Abstract
Background and objective
The B19 virus is mainly transmitted through the respiratory tract; however, studies have shown that it can also be transmitted through blood transfusions or plasma products. This study investigated B19V antibodies, DNA, and gene typing in blood donors at a central blood station in China to evaluate the status of B19V infection.
Materials and methods
A total of 7728 samples from Suzhou Blood Center were collected from July 2022 to April 2023. Samples were detected for the B19V DNA using real-time polymerase chain reaction. Furthermore, 893 selected samples were screened for the seroprevalence of B19V antibodies using enzyme-linked immunosorbent assay. The NS1-VP1u fragment of the B19V DNA-positive samples was amplified using nested PCR, and the sequences were determined. A B19V phylogenetic tree was constructed using neighborhood joint and maximum parsimony methods to discriminate genotypes using the NS1-VP1u sequences.
Results
The percentages of IgG, IgM, and DNA were 19.4 %, 1.9 %, and 0.09 %, respectively. IgG positivity increased with age, and there was a significant difference among the blood groups. The IgG levels of repeat donors were greater than those of first-time donors. There were no apparent differences in the IgM levels in all the participants. Genotyping revealed that the B19 genotype was 1.
Conclusions
The prevalence of B19V antibodies and DNA was lower in these areas than in rest of China, indicating that the risk of B19V transmission via transfusion may be relatively low. However, during transfusion, particular attention should be paid to the B19V-susceptible populations, especially those in high-risk groups.
Keywords: B19V, Blood donations, Viral load, Seroprevalence, Phylogenetic analysis
Human parvovirus B19 (B19V) is a small, single-stranded DNA virus with a lipid-free envelope. It belongs to the Parvovirus family and is a member of the Erythroparvovirus genus. This virus shows a marked preference for the human bone marrow and replicates only in erythroid progenitor cells [1]. The pathogenicity of B19V is related to tissue tropism. After B19V infection, most people exhibit mild or no symptoms, while serious complications may develop in high-risk groups such as pregnant women, patients who are immunocompromised, and those with decreased red cell survival due to haematological problems. Studies have shown that 33–51 % of pregnant women with infections can transmit B19V to their fetuses, increasing the risk of fetal edema, spontaneous abortion, or stillbirth, although infrequently [2,3]. In patients with immunodeficiencies, for example HIV infection, bone marrow transplantation, cancer chemotherapy, or radiotherapy, B19V may cause persistent anemia and failure of bone marrow transplantation, whereas in patients with potential haematological malignancies, B19V can cause transient aplastic crisis and other life-threatening conditions [4,5].
B19V primarily spreads through respiratory droplets, but can also be transmitted via blood or blood products [6]. Following infection, seroconversion occurs, with antibody prevalence ranging from 2 to 15 % in children, 30–60 % in adults, and >85 % in individuals aged ≥70 [7]. In the early stages of infection, patients are often asymptomatic or exhibit only mild cold symptoms characterized by a high viral load (1014copies/mL) [8]. As antibodies are produced, the concentration of B19V DNA in the peripheral blood decreases gradually but can persist for up to six months or longer, thus increasing the risk of transmission through transfusion. In adults, B19V infections may go unnoticed, causing many blood donors to donate because they are unaware of their positive infection status. In addition, B19V exhibits considerable resistance to pathogen removal processes, such as filtration, inactivation, solvent/detergent treatment, and pasteurization, posing a threat to the safety of plasma products [9]. Internationally, US Food and Drug Administration (FDA) has set an upper limit of 104 IU/mL to reduce the burden of B19V DNA for plasma pools. Plasma Protein Therapeutics Association (PPTA) also stipulates that the B19V DNA content in plasma raw materials used for producing anti-D antibodies for pregnant women must not exceed 104 IU/mL. Germany [10], Austria [10], and the Netherlands [11] have already initiated B19V screening. Since 2008, Japan [12] has replaced the receptor-mediated hemagglutination assay with a chemiluminescent enzyme immunoassay to detect B19V antigens. In China, there are currently no specific requirements for B19V screening, although some regions have investigated the presence of B19V antibodies or nucleic acids [[13], [14], [15]]. However, information on the prevalence of B19V among Chinese blood donors remains insufficient. This study focused on conducting B19V DNA and serological surveys in Suzhou, Jiangsu Province, Eastern China to complement the epidemiological data on B19V in the region and help monitor local B19V infections, which can help initiate measures to ensure blood safety.
1. Materials and methods
Study populations: Between July 2022 and April 2023, 7728 blood donation samples were collected for DNA testing from individuals aged 18–60 years with a median age of 33. Additionally, 893 blood donation specimens were randomly selected for Parvovirus B19V antibody testing, comprising 582 males and 311 females, aged 18–59 years, with a median age of 34. All blood donors had to comply with the “Health Screening Requirements for Blood Donors.” Briefly, 5 mL samples of both non-anticoagulant and anticoagulant blood were collected from each donor. Non-anticoagulant blood sample was centrifuged at 3500 rpm for 15 min, and anticoagulant blood was centrifuged at 4000 rpm for 20 min. The supernatants were used for enzymatic immunoassays and nucleic acid testing. All blood donor specimens in this study were tested for alanine transaminase (ALT), HBsAg, anti-HCV, anti-HIV, and anti-TP antibodies, and all results were negative. This study was approved by the Medical Ethics Committee of the Suzhou Blood Center, approval number: 202103, Date of approval: March 23, 2021.
Nucleic Acid Extraction: Aliquots of 100 μl plasma from each sample was pooled into minipools containing up to 5 samples per pool and 400 μL of 500 μL–pooled specimen was used for DNA extraction with the TGuide S32 Automated Nucleic Acid Extractor (Tiangen, Beijing, China). The extraction kit used was the magnetic bead-based Viral DNA Extraction Kit (Tiangen, Beijing, China), resulting in a final elution volume of 50 μL. All minipools that yielded a B19V DNA positive were resovled by individual testing, from which 400 μL from per sample was directly extracted. The DNA extraction was performed following the manufacturer's protocol and stored at −80 °C for further experiments. The purity and concentration of all DNA samples were measured using an SMA1000 UV Microspectrophotometer (Merinton, Beijing, China).
1.1. B19V detection and quantification of the viral load
Real-time polymerase chain reaction (PCR) was used to detect B19V DNA. The primers targeted the NS1 region. The specificity of the primers and probes was confirmed using NCBI BLAST, which amplified the three genotypes of B19V simultaneously. The forward primer, 5′-AATGCAGATG CCCTCCAC-3′ (nts 2083–2100); the reverse primer 5′-ATGATTCTCCTGAACTGGTCC-3′ (nts 2255–2275), and the probe 5′-FAM-AACCCCGCGCTCTAGTAC-TAMRA-3' (nts 2226–2243) were designed to target an amplification fragment of 193 bp (nts 2083–2275) on GenBank NC_000883.2. The 193bp NS1 fragment was inserted into the plasmid vector pUC-GW-Kan as a positive control. The amplification and detection were carried out on ABI7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) using Probe qPCR Mix (Takara Bio Incorporation, Kusatsu, Japan) comprising nucleic acid template (5 μL), Probe qPCR Mix (12.5 μL) (2 × ), both primers (100 μM) and probe (100 μM) (0.05 μL), and ROX (50 × ) (0.25 μL) to make up a total volume of 25 μL. PCR procedure included an initial stage of 95 °C for 5 min, followed by 45 two-step cycles of 5 s at 95 °C and 30 s at 60 °C. The FAM (Carboxyfluorescein) fluorescence channel was detected at 60 °C. An increase in fluorescence intensity of the reporter dye indicated a positive PCR result. The detection was repeated when the specimen exhibited the target fluorescence signal. Two positive controls (103 copies/μL) and one negative control were included in each run. The results were deemed valid only if both positive amplifications were observed in positive controls, and no amplification was detected in the negative control.
To quantify B19V DNA, the positive plasmid was decimally diluted by eight orders of magnitude (5 × 107∼5 copies/μL). The reaction conditions were identical to those of the aforementioned PCR using ABI7500 to generate a standard curve. The copy number of the target gene in the samples (copies/μL) was calculated based on the Ct values and then converted to the specimen concentration, which was the copy number multiplied by 125 (copies/mL).
To analyze the sensitivity and specificity, Probit analysis was conducted on at least 24 replicates of each dilution from a dilution series (5 × 103∼5copies/μL). Calculations were performed on log-transformed data, and the specificity was determined using the positive plasmid (50 copies/μL) and 80 B19V DNA-negative plasma samples, which contained HBV DNA +(200IU/mL), HCV RNA +(200IU/mL), and HIV RNA +(200IU/mL) plasma.,20 of each, purchased from Conchrestin (Wantai company, Beijing, China). The quantification of positive samples was initially expressed in copies/μL.
1.2. Serological tests
Briefly, 893 blood donations and B19V DNA-positive samples were tested for specific antibodies using a commercial kit for the qualitative detection of parvovirus IgG/IgM (Virion/Serion, Würzburg, Germany), following the manufacturer's instructions. According to the Enzyme-Linked Immunosorbent Assay (ELISA) kit, the results were interpreted as negative if < 3IU/mL, equivocal if 3–5 IU/mL, and positive if > 5 IU/mL for the IgG test results, and the results were evaluated as negative if < 13U/mL, equivocal if 13–17U/mL, and positive if > 17U/mL for the IgM test results. If the result was borderline, the assay was repeated and the final result was unambiguous. The 2nd international standard for Anti-Parvovirus B19 plasma (NIBSC code:01/602) was used for quantification.
1.3. Sequencing, phylogenetic analysis
B19V DNA sequencing was performed using nested PCR amplification of a 944-bp region spanning the NS1-VP1u junction. The following primers were used: P1F(5′-CACTATGAAAACT GGGCAATAAAC-3′,2035-2058nt,NC000883.2) and P1R (5′-CCAGGCT TGTGTAAGTCTT C-3′,2960–2979 nt,NC000883.2) for the first round, P2F(5′-ATAAACTACACTTTTGATTTC CCTG-3′,2053-2077 nt,NC_000883.2) and P1R for the second round. The PCR conditions were the same for the first and the second reaction which included: 1 cycle of 95 °C for 5 min, 30 cycles of 95 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min and 1 cycle of 72 °C for 5 min. The B19 virus NS1-VP1u genomic fragment was amplified, recovered, purified, and bi-directionally sequenced using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) on an ABI3730XL Genetic Analyzer (Applied Biosystems). The evolutionary tree was constructed using MEGA version 7.0 software with neighborhood joint (NJ) and Maximum Parsimony (MP) methods for phylogenetic analysis, using the reference sequences with GenBank accession numbers shown in the figure, and consistency was tested with 1000 bootstrap replicates. A bootstrap value of >75 % was considered plausible.
1.4. Statistical analysis
Statistical analysis was conducted using SPSS version 19 (IBM Corp., Armonk, NY, USA). Differences were analyzed using the chi-squared test, whereas Fisher's exact test was performed when the expectation was less than 5. Statistical significance was set at p < 0.05.
2. Results
2.1. Characteristics of the study population
In this study, 7728 blood donor specimens were tested by PCR, of which 63.87 % were from males. The age of the donors ranged from 18 to 60 years with a median age of 33 years, and most donors (73.9 %) were below 40 years. Blood groups A and O were 30.42 % and 30.82 % of all samples, respectively, whereas blood group AB was the lowest (9.37 % of all samples). Considering the frequency of donations, 41.91 % were first-time blood donors and 58.09 % were repeat blood donors (Table 1).
Table 1.
Characteristics of the study population.
| Characteristic | Number (%) | |
|---|---|---|
| Sex | Male | 4936 (63.87) |
| Female | 2792 (36.13) | |
| Total | 7728 | |
| Age (years) | 18–30 | 3013 (38.99) |
| 31–40 | 2701 (34.95) | |
| 41–50 | 1499 (19.40) | |
| ≥51 | 515 (6.66) | |
| Total | 7728 | |
| Blood group | A | 2351 (30.42) |
| B | 2271 (29.39) | |
| O | 2382 (30.82) | |
| AB | 724 (9.37) | |
| Total | 7728 | |
| Donation times | First | 3239 (41.91) |
| Repeat (≥2) | 4489 (58.09) | |
| Total | 7728 |
2.2. Performance of the B19V qPCR
The sensitivity and specificity of the B19V qPCR detection system were validated. The standard curve, established using plasmid pUC-GW-Kan with target gene concentrations ranging from 5 × 107 to 5 copies/μL, resulted in a linear equation y = −3.304X+40.828 and an R2 value of 0.997, indicating a good fit (Fig. 1a、Fig. 1b). The 95 % detection limit, determined by Probit regression analysis,was 6.5 copies/μL (Table 2). Specificity testing showed that the positive plasmid (50 copies/μL) was correctly identified as positive, and all 80 B19V DNA-negative plasma samples were confirmed negative.
Fig. 1.
Performance of the B19V qPCR. (a) Amplification plot obtained from a serial dilution (10 × ) of plasmid pUC-GW-Kan (5 × 107 to 5 copies/μL); (b) Linear standard curve generated from the amplification plot. The quantitative curve parameters obtained were suitable for quantifying B19V DNA: Slope, −3.304; Y-intercept, 40.828; R2 = 0.997.
Table 2.
Sensitivity of B19V DNA qPCR determined by plasmid dilution.
| Plasmid (copies/μL) | 5000 | 500 | 50 | 13.0 | 6.5 | 5 | 1.2 |
|---|---|---|---|---|---|---|---|
| Number of positive results | 24 | 24 | 24 | NT | NT | 22 | NT |
| Total | 24 | 24 | 24 | NT | NT | 24 | NT |
| Probit | 100 % | 99 % | 95 % | 92 % | 50 % |
NT: not tested.
2.3. Prevalence of B19V DNA and viral loads
Of the 7728 plasma samples, 0.09 % were positive for B19V DNA (95 % CI: 0.02–0.15 %). The majority of positive cases were males (71.4 % vs. 28.6 % females), aged between 19 and 43 years, with DNA levels ranging from 2.2 × 102–1.1 × 104 copies/mL, which is considered a low concentration. All DNA-positive specimens were positive for IgG antibodies, with most cases having a high concentration of >90 IU/mL (71.4 %). Additionally, all cases tested negative for IgM, with only one case classified as suspicious. Regarding the number of blood donations, there were five first-time donors and two repeat donors. One repeat donor donated blood up to 10 times between 2012 and 2023. The DNA concentration of this donor was 770 copies/ml, and the IgG concentration was 47 IU/mL. The characteristics of the B19V DNA blood donors are shown in Table 3.
Table 3.
B19V viremia in the studied population (n = 7728).
| Donor | Sex | Age | Blood Group | Times | Occupation | viral load (copies/mL) | IgG (IU/mL) | IgM | Destiny of blood components |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 43 | B | 1 | NA | 7.3 × 102 | 117.04 | – | Red cells Plasma |
| 2 | M | 23 | O | 4 | Banking staff | 5.8 × 103 | 252.18 | – | Red cells, Plasma |
| 3 | M | 20 | B | 1 | Medical staff | 2.2 × 102 | 96.6 | suspicious | Red cells, Plasma |
| 4 | M | 37 | O | 10 | Medical staff | 7.7 × 102 | 47 | – | Red cells, Plasma |
| 5 | M | 19 | O | 1 | NA | 4.3 × 103 | 276.55 | – | Red cells, Plasma |
| 6 | F | 23 | A | 1 | Medical staff | 1.1 × 104 | 191.32 | – | Red cells, Plasma |
| 7 | F | 21 | A | 1 | Medical staff | 3.7 × 103 | 39 | – | Red cells, Fresh frozen plasma |
NA, not available; M, male; F, female; -, negative.
2.4. Seroprevalence of parvovirus B19 antibodies
To determine the seroprevalence of B19V, B19V-specific antibodies (IgG/IgM) were investigated among 893 samples, of which 185 (20.7 %, 95 % CI: 18.1–23.4 %) specimens were tested positive. As shown in Table 4, Table 5, the prevalences of B19V IgG and IgM in this study were 19.4 % (95 % CI:16.8–22.0 %) and 1.9 % (95 % CI:1.0–2.8 %) respectively, with the former being significantly higher than the latter. There were 5 cases (0.56 %) with double antibody positivity. Of the samples tested, 14 (1.6 %) were equivocal for IgG, and six (0.7 %) were equivocal for IgM. There was no significant difference in the rates of IgG and IgM between the sexes (p > 0.05), and the rate of IgG positivity increased with age (p < 0.05), while the rate of IgM positivity did not show a significant difference across age groups (p > 0.05). Regarding the different blood types, the significant difference in IgG was found (p < 0.05), with blood-type O having the highest rate (24.4 %, 95 % CI: 29.5–29.3 %). However, IgM positivity did not vary significantly with the blood type (p > 0.05). Furthermore, first-time blood donors had lower IgG positivity than repeat donors (p < 0.05); however, there was no significant difference in IgM positivity (P > 0.05).
Table 4.
The prevalence of IgG according to different characteristics.
| N (%) | Positivity (%) | 95%CI | P (χ2) | ||
|---|---|---|---|---|---|
| Genders | Male | 582 (65.2) | 117 (20.1) | 16.8–23.4 | |
| Female | 311 (34.8) | 56 (18.0) | 13.7–22.3 | 0.450 (0.570) | |
| Age groups | 18~30 | 300 (33.6) | 34 (11.3) | 7.7–14.9 | |
| 31~40 | 348 (39.0) | 66 (19.0) | 14.8–23.1 | ||
| 41~50 | 178 (19.9) | 45 (25.3) | 18.8–31.7 | ||
| ≥51 | 67 (7.5) | 28 (41.8) | 29.7–53.9 | 0.000 (37.986) | |
| Blood types | A | 282 (31.6) | 57 (20.2) | 15.5–24.9 | |
| B | 225 (25.2) | 34 (15.1) | 10.4–19.8 | ||
| O | 295 (33.0) | 72 (24.4) | 19.5–29.3 | ||
| AB | 91 (10.2) | 10 (11.0) | 4.4–17.5 | 0.009 (11.624) | |
| Times | 1 | 320 (35.8) | 51 (15.9) | 11.9–20.0 | |
| ≥2 | 573 (64.2) | 123 (21.5) | 18.1–24.8 | 0.045 (4.000) | |
| Total | 893 | 173 (19.4) | 16.8–22.0 | ||
Table 5.
The prevalence of IgM in different characteristics.
| N (%) | Positivity (%) | 95 % CI | p | ||
|---|---|---|---|---|---|
| Genders | Male | 582 (65.2) | 9 (1.5) | 0.5–2.6 | |
| Female | 311 (34.8) | 8 (2.6) | 0.8–4.3 | 0.285a | |
| Age group | 18~30 | 300 (33.6) | 5 (1.7) | 0.2–3.1 | |
| 31~40 | 348 (39.0) | 9 (2.6) | 0.9–4.3 | ||
| 41~50 | 178 (19.9) | 2 (1.1) | 0–2.7 | ||
| ≥51 | 67 (7.5) | 1 (1.5) | 0–4.5 | 0.737a | |
| Blood types | A | 282 (31.6) | 5 (1.8) | 0.2–3.3 | |
| B | 225 (25.2) | 4 (1.8) | 0–3.5 | ||
| O | 295 (33.0) | 5 (1.7) | 0.2–3.2 | ||
| AB | 91 (10.2) | 3 (3.3) | 0–7.0 | 0.744a | |
| Times | 1 | 320 (35.8) | 7 (2.2) | 0.6–3.8 | |
| ≥2 | 573 (64.2) | 9 (1.6) | 0.5–2.6 | 0.505 (0.444) | |
| Total | 893 | 17 (1.9) | 1.0–2.8 | ||
:Fischer's exact test.
2.5. Genotype analysis of B19V
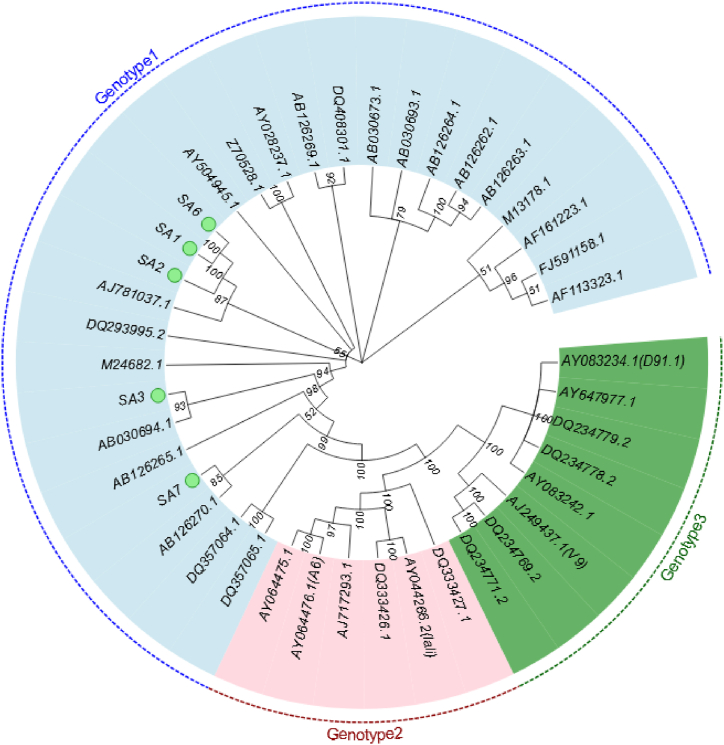
For phylogenetic purposes, the aligned sequences of the 926 bp fragment were analyzed to construct the phylogeny using the MP and NJ methods. Seven B19V DNA-positive isolates were amplified using nested PCR for NS1-VP1u. Of these, five isolates were successfully amplified, whereas two were not. The two methods provided the same and well-supported tree topologies; therefore, only the MP method is shown in Fig. 2, where all samples were clustered within genotype 1. Samples SA1, SA2, and SA6 were identical and sub-clustered with the German (AJ781037.1) isolate (bootstrap: 87 %), whereas SA3 and SA7 were closer to the Japanese (AB03694.1, AB126270.1) clade (bootstrap: 93 % and 85 %, respectively), suggesting that B19V genotype 1 was widespread and the most common genotype.
Fig. 2.
Phylogenetic analysis of human parvovirus B19V NS1-VP1u fragment (926 bp). The GenBank accession number of each reference sequence is shown at the tips. The green circle marked at the back represented our samples.
3. Discussion
This study aimed to evaluate the serological and DNA positivity of B19V among the blood donor population in Suzhou, eastern China.
In our survey, the percentage of positivity of B19V DNA was 0.09 % (95 % CI: 0.02–0.15 %), which was lower than that reported in other domestic studies [[13], [14], [15]]. This discrepancy may be due to the implementation of infection control measures to prevent the spread of respiratory viruses during the COVID-19 pandemic. However, these rates are comparable to those reported Netherlands (0.06 %) [11], South Korea (0.1 %) [16], and Portugal (0.18 %) [17], while some countries reported the lower prevalence (Japan, 0.01 % [18] and German, 0.013 % [19]). Previous studies indicated that the estimated proportion of B19V DNA positivity among voluntary blood donors ranged from 0.006 to 1.3 % [11,20,21], with significant variations across different countries and regions. These differences were attributed not only to variations in the test subjects, geographic regions, and seasons but also to the testing reagents used. Currently, besides commercialized Roche reagent kits, most B19V nucleic acid detection reagents are self-developed, and there is considerable disparity in their sensitivity and specificity, which increases the possibility of false positives and contamination. This study used real-time quantitative PCR to detect B19V DNA using primers designed specifically for the NS1 region, which is associated with virulence and is highly conserved compared to the VP1 and VP2 regions, thus reducing false negatives due to primer mismatches caused by mutations. Additionally, it can simultaneously detect all three genotypes of B19V, offering excellent sensitivity (95 % detection limit at 6.5 copies/μL) and a broad linear range (5–5 × 107 copies/μL). To minimize contamination and produce false-positive results, nucleic acid extraction and amplification were performed in separate rooms and ultraviolet irradiation was applied for over 30 min after detection.
Notably, after infection, B19 virus is not easily expulsed by the host and can persist at low levels in the plasma of healthy donors for an extended period. Studies have shown that one-year post-infection, viral DNA levels drop below 104 IU/mL; after two years, they are below 103 IU/mL and continue remaining at lower levels for several years [18,22]. The potential threat to blood safety posed by low-level viremia remains a topic of ongoing debate [23]. In our screening, we identified seven donors positive for B19V DNA. The viral load ranged from 220 to 11,000 copies/mL. According to FDA and PPTA guidelines on blood B19V DNA concentrations, the viral contamination of these blood products did not appear to pose a threat to the low-level viremia. However, two studies reported that the red blood cell products containing low levels of B19V DNA still result in transfusion-transmitted B19V despite the presence of potential neutralizing antibodies [24,25].
Among the seven samples tested, the highest concentration was 11,000 copies/ml. All samples tested positive for IgG antibodies, but not IgM, except for one suspected case. Given that the DNA level of this sample was only 220 copies/mL and was IgG-positive, it is likely that the IgM result was a false reaction. Based on the above findings, we can speculate that the seven donors may have had late-stage or persistent infections (DNA+/IgG+/IgM-). Persistent infection, often characterized by low levels of DNA with or without IgG, has been reported in various studies and can occur in both immunocompromised and immunocompetent patients, depending largely on the immune status of the recipients [23,26,27]. The mechanisms of persistent infection remain unclear, but one study suggested that a qualitative defect in the humoral response to B19V may be responsible [28]. Some individuals with persistent infections produce antibodies that are neither neutralizing nor are present in sufficient quantities for neutralization, suggesting that the presence of antibodies does not always provide protection against the virus. Moreover, the potential transmission threat posed by the persistence of B19V DNA, especially among high-risk groups, such as pregnant women, immunosuppressed recipients, and hematological patients, cannot be overlooked.
Serological antibody testing was conducted on 893 specimens to determine the prevalence of B19V infection. The prevalence of B19V IgG antibodies was 19.4 % (95 % CI:16.8–22.0 %), which was similar to that reported in domestic studies, such as Oushanhai et al. who reported 16.79 % [29], He Miao et al. who reported 24.6 % [14], and Yan Junxiong et al. who reported 25 % [13], but lower than that reported by Bao Haie et al. (43.36 %) [30] and Wei Qiang et al. (55.43 %) [31]. Globally, our data were lower than those of European countries, such as the Netherlands (60.9 %) [11], United Kingdom (60 %) [32], and Turkey (59.9 %) [33]. IgG positivity varied between 6 % and 82.3 %, with differences mainly due to factors such as age distribution, sample size, sampling time, viral epidemic cycles, and seasonality (with infection peaks in late winter and early spring) [16,21,34]. In our study, the positivity rate of B19V IgM was 1.9 % (95 % CI: 1.0–2.8 %), which was lower than that reported in other regions, further indicating a lower rate of B19V infection among local blood donors. Following infection with B19V, the viral load can reach 1012copies/mL, but it decreases within a week as IgM antibodies are produced. IgG antibodies gradually appear after 15 days, providing immunity for months or even a lifetime [35]. In this study, the rate of IgG positivity was 19.4 % (95 % CI: 16.8–22.0 %), indicating that approximately 80 % of donors were susceptible to the virus, posing a potential threat to blood safety. IgM is generally considered as a marker of acute infection. However, in this study, we did not detect B19V DNA in the IgM-positive specimens, potentially because of the following reasons: 1) false positives in specimen testing; 2) the fact that some blood donors maintained IgM for an extended period, typically 2–4 months, and in some cases up to six months, at which time the viral load may have decreased below the detection limit of nucleic acid testing; and 3) the fact that serological tests detected proteins, while nucleic acid tests detected DNA, leading to potential inconsistencies due to different targets. Additionally, our study revealed that IgM antibodies were not detected in B19V DNA-positive specimens. In contrast, Steven [36] detected IgM in 10 of 44 B19V DNA-positive specimens, whereas Guclu [33] detected B19V IgM in all five DNA-positive specimens. Our study's conclusions differed from these reports, possibly due to the small number of positive samples, and possibly because there is no inherent correlation between IgM and DNA.
Seroprevalence in blood donors is disputed across the sexes. Studies by Ke [14] and Adamo [37] indicated no significant difference between males and females, whereas researchers from Qatar and Turkish reported disparities [38,39]. In our study, the IgG positivity rate was greater in males (20.1 %, 95 % CI: 16.8–23.4 %) than in females (18.0 %, 95 % CI: 13.7–22.3 %), and the IgM positivity rate was greater in females than in males (2.6 %, 95 % CI: 0.8–4.3 % vs. 1.5 %, 95 % CI: 0.5–2.6 %); however, no difference was found. Regarding age groups, the B19V IgG seroprevalence increased with age, which is consistent with the findings of other studies [[38], [39], [40]], and in our study, younger donors (18–40 years) constituted 72.5 %, which aligns with the lower IgG antibody positivity rate (19.4 %, 95 % CI: 16.8–22.0 %). Studies suggest that the B19V IgM positivity rate reaches its peak between the ages of 18–30, while the lowest rates are observed between 41 and 50 [21]. However, our findings indicated increased IgM positivity (2.6 %, 95 % CI: 0.8–4.3 %) in the 31–40 age group and decreased IgM positivity (1.1 %, 95 % CI: 0–2.7 %) in the 41–50 age group, and there were no significant differences in IgM positivity across age groups (p > 0.05). Regarding the different blood types, there was a significant difference in IgG positivity rates (p < 0.05), with type O having the highest rate (24.4 %, 95 % CI: 19.5–29.3 %) and type AB having the lowest (11.0 %, 95 % CI: 4.4–17.5 %). However, no significant differences were observed in the IgM levels. Currently, there is a paucity of research investigating the relationship between B19V infection and blood groups. Healy [19] conducted a study examining the prevalence of Parvovirus B19 virus infection in relation to ABO and Rh blood group antigens among German blood donors. Their findings indicated that the ABO blood group may not be a significant risk factor for B19V infection. However, the infections they identified were all in Rh(D) positive donors. It is noteworthy that all the 7 B19 DNA-positive blood donors identified in our study were Rh(D) + donors (data not shown). It has been postulated that the Rh(D) phenotype may confer a viroprotective effect, as evidenced by recent observations in the context of COVE-19 infection [41]. Further evidence is required to ascertain whether the Rh antigen plays a role in B19V infectivity. Our study also found that frequent blood donors had a greater B19V IgG antibody positivity rate (21.5 %, 95 % CI: 18.1–24.8 %) than first-time donors (15.9 %, 95%CI: 11.9–20.0 %), although no significant difference was noted in IgM positivity. B19V is primarily transmitted via the respiratory route and the population is generally susceptible. The higher rate of B19V IgG antibody positivity in repeat donors may be related to their age. This is because the average age of repeat donors was 41.1 ± 9.6 years, compared to the mean age of 34.5 ± 9.3 years for first-time donors, which further substantiated the observation that the positivity for B19V IgG antibodies escalated with advancing age.
Human parvovirus B19 has been found to have three genotypes, as discovered by Servants [42], with approximately 10 % sequence divergence. We performed a nested amplification of the NS1-VP1u sequence in seven B19V DNA-positive specimens to determine the predominant genotype in the region. The five successfully amplified cases in this study were identified as genotype 1, consistent with the findings of several other studies in China [14,29], suggesting that genotype 1 may be predominant in China. Similar findings have been reported for blood donors in Iran [21] and the Netherlands [11]. Globally, the B19V genotype 1 is widespread (78 %) and accounts for most infections, whereas genotype 2 is more common in Europe, Vietnam, and Brazil. Genotype 3 has been detected in North and West Africa, which are endemic [43]. In this study, the target fragment could not be amplified in the two donors, possibly because of mismatches in the NS1-VP1u primers, as confirmed by Abdelrahman, who reported 22 DNA-positive specimens that failed to be amplified in their study [38]. In addition, low viral loads can lead to the failure of nested PCR amplification and partial sequencing of the NS1-VP1u genomic region.
B19V can be transmitted through infected blood products, with serious consequences in patients who are immunocompromised, have hematological disorders, and pregnant women. Consequently, it is imperative to ensure a supply of B19V-safe blood to risk-prone patients and populations. Our primary risk mitigation methods included ELISA (primarily for IgM) and nucleic acid testing (NAT). Despite the superior sensitivity of PCR, it is important to recognise that PCR only detects DNA fragments, which do not necessarily represent intact particles, which was confirmed by Molenaar [44]. In this study, B19V DNA was detected in DNA-positive samples before and after treatment with an endonuclease. He found that B19V DNA in the early stages of acute infection was not degraded, possibly indicating the presence of infectious viral particles, while after five months, DNA could not be detected after treatment, suggesting the presence of DNA remnants. Interestingly, a second transient peak of B19 DNA was observed in four donors between 6 and 12 months after the acute infection; however, the mechanisms involved remain unclear. Therefore, a comprehensive assessment that combines nucleic acid and serological test results is required. Countries such as Germany, Japan, and the Netherlands have already implemented various strategies for routinely screening blood donors for B19V, including pooled B19V NATs, chemiluminescent immunoassays, and blood from donors if B19V IgG is positive for at least 6 months. However, there is currently no established B19V screening strategy for blood donation in China. Given the lack of data on the prevalence of B19V, and the need to balance routine testing with other important public health priorities, further studies with larger sample sizes are recommended to determine the feasibility of parvovirus B19 screening in healthy blood donors.
Data availability statement
Data will be made available on request.
Ethics declarations
This study was reviewed and approved by the Medical Ethics Committee of the Suzhou Blood Center with the approval number: 202103, dated March 23, 2021.
Funding information
Suzhou Science and Technology Plan Project [SS202080]. Suzhou Health Science and Technology Project [GWZX202203]. Suzhou Medical Key Discipline Funding Project [SZXK202118] and Suzhou Youth Science &Technol Project [KJXW2023060, KJXW2022057].
CRediT authorship contribution statement
Rong Lu: Writing – original draft, Formal analysis, Data curation. Shuhong Xie: Writing – review & editing, Writing – original draft, Project administration. Zihao Xu: Supervision, Investigation. Zhen Liu: Visualization, Validation, Methodology. Jia Jiang: Validation, Investigation, Data curation. Longhai Tang: Validation, Supervision, Software. Yiming Jin: Supervision, Resources, Funding acquisition. Xiaoyan Fu: Supervision, Software, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yiming Jin, Email: jym98121@sohu.com.
Xiaoyan Fu, Email: 15962273374@163.com.
References
- 1.Morey A.L., Fleming K.A. Immunophenotyping of fetal haemopoietic cells permissive for human parvovirus B19 replication in vitro. Br. J. Haematol. 1992;82(2):302–309. doi: 10.1111/j.1365-2141.1992.tb06422.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown K.E. The expanding range of parvoviruses which infect humans. Rev. Med. Virol. 2010;20(4):231–244. doi: 10.1002/rmv.648. [DOI] [PubMed] [Google Scholar]
- 3.Gigi C.E., Anumba D.O.C. Parvovirus b19 infection in pregnancy - a review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;264:358–362. doi: 10.1016/j.ejogrb.2021.07.046. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y., Qiu J. Human parvovirus B19: a mechanistic overview of infection and DNA replication. Future Virol. 2015;10(2):155–167. doi: 10.2217/fvl.14.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchand S., Tchernia G., Hiesse C., Tertian G., Cartron J., Kriaa F., Boubenider S., Goupy C., Lecointe D., Charpentier B. Human parvovirus B19 infection in organ transplant recipients. Clin. Transplant. 1999;13(1 Pt 1):17–24. doi: 10.1034/j.1399-0012.1999.t01-1-130103.x. [DOI] [PubMed] [Google Scholar]
- 6.Satake M., Hoshi Y., Taira R., Momose S.Y., Hino S., Tadokoro K. Symptomatic parvovirus B19 infection caused by blood component transfusion. Transfusion. 2011;51(9):1887–1895. doi: 10.1111/j.1537-2995.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 7.Servant-Delmas A., Morinet F. Update of the human parvovirus B19 biology. Transfus. Clin. Biol. 2016;23(1):5–12. doi: 10.1016/j.tracli.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Gowland P., Fontana S., Stolz M., Andina N., Niederhauser C. Parvovirus B19 passive transmissi- on by transfusion of Intercept® blood system-treated platelet concentrate. Transfus Med Hemo- ther. 2016;43(3):198–202. doi: 10.1159/000445195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt I., Blümel J., Seitz H., Willkommen H., Löwer J. Parvovirus B19 DNA in plasma pools and plasma derivatives. Vox Sang. 2001;81(4):228–235. doi: 10.1046/j.1423-0410.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M., Themann A., Drexler C., Bayer M., Lanzer G., Menichetti E., Lechner S., Wessin D., Prokoph B., Allain J.P., Seifried E., Hourfar M.K. Blood donor screening for parvovirus B19 in Germany and Austria. Transfusion. 2007;47(10):1775–1782. doi: 10.1111/j.1537-2995.2007.01443.x. [DOI] [PubMed] [Google Scholar]
- 11.Kooistra K., Mesman H.J., de Waal M., Koppelman M.H., Zaaijer H.L. Epidemiology of high-level parvovirus B19 viraemia among Dutch blood donors, 2003-2009. Vox Sang. 2011;100(3):261–266. doi: 10.1111/j.1423-0410.2010.01423.x. [DOI] [PubMed] [Google Scholar]
- 12.Ikegawa M., Ohashi S., Minagi T., Okamoto H., Yunoki M. Screening for parvovirus B19 antigen through chemiluminescent enzyme immunoassay is equivalent to B19 nucleic acid amplification test-based screening of pooled plasma. Transfusion. 2021;61(8):2240–2244. doi: 10.1111/trf.16512. [DOI] [PubMed] [Google Scholar]
- 13.Yan J., Wu W., Zhou J., Liu J. Human parvovirus B19 detection in voluntary blood donors of Foshan City. Internet J. Lab. Med. 2016;37(8):1039–1040. [Google Scholar]
- 14.Ke L., He M., Li C., Liu Y., Gao L., Yao F., Li J., Bi X., Lv Y., Wang J., Hirsch M.L., Li W. The prevalence of human parvovirus B19 DNA and antibodies in blood donors from four Chinese blood centers. Transfusion. 2011;51(9):1909–1918. doi: 10.1111/j.1537-2995.2011.03067.x. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y.R., Li Z.P., Liang H.J., et al. Epidemiological study on HPV B19and viral load among volunteer blood donors in Guangzhou. Chin J Blood Transf. 2009;22:549–551. [Google Scholar]
- 16.Oh D.J., Lee Y.L., Kang J.W., Kwon S.Y., Cho N.S. Investigation of the prevalence of human parvovirus B19 DNA in Korean plasmapheresis donors. Korean J Lab Med. 2010;30(1):58–64. doi: 10.3343/kjlm.2010.30.1.58. [DOI] [PubMed] [Google Scholar]
- 17.Henriques I., Monteiro F., Meireles E., Cruz A., Tavares G., Ferreira M., Araújo F. Prevalence of Parvovirus B19 and Hepatitis A virus in Portuguese blood donors. Transfus. Apher. Sci. 2005;33(3):305–309. doi: 10.1016/j.transci.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Matsukura H., Shibata S., Tani Y., Shibata H., Furuta R.A. Persistent infection by human parvovirus B19 in qualified blood donors. Transfusion. 2008;48(5):1036–1037. doi: 10.1111/j.1537-2995.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 19.Healy K., Aulin L.B.S., Freij U., Ellerstad M., Brückle L., Hillmering H., Svae T.E., Broliden K., Gustafsson R. Prevalence of parvovirus B19 viremia among German blood donations and the relationship to ABO and rhesus blood group antigens. J. Infect. Dis. 2023;227(10):1214–1218. doi: 10.1093/infdis/jiac456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francois K.L., Parboosing R., Moodley P. Parvovirus B19 in South African blood donors. J. Med. Virol. 2019;91(7):1217–1223. doi: 10.1002/jmv.25450. [DOI] [PubMed] [Google Scholar]
- 21.Zadsar M., Aghakhani A., Banifazl M., Kazemimanesh M., Tabatabaei Yazdi S.M., Mamishi S., Bavand A., Sadat Larijani M., Ramezani A. Seroprevalence, molecular epidemiology and quantitation of parvovirus B19 DNA levels in Iranian blood donors. J. Med. Virol. 2018;90(8):1318–1322. doi: 10.1002/jmv.25195. [DOI] [PubMed] [Google Scholar]
- 22.Juhl D., Hennig H. Parvovirus B19: what is the relevance in transfusion medicine? Front. Med. 2018;5:4. doi: 10.3389/fmed.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefrère J.J., Servant-Delmas A., Candotti D., Mariotti M., Thomas I., Brossard Y., Lefrère F., Girot R., Allain J.P., Laperche S. Persistent B19 infection in immunocompetent individuals: implications for transfusion safety. Blood. 2005;106(8):2890–2895. doi: 10.1182/blood-2005-03-1053. [DOI] [PubMed] [Google Scholar]
- 24.Juhl D., Özdemir M., Dreier J., Görg S., Hennig H. Look-back study on recipients of Parvovirus B19 (B19V) DNA-positive blood components. Vox Sang. 2015;109(4):305–311. doi: 10.1111/vox.12295. [DOI] [PubMed] [Google Scholar]
- 25.Servant-Delmas A., Laperche S., Mercier M., Michel Y., Garbarg-Chenon A., Boyeldieu D., Pelissier E., Lefrère J.J. Limits of sequencing and phylogenetic analysis to assess B19V transmission by single-donor blood component. Vox Sang. 2011;100(2):254–255. doi: 10.1111/j.1423-0410.2010.01390.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee T.H., Kleinman S.H., Wen L., Montalvo L., Todd D.S., Wright D.J., Tobler L.H., Busch M.P. NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II). Distribution of parvovirus B19 DNA in blood compartments and persistence of virus in blood donors. Transfusion. 2011;51(9):1896–1908. doi: 10.1111/j.1537-2995.2010.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindblom A., Isa A., Norbeck O., Wolf S., Johansson B., Broliden K., Tolfvenstam T. Slow clearance of human parvovirus B19 viremia following acute infection. Clin. Infect. Dis. 2005 15;41(8):1201–1203. doi: 10.1086/444503. [DOI] [PubMed] [Google Scholar]
- 28.Juhl D., Görg S., Hennig H. Persistence of Parvovirus B19 (B19V) DNA and humoral immune response in B19V-infected blood donors. Vox Sang. 2014;107(3):226–232. doi: 10.1111/vox.12162. [DOI] [PubMed] [Google Scholar]
- 29.Ou Sh, Xie J., Zhang Y., Ni H., Song X. Prevalence of parvovirus B19 infection in Chinese xiamen area blood donors. J. Exp. Hematol. 2016;(5):1572–1576. doi: 10.7534/j.issn.1009-2137.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 30.Bao H., Yang B., Pan X., Nie x, Zhang X., Chen S., Zhang L., Lu J. A survey of human parvovirus B19 prevalence among blood donors in the three gorges region. J Clin Hematol(China) 2015;28(4):2 89–90. [Google Scholar]
- 31.Wei Q., Li Y., Wang J., Wang H., Qu J., Hong T. Prevalence of anti-human parvovirus B19 IgG antibody among blood donors in JiLin Province. Chin. J. Exp. Clin. Virol. 2006;20(2):60–62. [PubMed] [Google Scholar]
- 32.O'Bryan T.A., Wright W.F. Parvovirus B19 and C-reactive protein in blood bank donors: implications for hygiene hypothesis research. Lupus. 2010;19(13):1557–1560. doi: 10.1177/0961203310375438. [DOI] [PubMed] [Google Scholar]
- 33.Uskudar Guclu A., Yilmaz S., Baysallar M., Avci I.Y. Prevalence and quantity of parvovirus B19 DNA among blood donors from a regional blood center in Turkey. Transfus. Apher. Sci. 2020;59(4) doi: 10.1016/j.transci.2020.102775. [DOI] [PubMed] [Google Scholar]
- 34.Kleinman S.H., Glynn S.A., Lee T.H., Tobler L.H., Schlumpf K.S., Todd D.S., Qiao H., Yu M.Y., Busch M.P. National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study-II (NHLBI REDS-II). A linked donor-recipient study to evaluate parvovirus B19 transmission by blood component transfusion. Blood. 2009;114(17):3677–3683. doi: 10.1182/blood-2009-06-225706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young N.S. B19 parvovirus. Baillieres Clin Haematol. 1995;8(1):25–56. doi: 10.1016/s0950-3536(05)80231-8. [DOI] [PubMed] [Google Scholar]
- 36.Kleinman S.H., Glynn S.A., Lee T.H., Tobler L., Montalvo L., Todd D., Kiss J.E., Shyamala V., Busch M.P. Prevalence and quantitation of parvovirus B19 DNA levels in blood donors with a sensitive polymerase chain reaction screening assay. Transfusion. 2007;47(10):1756–1764. doi: 10.1111/j.1537-2995.2007.01341.x. [DOI] [PubMed] [Google Scholar]
- 37.Adamo M.P., Blanco S., Viale F., Rivadera S., Rodríguez-Lombardi G., Pedranti M., Carrizo H., Gallego S. Human parvovirus B19 frequency among blood donors after an epidemic outbreak: relevance of the epidemiological scenario for transfusion medicine. Heliyon. 2020 4;(6) doi: 10.1016/j.heliyon.2020.e03869. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelrahman D., Al-Sadeq D.W., Smatti M.K., Taleb S.A., AbuOdeh R.O., Al-Absi E.S., Al-Thani A.A., Coyle P.V., Al-Dewik N., Qahtani A.A.A., Yassine H.M., Nasrallah G.K. Prevalence and phylogenetic analysis of parvovirus (B19V) among blood donors with different nationalities residing in Qatar. Viruses. 2021;13(4):540. doi: 10.3390/v13040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Göral Ş., Yenicesu İ., Bozdayı G., Duyan Çamurdan A., Altay Koçak A. Parvovirus B19 seroprevalence in Turkish blood donors. Turk. J. Med. Sci. 2018;48(5):956–960. doi: 10.3906/sag-1802-150. [DOI] [PubMed] [Google Scholar]
- 40.Raturi G., Kaur P., Kaur G. Seroprevalence of human parvovirus B19 amongst North Indian blood donors - do current donor testing guidelines need a relook? Transfus. Apher. Sci. 2018;57(5):646–650. doi: 10.1016/j.transci.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Zietz M., Zucker J., Tatonetti N.P. Associations between blood type and COVID-19 infection, intubation, and death. Nat Commun. 2020;11(1):5761. [42. doi: 10.1038/s41467-020-19623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Servant A., Laperche S., Lallemand F., Marinho V., De Saint Maur G., Meritet J.F., Garbarg-Chenon A. Genetic diversity within human erythroviruses: identification of three genotypes. J. Virol. 2002;76(18):9124–9134. doi: 10.1128/JVI.76.18.9124-9134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hübschen J.M., Mihneva Z., Mentis A.F., Schneider F., Aboudy Y., Grossman Z., Rudich H., Kasymbekova K., Sarv I., Nedeljkovic J., Tahita M.C., Tarnagda Z., Ouedraogo J.B., Gerasimova A.G., Moskaleva T.N., Tikhonova N.T., Chitadze N., Forbi J.C., Faneye A.O., Otegbayo J.A., Charpentier E., Muller C.P. Phylogenetic analysis of human parvovirus b19 sequences from eleven different countries confirms the predominance of genotype 1 and suggests the spread of genotype 3b. J. Clin. Microbiol. 2009;47(11):3735–3738. doi: 10.1128/JCM.01201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molenaar-de Backer M.W., Russcher A., Kroes A.C., Koppelman M.H., Lanfermeijer M., Zaaijer H.L. Detection of parvovirus B19 DNA in blood: viruses or DNA remnants? J. Clin. Virol. 2016;84:19–23. doi: 10.1016/j.jcv.2016.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.