Abstract
Background
Performing spinal anaesthesia in elderly patients with ligament calcification or hyperostosis is challenging for novice practitioners. This pilot study aimed to compare the effectiveness of mixed reality-assisted spinal puncture (MRasp) with that of landmark-guided spinal puncture (LGsp) by novice practitioners in elderly patients.
Methods
In this pilot study, 36 patients (aged ≥65 years) scheduled for elective surgery under spinal anaesthesia by anaesthesiology residents were included. Patients were randomly assigned to the MRasp group (n = 18) or the LGsp group (n = 18). The outcomes included the number of needle insertion attempts, redirection attempts, passes, the rate of successful first-attempt needle insertion, the rate of successful first needle pass, the spinal puncture time, the total procedure time, and the incidence of perioperative complications.
Results
The median number of needle insertion attempts was significantly fewer in the MRasp group than in the LGsp group (1.0 vs 2.0, P = 0.023). The proportion of patients with successful first-attempt needle insertion was 72.2% in the MRasp group and 44.4% in the LGsp group (P = 0.176). The incidence of perioperative complications did not significantly differ between the two groups.
Conclusion
This pilot study found that novice practitioners made significantly fewer needle insertion attempts in the MRasp group compared to the LGsp group when performing spinal anaesthesia on elderly patients. A future randomized controlled trial (RCT) is warranted to validate its effectiveness.
Trial Registration
This trial was registered at https://www.chictr.org.cn/showproj.html?proj=178960 (ChiCTR-IPR-2300068520). Public title: Mixed reality-assisted versus landmark-guided spinal puncture in elderly patients: a randomized controlled pilot study. Principal investigator: Lei Gao. The registration date was February 22, 2023. The date of the first participant enrolment was February 27, 2023.
Keywords: augmented reality, computer simulation, mixed reality, spinal puncture
Plain Language Summary
We developed virtual spine-presenting technology and patented optimal trajectory design technology to assist in spinal puncture and reported that the median number of needle insertion attempts was significantly fewer in the mixed reality-assisted spinal puncture group than in the landmark-guided spinal puncture group.
Introduction
In the field of anaesthesia education, the spinal puncture technique is foundational for novice anaesthetists and holds significant importance in anaesthesia medical education. Traditional landmark-guided spinal puncture (LGsp) usually uses a blind technique. For example, during lumbar puncture, the operator manually palpated Tuffier’s line (the horizontal line connecting the superior iliac crests) as a surface landmark.1 However, for elderly patients with degenerative changes such as spinal stenosis and bone hyperplasia, spinal puncture may be challenging.2,3 Additionally, operator skill variability impacts the success rate of spinal puncture. For novices, performing spinal punctures in elderly patients via blind techniques is even more challenging and risk-raising. Therefore, visualization technology to help novice practitioners master spinal puncture skills is clinically important, especially for elderly patients. Ultrasonic technology might be an option for visualization guidance. However, it is difficult for novices to determine the anatomical structure and puncture angle on the basis of two-dimensional images. In addition, it is difficult for one operator to hold the ultrasonic probe and perform spinal puncture at the same time.
Mixed reality (MR), a novel visualization technology, merges real and virtual images in a unified display. It has been used in various fields, such as medical education and intraoperative navigation. A patented MR system was developed in our previous study.4 This system converts lumbar computed tomography (CT) images into three-dimensional representations through an MR head-mounted display (HoloLens 2nd, Microsoft, USA). Novices can visualize patients’ lumbar spine structures in the MR environment by wearing an MR head-mounted display. A patented trajectory design software (RM: 2023SR0486397) was designed to generate the optimal routes, automatically avoiding obstacles from the skin to the subarachnoid space. This pilot study aimed to explore the possibility of a mixed reality-assisted spinal puncture (MRasp) approach, encompassing virtual spine presentation and an optimal trajectory design, which may help novices reduce the difficulty of puncturing elderly patients with spinal degeneration.
Material and Methods
Aim
The effectiveness of MRasp with LGsp for novice residents in elderly patients was compared in this study. The results of this study provide a basis for the sample size calculation of subsequent randomized controlled studies.
Study Design
This pilot study was approved by the Ethics Committee of Huadong Hospital affiliated to Fudan University (IRB: 20220126; Chairperson Prof Yinghao Sha; approval date, November 9, 2022), and complied with the Declaration of Helsinki. All participants provided written informed consent. Registered in the Chinese Clinical Trial Registry (ChiCTR2300068520; principal investigator: Lei Gao; registration date, February 22, 2023) and was conducted between February and April 2023 at Huadong Hospital, Fudan University, Shanghai, China. The study adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines.5
Participants
Thirty-six eligible patients who met the following specific criteria were recruited: (1) scheduled for surgery with spinal anaesthesia, (2) aged ≥65 years, (3) American Society of Anaesthesiologists (ASA) grade I to II, and (4) had available lumbar CT data from preoperative examinations. Since most patients have undergone preoperative abdominal CT to confirm the diagnosis of the primary disease before surgery (eg, for patients undergoing prostate or bladder surgery), the CT data included lumbar spine imaging and could be utilized to generate virtual spine images. Therefore, there was no need for patients to undergo another CT scan to reconstruct virtual three-dimensional (3D) spine images.
The exclusion criteria were as follows: (1) had contraindications to spinal anaesthesia (eg, coagulopathy or recent anticoagulant therapy, hypovolemia, increased intracranial pressure, nervous system diseases, infection in the puncture area, or lack of cooperation); (2) had chronic severe lumbocrural pain or a history of lumbar surgery; and (3) refused to participate in the study.
Six anaesthesiology residents (D.-F.J., L.W., H.G., M.-Q.Y., Z.-S.J., and X.D). conducted the spinal punctures. To mitigate puncture risk, an experienced anaesthesiologist (with more than ten years of LGsp expertise and more than 100 MRasp procedures annually) served as a supervisor.
Randomization and Blinding
Patients (n = 36) were randomly assigned to the MRasp (n = 18) or LGsp (n = 18) group by an independent statistician Z.-C.J. via computer-generated random numbers. This approach ensured a balanced allocation of residents and patients in both arms. Y.-D. X was in charge of enrolling participants, and L.G. was in charge of assigning participants to the intervention. Concealed allocation was maintained via sequential numbers and was maintained by a study nurse who was not involved in data collection before patient recruitment. The sealed opaque envelopes were opened by the researcher after patient selection and consent. On the basis of the allocation, the operators performed spinal punctures on six patients each (three per group), maintaining randomization integrity.
This was a single-blind study. The operator was aware of the group allocation when wearing MR glasses in the MRasp group. Throughout the procedure, only patients remained blinded to the group allocation.
Study Interventions
Patients were positioned in a lateral decubitus posture, and their arms were wrapped around the knees during the procedures. Standard monitors (noninvasive blood pressure, 5-lead electrocardiogram, and pulse oximetry) and a 2 L/min mask oxygen flow were used. Intravenous access was secured, and 1–2 mg of intravenous midazolam was provided as needed for anxiolysis in all patients.6
Aseptic techniques were used strictly for both groups. A 25G/11.5 mm-gauge CSE kit was used for spinal anaesthesia (Yixin Medical, Shanghai, China). Upon cerebrospinal fluid (CSF) outflow detection, 0.5% ropivacaine (12–18 mg) was injected.
In the MRasp group, the procedure comprised seven steps:
Virtual 3D image reconstruction and display - This step was conducted by the researcher the day before surgery. The key technology is shown in the “Development of MRasp Technology” section below. Each virtual image reconstruction and uploading process lasted approximately thirty minutes.
Learning the MRasp operating system - Six anaesthesiology residents, selected as the operators in this pilot study, needed to learn the use of the MRasp operating system before spinal puncturing. They needed to adapt to the visual environment of augmented reality, follow the prompts provided by the interface in the HoloLens, and select the specified virtual model for observation. The key operational steps for gesture control and trajectory design (as explained below in the “Development of MRasp Technology” section) enabled autonomous manipulation of virtual images and observation of the automatically designed trajectory. The prompts provided by the interface in the HoloLens were easy to learn. The researcher assisted the novice students in this step to help them master the operating skills. Each novice took approximately thirty minutes to master the skills with the assistance of the researcher.
Previewing - The operator reviewed the 3D image and optimal trajectory in the HoloLens, assessing the entry point, angle, and depth before puncture. This process took approximately 2–5 minutes for each case.
Patient positioning and video recording - The patient was positioned laterally, the puncture kit was opened, and video recording was initiated.
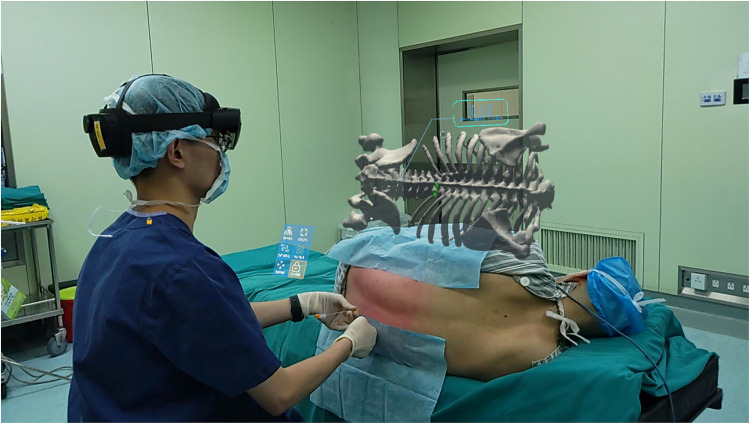
Virtual image adjustment - The operator put on the HoloLens again, and aligned the virtual image right above the patient (Figure 1).
Disinfection and local anaesthesia - The operator wore aseptic gloves, disinfected the puncture area, draped the patient with sterile surgical towels, and injected local anaesthesia.
Needle insertion - The operator executed the puncture following trajectory guidance, concluding video recording upon CSF outflow.
Figure 1.
MR visual presentation at puncture time with MRasp technology. The virtual image was fixed above the real body in space, and the trajectory was displayed on the image.
In the LGsp group, the procedure consisted of three steps:
Patient positioning and video recording - The mirroring step (4) in the MRasp group.
Disinfection and local anaesthesia - mirroring step (6) in the MRasp group.
Needle insertion - The operator executed the spinal puncture using the blind technique via the median approach. Video recording concluded upon CSF outflow observation.
Development of MRasp Technology
MR Hardware
This study utilized the 2nd Gen HoloLens, which is a Microsoft-developed MR head-mounted display.7 By incorporating 3D diffraction display technology, HoloLens projects virtual object images into human eyes through transparent light-guided lenses. This process combines virtual images with real environmental light, creating a comprehensive visual experience.8,9
Virtual 3D Image Reconstruction and Display
Preoperative CT data were acquired via a Siemens Somatom Force scanner (tube voltage, 120 kV; tube voltage care dose, 4D; slice thickness, 1 mm; helical mode; Siemens Healthcare, Erlangen, Germany). The patented medical MR system (patent application no. 201910767511.9) transformed digital imaging and communications in medicine (DICOM)-formatted images into holographic lumbar spine images through stitching, stripping, surface reduction, and synthesis. Virtual 3D images were stored and displayed in the system, with the data synchronized to the HoloLens for operator viewing and manipulation.4
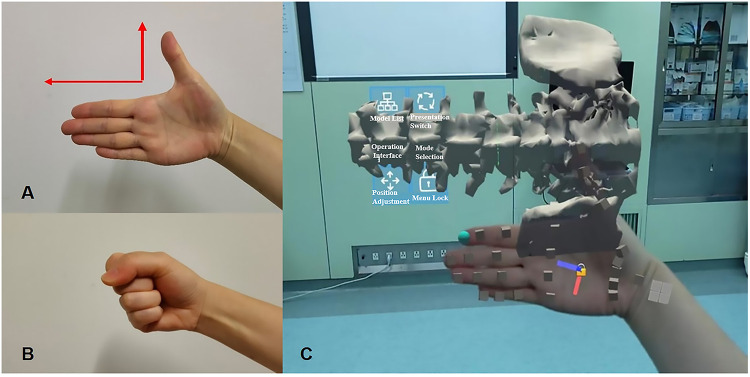
Gesture control software (RM: 2023SR0486398) was developed, patented, and employed for swift manipulation of virtual images. Two premastered gestures facilitated this process. First, the direction gesture involved an open palm, and the superior direction of the patient’s lumbar spine was determined on the basis of the position of the index finger (Figure 2A). For example, when the operator raised the right palm, the image was consistent with the direction of the index finger and presented as the left lateral position (Figure 2C). When the operator changed the right palm to the left palm, the virtual image was synchronously converted to the right lateral position. The image was then moved with the operator’s palm until it was positioned above the patient’s body (Figure 1). Second, the lock/unlock gesture is accomplished by making two fists in two seconds (Figure 2B), swiftly immobilizing or releasing the virtual image, allowing hands-free observation during spinal puncture.
Figure 2.
The gesture software used to convert and immobilize the virtual image. (A) Direction gesture. Palm opened and inwards. The direction of the index finger represented the superior direction of the patient’s real lumbar spine. The HoloLens quickly recognized the gesture and automatically adjusted the superior direction of the virtual spine image to be consistent with the direction of the index finger. The virtual image could follow the movement of the palm close to the patient’s body. (B) Lock/unlock gesture. Make two fists in two seconds. The HoloLens automatically fixed the virtual image, which would no longer move with the palm. (C) The visual effect of the image conversion functions conducted by the gestured software in the HoloLens.
Puncture Trajectory Design
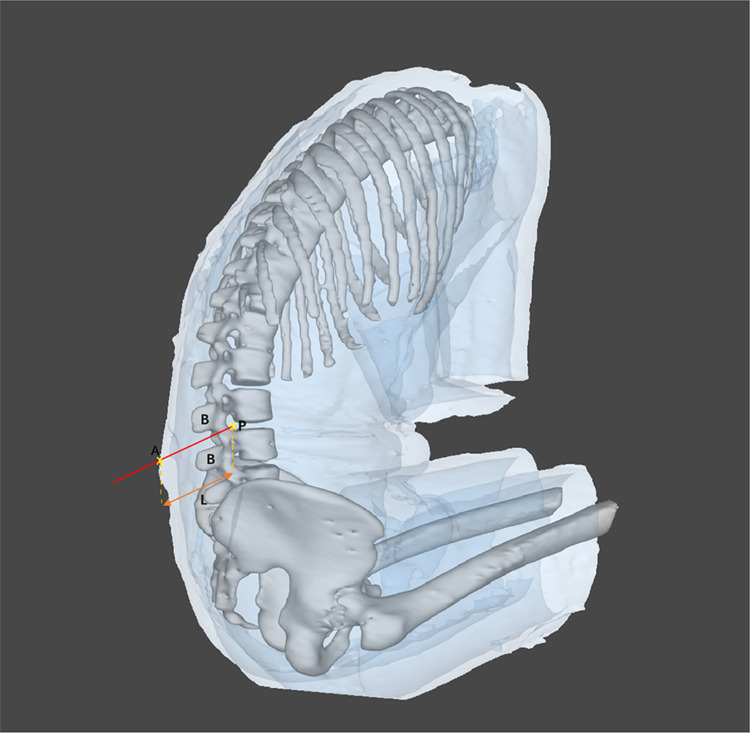
In this study, Huadong MRsp pathway software (RM: 2023SR0486397) was developed and copyrighted to autonomously design an optimal trajectory for assisting in spinal puncture, as illustrated in Figure 3. Following 3D lumbar spine image reconstruction, the subarachnoid space at the L3-L4 intervertebral space was pinpointed as the target point P. The operator identified the corresponding skin area in the HoloLens as entry zone A. All bony obstacles on the trajectory from A to P were designated obstacle B. The algorithm computed the shortest trajectory between A and P, avoiding obstacle B. The operator visualized the trajectory, needle insertion depth (L), and trajectory angle in the HoloLens (Figure 4).
Figure 3.
Automatic trajectory design of MRasp. The optimal spinal puncture trajectory for MRasp was calculated automatically by using the Reacool medical mixed reality system and Huadong MRsp pathway algorithm. A: The skin zone corresponds to the spinous process of L3-L4 (entry zone). P: The subarachnoid space corresponding to the intervertebral space of L3-L4 (target point). B: All bony obstacles, such as the vertebral lamina, pedicle, articular process, spinous process, and irregular osteoproliferation, on the trajectory from A to P. L: The shortest distance between zone A and point P.
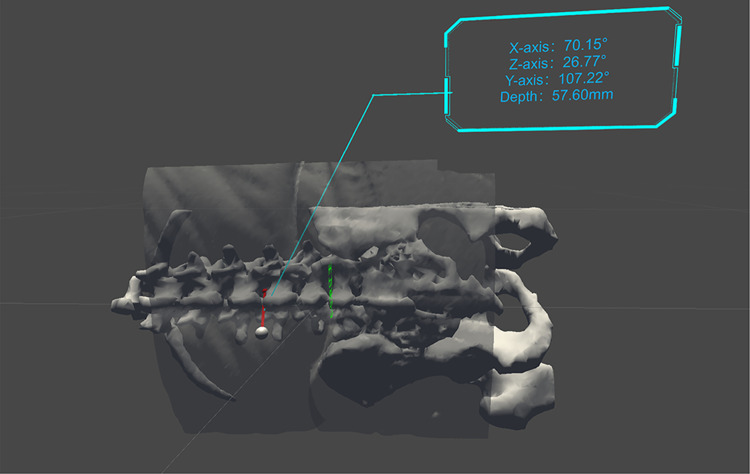
Figure 4.
Three-dimensional visual effects of the MRasp. Relationships between the optimal spinal puncture trajectory and the 3D virtual lumbar spine. The upper right corners of both A and B display the angle between the trajectory and the X, Y, and Z axes, respectively, and the needle insertion depth.
Study Outcomes
All the procedures were recorded on video. Two researchers documented the following observational indicators after surgery:
Number of needle insertion attempts: The number of separate skin punctures performed by a needle was counted.
Number of redirection attempts: number of needle redirections through the soft tissue.
Number of passes: sum of needle insertion attempts and redirection attempts.
Rate of successful first-attempt needle insertion: the rate of success if CSF outflows through a single puncture point, allowing needle redirection through the soft tissue.
Rate of successful first needle pass: the rate of success if initial insertion yields CSF outflow without redirection.
Spinal puncture time: Duration from first needle insertion to CSF outflow.
Total procedure time: Time from HoloLens-donning to CSF outflow for the MRasp group (including steps 5–7 in the MRasp group); time from glove-wearing to CSF outflow for the LGsp group (including steps 2–3 in the LGsp group).
Change to the paramedian approach: A landmark-guided paramedian approach was implemented if three insertion attempts failed for the MRasp group or the LGsp group via the median approach.
Selection of another lumbar segment (L2-L3): If the paramedian approach failed.
Conversion to the supervisor: If the resident failed the L2-L3 puncture, the supervisor would take over the procedure.
Conversion to general anaesthesia: Implemented if the supervisor failed.
The following vital signs were recorded: mean arterial pressure, heart rate, and oxygen saturation during puncture.
Procedural adverse reactions: Recorded instances, including severe pain, a numerical rating scale (NRS) score ≥ 7, radiating pain, and subcutaneous hematomas (diameter > 10 mm).
Postoperative complications: Patients were assessed by a nurse anaesthetist, including headache, intraspinal hematoma, nerve damage, paraplegia, and persistent pain at the puncture site on the first day after surgery and the discharge day.
Statistical Analysis
The data were analysed with SPSS 26.0 (IBM Corporation, NY, USA) following the intention-to-treat principle. The Shapiro–Wilk test was used to assess the normality of continuous data. Normally distributed data (means ± SDs) were compared with an independent sample t test, whereas nonnormally distributed data (medians [interquartile ranges]) were compared with the Mann–Whitney U-test. Nonnormally distributed data were reported with minimum and maximum values for the number of passes and attempts, time variables, pain, and conversion to alternative solutions. Categorical data [n (%)] were compared via the X2 test or Fisher’s exact test. A two-tailed P value <0.05 was considered to indicate statistical significance.
Results
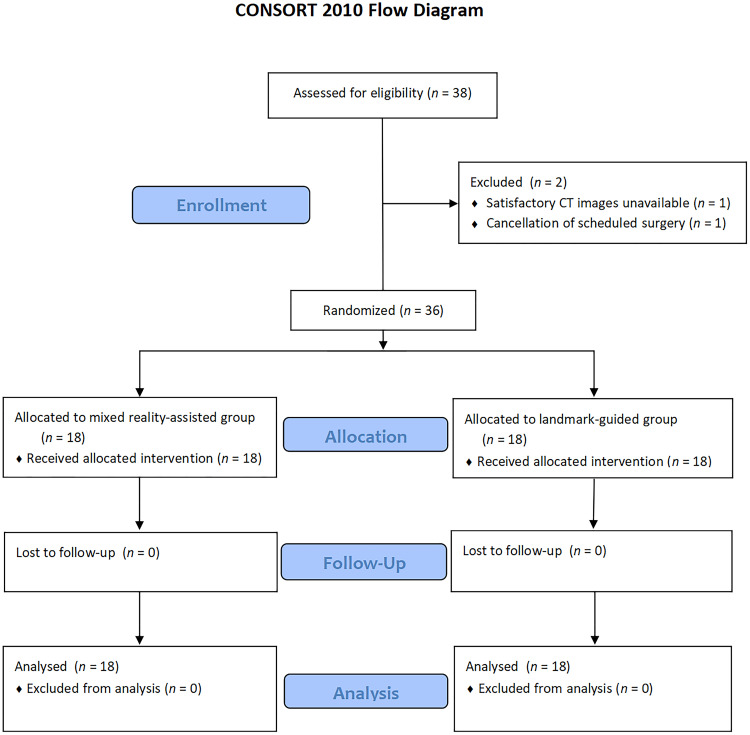
After being admitted to the hospital and completing the preoperative examination, 38 elderly patients were recruited and assessed for eligibility. Among them, 36 patients were randomized and completed the study from February to April 2023 (Figure 5). Two patients were excluded because of the unavailability of satisfactory CT images (n = 1) or cancelled surgery (n = 1). All follow-up visits were conducted on each patient’s first day after surgery and on the discharge day.
Figure 5.
Flow diagram of the study according to the CONSORT 2010 guidelines.
The baseline characteristics were comparable between the MRasp and LGsp groups (Table 1). The outcomes and alternatives are presented in Table 2. The median number of needle insertion attempts was significantly lower in the MRasp group than in the LGsp group (1.0 vs 2.0, P < 0.05). The first-attempt needle insertion success rate was 13 (72.2%) in the MRasp group and 8 (44.4%) in the LGsp group (P =0.176). The initial data suggested lower redirection attempts, passes, successful first needle pass rate, spinal puncture time, and total procedure time in MRasp group, but these differences were not statistically significant. There was no significant difference in the use of alternatives. Among LGsp patients, 33.3% required paramedian approach conversion, whereas 5.6% of MRasp patients required paramedian approach conversion. L2-L3 segment change occurred in 16.7% (LGsp) of patients and none (MRasp), and anaesthesiologists changed in 11.1% (LGsp) of patients and none (MRasp) of patients. No patients required general anaesthesia conversion.
Table 1.
Baseline Patient Characteristics
| MRasp group (n = 18) |
LGsp group (n = 18) |
P value | |
|---|---|---|---|
| Age (y) | 75.00 [70.75 to 78.25] | 72.50 [67.00 to 77.00] | 0.132b |
| Height (cm) | 164.28 ± 7.54 | 164.50 ± 8.12 | 0.933a |
| Weight (kg) | 63.94 ± 7.51 | 63.78 ± 8.68 | 0.951a |
| BMI (kg/m2) | 23.74 [21.46 to 25.99] | 22.95 [22.38 to 24.53] | 0.602b |
| Sex (male/female) | 12/6 | 9/9 | 0.310c |
| ASA Classification | 0.603c | ||
| I | 1 (5.56%) | 3 (16.67%) | |
| II | 17 (94.44%) | 15 (83.33%) | |
| Platelet (×109/L) | 220.39 ± 55.73 | 217.67 ± 53.55 | 0.692a |
| PT (s) | 11.47 ± 1.20 | 11.13 ± 0.84 | 0.332a |
| APTT (s) | 32.05 [28.80 to 33.03] | 30.80 [28.18 to 33.73] | 0.669b |
| MBP (mmHg) | 94.28 ± 14.63 | 101.94 ± 15.32 | 0.134a |
| HR (bmp) | 70.00 ± 10.28 | 74.17 ± 13.61 | 0.307a |
| SPO2 (%) | 98.00 [97.00 to 99.25] | 99.00 [98.00 to 100.00] | 0.272b |
Notes: Data are presented as the mean ± standard deviation, median [interquartile range], or n (%).
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; PT, prothrombin time; APTT, activated partial thromboplastin time; MBP, mean arterial pressure; HR, heart rate; SPO2, pulse oxygen saturation. aIndependent-sample t test; bMann‒Whitney U-test; cFisher’s exact test.
Table 2.
Outcomes and Alternatives of Spinal Puncture
| MRasp group (n = 18) |
LGsp group (n = 18) |
P value | |
|---|---|---|---|
| Number of needle insertion attempts | 1.00 [1.00 to 2.00] | 2.00 [1.00 to 2.25] | 0.023a |
| Number of redirection attempts | 2.00 [0.00 to 3.00] | 3.00 [0.00 to 8.00] | 0.111a |
| Number of passes | 3.00 [1.00 to 4.25] | 5.00 [1.00 to 10.25] | 0.103a |
| Rate of successful first-attempt needle insertion, n | 13 (72.22%) | 8 (44.44%) | 0.176b |
| Rate of successful first needle pass, n | 8 (44.44%) | 5 (27.78%) | 0.489b |
| Spinal puncture time (s) | 105.50 [44.75 to 166.50] | 160.00 [54.00 to 332.50] | 0.261a |
| Total procedure time (s) | 255.00 [194.25 to 368.25] | 316.00 [239.75 to 415.00] | 0.195a |
| Change to paramedian approach, n | 1 (5.56%) | 6 (33.33%) | 0.088b |
| Change lumbar segment, n | 0 (0.00%) | 3 (16.67%) | 0.229b |
| Conversion to the supervisor, n | 0 (0.00%) | 2 (11.11%) | 0.486b |
| Conversion to general anesthesia, n | 0 (0.00%) | 0 (0.00%) | – |
Notes: Data are presented as the median [interquartile range] or n (%). aMann–Whitney U-test; bFisher’s exact test.
Table 3 shows the perioperative complications for both groups. In the LGsp group, one patient experienced radiating pain during the puncture. No patient in either group experienced severe pain during the puncture. In all patients followed up for 24 hours after surgery and on the discharge day, there was no occurrence of subcutaneous hematomas, headache with low cranial pressure, intraspinal hematoma, nerve damage, or paraplegia.
Table 3.
Perioperative Complications
| MRasp group (n = 18) |
LGsp group (n = 18) |
P valuea | |
|---|---|---|---|
| Severe pain during puncturing, n | 0 (0.00%) | 0 (0.00%) | – |
| Radiates pain during puncturing, n | 0 (0.00%) | 1 (5.56%) | 1.000 |
| Subcutaneous hematomas, n | 0 (0.00%) | 0 (0.00%) | – |
| Headache with low cranial pressure, n | 0 (0.00%) | 0 (0.00%) | – |
| Intraspinal hematoma, n | 0 (0.00%) | 0 (0.00%) | – |
| Nerve damage, n | 0 (0.00%) | 0 (0.00%) | – |
| Paraplegia, n | 0 (0.00%) | 0 (0.00%) | – |
Notes: Data are presented as n (%). aFisher’s exact test.
Discussion
The success rate of lumbar puncture in elderly patients is usually influenced by two main factors: the operator’s skills and the degree to which the spine degenerates.10 Novice trainees often face a long learning curve when blind puncture techniques are used to master lumbar puncture.11 When faced with difficult punctures in elderly patients, repeated attempts may cause soft tissue damage. The traditional teaching method often involves trainees practising on simulated models, which only helps students understand the puncture steps. However, the spinal structure of each patient is different in detail, while the simulated model’s spinal structure remains immutable Therefore, this pilot study attempted to use MRasp in elderly patients during their clinical practice. The characteristic of MRasp is that each reconstructed image and the guiding trajectory reflect the real spinal structure of a particular patient. This approach helps beginners visually observe the individualized spinal skeletal structure of each patient. Before the procedure, learners can gain a three-dimensional, comprehensive understanding of the puncture entry points, direction, depth, and potential spinal bony proliferations or degenerative changes along the pathway. This virtual 3D spine image is presented above the patient’s real body via a self-developed medical mixed reality system and proprietary manual control and trajectory design software. The operator can observe the virtual image and the guiding trajectory during a spinal puncture. Most elective surgery patients had already undergone CT scans during their preoperative workup. The existing CT scan results can be used to reconstruct the 3D hologram images of the spine needed for the MR system, thereby avoiding further radiation exposure.
In this pilot study, anaesthesiology residents undergoing standardized training were selected as operators. The number of needle insertion attempts and the rate of successful first-attempt needle insertion, along with other relevant outcome indicators and complications, were compared between the MRasp and LGsp groups. This comparison aimed to preliminarily examine the clinical effectiveness of MRasp technology by novice practitioners in elderly patients. Compared with the LGsp group, the MRasp group had significantly fewer needle insertion attempts (1.0 vs 2.0). Although the rate of successful first-attempt needle insertion, number of redirection attempts, number of passes, and spinal puncture time were not significantly different in this pilot study with a small sample size, they still showed a lower trend in the MRasp group than in the LGsp group clinically. Observing the reconstructed spinal image and the guiding trajectory might help the operator determine the puncture point and angle. In the next stage, we will conduct a formal randomized controlled trial on the basis of the sample size calculated in this pilot study to observe the differences in all these observational indicators between the two groups. MRasp is considered a promising technology for assisting with spinal puncture. To our knowledge, this study is creative work in which MR technology has been used to assist spinal puncture in elderly patients.
Other studies have explored the application of virtual reality (VR) or augmented reality (AR) technologies in medical teaching and model navigation, demonstrating their potential for improving procedural training and accuracy. Vrillon et al reported their experience with a 3D video for lumbar puncture training and found VR support could increase knowledge retention and skill acquisition in association to lumbar puncture simulation training.12 Gibby et al used MR technology to guide the insertion of a percutaneous pedicle screw in a lumbar spine silicone model. The study reported an average registration error of 0.25 cm between the virtual image and the model.13 The model, which has the shape of a regular hexagonal column, more easily overlaps with the virtual image. Reinacher et al evaluated the feasibility of an AR-guided approach for neuraxial access in a randomized phantom-based study. The AR technique showed a significantly greater success rate (82.5%) than did the conventional approach (40%), indicating the potential for AR to improve procedural outcomes.14 In the present study, the MRasp was employed to assist lumbar puncture in elderly patients who had more complex and variable structures than traditional models (eg, manikins or hexagonal column models). The MRasp allows operators to observe the reconstructed virtual lumbar vertebra, enabling direct observation of individual differences such as bone deformities, hyperplasia, and vertebral space stenosis. Moreover, the Reacool medical MR system automatically calculates and displays the optimal spinal puncture trajectory, assisting trainees in recommending the angle and depth during the procedure. This technique offered a more personalized, comprehensive learning and practice environment for beginners.
Recently, there has been growing interest in the use of ultrasound-assisted techniques for accessing difficult neuraxial blocks.10,15,16 Research by Coviello et al reported that ultrasound-assisted spinal anaesthesia performed by novice practitioners improved the success rate and reduced complications.10 However, the lack of three-dimensional figures in ultrasonic images creates challenges for novices when attempting to master the spinal anaesthesia technique. Furthermore, as the lumbar puncture procedure requires both hands, the use of ultrasound-guided techniques is challenging when performing the procedure independently. With MRasp technology, three-dimensional images of the spine can be vividly displayed in an MR environment, providing a more user-friendly approach and allowing novice practitioners to perform the procedure independently.
In this study, the cost of this MRasp device (including the headset HoloLens 2nd and the MRasp software kit) was CNY 58,000. Further clinical studies are needed to confirm its efficacy before it can be formally introduced to the market. As it is a one-time payment device with no disposable consumables, if formally deployed for clinical use in the future, it is anticipated that the fee will be approximately CNY 100 per person or not exceed the cost of ultrasound-guided procedures. In the long term, given the rapid developments in augmented reality and artificial intelligence, this technology will have broad applications not only in the field of anaesthesiology for visualized puncture procedures but also in the entire discipline of surgery.
This study has several limitations. First, the operators and the observers were nonblinded because of the nature of the study. However, the procedures were recorded on video, with two researchers documenting the observational indicators to mitigate observer bias. Second, in China, lumbar punctures are mostly performed in the lateral decubitus position, unlike the sitting position, which is more likely to be used in other countries. Therefore, the effectiveness of MRasp technology should be further studied in different positions. Finally, as a pilot study, the small sample size limits the generalizability of the findings. However, this set of results can provide the basis for calculating the sample size for a formal randomized controlled study to further validate the effectiveness of MRasp. More clinical research is needed before it can enter the market and become available to all anaesthesiologists.
Conclusions
MRasp technology aids novice trainees in spinal punctures by guiding the shortest obstacle-free route to access the subarachnoid space. The pilot study revealed significantly fewer needle insertion attempts in the MRasp group than in the LGsp group, demonstrating its promise in the medical education field in anaesthesia. A randomized controlled trial with an adequate sample size will further confirm the validity of MRasp in the future. MRasp may become a promising visualization technique for assisting with spinal puncture.
Acknowledgments
The authors would like to acknowledge Zhichao Jin (Department of Health Statistics, Second Military Medical University, Shanghai, China) for his assistance with the statistical consultation.
Funding Statement
This work was supported by the Shanghai Municipal Health Commission (Grant No. 2022JC025), the Project of Health and Medical Research of Shanghai (Grant No. 202240045), the Clinical Research Program of Huadong Hospital (Grant No. HDLC2022011), the Project of Science and Technology Commission of Shanghai Municipality (Grant No. 20Y11900200), the National Natural Science Foundation of China (Grant No. 82271286), and the Huadong Hospital Excellent Project (Grant No. ZDXK2210).
Abbreviations
MR, mixed reality; MRasp, mixed reality-assisted spinal puncture; LGsp, landmark-guided spinal puncture; ASA, American Society of Anaesthesiologists; CSF, cerebrospinal fluid; 3D, three dimensions; DICOM, digital imaging and communications in medicine; CT, computed tomography.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This pilot study was approved by the Ethics Committee of Huadong Hospital affiliated to Fudan University (IRB: 20220126; Chairperson Prof Yinghao Sha; approval date, November 9, 2022), and all participants provided written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Duniec L, Nowakowski P, Kosson D, Łazowski T. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther. 2013;45(1):1–6. doi: 10.5603/ait.2013.0001 [DOI] [PubMed] [Google Scholar]
- 2.Stendell L, Lundstrøm LH, Wetterslev J, Itenov TS, Rosenstock CV. Risk factors for and prediction of a difficult neuraxial block: A cohort study of 73,579 patients from the Danish anaesthesia database. Reg Anesth Pain Med Sep-Oct. 2015;40(5):545–552. doi: 10.1097/aap.0000000000000293 [DOI] [PubMed] [Google Scholar]
- 3.de Filho GR, Gomes HP, da Fonseca MH, Hoffman JC, Pederneiras SG, Garcia JH. Predictors of successful neuraxial block: a prospective study. Eur J Anaesthesiol. 2002;19(6):447–451. doi: 10.1017/s0265021502000716 [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Gao L, Shi Q, et al. Accuracy Evaluation trial of mixed reality-guided spinal puncture technology. Ther Clin Risk Manag. 2023;19:599–609. doi: 10.2147/tcrm.S416918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232 [DOI] [PubMed] [Google Scholar]
- 6.Conway A, Rolley J, Sutherland JR. Midazolam for sedation before procedures. Cochrane Datab Syst Rev. 2016;(5):Cd009491. doi: 10.1002/14651858.CD009491.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gsaxner C, Li J, Pepe A, et al. The HoloLens in medicine: a systematic review and taxonomy. Med Image Anal Apr. 2023;85:102757. doi: 10.1016/j.media.2023.102757 [DOI] [PubMed] [Google Scholar]
- 8.Tepper OM, Rudy HL, Lefkowitz A, et al. Mixed reality with hololens: where virtual reality meets augmented reality in the operating room. Plast Reconstr Surg. 2017;140(5):1066–1070. doi: 10.1097/prs.0000000000003802 [DOI] [PubMed] [Google Scholar]
- 9.Shinbane JS. Editorial commentary: current reality and future evolution of virtual, augmented, and mixed realities for cardiovascular application. Trends Cardiovasc Med. 2020;30(3):149–150. doi: 10.1016/j.tcm.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Coviello A, Iacovazzo C, Piccione I, et al. Impact of ultrasound-assisted method on success rate of spinal anesthesia performed by novice trainees: a retrospective comparative study. J Pers Med. 13(10). doi: 10.3390/jpm13101515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 21(1):18. doi: 10.1186/s12909-020-02452-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 22(1):244. doi: 10.1186/s12909-022-03317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibby JT, Swenson SA, Cvetko S, Rao R, Javan R. Head-mounted display augmented reality to guide pedicle screw placement utilizing computed tomography. Int J Comput Assist Radiol Surg. 2019;14(3):525–535. doi: 10.1007/s11548-018-1814-7 [DOI] [PubMed] [Google Scholar]
- 14.Reinacher PC, Cimniak A, Demerath T, Schallner N. Usage of augmented reality for interventional neuraxial procedures: a phantom-based study. Eur J Anaesthesiol. 40(2):121–129. doi: 10.1097/eja.0000000000001755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chin KJ, Perlas A, Chan V, Brown-Shreves D, Koshkin A, Vaishnav V. Ultrasound imaging facilitates spinal anesthesia in adults with difficult surface anatomic landmarks. Anesthesiology. 2011;115(1):94–101. doi: 10.1097/ALN.0b013e31821a8ad4 [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb M, Holladay D, Peksa GD. Ultrasound-assisted lumbar punctures: a systematic review and meta-analysis. Acad Emerg Med. 2019;26(1):85–96. doi: 10.1111/acem.13558 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.