Abstract
Skin infections considered as one of the predominant disorders that could greatly influence humans. Topical drug delivery is believed to be an effective substitute to systemically delivered medication for skin disorders management. Erythromycin has been proven to retain anti-bacterial activity. Based on that, the aim of existent study is to develop a proper nanocarrier, namely; nanoemulsion using tea tree oil including Erythromycin. Applying quality by design approach, the optimized nanoemulsion was selected based on number of independent variables namely; particle size and in vitro release study. Yet, in order to get appropriate topical application, the optimized nanoemulsion was combined with previously prepared hydrogel base to provide Erythromycin based nanoemulgel. The developed nanoemulgel was assessed for its organoleptic and physical characters to ensure its suitability for topical application. Stability study was implemented over three months after being kept in two distinct environments. Eventually, the antibacterial behavior of the preparation was investigated on MRSA to verify the expected antibacterial improvement and validate the effectiveness of the developed nanocarrier. The formulation showed consistent appearance, with pH (6.11 ± 0.19), viscosity (10400 ± 1275 cP), spreadability (54.03 ± 2.3 mm), extrudability (80.36 ± 3.15 g/cm2) and drug content (99.3 ± 0.46 %) that seemed to be satisfied for topical application. It could provide 48.1 ± 4.2 % releases over 6 h in addition to be stable at room temperature and at refrigerator. Ultimately, the formula showed a significant antibacterial activity against MRSA proving the combination and the nanocarrier effectiveness.
Keywords: Erythromycin, Natural oil, Tea tree oil, Nanocarrier, Optimization, Topical drug delivery, Anti-bacterial
1. Introduction
The human skin is deliberated the biggest organ in the body that serves as a defense mechanism against foreign objects and infections (Haftek, Coutanceau, and Taïeb 2013). Topical delivery is the most common system for delivering drugs across the skin, providing comfortable, quick, and safe way. This method is recommended owing to its convenience and the ability to avoid gastrointestinal problems associated with oral medications (Leppert et al. 2018). These medications can be delivered topically in a form of various formulations such as creams, gels, and ointments (Barnes et al. 2021). Numerous techniques have been employed in recent decades to maximize medication delivery over the skin (Narasimha Murthy and Shivakumar 2010). Topical formulations can incorporate various medications for several purposes, including anti-oxidant, anti-cancer, anti-inflammatory, analgesic, anti-fungal, and antibacterial agents (Elsewedy et al., 2022a).
Antibacterial agents are among the most frequently used medicinal substances in the world for managing several infectious disorders (Leekha, Terrell, and Edson 2011). Moreover, it has been shown that these topical antibiotics accomplish a significant contribution to the management of skin infections (Bandyopadhyay 2021). One of the efficiently used antibiotics is Erythromycin (EM), a macrolide antibacterial agent efficient against several Gram-positive and Gram-negative bacterial strains (Jelić and Antolović 2016). However, it was revealed that the resistance of Methicillin-resistant Staphylococcus aureus (MRSA) toward EM could reach high percentage (Hu et al., 2018, Hu et al., 2023). Originally, EM was obtained from the fermentation products of Streptomyces erythraeus (Li et al. 2021). As most of macrolides, EM exhibits its antibacterial activity by reversibly attaching to the 50S ribosomal subunit and influencing the synthesis of proteins leading to bacterial death (Jelić and Antolović 2016). However, there are some obstacles that restrict its oral administration, mainly; its unpleasant taste, poor solubility, low bioavailability, instability in stomach pH and possessing short half-life (Mahama and Songuigama, 2020, Pignatello et al., 2011). These limitations make it necessary to search for other route of administration, principally; EM topical delivery. Although topical application can be beneficial in lowering systemic adverse effects and enhancing compliance of the patient, it is still challenging to control the poor penetration of drugs through the skin (Raina et al. 2023). Among the most effective strategies to address these challenges is the use of nanoparticulate carriers, which effectively delivers medication through the skin and gets around the drawbacks of conventional formulations.
Developing nanoparticulate is part of nanotechnology that focuses on creating carriers in a nano-range of sizes (Khan, Saeed, and Khan 2019). It offers promising potential in drug delivery that can target specific sites of action providing high therapeutic effectiveness with minimal undesirable reaction (Alhasso, Ghori, and Conway 2023). Nanocarriers are among different nanoparticulate developed for drug delivery owing to their ability to accept a wide range of medications and overcome their solubility issue (Ghasemiyeh and Mohammadi-Samani 2018). They are colloidal carriers that available in various forms such as solid lipid nanoparticles, liposomes, niosomes and nanoemulsions (Buya et al. 2021). Nanoemulsions (NEs) are formed of two immiscible liquids that are dispersed to form either water-in-oil (W/O) or oil-in-water (O/W) NE stabilized by a proper surfactant (Singh et al. 2017b). There are several ways to deliver these NEs, such as oral, intravenous, intranasal, pulmonary, ophthalmic, and topical route (Singh et al. 2017a). They possess lot of advantages such as better stability and bioavailability, ability of incorporating hydrophilic or lipophilic medications, providing better loading capacity (Elsewedy et al., 2021a, Elsewedy et al., 2021b). These factors make it a valuable substitute for other less stable nanocarriers like liposomes and vesicles (Preeti et al. 2023). Despite all these benefits, topical application of NE is inadequate due to its low viscosity that leads to low spreadability as well (Donthi et al. 2023). Therefore, there was a necessity to find a way for better and proper application of topical preparation by converting NE into more viscous formulation termed as Nanoemulgel (NEG) using gelling agents. Lately, there has been an increase in the usage of topical NEGs due to their improved patient acceptability, lack of gastrointestinal side effects, ease of application, and favorable therapeutic and safety profile (Bujubarah et al. 2023).
It was reported that an effective strategy for treating a variety of infectious disease is to apply a combination therapy. This therapy involves combining two or more medicinal agents that might be natural or synthetic (Iqubal et al. 2021). Using natural products has been widely spread due to their great effectiveness and safety (Elsewedy et al. 2022). Melaleuca alternifolia found in Australia is the source of important natural oil known as tea tree oil (TTO). It was proven to have great antibacterial activity when applied topically due to its monoterpenoid constituents, which is mainly attributed to Terpinen-4-ol (Bujubarah et al. 2023). Accordingly, TTO would significantly improve the antibacterial behavior of EM when combined together.
In this context, the present study sought to explore NEG containing EM as a credible nanocarrier. Therefore, the optimized EM based NE was developed using quality by design approach and then incorporated into pre-formulated gel to provide EM based NEG. The developed formula was screened for its physical properties and other parameters in addition to evaluating its antibacterial effect especially against MRSA.
2. 2- material and methods
2.1. Material
Tea tree oil was supplied from NOW® Essential Oils (NOW Foods, Bloomingdale, IL, USA). Erythromycin, Tween 80, and hydroxypropylmethyl cellulose (HPMC) were bought from Sigma-Aldrich (St. Louis, MO, USA). Diethylene glycol monoethyl ether (Transcutol® P) was bought from Gattefosse SAS (Saint-Priest, Cedex, France). All other reagents were of analytical quality.
2.2. Optimization study of variables
One of the most widely used factorial design options is central composite design (CCD). thus it was applied in the current optimization process using Design-Expert version 12.0 software (Stat-Ease, Minneapolis, MN, USA). The input factors for the study was determined as oil concentration (X1) and surfactant concentration (X2) while the responses that were chosen, namely; particle size (Y1) and in vitro release (Y2) in order to offer (22) factorial design as shown in Table 1. Both responses were selected to be the investigated response owed to their great impact on the obtained formulations. Particle size of the formulation can impact the rate of absorption since the smaller the particles the faster the absorption. As well, the response of in vitro release is very vital parameter to be deliberated due to its influence on the formulation’s efficacy (Weng, Tong, and Chow 2020). Based on the design, ten experimental runs (N1–N10) were proposed and prepared as displayed in Table 2. To estimate the effect of variables on the responses, the design plotted certain graphs like 3D surface and contour graphs further to some mathematical formulas used for emphasizing the data and the model. Regarding the check of the statistical aspects of the model, analysis of variance test (ANOVA) was used (Elsewedy et al. 2023).
Table 1.
Factors and response variables for CCD:
| Factors | Symbol | Levels | |
|---|---|---|---|
| (−1) | (+1) | ||
| TTO (g) | X1 | 1.5 | 2.5 |
| Tween 80 (g) | X2 | 0.5 | 1 |
Table 2.
Data for selected factors and their observed responses for various prepared EM-based-NE formulations.
| NE | Selected factors | Observed responses | ||
|---|---|---|---|---|
| X1 (g) | X2 (g) | Y1 (nm) | Y2 (%) | |
| N1 | 2 | 0.75 | 302 ± 2.8 | 66.0 ± 2.0 |
| N2 | 1.5 | 0.5 | 252 ± 2.6 | 79.0 ± 2.4 |
| N3 | 2 | 0.5 | 326 ± 3.1 | 60.0 ± 2.0 |
| N4 | 2.5 | 0.75 | 394 ± 3.6 | 50.2 ± 2.2 |
| N5 | 1.5 | 0.75 | 225 ± 3.5 | 82.3 ± 1.3 |
| N6 | 1.5 | 1 | 214 ± 2.7 | 86.0 ± 2.4 |
| N7 | 2.5 | 0.5 | 411 ± 3.7 | 46.0 ± 2.0 |
| N8 | 2.5 | 1 | 378 ± 3.0 | 53.4 ± 1.4 |
| N9 | 2 | 1 | 283 ± 3.2 | 71.5 ± 1.7 |
| N10 | 2 | 0.75 | 305 ± 2.6 | 64.5 ± 1.9 |
X1: TTO (g); X2: Tween 80 (g); Y1: particle size (nm) and Y2: In vitro release (%).
2.3. Development of EM-based-NE
NEs having EM were developed using various amounts of TTO and tween 80 as exhibited in Table 2. Brifely, NE was prepared by disolving EM in specified concentration of TTO with the addition of 500 mg Transcutol® P that acted as a solubilizing agent and stirr well using a classic advanced vortex mixer (VELP Scintifica, Italy) till the formation of oily phase. Regarding the aqueous phase, specific concentration of tween 80 was added to water and mixed well using same vortex mixer. The two phases were combined together and the final volume was adjusted to 10 mL and then the mixture was subjected to homogenization for 10 mints utilizing a high shear homogenizer (T 25 digital Ultra-Turrax, IKA, Staufen, Germany) at 10,000 rpm till homogenous NE was attained (Shehata and Elsewedy 2022). A matrix of eleven experimental runs was presented in Table 2.
2.4. Description of the formulated EM-based-NE
2.4.1. Particle size and size distribution (PDI)
The particle size and PDI of the generated EM-based-NE were measured using a disposable cuvette by diluting a sample of 5 µL from each formula to 3 mL of distilled water. The dynamic light scattering technique was employed together with a Malvern zetasizer (Nanoseries, zs; Malvern Instruments, Malvern, UK) to conduct the measurement, which was observed at 25 °C at an angle of 90° (Elsewedy et al., 2022a).
2.4.2. Investigation of in vitro release
In vitro release study is an essential test for evaluating the quality, safety, and effectiveness of drug delivery systems besides predicting formulations’ stability (Weng, Tong, and Chow 2020). The study was performed for detecting the percentage of EM released from each of the produced NE formulations using dissolution system (ERWEKA, GmbH, Heusenstamm, Germany). A glass tube was attached to the apparatus from one side and on the other side covered with a cellophane membrane (MWCO 2000–15,000) that holds a sample of the formulation under examination. The tubes were suspended in 500 mL of phosphate buffer of pH 5.5 to mimic the pH of the skin at a persistent temperature 32 ± 0.5 ◦C. When the system was turned on, its spinning speed was set at 50 rpm. Regularly, a 3 mL sample is removed and replaced with an equivalent volume of new buffer at predetermined intervals (0.25, 0.5, 1, 2, 3, up to 6 h). (JENWAY 6305, Bibby Scientific Ltd, Staffs, UK) spectrophotometer was used to perform a spectrophotometric analysis of the withdrawn sample at λmax 285 nm (Shehata et al., 2022). Every experiment was run three times, with a mean value ± SD for each.
2.5. Study of Drug-Excipient compatibility (Fourier transform infrared spectroscopy)
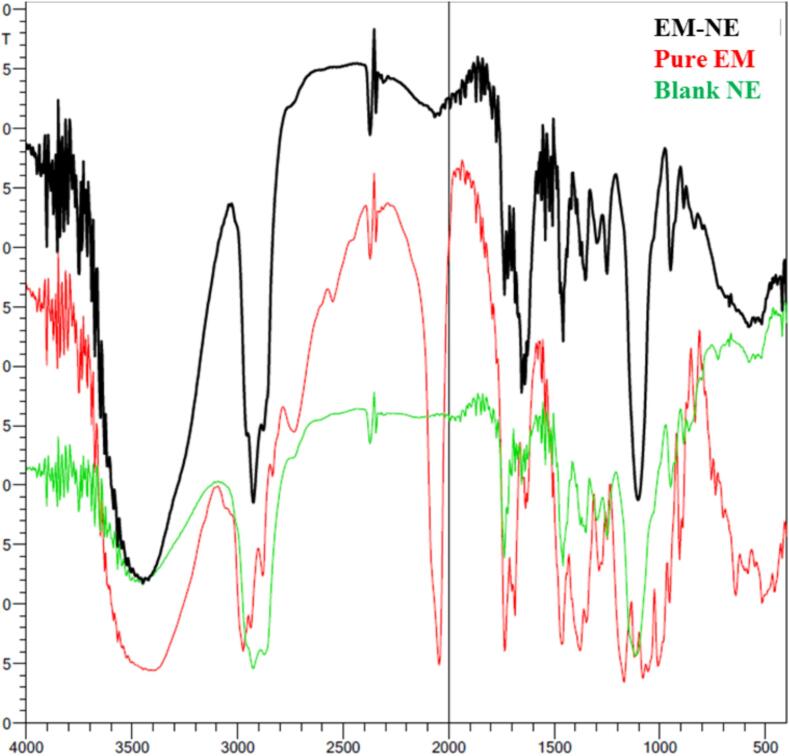
Determining the drug-excipient compatibility in developed formulations require performing Fourier transform infrared spectroscopy (FTIR) analysis using FTIR spectrophotometer (FTIR spectrophotometer, SHIMADZU, IRAFFINITY-1S, Japan). The KBr pellet method was used for the analysis where the dried KBr plate was utilized to apply the examined sample. The spectra for all investigated formulations; pure EM, blank NE, and EM-based-NE, were examined on spectra from 4000 and 400 cm−1 (Bujubarah et al. 2023).
2.6. Development of topical EM-based-NEG
As previously stated, the utilization of viscous formulations for topical application is more recommended since it offers sufficient and longer contact time with the skin, which would positively affect the efficiency (Müller et al. 2019). Accordingly, the optimized EM-based-NE formulation was combined with a gel preparation that was previously prepared. HPMC was selected as a gelling agent since it has no pharmacological activity, could attain clear gel, in addition to providing stable viscosity over long term (Nugrahaeni and Okvianida 2022). Mainly, 4 % w/w of HPMC gelling agents was distributed into 15 mL of distilled water and agitated with magnetic stirrer (Jeio Tech TM-14SB, Medline Scientific, Oxfordshire, UK) to prepare the gel. Afterward, the formulated gel was added and mixed with the optimized EM-based-NE for 5 min using a Heidolph mixer (Heidolph RZR 1, Heidolph Instruments, Schwabach, Germany). Using the same procedure, a blank NEG devoid of EM was created in order to assess the efficacy of the EM-based-NEG.
2.7. Characterization of topical EM-based-NEG
2.7.1. Organoleptic assessment
Visual examination of the physical attributes, such as color, homogeneity, and appearance, was conducted in order to look for any potential phase separation or agglomeration (Szulc-Musioł et al., 2023).
2.7.2. pH value measurement
To avoid triggering irritation, formulations intended for topical use should have a pH that is quite similar to human skin. Therefore, a calibrated pH meter (MW802, Milwaukee Instruments, Szeged, Hungary). was used to measure the pH of the formulation at room temperature by dipping its glass electrode into a sufficient volume of the formulation (Sharma and Tailang 2020).
2.7.3. Viscosity evaluation
Utilizing a Brookfield viscometer (DV-II+Pro, Middleboro, USA), the viscosity of the prepared EM-based-NEG was measured using a spindle R5 operated at 25 ± 0.5 °C and rotated at 0.5 rpm (Bujubarah et al. 2023).
2.7.4. Spreadability evaluation
The spreadability of EM-based-NEG was assessed since it is essential to apply the formulation evenly over the skin. A 500 mg load was fixed for one minute over the system after placing (1 gm) of the formulation in the middle of two glass slides measuring (25 cm × 25 cm). Applying the formulation to the slide allowed for the measurement of the diameter to determine how well the preparation could spread across the skin.
2.7.5. Extrudability assessment
Extrudability is very essential property to be assessed for any topical preparations. It represents the capability of any topical formulation to be shaped into a ribbon when being extruded from a collapsible tube. Presently, when the collapsed tube's crimped end was pressed, the weight in grams was determined that allow the gel ribbon of at least half a centimeter to be extruded out. The extrudability (g/cm2) was calculated using the formula below:
Extrudability = Weight (g) required to extrude topical preparation out of tube/ Area (cm2)
2.8. Drug content
After being diluted with 100 mL of phosphate buffer (pH 5.5), one gram of EM-based-NEG was run through 0.45 micro-syringe filters for filtration. Using a spectrophotometric assay at λmax 285 nm, the drug content was determined. The following equation was employed to calculate the drug content of the blank sample applying the same procedure:
Drug content %= (Actual drug amount / Theoretical) x 100
2.9. Investigating in vitro release from ER-based-NEG
To evaluate the percentage of release from the developed EM-based-NEG, the same methodology used to determine the in vitro release from the optimized ER-based-NE was applied. ERWEKA dissolution system was utilized to carry out the procedure.
2.10. Kinetic study
The method by which the drug could be released from the formulated preparation is explained by kinetic studies. Zero-order reaction, first-order, Higuchi, and Korsmeyer–Peppas modeling are some of these kinetic modeling systems. Each model provides a unique illustration of the relationship between the drug concentration and the time, allowing the most fitting model with the maximum value of correlation R2 to be established. Generally, the relationship between drug concentrations and time is exhibited by zero-order kinetics, while the relationship between log concentrations and time is clearly shown by first-order kinetics. The link between drug concentrations and the square root of time (t0.5) was depicted by the Higuchi equation. However, the model appeared to follow the Korsmeyer–Peppas equation if there was a link between log concentration and log time (Yeo et al. 2021). The following formulas illustrate the different models as follow:
Zero order kinetic C=C0 + kt;
First order kinetic C=C0 × ekt;
Higuchi kinetic C=k × t0.5;
Korsmeyer-Peppas kinetic C=k × tn;
The value of (C) at time zero was indicated by (C0). The release constant is denoted by (k), and the permeation rate exponent is presented by (n) (Elsewedy et al. 2024).
2.11. Stability study
Examining the stability of the formulation is very crucial since it provides information about the characters of the preparation and whether they changed over time under specific conditions. EM-based-NEG was assessed for number of characteristics, such as physical appearance, pH, viscosity, spreadability, and extrudability as well as the in vitro release investigation. After maintaining the formulation at two distinct temperatures 4 ± 2 °C and 25 ± 2 °C for one and three months. The International Conference on Harmonization (ICH) was followed in conducting the examination. While fresh withering, the formulation was inspected and contrasted with the identical preparation while it was fresh (Elsewedy et al. 2022).
2.12. Antibacterial activity
Using bacterial strain, the disc diffusion technique was applied to examine the impact of the developed formulations on the bacterial growth. The American Type Culture Collection (ATCC) provided the strain of MRSA bacterium (methicillin-resistant Staphylococcus aureus) that was used in the study. Briefly, Moller Hinton Agar was made in a petri dish and distributed to create the bacterial culture medium. Each petri dish has three 6 mm-diameter wells that need to be filled with the mixture under examination. Wells were filled with EM-based-NEG, blank NEG, and EM suspension and then were incubated for 24 h at 37 ± 1 °C. Measuring the inhibition zone’s diameter offers insight into the potential antibacterial efficacy of the formulation (Hombach, Zbinden, and Böttger 2013). Every experiment had a mean value ± SD and was conducted in triplicate.
2.13. Morphology of treated bacterial cells
Using scanning electron microscopy, the bacterial strain’s morphology prior and following the treatment with the proposed formulation was examined in order to verify the antibacterial effect of EM-based-NEG. Checking biological material using an electron microscope is a critical issue and require extensive processing at which the specimen should kept in a high vacuum to be photographed using SEM. In brief, 100 μg of EM-based-NEG was incubated for 1 h in combination with Mueller-Hinton broth (100 μL), and the strain (about 1.5 × 106 CFU/mL) (10 μL). Subsequently, the mixture was centrifuged for 20 min at 15,000 rpm to eliminate the supernatant and maintain the pellets dispersed in regular saline. A 50 μL aliquot of the suspension was spread out across the slide and left to dehydrate. After three hours of fixing in 3 % glutaraldehyde, the sample was examined under a scanning electron microscope to examine its morphology. Before treating with the examined formulation, the bacteria was examined alone as a control using a scanning electron microscope at 30 kV (Elsewedy et al., 2022b).
2.14. Statistical analysis
In the current study, each experiment was carried out a minimum of three times, and the results were displayed as the mean ± SD. The statistical significance was ascertained using the one-way analysis of variance (ANOVA) test when the p value was less than 0.05. The statistical analysis was detected using SPSS statistics software, version 9 (IBM Corporation, Armonk, NY).
3. Results
3.1. Optimization study of variables
CCD served as a tool for suggesting various NE formulations based on the influence of certain independent factors on the response variables as shown in Table 1. Ten NE formulations were suggested by the software and subjected to various analyses. For X1 and X2, the model F-value was determined to be 711.17 and 678.31, respectively, indicating a significant model. Additionally, the majority of the model terms had P-values less than 0.05, indicating the presence of significant model variables. Additionally, lack of fit is another element to take into account as it indicates that the model fits. It is necessary for other parameter to be non-significant such as the lack of fit. According to the outcomes, lack of fit for Y1 and Y2 was non-significant, with values of 0.3855 and 0.6159 and F-values of 3.22 and 1.13, respectively.
3.2. Characterization of the developed ER-based-NE
3.2.1. Effect of the selected factors on the particle size
Based on information shown in Table 2, it was demonstrated that the particle sizes of all formulated EM-based-NE varied from 214 ± 2.7 to 411 ± 3.7 nm. The concentration of each independent factor in the formulation is responsible for this variance in particle size. It was noted that a higher amount of TTO in the formula would produce larger particles, whereas a lower amount would produce NE with smaller particles. However, with regard to variable X2, it was observed that NE with smaller particle sizes would be produced upon using a greater amount of surfactant while maintaining a constant amount of oil. On the other hand, a larger size would result from utilizing a lower amount of surfactant while maintaining the same amount of oil. The obtained data were also certified by certain mathematical formulas generated from CCD that illustrates the quadratic model of the design. This equation confirms the direct relationship between X1 and Y1. In addition to the inverse relationship between X2 and Y1 response that is evident in the following mathematical equation
| (1) |
Commonly, it is well defined that formulas carrying a positive sign denotes a affirmative increase in the investigated response; however, a negative sign in front of the data indicates a reduction on that response. Accordingly, equation (1) showed that X1 has a positive sign, indicating that X1 and Y1 are working synergistically to increase the particle size. On the other hand, the antagonistic relationship between X2 and Y1 was indicated by the negative sign that was observed in front of X2, meaning that increasing tween 80 amount would reduce the particle size.
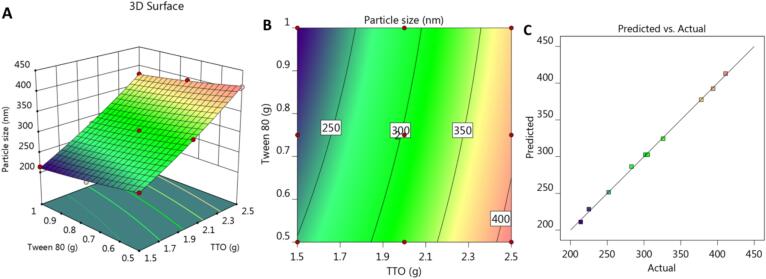
Furthermore, some graphs were created by the design and provide further illustration and confirmation of the data. 3D response surface plots and 2D contour graphs were generated as seen in Fig. 1A and 1B, respectively. Additionally, as shown in Fig. 1C and Table 3, the adjusted and anticipated R2 values for Y1 response were 0.9975 and 0.9891, respectively, showing a correlation between each other. Since there is less than 0.2 of a discrepancy between these figures, it was confirmed that they are in reasonable agreement with one another. Additionally, it was presented that the adequate precision was (75.2530), indicating a sufficient signal suitable for navigating the design space.
Fig. 1.
(A) 3D response surface plot, (B) 2D contour graph and (C) linear correlation plot between predicted against actual values for explaining the influence of variables X1 and X2 on the particle size response (Y1).
Table 3.
Fit statistics:
| R | ||
|---|---|---|
| Y1 | Y2 | |
| R2 | 0.9989 | 0.9949 |
| Adjusted R2 | 0.9975 | 0.9934 |
| Predicted R2 | 0.9891 | 0.9895 |
| Adequate precision | 75.2530 | 67.1426 |
3.2.2. Effect of the selected factors on the in vitro release study
The goal of the in vitro investigation was to quantify the amount of EM emitted from the developed NE formulations. The percentage of EM released was ranged from 46 ± 2.0 to 86 ± 2.4 %, as shown clearly in Table 2. According to the results obtained, it can be established that a lower amount of TTO was used to achieve higher in vitro release of EM. On the other side, it was shown that raising the surfactant amount would raise the percentage of EM released, even when the amount of TTO remained constant.
The obtained linear design could produce the following mathematical equation, which was verified by the negative sign, to demonstrate that (X1) was inversely related to variable Y2. Conversely, variable X2 showed a direct relationship with response Y1, as indicated by the positive sign in front of it. Equation (2) exhibited the previous verification as follow:
Y2 = 65.89–––16.2833 * X1 + 4.31667 * X2
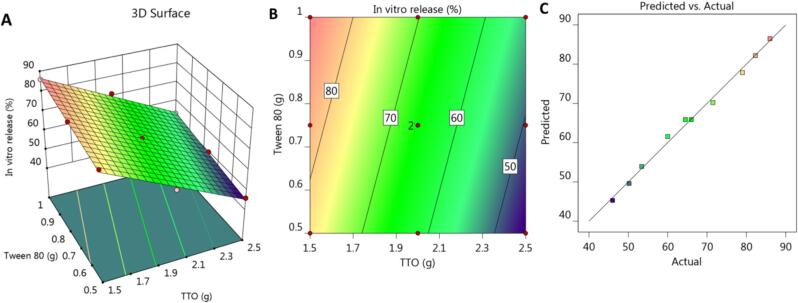
Several graphs were produced by CCD to further interpret the obtained data, showing the relationship between the studied variables, X1 and X2, and the observed in vitro release response Y2. The 3D-response surface plot shown in Fig. 2A illustrates the significant negative influence of variable (X1) in addition to the positive effects of variable X2 on Y2 response. In addition to the 2D contour plot, shown in Fig. 2B that interprets the same findings. Furthermore, according to the information in Table 3 and Fig. 2C, the adjusted and predicted R2 for the Y2 response were 0.9934 and 0.9895, respectively, which appeared to be quite near to one another given that the difference between them was less than 0.2. This suggests that both values were reasonably in accord with one another. Besides, the adequate precision was (67.1426), indicating a sufficient signal for navigating the design space.
Fig. 2.
(A) 3D response surface plot, (B) 2D contour graph and (C) linear correlation plot between predicted against actual values for explaining the influence of variables X1 and X2 on the in vitro release response (Y2).
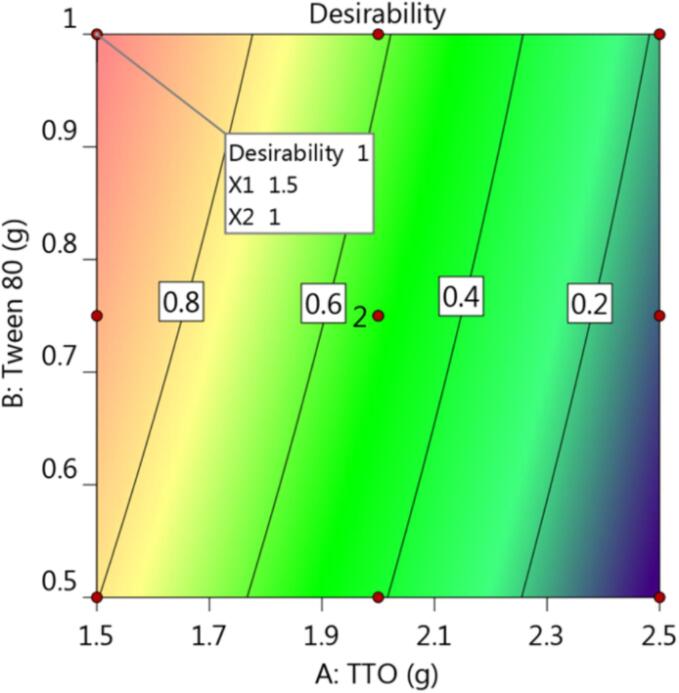
3.3. Optimization and validation of CCD
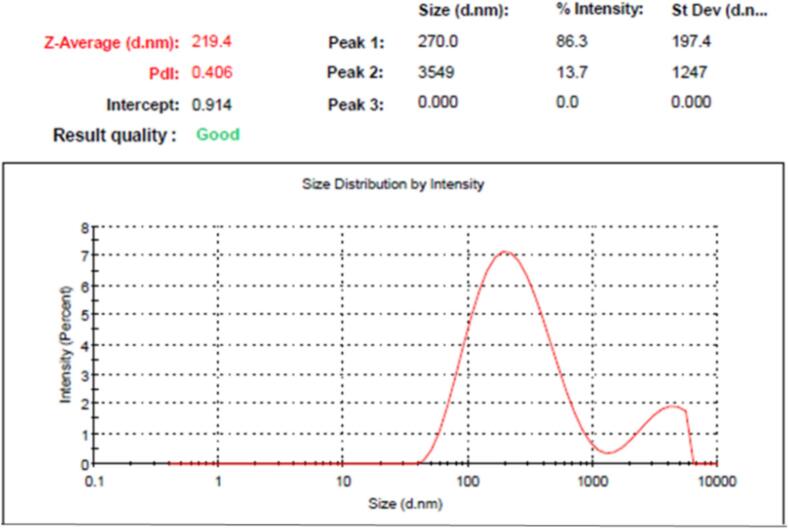
Liable to the desirability function, generated graph models, and numerical optimization, the optimal formula could be chosen. The responses were intended to be directed toward two essential goals for minimizing particle size, and maximizing in vitro drug release from NE formulation. As stated in Table 4 and Fig. 3, the quantity of TTO was directed to be 1.5 g, and amount of tween 80 to be 1 g based on the prior arrangement. These supposed proportions of the chosen factors could yield the highest desirability value, which appear to be 1. The optimized EM-based-NE formulation was produced using the suggested amounts, and it will be assessed and compared with calculated values that have been anticipated by the design. It was clearly evident that the observed and anticipated values were noticeably near to each other. The particle size of the selected EM-based-NE formulation (219.4 ± 3.7) was displayed in Fig. 4. Furthermore, its relative PDI value was 0.406 ± 0.22, indicating that the distribution of particle size is contained within a slim range of sizes, which is thought to be a sign of stability (Elsewedy et al., 2022a).
Table 4.
Predicted and observed value for the optimized em-based-ne formulation.
| Selected factor | Constraint | |
|---|---|---|
| TTO (g) | In range | |
| Tween 80 (g) | In range | |
| Response | Predicted values | Observed values |
| Particle size (nm) | 211.04 ± 3.46 | 219.4 ± 3.7 |
| In vitro release (%) | 86.49 ± 1.12 | 84.6 ± 2.4 |
Fig. 3.
(A) Desirability graph showing the values of independent variable required to produce the higher desirability value.
Fig. 4.
Particle size of optimized EM-based-NE formulation.
3.4. Study of Drug-Excipient compatibility (Fourier transform infrared spectroscopy)
Checking the incompatibility that might present between components of the formulation was performed via FTIR study. It was apparent in Fig. 5 that certain distinctive peaks related to the pure drug EM were present while other peaks were absent from the EM-based-NE formulation. The distinctive peaks of EM were at 3520 cm−1 corresponding to the OH groups, 2970 cm−1 for – CH2, 1715 cm−1 related to C=O, 1630 cm−1 correlated with OH groups, 1370 and 1270 cm−1 associated with OH, and 1190 cm−1 corresponding to C-O-C.
Fig. 5.
FTIR chart demonstrating the spectra of pure EM, blank NE and optimized EM-based-NE formulation.
3.5. Development of topical EM-based-NEG
Topical EM-based-NEG was developed via mixing the optimized ER-NE with a HPMC hydrogel that was previously prepared. A number of characterizations were conducted on that formulation to evaluate its efficacy as a topical antibacterial formulation.
3.6. Characterization of topical EM-based-NEG
3.6.1. Organoleptic assessment
The developed topical EM-based-NEG was physically examined, and it was apparent that the formula was acceptable, exhibiting a uniform, smooth, and even texture.
3.6.2. pH value
It is well known that in order for topical medications to be calming, comforting, and nonirritating, their pH value should be extremely near to that of skin, in the range of 5–6 (Elsewedy et al. 2023). Based on the information shown in Table 5, the pH of the manufactured EM-based-NEG was ideal and could protect against skin irritation.
Table 5.
Characterization of topical EM-based formulations:
| Parameter | EM-based-NEG |
|---|---|
| Organoleptic properties | Consistent and uniform |
| pH | 6.11 ± 0.19 |
| Viscosity (cP) | 10400 ± 1275 |
| Spreadability (mm) | 54.03 ± 2.3 |
| Extrudability (g/cm2) | 80.36 ± 3.15 |
3.6.3. Viscosity
Based upon the guidelines of pharmacopoeia, viscosity is very essential parameter to be considered for formulations applied topically (Chang et al. 2013). Consequently, topical formulations must have the proper viscosity for efficient and easier skin application. As a result, Table 5 shows that the viscosity of EM-based-NEG was assessed and proven to meet the ideal characteristics of topical applications.
3.6.4. Spreadability
Similarly, for topical formulations, spreadability is a crucial factor that needs to be assessed. The topical preparation can be applied over the skin more easily if possess a lower spreadability value (Djiobie Tchienou et al. 2018). As clear in Table 5, the developed EM-based-NEG spreadability was within a reasonable range for skin smearing.
3.6.5. Extrudability
In order to guarantee patient compliance with semisolid preparations, extrudability is one of the critical factors that should be considered and estimated (Khan et al. 2022). As illustrates in Table 5, the formulated EM-based-NEG demonstrated perfect extrudability, making it suitable for topical application.
3.7. Drug content
Following a drug content evaluation, the results revealed that the EM was distributed equivalently throughout the developed formulation since it appeared to be 99.3 ± 0.46 %.
3.8. Investigation of in vitro release from EM-based-NEG
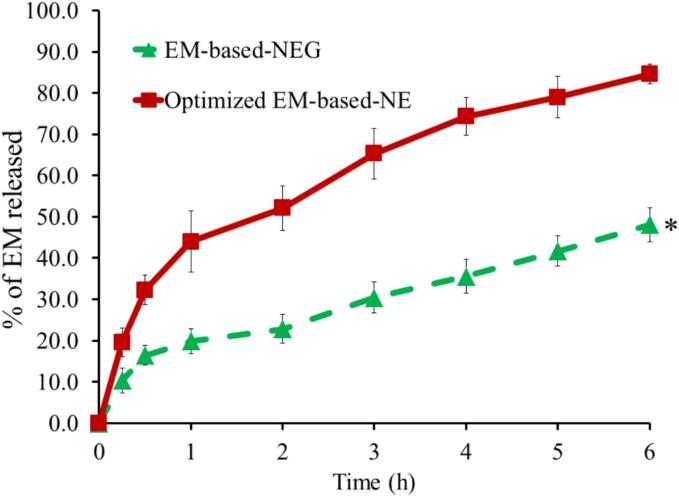
The in vitro release of EM from the optimized EM-based-NE and the formulated EM-based-NEG was estimated, as outlined in Fig. 6. The study was lasted for six hours and revealed a significant difference (p < 0.05) in the release of EM from the developed NEG (48.1 ± 4.2 %) and the optimized NE (84.6 ± 2.4 %). Despite the obtained results that the optimized EM-based-NE appears to release more drug than the EM-based-NEG, the NEG formulation was found to be more preferable..
Fig. 6.
In vitro release of EM from optimized NE and developed NEG formulation in phosphate buffer pH 5.5 at 32 °C±0.5. The findings are displayed as mean ± SD (n = 3). * P<0.05 compared to optimized EM-based-NE formulation.
3.9. Kinetic study
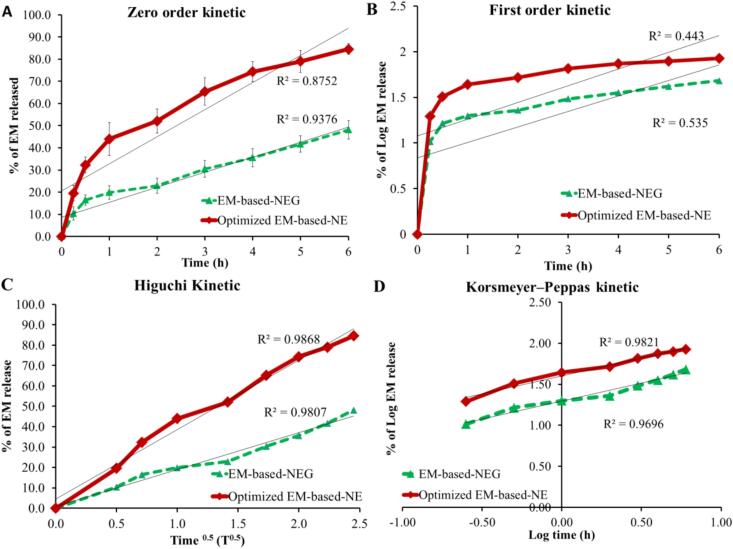
In order to determine the exact strategy by which EM was released from NE and NEG formulations, various kinetic modeling was examined. A drug release against time curve was created and the utmost linear plot with highest value of (R2) were identified. Accordingly, the study exhibited that EM release from each of the two formulations under investigation obeyed Higuchi kinetic modeling. Since this model provide the most linear correlation and the greatest R2 value, 0.9868, and 0.9807 for EM-based-NE and EM-based-NEG, respectively if compared to other kinetic modeling (Fig. 7).
Fig. 7.
Kinetic analysis of EM released from NE and NEG formulations in terms of (A) zero order, (B) first order, (C) Higauchi, and (D) Korsmeyer-Peppas kinetic model.
3.10. Stability study
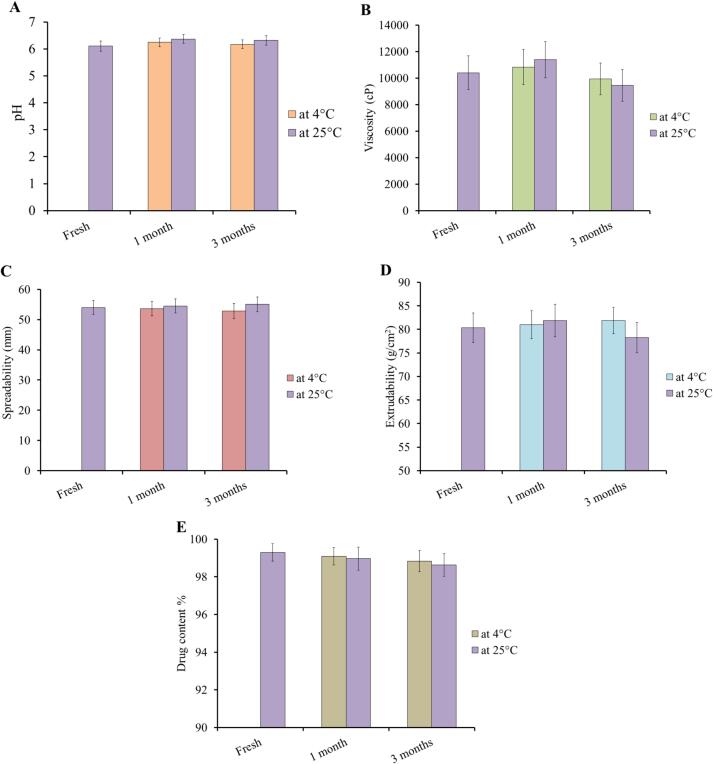
Investigating the stability of the preparation is an important step in figuring out the best storage conditions and emphasizing the quality of the formulation (Osel et al. 2021). During examining stability of the prepared EM-based-NEG, a number of parameters were studied including pH, viscosity, spreadability and extrudability. As seen in Fig. 8, the EM-based-NEG stability was verified by storing it under two distinct conditions for one and three months at 4 ± 2 °C and at 25 ± 2 °C. The results showed that there was no significant difference in the parameters assessed (P<0.05).
Fig. 8.
Stability of the developed EM-based-NEG upon storage for 1 and 3 months at two distinct conditions; at 4 °C and 25 °C with regard to A) pH, B) viscosity, C) spreadability, D) extrudability, and E) drug content.
3.11. Antibacterial activity
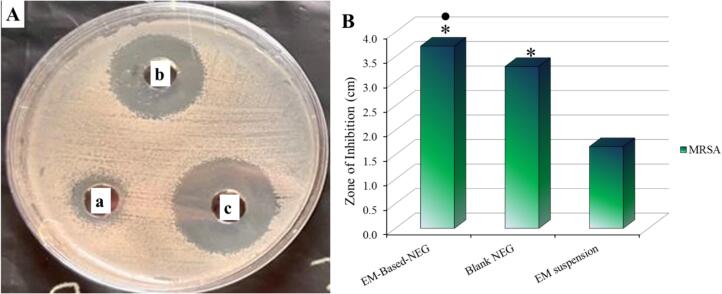
Investigating the antibacterial activity of the formulated EM-based-NEG against MRSA bacterial strain was conducted using disc diffusion method. This is accomplished simply by measuring the inhibitory zone that was formed as a result of the formulation influence over the bacteria. The inhibitory zone created by blank NEG, EM suspension, and EM-based-NEG was evaluated, as shown in Fig. 9A and 9B. A significant inhibitory zone against MRSA was demonstrated for EM-based-NEG if compared to blank NEG. Additionally, a significant difference was detected between EM-based-NEG and the EM suspension (P<0.05). Moreover, it's remarkable to note that, the antibacterial activity of blank NEG was shown to exhibit a notable suppression of bacterial growth in the media.
Fig. 9.
A) Diameter of the inhibition zone produced by the antibacterial influence of the investigated formulations: (a) EM suspension; (b) blank NEG; and (c) EM-based-NEG on MRSA bacterial strain, and B) Antibacterial activity of the investigated formulations against bacterial strain (MRSA). Values are shown as mean ± SD. * (P<0.05) compared to EM suspension; and ● (P<0.05) compared to blank NEG.
3.12. Morphology of treated bacterial cells
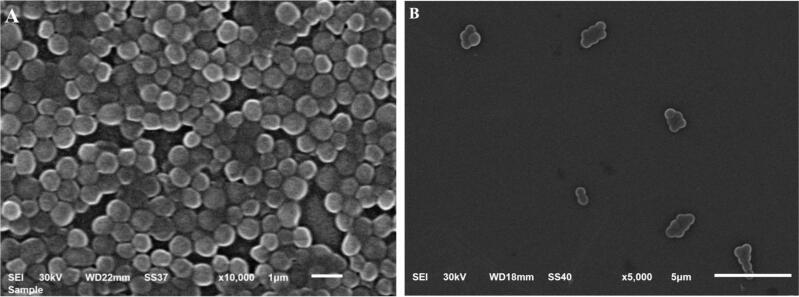
SEM was used to analyze the bacterial cells' morphology after they have been exposed to EM-based-NEG formulation as shown in Fig. 10. The images clearly show that, the control bacterial cells (Fig. 10A) had more cells adhering to their surface, which was not present in the treated cells (Fig. 10B). The results of the treatment with the EM-based-NEG formulation exhibited a significant decrease in the number of bacterial cells in addition to a significant alteration in the bacterial shape.
Fig. 10.
SEM images demonstrating the shape of the bacterial biofilm formed after being incubated for 24 h and its inhibition. A) Control MRSA; B) MRSA treated with EM-based-NEG.
4. Discussion
The results obtained from current investigation could highlight the importance of CCD in providing optimum formulation in addition to the importance of combination therapy in maximizing the antibacterial influence. Accordingly, upon investigating the effect of the selected factors on the particle size response, it was noted that the reason of obtaining higher particle size upon using higher amount of TTO could be owed to an increase in the dispersed phase (Artiga-Artigas et al. 2019). However, getting smaller particle size following utilization of higher amount of surfactant was attributed to the interfacial tension phenomena since a preparation with a higher surfactant concentration would have a lower interfacial tension, which would increase surface stabilization, avoids particle coalescence and aggregate formation leading to smaller particles (Ogunmokun and Wallach 2024). Furthermore, while investigating the effect of the selected factors on the second response; the in vitro release study, it was observed that higher in vitro release of EM was achieved by using a lower amount of TTO. The reason behind that returned to smaller particle size that was attained while using a lower TTO amount that would offer a large surface area so it would allow for large amount of drug to be released. On the other hand, higher amount of surfactant would increase the percentage of drug released. That might be explained by a reduction in particle size and an increase in surface area that would raise the proportion of in vitro release upon the addition of surfactant.
Regarding FTIR study for the optimized EM-based-NE formula, pure EM and blank NE, it was found that certain distinctive peaks corresponding to the pure EM were present indicating the existence of the drug in the EM-NE formulation. Other peaks were absent from the EM-based-NE formulation signifying that the drug is successfully embedded inside the nanocarrier.
The optimized EM-based-NE was incorporated into a hydrogel that had been prepared previously to obtain more viscous formulation owing to the effectiveness of viscous preparations in topical use. The developed EM-based-NEG was evaluated for various characterizations in addition to the in vitro release study that provided a larger drug release from NE formulation if compared to NEG. In fact, the inclusion of a gelling agent in the NEG preparation played a role in the reduced release when compared to the optimized NE formulation. This is definitely attributed to the greater viscosity the NEG owed as a result of having a gelling agent (Binder et al. 2019). However, EM-based-NEG was found to be more preferable than the optimized EM-based-NE This is attributed to the appropriate viscosity and spreadability of NEG formulation that would improve the topical application and increase patient compliance (Bayan et al., 2023).
Concerning with the kinetic study, it was found that the most linear plot with highest value of (R2) was for Higuchi modeling. It is well known that when the drug diffuses from its lipid matrix type while applying regulated conditions, it is claimed that the drug released according to Higuchi kinetic modeling (Mircioiu et al. 2019). In addition, Higuchi modeling is best model describing the drug dissolution from topical and transdermal preparation (Damodharan 2020).
Great stability of the developed EM-based-NEG upon storage for 3 months at two different conditions was observed. Actually, it was reported previously that the addition of gelling agent to the nanoemulsion formulations would improve the stability via increasing their viscosity (Alhasso, Ghori, and Conway 2023).
Concerning with the antibacterial study, the significant inhibitory zone against MRSA in case of EM-based-NEG if compared to blank NEG, indicated its great activity against these bacteria P<0.05. Additionally, the significant difference that detected between EM-based-NEG and the EM suspension (P<0.05), indicted that the developed formulation could inhibit the growth of the bacteria more significantly than EM suspension. This finding emphasized the previous fact that MRSA showed certain resistant influence against EM (Hu et al. 2023). This result owed to the presence of TTO in EM-based NEG formulation that provides more bacterial inhibition than the drug alone. Moreover, the antibacterial activity of blank NEG was likely because the formulation contain TTO. This result would emphasize the well-known fact regarding TTO and its antimicrobial properties (Wei et al. 2022). Terpinen-4-ol, the primary ingredient in TTO, is said to be the basis of this behavior (Elsewedy et al., 2022b). In summary, the results of this study suggest that the higher antimicrobial activity of EM-based-NEG could be due to the combination of EM and TTO, which enhanced the antibacterial activity of EM. Moreover, the findings would emphasis that the combination therapy is one of the possible options for treating the bacterial infections (Wei et al. 2022).
These results were further emphasized by studying the morphology of the bacteria alone and after treated with the studied formulation. Numerous researches stated that different antibiotics have the capability to cause morphological changes in the bacterial cells, including altering in their size, and curvature (Cylke, Si, and Banerjee 2022). Mostly, the formulation containing antibacterial agents could target the cellular membrane resulting in its destruction or inhibit its synthesis, which would reduce the total growth rate of the bacterial cell (Vadia et al. 2017). The significant decrease in the number of bacterial cells as well as the significant alteration in the shape of the bacteria following treatment with EM-based-NEG formulation indicated the effectiveness of the formulation as an antibacterial agent. More investigation needs to be conducted, including; animal experiment in order to provide more confirmation about the obtained antibacterial influence of erythromycin.
Finally, since the clinical implications of erythromycin encompass its various therapeutic uses such as; acne treatment, ophthalmic infections, gastroparesis treatment and prophylactic use. Our finding could enhance the pharmacological activity and decrease the side effect of traditional formulations of erythromycin; however, several investigations are necessary to confirm such speculations.
5. Conclusion
Nanoemulsion has been successfully used to encapsulate erythromycin, which is considered a potential nanocarrier for delivering drugs. Central composite design tool was used to develop a number of nanoemulsion formulations. In accordance with a particular parameter's values, the ideal formula was selected. For simple and efficient topical application, the optimized nanoemulsion formulation was incorporated into gel providing nanoemulgel. The developed erythromycin-based-nanoemulgel exhibited satisfactory physical properties appropriate for topical application. As well, the developed formulation prepared using tea tree oil demonstrated significant antibacterial action, which augments the effect of erythromycin in inhibiting bacterial growth. In conclusion, the study found that erythromycin based nanoemulgel containing tea tree oil enhanced the antibacterial activity of erythromycin in a synergistic manner and overcome the problem of bacterial resistant against antibiotics.
Funding
The authors are thankful to the Researchers Supporting Project number (RSP2024R146) at King Saud University, Riyadh, Saudi Arabia, for supporting this research financially. This research was funded by Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, grant number GRANT 6000. The authors are also thankful to AlMaarefa University for supporting this research.
CRediT authorship contribution statement
Heba S. Elsewedy: Writing – original draft, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Sultan Alshehri: Visualization, Validation, Supervision, Conceptualization. Naheda S. Alsammak: Software, Methodology, Investigation. Nada F. Abou Chahin: Writing – original draft, Resources, Methodology, Investigation. Manal S. Alotaibi: Validation, Software. Rehab A. Alshammari: Software, Resources, Project administration, Methodology. Tamer M. Shehata: Writing – review & editing, Visualization, Validation, Supervision, Investigation, Formal analysis. Bandar Aldhubiab: Validation, Supervision, Formal analysis, Conceptualization. Wafaa E. Soliman: Visualization, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to acknowledge the Researchers Supporting Project number (RSP2024R146) at King Saud University, Riyadh, Saudi Arabia, and Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, grant number GRANT 6000 for extending financial support to do this research project. The authors would like to express sincere gratitude to AlMaarefa University for supporting this research.
Contributor Information
Heba S. Elsewedy, Email: hsewedy@um.edu.sa.
Sultan Alshehri, Email: Salshehri1@ksu.edu.sa.
Tamer M. Shehata, Email: tshehata@kfu.edu.sa.
Wafaa E. Soliman, Email: weahmed@kfu.edu.sa.
References
- Alhasso, Bahjat, Muhammad U. Ghori, and Barbara R. Conway. 2023. “Development of a Nanoemulgel for the Topical Application of Mupirocin.” In Pharmaceutics. [DOI] [PMC free article] [PubMed]
- Artiga-Artigas M., Montoliu-Boneu J., Salvia-Trujillo L., Martín-Belloso O. Factors affecting the formation of highly concentrated emulsions and nanoemulsions. Colloids Surf A Physicochem. Eng. Asp. 2019;578 [Google Scholar]
- Bandyopadhyay D. Topical Antibacterials in Dermatology. Indian J. Dermatol. 2021;66:117–125. doi: 10.4103/ijd.IJD_99_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes T.M., Mijaljica D., Townley J.P., Spada F., Harrison I.P. Vehicles for drug delivery and cosmetic moisturizers: Review and comparison. Pharmaceutics. 2021;13:2012. doi: 10.3390/pharmaceutics13122012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayan, Mohammad F., Balakumar Chandrasekaran, and Mohammad H. Alyami. 2023. “Development and Characterization of Econazole Topical Gel.” In Gels. [DOI] [PMC free article] [PubMed]
- Binder L., Mazál J., Petz R., Klang V., Valenta C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Skin Res. Technol. 2019;25:725–734. doi: 10.1111/srt.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujubarah, Mahdi M., Heba S. Elsewedy, Tamer M. Shehata, and Wafaa E. Soliman. 2023. “Formulation by Design of an Innovative Tea Tree Oil Nanoemulgel Incorporating Mupirocin for Enhanced Wound Healing Activity.” In Applied Sciences.
- Buya A.B., Witika B.A., Bapolisi A.M., Mwila C., Mukubwa G.K., Memvanga P.B., Makoni P.A., Nkanga C.I. Application of Lipid-Based Nanocarriers for Antitubercular Drug Delivery: A Review. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13122041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R.-K., Raw A., Lionberger R., Lawrence Yu. Generic development of topical dermatologic products: formulation development, process development, and testing of topical dermatologic products. AAPS J. 2013;15:41–52. doi: 10.1208/s12248-012-9411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cylke C., Si F., Banerjee S. Effects of antibiotics on bacterial cell morphology and their physiological origins. Biochem. Soc. Trans. 2022;50:1269–1279. doi: 10.1042/BST20210894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodharan N. Mathematical modelling of dissolution kinetics in dosage forms. Research Journal of Pharmacy and Technology. 2020;13:1339–1345. [Google Scholar]
- Donthi M.R., Munnangi S.R., Krishna K.V., Saha R.N., Singhvi G., Dubey S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics. 2023;15 doi: 10.3390/pharmaceutics15010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsewedy H.S., Al-Dhubiab B.E., Mahdy M.A., Elnahas H.M. Basic Concepts of Nanoemulsion and its Potential application in Pharmaceutical, Cosmeceutical and Nutraceutical fields. Research Journal of Pharmacy and Technology. 2021;14:3938–3946. [Google Scholar]
- Elsewedy, Heba S, Bandar E Al Dhubiab, Mahmoud A Mahdy, and Hanan M Elnahas. 2021. 'A review article on the basic concepts of drug delivery systems as targeting agents', Int. J. Pharma Med. Biol. Sci, 10: 23-29.
- Elsewedy, Heba S., Tamer M. Shehata, Mervt M. Almostafa, and Wafaa E. Soliman. 2022. “Hypolipidemic Activity of Olive Oil-Based Nanostructured Lipid Carrier Containing Atorvastatin.” In Nanomaterials. [DOI] [PMC free article] [PubMed]
- Elsewedy, Heba S., Tamer M. Shehata, and Wafaa E. Soliman. 2022a. “Shea Butter Potentiates the Anti-Bacterial Activity of Fusidic Acid Incorporated into Solid Lipid Nanoparticle.” In Polymers. [DOI] [PMC free article] [PubMed]
- Elsewedy, Heba S., Tamer M. Shehata, and Wafaa E. Soliman. 2022b. “Tea Tree Oil Nanoemulsion-Based Hydrogel Vehicle for Enhancing Topical Delivery of Neomycin.” In Life. [DOI] [PMC free article] [PubMed]
- Elsewedy, Heba S., Tamer M. Shehata, Nashi K. Alqahtani, Hany E. Khalil, and Wafaa E. Soliman. 2023. “Date Palm Extract (Phoenix dactylifera) Encapsulated into Palm Oil Nanolipid Carrier for Prospective Antibacterial Influence.” In Plants. [DOI] [PMC free article] [PubMed]
- Elsewedy, Heba S., Tamer M. Shehata, Shaymaa M. Genedy, Khuzama M. Siddiq, Bushra Y. Asiri, Rehab A. Alshammari, Sarah I. Bukhari, Adeola T. Kola-Mustapha, Heba A. Ramadan, and Wafaa E. Soliman. 2024. “Enhancing the Topical Antibacterial Activity of Fusidic Acid via Embedding into Cinnamon Oil Nano-Lipid Carrier.” In Gels. [DOI] [PMC free article] [PubMed]
- Ghasemiyeh P., Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res. Pharm. Sci. 2018;13:288–303. doi: 10.4103/1735-5362.235156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haftek M., Coutanceau C., Taïeb C. Epidemiology of “fragile skin”: results from a survey of different skin types. Clin. Cosmet. Investig. Dermatol. 2013;6:289–294. doi: 10.2147/CCID.S55223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach M., Zbinden R., Böttger E.C. Standardisation of disk diffusion results for antibiotic susceptibility testing using the sirscan automated zone reader. BMC Microbiol. 2013;13:1–8. doi: 10.1186/1471-2180-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Demei Zhu Fu., Wang, Wang Minggui. Current status and trends of antibacterial resistance in China. Clin. Infect. Dis. 2018;67:S128–S134. doi: 10.1093/cid/ciy657. [DOI] [PubMed] [Google Scholar]
- Hu Y., Ouyang L., Li D., Deng X., Hongbo Xu., Zhijian Yu., Fang Y., Zheng J., Chen Z., Zhang H. The antimicrobial activity of cethromycin against Staphylococcus aureus and compared with erythromycin and telithromycin. BMC Microbiol. 2023;23:109. doi: 10.1186/s12866-023-02858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqubal, Mohammad Kashif, Sadaf Saleem, Aiswarya Chaudhuri, Ashif Iqubal, Jasjeet K. Narang, Javed Ali, and Sanjula Baboota. 2021. 'Chapter 1 - Combination therapy: past, present, and future.' in Sanjula Baboota and Javed Ali (eds.), Nanocarriers for the Delivery of Combination Drugs (Elsevier).
- Jelić, D., and R. Antolović. 2016. 'From Erythromycin to Azithromycin and New Potential Ribosome-Binding Antimicrobials', Antibiotics (Basel), 5. [DOI] [PMC free article] [PubMed]
- Khan B.A., Ahmad S., Khan M.K., Hosny K.M., Bukhary D.M., Iqbal H., Murshid S.S., Halwani A.A., Alissa M., Menaa F. Fabrication and Characterizations of Pharmaceutical Emulgel Co-Loaded with Naproxen-Eugenol for Improved Analgesic and Anti-Inflammatory Effects. Gels. 2022;8 doi: 10.3390/gels8100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931. [Google Scholar]
- Leekha S., Terrell C.L., Edson R.S. General principles of antimicrobial therapy. Mayo Clin. Proc. 2011;86:156–167. doi: 10.4065/mcp.2010.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert W., Malec-Milewska M., Zajaczkowska R., Wordliczek J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules. 2018;23 doi: 10.3390/molecules23030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.-Q., Lei H.-M., Qian-Yi Hu., Li G.-H., Zhao P.-J. Recent advances in the synthetic biology of natural drugs. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.691152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahama O., Songuigama C. Pharmacochemical aspects of the evolution from erythromycin to neomacrolides, ketolides and neoketolides. Open Journal of Medicinal Chemistry. 2020;10:57–112. [Google Scholar]
- Mircioiu C., Voicu V., Anuta V., Tudose A., Celia C., Paolino D., Fresta M., Sandulovici R., Mircioiu I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics. 2019;11:140. doi: 10.3390/pharmaceutics11030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R.H., Hespeler D., Jin N., Pyo S.M. smartPearls–Novel physically stable amorphous delivery system for poorly soluble dermal actives. Int. J. Pharm. 2019;555:314–321. doi: 10.1016/j.ijpharm.2018.11.018. [DOI] [PubMed] [Google Scholar]
- Narasimha Murthy S., Shivakumar H.N. In: Handbook of Non-Invasive Drug Delivery Systems. Kulkarni V.S., editor. Boston; William Andrew Publishing: 2010. CHAPTER 1 - Topical and Transdermal Drug Delivery. [Google Scholar]
- Nugrahaeni, Fitria, and Redina Okvianida. 2022. “The effect of HPMC concentration as a gelling agent on color stability of copigmented blush gel extract of purple sweet (Ipomoea batatas (L.) Lam.).” In IOP Conference Series: Earth and Environmental Science, 012070. IOP Publishing.
- Ogunmokun F.A., Wallach R. Effect of surfactant surface and interfacial tension reduction on infiltration into hydrophobic porous media. Geoderma. 2024;441 [Google Scholar]
- Osel N., Planinšek Parfant T., Kristl A., Roškar R. Stability-Indicating Analytical Approach for Stability Evaluation of Lactoferrin. Pharmaceutics. 2021;13 doi: 10.3390/pharmaceutics13071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatello R., Mangiafico A., Ruozi B., Puglisi G., Furneri P.M. Amphiphilic Erythromycin-Lipoamino Acid Ion Pairs: Characterization and In Vitro Microbiological Evaluation. AAPS PharmSciTech. 2011;12:468–475. doi: 10.1208/s12249-011-9605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preeti S., Sambhakar R., Malik S., Bhatia A.A., Harrasi C., Rani R., Saharan S.K., Geeta, Sehrawat R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica (cairo) 2023;2023:6640103. doi: 10.1155/2023/6640103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina, Neha, Radha Rani, Vijay Kumar Thakur, and Madhu Gupta. 2023. 'New Insights in Topical Drug Delivery for Skin Disorders: From a Nanotechnological Perspective', ACS omega. [DOI] [PMC free article] [PubMed]
- Sharma P., Tailang M. Design, optimization, and evaluation of hydrogel of primaquine loaded nanoemulsion for malaria therapy. Future Journal of Pharmaceutical Sciences. 2020;6:1–11. [Google Scholar]
- Shehata, Tamer M., and Heba S. Elsewedy. 2022. “Paclitaxel and Myrrh oil Combination Therapy for Enhancement of Cytotoxicity against Breast Cancer; QbD Approach.” In Processes.
- Shehata, Tamer M., Mervt M. Almostafa, and Heba S. Elsewedy. 2022. “Development and Optimization of Nigella sativa Nanoemulsion Loaded with Pioglitazone for Hypoglycemic Effect.” In Polymers. [DOI] [PMC free article] [PubMed]
- Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., Chourasia M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Szulc-Musioł, Beata, Wioletta Siemiradzka, and Barbara Dolińska. 2023. “Formulation and Evaluation of Hydrogels Based on Sodium Alginate and Cellulose Derivatives with Quercetin for Topical Application.” In Applied Sciences.
- Djiobie Tchienou, Gertrude E., Roli K. Tsatsop Tsague, Therese F. Mbam Pega, Vera Bama, Albert Bamseck, Selestin Dongmo Sokeng, and Martin B. Ngassoum. 2018. “Multi-Response Optimization in the Formulation of a Topical Cream from Natural Ingredients.” In Cosmetics.
- Vadia S., Tse Jessica L., Lucena R., Yang Z., Kellogg D.R., Wang J.D., Levin P.A. Fatty acid availability sets cell envelope capacity and dictates microbial cell size. Curr. Biol. 2017;27(1757–67):e5. doi: 10.1016/j.cub.2017.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Tian Q., Zhao X., Liu X., Husien H.M., Liu M., Bo R., Li J. Tea Tree Oil Nanoemulsion Potentiates Antibiotics against Multidrug-Resistant Escherichia coli. ACS Infect. Dis. 2022;8:1618–1626. doi: 10.1021/acsinfecdis.2c00223. [DOI] [PubMed] [Google Scholar]
- Weng J., Tong H.H.Y., Chow S.F. In Vitro Release Study of the Polymeric Drug Nanoparticles: Development and Validation of a Novel Method. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo E., Yew Chieng C.J., Choudhury H., Pandey M., Gorain B. Tocotrienols-rich naringenin nanoemulgel for the management of diabetic wound: Fabrication, characterization and comparative in vitro evaluations. Curr Res Pharmacol Drug Discov. 2021;2 doi: 10.1016/j.crphar.2021.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]