 | 100 | 6.07 | 6.40 | 2.92 | 3.11 |
| 6 | 5 | 10 | 100 | 3.67 | 4.25 | 3.96 | 4.01 |
| 7 | 5 | 30 | 100 | 6.87 | 6.40 | 3.33 | 3.11 |
| 8 | 5 | 30 | 150 | 3.54 | 3.53 | 2.48 | 2.41 |
| 9 | 4 | 10 | 150 | 1.93 | 1.46 | 2.33 | 2.30 |
| 10 | 5 | 50 | 100 | 7.07 | 6.74 | 3.68 | 3.39 |
| 11 | 5 | 30 | 100 | 6.81 | 6.40 | 2.96 | 3.11 |
| 12 | 5 | 30 | 100 | 6.26 | 6.40 | 3.38 | 3.11 |
| 13 | 6 | 10 | 150 | 2.07 | 2.31 | 3.98 | 3.96 |
| 14 | 5 | 30 | 50 | 3.10 | 3.37 | 2.98 | 2.81 |
| 15 | 6 | 50 | 150 | 4.70 | 4.78 | 3.54 | 3.58 |
| 16 | 5 | 30 | 100 | 6.77 | 6.40 | 2.93 | 3.11 |
| 17 | 6 | 50 | 50 | 4.40 | 4.80 | 2.69 | 2.78 |
| 18 | 5 | 30 | 100 | 6.12 | 6.40 | 2.64 | 3.11 |
| 19 | 4 | 10 | 50 | 1.27 | 1.13 | 3.88 | 3.89 |
| 20 | 6 | 30 | 100 | 7.53 | 7.03 | 3.58 | 3.48 |
3.1.1. Biomass production optimization
The ANOVA for mycelium biomass production is presented in Table 3. The predicted coefficient determination indicates that 96.83 % (R2 = 0.9683) of the variability in the actual response can be explained using this model. The model is significant (p < 0.005). The adjusted coefficient determination value (Adj. R2 = 0.9397) implies the significance of the model and is in reasonable agreement with the predicted R2 value (0.7553), with a difference of less than 0.2. The model for biomass yield was regressed by considering the actual variables and is expressed in Eq (1).
| Biomass = − 4.16075 −1.94516 × Initial pH + 0.143333 × Glucose + 0.220156 × Agitation + 0.0184906 × Initial pH × Glucose + 0.00582375 × Initial pH × Agitation − 0.000379712 × Glucose × Agitation + 0.130877 × Initial pH2 − 0.00225856 × Glucose2 − 0.00118147 × Agitation2 | (1) |
Table 3.
Analysis of variance (ANOVA) for the experimental outcomes of the CCD quadratic model applied to Ganoderma lucidum mycelium biomass.
| Source | Sum of Squares | df | Mean Square | F-value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 81.79 | 9 | 9.09 | 33.9 | <0.0001a | significant |
| A-Initial pH | 2.51 | 1 | 2.51 | 9.35 | 0.0121a | significant |
| B-Glucose | 15.53 | 1 | 15.53 | 57.92 | <0.0001a | significant |
| C-Agitation | 0.0632 | 1 | 0.0632 | 0.2356 | 0.6378 | |
| AB | 1.09 | 1 | 1.09 | 4.08 | 0.0710 | |
| AC | 0.6783 | 1 | 0.6783 | 2.53 | 0.1428 | |
| BC | 1.15 | 1 | 1.15 | 4.3 | 0.0648 | |
| A2 | 0.0471 | 1 | 0.0471 | 0.1757 | 0.6839 | |
| B2 | 2.24 | 1 | 2.24 | 8.37 | 0.016 | significant |
| C2 | 23.99 | 1 | 23.99 | 89.5 | <0.0001a | significant |
| Residual | 2.68 | 10 | 0.2681 | |||
| Lack of Fit | 1.99 | 5 | 0.3981 | 2.88 | 0.135 | not significant |
| Pure Error | 0.6904 | 5 | 0.1381 | |||
| Cor Total |
84.47 |
19 |
||||
| Std. Dev. = 0.5178 | R2 = 0.9683 | Adeq Precision = 16.9985 | ||||
| Mean = 4.54 | Adjusted R2 = 0.9397 | |||||
Significant value.
From the model, glucose (B) shows the strongest effect (p < 0.0001) on biomass, while initial pH (A) shows a significant effect at p < 0.05. The quadratic terms of glucose (B2) and agitation (C2) show significant effect at p < 0.05 on the production of mycelium biomass. However, negative effects are shown by agitation (C) and the quadratic terms (A2, AB, AC and BC). Fig. 1 displays the combined effect of initial pH, glucose concentration and agitation in three-dimensional (3D) surface. Fig. 1a shows the effect of initial pH (A) and starting glucose concentration (B), Fig. 1b shows the effect of A and agitation rate (C), and Fig. 1c shows the effect of B and C on biomass production. Fig. 1a indicates that an increase in both pH and glucose levels corresponds to a simultaneous increase in biomass production, suggesting a collaborative effect between pH and glucose. Fig. 1b demonstrates that maintaining agitation at an intermediate level, along with an increase in pH, leads to a higher production of biomass. Meanwhile, in Fig. 1c, it is observed that the middle rate agitation coupled with an increase in glucose concentration, results in a higher production of biomass. The maximum biomass yield was obtained at initial pH 5.88, 44.88 g/L glucose concentration and 97.23 rpm.
Fig. 1.
Response surface curve (3D plot) of mycelium biomass from Ganoderma lucidum showing the interaction between (a) Initial pH and glucose, (b) Initial pH and agitation, (c) Glucose and agitation.
The initial pH of the medium affects the availability and solubility of nutrients required for mycelial growth, thus influencing the consumption rates during fermentation [18]. Glucose serves as the primary carbon source for mycelial growth and development. Therefore, the availability and concentration of glucose in fermentation medium directly impact the mycelium biomass production [19]. Low agitation speed limits the dissolved oxygen levels in the medium, hindering mycelium oxygenation and consequently affecting mycelium growth. Conversely, excessively high rotational speeds can induce mechanical stress on the mycelial structures, leading to mycelial autolysis and reducing biomass productivity [20].
3.1.2. Optimization of lipid production
The ANOVA data for lipid production of Ganoderma lucidum is shown in Table 4. The predicted coefficient determination indicates that 90.01 % (R2 = 0.9001) of the variability in the actual response can be explained using this model. The p-value was 0.0020 (p < 0.005), indicating that the model was significant. The adjusted coefficient determination value (Adj. R2 = 0.8102) indicates the significance of the model and is in reasonable agreement with the predicted R2 value (0.7226), with a difference is less than 0.2. The regression model based on the actual factor of lipid can be expressed using Eq (2).
| Lipid = 5.87637 − 0.474875 × Initial pH − 0.104437 × Glucose − 0.0095075 × Agitation − 0.0041875 × Initial pH × Glucose + 0.0078 × Initial pH × Agitation + 0.00020625 × Glucose × Agitation + 0.0175 × Initial pH2 + 0.0014875 × Glucose2 − 0.000198 × Agitation2 | (2) |
Table 4.
Analysis of variance (ANOVA) for the experimental outcomes of the CCD quadratic model for total lipid production.
| Source | Sum of Squares | df | Mean Square | F-value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 5.46 | 9 | 0.6061 | 10.01 | 0.0006a | significant |
| A-Initial pH | 1.26 | 1 | 1.26 | 20.76 | 0.0010a | significant |
| B-Glucose | 0.961 | 1 | 0.961 | 15.88 | 0.0026a | significant |
| C-Agitation | 0.3842 | 1 | 0.3842 | 6.35 | 0.0304a | significant |
| AB | 0.0561 | 1 | 0.0561 | 0.9271 | 0.3583 | |
| AC | 1.22 | 1 | 1.22 | 20.1 | 0.0012a | significant |
| BC | 0.3403 | 1 | 0.3403 | 5.62 | 0.0392a | significant |
| A2 | 0.0008 | 1 | 0.0008 | 0.0139 | 0.9084 | |
| B2 | 0.9736 | 1 | 0.9736 | 16.08 | 0.0025a | significant |
| C2 | 0.6738 | 1 | 0.6738 | 11.13 | 0.0075a | significant |
| Residual | 0.6053 | 10 | 0.0605 | |||
| Lack of Fit | 0.2114 | 5 | 0.0423 | 0.5368 | 0.7444 | not significant |
| Pure Error | 0.3938 | 5 | 0.0788 | |||
| Cor Total |
6.06 |
19 |
||||
| Std. Dev. = 0.246 | R2 = 0.9001 | Adeq Precision = 10.048 | ||||
| Mean = 3.16 | Adjusted R2 = 0.8102 | |||||
Significant value.
From the model, both initial pH (A) and glucose concentration (B) showed a significant effect at p < 0.005. Agitation (C) shows a significant effect at p < 0.05. Among the three variables, initial pH showed the highest significant (p = 0.0010), followed by glucose concentration (p = 0.0026) and agitation rate (p = 0.0304). The quadratic terms (AC, BC, B2 and C2) also showed a significant effect (p < 0.05) on total lipid production. However, negative effects are shown by quadratic terms (AB and A2). Fig. 2 displays the merged effect of initial pH, glucose concentration and agitation in response surface curve. Fig. 2a shows the effect of initial pH (A) and starting glucose concentration (B), Fig. 2b shows the effect of A and agitation (C) and Fig. 2c shows the effect of B and C on total lipid production. Fig. 2a and c shows that decreasing in glucose concentration leads to an increase in total lipid production. By increasing the initial pH as depicted in Fig. 2a and c, leads to an increase in the total lipid yield. According to Fig. 2b and c, the agitation rate significantly impacts the total lipid yield. Both figures demonstrate that total lipid production decreases at both lower and higher agitation rates, with the most favourable lipid yield observed within the 90–110 rpm range. The maximum total lipid yield was obtained at initial pH 4.68, 10.11 g/L glucose concentration and 67.92 rpm.
Fig. 2.
Response surface curve (3D plot) of total lipid production from Ganoderma lucidum mycelium biomass showing the interaction between (a) Initial pH and glucose, (b) Initial pH and agitation, (c) Glucose and agitation.
The rate of agitation would influence the state of mycelium cells, leading to both positive and negative effects. Positive impact caused by agitation is enhancing oxygen and nutrient solubility, ensuring their uniform distribution, which in turn fosters increased lipid production. On the other hand, an excessively high agitation rate can have a detrimental impact by inducing shear stress and damaging the mycelium cells [21]. For example, Adriana Tita and Miftahul [22] suggests that maintaining the agitation rate at 100 rpm, ranging from 0 to 200 rpm, resulted in an optimal increased in lipid production of fungal BR 2.2 isolate. Moreover, a higher lipid yield is observed at a lower glucose concentration might be due to the nutrient limitation or starvation, which caused an environment stress that prompts cells to accumulate lipid as a response. The impact of initial medium pH on lipid production could be because of its influence on cell growth and nutrient uptake [23].
3.2. Verification of the optimized conditions
After construction of the biomass and total lipid CCD quadratic model, the biomass and total lipid of Ganoderma lucidum was optimized using Design Expert software. Table 5 shows the optimized conditions applied to verify the mycelium biomass and total lipid production in statistical models. To validate the strength and accuracy of the model under Eq (1) and Eq (2), various experiments were performed. The validation experiments were performed in 500-mL shake flasks and 3 L Air-L-Shaped Bioreactor under controlled conditions. The biomass and total lipid obtained in shake flasks were 8.66 g/L and 3.62 g/L, respectively, under maximised conditions. Maximising both biomass and total lipid production was achieved under conditions of initial pH 6, 50 g/L glucose concentration and agitation speed at 113.40 rpm. This resulted in 8.33 g/L of biomass and 2.17 g/L of total lipid in shake flasks, whereas ALSB produced 5.32 g/L of biomass and 2.35 g/L of total lipid. The total lipid production in ALSB was 1.1-fold higher than in the shake flaks. However, the biomass production in ALSB was lower than in shake flasks. The fermentation parameters, including dissolved oxygen and agitation rate, affected the production of both biomass and lipid in ALSB. The use of L-shaped impeller and supplied of oxygen in ALSB provided aeration and potentially resulting in increased lipid production by the mycelium [14,24]. The study conducted by Saad et al. [25] on the lipid production of Cunninghamella bainieri 2A1 reported that aeration facilitates the nutrient mixing and supplies dissolved oxygen in the fermentation medium, resulting in increased lipid production. Aeration also positivity impacts the consumption of sugar by lipid-producing fungi. However, the biomass produced in ALSB in this study was lower, likely due to suboptimal parameters for biomass production [11]. Additionally, Fig. 3 depicts the upstream and downstream processes of Ganoderma lucidum submerged liquid fermentation in 500 mL-shake flasks and 3 L ALSB for biomass and lipid production. Fig. 3a depicted the first seed culture in shake flasks while Fig. 3b showed the second seed culture fermentation in a 3 L ALSB. On the other hand, Fig. 3c and d, depicted dried mycelium biomass and ground mycelium biomass. Ganoderma lucidum lipid collected after removing excessive solvent using rotary evaporator is shown in Fig. 3e followed by the final obtained lipid in Fig. 3f.
Table 5.
Validation of the model with the optimized conditions.
| To maximize | Variables |
Predicted Responses |
Actual Responses |
||||
|---|---|---|---|---|---|---|---|
| Initial pH | Glucose (g/L) | Agitation (rpm) | Biomass (DCW g/L) | Total Lipid (%) | Biomass (DCW g/L) | Total Lipid (%) | |
| Biomass | 5.88 | 44.89 | 97.23 | 7.60 | – | 8.66 (SF)a | – |
| Total lipid | 4.68 | 10.11 | 67.92 | – | 4.00 | – | 3.62 (SF) |
| Biomass and Total lipid | 6.00 | 50.00 | 113.40 | 7.53 | 3.75 | 8.33 (SF) 5.32 (ALSB)a |
2.17 (SF) 2.35 (ALSB) |
SF = 500 mL shake flask, ALSB = 3 L Air-L-Shaped Bioreactor.
Fig. 3.
The cultivation Ganoderma lucidum in submerged liquid fermentation (SLF) and its products: (a) first seed culture in shake flasks, (b) second seed culture fermentation in a 3 L ALSB, (c) dried mycelium biomass, (d) ground mycelium biomass, (e) Ganoderma lucidum lipid collected after removing excessive solvent using rotary evaporator, and (f) Ganoderma lucidum lipid.
3.3. Biodiesel properties of Ganoderma lucidum FAME (Ganodiesel)
Table 6 presents a comparison of biodiesel properties between Ganoderma lucidum FAME (Ganodiesel) and international standards. The findings indicate that Ganodiesel aligns with the biodiesel properties outlined by the US (ASTM D6751) and EU (EN 14214) standards. Specifically, the parameters including kinematic viscosity (4.5 mm2/s), ignition point (172 °C), cloud point (−1.5 °C), density (835 kg/cm3), oxidation stability (8 h), acid value (0.35 mg KOH/g), FAME content (97.2 %), and total glycerol content (0.18 %) are found to fall within the prescribed range established by the international standards.
Table 6.
The biodiesel properties of Ganoderma lucidum FAME in comparison with international standards [16,17].
| Properties | Unit | Method | FAME (Ganodiesel) | EU (EN 14214) | US (ASTM D6751) |
|---|---|---|---|---|---|
| Kinematic viscosity (40 °C) | mm2/s | ASTM D445 | 4.5 | 3.5–5.0 | 1.9–6.0 |
| Cloud point | °C | ASTM D6749 | −1.5 | – | −3.0 to 12.0 |
| Ignition point | °C | ASTM D93 | 172 | ≥120 | ≥130 |
| Density | kg/cm3 | ASTM D4052 | 835 | <850 | 860–900 |
| Oxidation stability | h | EN 14112 | 8 | ≥6 | ≥3 |
| Acid value | mg KOH/g | EN 14104 | 0.35 | <0.8 | <0.5 |
| FAME Content | wt% | EN 14103 | 97.2 | aMin 96.5 | – |
| Total Glycerol | wt% | EN 14105 | 0.18 | aMax 0.25 | Max 0.24 |
| Monoacylglycerol | wt% | EN 14105 | 0.45 | Max 0.8 | – |
| Diacylglycerol | wt% | EN 14105 | 0.18 | Max 0.2 | – |
| Triacylglycerol | wt% | EN 14105 | 0.12 | Max 0.2 | – |
Min - minimum; *Max – maximum; - no standard limits classified by ASTM standards.
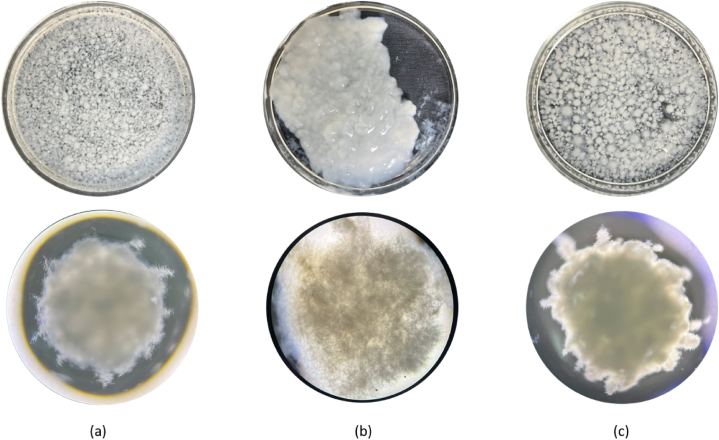
3.4. Pellet morphology
During the verification process, the pellet morphology for maximising levels of biomass and lipid for Ganoderma lucidum was observed both macroscopically and microscopically, as depicted in Fig. 4. The findings indicate that the shape of mycelial pellets varies under different cultivation parameters, with each response favouring distinct morphology characteristics. In Fig. 4a, the formation of small and starburst-like globular pellets with loosely branching outer layer were observed. This result aligns with literature reports, where small-compact pellets are noted to favour high biomass production [13,26]. Fig. 4b shows the formation of clumped and irregular-shaped pellets with smooth hairy surface, favouring lipid production. Previous research indicates that stress influences pellet morphology, causing mycelium aggregation into pellet formations, potentially due to self-immobilization. This clumping provides fungus protection in the liquid medium, and is consequently linked to enhanced lipid production [26]. Furthermore, Fig. 4c depicts that culture conditions optimizing both biomass and total lipid production tend to favour larger-sized pellets with the formation of starburst-like and thick-branched structures.
Fig. 4.
Macroscopic (above) and microscopic (below) (under microscope 4× objective lens) for morphological analysis of mycelium pellets of Ganoderma lucidum in optimized fermentation parameters for responses (a) biomass, (b) total lipid, and (c) biomass and total lipid.
3.5. Challenges of Ganodiesel production and comparisons to other sources
3.5.1. Comparison of the current study with the literature
A comparison of the production of biomass and lipid through submerged liquid fermentation between different Ganoderma lucidum species is shown in Table 7. The biomass and lipid yield vary depending on the strain, although the application of RSM led to increased production of Ganoderma lucidum products in all optimization studies. To the best of our knowledge, no previous studies have focused on optimizing the media compositions and fermentation parameters for lipid production in Ganoderma lucidum. The data presented in this study utilized RSM, providing insights into the experimental parameters for lipid production of Ganoderma lucidum.
Table 7.
The literature comparison for the optimization for Ganoderma lucidum using submerged liquid fermentation.
| Species | Optimization method | Cultivation method | Working volume (mL) | Initial pH | Glucose concentration (g/L) | Agitation (rpm) | Biomass (g/L) | EPS (g/L) | ENS (g/L) | Lipid Yield (% w/w) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
G. lucidum QRS 5120 |
RSM | Shake Flask ALSB |
200 2000 |
6 | 50 | 113.40 | 8.33 5.32 |
⁎NA | NA | 2.17 2.35 |
Current study |
|
G. lucidum QRS 5120 |
RSM | ALSB | 2000 | 4 | 30 | 110 | 7.90 | 4.6 | NA | NA | [11] |
| G. lucidum QRS 5120 | RSM | Shake Flask | 200 | 4 | 26.5 | 100 | 5.19 | 2.64 | 1.52 | NA | [12] |
| G. lucidum BGF4A1 | RSM | Shake Flask | 200 | 5.26 | 50 | – | 3.12 | 1.96 | NA | NA | [5] |
| G. lucidum 0201 | One-factor-at-a-time (OFAAT) | Shake Flask | 100 | 5 | 15 | – | 3.68 | NA | NA | NA | [27] |
| G. lucidum CAU5501 | OFAAT and orthogonal matrix method | Shake Flask | 1200 | – | 50 | 150 | 7.235 | 1.723 | NA | NA | [28] |
⁎NA= not available; EPS= exopolysaccharides; ENS= endopolysaccharides.
Furthermore, Table 8 shows the biodiesel production from first to third generations of feedstocks. For example, the biodiesel yield from Jatropha curcas seeds (95.0 %) reported by Kartika et al. [29] does not meet the biodiesel standard requirement of 96.5 %. However, biodiesel derived from Jatropha seeds as reported by Abdalla et al. [30] achieved a yield of 99.7 %. The efficiency of biodiesel production is highly dependent on various factors, including the strains used, selection of catalyst and alcohol type, and temperature during transesterification processes [31]. In this study, the Ganodiesel yield of fatty acid methyl esters (FAME) is achieved at 97.2 %, meeting the minimum requirement of 96.5 %. The other properties of Ganodiesel also meet the biodiesel standards, despite exhibiting a lower lipid yield compared to other sources. Additionally, utilizing a bioreactor simplifies both cultivation and biomass harvesting, requiring less water and minimizing waste products, thereby improving the effectiveness of Ganoderma lucidum as a biodiesel feedstock. However, challenges such as optimizing lipid yield and scaling up production processes need to be addressed to enhance the competitiveness of Ganoderma lucidum-derived biodiesel.
Table 8.
The different generations of feedstocks for biodiesel production.
| Feedstocks | Sources | Lipid extraction method | Lipid yield (wt%) | Biodiesel (FAME) yield (%) | References |
|---|---|---|---|---|---|
| Third-generation | Ganoderma lucidum mycelium | Solvent extraction | 3.6 | 97.2 | Current study |
| Third-generation | Aurantiochytrium sp. UMACC-T023 | Bligh and Dyer method | 14.9 | 97.1 | [24] |
| Third-generation | Spirogyra algae | Soxhlet extraction | 22.7 | 96.2 | [32] |
| Second-generation | Jatropha curcas seeds | Soxhlet extraction | 39.0 | 95.0 | [30] |
| Second-generation | Jatropha seeds | Solvent extraction | 39.4 | 99.7 | [29] |
| Second-generation | Millettia ferruginea seeds | Soxhlet extraction | 46.2 | 98.1 | [33] |
| Second-generation | Prosopis julifera seed | Soxhlet extraction | 32.5 | 94.2 | [34] |
| First-generation | Rapeseed oil | – | – | 90.9 | [35] |
| First-generation | Sunflower oil | – | – | 95.5 | [35] |
3.5.2. Challenges and limitation of Ganodiesel production
To establish lipid production from Ganoderma lucidum as a feasible bioenergy source, several challenges and limitations need to be tackled.
-
i.
Lipid yield: The primary challenge lies in achieving high lipid yields from liquid-cultivated Ganoderma lucidum mycelium, despite the biodiesel properties complied the international standards.
-
ii.
Scale-up challenges: Scaling up liquid cultivation and transesterification processes from laboratory to industrial levels presents technical challenges, including maintaining consistency, ensuring contamination control, and optimizing productivity [36].
-
iii.
Economic viability: The cost-effectiveness of liquid cultivation methods for Ganoderma lucidum, coupled with lipid extraction and biodiesel production processes, needs to be evaluated. High production cost may hinder the competitiveness of Ganoderma lucidum as a biodiesel feedstock compared to other sources and traditional fossil fuels [14].
-
iv.
Environmental sustainability: Assessing the environmental impacts of liquid-cultivated Ganoderma lucidum production, including water consumption, waste management and energy inputs, is essential for ensuring overall sustainability. Also, minimizing environmental footprints and adopting eco-friendly practices during the processes are imperative [36].
3.6. Conclusion and future perspective
This study presented that optimizing the fermentation condition of Ganoderma lucidum resulted in a high biomass (5.32 g/L) and total lipid (2.35 %) under optimized conditions of initial medium pH 6, 50 g/L glucose and agitation at 113.40 rpm. The glucose concentration and initial pH significantly influenced biomass production, while glucose concentration, initial pH and agitation has a significant effect on lipid production. Additionally, the biodiesel properties of Ganodiesel were in accordance with ASTM D6751 and EN 14,214 standards. Therefore, this study provides a groundwork for the potential lipid production of liquid-cultivated Ganoderma lucidum mycelium and its application in the field of bioenergy, aligning with Sustainable Development Goal 7 (Affordable and Clean Energy). Lipids produced from Ganoderma lucidum mycelium can potentially serve as a new renewable and sustainable feedstock for biodiesel production, thereby promoting clean energy alternatives to fossil fuels.
In term of future perspectives, further research could focus on further enhancing the process by employing the optimum parameters of Ganoderma lucidum growth in ASLB as stated in Ref. [11], and associated with the optimization of lipid production to maximize both biomass and lipid in ALSB. Additionally, there is an opportunity to refine lipid extraction techniques, including the selection of solvents and the ratio, to improve overall efficiency. These research directions could help advance the practical utilization of Ganoderma lucidum in bioenergy, thereby supporting efforts towards achieving SDG7.
Funding
The authors declare financial support was received for the research, authorship or publication of this article. This research was funded by the Ministry of Higher Education (MOHE) Malaysia and Universiti Malaya under the Fundamental Research Grant Scheme (FRGS/1/2022/STG01/UM/02/2) (FRGS: FP062-2022), ''Advanced Landless Biodiesel From Oleaginous Microorganism Biomass (ALBOMB) Grant” (PV055-2022) as well as (ST037-2023).
Data availability statement
The data is available upon request to the corresponding author.
CRediT authorship contribution statement
Teik Chee Lim: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Data curation. Zul Ilham: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Wan Abd Al Qadr Imad Wan-Mohtar: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Investigation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Zul Ilham reports financial support was provided by Malaysia Ministry of Higher Education. Zul Ilham reports financial support and administrative support were provided by University of Malaya (ST037-2023). Wan Abd Al Qadr Imad Wan-Mohtar reports financial support was provided by Advanced Landless Biodiesel From Oleaginous Microorganism Biomass (ALBOMB) Grant (PV055-2022). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Ministry of Higher Education (MOHE) Malaysia and Universiti Malaya under the Fundamental Research Grant Scheme (FRGS/1/2022/STG01/UM/02/2) (FRGS: FP062-2022), ''Advanced Landless Biodiesel From Oleaginous Microorganism Biomass (ALBOMB) Grant” (PV055-2022) as well as (ST037-2023).
References
- 1.Bhattacharyya S. Transesterification of Yellow Oleander seed oil, its utilization as biodiesel and performance evaluation. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09250. https://www.sciencedirect.com/science/article/pii/S2405844022005382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiehbadroudinezhad M., Merabet A., Ghenai C., Abo-Khalil A.G., Salameh T. The role of biofuels for sustainable MicrogridsF: a path towards carbon neutrality and the green economy. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e13407. https://www.sciencedirect.com/science/article/pii/S240584402300614X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goh B.H.H., Ong H.C., Cheah M.Y., Chen W.-H., Yu K.L., Mahlia T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: a critical review. Renew. Sustain. Energy Rev. 2019;107:59–74. https://www.sciencedirect.com/science/article/pii/S1364032119301054 [Google Scholar]
- 4.Quah R.V., Tan Y.H., Mubarak N.M., Khalid M., Abdullah E.C., Nolasco-Hipolito C. An overview of biodiesel production using recyclable biomass and non-biomass derived magnetic catalysts. J. Environ. Chem. Eng. 2019;7 https://www.sciencedirect.com/science/article/pii/S2213343719303422 [Google Scholar]
- 5.Hassan N.A., Supramani S., Azzimi Sohedein M.N., Ahmad Usuldin S.R., Klaus A., Ilham Z., Chen W.-H., Wan-Mohtar W.A.A.Q.I. Efficient biomass-exopolysaccharide production from an identified wild-Serbian Ganoderma lucidum strain BGF4A1 mycelium in a controlled submerged fermentation. Biocatal. Agric. Biotechnol. 2019;21 https://www.sciencedirect.com/science/article/pii/S1878818119306826 [Google Scholar]
- 6.Zhang X.-Y., Li B., Huang B.-C., Wang F.-B., Zhang Y.-Q., Zhao S.-G., Li M., Wang H.-Y., Yu X.-J., Liu X.-Y., Jiang J., Wang Z.-P. Production, biosynthesis, and commercial applications of fatty acids from oleaginous fungi. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.873657. https://www.frontiersin.org/articles/10.3389/fnut.2022.873657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fazili A.B.A., Shah A.M., Zan X., Naz T., Nosheen S., Nazir Y., Ullah S., Zhang H., Song Y. Mucor circinelloides: a model organism for oleaginous fungi and its potential applications in bioactive lipid production. Microb. Cell Factories. 2022;21:29. doi: 10.1186/s12934-022-01758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvatore M.M., Elvetico A., Gallo M., Salvatore F., DellaGreca M., Naviglio D., Andolfi A. Fatty acids from Ganoderma lucidum spores: extraction, identification and quantification. Appl. Sci. 2020;10:3907. https://www.mdpi.com/2076-3417/10/11/3907 [Google Scholar]
- 9.Sharipova D.A., Kopitsyn D.S., Ziangirova M.Y., Novikov A.A., Vinokurov V.A. Fatty acid composition of basidiomycetes lipids - a promising feedstock for obtaining biodiesel. Chem. Technol. Fuels Oils. 2016;52:255–260. doi: 10.1007/s10553-016-0701-6. [DOI] [Google Scholar]
- 10.Mohamad Jahis B.M., Ilham Z., Supramani S., Sohedein M.N.A., Ibrahim M.F., Abd-Aziz S., Rowan N., Wan-Mohtar W.A.A.Q.I. Ganodiesel: a new biodiesel feedstock from biomass of the mushroom Ganoderma lucidum. Sustainability. 2022;14 https://www.mdpi.com/2071-1050/14/17/10764 [Google Scholar]
- 11.Supramani S., Rejab N.A., Ilham Z., Ahmad R., Show P.-L., Ibrahim M.F., Wan-Mohtar W.A.A.Q.I. Performance of biomass and exopolysaccharide production from the medicinal mushroom Ganoderma lucidum in a new fabricated air-L-shaped bioreactor (ALSB) Processes. 2023;11:670. https://www.mdpi.com/2227-9717/11/3/670 [Google Scholar]
- 12.Sugenendran S., Rahayu A., Zul I., Mohamad Suffian Mohamad A., Anita K., Wan Abd Al Qadr Imad W.-M. Optimisation of biomass, exopolysaccharide and intracellular polysaccharide production from the mycelium of an identified Ganoderma lucidum strain QRS 5120 using response surface methodology. AIMS Microbiol. 2019;5:19–38. doi: 10.3934/microbiol.2019.1.19. https://www.aimspress.com/article/doi/10.3934/microbiol.2019.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullah N.R., Sharif F., Azizan N.H., Hafidz I.F.M., Supramani S., Usuldin S.R.A., Ahmad R., Wan-Mohtar W. Pellet diameter of Ganoderma lucidum in a repeated-batch fermentation for the trio total production of biomass-exopolysaccharide-endopolysaccharide and its anti-oral cancer beta-glucan response. AIMS Microbiol. 2020;6:379–400. doi: 10.3934/microbiol.2020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usuldin S.R.A., Ilham Z., Jamaludin A.A., Ahmad R., Wan-Mohtar W.A.A.Q.I. Enhancing biomass-exopolysaccharides production of lignosus rhinocerus in a high-scale stirred-tank bioreactor and its potential lipid as bioenergy. Energies. 2023;16:2330. https://www.mdpi.com/1996-1073/16/5/2330 [Google Scholar]
- 15.Redzwan G., Amin M.M., Zulkarnain N.N., Mansor M.R.A., Annuar M.S.M., Ilham Z. Extrication of biodiesel feedstock from early stage of food waste liquefaction. J. Mater. Cycles Waste Manag. 2017;19:676–681. doi: 10.1007/s10163-015-0463-y. [DOI] [Google Scholar]
- 16.B.E. 14214:2012+A2:2019 . British Standards Institutions (BSI); London, UK: 2019. Liquid Petroleum Products. Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications. Requirements and Test Methods. [Google Scholar]
- 17.ASTM D. D6751-08 . ASTM International; West Conshohocken, PA, USA: 2018. Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. [Google Scholar]
- 18.Arana-Gabriel Y., Burrola-Aguilar C., Alcalá-Adán A., Carmen Z., Estrada-Zúñiga M. Mycelial growth of the edible wild mushrooms Floccularia luteovirens in different culture mediums and pH. Agro Productividad. 2020;13:33–38. [Google Scholar]
- 19.Pessoa V.A., Soares L.B.N., Silva G.L., Vasconcelos A.S., Silva J.F., Fariña J.I., Oliveira-Junior S.D., Sales-Campos C., Chevreuil L.R. Production of mycelial biomass, proteases and protease inhibitors by Ganoderma lucidum under different submerged fermentation conditions. Braz. J. Biol. 2023;83 doi: 10.1590/1519-6984.270316. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim D., Weloosamy H., Lim S.H. Effect of agitation speed on the morphology of Aspergillus Niger HFD5A-1 hyphae and its pectinase production in submerged fermentation. World J. Biol. Chem. 2015;6:265–271. doi: 10.4331/wjbc.v6.i3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miranti A., Arbianti R., Utami T.S. IOP Conf. Ser.: Earth Environ. Sci. 2018. Effect of pH, temperature and medium agitation rate in production of AA, DHA, EPA from Aspergillus oryzae with submerged fermentation. 105. [DOI] [Google Scholar]
- 22.Adriana Tita S., Miftahul I. Proceedings of the 3rd International Conference on Biology, Science and Education (IcoBioSE 2021) Atlantis Press; 2023. The effect of various agitation speeds on lipid production from fungal BR 2.2 isolate; pp. 544–550. [Google Scholar]
- 23.Robles-Iglesias R., Naveira-Pazos C., Fernández-Blanco C., Veiga M.C., Kennes C. Factors affecting the optimisation and scale-up of lipid accumulation in oleaginous yeasts for sustainable biofuels production. Renew. Sustain. Energy Rev. 2023;171 https://www.sciencedirect.com/science/article/pii/S1364032122009248 [Google Scholar]
- 24.Sohedein M.N.A., Wan-Mohtar W.A.A.Q.I., Hui-Yin Y., Ilham Z., Chang J.-S., Supramani S., Siew-Moi P. Optimisation of biomass and lipid production of a tropical thraustochytrid Aurantiochytrium sp. UMACC-T023 in submerged-liquid fermentation for large-scale biodiesel production. Biocatal. Agric. Biotechnol. 2020;23 https://www.sciencedirect.com/science/article/pii/S1878818119308527 [Google Scholar]
- 25.Saad N., Abdeshahian P., Kalil M.S., Wan Yusoff W.M., Abdul Hamid A. Optimization of aeration and agitation rate for lipid and gamma linolenic acid production by Cunninghamella bainieri 2A1 in submerged fermentation using response surface methodology. Sci. World J. 2014;2014 doi: 10.1155/2014/280146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Supramani S., Jailani N., Ramarao K., Mohd Zain N.A., Klaus A., Ahmad R., Wan-Mohtar W.A.A.Q.I. Pellet diameter and morphology of European Ganoderma pfeifferi in a repeated-batch fermentation for exopolysaccharide production. Biocatal. Agric. Biotechnol. 2019;19 https://www.sciencedirect.com/science/article/pii/S1878818118309885 [Google Scholar]
- 27.Shah P., Modi H. Optimization of culture conditions for biomass production of Ganoderma lucidum. Int.J.Curr.Microbiol.App.Sci. 2018;7:1882–1889. [Google Scholar]
- 28.Yuan B., Chi X., Zhang R. Optimization of exopolysaccharides production from a novel strain of Ganoderma lucidum CAU5501 in submerged culture. Braz. J. Microbiol. 2012;43:490–497. doi: 10.1590/S1517-83822012000200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amalia Kartika I., Yani M., Ariono D., Evon P., Rigal L. Biodiesel production from jatropha seeds: solvent extraction and in situ transesterification in a single step. Fuel. 2013;106:111–117. https://www.sciencedirect.com/science/article/pii/S0016236113000306 [Google Scholar]
- 30.Abdalla A., Rusli H., Amran M.B. Biodiesel production from sudanese jatropha curcas seed by the alkali-catalyzed transesterification process and its analysis by gas chromatography. Helium: J. Appl. Chem. 2023;2:58–63. [Google Scholar]
- 31.Nur Aishah R., Salina Mat R., Maryam Mohamed R., Nur Amalina Mohd A. Optimization of the biodiesel production via transesterification reaction of palm oil using response surface methodology (RSM): a review. MJoSHT. 2022;8:58–67. https://mjosht.usim.edu.my/index.php/mjosht/article/view/292 [Google Scholar]
- 32.Teku K., Prasad L.S.V., Kolakoti A. Preparation and physicochemical properties of naturally grown green Spirogyra algae biodiesel. Chem. Ind. Chem. Eng. Q. 2022;29:15. 15. [Google Scholar]
- 33.Ayalew L.A., Tizazu B.Z. Valorization of indigenous Millettia ferruginea seed oil for biodiesel production: parametric optimization, physicochemical characterization, engine performance and emission characteristics. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e11601. https://www.sciencedirect.com/science/article/pii/S2405844022028894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hundie K.B., Akuma D.A. Optimization of biodiesel production parameters from Prosopis julifera seed using definitive screening design. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e08965. https://www.sciencedirect.com/science/article/pii/S2405844022002535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan E., Ozaltin K., Spagnuolo D., Bernal-Ballen A., Piskunov M.V., Di Martino A. Biodiesel from rapeseed and sunflower oil: effect of the transesterification conditions and oxidation stability. Energies. 2023;16:657. https://www.mdpi.com/1996-1073/16/2/657 [Google Scholar]
- 36.Babadi A.A., Rahmati S., Fakhlaei R., Barati B., Wang S., Doherty W., Ostrikov K. Emerging technologies for biodiesel production: processes, challenges, and opportunities. Biomass Bioenergy. 2022;163 https://www.sciencedirect.com/science/article/pii/S0961953422001830 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available upon request to the corresponding author.