Abstract
The presence of hexavalent chromium species (CrVI) in wastewater from manufacturing industries such as electroplating and leather production can pose serious health hazards. To address these concerns, this study developed a novel adsorbent based on activated carbon as the primary material to attract CrVI. Activated carbon has been modified with several other components to improve its comprehensive performance, including adsorption capacity, chemical stability, collectability, and reusability. Specifically, decoration with magnetite nanoparticles made it possible to collect the adsorbent magnetically and reuse it several times. On the one hand, the addition of chitosan not only increased the chemical stability of activated carbon, especially under acidic conditions, but also enhanced the Cr adsorption capacity at pH higher than 4, where adsorption of only activated carbon was significantly decreased, probably because the protonated amino groups attracted chromate anions. In addition, the co-existence of tannic acid did not increase the adsorption capacity significantly but appeared to promote the reductive adsorption of CrVI, where the reduction of CrVI means lowering the toxicity of Cr species. It was demonstrated that activated carbon modified with magnetite, chitosan, and tannic acid exhibited superior comprehensive performance that could be repeatedly used over a wide pH range as compared to the parent activated carbon.
Keywords: Chromium removal, Additive modification, Removal over wide pH
Graphical abstract
Highlights
-
•
Modified activated carbons showed efficient CrVI removal under various conditions.
-
•
The presence of chitosan enhanced the adsorption capacity especially at neutral to basic pH.
-
•
Tannic acid promoted the reductive adsorption of CrVI and improved the reusability.
1. Introduction
Hexavalent chromium (CrVI) pollution of water bodies is a serious environmental and public health issue, especially in underdeveloped nations, where industrial controls may be less strict. The tannery sector is one of the main sources of CrVI contamination, owing to the substantial use of Cr compounds in leather processing. Chromium compounds are used in tanning to stabilize animal hides, making them more durable and less susceptible to decomposition. However, this process generates significant amounts of wastewater containing high levels of CrVI, which, if not properly treated, can be discharged into water bodies, leading to severe environmental and health consequences. CrVI is highly toxic, carcinogenic, and poses risks such as kidney and liver damage, reproductive issues, and DNA damage when present in drinking water sources [1]. Hence, the development of efficient, affordable, and scalable adsorbents is vital, because conventional techniques for eliminating CrVI from wastewater are expensive and ineffective. Traditional methods for CrVI removal, such as chemical precipitation, ion exchange, membrane filtration, and electrochemical reduction, often come with significant drawbacks [[2], [3], [4]]. Chemical precipitation generates large volumes of sludge that requires further treatment and disposal, in addition to operational costs and environmental concerns. While ion exchange and membrane filtration processes are effective, they are typically cost-prohibitive for large-scale applications, especially in developing countries. Electrochemical reduction methods, however, require high energy inputs and sophisticated equipment, making them impractical for widespread use in resource-limited settings.
Adsorption is a highly attractive alternative because of its simplicity, cost-effectiveness, and versatility. The adsorption process involves the adhesion of CrVI ions onto the surface of an adsorbent material, which can then be separated from the treated water. This method offers several advantages such as cost-effectiveness, high efficiency, simplicity in operation, scalability, and environmental friendliness [5]. Given these advantages, adsorption appears to be one of the best options for treating CrVI contamination, particularly in the context of developing countries. Activated carbon (AC) is a widely used adsorbent due to its high surface area, porosity, and ability to adsorb a variety of contaminants [6,7]. These characteristics make AC highly effective in removing pollutants from water, including heavy metals, such as CrVI. The high surface area provides ample sites for contaminant binding, whereas the porous structure facilitates the diffusion of contaminants into the adsorbent material. Additionally, AC is abundant and can be produced from various low-cost, locally available biomass sources, making it an ideal candidate for water treatment in developing countries, where financial and technological resources may be limited. However, AC alone may not provide sufficient removal efficiency for CrVI, owing to the specific chemical properties of CrVI and its interaction with the carbon surface. Additionally, separating AC from treated water can be challenging, making recovery and reuse difficult, and potentially reducing the cost-effectiveness of the treatment process.
To address these challenges, the present study aimed to enhance the performance of AC by combining it with magnetic chitosan and tannic acid. The incorporation of magnetic-chitosan into the composite material offers a significant advantage: it allows for easy separation of the adsorbent from aqueous solutions using an external magnetic field [8]. Magnetic-chitosan was created by embedding magnetic nanoparticles within a chitosan matrix, providing the composite material with magnetic properties. This facilitates the quick and efficient recovery of the adsorbent after the treatment process, enabling repeated use and reducing operational costs. The amino groups of chitosan can react to form a complex with Cr, making it suitable for use as an adsorbent for Cr. Tannic acid, a naturally occurring polyphenol, is included in the composite to enhance the adsorption capacity and stability of the material [9,10]. Tannic acid has a strong affinity for heavy metals because of its multiple hydroxyl and carboxyl groups, which can form stable complexes with metal ions such as CrVI. By integrating tannic acid into the composite, the number of adsorption sites for CrVI increased, leading to improved removal efficiency. Furthermore, tannic acid can enhance the stability of the adsorbent, making it more durable and effective over multiple cycles of use [11].
Previous studies have shown the potential of composite materials to improve adsorption efficiency. For example, Li et al. developed a magnetic ZnFe2O4/activated carbon composite that demonstrated high adsorption capacities for antibiotics from aqueous solutions [9]. The addition of magnetic components enabled the simple recovery of the adsorbent using a magnetic field. However, their research did not include comprehensive environmental testing to evaluate the performance of the composite under various conditions, such as changes in pH, temperature, and the presence of competing ions, which are essential for practical applications. Similarly, Zheng et al. explored a metal-organic framework (MOF) for CrVI removal, emphasizing enhanced hydrogen bond interactions for increased efficiency and stability. Their work highlighted the high selectivity and efficiency of MOFs in adsorbing CrVI. However, this study also highlighted the need for a detailed analysis under various environmental conditions. Additionally, long-term stability assessments were not conducted, leaving questions about the MOF's durability and practicality for real-world applications [12].
By developing a composite adsorbent that leverages the strengths of AC, magnetic chitosan, and tannic acid, this project aims to produce a high-performance material for CrVI removal from tannery wastewater. This approach addresses the gaps identified in previous studies and leverages innovative material designs to provide a sustainable and cost-effective solution for water treatment in developing countries. The anticipated outcomes include an enhanced adsorption capacity, ease of recovery, robust performance under diverse conditions, and long-term environmental and economic benefits.
2. Experimental
All materials used here, chitosan, commercial activated carbon, FeCl2⋅4H2O, FeCl3⋅6H2O, a commercial mixed alcohol solution (ethanol 85 %, methanol 5 % and n-propyl alcohol 10 %), potassium tripolyphosphate (KTPP), potassium dichromate (K2Cr2O7), tannic acid (C76H52O46), sodium hydroxide (NaOH), hydrochloric acid (HCl, 37 %), acetic acid (CH3COOH, 50 %), hydroxylamine, phenanthroline (C12H8N2⋅H2O), potassium permanganate (KMnO4), disodium dihydrogen ethylenediaminetetraacetate (Na2EDTA), 1,5-dyphenilcarbazide (C13H14N4O), sulfuric acid (H2SO4, 98 %), urea, acetone, ammonia solution, and hydrogen peroxide (H2O2, 30 %), were an analytical grade obtained from Fujifilm Wako Pure Chemical Corporation, Osaka, and were used without further purification.
Magnetite nanoparticles were synthesized using a conventional co-precipitation method [13]. Briefly, FeCl3 and FeCl2 were dissolved in deionized water (30 ml) at 318 K to obtain an orange solution with FeCl3 and FeCl2 concentrations of 315 and 157 mM, respectively. Then, 4 ml of a 28 % NH3 solution was added dropwise to the orange solution at 353 K under gentle shaking to induce co-precipitation. The mixture was then aged for 12 h at the same temperature. The precipitate was filtered and washed several times with DI water. The resulting powdered products (Fe3O4, magnetite) were stored in ethanol before use.
The modification of activated carbon with magnetite nanoparticles, chitosan, and tannic acid was carried out using the following procedure. Initially, the activated carbon was dispersed in a solution (30 ml) containing 157 mM FeCl2 and 315 mM FeCl3, and left for an hour. Then, 4 ml of a 28 % NH3 solution was added to the mixture to produce magnetite nanoparticles and stirred for another hour. Subsequently, 1 % chitosan solution in 0.3 % acetic acid (100 ml) was added, and the mixture was stirred for an hour before the addition of 0.1 % KTPP solution (40 ml), followed by further stirring for 24 h, where KTPP acted as a crosslinker for chitosan chains. After the aging process was complete, the mixture was neutralized by washing with deionized water and dried in a vacuum dryer at 378 K. However, in the case of activated carbon with tannic acid, prior to the procedures described above, modification with tannic acid was conducted as follows [14]. Activated carbon (1 g) was oxidized by immersion in 30 % H2O2 (100 ml) for 24 h at ambient temperature, followed by washing with DI water and drying in a vacuum oven at 378 K for 12 h. Then, 1 g of oxidized activated carbon was mixed with 100 ml of 85 mg/L tannic acid and the mixture was allowed to stand for 24 h at ambient temperature. The solid was recovered via filtration and dried in a vacuum oven at 378 K. The composition of the modified activated carbon is listed in Table 1.
Table 1.
Composition, specific surface area, average pore size, and Fe leakage percentage of the samples used for CrVI removal.
| Sample | Weight ratio of components |
SSA (m2/g) | Fe leakage (%) | |||
|---|---|---|---|---|---|---|
| Activated carbon (A) | Magnetite (M) | Chitosan (C) | Tannic acid (T) | |||
| A | 1 | 974 | ||||
| MA | 1 | 1 | 568 | 0 | ||
| CMA | 1 | 1 | 1 | 869 | 6 | |
| CMAT | 1 | 1 | 1 | 0.009 | 688 | 10 |
| AT | 1 | 0.009 | 697 | |||
| CM | 1 | 1 | 63 | 9 | ||
The prepared samples were characterized using a Fourier-transform infrared (FTIR) spectrometer, Raman spectrometer, vibrating sample magnetometer (VSM), and X-ray diffractometer with Cu-Kα radiation. The surface morphologies of the samples were observed using FE–FE-SEM. Specific surface area was determined with a BET surface area measurement system by N2 adsorption.
Adsorption performance was evaluated at various pH values and times. The pH was adjusted by dropwisely adding 0.1 M HCl or NaOH. 0.5 g of adsorbent (A, MA, CMA, CMAT) was added to 100 ml of 100 ppm CrVI (K2Cr2O7) and gently shaken at 298 K. A small portion of this solution was collected from the reactor at regular intervals. After filtering the solid, the quantitative analysis of chromium after adsorption was performed using the diphenyl carbazide method. Absorbance was measured at 540 nm using a UV-VIS spectrophotometer. Compared with a calibration curve prepared with standard solutions in advance, the concentration of CrVI in the solution could be estimated.
3. Results and discussion
To achieve magnetic separation of the activated carbon-based adsorbent after adsorption, magnetite nanoparticles were deposited on activated carbon using the coprecipitation method. Fig. 1 shows the XRD pattern (a) and magnetization curve (b) of magnetite nanoparticles precipitated in a mixed solution of FeCl3 and FeCl2. As shown in Fig. 1a, several diffraction lines assigned to Fe3O4 (JCPDS 01-088-0315) appeared [13,15], suggesting the formation of magnetite nanoparticles with moderate crystallinity. The crystallite size calculated for the (311) diffraction line using the Scherrer equation was 5.5 nm. In fact, a number of magnetite nanoparticles less than 10 nm was observed in the TEM image of CMAT (Fig. 1S). The magnetization curve (Fig. 1b) shows typical superparamagnetic properties frequently seen in nano-sized particles of ferromagnetic materials, that is, smaller saturated magnetization (82 emu/g) than bulk Fe3O4 and less residual magnetizations [16]. Consequently, it was confirmed that magnetite nanoparticles formed through coprecipitation possessed sufficient magnetization, which is capable of magnetic separation of activated carbon.
Fig. 1.
(a) X-ray diffraction pattern and (b) magnetization curve of magnetite nanoparticles (0.1 g).
Because the adsorption performance of activated carbon is improved by modification with several other components, chitosan and/or tannic acid were hybridized in addition to magnetite, and the performance was compared with that of bare active carbon. As shown in Table 1, the samples containing multiple components were expressed by taking the initial letters of the components (activated carbon: A, magnetite: M, chitosan: C, tannic acid: T) contained in them. For example, activated carbon modified with magnetite and chitosan is referred to as CMA. The specific surface areas (SSA) of the samples estimated by N2 gas adsorption at 77 K are summarized in Table 1. The N2 adsorption-desorption isotherms for all samples (Fig. 2S) were classified as type I in the IUPAC classification, implying the existence of micropores. The behavior has been frequently reported for various activated carbons with different origins [7,17]. As expected, the pristine activated carbon (A) had the maximum SSA and the modification with magnetite, chitosan and tannic acid reduced the SSA. However, the SSA reduction after the modification was not significant, especially for CMA (869 m2/g) and CMAT (669 m2/g), even though the SSA of CM was very small (63 m2/g) and a large quantity of additional components (C or M) was present (weight ratio: A:C or M = 1:1)).
Fig. 2.
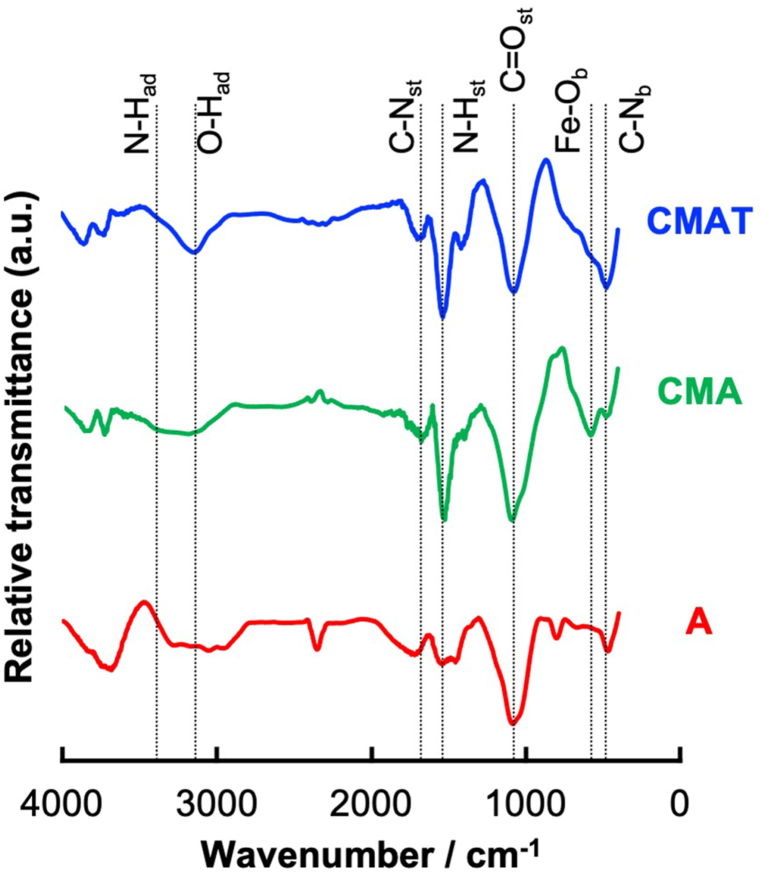
FT-IR spectra of activated carbon (A), CMA, and CMAT. The subscripts of the chemical bonds indicate the vibration modes: st, stretching; ad, angular deformation; and b, bending.
Fig. 2 shows the FT-IR transmission spectra of several samples containing multiple components (CMA and CMAT) with activated carbon (A). As a result of magnetite deposition on A, a broad band assigned to Fe–O bending (Fe3O4) appears at approximately 600 cm−1. Additionally, CMA possess the peaks based on the existence of amino groups in chitosan (3200-3400 cm−1 for angular deformation and 1560 cm−1 for stretching of N–H) [8,18]. The relative sharp band assigned to phenolic OH groups (OHad) was observed for CMAT owing to the presence of tannic acid.
Fig. 3 shows the SEM images of the synthesized powders of (a) pristine activated carbon (A), (b) magnetite (M), (c) CMA, (d) AT, and (e) CMAT at two different magnifications. As the crystallite size of magnetite was estimated to be approximately 5.5 nm from the XRD pattern (Fig. 1a), the magnetite particles in (b) seem to aggregate with each other to form large secondary particles. After the deposition of magnetite and chitosan (CMA), the active carbon flakes were surrounded by magnetite aggregates and chitosan (Fig. 3c). In contrast, the pleated surface of activated carbon disappeared and became smooth after interacting with tannic acid (Fig. 3d AT). Because activated carbon pre-oxidized with H2O2 would have enhanced reactivity owing to the increased population of phenolic groups, tannic acid could homogeneously cover the surface of activated carbon through dehydration condensation between the phenolic groups, resulting in the formation of a relatively flat surface. The surface area of activated carbon was significantly reduced by modification with tannic acid, as shown in Table 1. The addition of magnetite and chitosan to AT caused the precipitation of aggregated magnetite and chitosan on the smooth surface of AT (Fig. 3e, CMAT), similar to CMA.
Fig. 3.
SEM images of (a) activated carbon (A), (b) magnetite (M), (b) MA, (c) CMA, (d) AT, and (e) CMAT.
The coexistence of magnetite nanoparticles in functional materials, such as adsorbents and catalysts, that are utilized in solutions can realize magnetic separation under a magnetic field from external magnets, inducing repeated use of the materials after an appropriate regeneration process. However, the adsorbents for CrVI are maximized in acidic solutions, and the dissolution of magnetite may reduce the recovery efficiency of the absorbent during repeated procedures. The coating of magnetite particles with chemically robust layers, such as silica, is frequently accomplished to avoid this drawback. To determine how the magnetite nanoparticles in our adsorbents dissolved in an acidic solution, leaching tests of iron in the adsorbents were conducted as follows. The samples (0.5 g) were dispersed in a diluted HCl solution (100 mL) at pH 2, and the mixtures were stirred for 24 h at ambient temperature. The supernatants of the solutions were collected by centrifugation and their iron concentrations were determined using the 1,10-phonenantrolline method. The leakage percentage of Fe is listed in Table 1. The magnetite on activated carbon (MA) did not dissolve in the solution, but the samples containing chitosan (CM, CMA, and CMAT) were allowed to release a small amount of iron (9, 6, and 10 %, respectively). This indicates that the presence of chitosan significantly enhanced the detachment of magnetite from the absorbents. The solubility of chitosan has been reported to be pH-dependent and increases with decreasing pH [15]. Accordingly, chitosan dissolves easily in acidic solutions. Because the synthetic route of the adsorbent, which includes both magnetite and chitosan, is initiated by the hybridization of magnetite with chitosan, most magnetite nanoparticles can be deposited on chitosan. As a result, magnetite appears to be eliminated with chitosan rather than chemical dissolution at low pH. During the crosslinking of chitosan with KTPP, negatively charged phosphate groups (-HPO3-) at both ends of a KTTP molecule interacted with the positively charged amino groups (-NH3+) of chitosan to form a three-dimensional network of chitosan chains, and magnetite nanoparticles were incorporated into the mesh structure. Therefore, chitosan would be easily released into the acidic solution accompanied by magnetite nanoparticles without dissolving to Fe2+ and/or Fe3+ because distinct chemical bonds between chitosan and activated carbon were not produced. This was also confirmed by the fact that no iron dissolution (i.e., no dissolved Fe2+/3+) was detected in the MA-containing solution. As the presence of chitosan might improve the adsorption performance for CrVI despite the detachment of a small part of magnetite, adsorption tests of the samples fabricated for CrVI were carried out under several pH conditions.
Fig. 4a shows the adsorption transients of activated carbon (A) and its derivatives (MA, CMA, and CMAT) for 100 ppm CrVI at pH 2 and 298 K, where the dosage of adsorbates was set to be 0.5 g. As expected, the adsorption percentage of CrVI increased drastically and reached 100 % within 60 min, except for MA, which had the smallest specific surface area among the samples. The rapid adsorption in acidic solutions could be due to the abundant positively charged chemical groups (-NH3+, –OH2+, etc.) on the surface of the activated carbon and/or the modifiers, which can form tight electrostatic interactions with chromate anions (Cr2O72−, HCrO4−). Fig. 4b shows the pH dependence of the percentage removal of CrVI. At pH 4, the highest Cr removal rate was achieved using A (98 %), followed by CMA (53 %), MA (34 %), and CMAT (17 %). According to Table 1, as CMA includes only half the weight of activated carbon compared to A, the adsorption capacities per unit weight of activated carbon of A and CMA are almost comparable. Interestingly, the adsorption % of CMA exceeded that of A above pH 6. The highest removal efficiency of CMA may originate from the presence of chitosan with numerous amino groups attracting these anions, which is the dominant form of CrVI at neutral to weakly alkaline pH. Because the dissolution of chitosan from CMA or CMAT under acidic conditions was suppressed at pH 6 and 8, a desirable effect of chitosan addition appeared. Even though tannic acid with many phenolic groups would improve CrVI removal [18], the expected effect could not be found, probably because of the relatively small SSA of CMAT (688 m2/g) compared with that of A (974 m2/g) and CMA (869 m2/g). Consequently, it was demonstrated that the modification of activated carbon with chitosan is effective in enhancing the adsorption amount of CrVI, especially in the neutral to weakly alkaline pH region. Compared with other studies, the adsorption capacity of this material is still superior and promising, as shown in Table 2.
Fig. 4.
(a) Adsorption transients of activated carbon (A), MA, CMA, and CMAT for 100 ppm CrVI at pH 2 and 298 K and (b) their CrVI removal (%) at various pH values after 24 h.
Table 2.
Cr removal comparison using different materials.
| No. | Materials | Optimum pH | Adsorption capacity (mg/g) | Contact time (min) | Reference |
|---|---|---|---|---|---|
| 1 | Chitosan–magnetic biochar (from loofah) | 3 | 28 | n.a | [8] |
| 2 | Spent tea leaves treated with ascorbic acid | 2 | 93 | n.a | [19] |
| 3 | Magnetic–activated carbon from rice husk | 2 | 10 | 120 | [15] |
| 4 | Commercial activated carbon from wood | 2 | 242 | n.a | [7] |
| 5 | CMAT | 2 | 200 | 90 | this study |
Previous studies have proven that CrVI adsorption on activated carbon occurs through several routes, including ion exchange, electrostatic adsorption, precipitation, and complexation [7,15]. Furthermore, a considerable quantity of CrVI adhering to the surface is reduced to CrIII by phenolic and carboxyl groups acting as reductants. Fig. 5 shows the Cr 2p XPS spectra of activated carbon (A) after Cr removal at pH 2, followed by thorough washing with pure water. The spectrum of A did not have any peaks assigned to Cr 2p, implying that most of the CrVI adsorbed to A was desorbed during washing with pure water, which increased the surface pH to more than 2 and induced weak interactions between CrVI and A. In contrast, the spectrum of CMA exhibited two distinct peaks of Cr 2p1/2 and 2p2/3. As a result of peak deconvolution of Cr 2p3/2 (579.9 eV for CrVI (K2Cr2O7) and 577.4 eV for CrIII (Cr (OH)3)), molar% of CrVI and CrIII was estimated to be 23 and 77 % respectively, suggesting the reductive adsorption of CrVI followed by production of Cr(OH)3. In the case of CMA, Fe2+ in magnetite may also participate in the reduction of CrVI [20]. According to the XPS spectra CMAT after adsorption at pH 2, molar% of CrIII on the adsorbent was further increased to 80 %, indicating that tannic acid contributed to the reductive adsorption of CrVI [9].
Fig. 5.
Cr 2p XPS spectra of activated carbon (A) and CMA after CrVI removal at pH 2.
To study the durability of the adsorbents, the CrVI removal (pH 2) and regeneration processes were repeated several times for A, CMA, and CMAT. The changes in the removal% of CrVI are shown in Fig. 6, where the regeneration (desorption of Cr) was conducted by immersing the spent adsorbents in a Na2EDTA solution for 24 h at room temperature because Na2EDTA has been reported to have a better ability to release Cr from adsorbent [21]. Even after three cycles, CMA and CMAT retained more than 90 % of CrVI removal. In particular, except for the initial reduction, CMAT showed almost constant CrVI removal of approximately 90 % until four cycles, and hence, more prominent adsorption stability was anticipated as compared with the tendency of CMA after the second cycle (gradual drop in removal%). In contrast, the CrVI removal% of A gradually decreased every cycle and deteriorated to 72 %, which was probably due to the reduced number of surface chemical groups contributing to the adsorption. However, the C 1s XPS analysis of activated carbon (A) (Fig. 3S) imply that the population of chemical groups (-C-O, -O-C=O, etc.) participating in the reductive adsorption of CrVI were increased after the removal. Hence, although the decreased CrVI removal% for A would be caused by irreversible adhesion of unknown contaminants, these results definitely demonstrate that modification, especially with chitosan, improves the reusability of pristine A and that the adsorbent can be used even after three cycles without serious deterioration.
Fig. 6.
Changes in CrVI removal% of activated carbon (A), CMA, and CMAT during removal and regeneration cycling.
4. Conclusions
The modification of activated carbon with several substances, including magnetite, chitosan, and tannic acid, could improve the adsorption behavior of toxic hexavalent chromium ions (CrVI) based on the desirable chemical and/or physical properties of each additive. Unsurprisingly, the presence of magnetite made magnetic separation possible despite the reduced specific surface area. Hybridization of chitosan with rich NH2 (-NH3+) groups, which act as adsorption centers for CrVI anions (Cr2O72− or HCrO4−), enhanced the reductive removal of CrVI to CrIII at pH higher than 4, where the adsorption of activated carbon itself was significantly decreased. In other words, the CrVI removal performance of activated carbon can be strengthened, especially in the weakly acidic to basic pH range. Moreover, the presence of chitosan enhanced the reusability during the CrVI removal and regeneration cycles because the chitosan coating compensated for the reduction in adsorption sites on the activated carbon, similar to the above. On the other hand, even though the addition of tannic acid with numerous hydroxyl groups reduced the specific surface area, its superior recycling ability, equivalent to that of chitosan, was confirmed at pH 2. As the hybrid materials proposed here show adsorption capacity for anions over a wide pH range, future efforts will be devoted to the reductive collection of complex anions, including noble metals, such as Ru, Ir, Pt, Au, and Pd.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Rizki Ainuna Wijaya: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Osamu Nakagoe: Writing – review & editing, Formal analysis, Data curation. Hideaki Sano: Writing – review & editing, Formal analysis, Data curation. Shuji Tanabe: Writing – review & editing, Supervision, Methodology, Investigation, Conceptualization. Kai Kamada: Writing – review & editing, Validation, Supervision, Methodology, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35557.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sawalha H., Alsharabaty R., Sarsour S., Al-Jabari M. Wastewater from leather tanning and processing in Palestine: characterization and management aspects. J. Environ. Manag. 2019;251 doi: 10.1016/j.jenvman.2019.109596. [DOI] [PubMed] [Google Scholar]
- 2.Liu M., Ma J., Lyu B., Gao D., Zhang J. Enhancement of chromium uptake in tanning process of goat garment leather using nanocomposite. J. Clean. Prod. 2016;133:487–494. doi: 10.1016/j.jclepro.2016.04.156. [DOI] [Google Scholar]
- 3.Xing X., Alharbi N.S., Ren X., Chen C. A comprehensive review on emerging natural and tailored materials for chromium-contaminated water treatment and environmental remediation. J. Environ. Chem. Eng. 2022;10 doi: 10.1016/j.jece.2022.107325. [DOI] [Google Scholar]
- 4.Nur-E-Alam M., Mia M.A.S., Ahmad F., Rahman M.M. An overview of chromium removal techniques from tannery effluent. Appl. Water Sci. 2020;10 doi: 10.1007/s13201-020-01286-0. [DOI] [Google Scholar]
- 5.Rashid R., Shafiq I., Akhter P., Iqbal M.J., Hussain M. A state-of-the-art review on wastewater treatment techniques: the effectiveness of adsorption method. Environ. Sci. Pollut. Res. Int. 2021;28:9050–9066. doi: 10.1007/s11356-021-12395-x. [DOI] [PubMed] [Google Scholar]
- 6.Lach J. Adsorption of chloramphenicol on commercial and modified activated carbons. Water. 2019;11 doi: 10.3390/w11061141. [DOI] [Google Scholar]
- 7.Wang H., Wang W., Zhou S., Gao X. Adsorption mechanism of Cr(VI) on woody-activated carbons. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao F., Cheng J., Cao W., Yang C., Chen J., Luo Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019;540:579–584. doi: 10.1016/j.jcis.2019.01.068. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X., Long W., Peng L., Xu T., He F., Tang Y., Zhang W. Reductive immobilization of Cr(VI) in contaminated water by tannic acid. Chemosphere. 2022;297 doi: 10.1016/j.chemosphere.2022.134081. [DOI] [PubMed] [Google Scholar]
- 10.Yin L., Mi N., Yao Y.-r., Li J., Zhang Y., Yang S.-g., He H., Hu X., Li S.-y., Ni L.-x. Efficient removal of Cr(VI) by tannic acid-modified FeS nanoparticles: performance and mechanisms. Water Sci. Eng. 2021;14:210–218. doi: 10.1016/j.wse.2021.08.006. [DOI] [Google Scholar]
- 11.Mim S., Hashem M.A., Maoya M. Adsorption-oxidation process for dyestuff removal from tannery wastewater. Environ. Nanotechnol. Monit. Manag. 2024;21 doi: 10.1016/j.enmm.2023.100911. [DOI] [Google Scholar]
- 12.Zheng M., Zhao X., Wang K., She Y., Gao Z. Highly efficient removal of Cr(VI) on a stable metal–organic framework based on enhanced H-bond interaction. Ind. Eng. Chem. Res. 2019;58:23330–23337. doi: 10.1021/acs.iecr.9b04598. [DOI] [Google Scholar]
- 13.Obaidat I.M., Nayek C., Manna K., Bhattacharjee G., Al-Omari I.A., Gismelseed A. Investigating exchange bias and coercivity in Fe(3)O(4)-gamma-Fe(2)O(3) core-shell nanoparticles of fixed core diameter and variable shell thicknesses. Nanomaterials. 2017;7 doi: 10.3390/nano7120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin J.F., Heng Z.W., Teoh H.C., Chong W.C., Pang Y.L. Recent development of magnetic biochar crosslinked chitosan on heavy metal removal from wastewater - modification, application and mechanism. Chemosphere. 2022;291 doi: 10.1016/j.chemosphere.2021.133035. [DOI] [PubMed] [Google Scholar]
- 15.Sinha R., Kumar R., Abhishek K., Shang J., Bhattacharya S., Sengupta S., Kumar N., Singh R.K., Mallick J., Kar M., Sharma P. Single-step synthesis of activated magnetic biochar derived from rice husk for hexavalent chromium adsorption: equilibrium mechanism, kinetics, and thermodynamics analysis. Groundwater Sustain. Dev. 2022;18 doi: 10.1016/j.gsd.2022.100796. [DOI] [Google Scholar]
- 16.Dabagh S., Haris S.A., Isfahani B.K., Ertas Y.N. Silver-decorated and silica-capped magnetite nanoparticles with effective antibacterial activity and reusability. ACS Appl. Bio Mater. 2023;6:2266–2276. doi: 10.1021/acsabm.3c00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong X., Li W., Wang K., Hu J. Study of the adsorption of Cr(VI) by tannic acid immobilised powdered activated carbon from micro-polluted water in the presence of dissolved humic acid. Bioresour. Technol. 2013;141:145–151. doi: 10.1016/j.biortech.2013.01.166. [DOI] [PubMed] [Google Scholar]
- 18.Marques Neto J.O., Bellato C.R., Silva D.C. Iron oxide/carbon nanotubes/chitosan magnetic composite film for chromium species removal. Chemosphere. 2019;218:391–401. doi: 10.1016/j.chemosphere.2018.11.080. [DOI] [PubMed] [Google Scholar]
- 19.Zaib Q., Kyung D. Optimized removal of hexavalent chromium from water using spent tea leaves treated with ascorbic acid. Sci. Rep. 2022;12:8845. doi: 10.1038/s41598-022-12787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao P., Xu J., Shi H., Du F., Du H., Li G. Simultaneous Cr(VI) reduction and Cr(III) sequestration in a wide pH range by using magnetic chitosan-based biopolymer. Int. J. Biol. Macromol. 2023;253 doi: 10.1016/j.ijbiomac.2023.127398. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H., Xiao R., Li R., Ali A., Chen A., Zhang Z. Enhanced aqueous Cr(VI) removal using chitosan-modified magnetic biochars derived from bamboo residues. Chemosphere. 2020;261 doi: 10.1016/j.chemosphere.2020.127694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.