Abstract
Antibiotic overuse in poultry feeds has disastrous implications; consequently, long-term alternatives must be developed. As a result, the current study aims to assess the impact of Aspergillus niger filtrate (ANF) high in organic acids grown on agro-industrial residue of faba bean (AIRFB) on quail diet, as well as their influence on bird productivity, digestion, carcass yield, blood chemistry, and intestinal microbiota. A total of 240 Japanese quails (aged 7 d) were used in this study, divided equally among 5 experimental groups with 48 quails each. Group 1 (G1) received a basal diet without any ANF, group 2 (G2) received a basal diet supplemented with 0.5 mL ANF/kg diet, group 3 (G3) received a basal diet supplemented with 1.0 mL ANF/kg diet, group 4 (G4) received a basal diet supplemented with 1.5 mL ANF/kg diet, and group 5 (G5) received a basal diet supplemented with 2 mL ANF/kg diet. The performance parameters were monitored at 1 to 3, 3 to 5, and 1 to 5 wk. Adding ANF increased body weight at 3 and 5 wk, as well as body weight gain at 1 to 3, 3 to 5, and 1 to 5 wk, compared to the control diet. The ANF fed quails had the highest feed conversion ratio compared to the control group. The addition of ANF to the quail diet had no effect on the weight of the carcass, gizzard, heart, liver, giblets, or dressing; however, it did lower triglycerides, low-density lipoprotein, and very low-density lipoprotein while increasing high-density lipoprotein levels. The quail groups that received ANF had enhanced immunological indices such as IgG, IgM, IgA, and lysozymes. It also increased the levels of superoxide dismutase and total antioxidant contents, as well as catalase, and digestive enzymes such as protease, amylase, and lipase. However, it lowered the blood MDA levels compared to control. It has been demonstrated that the total gut microbiota, Escherichia coli, total coliforms, and the population of Salmonella are all reduced in ANF-fed quails. Histological examination of ANF quails' liver and intestinal sections revealed normal hepatic parenchyma, typical leaf-like intestinal villi, and comparatively short and frequently free lumina. In conclusion, Japanese quail showed improvements in performance, digestive enzymes, antioxidant indices, immunity, and capacity to reduce intestinal pathogenic bacteria after consuming diet supplemented with ANF.
Key words: Agricultural wastes, growth performance, intestinal pathogens, Japanese quail, organic acids, organic poultry
INTRODUCTION
Poultry feed additives and vitamins have effectively improved public health performance (Alghamdi et al., 2024, Zhou et al., 2024). Organic products/foods have grown demand in some countries due to its effectiveness in reducing the symptoms of various ailments and improving consumer public health (Vlaicu et al., 2023). Among the dietary supplements that are used in poultry nutrition are organic and inorganic acids (Ferronato and Prandini, 2020; Salem et al., 2023).
Aspergillus niger is a vital fungus in the industrial sector, as it plays a crucial role in the production of succinic acid and citric acid. Furthermore, it may produce valuable compounds through fermentation, making it a vital participant in many biotechnological applications (Upton et al., 2020; Papadaki and Mantzouridou, 2024). The production process should prioritize environmental sustainability by utilizing cost-effective and easily accessible agro-industrial waste materials, such as citric acid produced primarily through microbial fermentation using A. niger (Show et al., 2015; Stefanolo et al., 2024).
A. niger is easily manipulated, ferments a wide range of cost-effective raw materials, and produces valuable yields (Themelis and Tzanavaras, 2001). There is a developing desire for converting to a more profitable fermentation method that leverages cheap substrates and underutilized resource streams, therefore more effective strain development approaches are essential (Show et al., 2015). Lately, Mahgoub et al. (2022) demonstrated that Aspergillus japonicus can transform a mixture of plant biomass residues, and this strain could form organic acids and cellulase enzymes. Aspergillus spp. has the potential to be evaluated as a bio-converter of sugarcane bagasse wastes into organic acids. Furthermore, Aspergillus spp. could be a unique source of mycological cellulase, providing a practical technique for minimizing ecological pollution (Mahgoub et al., 2022).
In addition, A. niger can use its metabolism to transform most feedstock into a wide variety of desirable chemical compounds (Mahgoub et al., 2022). The tools needed to develop strains exhibiting the desired potential are established by molecular sequencing and alterations in focused engineering approaches (Upton et al., 2020). Using solid-state fermentation circumstances for agro-industrial residues such as rice straw by A. niger resulted in the maximum cellulase formation (Mrudula and Murugammal, 2011).
Applying these fermentation environments to wheat straw and rice, with either a single injection of A. niger or a dual injection of Phanerochaete chrysogenum, led to a weight loss of over 40% in the fermented straw. Along with this loss, the hydrolysate of the fermented straw produced soluble phosphorus (P) and glucose (Saber et al., 2010).
Apata (2011) reported that the use of A. niger in the solid-state fermentation of Terminalia catappa fruit meal could enhance the fruit's nutritional qualities while reducing its natural tannin and phytic acid contents. It also exhibits great potential as a poultry feed ingredient which can be added to broiler chicken ration at a rate of 180 g kg−1, substituting 40% of the dietary maize level without compromising growth performance, apparent nutrient digestibility, or serum biochemical constituents (Apata, 2011).
In the chicken industry, short-chain organic acids like formic, acetic, butyric, and propionic acids, as well as malic, lactic, fumaric, tartaric, and citric acids, are mainly used (Salem et al., 2023). Organic acids have received much attention as feasible substitutes for antibiotic growth promoters due to their influence on microorganisms (Khan and Iqbal, 2016). They also reduce the pH in the intestines, which helps chickens absorb nutrients better (Mosa et al., 2023). In several animals, organic acids promote growth rate, feed efficiency, and disease resistance (Salem et al., 2023).
In addition, formic acid was added to broiler water and feed to increase performance indices and the bacteria in the intestine (Broom, 2015). Broilers fed 0.5 % citric acid exhibited enhanced immunological parameters, according to Chowdhury et al. (2009), while organic acids supplementation increased the immune function of hens, according to Abdel-Fattah et al. (2008).
The inclusion of organic acids in Japanese quail diets is believed to have benefits, but how different concentrations of A. niger filtrate (ANF) affect the physiology, feed consumption, and production of growing quails remain largely unknown. As a result, the purpose of the current study was to determine the effect of adding various quantities of A. niger grown on agro-industrial residue of faba bean (AIRFB) to quail diet on performance, carcass production, digestive enzymes, hematological chemistry, and gut microbiota in developing quail.
MATERIALS AND METHODS
Fungal Strains and AIRFB
The rhizosphere soil samples were collected at 10 to 15 cm depth of faba bean plants. The collected soil samples were mixed, and the representative samples were transferred to the laboratory and kept at 4°C for further use (Lokesh et al., 2016).
Ten gm of representative soil samples were homogenized in 90 ml of sterilized saline solution. Then serial dilutions (10−2 to 10−6) were plated on Pikovskaya's agar medium containing (5 g of Ca3(PO4)2, 10 g glucose, 0.5 g of yeast extract, 0.5 g (NH4)2SO4, 0.2 g KCl, 0.1 g MgSO4 7H2O, and trace of MnSO4 7H20 and FeSO4 and 20 g agar dissolved in 1000 mL of distilled and pH 7.2) and incubated at room temperature for 7 d. Plates were examined for solubilizing zones around the fungal colony. A colony showing a solubilizing clear zone was picked and subcultured for further use (Jain and Singh, 2015) Twelve fungal colonies were selected and identified.
The fungal isolates were identified based on their morphological characteristics, including colony morphology, conidiophore diameter, phialide shape, and conidia shape. The phosphate solubilizing fungal isolates were identified as A. niger. The isolates were confirmed by identification at the gene level through isolating DNA and using PCR to detect genes using 18S rRNA gene sequence analysis. Sequencing was performed via the automated DNA sequencer (ABI Prism 3130 Genetic Analyzer by Applied Biosystems, Hitachi, Japan). Genomic DNA was obtained by the hexadecyltrimethylammonium bromide technique, and the integrity and level of purified DNA were established by agarose gel electrophoresis. All the A. niger strains were maintained by sub-culturing on potato dextrose agar (Lab M Limited, Lancashire, UK) and stored at 4 °C (Alsubhi et al., 2022).
The samples of AIRFB were dried at 70°C for 48 h and minced in an electric grinder. The AIRFB samples were 45.87 ± 0.87% organic carbon, 1.49 ± 0.56% total nitrogen, 30.67% C/N ratio, 0.38% P, and 1.36% total potassium (K).
Production of Organic Acids by Solid-State Fermentation
The fungus was cultivated on potato dextrose broth medium (PDB) (Lab M Limited) composed of 200 g potato, 20 g dextrose, in 1 liter distilled water, adjusted at pH 5.
The flasks were inoculated with 1 mL of the fungal inoculum with 1.5 × 108 spore/mL. The inoculated flasks were incubated at 28°C for 5 d on a rotary shaking at 140 rpm and the biomass of the fungus was isolated by filtration. The inoculum for solid-state fermentation studies was approximately adjusted to be 1.5 × 108 spore/mL.
The media of Chang et al. (2006) was used to determine cellulase formation and RP solubilization under solid-state fermentation conditions. The medium consists of 5 g of AIRFB ground faba bean straw and 15 mL of salt solution (1.6 g/L (NH4)2SO4, 4.0 g/L KH2PO4, and 1.0 g/L MgSO4). The 100 mg P2O5 from rock phosphate (RP) was used as the sole P resource as an alternative to KH2PO4 and supplemented singly to every flask before autoclaving. Inoculation was done by applying 5% (v/w) from the spore suspension of A. niger approximately 1.5 × 108 spore/mL of the examined fungi. The start moisture was accustomed to 65%. The substances were thoroughly mixed and the optimal solid-state fermentation circumstances was as recommended by Mahgoub et al. (2022). The flasks were kept at 28°C, and 50 mL of distilled water was supplemented to every flask, mixed for 30 min on a rotary shaker at 140 rpm and filtered using filter paper (Whatman No. 1 Buckinghamshire, United Kingdom) (Abu Yazid et al., 2017, El-Sawah et al., 2001, Kumari et al., 2008).
Phytase activity was determined as the enzyme quantity forming 1 µmole of inorganic P/mL/min. The filtrate was checked for pH, titratable acidity (TA), the formation of glucose, soluble P, and phytase activities in the supernatant acquired via 5 min centrifugation at 4,000 rpm. The filtrate was used as rough enzyme (RE) acclimatization to assess cellulase activities (CMC-ase, FP-ase, and β-glucosidase), and for the amount of glucose produced using glucose oxidase kit (Spainreact Co., Girona, Spain). The growth of fungi was defined in terms of the dry weight (DW) of mycelia (mg/flask) (Narasimha et al., 2006). The AIRFB were dried using the oven at 80°C.
Determination of Organic Acids Production Using High-Performance Liquid Chromatography (HPLC)
Utilizing an Agilent 1100 HPLC system (Agilent Technologies, Inc., Santa Clara, California, USA) the organic acids in the aquatic extracts of AIRFB were analyzed. The HyperREZ XP carbohydrate H (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) 8 μm column 300 × 7.7 mm (Part Number: 69008-307780) was used, with a mobile stage of 5 mM H2SO4, a flow rate of 0.6 mL/min, and a column temperature of 55°C. The samples applied for the HPLC analysis had an inoculation volume of 5 μL of the terminal extract. At 280 nm, UV absorbance was used for recognition (Mahgoub et al., 2022). Their identities were determined by comparing the retention period of the organic acids with those of known organic acids. Ascorbic acid was identified and quantified utilizing a Hypersil GOLD PFP column (Thermo Fisher Scientific Inc.), (35,405-246,630, 5 µm × 150 × 4.6 mm) (Mahgoub et al., 2022).
The mobile phase was adjusted at 1.0 mL /min, utilizing acetonitrile and water containing 0.1% formic acid as mobile stages A and B, respectively. The column was inoculated with 20 L at a temperature of 25°C using a thermostat. The amount of ascorbic acid was quantified using a fluorescence detector that functioned at a 250 nm excitation wavelength and a 410 nm emission wavelength.
The organic acids were measured by signal to the peak zone and detention period obtained for the original standards for different organic acids (i.e., citric, ascorbic, acetic, formic, levulinic, itaconic, maleic, succinic and oxalic acids) in the mobile stage which tested singly and mixed in the concentration of 10 mg/mL (Mahgoub et al., 2022).
Quails, Diets, and Trial Design
A total of 240 Japanese quails aged 7 d with an average weight of 29.95 g were assigned randomly to 5 experimental groups, each with 4 replications of 12 quails. This experiment lasted 35 d. G1) control group received basal diet without ANF, G2) basal diet + 0.5 mL ANF/kg diet, G3) basal diet + 1 mL ANF/kg diet, G4) basal diet + 1.5 mL ANF/kg diet, and G5) basal diet + 2 mL ANF/kg diet. A completely randomized procedure was used to conduct the current investigation. We used a litter model to raise all of the chicks. The litter was rice husk, and the shed was well ventilated. The birds were given free access to food and water in batteries that had three decks and two sections of cages with automated watering. The basal diet is presented in Table 1 and the calculated analysis were determined (National Research Council, 1994). The protocols established by the Local Experimental Animal Care Committee were followed during the experimental procedures, Zagazig University, Zagazig, Egypt. The ethical approval code was ZU. IACUC/2/ F/394/2023.
Table 1.
Ingredients and nutrient contents of the basal diet of growing Japanese quail.
| Items | (g/kg) |
|---|---|
| Ingredient | |
| Maize (8.5%) | 518.00 |
| Maize gluten meal (62 %) | 52.10 |
| Soybean meal (44%) | 367.00 |
| Soybean oil | 29.00 |
| Limestone | 7.00 |
| Di-calcium phosphate | 16.50 |
| Salt | 3.00 |
| L-Lysine | 1.30 |
| Dl-Methionine | 1.10 |
| Premix* | 3.00 |
| Choline chloride | 2.00 |
| Total | 1000 |
| Metabolizable energy (MJ/kg) | 12.53 |
| Crudeprotein (g/kg) | 240 |
| Ca (g/kg) | 8.00 |
| Non-phytatephosphorus (g/kg) | 4.50 |
| Lysine (g/kg) | 13.00 |
| S amino acids (g/kg) | 9.20 |
Growth vitamin and Mineral premix. Each 2.5 kg consists of: vitamin A, 12000 IU; vitamin D3, 2000 IU; vitamin E, 10 g; vitamin k3, 2 g; vitamin B1, 1000 mg ; vitamin B2, 49 g; vitamin B6, 105 g; vitamin B12, 10 mg; pantothenic acid, 10 g; niacin, 20 g , folic acid, 1000 mg ; biotin, 50 g; choline chloride, 500 mg, Fe, 30 g; Mn, 40 g; Cu, 3 g; Co, 200 mg; Si, 100 mg; and Zn, 45 g.
Determination of Growth Performance and Carcass Characteristics
Weights of birds were taken at 1, 3, and 5 wk old to determine body weight (BW), and body weight gain (BWG). During the experiment, feed intake (FI) and feed conversion rate (FCR) were estimated. At wk 5, 5 birds from each group were chosen randomly and ethically slaughtered for carcass evaluation (Zhou et al., 2024).
Assay of Digestive Enzymes
According to the procedures of Najafi et al. (2006), the activity of lipase, amylase, and protease in the ileum contents of birds at 35 d old were measured (5 birds/ group) (Najafi et al., 2005; 2006).
Evaluation of Blood Biochemical Parameters
Blood samples from slain quails were collected into sterile tubes and centrifuged for 15 min with plasma preserved for examination. Kits from Spectrum Company (Cairo, Egypt) were used to determine all biochemical blood parameters (lipid profile, hepatic, renal functions, and immune parameters). A spectrophotometer (UV-2101/3101 PC; Shimadzu Corporation, Analytical Instruments Division, Kyoto, Japan) was used to determine the antioxidant properties.
Assessment of Caecal Bacterial Load
To collect caecal content, three birds from each replication were slain at 35 d old. After separating the caeca, the contents were carefully collected in sterile cups for aseptic handling. The samples were kept at 4°C until the microbial population was enumerated. About 25 g were obtained to assess the total bacterial count (TBC), total coliforms, Escherichia coli, Salmonella and lactic acid bacteria. TBC was determined on nutrient agar medium (HiMedia Laboratories Pvt. Ltd., Mumbai, India); (ii) E. coli on eosin methylene blue (EMB agar) (LHiMedia Laboratories); (iv) total coliforms on MacConkey agar medium (HiMedia Laboratories); (v) Salmonella spp. on xylose lysine decarboxylase agar (XLD agar) (HiMedia Laboratories), and (iv) lactic acid bacteria on MRS agar (HiMedia Laboratories). The plates were incubated at 30°C in the dark for 3 days and results were expressed as log10 the number of colony-forming units (CFU) per gram of dry caecal weight (Log10 CFU).
Histological Investigation
Intestinal and hepatic specimens were collected and subsequently preserved in formalin (10%) for 2 d. Subsequently, the specimens were dehydrated in different ethanol concentrations in ascending order (70, 80, 95, and 100%), followed by xylene clearance and paraffin embedding. The paraffin thickness was adjusted to 5 µm using a microtome (Leica Camera AG, Wetzlar, Germany). Following section preparation, the specimens were subjected to standard histological hematoxylin and eosin staining procedures, followed by microscopic examination (Suvarna et al., 2013). The sections were examined using an optical microscope (Olympus BH-2, Olympus Optical Co. Ltd, Tokyo, Japan), a digital camera, and software (Jenoptik ProgRes Camera, C12plus, Frankfurt, Germany).
Statistical Analysis
The study statistics were performed by one-way ANOVA utilizing SAS (v 9.4). All examined means (treatments) were compared using the Tukey's test, ensuring significance at P < 0.05.
RESULTS
Cellulase Activity of A. niger From AIRFB Under Solid-State Fermentation Conditions
A. niger was selected and tested based on RP solubilization. A. niger showed good multiplication and good sporulation on AIRFB growth medium compared to the control. The time-course profile of FP-ase, CMC-ase, and β-glucosidase production, as well as soluble P within the solid-state fermentation period, performed to be identical and these enzymes elevated below the same circumstances by A. niger. Under the treatments, the formation of the tested enzymes and the RP solubilization process reached a maximum at the end of experiments (4 wk). Cellulase formation was quantitatively assessed on the broth medium and the activity of cellulase enzymes was found to be 280 cellulase unit. The results revealed that A. niger lowered the end culture pH to the acidic side. In addition, phytase activities and the production of soluble P from RP were observed. The production of soluble P was 32 µg/mL in the culture filtrates, indicating that the fungus has a potent system for RP solubilization.
Organic Acids Contents in ANF
The efficient bioconversion of AIRFB into sugars and the acidic nature of the hydrolysate, indicated by (pH, TA, the releasing of glucose, soluble phosphorus (soluble P), and phytase measurements), enhanced the distinction of organic acids that may be formed because of conversation action of A. niger.
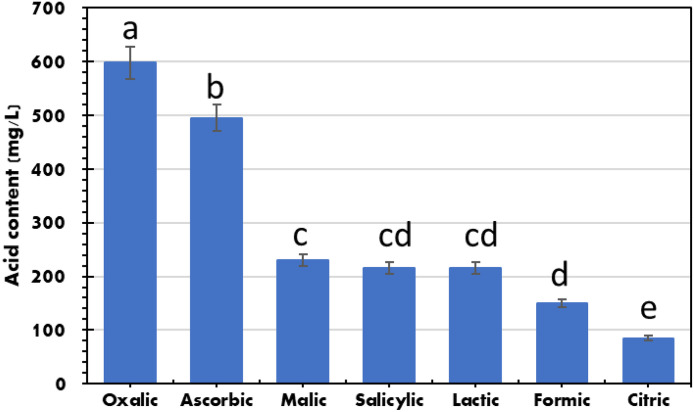
Seven organic acids (oxalic, ascorbic, maleic, salicylic, lactic, formic, and citric) in the filtrate of ANF were detected and quantified utilizing HPLC (Figure 1).
Figure 1.
Organic acid production (mg/g straw) during solid-state fermentation of agro-industrial residue of faba bean (AIRFB) and rock phosphate by Aspergillus niger. Mean values followed by different letters are significantly (P < 0.05) different from each other according to Tukey test. Bars represent standard deviation.
Effect of ANF on Growth-Related Parameters
In addition to feed consumption at all ages, adding ANF at varied doses (G2, G3, G4, and G5) to the quail diet resulted in a significant shift in growth indices, as shown in Table 2. Adding ANF up to 1 mL/kg to the diet elevated BWG at at 1 to 3, 3 to 5, and 1 to 5 wk (Table 2). During 1 to 3 and 1 to 5 wk of age, birds consume 0.5, 1, 1. 5, and 2 mL ANF/kg diet consumed less ration than control birds. Low feed consumption is likely due to the acid's strong flavor, which would have lowered the rations' palatability and reduced FI. The quails supplied organic acids ration had the highest FCR at 1 to 3, 3 to 5, and 1 to 5 wk of age (Table 2). The ANF fed quails had the highest FCR compared to the control group G1 (Table 2).
Table 2.
Effect of dietary supplementation with Aspergillus niger culture filtrate on growth performanceof Japanese quail.
| Characteristics |
Aspergillus niger culture filtrate (mL/kg diet) |
Standard error of the mean | P value | ||||
|---|---|---|---|---|---|---|---|
| 0 (G1) | 0.5 (G2) | 1 (G3) | 1.5 (G4) | 2 (G5) | |||
| Body weight (g) | |||||||
| 1 wk | 29.10a | 29.14a | 29.13a | 29.15a | 29.10a | 0.320 | 0.9999 |
| 3 wk | 95.79c | 111.90a | 103.68b | 108.81ab | 103.51b | 1.595 | 0.0004 |
| 5 wk | 186.21c | 196.09ab | 201.72a | 201.42a | 191.05bc | 1.747 | 0.0004 |
| Body weight gain (g/d) | |||||||
| 7–21d | 4.76c | 5.91a | 5.32b | 5.69a | 5.32b | 0.105 | 0.0002 |
| 21–35d | 6.46bc | 6.01d | 7.00a | 6.62b | 6.25cd | 0.092 | 0.0005 |
| 7–35d | 5.61c | 5.96ab | 6.16a | 6.15a | 5.78bc | 0.063 | 0.0005 |
| Feed intake (g/d) | |||||||
| 7–21d | 15.42a | 15.86a | 13.70a | 15.14a | 14.96a | 0.465 | 0.0753 |
| 21–35d | 21.07a | 18.73a | 19.25a | 19.47a | 20.24a | 0.608 | 0.1682 |
| 7–35d | 18.25a | 17.29a | 16.47a | 17.30a | 17.60a | 0.429 | 0.1997 |
| Feed conversion ratio (g feed/g gain) | |||||||
| 7–21d | 3.24a | 2.69b | 2.57b | 2.66b | 2.82b | 0.085 | 0.0029 |
| 21–35d | 3.26a | 3.12ab | 2.75c | 2.94bc | 3.24a | 0.074 | 0.0111 |
| 7–35d | 3.25a | 2.90bc | 2.67c | 2.81bc | 3.04ab | 0.075 | 0.0044 |
Values with the same letter in a row are not significantly (P > 0.05) different according to Tukey test.
Effect of ANF on Carcass Traits
The administration of ANF at varied doses (G2, G3, G4, and G5) did not affect the carcass, heart, liver, gizzard, giblets, or dressing (P > 0.05; Table 3). In birds treated with 2 mL ANF/kg diet (G5), the carcass % was 68.22, liver % was 2.33, gizzard % was 2.38, heart % was 1.00, giblet % was 5.70, and dressing % was 73.92 (Table 3).
Table 3.
Effect of dietary supplementation with Aspergillus niger culture filtrate on carcass traits of Japanese quail.
| Characteristics |
Aspergillus niger culture filtrate (mL/kg diet) |
Standard error of the mean | P value | ||||
|---|---|---|---|---|---|---|---|
| 0 (G1) | 0.5 (G2) | 1 (G3) | 1.5 (G4) | 2 (G5) | |||
| Carcass % | 68.06a | 72.7a | 72.87a | 74.42a | 68.22a | 2.800 | 0. 4883 |
| Liver % | 3.33a | 2.82a | 2.84a | 3.21a | 2.33a | 0.377 | 0.5028 |
| Gizzard % | 2.12a | 2.38a | 2.71a | 2.69a | 2.38a | 0.303 | 0.6870 |
| Heart % | 1.23a | 1.07a | 1.00a | 1.22a | 1.00a | 0.152 | 0.6931 |
| Giblets % | 6.68a | 6.26a | 6.55a | 7.12a | 5.70a | 0.532 | 0.5385 |
| Dressing % | 74.74a | 78.96a | 79.42a | 81.54a | 73.92a | 2.932 | 0.4247 |
Values with the same letter in a row are not significantly (P > 0.05) different according to Tukey test.
Effect of ANF on Digestive Enzymes
The digestive enzymes in quail diets containing varied doses of ANF (G2, G3, G4, and G5) are significantly affected (Table 4). In comparison to the control G1, adding up to 2 mL ANF/kg diet to quail feed boosted digestive enzyme activity like protease (P = 0.0001), amylase (P = 0.0007), and lipase (P = 0.0004) (Table 4).
Table 4.
Effect of dietary supplementation with Aspergillus niger culture filtrate on digestive enzymes of Japanese quail.
| Characteristics |
Aspergillus niger culture filtrate (mL/kg diet) |
Standard error of the mean | P value | ||||
|---|---|---|---|---|---|---|---|
| 0 (G1) | 0.5 (G2) | 1 (G3) | 1.5 (G4) | 2 (G5) | |||
| Protease (IU/L) | 0.23b | 0.95a | 1.10a | 1.13a | 0.91a | 0.074 | <.0001 |
| Amylase (IU/L) | 5.22c | 14.90ab | 16.90a | 11.84b | 11.44b | 1.199 | 0.0007 |
| Lipase (IU/L) | 3.51c | 12.43a | 8.78b | 12.38a | 7.50b | 0.965 | 0.0004 |
Values with the same letter in a row are not significantly (P > 0.05) different according to Tukey test.
Effect of ANF on Blood-Related Parameters
Except for creatinine level, all hepatic and renal functions (total protein, albumin, globulin, albumin/globulin ratio, aspartate aminotransferase (AST), alanine aminotransferase (ALT), Lactate dehydrogenase (LDH), and urea) revealed statistical differences, as shown in Table 5. AST (P = 0.0014), ALT (P < 0.0001), LDH (P = 0.0016), and urea (P = 0.0020) levels were considerably lower in quails given varied doses of ANF (G2, G3, G4, and G5) (Table 5). Except for 1.5 mL ANF/kg diet, supplementation of ANF increased total protein and albumin contents (Table 5).
Table 5.
Effect of dietary supplementation with Aspergillus niger culture filtrate on liver and kidney functions of Japanese quail.
| Characteristics |
Aspergillus niger culture filtrate (mL/kg diet) |
Standard error of the mean | P value | ||||
|---|---|---|---|---|---|---|---|
| 0 (G1) | 0.5 (G2) | 1 (G3) | 1.5 (G4) | 2 (G5) | |||
| Albumin (g/dL) | 1.60b | 1.99a | 2.04a | 1.61b | 1.95a | 0.076 | 0.0098 |
| Globulin (g/dL) | 1.36b | 1.72a | 1.79a | 1.66a | 1.61a | 0.055 | 0.0036 |
| Albumin globulin ratio (%) | 1.18a | 1.15a | 1.14a | 0.97b | 1.20a | 0.035 | 0.0220 |
| Total protein (g/dL) | 2.96c | 3.71a | 3.83a | 3.27bc | 3.55ab | 0.118 | 0.0064 |
| Aspartate aminotransferase (AST) (IU/L) | 228.90a | 219.60a | 177.20b | 187.35b | 194.11b | 6.078 | 0.0014 |
| Alanine aminotransferase (ALT) (IU/L) | 11.26a | 8.88b | 9.06b | 9.56b | 7.18c | 0.257 | <.0001 |
| Lactate dehydrogenase (LDH) (IU/L) | 236.17a | 217.33ab | 210.50ab | 142.00c | 179.50b | 10.998 | 0.0016 |
| Urea (mg/dL) | 7.77a | 6.42b | 6.27b | 5.36c | 6.82b | 0.262 | 0.0020 |
| Creatinine (mg/dL) | 0.34a | 0.30a | 0.31a | 0.33a | 0.34a | 0.018 | 0.4958 |
Values with the same letter in a row are not significantly (P > 0.05) different according to Tukey test.
The administration of ANF at varied doses (G2, G3, G4, and G5) significantly reduced triglycerides, low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL), while increasing high-density lipoprotein (HDL) levels (Table 6). Conversely, there was no significant difference in total cholesterol levels across treatments (P > 0.1011) (Table 6). In Table 7, there were significant changes in antioxidant indices connected to A. niger filtrate. The administration of ANF at varied doses (G2, G3, G4, and G5) significantly increased total antioxidant contents, superoxide dismutase, catalase, malondialdehyde, lysozyme, immunoglobin M (IgM), immunoglobin G (IgG), immunoglobin A (IgA), while reducing the level of malondialdehyde compared to the control group G1 (Table 7).
Table 6.
The impact of dietary supplementation with Aspergillus culture filtrate on the lipid profile of Japanese quail.
| Characteristics |
Aspergillus niger culture filtrate (mL/kg diet) |
Standard error of the mean | P value | ||||
|---|---|---|---|---|---|---|---|
| 0 (G1) | 0.5 (G2) | 1 (G3) | 1.5 (G4) | 2 (G5) | |||
| Total cholesterol (mg/dL) | 208.34a | 194.75a | 197.02a | 194.9a | 188.93a | 7.426 | 0.5146 |
| Triglycerides (mg/dL) | 215.50a | 174.15b | 167.00bc | 159.90bc | 132.75c | 9.757 | 0.0064 |
| High-density lipoprotein (mg/dL) | 50.83d | 68.12bc | 80.34a | 75.28ab | 64.56c | 2.746 | 0.0002 |
| Lowe-density lipoprotein (mg/dL) | 114.41a | 91.80bc | 83.28c | 87.64bc | 97.83b | 3.244 | 0.0005 |
| Very low-density lipoprotein (mg/dL) | 43.10a | 34.83b | 33.40bc | 31.98bc | 26.55c | 1.951 | 0.0064 |
Values with the same letter in a row are not significantly (P > 0.05) different according to Tukey test.
Table 7.
Effect of dietary supplementation with Aspergillus niger culture filtrate on immunity and antioxidants of Japanese quail.
| Parameters |
Aspergillus niger culture filtrate (mL/kg diet) |
Standard error of the mean | P value | ||||
|---|---|---|---|---|---|---|---|
| 0 (G1) | 0.5 (G2) | 1 (G3) | 1.5 (G4) | 2 (G5) | |||
| Immunity | |||||||
| Lysozyme (U/mL) | 0.13b | 0.30a | 0.28a | 0.20b | 0.32a | 0.022 | 0.0016 |
| Immunoglobin M (IgM, mg/dL) | 0.44c | 0.87a | 0.84a | 0.61b | 0.67b | 0.069 | 0.0233 |
| Immunoglobin G (IgG, mg/dL) | 0.85c | 1.45ab | 1.53a | 1.25b | 1.25b | 0.070 | 0.0009 |
| Immunoglobin A (IgA, mg/dL) | 0.66b | 1.02a | 1.07a | 1.10a | 0.90a | 0.065 | 0.0054 |
| Antioxidants | |||||||
| Total antioxidant content (ng/mL) | 0.08c | 0.27a | 0.19ab | 0.13bc | 0.25a | 0.021 | 0.0016 |
| Superoxide dismutase (U/mL) | 0.10c | 0.29a | 0.21b | 0.24ab | 0.27a | 0.014 | 0.0001 |
| Catalase (ng/mL) | 0.08c | 0.27a | 0.21ab | 0.14bc | 0.27a | 0.021 | 0.0014 |
| Malondialdehyde (nmol/mL) | 0.43a | 0.23b | 0.26b | 0.20b | 0.21b | 0.022 | 0.0002 |
| Glutathione reductase (ng/mL) | 0.21a | 0.24a | 0.22a | 0.23a | 0.28a | 0.040 | 0.7854 |
Values with the same letter in a row are not significantly (P > 0.05) different according to Tukey test.
Effect of ANF on the Caecal Bacterial Load
Supplementation of the basal diet with ANF (quails in groups G2, G3, G4, and G5) significantly reduced the population of E. coli (P < 0.0001), coliform (P < 0.0001), and Salmonella (P < 0.0001) comapred to the control group (G1) (Table 8). On the other hand, supplementation of the basal diet with ANF in quails in groups G2, G3, G4, and G5 significantly increased the population of Lactobacillus (Table 8) with the higher Lactobacillus count detected with 2 mL ANF/kg diet (Table 8).
Table 8.
Effect of dietary supplementation with Aspergillus niger culture filtrate on bacterial contents of Japanese quail.
|
Aspergillus niger culture filtrate (mL/kg diet) |
Standard error of the mean | P value | |||||
|---|---|---|---|---|---|---|---|
| Caecal count (Log10 CFU/g) | 0 (G1) | 0.5 (G2) | 1 (G3) | 1.5 (G4) | 2 (G5) | ||
| Total bacterial count | 9.11a | 9.16a | 8.83c | 9.02b | 9.16a | 0.009 | <0.0001 |
| Total coliform | 5.25a | 4.83b | 4.25d | 3.14e | 4.39c | 0.023 | <0.0001 |
| Escherichia coli | 4.21a | 3.74c | 4.04b | 3.09e | 3.26d | 0.034 | <0.0001 |
| Salmonella | 3.25a | 3.17a | 2.88b | 1.81d | 2.41c | 0.040 | <0.0001 |
| Lactobacillus | 7.10d | 8.10b | 7.73c | 8.12b | 8.31a | 0.025 | <0.0001 |
Values with the same letter in a row are not significantly (P > 0.05) different according to Tukey test.
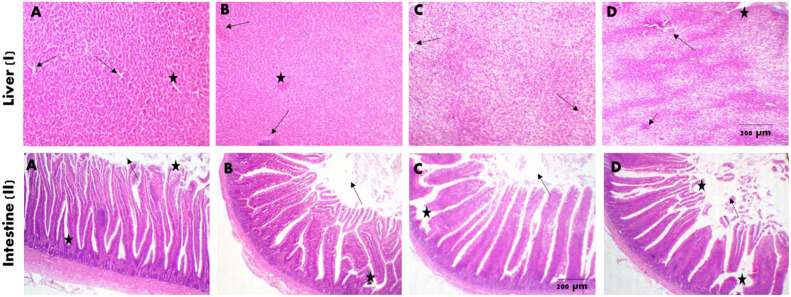
Histological Investigation
The histological examination of the quail liver and all intestinal segments from the control group (G1, basal diet without ANF), revealed typical hepatic structures, such as hepatic parenchyma (comprising the central vein, portal trades, sinusoids, and hepatic cords) (Figure 2IA). Quail intestine sections exhibited typical leaf-like intestinal villi, although they were relatively short and frequently contained free lumina (Figure 2IIA). Hepatic sections from G2 (basal diet supplemented with 0.5 mL ANF/kg diet) exhibited typical hepatic parenchyma without any aggregations of cells (Figure 2IB). Although healthy tall villi, free lumen, and limited goblet cell metaplasia without denudations were observed in all examined intestinal sections (Figure 2IIB).
Figure 2.
Photomicrograph of the quail liver (I) and intestines (II) stained with hematoxylin and eosin (X200). (A) control group (G1) without Aspergillus niger filtrate, (B) quail groups received 0.5 mL of A. niger filtrate/kg diet (G2), (C) quail groups received 1.0 mL of A. niger filtrate/kg diet (G3), (D) quail groups received 2.0 mL of A. niger filtrate/kg diet (G5).
The histological sections of the livers of quail group 3 (basal diet supplemented with 1.0 mL ANF/kg diet) indicated the presence of normal hepatic parenchyma with minor fatty changes in the central lobes (Figure 2IC). Figure 2IIC illustrates intestinal sections revealing healthy, erect villi, a free lumen, and restricted goblet cell metaplasia.
In the majority of liver sections examined, the quail group 5 (basal diet supplemented with 2.0 mL ANF/kg diet) exhibited multifocal intestinal lymphocytic aggregation surrounded by mildly degenerated hepatocytes. Additionally, sinusoid spaces were lost due to acute cell swelling (Figure 2ID). The lumina of the intestine exhibited a modest fusion of villi and residual desquamation portions, giving the impression of height (Figure 2IID).
DISCUSSION
The poultry industry is essential and has a tremendous economic return to most countries globally (Banday et al., 2023; Alghamdi et al., 2024; Salama et al., 2024). Therefore, saving feed and using products that reduce the cost of feed and preserve the health of birds and the environment is of great importance and is considered a focus point for most current research (Abd El-Hack et al., 2022; El-Saadony et al., 2022).
The discovery of antibiotics has been one of the most significant milestones in medical science and has played a pivotal role in advancing the progress and performance of the livestock and poultry sector (El-Hack et al., 2022, El-Saadony et al., 2022). Antibiotics have been used in poultry diets at sub-therapeutic doses for growth promotion and therapeutic levels for disease treatment (Banday et al., 2023). Inappropriate and indiscriminate use of antimicrobials leads to antibiotic resistance and transmission of resistant genes directly or through bacteria from animal feed or the environment (Salama et al., 2024). Numerous feed supplements have been proposed over the years as antibiotic alternatives to improve poultry birds' health status, welfare and productive performance (Alghamdi et al., 2024). However, organic acids have emerged as a potential antibiotic replacement due to their antimicrobial nature (Du et al., 2024). Although organic acids are abundant in nature as conventional components of animal and plant tissues, few of them are produced by microbial fermentation in the hindgut of animals (Ebeid and Al-Homidan, 2022).
Interestingly, supplementation of several types of organic acids, such as formic acid, lactic acid, fumaric acid, butyric acid, citric acid and tartaric acid, have been shown to improve poultry birds' health status and production performance significantly (Du et al., 2024, Ebeid and Al-Homidan, 2022). At the same time, organic acids has been investigated for their multiple therapeutic effects on pathological disorders, such as antimicrobial, antifungal, antiprotozoal, and anticoccidial agents (Ebeid and Al-Homidan, 2022). In addition, organic acids protects by improving the immune system, physiology, and morphology of the gastrointestinal tract (Du et al., 2024).
Therefore, the current investigation aimed to assess ANF growth onto AIRFB on quail with the evaluation of their productivity, digestion, carcass yield, intestinal microbiota, and blood chemistry.
In the current work, A. niger was chosen and tested on the base of RP solubilization; A. niger showed good growth and strong sporulation on the growth medium compared to the control. From the previous work by Mahgoub et al. (2022), the incubation periods (4 wk) were selected to produce organic acids, concentrations of P (100 mg P2O5 from RP), and the inoculum ratios (5 %, v/w) with 0.5 g from faba bean residuals. Saber et al. (2010) reported that the concentration of P2O5 was 75 mg under the lower levels of RP under solid-state fermentation conditions involving Phanerochaete chrysogenum, A. niger, rice, and wheat straw. Additionally, Youssef (2017) demonstrated that the molds Botrytis sp. MY29, Penicillium purpurogenum MY48, A. niger MY55, and A. niger MY81 exhibited the highest levels of phosphate solubilization and cellulolytic activity under solid-state fermentation conditions.
The cellulase activities of A. niger were valuable in the bioconversion of cellulosic residues. Youssef (2017) noticed that A. niger MY55 and A. niger MY81 were the most fungi that have both cellulolytic activities and P solubilization under solid-state fermentation requirements. Different organic acids have been produced by A. niger (Youssef et al., 2017). The enzyme activities are accompanied by the fact that the enzyme production in various microbes reached the maximum level in the log phase (Narsaleet al., 2018; Mahgoub et al., 2022).
The bio-somatization of RP is a multifaceted procedure due to the intricate configuration of established particle sizes. Previous studies have demonstrated a strong correlation between the solubility of insoluble P and a decrease in the pH of the culture media. In this study, the pH in the end filtrate reached 4.0. According to Abu Yazid et al. (2017), the metabolic process of carbon and nitrogen resources impacts total organic acids formation and decreases the culture media pH, which has an extension impact on the solubilization of insoluble P. The earlier evidence for the efficient bioconversion of faba bean straw into sugars and the acidic nature of the hydrolysate, denoted by pH, TA, the releasing of glucose, soluble P, and phytase, enhanced the differentiation of organic acids that may be formed because of conversation action of A. niger.
In the current work, the addition of ANF to basal diets at all ages, resulted in a significant shift in growth indices. This finding is consistent with the investigation conducted by Garca et al. (2007), which observed that quails' development rate was enhanced by adding organic acids up to 10,000 ppm.
The use of CH₃COOH (30 g/kg ration) enhanced the growth rate in broilers (Saleem et al., 2020). Organic acids may improve the ileum's digestibility of dry matter and rest nutrients, improving growth metrics (Hernández et al., 2006). The chickens fed a ration with organic acids like butyric, lactic, and fumaric acids had the best FCR. The current study's enhancement in ration utilization could be owed to better absorption of the nutrients and utilization, resulting in enhanced BW in the birds consumed a ration supplemented with organic acids as fumaric acids, lactic, and butyric (Adil et al., 2011).
Broiler hens fed 3 g/kg organic acids (ammonium salt of formic acid) exhibited lower feed consumption, which agrees with our findings; nonetheless, this addition improved growth measurements. These statements are backed up by the findings of Abdel-Fattah et al. (2008), who discovered that adding 1.5 and 3% acetic or citric acids to the ratio improved growth indices. Following Chamba et al. (2014), chickens given 700 ppm organic acids acquired greater weight and had a higher FCR during the growth and finishing stages. Organic acids may be responsible for the improvements in performance parameters because they improve the intestinal ability to uptake nutrients by improving the construction and action of the villi, as well as digestive enzymes, resulting in better nutrient absorption such as proteins, carbohydrates, and minerals (Nair and Johny, 2019).
A. niger-produced dietary organic acids and other chemicals did not affect the carcass, heart, liver, gizzard, giblets, or dressing. Our findings are in accordance with those of Brzóska et al. (2013), who reported that at 3, 6, and 9 g/kg rations, the weights of the gizzard, liver, carcass, breast muscles, abdominal fat, and leg were unaffected. Broiler carcass features (breast, thigh, heart, gizzard, and liver) were unaffected by the organic acids supplement NufocidL (1 mL/L drinking water) (Heidari et al., 2018). In the same way, lactic acid supply did not affect the edible sections of broilers. Some investigations indicated that organic acids had no impact on broiler carcass yield or dressing characteristics, confirming the effect of organic acids (Kopeck et al., 2012; Ghasemi et al., 2014). All these findings supported our observations in the current study.
In the current investigation, the digestive enzymes in quail diets containing ANF are significantly affected. In comparison to the control, adding up to 2 mL ANF/kg diet to quail feed boosted digestive enzyme activity like protease, amylase, and lipase. According to Dittoe et al. (2018), organic acids can enhance poultry digestive physiology, nutritional digestibility, and proteolytic enzyme activity, stimulate pancreatic excretions, and balance the intestinal flora. Furthermore, when organic acids were added at the dose of 0.30 g/kg, endogenous digestive enzyme activity in the gut was greater (Liu et al., 2017). Likewise, a mixture of sorbic acid, fumaric acid, and thymol could be utilized efficiently in broiler chicken feeds to boost trypsin and lipase levels as well as intestinal morphology, particularly during the growth stage (Yang et al., 2018).
From the current investigation, all hepatic and renal functions (total protein, albumin, globulin, albumin to globulin ratio, AST, ALT, LDH, and urea) revealed statistical differences except for creatinine level. AST, ALT, LDH, and urea levels were considerably lower in quails given ANF (0.5–2 mL ANF/kg diet). In the current study, except for 1.5 mL ANF, the supplementation of ANF increased total protein and albumin. In quails-fed meals containing ANF, plasma globulin levels were considerably higher than in controls. Organic acid supplementation (40 g citric/kg diet) showed no effect on liver enzyme activity ALT and AST, according to Ahmad et al. (2018) that explained the normal hepatic structures, such as hepatic parenchyma (comprising the central vein, portal trades, sinusoids, and hepatic cords) in the first 4 groups.
In the current study ANF supplementation lowered plasma triglycerides, LDL, and VLDL while increasing HDL. Nevertheless, there was no significant difference in plasma total cholesterol levels across treatments (P > 0.1011). Compared to control birds, organic acid-treated birds exhibited the lowest levels of blood total lipids, cholesterol, and LDL (Kamal and Ragaa, 2014), confirming our findings of plasma lipid index. Organic acids also lowered blood cholesterol, LDL cholesterol, and total lipids in broiler diets (Youssef et al. 2017; Naveenkumar et al., 2018). Adding citric acid to quail meals lowered cholesterol, LDL, and VLDL when compared to the control birds (Ahmad et al., 2018). Lower ration consumption throughout the growth stage and lower fat intake due to fat depletion may assist in lowering blood lipid levels, according to Abdel-Fattah et al. (2008).
When contrasted to the control and other levels (1, 1.5, or 2 mL ANF/kg diet), in quail ration enhanced immunological index (IgA, IgM, IgG, and lysozymes) (Table 7). According to Chowdhury et al. (2009), using 0.5% citric acid in broiler feed enhances immunological conditions. Organic acids in the poultry feed, according to Dibner and Buttin (2002) have a favorable effect on chicken immunological indices. Organic acids increased the immunological activity of broiler hens, according to Abdel-Fattah et al. (2008).
Adding a combination of organic acids to broiler ratio led to a linear rise in IgG levels in a similar vein (Nguyen et al., 2018). In birds given 0.30 g/kg organic acids and thymol essential oil during the grower or finisher phases, Yang et al. (2018) demonstrated an elevation in spleen size, suggesting improved immunological parameters. Furthermore, the organic acids (0.30 g/kg diet) therapy increased IgA levels in the ileal mucosa (Liu et al., 2017).
Quails fed ration supplemented with ANF in the present investigation had higher superoxide dismutase, total antioxidants and catalase activities than control quails. The quails given ANF had decreased malondialdehyde levels in their blood. The antioxidant markers utilized in our study were supported by the findings of Ahmad et al. (2018), which showed that GPX activity was higher in the organic acids group. At slaughter, there was no significant difference in total antioxidants between acidified diet chickens and control chickens (Abudabos et al., 2017).
Dietary organic acids supplementation could enhance gut structure integrity, physiological function, intestinal immunity, and health. Organic acid products have also improved the antioxidative status by activating antioxidative enzymes and reducing lipid peroxidation. Moreover, dietary organic acids can stimulate humoral- and cell-mediated immunity and regulate cytokines in the host. Organic acids physiological and immunological benefits are translated into various improvements in growth performance, laying performance, egg quality, meat quality, and the products' safety. Interestingly, organic acids positively impacts alleviating heat stress in poultry (Ebeid and Al-Homidan, 2022).
Avian salmonellosis and colibacillosis are serious pathogens inducing financial losses in the poultry sector (Elsayed et al., 2023; Yousef et al., 2023). From our investigation, birds fed meals supplemented with ANF had lower E. coli , coliform, and Salmonella colonization than the control group. The maximum total bacterial count and Lactobacillus were reported with 2 mL ANF/kg diet and the lowest total bacterial count and Lactobacillus were found with 1 mL ANF/kg diet. Organic acids have been successfully added to chicken rations to reduce harmful bacteria, mostly Salmonella spp. and mycotoxins formed by fungi (Dittoe et al., 2018). This current research is in accordance with the findings of Attia et al. (2018), who found that dietary organic acids reduce pathogenic bacteria count due to their antibacterial effect by making pH unsuitable for pathogenic growth in the gut while increasing beneficial bacteria and improving the solubility of feed ingredients, digestion and absorption of nutrients. According to Nair and Johny (2019), organic acids in broiler chicken diminish the number of Salmonella and members of the family Enterobacteriaceae.
Furthermore, several other investigations have shown that formic and propionic acids have a microbiological impact on Salmonella spp., coliforms, and E. coli in the gut of birds (Ruhnke et al., 2015; Gowda and Shivakumar, 2019). Regassa and Nyachoti (2018) reported that feeding with butyric acid can prevent Salmonella enteritidis infection in chicken intestines. Organic acids in broiler feeds, according to Nair and Johny (2019), reduced vertical transmission of illnesses like Salmonella. The population of E. coli were decreased linearly in chickens fed organic acids diets, but the count of Lactobacillus were elevated (Nguyen et al., 2018). This may explains the normal and healthy tall villi, free lumen, and limited goblet cell metaplasia without denudations observed in all examined intestinal sections in groups 1, 2, 3 and 4 that received 0, 0.5, 1.0, and 1.5 mL ANF/kg diet.
CONCLUSIONS
Supplementation of Japanese quail basal diet with ANF grown onto AIRFB boosts quails' growth and health. Seven organic acids, including oxalic, ascorbic, maleic, salicylic, lactic, formic, and citric, were detected from the growth of A. niger growth on AIRFB. These organic acids showed improvement in the nutritional characteristics of quails' diet, improved digestive enzymes, nutrient digestibility, immunity, antioxidant indices, minimized intestinal pathogenic bacteria and improved intestinal health of the treated birds.
DISCLOSURES
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups (RGP 2/491/44).
Funding: This research was funded by Abu Dhabi Department of Education and Knowledge, grant number 21S105, and UAEU Program for Advanced Research grant number 12S169 to KE-T.
Author Contributions: Conceptualization, H.K.A.-G., F.M.R., M.A., O.S., N.A., H.M.S., K. A. E.-T., and S.M., formal analysis, E.H.I., M.Y.A., M.M.A.-Q., M.T.E.-S., and A.M.S., investigation, H.K.A.-G., F.M.R., M.A., O.S., N.A., H.M.S., K. A. E.-T., S.M., E.H.I., M.Y.A., M.M.A.-Q., M.T.E.-S., and A.M.S., data curation, H.K.A.-G., F.M.R., M.A., O.S., N.A., H.M.S., and S.M., writing original draft preparation, H.K.A.-G., F.M.R., M.A., O.S., N.A., H.M.S., K. A. E.-T., S.M., E.H.I., M.Y.A., M.M.A.-Q., M.T.E.-S., and A.M.S., writing final manuscript and editing, H.K.A.-G., F.M.R., M.A., O.S., N.A., H.M.S., K. A. E.-T., and S.M., visualization and methodology, H.K.A.-G., F.M.R., M.A., O.S., N.A., H.M.S., S.M., E.H.I., M.Y.A., M.M.A.-Q., M.T.E.-S., and A.M.S. All authors have read and agreed to the published version of the manuscript.
REFERENCES
- Abd El-Hack M.E., El-Saadony M.T., Salem H.M., El-Tahan A.M., Soliman M.M., Youssef G.B.A., Taha A.E., Soliman S.M., Ahmed A.E., El-Kott A.F., Al Syaad K.M., Swelum A.A. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird's health and production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Fattah S.A., El-Sanhoury M.H., El-Mednay N.M., Abdel-Azeem F. Thyroid activity, some blood constituents, organs morphology and performance of broiler chicks fed supplemental organic acids. Int. J. Poult. Sci. 2008;7:215–222. [Google Scholar]
- Abu Yazid N., Barrena R., Komilis D., Sánchez A. Solid-state fermentation as a novel paradigm for organic waste valorization: a review. Sustainability. 2017;9:224. [Google Scholar]
- Abudabos A.M., Alyemni A.H., Dafalla Y.M., Al-Owaimer A.N. Effect of organic acid blend and Bacillus subtilis on growth, blood metabolites and antioxidant status in finishing broilers challenged with Clostridium perfringens. J. Anim. Plant Sci. 2017;27:1101–1107. [Google Scholar]
- Adil S., Banday M.T., Bhat G.A., Qureshi S.D., Wani S.A. Effect of supplemental organic acids on growth performance and gut microbial population of broiler chicken. Livestock Res. Rural. Dev. 2011;23:1–8. [Google Scholar]
- Ahmad E.A.M., Abdel-Kader I.A., Abdel-Wahab A.A. Organic acids as potential alternate for antibiotic as growth promoter in Japanese quail. Egypt. Poult. Sci. 2018;38:359–373. [Google Scholar]
- Alghamdi M.A., Reda F.M., Mahmoud H.K., Bahshwan S.M.A., Salem H.M., Alhazmi W.A., Soror A.S., Mostafa N.G., Attia S., Mohamed M.D.A., Saad A.M., El-Tarabily K.A., Abdelgeliel A.S. The potential of Spirulina platensis to substitute antibiotics in Japanese quail diets: impacts on growth, carcass traits, antioxidant status, blood biochemical parameters, and cecal microorganisms. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsubhi N.H., Al-Quwaie D.A., Alrefaei G.I., Alharbi M., Binothman N., Aljadani M., Qahl S.H., Jaber F.A., Huwaikem M., Sheikh H.M., Alrahimi J., Abd Elhafez A.N., Saad A. Pomegranate pomace extract with antioxidant, anticancer, antimicrobial, and antiviral activity enhances the quality of strawberry-yogurt smoothie. Bioengineering. 2022;9:735. doi: 10.3390/bioengineering9120735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apata D.F. Effect of Terminalia catappa fruit meal fermented by Aspergillus niger as replacement of maize on growth performance, nutrient digestibility, and serum biochemical profile of broiler chickens. Biotechnol. Res. Int. 2011;2011 doi: 10.4061/2011/907546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia F.A. Effect of organic acids supplementation on nutrients digestibility, gut microbiota and immune response of broiler chicks. Egypt. Poult. Sci. 2018;38:223–239. [Google Scholar]
- Banday M.T., Adil S., Sheikh I.U., Hamadani H., Qadri F.I., Sahfi M.E., Sait H.S., Abd El-Mageed T.A., Salem H.M., Taha A.E., T.El-Saadony M. The use of silkworm pupae (Bombyx mori) meal as an alternative protein source for poultry. World’s Poult. Sci. J. 2023;79:119–134. [Google Scholar]
- Broom L.J. Organic acids for improving intestinal health of poultry. World’s Poult. Sci. J. 2015;71:630–642. [Google Scholar]
- Brzóska F., Śliwiński B., Michalik-Rutkowska O. Effect of dietary acidifier on growth, mortality, post-slaughter parameters and meat composition of broiler chickens. Ann. Anim. Sci. 2013;13:85–96. [Google Scholar]
- Chamba F., Puyalto M., Ortiz A., Torrealba H., Mallo J.J., Riboty R. Effect of partially protected sodium butyrate on performance, digestive organs, intestinal villi and E. coli development in broilers chickens. Int. J. Poult. Sci. 2014;13:390–396. [Google Scholar]
- Chang X., Minnan L., Xiaobing W., Huijuan X., Zhongan C., Fengzhang Z., Liangshu X. Screening and characterization of the high cellulase producing strain Aspergillus glaucus XC9. Front. Biol. China. 2006;1:35–40. [Google Scholar]
- Chowdhury R., Islam K.M., Khan M.J., Karim M.R., Haque M.N., Khatun M., Pesti G.M. Effect of citric acid, avilamycin, and their combination on the performance, tibia ash, and immune status of broilers. Poult. Sci. 2009;88:1616–1622. doi: 10.3382/ps.2009-00119. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Buttin P. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 2002;11:453–463. [Google Scholar]
- Dittoe D.K., Ricke S.C., S.Kiess A. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 2018;5:216. doi: 10.3389/fvets.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Sarwar I., Ahmad S., Suheryani I., Anjum S., Andlib S., Kakar M.U., Arain M.A. Organic acids in poultry industry: a review of nutritional advancements and health benefits. World’s Poult. Sci. J. 2024;80:133–153. [Google Scholar]
- Ebeid T.A., Al-Homidan I.H. Organic acids and their potential role for modulating the gastrointestinal tract, antioxidative status, immune response, and performance in poultry. World’s Poult. Sci. J. 2022;78:83–101. [Google Scholar]
- El-Saadony M.T., Salem H.M., El-Tahan A.M., Abd El-Mageed T.A., Soliman S.M., Khafaga A.F., Swelum A.A., Ahmed A.E., Alshammari F.A., Abd El-Hack M.E. The control of poultry salmonellosis using organic agents: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sawah M.M.A., El-Rafey H.H., Saber W.I.A. Reduction of phytic acid content in soy bean by A. niger phytase immobilized into gelatin film by cross-linking. J. Agric. Sci. Mansoura Univ. 2001;26:5165–5175. [Google Scholar]
- Elsayed M.M., El-Basrey Y.F.H., El-Baz A.H., Dowidar H.A., Shami A., Al-Saeed F.A., Alsamghan A., Salem H.M., Alhazmi W.A., El-Tarabily K.A., Khedr M.H.E. Ecological incidence, genetic diversity, multidrug resistance of Salmonella enteritidis recovered from broiler and layer chicken farms. Poult. Sci. 2023;103 doi: 10.1016/j.psj.2023.103320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferronato G., Prandini A. Dietary supplementation of inorganic, organic, and fatty acids in pig: a review. Animals. 2020;10:1740. doi: 10.3390/ani10101740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V., Catala-Gregori P., Hernandez F., Megias M.D., Madrid J. Effect of formic acid and plant extracts on growth, nutrient, digestibility, intestine mucosa morphology, and meat yield of broilers. J. Appl. Poult. Res. 2007;16:555–562. [Google Scholar]
- Ghasemi H.A., Akhavan-Salamat H., Hajkhodadadi I., Khaltabadi-Farahani A.H. Effects of dietary organic acid blend supplementation on performance, intestinal morphology and antibody-mediated immunity in broiler chickens. Acta Advances Agri. Sci. 2014;2:64–74. [Google Scholar]
- Gowda V., Shivakumar S. In: Biotechnological Applications of Polyhydroxyalkanoates. Kalia V., editor. Springer; Singapore: 2019. Novel biocontrol agents: short chain fatty acids and more recently, polyhydroxyalkanoates; pp. 323–345. [Google Scholar]
- Heidari M.R., Sadeghi A.A., Rezaeipour V. Effects of acidifier supplementation and toxin binder on performance, carcass, blood metabolites, intestinal morphology and microbial population in broiler chickens. Iran. J. Appl. Anim. Sci. 2018;8:469–476. [Google Scholar]
- Hernández F., García V., Madrid J., Orengo J., Catalá P., Megías M.D. Effect of formic acid on performance, digestibility, intestinal histomorphology and plasma metabolite levels of broiler chickens. Br. Poult. Sci. 2006;47:50–56. doi: 10.1080/00071660500475574. [DOI] [PubMed] [Google Scholar]
- Jain P., Singh D. Study on the role of phosphate solubilising fungi in phosphorus bioavailability and growth enhancement of potato. Chem. Sci. Rev. Lett. 2015;4:101–108. [Google Scholar]
- Kamal A.M., M.Ragaa N. Effect of dietary supplementation of organic acids on performance and serum biochemistry of broiler chicken. Nat. Sci. 2014;12:38–45. [Google Scholar]
- Khan S.H., Iqbal J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016;44:359–369. [Google Scholar]
- Kopecký J., Hrnčár C., Weis J. Effect of organic acids supplement on performance of broiler chickens. Anim. Biotechnol. 2012;45:51–54. [Google Scholar]
- Kumari A., Kapoor K.K., Kundu B.S., Mehta R.K. Identification of organic acids produced during rice straw decomposition and their role in rock phosphate solubilization. Plant Soil Environ. 2008;54:72–77. [Google Scholar]
- Liu Y., Yang X., Xin H., Chen S., Yang C., Duan Y., Yang X. Effects of a protected inclusion of organic acids and essential oils as antibiotic growth promoter alternative on growth performance, intestinal morphology and gut microflora in broilers. Anim. Sci. J. 2017;88:1414–1424. doi: 10.1111/asj.12782. [DOI] [PubMed] [Google Scholar]
- Lokesh S.T., Naveen Kumar K.J., Thippeswamy B. Screening of efficient phosphate solubilizing fungi from mine soil and effect of phosphofungi on seed germination and vigour index of ground nut (Arachis hypogaea L.) and green gram (Vigna radiata L.) Int. J. Biol. Res. 2016;4:288–294. [Google Scholar]
- Mahgoub S.A., A.Kedra E.G., Abdelfattah H.I., Abdelbasit H.M., A.Alamoudi S., Al-Quwaie D.A., Selim S., Alsharari S.S., Saber W.I.A., El-Mekkawy R.M. Bioconversion of some agro-residues into organic acids by cellulolytic rock-phosphate-solubilizing Aspergillus japonicus. Fermentation. 2022;8:437. [Google Scholar]
- Mosa M.I., Salem H.M., Bastamy M.A., Amer M.M. Pathogenic and non-pathogenic factors; especially infectious bursal disease viruses; affect chicken digestive system microbiota and methods of its evaluation and recovery: a review. Egypt. J. Vet. Sci. 2023;54:733–760. [Google Scholar]
- Mrudula S., Murugammal R. Production of cellulase by Aspergillus niger under submerged and solid-state fermentation using coir waste as a substrate. Braz. J. Microbiol. 2011;42:1119–1127. doi: 10.1590/S1517-838220110003000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair D.V.T., Johny A.K. In: Food Safety in Poultry Meat Production. Food Microbiology and Food Safety. Venkitanarayanan K., Thakur S., Ricke S., editors. Springer; Cham, Switzerland: 2019. Salmonella in poultry meat production; pp. 1–24. [DOI] [Google Scholar]
- Najafi M.F., Deobagkar D., Deobagkar D. Purification and characterization of an extracellular α-amylase from Bacillus subtilis AX20. Protein Exp. Purif. 2005;41:349–354. doi: 10.1016/j.pep.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Najafi M.F., Deobagkar D.N., Mehrvarz M., Deobagkar D. Enzymatic properties of a novel highly active and chelator resistant protease from a Pseudomonas aeruginosa PD100. Enz. Microb. Technol. 2006;39:1433–1440. [Google Scholar]
- Narasimha G., Sridevi A., Buddolla V., M.Subhosh C., Rajasekhar R.B. Nutrient effects on production of cellulolytic enzymes by Aspergillus niger. Afr. J. Biotechnol. 2006;5:472–476. [Google Scholar]
- Narsale P.V., Patel S.R., Acharya P. Role of Aspergillus flavus on biodegradation of lignocellulosic waste millet straw and optimization parameters for enzyme hydrolysis and ethanol production under solid state fermentation. Int. J. Curr. Microbiol. App. Sci. 2018;7:429–445. [Google Scholar]
- National Research Council . Nutrient Requirements of Poultry: Ninth Revised. Edition. The National Academies Press; Washington, DC, USA: 1994. [DOI] [Google Scholar]
- Naveenkumar S., Karthikeyan N., Narendra Babu R., Veeramani P., Sivarama Krishnani S., Srinivasan G. Effect of calcium propionate and coated sodium butyrate as an alternative to antibiotic growth promoters on the serum profile of commercial broiler chicken. Int. J. Chem. Stud. 2018;6:36–39. [Google Scholar]
- Nguyen D.H., Lee K.Y., Mohammad M., kim I.H. Evaluation of the blend of organic acids and medium-chain fatty acids in matrix coating as antibiotic growth promoter alternative on growth performance, nutrient digestibility, blood profiles, excreta microflora, and carcass quality in broilers. Poult. Sci. 2018;97:4351–4358. doi: 10.3382/ps/pey339. [DOI] [PubMed] [Google Scholar]
- Papadaki E., Mantzouridou F.T. Αpplication of Aspergillus niger for extracellular tannase and gallic acid production in non-sterile table olive processing wastewaters. Waste Biomass Valor. 2024;15:1199–1212. [Google Scholar]
- Regassa A., Nyachoti C.M. Application of resistant starch in swine and poultry diets with particular reference to gut health and function. Anim. Nutr. 2018;4:305–310. doi: 10.1016/j.aninu.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhnke I., Röhe I., Goodarzi Boroojeni F., Knorr F., Mader A., Hafeez A., Zentek J. Feed supplemented with organic acids does not affect starch digestibility, or intestinal absorptive or secretory function in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2015;99:29–35. doi: 10.1111/jpn.12313. [DOI] [PubMed] [Google Scholar]
- Saber W.I.A., El-Naggar N.E., AbdAl-Aziz S.A. Bioconversion of lignocellulosic wastes into organic acids by cellulolytic rock phosphate-solubilizing fungal isolates grown under solid-state fermentation conditions. Res. J. Microbiol. 2010;5:1–20. [Google Scholar]
- Salama T.M., Kamal M.A., Abdelfatah S.H., Salem H.M., Mohamed F.F. Ameliorative effect of a novel enzymatic detoxifier against natural field levels of mycotoxins in the broiler chicken diet. J. Adv. Vet. Res. 2024;14:228–234. [Google Scholar]
- Saleem K., Saima A.R., Pasha T.N., Mahmud A., Hayat Z. Effects of dietary organic acids on performance, cecal microbiota, and gut morphology in broilers. Trop. Anim. Health Prod. 2020;52:3589–3596. doi: 10.1007/s11250-020-02396-2. [DOI] [PubMed] [Google Scholar]
- Salem H.M., Saad A.M., Soliman S.M., Selim S., Mosa W.F.A., Ahmed A.E., Al Jaouni S.K., Almuhayawi M.S., Abd El-Hack M.E., El-Tarabily K.A., El-Saadony M.T. Ameliorative avian gut environment and bird productivity through the application of safe antibiotics alternatives: a comprehensive review. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Show P.L., Oladele K.O., Siew Q.Y., Zakry F.A., Lan J.C.W., Ling T.C. Overview of citric acid production from Aspergillus niger. Front. Life Sci. 2015;8:271–283. [Google Scholar]
- Stefanolo J.P., Segura V., Grizzuti M., Heredia A., Comino I., Costa A.F., Puebla R., Temprano M.P., Niveloni S.I., de Diego G., Oregui M.E., Smecuol E.G., de Marzi M.C., Verdú E.F., Sousa C., Bai J.C. Effect of Aspergillus niger prolyl endopeptidase in patients with celiac disease on a long-term gluten-free diet. World J. Gastroenterol. 2024;30:1545–1555. doi: 10.3748/wjg.v30.i11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna S.K., Layton C., Bancroft J.D. Bancroft's Theory and Practice of Histological Techniques. 7th ed. Churchill Livingstone. Elsevier; England: 2013. p. 603 p. [Google Scholar]
- Themelis D.G., Tzanavaras P.D. Reagent-injection spectrophotometric determination of citric acid in beverages and pharmaceutical formulations based on its inhibitory effect on the iron (III) catalytic oxidation of 2,4-diaminophenol by hydrogen peroxide. Anal. Chim. Acta. 2001;428:23–30. [Google Scholar]
- Upton D.J., McQueen-Mason S.J., Wood A.J. In silico evolution of Aspergillus niger organic acid production suggests strategies for switching acid output. Biotechnol. Biofuels. 2020;13:27. doi: 10.1186/s13068-020-01678-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaicu P.A., Untea A.E., Varzaru I., Saracila M., G.Oancea A. Designing nutrition for health-incorporating dietary by-products into poultry feeds to create functional foods with insights into health benefits, risks, bioactive compounds, food component functionality and safety regulations. Foods. 2023;12:4001. doi: 10.3390/foods12214001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Xin H., Yang C., Yang X. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr. 2018;4:388–393. doi: 10.1016/j.aninu.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef H.M.Y., Hashad M.E., Osman K.M., Alatfeehy N.M., Hassan W.M.M., Elebeedy L.A., Salem H.M., Shami A., Al-Saeed F.A., El-Saadony M.T., El-Tarabily K.A., Marouf S. Surveillance of Escherichia coli in different types of chicken and duck hatcheries: one health outlook. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef I.M.I., Mostafa A.S., Abdel-Wahab M.A. Effects of dietary inclusion of probiotics and organic acids on performance, intestinal microbiology, serum biochemistry and carcass traits of broiler chickens. J. World Poult. Res. 2017;7:57–71. [Google Scholar]
- Zhou L., Abouelezz K., Momenah M.A., Bajaber M.A., Baazaoui N., Taha T.F., Awad A.E., Alamoudi S.A., Beyari E.A., Alanazi Y.F., Allohibi A., Saad A.M. Dietary Paenibacillus polymyxa AM20 as a new probiotic: improving effects on IR broiler growth performance, hepatosomatic index, thyroid hormones, lipid profile, immune response, antioxidant parameters, and caecal microorganisms. Poult. Sci. 2024;103 doi: 10.1016/j.psj.2023.103239. [DOI] [PMC free article] [PubMed] [Google Scholar]