Highlights
-
•
Impact of biofertilizers on soil microbial diversity and functionality.
-
•
Role of microbial-based bio-inoculum in improving plant growth and soil health.
-
•
Growth-promoting attributes of biofertilizer and its effectiveness over inorganic fertilizers for sustainable agriculture.
-
•
Prospects of commercialization, bioeconomy and production of biofertilizers for the development of agricultural productivity and socioeconomic status.
Keywords: Bio-inoculum, PGPR, Plant growth promotion, Soil health, Sustainable agriculture
Abstract
The adoption of sustainable agricultural practices is increasingly imperative in addressing global food security and environmental concerns, with microbial based bio-inoculums emerging as a promising approach for nurturing soil health and fostering sustainable crop production.This review article explores the potential of microbial based bio-inoculumsor biofertilizers as a transformative approach toenhance plant disease resistance and growth. It explores the commercial prospects of biofertilizers, highlighting their role in addressing environmental concerns associated with conventional fertilizers while meeting the growing demand for eco-friendly agricultural practices. Additionally, this review discusses the future prospects of biofertilizers, emphasizing the ongoing advancements in biotechnology and formulation techniques that are expected to enhance their efficacy and applicability. Furthermore, this article provides insights into strategies for the successful acceptance of biofertilizers among farmers, including the importance of quality control, assurance, and education initiatives to raise awareness about their benefits and overcome barriers to adoption. By synthesizing the current research findings and industrial developments, this review offers valuable guidance for stakeholders seeking to exploit the potential of biofertilizers or beneficial microbes to promote soil health, ensure sustainable crop production, and addressing the challenges of modern agriculture.
Graphical abstract
1. Introduction
The Green Revolution, characterized by advancements in crop productivity through high-yielding varieties, modern farming techniques, and chemical fertilizers, has raised concerns about environmental degradation and soil health (Swaminathan, 2006; Pramanik et al., 2023). In response, biotechnological approaches utilizing beneficial bacteria have gained traction (Biswas et al., 2021, 2022; Chattaraj et al., 2023a, 2023b; Ganguly et al., 2024). The integration of biofertilizers into agricultural practices has emerged as a promising strategy, offering eco-friendly alternatives to chemical fertilizers (Nad et al., 2024). Derived from natural sources like bacteria, fungi, and algae, biofertilizers enhance soil fertility, nutrient cycling, and plant growth, aligning with sustainable agriculture principles (Chakraborty and Akhtar, 2021; Mitra et al., 2021a; Nosheen et al., 2021; Kumar et al., 2022). Additionally, biofertilizers can remediate heavy metals present in the soil which are hazardous to the environment (Haroun et al., 2023; Sen et al., 2023; Chattaraj et al., 2024a). Biofertilizers play important role in nitrogen fixation, phosphorus solubilization and potassium mobilizationwhich complement the goals of the Green Revolution while promoting sustainable agriculture and resilience to climate change (Khoshru et al., 2023b; Kaur and Purewal, 2019). Biofertilizers, originating with "Nitragin" in 1895, address economic challenges of conventional methods, providing a cost-effective and sustainable solution (Riaz et al., 2020). In 2022, the global biofertilizers market reached a value of approximately USD 2.15 billion, and it is expected to reach approximately USD 6.83 billion by 2032, with a compound annual growth rate (CAGR) of 12.3 %, driven by the increasing demand for organically produced plants and vegetables (Precedence Research, 2023).The Indian biofertilizer market is projected to be valued at approximately USD 10.63 million in 2024 and is anticipated to achieve a valuation of about USD 16.5 million by 2029, with a CAGR of around 9.19 % over the forecast period from 2024 to 2029 (Gii research, 2024). The perspectives on the production, distribution, and access to biofertilizers can be portrayed with enhancing agricultural productivity, soil health, and socioeconomic development while aligning with climate change mitigation efforts (Ajmal et al., 2018). Moreover, from an economic perspective, biofertilizers are lauded for their cost-effectiveness compared to chemical fertilizers (Tiwari et al., 2004; Hassanpour et al., 2021). The viewpoints collectively underscore the potential of biofertilizers to contribute to a more sustainable and prosperous agricultural system. Biofertilizers can mitigate soil erosion, conserve water resources, and enhance biodiversity by promoting healthy and resilient agroecosystems (Riaz et al., 2020). This ecological approach to farming not only benefits the environment but also safeguards the long-term viability of agricultural production systems, ensuring food security for future generations (Garrity et al., 2010). In many developing countries, where agriculture remains the backbone of the economy and the primary source of livelihood for millions of people, the adoption of biofertilizers can have transformative effects on rural communities (Sarkar et al., 2022; Angom and Viswanathan, 2023). Biofertilizers, as sustainable agricultural inputs, empower farmers to enhance productivity and income while improving overall well-being (Mohanand Reddy, 2020). Additionally, decentralized production and distribution of biofertilizers create rural employment opportunities, stimulate local economies, and contribute to poverty reduction (Cong and Thomsen, 2021). Agricultural enterprises require improved marketing strategies to adapt to market conditions and enhance competitiveness, while farmer training in biofertilizer applications is essential for successful adoption (Lohosha et al., 2023). The inclusive adoption of biofertilizers promotes social cohesion and economic prosperity, representing a paradigm shift in agricultural practices with holistic and sustainable solutions (Barragán-Ocañaand and del Carmen del-Valle-Rivera, 2016; Ray et al., 2020). Realizing the full potential of biofertilizers necessitates collaborative efforts among policymakers, researchers, and farmers to overcome adoption barriers, raise awareness, and invest in research and development (Parida, 2016). Embracing sustainability and innovation can address biofertilizers to create a resilient, inclusive, and environmentally sustainable agricultural future (Sultan and El–Qassem, 2021; Prabhu et al., 2022). Hence, this review aims to assess the efficacy of biofertilizers in enhancing soil health and crop productivity and to provide recommendations for sustainable adoption and commercialization of biofertilizer.
2. Bioinoculum for sustainable agriculture
Bioinoculum represents a pivotal component of sustainable agriculture and offers a natural and environmentally friendly alternative to chemical fertilizers (Sarker et al., 2022; Table 1). These microbial formulations harness beneficial microorganisms such as bacteria, fungi, and algae to enhance soil fertility, nutrient availability, and plant health (Yadav and Smritikana Sarkar, 2019). By fostering symbiotic relationships with crops, nitrogen-fixing biofertilizers facilitate the conversion of atmospheric nitrogen into plant-available forms, promoting robust growth while reducing the need for synthetic nitrogen fertilizers (Singh, 2022). Similarly, phosphorus-solubilizing biofertilizers aid in releasing bound phosphorus in the soil, ensuring optimal uptake by plants for essential functions such as root development and flowering (Etesami, 2020). Furthermore, biofertilizers contribute to the restoration of soil biodiversity and health by promoting microbial diversity and activity, thus improving the soil structure, water retention, and nutrient cycling (Nosheen et al., 2021). Embracing biofertilizers in agricultural practices not only enhances crop productivity and resilience but also mitigates environmental impacts, fostering long-term sustainability and resilience in agricultural systems. The genera Azotobacter, Glomus, Clostridium, Bacillus, Rhizobium, Azospirillum, Boletus, Pseudomonas, Priestia, Laccaria, and Pezizella are regarded as the major bioinoculants for biofertilisers (Ortega-Urquieta et al., 2022; Nosheen et al., 2021; Table 1).
Table 1.
Insights into various biofertilizers: organisms, beneficial modes, and crop application practices.
| Types of biofertilizer | Organism used | Mode of beneficial action | Applied on crops | References |
|---|---|---|---|---|
| Nitrogenfixing biofertilizers | Rhizobium, Azotobacter, Azospirillum, Bradyrhizobium, and Sinorhizobium | Nitrogen-fixing bacteria play a crucial role in enhancing soil fertility by converting atmospheric nitrogen into a plant-usable form. Rhizobium forms symbiotic associations with leguminous plants, while Azotobacter establishes free-living associations in the rhizosphere | Cereals and wheat, rice, maize, pulses and oilseeds such as soybeans and peanuts. | Bhattacharjee et al., 2008 |
| Phosphorus-solubilizing biofertilizers | Rhizobium sp., Priestia sp., Enterobacter sp., Mycorrhizal fungi, Streptomyces sp., Bacillus megaterium, Azotobacter chroococcum, Pseudomonas fluorescent, Penicillium bilaii, Bacillus circulans, Bacillus subtilis, and Pseudomonas striata | Phosphorus enhances plant growth and development, including photosynthesis, root and stem strength, flower and seed formation, crop quality, energy production, and disease resistance. It also supports processes like root growth, cell division, nitrogen fixation in legumes, sugar conversion to starch, and genetic trait transmission. Adequate phosphorus availability is crucial for early plant reproductive development. | Mustard, Peanut and legumes, Sugar beet, Walnut, Rice, Aubergine, Chili, Soybean, Maize, Sugarcane, Apple, Chickpea, Oil palm, Potatoes, Wheat | Ortega-Urquieta et al., 2022; Kalayu, 2019; Panneerselvam et al., 2021b |
| Potash biofertilizers | Bacillus, Actinomycetes, Pseudomonads | These biofertilizers enhance the soil rhizosphere. The microorganisms aid in enhancing soil nutrient and moisture retention, nutrient cycling, stress tolerance, and crop yield, while also conferring disease resistance to plants. They are also capable of releasing potassium from mineral sources in the soil. Potassium-solubilizing bacteria serve various functions, including safeguarding plant ions from salinity by enhancing growth-related physiological processes like stomatal conductance, electrolyte leakage, and lipid peroxidation. | Rice, Maize,wheat, Tomato, Potato | Deilamirad et al., 2017; Ali et al., 2021; Gautam et al., 2022; Mitra et al., 2022; Panneerselvam et al., 2021a |
| Sulfur-oxidizing biofertilizers | Thiobacillus, Thiothrix, Sulfolobus, Thermothrix, and Beggiatoa | Sulfur-oxidizing bacteria are utilized as biofertilizers to enhance sulfur availability in the soil. These bacteria oxidize elemental sulfur or sulfide minerals, converting them into sulfate forms that plants can readily absorb. | Rice, Wheat, Chickpea, Pigeonpea, Garlic | Youssif et al., 2015; Ranadev et al., 2023; Jabbar and Al-Ziyadi, 2021; Malviya et al., 2022; Patel et al., 2024 |
| Biofertilizers containing bacterial micronutrients | Micronutrient-producing bacteria | Certain microorganisms are used to enhance the availability of micronutrients such as iron, zinc, and manganese in the soil. Siderophore-producing bacteria secrete organic compounds called siderophores, which chelate micronutrients, making them more accessible to plants. Biofertilizers containing siderophore producing microorganisms shield plants from bio-surfactants and enzymes that degrade cell walls. | Mushtaq et al., 2021; Waqeel and Khan, 2022 | |
| Compost added biofertilizers | Bacillus cereus AR156, Bacillus subtilis SM21, and Serratia sp. XY21, Plant growth-promoting rhizobacteria, Mycorrhiza | It containsbiosoal, tea compost, vermicompost, compost of farming waste including used up substrate of Pleurotus ostreatus/Volvariella volvacea, chicken manure, and inorganic fertilizer. These biofertilizers enrich the soil with a diverse microbial community, improving soil structure, nutrient cycling, and overall fertility. | Saffron,cotton,Wheat, Capsicum annuum | Zewail and Ahmed, 2015; Yu et al., 2019; Jami et al., 2020 |
| Phosphate-fixing biofertilizers | Bacillus sp., Pseudomonas sp., Vesicular arbuscular mycorrhizae, Phosphate solubilizing bacteria | These bacteria produce organic acids and enzymes that break down complex phosphorus compounds, making phosphorus more accessible to plants. Phosphate-fixing biofertilizers are widely used for fruits and vegetables, as well as for cereals and grains in phosphorus-deficient soils. | Rice, wheat, ground nut | Wahane et al., 2022; Panneerselvam et al., 2019; Mitra et al., 2014 |
3. Mode of action of nitrogen-fixing biofertilizers for enhancing soil fertility and plant growth
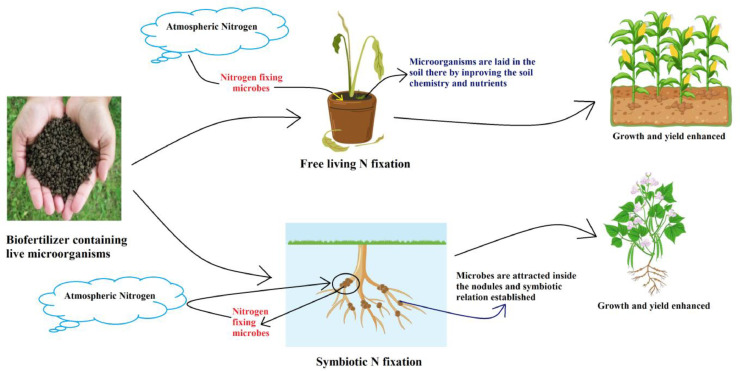
Understanding the intricate mode of action of nitrogen-fixing biofertilizers reveals their pivotal role in fortifying soil fertility and fostering optimal plant growth (Fig. 1). These biofertilizers contain nitrogen-fixing bacteria such as Azotobacter, Beijerinckia, Clostridium, Klebsiella, Rhizobium, and Azospirillum which can convert atmospheric nitrogen (N2) into plant-available forms such as ammonia (NH3) or nitrate (NO3−) (Fatima et al., 2019). Rhizobium species form symbiotic relationships with leguminous plants, forming nodules on their roots where nitrogen fixation occurs. Azotobacter and Azospirillum, on the other hand, colonize the rhizosphere of various crops, promoting nitrogen fixation and stimulating root development (Mehboob et al., 2013).Nitrogen-fixing bacteria exhibit versatility in their ecological roles, existing as either free-living entities or in symbiotic relationships with host plants, showcasing their adaptability across diverse environmental contexts.
Fig. 1.
Types of nitrogen fixation and their beneficial mode of action to the plant.
3.1. Symbiotic association
Symbiotic nitrogen fixation in plants represents a sophisticated biological partnership between certain plants and nitrogen-fixing bacteria, most prominently exemplified by the legume-rhizobial symbiosis (Fig. 1). This intricate relationship relies on specific genetic signaling pathways and molecular dialogues between the plant host and the bacterial symbiont. Initially, host plants release specific flavonoids into the rhizosphere, triggering bacterial nodulation (nod) gene expression in compatible rhizobial strains. In response, rhizobia produce nodulation factors, which are signaling molecules that elicit root hair curling and nodule formation (Limpens and Bisseling, 2009). Within nodules, bacteria differentiate into bacteroids, a state marked by genetic changes that enable nitrogen fixation. Concurrently, the plant genetic machinery orchestrates the development and maintenance of nodules by regulating the oxygen and nutrient supply to bacteroids. This intricate genetic interplay ensures the efficient establishment of a symbiotic relationship in which plants gain access to biologically fixed nitrogen, while providing rhizobia with a hospitable niche. Through a complex network of genetic interactions, symbiotic nitrogen fixation exemplifies the remarkable ability of nature to optimize nutrient acquisition in plants, thus enriching soil fertility and supporting sustainable agriculture. In most legume nodules, rhizobial bacteria that fix nitrogen (N2) are found within specialized structures that resemble organelles within their host root cells. The symbiotic relationship between plants and nodules involves numerous interconnected processes, including nitrogen and carbon metabolism, oxygen diffusion within nodules, management of oxidative stress, and phosphorus levels (Ray et al., 2024). These processes, which intricately regulate N2 fixation and are finely coordinated at the whole-plant level, were extensively examined in this study. Among the pathways crucial to this regulation is the carbonic anhydrase-phosphoenolpyruvate carboxylase-malate dehydrogenase pathway, which plays a pivotal role in various aspects of symbiotic N2 fixation (Schwember et al., 2019). Various nitrogen compounds and enzymes, such as glutamate, glutamine, glutamine synthetase, aspartate, asparagine, ureides, polyamines, and proline, are implicated in the regulationof N2 fixation rates. Despite substantial indirect evidence, no definitive proof of a specific compound or mechanism controlling nodule N2 fixation has been established. Additionally, components such as ureid permeases (UPS1) in Phaseolus vulgaris and amino acid permease (AAP6) in pea nodules play crucial roles in the regulation of N metabolism and organic N transport, with AAP6 particularly vital for overcoming vascular tissue barriers (Schwember et al., 2019). Downregulation of AAP6 leads to reduced N export, nodular N accumulation, decreased shoot N content, and stimulated N2 fixation, indicating a potential phloem-mobile N-deficiency signal induced by leaf N status (Schwember et al., 2019). A limited subset of plant species from four orders, Fabales, Fagales, Cucurbitales, and Rosales (referred to as the FaFaCuRo clade), have developed the capacity for a mutually advantageous relationship known as the nitrogen-fixing root nodule symbiosis (RNS). Root nodule symbioses, exemplified by legumes and actinorhizal plants, represent intricate partnerships between plants and nitrogen-fixing bacteria, which facilitate nutrient exchange and promote ecosystem sustainability. Root nodule symbioses are unique to plants in the FaFaCuRo clade and involve rhizobia, nodule anatomy, and metabolism, enabling legumes to thrive globally in various cropping systems (Pankievicz et al., 2019).
3.2. Free living (nonsymbiotic)
Free-living nitrogen fixation in plants is a fascinating process wherein certain diazotrophic bacteria, such as Azotobacter and Cyanobacteria, independently fix atmospheric nitrogen into biologically available forms without symbiotic associations with host plants (Fatima et al., 2019). This phenomenon involves a diverse array of genetic mechanisms that enable bacteria to assimilate and reduce atmospheric nitrogen. Key genetic components include nitrogenase enzymes, encoded by nif genes, which catalyze the conversion of dinitrogen gas (N2) into ammonia (NH3). Additionally, genes responsible for regulating nitrogenase activity and protecting them from oxygen-mediated inactivation play crucial roles (Pedrosa and Yates, 1988). Furthermore, diazotrophs possess genetic machinery for the synthesis of specialized structures such as heterocysts in cyanobacteria, which create microaerobic conditions favoring nitrogenase activity (Issa et al., 2014). These intricate genetic adaptations enable free-living nitrogen-fixing bacteria to thrive in diverse environments, contributing significantly to global nitrogen cycling, and influencing soil fertility and plant growth by providing bioavailable nitrogen sources. Non-symbiotic heterotrophic N fixation occurs in the soil and leaf litter (Reed et al., 2013; Fig. 1).
4. The complex interplay between root and stem nodules and microorganisms in biofertilizer
Root and stem nodules are intricately linked with biofertilizers becauseof their role in nitrogen fixation, which is a vital process in soil fertility and plant nutrition. Biofertilizers contribute to nitrogen fixation in both root and stem nodules, thereby enhancing soil fertility and promoting plant growth (Bhat et al., 2015).Plant-associated microorganisms have developed the capacity to autonomously synthesize gibberellin (GA) phytohormones to influence their hosts (Nett et al., 2022). Symbiotic nitrogen-fixing bacteria (rhizobia) residing within legume root nodules can produce GA, suggesting a role in symbiosis. Although the bacterial GA biosynthetic operon has been identified, the final metabolic gene (cyp115) is typically absent in rhizobia, resulting in the production of only the penultimate intermediate, GA9. Functional GA3-oxidases (GA3ox) are expressed within soybean (Glycine max) nodules, enabling the conversion of GA9, produced by the enclosed rhizobial symbiont Bradyrhizobium diazoefficiens to bioactive GA4. Rhizobia-derived GA induces an increase in nodule size and a reduction in nodule number (Nett et al., 2022). Within these nodules, bacteria convert atmospheric nitrogen into ammonia, which can be used by the host plant as a nutrient source. Biofertilizers containing compatible strains of nitrogen-fixing bacteria can be applied to the soil to establish or enhance symbiotic relationships with leguminous plants, leading to the formation of root nodules and increased nitrogen fixation. By inoculating the soil with these biofertilizers, farmers can improve soil fertility, reduce the need for synthetic nitrogen fertilizers, and sustainably enhance crop yield. Hata et al. (2023) reported that intercellular spaces within leguminous root nodules can harbor both compatible and incompatible rhizobia. Transmission electron microscopy was employed to observe Mesorhizobium loti within the intercellular spaces of actively functioning wild-type nodules in Lotus japonicus. Although compatible intercellular colonization by rhizobia has been documented during nodule development in various legume species and certain mutants. Hata et al. (2023) indicated that this mode of colonization may be more prevalent in the nodules of leguminous plants. Similarly, stem nodules, which occur in certain woody legume species such as Dalbergia and Aeschynomene sp., also facilitate nitrogen fixation through symbiotic interactions with nitrogen-fixing bacteria (de Faria et al., 2020). Although stem nodules are less common than root nodules, they play a crucial role in nitrogen cycling and soil fertility in certain ecosystems. Biofertilizers containing nitrogen-fixing bacteria compatible with woody legumes can promote the formation of stem nodules, thereby increasing nitrogen fixation and nutrient availability in the soil. This, in turn, benefits not only the host plants but also the neighboring crops and overall health of the agroecosystem.Both root and stem nodules serve as sites for nitrogen fixation facilitated by beneficial microorganisms, which are the central components of biofertilizers (Kca et al., 2021). By harnessing the symbiotic relationships between nitrogen-fixing bacteria and host plants, biofertilizers can contribute to sustainable agriculture by improving soil fertility, reducing environmental impacts, and enhancing crop productivity (de Novais et al., 2020). Additionally, the use of biofertilizers containing nitrogen-fixing bacteria can promote the development of nitrogen-fixing nodules in both roots and stems, further enriching the soil with valuable nutrients and supporting the growth of healthy, resilient plants. Thus, the linkage between root nodules, stem nodules, and biofertilizers underscores the importance of microbial contributions to soil fertility and agricultural sustainability (Wei et al., 2024).
5. Role of microorganism in biofertilizer for sustainable agriculture
5.1. Rhizobium
Rhizobium, vital for biofertilizers, enhances soil fertility by symbiotic nitrogen fixation, forming nodules in leguminous plants (Rai, 2006; Kumar et al., 2020). Beyond nitrogen fixation, rhizobia exhibit plant growth-promoting properties through mechanisms such as metal solubilization and siderophore activity, thereby enhancing soil nutrient bioavailability (Kumar et al., 2019). The combined action of phytohormones, enzymes, and siderophores promotes plant growth, nutrient uptake, and phytoremediation. Additionally, rhizobia contribute to biocontrol by antagonizing pathogens through antibiosis, parasitism, or competitive nutrient uptake, positioning them as vital assets for sustainable agriculture worldwide (Kumar et al., 2019). As a biofertilizer, Rhizobium inoculants are applied to the soil or seeds, facilitating the establishment of symbiotic associations with leguminous crops such as soybeans, peas, and alfalfa. By harnessing the natural capabilities of Rhizobium bacteria, biofertilizers promote sustainable agriculture by reducing reliance on synthetic nitrogen fertilizers, improving soil fertility, and enhancing crop yield (Mia and Shamsuddin, 2010). Moreover, the use of Rhizobium biofertilizers contributes to environmental sustainability by minimizing nitrogen runoff and greenhouse gas emissions associated with conventional fertilization practices (Abd-Alla et al., 2023). Dong et al. (2024) mentioned that symbiotic nitrogen fixation accounted for 58.1–84.9 % of nitrogen uptake in hairy vetch, leading to improved crop yield and reduced nitrous oxide emissions in paddy fields. Thus, Rhizobium biofertilizers exemplify the potential of microbial solutions to address key challenges in modern agriculture while promoting long-term soil health and agricultural productivity.
5.2. Azotobacter
Azotobacter holds significant promise as a biofertilizer becauseof its ability to enhance soil fertility and support plant growth in diverse agricultural settings (Barman et al., 2019) and over a century, they have served as biofertilizers. They perform aerobic nitrogen fixation, produce plant hormones, facilitate phosphate solubilization, and suppress phytopathogens. Wild-type Azotobacters have been linked to enhanced yields across a diverse range of crops, including cereals (wheat, pearl millet, oat, corn, barley, rice, and sorghum), oilseeds (mustard and sunflower), vegetables (tomato, potato, carrot, eggplant, onion, chili, beans, and sugar beet), fruits (mango and sugarcane), fiber crops (jute and cotton), and trees (oak) (Das, 2019). When applied as a biofertilizer, Azotobacter inoculants colonize the rhizospheres of various crops, promote nitrogen fixation, and stimulate root development. Unlike symbiotic nitrogen-fixing bacteria, Azotobacter operates independently of plant roots, making it suitable for a wide range of crops, including non-leguminous species. Azotobacter biofertilizers enhance nutrient availability, improve soil structure, and contribute to sustainable agriculture by reducing the need for synthetic nitrogen fertilizers, minimizing environmental impacts, and enhancing crop yields (Wani et al., 2016). Furthermore, Azotobacter biofertilizers exhibit resilience to adverse soil conditions, making them particularly valuable in marginal or degraded soils where nutrient deficiencies are common. By harnessing the nitrogen-fixing capabilities of Azotobacter bacteria, biofertilizers offer a cost-effective and environmentally friendly solution to improve soil fertility, promote crop productivity, and support the transition towards more sustainable agricultural practices. The genetics underlying the role of Azotobacter as a biofertilizer is multifaceted and encompasses a range of genes responsible for nitrogen fixation, nutrient metabolism, and environmental adaptation. The nitrogenase enzyme complex, encoded by nif genes, is the core of Azotobacter’s ability to fix nitrogen. These genes orchestrate a complex process of converting atmospheric nitrogen (N2) into ammonia (NH3), a form that is usable by plants. The regulatory components NifA and NifL, along with the nitrogen-control proteins GlnD and GlnK, form a system that integrates signals related to cellular redox, energy, and nitrogen. This system regulates the initiation of nif gene expression byinteracting with the σ54-containing RNA polymerase (Poza‐Carrión et al., 2015). Additionally, Azotobacter possesses genes involved in carbon and energy metabolism, as well as nutrient uptake and utilization, which contribute to its survival and proliferation in soil environments. Furthermore, genes related to stress response mechanisms enable Azotobacter to thrive under various environmental conditions, including drought, salinity, and low nutrient availability (Bandyopadhyay et al., 2022). Understanding the genetic mechanisms underlying Azotobacter nitrogen-fixing abilities is essential for optimizing its use as a biofertilizer, enhancing its efficiency, and expanding its applicability to different agricultural systems and soil types. Through genetic research and manipulation, scientists aim to unlock the full potential of Azotobacter as a sustainable solution for improving soil fertility and promoting crop productivity in diverse farming environments.
5.3. Blue-green algae
Algae offer a promising avenue for sustainable biotechnology because of their nutritional value (Chattaraj et al., 2024b) and the presence of bioactivecompounds that confer antimicrobial properties on the host (Chattaraj et al., 2022; Chattaraj and Das Mohapatra, 2023). Recently, algae have been exploited in agriculture as biofertilizers because of their ability to fix atmospheric nitrogen and enhance soil fertility (Gupta et al., 2013). Cyanobacteria can be used to promote plant growth and productivity in agricultural soils. Moreover, cyanobacteria contribute to soil health by enriching it with organic matter and essential nutrients. Their ability to thrive under diverse environmental conditions, including nutrient-poor soils and aquatic environments, makes them particularly valuable for improving soil fertility in marginal land. Additionally, cyanobacteria exhibit resilience to environmental stresses, such as drought and salinity, further enhancing their suitability as biofertilizers in challenging agricultural landscapes (Gautam et al., 2021). By harnessing the nitrogen-fixing capabilities of cyanobacteria, biofertilizers offer a sustainable and environmentally friendly solution to enhance soil fertility, promote crop productivity, and reduce the dependence on chemical fertilizers in agriculture. The genetics underlying blue-green algae, also known as cyanobacteria, as biofertilizers is complex and diverse, reflecting their adaptability and ecological significance. Cyanobacteria possess a wide array of genes that govern various physiological processes crucial for their function as biofertilizers. Of particular importance are genes encoding nitrogenase enzymes that are responsible for nitrogen fixation (Bothe et al., 2010). Additionally, Cyanobacteria harbor genes involved in photosynthesis, carbon fixation, and nutrient metabolism, which contribute to their ability to harness solar energy and assimilate carbon and other essential nutrients. Cyanobacteria possess genes encoding stress response mechanisms that allow them to thrive under diverse environmental conditions, including nutrient-poor soils and fluctuating water availability. Understanding the genetic makeup of cyanobacteria is essential for harnessing their potential as biofertilizers, optimizing their performance, and expanding their applications in sustainable agricultural practices. Through genetic engineering and manipulation, researchers aim to enhance nitrogen-fixing efficiency and stress tolerance of cyanobacteria, ultimately contributing to improved soil fertility, enhanced crop yields, and sustainable agricultural production systems. Several species of blue-green algae and cyanobacteria have been applied as biofertilizers in agriculture. Commonly used cyanobacterial species include the following.
-
a.
Anabaena:Anabaena species are filamentous cyanobacteria that are known for their ability to fix atmospheric nitrogen, and they contain specialized structures called heterocysts that are responsible for nitrogen fixation. These heterocysts provide an anaerobic environment suitable for nitrogenase activity. In agricultural systems, Anabaena species are commonly applied as biofertilizers in rice paddies and other crops, where they contribute to soil fertility by supplying plants with essential nitrogen nutrients (Zulkefli and Hwang, 2020).
-
b.
Nostoc:Nostoc is a genus of cyanobacteria found in various terrestrial and aquatic habitats including soil, freshwater, and marine environments. Cyanobacteria have traditionally been used as biofertilizers because of their ability to fix atmospheric nitrogen and improve soil fertility. Nostoc colonies consisted of multicellular filaments enclosed in gelatinous sheaths. Within these colonies, specialized cells, called heterocysts, perform nitrogen fixation by converting atmospheric nitrogen into ammonium ions. Additionally, Nostoc species produce mucilage, a sticky substance that aids soil aggregation and moisture retention, further enhancing soil fertility and structure (Joshi et al., 2020).
-
c.
Spirulina:Spirulina is a genus of cyanobacteria that has gained popularity as a nutritional supplement owing to its high protein content and nutrient-rich profile. Although primarily cultivated for human consumption, Spirulina can also be utilized as a biofertilizer in agriculture. Spirulina species can fix atmospheric nitrogen, thus providing a sustainable source of nitrogen for plant growth. Moreover, Spirulina biomass contains a range of nutrients, including vitamins, minerals, and amino acids, which can contribute to soil fertility and plant nutrition when applied as a biofertilizer (Alghamdi et al., 2023).
-
d.
Aulosira:Aulosira species are filamentous cyanobacteria commonly found in soil and aquatic habitats. These cyanobacteria exhibit nitrogen-fixing capabilities, which contribute to soil fertility and ecosystem nitrogen cycling. Aulosira filaments contain specialized cells known as heterocysts, where nitrogen fixation occurs. Through the activity of nitrogenase enzymes, Aulosira converts atmospheric nitrogen into ammonia, which can be utilized by plants for growth and development. In agricultural systems, Aulosira species have been investigated for their potential use as biofertilisers to enhance soil fertility and promote sustainable crop production (Mohan and Kumar, 2019).
5.4. Clostridium
Clostridium species are promising biofertilizers owingto their unique ability to fix atmospheric nitrogen through biological nitrogen fixation. The diverse genus Clostridium consists of gram-positive, mesophilic, and anaerobic species with recent updates in taxonomic classification. These bacteria play vital roles in various environments and contribute to agroecology by promoting plant growth, participating in industrial processes, and replacing harmful chemicals (Figueiredo et al., 2020). Their beneficial effects include biological nitrogen fixation and phosphate solubilization in soils, along with industrial outputs such as biohydrogen, acetone, biobutanol, and biofuels, showcasing their significant potential to mitigate environmental impacts and enhance agroecological systems (Figueiredo et al., 2020). The genetics underlying the role of Clostridium as a biofertilizer is multifaceted and involves a range of genes responsible for nitrogen fixation and metabolic pathways. Clostridium species possess nitrogenase genes organized into operons, such as nifHDK, which encodes the structural components of the nitrogenase complex, and nifENB, which encodes proteins involved in the assembly and maturation of nitrogenase. Clostridium bacteria harbor genes involved in carbon metabolism, energy production, and stress response mechanisms, which enable them to thrive under diverse environmental conditions and contribute to their effectiveness as biofertilizers. Understanding the genetic basis of Clostridium's nitrogen-fixing capabilities is essential for harnessing their potential as sustainable alternatives to chemical fertilizers, enhancing soil fertility, and promoting agricultural crop productivity (Koirala and Brözel, 2021).
5.5. Phosphate solubilizing microbes for sustainable agriculture
Phosphate-solubilizing bacteria (PSB) play a pivotal role in sustainable agriculture by offering a natural and eco-friendly solution to enhance soil phosphorus availability and promote plant growth (Khoshru et al., 2023c). These beneficial microorganisms possess enzymatic machinery to solubilize insoluble forms of phosphorus in the soil, making it more accessible to plants. PSB produces organic acids, such as citric, gluconic, and oxalic acids, as well as phosphatases and other enzymes that break down complex phosphorus compounds and release soluble phosphate ions (H2PO4− and HPO42−) that plants can absorb (Rawat et al., 2021; Yin et al., 2015). By improving phosphorus uptake, PSB biofertilizers enhance root development, nutrient assimilation, and overall plant vigor. Moreover, PSB contribute to soil health and fertility by promoting microbial activity, organic matter decomposition, and nutrient cycling (Mitra et al., 2023b; Mitra et al., 2023d). The use of PSB biofertilizers reduces the need for chemical phosphorus fertilizers, mitigates environmental pollution, and reduces the production costs for farmers. Furthermore, PSB biofertilizers are well-suited for organic farming systems, where they support sustainable soil management practices and minimize reliance on synthetic inputs. With advances in research, harnessing the potential of phosphate-solubilizing bacteria holds great promise in improving soil fertility, enhancing crop productivity, and fostering environmentally sustainable agriculture. Various PSB and fungi contribute to soil fertility and plant growth in agriculture. Bacillus species produce organic acids and enzymes, enhancing phosphorus availability (Saiyad et al., 2015). Pseudomonas sp., including Pseudomonas fluorescens and Pseudomonas putida, produce organic acids and siderophores, improving phosphorus uptake (Pastor et al., 2014). Again, Enterobacter cloacae, release phosphorus from soil compounds, benefiting plant growth (Singh, 2018). Some strains like Rhizobium leguminosarum biovar. phaseoli is a phosphate solubilizing bacteria that enhances the growth of maize and lettuce(Chabot et al., 1996). Phosphate-solubilizing fungi such as Aspergillus spp. (Aspergillus niger and Aspergillus flavus) and Penicillium spp. (Penicillium oxalicum, Penicillium citrinum, and Penicillium chrysogenum), along with Trichoderma spp. (Trichoderma harzianum, Trichoderma viride and Trichoderma asperellum) and Mycorrhizal fungi, enhance phosphorus availability and plant growth in agricultural systems (David et al., 2023; Lei et al., 2017; Paul and Rakshit, 2021).
5.6. Plant beneficial viruses
Beneficial viruses that increase the aesthetic appeal of ornamental plants are among the most studied and extensively used plants. The first of many exquisite viruses was the tulip-breaking virus, but numerous other valuable ornamentals have value, at least partially, to the viruses that infect them (Valverde et al., 2012). Other examples of beneficial plant viruses include a number of acute viruses such as the white clover cryptic virus (family Partitiviridae), which can suppress nodulation in legumes when sufficient nitrogen is present, and persistent viruses such as the cucumber mosaic virus (family Bromoviridae), tobacco rattle virus (family Virgaviridae), and tobacco mosaic virus (family Virgaviridae), which confer tolerance to drought and freezing temperatures in several different crops (Roossinck, 2011).Mildly symptomatic plant virus strains have been utilized for cross-protection against more pathogenic strains,and pathogen-derived transgenic resistance techniques have exploited this characteristic. Although this is not always the case, endogenous pararetroviruses can occasionally offer defence against closely related viruses (Roossinck, 2011). Various insect viruses, including nucleopolyhedrosis viruses (NPV), entomopoxviruses, granulosis viruses, cytoplasmic polyhedrosis viruses, and non-occluded viruses, have shown promise in naturally controlling harmful insects that affect horticultural plants (Cohen, 1981). These viruses are typically dispersed in water and sprayed onto plants for protection; however, alternative methods such as autodissemination, induction of epizootics, colonization, and environmental manipulation have also been explored. NPVs have been particularly effective in controlling pests such as gypsy moths, European spruce saw flies, cabbage looper, Douglas-fir tussock moths, and coconut palm rhinoceros beetles (Cohen, 1981). Additionally, granulosis and entomopox viruses have been utilized with promising results against pests, such as the imported cabbage worm and the spruce budworm, while a non-inclusion virus was effective against the citrus red mite (Cohen, 1981).Understanding inter-viral relationships in mixed infections and virus-drought interactions is crucial for agriculture and natural vegetation. These relationships can be additive or antagonistic, suggesting that viruses may have neutral or even positive effects on drought-stressed plants or that drought can enhance plant resistance to viruses (Sadras et al., 2024). The dynamics of inter-virus and virus-drought interactions are influenced by factors such as the virus species, host plant traits, timing of infection, plant age, and environmental conditions. This trait dependence highlights the importance of resource allocation in plants, necessitating further experimental and theoretical research (Sadras et al., 2024). Such research can progress through effective theory modeling system behavior without specifying all underlying causes and mechanistic theory considering the nuanced influence of plant phenotype on inter-virus relations, the impact of drought timing and intensity on plant phenotype modulation by viruses, and the role of both soil moisture and atmospheric conditions in drought stress (Sadras et al., 2024). Plant pathogenic fungi pose ongoing threats to agriculture, causing significant economic losses and reducing crop yield. Managing these diseases is crucial, and mycoviruses have emerged as promising biocontrol agents that alter fungal physiology and interactions with host plants. The main mycovirus families reported by the International Committee on Taxonomy of Viruses include Botourmiaviridae, Fusariviridae, Mitoviridae, and Hypoviridae for single-stranded positive-sense RNA genomes, and Chrysoviridae for double-stranded RNA genomes (Contreras-Soto and Tovar-Pedraza, 2024). The mycoviruses specific for Sclerotinia sclerotiorum include Sclerotinia sclerotiorum mycoreovirus 4, Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1, Sclerotinia sclerotiorum debilitation-associated RNA virus, and Sclerotinia sclerotiorum partitivirus 1, offer specificity, efficacy, and environmental safety, positioning them as valuable tools in crop protection (Contreras-Soto and Tovar-Pedraza, 2024). With over 250 mycoviruses reported in S. sclerotiorum, their potential as effective management strategies against major plant diseases worldwide is evident (Contreras-Soto and Tovar-Pedraza, 2024).
6. Mode of application of biofertilizer
The mode of application of biofertilizers is a crucial factor that influences their effectiveness in enhancing soil fertility and promoting plant growth. Various methods, including soil application, seed treatment, foliar spraying, and drip irrigation, have been employed to deliver biofertilizers to crops and the soil (Fig. 2).
Fig. 2.
Modes of application of biofertilizers and their benefits.
6.1. Seed treatment
Seed treatment involves coating the seeds with biofertilizer formulations containing beneficial microorganisms prior to planting. This mode of application ensures direct contact between the biofertilizer and developing roots, facilitating early colonization and the establishment of symbiotic relationships (Binodh et al., 2022). Seed-applied biofertilizers are particularly effective for crops with small seeds and shallow root systems such as grains, pulses, and vegetables. In addition, seed treatments offer convenience and ease of application, making them popular choices among farmers. Paul and Rakshit (2021) explored the impact of seed bio-priming using Trichoderma viride strain BHU-2953 to improve soil phosphorus solubilization and uptake in soybeans. Azotobacter and phosphorus-solubilizing bacteria have been used as seed treatments to obtain higher yields (Kalita et al., 2019). Pathak and Chakraborti (2014) treated maize seeds with Azospirillum spp. and found increased germination rates. Bharathi et al. (2013) treated Sesamum indicum seeds with Trichoderma + Pseudomonas and observed that the formulation was effective in reducing pathogenic fungal infections and seedling mortality (Fig. 2).
6.2. Soil treatment
Soil treatment involves the application of biofertilizers directly to the soil, either as liquid drenches, granular formulations, or as soil inoculants. This mode of application allows for widespread distribution of beneficial microorganisms throughout the rhizosphere, thereby promoting soil microbial diversity and activity. Soil-applied biofertilizers are suitable for a wide range of crops and soil types and can improve soil fertility, nutrient cycling, and overall soil health (Yang et al., 2022). Rathnathilaka et al. (2023) found that applying biofilm biofertilizers to the soil enhanced soil nutrient levels and microbial populations, resulting in higher rice grain yields. Their study revealed a notable correlation between a soil quality index developed specifically for biofilm biofertilizer application and rice grain yield, underscoring the significant role of soil in promoting environmentally sustainable rice cultivation. They are often used during land preparation or incorporated into irrigation systems for efficient nutrient delivery (Fig. 2).
6.3. Others
In addition to seed and soil treatments, biofertilizers can be applied using other methods, such as foliar sprays, root dips, and fertigation systems (Fig. 2). Foliar application involves spraying biofertilizer solutions onto the leaves of plants, where they are directly absorbed through stomata or epidermal cells. Root dips involve immersing seedlings or transplants in biofertilizer solutions before planting to promote rapid root colonization and establishment. Garg et al. (2022) examined how the application method of PGPR, either through soil application or root dip of seedlings, influenced the performance of onion across three distinct agro-climatic zones in Indian Punjab. Fertigation, however, involves injecting biofertilizer solutions into irrigation systems and delivering nutrients directly to plant roots through irrigation water. These alternative application methods offer flexibility and precision in nutrient delivery, allowing farmers to customize biofertilizer applications based on crop needs, growth stage, and environmental conditions (Thomas and Singh, 2019). Rahimzadeh et al. (2016) investigated the impact of bio-fertilizer on essential oil yield and components extracted from Dracocephalum moldavica L. using a nanoscale injection technique. Latif and Mustafa (2019) injected plants with liquid inoculant bacteria (16 ml per plant) to achieve 100 % inoculation.
7. Plant growth promoting rhizobacteria (PGPR): a newer approach
Plant growth-promoting rhizobacteria (PGPR) are multi-beneficial bacteria enhancing plant growth through mechanisms like phosphate solubilization and biofilm inhibition (Khoshru et al., 2023a; Bhattacharyya and Jha, 2012). Understanding their mechanisms, categorized into direct and indirect, is crucial for manipulating rhizospheric flora to promote sustainable agriculture (Goswami et al., 2016). PGPRs have been reported in various genera such as Bacillus, Azospirillum, Pseudomonas, Acinetobacter, Enterobacter, Rhizobium, Burkholderia, Aeromonas, Agrobacterium, Mesorhizobium, Caulobacter, Serratia, Allorhizobium, Chromobacterium, Paenibacillus, Arthrobacter, Frankia, Pantoea, Azoarcus, Bradyrhizobium, Delftia, Flavobacterium, Klebsiella, Streptomyces, Gluconacetobacter, Micrococcus, Thiobacillus (Basu et al., 2021).
7.1. Genetics involved in PGPR
Plant Growth-Promoting Rhizobacteria (PGPR) exerts beneficial effects on plant growth and development through various genetic mechanisms. The genetics involved in PGPR function encompass a range of traits that are crucial for plant-beneficial activities. Key genetic elements include genes encoding plant growth regulators, such as auxins, cytokinins, and gibberellins, which facilitate enhanced root growth and nutrient uptake. PGPR also harbor genes responsible for producing siderophores, facilitating iron uptake by both bacteria and plants. Moreover, genessuch aspyrroloquinoline quinone synthase (pqq), which isinvolved in phosphate solubilization, contributes to increased phosphorus availability to plants. In addition to its significant function in solubilizing phosphorus, pqq has been noted for its strong capacity to enhance the growth of both bacteria and plants. Moreover, it possesses antioxidant properties and is closely linked to the generation of antimicrobial compounds along with the stimulation of systemic plants (Meyer et al., 2011). Furthermore, PGPR possess genes encoding enzymes such as ACC deaminase, which reduce ethylene levels in plants, thus alleviating stress responses (Raghuwanshi and Prasad, 2018; Ruparelia et al., 2022). Genetic determinants associated with nitrogen fixation by some PGPR strains enable the conversion of atmospheric nitrogen into plant-available forms. Additionally, the genetic makeup of PGPR influences its ability to produce antimicrobial compounds, thereby suppressing pathogenic organisms and promoting plant health (Rabari et al., 2023). Horizontal gene transfer (HGT) plays a pivotal role in shaping the genetic diversity of PGPR populations by facilitating the acquisition of beneficial traits and adaptation to diverse environments. Mobile genetic elements play a crucial role in disseminating genes within bacterial communities and enhancing their adaptability, survival, and capacity to thrive under diverse environmental conditions. Bacterial conjugative plasmids that carry resistance genes, degradative genes, and stress tolerance mechanisms (Maheshwari et al., 2017). The biofilm growth mode of bacteria further facilitates gene exchange, boosting their fitness and competitive advantages. Microcosm studies have identified various factors that influence HGT in the soil. Recognizing the significance of HGT, a deeper understanding of the genetic mechanisms in the rhizosphere holds promise for leveraging naturally engineered bacteria towards sustainable agricultural practices (Maheshwari et al., 2017). Bruto et al. (2014) reported that HGT had significant effects on plant-beneficial functions, contributing to gene distribution in different PGPR in Proteobacteria.Understanding the genetics underlying PGPR functionality is crucial for harnessing its potential in sustainable agriculture practices aimed at enhancing crop productivity, nutrient use efficiency, and stress tolerance, while reducing reliance on chemical inputs, thereby contributing to environmentally friendly and economically viable agricultural systems (Table 2). Genetic engineering of Azotobacter provides solutions to the different difficulties faced in agriculture. Das (2019) discussed that apart from the structural genes encoding nitrogenase and other associated proteins, the chromosomes of Azotobacter vinelandii also harbor regulatory genes, namely nifL and nifA. NifA plays a crucial role, as it must bind upstream of the promoters of all nif operons to facilitate their expression. Upon activation by oxygen or ammonium, NifL interacts with NifA to neutralize its function. Notably, nitrogen fixation has been boosted through the deletion of nifL and by placing nifA under the control of a constitutive promoter, yielding a strain capable of continuous nitrogen fixation, even in the presence of urea fertilizer (Das, 2019). Furthermore, the introduction of additional copies of nifH (the gene encoding the Fe protein of nitrogenase) in A. vinelandii has been shown to enhance nitrogen fixation. Genetic modifications, including deletion of the urease gene complex ureABC, disruption of the ammonia transport gene amtB, and regulation of glutamine synthase gene expression, have been implemented to improve urea and ammonia excretion. Moreover, the introduction of the glucose dehydrogenase gene leads to the production of gluconic acid, thereby enhancing phosphate solubilization (Das, 2019).
Table 2.
Antimicrobial genes in PGPR and their roles in disease management for sustainable agriculture.
| Antimicrobial gene | Bacteria | Function | Host plant | References |
|---|---|---|---|---|
| Novel biosynthetic gene clusters (BGCs), includingNRPS-PKS hybrid, polyketide synthetase (PKS), nonribosomal peptide synthetase (NRPS) and terpene BGCs, and by BAGEL4 | B. pumilus, B. altitudinis and Brevibacillus, | Antagonistic againstMagnaporthe oryzae Guy11 (fungal pathogen that causes severe blast disease), Xanthomonas translucens pv. graminis LMG587 (causes bacterial wilt in plants). Strains B. velezensis MG33,B. subtilis subsp. subtilis MG27, Brevibacilluslaterosporus MG64 and B.velezensis MG43 can antagonize C. purpurea f. secalis and P. chartarum, while B. altitudinis MG75 and B. pumilus MG84 showed activity against C. purpurea f. secalis. | Perennial ryegrass seedlings | Li et al., 2020b |
| Fengycingenes, iturin, bacillomycin andSurfactin | B. amyloliquefaciens BSC6, B. cereus BSC5, B. subtilis BScnTNAU2 | Showed high antagonism towards Fusarium Wilt caused by Fusarium oxysporum f. sp. dianthi | Carnation | Kumar et al., 2014 |
| Subtilin, bacillibactin,surfactin, NRPs,fengycin, bacteriocins, terpenes, PKs-NRPs, PKs | Paenibacillus xylanexedens B22a, Bacillus endophyticus 2DT and MF126, Bacillus firmus IAM 12,464, Bacillus cereus ATCC14579, Bacillus aryabhattai B8W22, Bacillus megaterium NBRC 15,308, Bacillus velezensis FZB42 or SQR9, Bacillus subtilis BSn5 and NCD-2. | Antimicrobial activity against Pseudomonas syringae, Erwinia carotovora, Botrytiscinerea sp., Rhizoctonia solani, Phytophthora infestans, Verticillium dahliae, | Tomato | Zhou et al., 2021 |
| Cell wall degrading enzymes (cellulase, chitinase, and protease) antibiotics (Phenazineiturin, DAPG, fengycin, surfactins, etc.),plant growth promotion enzymes and hormones (phosphates, nitrogen fixation, ACC-deaminase, indole-3-acetic acid), N-acyl-homoserine lactones and siderophores | B. subtilis strain RMB5 and P. aeruginosa strain FB2 | Exhibit antifungal activity against Fusarium oxysporum, Fusarium moniliforme, Rhizoctonia solani, Colletotrichum gloeosporioides, Colletotrichum falcatum, Aspergillus niger, and Aspergillus flavus. | Maize, rice, wheat, potato, sunflower and soybean | Ali et al., 2020 |
| Genes hcnABC (hydrogen cyanide) and phlACBD (2,4-diacetylphloroglucinol) | P. fluorescens F113, P. protegens CHA0 and in various PGPR Pseudomonads | Synthesis of antimicrobial compounds | Bruto et al., 2014 | |
| Genes for phenazine-1-carboxylic acid, 2,4-diacetyl phloroglucinol, zwittermicin-A, oomycin, pyrrolnitrin, pyoluteorin, kanosamine, and pantocin | Pseudomonas, Azospirillum, Bacillus, Rhizobium, and Serratia species | Biocontrol of plant diseases | Kenawy et al., 2019 | |
| Host pattern recognition receptors (PRR), histone deacytylases, microbial effector proteins, nod-like receptor (NLR), microRNA, mitogen activated protein kinase (MAPK) | Different PGPR | Crucial controllers of gene expression reprogramming in plant defense mechanisms, pathogen virulence, and the exchange of signals in plant-microbe interactions. | Different plants | Bukhat et al., 2020 |
8. Mycorrhizal fungi as biofertilizers
Mycorrhizal fungi are integral components of soil ecosystems and form mutualistic symbiotic associations with the roots of most terrestrial plants (Panneerselvam et al., 2019; Mitra et al., 2023c). These fungi play a crucial role in nutrient acquisition, particularly phosphorus, in host plants (Alloun and Mitra, 2023). As biofertilizers, mycorrhizal fungi contribute to sustainable agriculture by enhancing soil fertility, promoting plant growth, and improving crop yields. Mycorrhizal associations involve intricate genetic interactions between the fungal symbiont and the plant host (Adeyemi et al., 2023). The genetic basis of mycorrhizal symbiosis includes genes encoding signaling molecules, transporters, and metabolic enzymes that mediate communication and nutrient exchange between fungi and plants. Notably, mycorrhizal symbiosis is regulated by a suite of genes in both partners, allowing for precise coordination and mutualistic benefits (Mitra et al., 2023a; Table 3).
Table 3.
Crop specific application of different mycorrhizal inoculants.
| Mycorrhizal fungi as biofertilizer | Applied in crops | Dosages | Outcomes | References | |||
|---|---|---|---|---|---|---|---|
| Glomeraceae, Claroideoglomeraceae, Paraglomeraceae | Oryza sativa | The application of the mycorrhizal fungiboosted the growth of rice | Wang et al., 2015 | ||||
| Arbuscular Mycorrhizalfungi (AMF) | Oryza sativa | AMF has the propensity to raise the harvest index and may quicken the transfer of N and P from shoots and/or soils to rice grains, especially in flooded situations. | Solaiman and Hirata, 1995 | ||||
| Acaulospora, Rhizoglomus, Entrophospora, Claroideoglomus, Funneliformis and Gigaspora | Oryza sativa | Utilizing AM inocula in the corresponding habitats boost plant production and growth. | Xavier Martins and Rodrigues, 2020 | ||||
|
Glomeromycotan Glomerales Rhizophagusirregularis |
Oryza sativa | Enhances the production of rice. The AMF interactions with rice plants indicate the function of strigolactone (SLs) in presymbiotic molecular communication, mitochondrial energy metabolism, spore germination, and hyphal branch stimulation. | Mitra et al., 2021b, 2021c, 2024 | ||||
| Funneliformismosseae, F. geosporus, Claroideoglomusclaroideum, Glomus microaggregatum, and Rhizophagus irregularis | Oryza sativa | The inoculum had a spore density of approximately 10 spores per gram of inoculum. | Plants with higher AMF colonization show higher stomatal conductance and chlorophyll fluorescence, especially under drought, indicating nutrient and hormone-driven pathways for drought tolerance in rice. | Chareesri et al., 2020 | |||
| AMF | Oryza sativa | The arbuscular mycorrhizal mutualism in rice fields enhances overall growth of rice plant. | Bao et al., 2022 | ||||
| Glomus intraradices | Maize | The AMF inoculum comprised 100 AMF infective propagules g−1 of product. The inoculum was applied at 25 kg ha−1. | Integrated nutrient management systems after the utilization of AMF inoculation. | Cozzolino et al., 2013 | |||
| AMF | Maize | Pomotes soil mycorrhizal activity and early mycorrhizal colonization of the next crop. | Njeru et al., 2014 | ||||
| Vesicular-arbuscular mycorrhizal (VAM) fungi | Maize | The level of maize mycorrhizal infection was connected with growth and yield of maize. | Boswell et al., 1998 | ||||
| AMF (Glomus mosseae, Glomus intraradices) |
Maize | Prior to seed sowing, 900 g of inoculum (25 spores per gram) had been incorporated 5 cm beneath the seeds in each row of 5 m length. | The application of AMF greatly boosted the output of dry matter and green matter, even with restricted watering. The application of AMF caused the leaf and stem ratios to rise while the ear ratios decreased. | Celebi et al., 2010 | |||
| Glomale fungi | Maize | Bestowed drought tolerance properties. | Jefwa et al., 2006 | ||||
| AMF | Maize | Maize growth using sterile substrate and non-sterile soil from native AMF communities promotes robust growth and blossoming, promoting local AMF usage in maize production farming techniques. | Alvarado-Herrejón et al., 2019 | ||||
| AMF | Wheat | AMF counteract the loss of biological fertility in soils, and provide a way to lessen the impact of biotic and abiotic stress. | Ganugi et al., 2019 | ||||
|
Glomus mosseae Glomus etunicatum |
Wheat | In native field soil about 3 spores per 100 g−1of air-dried soil has been added. | Mitigate the impacts of drought stress on wheat cultivated under field circumstances in semiarid regions. Also enhanced growth, yield, and nutrient uptake in wheat plants. | Al-Karaki et al., 2004 | |||
| AMF | Wheat | AMF inoculation improves wheat yields compared to conventional systems, potentially reducing fertilizer inputs after field validation. | Sharma et al., 2011 | ||||
| AMF | Wheat | Berruti et al., 2018 | |||||
| AMF (Glomus intraradices) |
Garlic Mustard | Foreign AM fungi and Alliariapetiolata can negatively impact local AM fungi, affecting their growth and reducing diversity in host roots. | Koch et al., 2011 | ||||
| AMF | Mustard | Improve plant heavy metal tolerance and accumulation through plant-microbe systems based on genotypes of plants and microsymbionts that are tolerant of Cd. | Belimov et al.,2020 | ||||
| AMF | Onion | A greater number of phylotypes were found in a few organic and conventional areas, including those connected to the genera Glomus, Archaeospora, and Paraglomus. | Galván et al., 2009 | ||||
| VAM (Gigaspora margarita, Glomus spp., Glomus fasciculatus Ger-. Demann, Glomus tenuis, F4, F11, FlO, NP9) |
Onion | Mycorrhizal fungi significantly stimulated onion growth and P uptake in sterilised and unsterilised soils, with Glomus spp. being the most efficient inoculants in Patumahoe and Horotiu soils. | Powell et al., 1982 | ||||
| Glomus etunicatum (AMF) | Sweet sorghum | To cultivate mycorrhizal plants, 100 g of an air-dried inoculum of Claroideoglomusetunicatium BEG168 was added to each container that held 900 g of soil. | Arbuscular mycorrhizal fungi enhances the overall growth of Sweet Sorghum cultivated in a molybdenum contaminated soil | Shi et al., 2020 | |||
| VAM | Sorghum | Enhances production | Raju et al.,1990 | ||||
| AMF | Sorghum | Mycorrhizal colonization has a detrimental influence on Striga germination, attachment, and emergence. | Lendzemo et al., 2007 | ||||
| AMF G. mosseae G. intraradices |
Mung bean | The mycorrhizal plants produced a larger seed output (161 g/m2), as well as higher levels of leaf phosphorus, leaf nitrogen, chlorophyll index, proline, total soluble carbohydrates content, relative water content, root length, root volume, root dry weight, and root/shoot weight ratio. | Habibzadeh et al., 2015 | ||||
| AMF | Mung bean | Interactions between mung bean, root-lesion nematode Pratylenchusthornei, beneficial symbionts arbuscular mycorrhizal fungi (AMF), and nitrogen-fixing Bradyrhizobium bacteria, enhance nutrition, growth, and seed yield. | Gough et al., 2021 | ||||
| AMF | Potatoes | Enhanced the potato production in soil with elevated phosphorus. | Douds et al., 2007 | ||||
| AMF | Soybean | AMF enhances the growth of soybean seeds. | Gabor et al., 1997 | ||||
The genetics underlying mycorrhizal symbiosis involve complex interactions between the fungal symbiont and the plant host, coordinating the establishment and maintenance of the mutualistic association. In arbuscular mycorrhizal (AM) symbiosis, key genetic determinants include genes encoding signaling molecules, transporters, and metabolic enzymes that mediate communication and nutrient exchange between the fungus and plant (Panneerselvam et al., 2019). For example, plant-derived strigolactones induce the germination of AM fungal spores and stimulate fungal hyphal branching, facilitating colonization of the plant root. In turn, fungal-derived Myc factors trigger plant signaling pathways, leading to the formation of arbuscules, specialized structures within root cells where nutrient exchange occurs (Hajiboland and Ahammed, 2024). Additionally, both fungal and plant genomes contain genes involved in nutrient transport and metabolism, such as phosphate transporters and enzymes involved in phosphorus metabolism, which are essential for nutrient exchange and for mutualistic benefits. In ectomycorrhizal (ECM) symbiosis, the genetic basis of the association is more complex because of the involvement of a diverse array of fungal and plant partners. ECM fungi produce a wide range of secreted proteins, including carbohydrate-active enzymes (CAZymes), proteases, and secondary metabolites, which play key roles in nutrient acquisition, symbiotic signaling, and host colonization (Genre et al., 2020). These genes are often organized into clusters or operons that are regulated by environmental and host signals. On the plant side, genetic factors involved in symbiotic recognition and signaling pathways, such as the symbiosis receptor-like kinase (SYMRK) gene and common symbiosis pathway (CSP), are essential for the establishment and maintenance of ECM symbiosis (Radhakrishnan et al., 2020). Furthermore, genes encoding plant defence responses and immune mechanisms may play a role in regulating the balance between mutualism and parasitism in symbiotic interactions. Overall, the genetics of mycorrhizal symbiosis are characterized by intricate molecular interactions between the fungal symbiont and the plant host, mediated by a diverse array of genes and signaling pathways. Understanding these genetic mechanisms is essential for elucidating the molecular basis of mycorrhizal symbiosis, optimizing the performance of mycorrhizal biofertilisers, and harnessing the potential of these symbiotic associations for sustainable agriculture and environmental conservation (Priyadarshini et al., 2023; Alloun et al., 2023).
The mode of action of mycorrhizal fungi as biofertilizers primarily revolves around their ability to extend the plant root system through the formation of a network of fungal hyphae, known as the mycelium. This mycelial network explores a larger volume of soil, effectively increasing plant access to water and nutrients, including phosphorus, nitrogen, and micronutrients.The hyphae of the fungus and plant have a larger surface area of interaction owing to penetrating hyphae. Increased contact between the two allows for higher nutrient flow (Panneerselvam et al., 2023). Additionally, mycorrhizal fungi produce enzymes such as acid phosphatases and proteases. These enzymes cansolubilize organic and inorganic phosphorus compounds in the soil, making phosphorus more readily available to the plant host. Furthermore, mycorrhizal fungienhance plant tolerance to environmental stresses, such as drought, salinity, and heavy metal toxicity, through mechanisms involving osmotic adjustment, antioxidant production, and hormone signaling pathways (Kapoor et al., 2012).
9. Impact of biofertilizers on soil microbial diversity and functionality
Biofertilizers, which are considered eco-friendly alternatives to chemical fertilizers, are increasingly used in agriculture to mitigate environmental contamination (Kour et al., 2020). Soil biodiversity is crucial for fostering healthy soil ecosystems and promoting robust plant growth. Beneficial microbes are instrumental in maintaining the soil fertility and nutrient cycles.The precise mechanism for enhancing plant growth remains unclear, and pre-deployment investigations have focused on targeted effects. However, their impact on indigenous microbial communities is often overlooked, despite the potential for significant alterations post-application, highlighting the need for comprehensive studies to optimize their effectiveness in crop rhizospheres. Metagenomic analyses provide deep insights into the intricate dynamics of soil microbial communities in response to biofertilizer application (Li et al., 2023; Iquebal et al., 2022). These analyses revealed substantial shifts in microbial diversity and functionality, indicating the enrichment of beneficial taxa that are crucial for nutrient cycling, disease suppression, and plant growth promotion (Pang et al., 2021; Lutz et al., 2020; Wang et al., 2023). By elucidating the genomic composition and metabolic potential of soil microbiota, metagenomic studies offer a comprehensive understanding of how biofertilizers modulate microbial communities and ecosystem processes (Trivedi et al., 2021). This highlights the intricate interactions between biofertilizers and indigenous soil microbes and elucidates the mechanisms underlying enhanced nutrient availability and plant health (Vishwakarma et al., 2020). Leveraging these insights, researchers can fine-tune biofertilizer formulations and application regimes to optimize soil health and bolster sustainable crop production practices. Yang et al. (2022) employed high-throughput quantitative polymerase chain reaction (HT-qPCR) to examine the impact of biofertilizer application over three years on soil antibiotic resistance genes. The study found that biofertilizer application had no significant effect on the relative abundance of antibiotic resistance genes in the soil, likely because of the intricate soil environment and competition between exogenous and indigenous microorganisms. Additionally, biofertilizers have been observed to alter the soil microbial community structure by modifying soil properties. Li et al. (2020a) performed metagenomic analysis and investigated the probable reason for the significant increases in sugarcane yield and sugar content under organic fertilizer treatment. Enhanced soil nutrient status and microbial communities (Chloroflexi, Acidobacteria, Proteobacteria, and Gemmatimonadetes) likely contribute to these effects, highlighting the potential of organic fertilizers for sustainable sugarcane production. Wu et al. (2020) observed higher bacterial diversity in soil treated with increased biofertilizer and reduced chemical fertilizer than in unfertilized and conventionally fertilized soils over two years. Zainuddin et al. (2022) found that the application of BF70 (containing 70 % biofertilizer) led to the highest microbial diversity, with 59 isolated bacteria and 45 isolated fungi. However, the highest bacterial diversity (H’) was noted in the soil treated with BF100 (containing 100 % biofertilizer) (3.4723). Biofertilizers containing beneficial microbes enhance the availability of soil microflora upon application (Seenivasagan and Babalola, 2021). The undisturbed conditions of biofertilizers positively influence bacterial activity and sustainability compared to chemical inputs. Additionally, biofertilizer application significantly boosted soil bacterial diversity, abundance, and viability. Zainuddin et al. (2022) found thatafter biofertilizer application, Cupriviadus sp. from the β-proteobacteria group dominated bacterial diversity, followed by Microbacterium sp. from Actinobacteria, aiding nutrient acquisition and disease protection. Actinobacteria phylum bacteria contribute to organic matter turnover and soil ecological cycles (Zainuddin et al., 2022). BF70 treatment recorded the highest fungal diversity (H’) and isolated 45 fungi, with Ascomycota being prevalent, particularly Aspergillus sp., Hypocrea sp. and Trichoderma sp. These fungi facilitate plant growth, act as biocontrol agents, and contribute to carbon cycling (Zainuddin et al., 2022). The abundance of fungi in BF70-treated soil correlates with increased vegetative measurements in plants owing to enhanced nutrient availability (Yadav et al., 2021). Bhattacharjee and Dey (2014) also supported this finding, indicating that biofertilizer application with reduced chemical fertilizers enhances soil nutrient availability, microbial biomass, richness, and overall soil health.
10. Commercial prospects of biofertilizers
The commercial prospects of biofertilizers have garnered significant attention in recent years becauseof their potential to revolutionize agricultural practices and promote sustainability (Yadav and Yadav, 2024). The biofertilizer market can be divided according to the type of microorganisms, crop, biofertilizer product, application method, and primary region of use (Joshi and Gauraha, 2022). Globally, governments are increasingly investing in initiatives to promote the adoption of biofertilizers (Kumawat et al., 2021). These initiatives often include subsidies, tax incentives, and educational programs aimed at raising farmers'awareness about the benefits of biofertilizers. Moreover, governments may also offer research grants to support scientific advancements in biofertilizer technology (Mawar et al., 2021). Scientific initiatives haveplayed a crucial role in enhancing the efficacy and applicability of biofertilizers (Bhardwaj et al., 2014). Researchers are continually exploring new microbial strains with superior nutrient-fixing abilities and developing innovative formulations to improve biofertilizer performance under diverse environmental conditions. Advances in biotechnology have enabled the genetic engineering of microbes to enhance their nutrient mobilization and stress tolerance capabilities, thereby expanding the commercial potential of biofertilisers (Sudheer et al., 2020). The rising global demand for organic and sustainable agricultural practices has further boosted the commercial prospects of biofertilisers. Consumers are increasingly seeking food products cultivated using environmentally friendly methods, creating a lucrative market for biofertilizer manufacturers (Joshi and Gauraha, 2022). Moreover, growing awareness of the adverse environmental impacts of chemical fertilizers, such as soil degradation and water pollution, encourages farmers to adopt biofertilizers as eco-friendly alternatives. Overall, with continued government support and scientific advancements, the commercial future of biofertilizers appears promising, offering sustainable solutions to address the challenges of modern agriculture while ensuring long-term environmental stewardship (Yadav and Yadav, 2024; Kumawat et al., 2021). Market dynamics demand from farmers, the regulatory environment, technological development, and competition from agricultural inputs influence the production, sale, and distribution of bioinput in one specific form or another. The use of bio-inputs and their specific market characteristics may vary significantly from one region to another, based on differences between agricultural countries, soil type, nature, and government regulation. Progress in targeting the market based on microbial-based bio-inoculant development and opportunities may open up for investment in the lab, production plant, network of distribution, and marketing. An investor can evaluate the expected return based on market size, growth potential, competitive factors, and risks based on regulatory risk. Environmental externalities refer to the impact of microbial-based bioinoculants on the environment. They have economic impacts through management, the carbon market, and valuation of the service system. Because biofertilizers are produced, distributed, and sold through a broad value chain, economic analysis may cover multiple stakeholders, including research institutions, biotechnology companies, manufacturers, retailers, distributors, and farmers. This may include an investigation of the maximum profitability level at each value chain stage, efficiency of multiple processes, and opportunities for optimal value addition. Government policies, incentives, subsidies, and regulations significantly influence the economics of microbial-based bio-inoculants. Thus, policies of R&D support, market access, certification, and sustainable practice adoption affect the associated cost, affordability, and general competence in predatory market environments. Bio-inoculants are sold to final consumers through diverse sales channels, including agricultural input retailers, distributors, cooperatives, online platforms, and zero- and three-level traders. Distribution networks are more or less complex depending on the demand and market size, availability of supporting infrastructure such as transportation, warehouses, information technologies, transaction services, geographic extent of the market, nature of the customer's institutions, level of their organization, etc. Conviction of the buyer to use microbial-based bio-inoculant products is a complex marketing process that includes appealing to buyers to sensitize them about the advantages, giving them a response on how and when to use, pricing, and enabling the buyer to adopt and implement a product. Marketers may use different methods or devices to sell products and integrate sales and advertising to conduct sales promotions. However, customers are likely to respond to a sales pitch when meeting their personal requirements or needs. Finally, the sales team must be proactive in engaging in mutually satisfying consultative sales practices. The distribution, sale, and use of microbial-based bioinoculants should comply with national rules and regulations. Therefore, a company seeking to sell such products in a given country must register the product with the government and abide by product labeling and advertising rules. Compliance with the environmental criteria for production and use in safety concerns is also necessary.Farmers can be trained through workshops, on-farm sessions, and field demonstrations to provide practical knowledge on biofertilizer application techniques, dosage, and timing. Educational materials such as brochures and videos can supplement this training. Continuous support and troubleshooting assistance should be offered as farmers adopt biofertilizers. For biofertilizer producers, technical training in fermentation processes, quality control, and safety protocols is essential. Additionally, they require guidance on optimizing production methods and minimizing environmental impacts. Market development strategies, such as branding and distribution training, can help producers effectively market their products. By implementing these comprehensive training programs, both farmers and biofertilizer producers can maximize the benefits of biofertilisers for sustainable agriculture.
11. Biofertilizer to improve bioeconomy
Biofertilizers, offering sustainable alternatives to chemical fertilizers, can enhance agricultural productivity, soil health, and socioeconomic development, thereby contributing significantly to the bioeconomy (Sadhukhan et al., 2018; Kour et al., 2020; Puglia et al., 2021). Additionally, biofertilizers contribute to carbon sequestration and greenhouse gas mitigation, aligning with global efforts to address climate change and promote sustainable development (Ajmal et al., 2018). The production and use of biofertilizers create opportunities for innovation, research, and technology development in biotechnology, microbiology, and agricultural sciences, driving economic growth and job creation in related industries (Singh, 2017). By reducing dependency on imported chemical fertilizers, biofertilizers can enhance national food security and resilience to external shocks, thereby strengthening the overall economic stability of countries (Sarker et al., 2023). Overall, the widespread adoption of biofertilizers can lead to a more sustainable, inclusive, and resilient bioeconomy that benefits both the environment and society. Joshi and Gauraha (2022) forecasted that the biofertilizer market would experience a Compound Annual Growth Rate (CAGR) of nearly 14 % by 2023. In 2016, the global biofertilizer market was valued at USD 1106.4 million, and it is anticipated to expand at a rate of 14.2 % to reach USD 3124.5 million by the ending of 2024 (Joshi and Gauraha, 2022).From an economic perspective, the progress in microbial-based bioinoculants for soil health and sustainable crop production can be examined through several mechanisms. Cost and benefit analysis can be performed to assess the economic evaluations and compare the monetary value of all costs to develop, produce, distribute, and apply microbial-based bio-inoculants and the monetary value of the benefits obtained from enhanced soil health, increased crop yield, and reduced use of other inputs.Tiwari et al. (2004) noted that utilizing biofertilizers presents significant economic advantages as they are more cost-effective than chemical fertilizers, boasting a benefit-cost ratio of 10:1.Hassanpour et al. (2021) proposed that the most effective and beneficial fertilizer regimen for corn cultivation involves the simultaneous application of biofertilizer and enriched granular organic fertilizer. This approach yields a net profit of 71.5 million rials and a harvest of 12.5 tons per hectare, which has the potential to greatly improve the bioeconomy.
12. Biofertilizer versus inorganic fertilizer
A comparison between biofertilizers and inorganic fertilizers presents a nuanced view of agricultural practices by weighing the benefits and drawbacks of each approach. Inorganic fertilizers, which are typically composed of synthetic nutrients, are readily available and provide a rapid boost to plant growth (Suhag, 2016). They are easy to apply and offer precise control over nutrient levels, making them popular among farmers seeking immediate results. However, the excessive use of inorganic fertilizers can lead to soil degradation, nutrient imbalances, and environmental pollution due to runoff. However, biofertilizers harness the ability of beneficial microorganisms to enhance soil nutrient availability. They improve soil structure, promote nutrient cycling, and contribute to long-term soil fertility and sustainability (Singh et al., 2016). Despite these advantages, biofertilizers have not yet gained widespread acceptance by farmers for several reasons (Carvajal-Muñoz and Carmona-Garcia, 2012). First, there is a lack of awareness and understanding of biofertilizer technology among farmers, leading to skepticism about its effectiveness compared with inorganic fertilizers. The inconsistent performance of biofertilizers under different soil and environmental conditions poses a challenge to their adoption (Malusà et al., 2016). Farmers often prefer the reliability and immediate results offered by inorganic fertilizers and fear potential yield losses associated with experimenting with new agricultural inputs (Michelson et al., 2023). Likewise, the availability and affordability of inorganic fertilizers, coupled with existing agricultural policies and subsidy programs that favor their use, further hinder the adoption of biofertilizers (Raimi et al., 2021, 2017; Kumawat et al., 2021). Overcoming these barriers requires concerted efforts by governments, researchers, and agricultural extension services to educate farmers about the benefits of biofertilizers, improve their efficacy and reliability through research and development, and create supportive policy frameworks that incentivize sustainable agricultural practices (Giles et al., 2021; Chartres and Noble, 2015; Rigby et al., 2001). Singhalage et al. (2019) discussed the economic advantages of strawberry cultivation using monocultures of Aspergillus sp. and Enterobacter sp. as well as their mixed cultures (fungal-bacterial biofilm; BF), compared with the recommended dosage of chemical fertilizers (CFs) in a pot experiment. In terms of productivity, strawberries yielded a 152 % higher profit when treated with BF alongside 39 % CFs compared to 100 % CFs treatment. Additionally, coupling Aspergillus sp. and Enterobacter sp. with 100 % and 34 % CFs, respectively, resulted in 102 % and 66 % higher profits, respectively. These findings clearly demonstrate the effectiveness of BF as a biofertilizer in reducing dependency on CFs in strawberry farming (Singhalage et al., 2019). Employing biological fertilizers in flat rice fields have the potential to enhance both land and crop productivity (Nurkhaida et al., 2021). These fertilizers are beneficial for boosting the growth and yield of various crops, including rice, soybeans, chilies, vegetables, and sugarcane. Utilizing the biofertilizer Agrimeth in lowland and upland rice cultivation can result in a 50 % reduction in the use of inorganic fertilizers, such as nitrogen, phosphorus, and potassium, consequently augmenting the income of rice farmers (Nurkhaida et al., 2021). Furthermore, the adoption of biofertilizers can lead to a similar 50 % decrease in the application of N, P, and K fertilizers to horticultural crops. Additionally, incorporating biofertilizers has been shown to elevate rice yields by 1.4 tons per hectare, equating to a 20.77 % increase, while concurrently decreasing the reliance on inorganic fertilizers by up to 25 % (Nurkhaida et al., 2021).
13. Future prospects to boost up the market of biofertilizer
The prospects of biofertilizers are promising for revolutionizing agricultural practices worldwide. As global populations continue to grow and concerns about environmental sustainability escalate, there is increasing recognition of the need for sustainable agricultural solutions (Joshi and Gauraha, 2022). Biofertilizers, offering a sustainable alternative to chemical fertilizers, address soil degradation concerns and greenhouse gas emissions while advancing with technological improvements (Kumawat et al., 2021; Joshi and Gauraha, 2022; Alnaass et al., 2023). Enhancing the quality of biofertilisers involves a multifaceted approach that encompasses various processes. Quality control measures are essential throughout the production process, including sourcing of raw materials, microbial inoculum preparation, and formulation to ensure consistency and effectiveness (Jenkinsand Grzywacz, 2000; García de Salamone et al., 2019). Quality assurance protocols, such as regular testing for microbial viability, nutrient content, and absence of contaminants, are crucial for maintaining product integrity and meeting regulatory standards (Stephens and Rask, 2000; Okpalaand Korzeniowska, 2023). Encapsulation of microbes within protective matrices or carriers enhances their survival and efficacy in the soil, prolongs their shelf life, and improves their delivery to plant root zones (Szopa et al., 2022). The production of encapsulated microbes for the fortification of biofertilizers is shown in Fig. 3. Additionally, optimizing fermentation and formulation techniques, selecting compatible microbial strains, and fine-tuning nutrient compositions will contribute to the development of high-quality biofertilizers with reliable performance and agronomic benefits (Sharma et al., 2023). Through meticulous attention paid to these processes, biofertilizer manufacturers can ensure the delivery of premium products that support sustainable agriculture and environmental stewardship. Advancements in biotechnology have facilitated the development of more effective biofertilizer formulations that enhance nutrient uptake and crop yields. The increasing adoption of organic farming methods further propels the demand for biofertilizers, as consumers prioritize health and environmental consciousness. Government initiatives promoting organic agriculture and subsidies for biofertilizer production have contributed to market growth. Governments can provide financial incentives, such as subsidies and grants, to encourage farmers to adopt biofertilizers, making them more affordable and accessible. Additionally, regulatory measures can be implemented to promote the use of biofertilizers, including setting standards for organic farming and certification programs. Investing in R&D can further enhance the efficacy of biofertilizers, leading to increased confidence among farmers and consumers. Moreover, government policies supporting sustainable agricultural practices and environmental conservation can create a conducive environment for the growth of the biofertilizer market.
Fig. 3.
Encapsulation of beneficial microbes for their long-term viability in biofertilizer.
14. Conclusion
Bioinoculants offer a sustainable solution for modern agriculture by harnessing beneficial microorganisms, such as bacteria, fungi, and algae. They naturally enhance soil fertility, boost plant growth, and increase crop yield by fixing nitrogen, solubilizing phosphorus, and mobilizing nutrients. Biofertilizers mitigate the environmental issues caused by chemical fertilizers, such as soil degradation and water pollution. As agriculture moves towards sustainability, the widespread use of biofertilizers promotes agroecological principles, improves food security, and helps tocombat climate change. This study underscores promising prospects for the commercialization of biofertilizers, driven by the growing demand for sustainable agricultural practices. Effective market policies and government support can further catalyze their adoption, creating opportunities for job growth in the production, research, and distribution sectors within the burgeoning biofertilizer industry.However, further research, education, and infrastructure development are needed to realize the full potential of biofertilizers and to effectively integrate them into agricultural systems worldwide. Through collaborative efforts among scientists, policymakers, farmers, and consumers, biofertilisers can play a crucial role in shaping a sustainable and resilient future for global agriculture.
CRediT authorship contribution statement
Aurodeepa Samantaray: Writing – review & editing, Data curation, Writing – original draft. Sourav Chattaraj: Writing – review & editing, Data curation, Writing – original draft. Debasis Mitra: Writing – review & editing, Data curation, Writing – original draft. Arindam Ganguly: Writing – review & editing, Data curation, Writing – original draft. Rahul Kumar: Writing – review & editing, Data curation, Writing – original draft. Ashish Gaur: Writing – review & editing, Supervision, Investigation. Pradeep K.Das Mohapatra: Writing – review & editing, Supervision, Investigation. Sergio de los Santos-Villalobos: Writing – review & editing, Supervision, Investigation. Anju Rani: Writing – review & editing, Supervision, Investigation. Hrudayanath Thatoi: Writing – review & editing, Supervision, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful to Siksha ‘O’ Anusandhan (Deemed to be University), India; Graphic Era (Deemed to be University), India; Bankura Sammilani College, India; Raiganj University, India; and Instituto Tecnológico de Sonora, Mexico for their support.
Data availability
Data will be made available on request.
References
- Abd-Alla M.H., Al-Amri S.M., El-Enany A.W.E. Enhancing rhizobium–legume symbiosis and reducing nitrogen fertilizer use are potential options for mitigating climate change. Agriculture. 2023;13(11):2092. [Google Scholar]
- Adeyemi, N.O., Emmanuel, O.O. and Mitra, D., 2023. 17 AM fungi interactions in rice seedling production. Arbuscular Mycorrhizal Fungi: For Nutrient, Abiotic and Biotic Stress Management in Rice, p.131.
- Ajmal M., Ali H.I., Saeed R., Akhtar A., Tahir M., Mehboob M.Z., Ayub A. Biofertilizer as an alternative for chemical fertilizers. J. Agric. Allied Sci. 2018;7(1):1–7. [Google Scholar]
- Alghamdi S.A., Alharby H.F., Abdelfattah M.A., Mohamed I.A., Hakeem K.R., Rady M.M., Shaaban A. Spirulina platensis-inoculated humified compost boosts rhizosphere soil hydro-physico-chemical properties and Atriplex nummularia forage yield and quality in an arid saline calcareous soil. J. Soil Sci. Plant Nutr. 2023;23(2):2215–2236. [Google Scholar]
- Ali M.M., Petropoulos S.A., Selim D.A.F.H., Elbagory M., Othman M.M., Omara A.E.D., Mohamed M.H. Plant growth, yield and quality of potato crop in relation to potassium fertilization. Agronomy. 2021;11(4):675. [Google Scholar]
- Ali S., Hameed S., Shahid M., Iqbal M., Lazarovits G., Imran A. Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol. Res. 2020;232 doi: 10.1016/j.micres.2019.126389. [DOI] [PubMed] [Google Scholar]
- Al-Karaki G., McMichael B.Z.A.K.J., Zak J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza. 2004;14:263–269. doi: 10.1007/s00572-003-0265-2. [DOI] [PubMed] [Google Scholar]
- Alloun, W. and Mitra, D., 2023. A sustainable approach for enhancing phosphorous and nitrogen use efficiency in rice cultivation. Arbuscular Mycorrhizal Fungi: For Nutrient, Abiotic and Biotic Stress Management in Rice, p.27.
- Alloun, W., Sinha, S. and Mitra, D., 2023. 21 AM fungi production upscaling, government regulations, marketing. Arbuscular Mycorrhizal Fungi: For Nutrient, Abiotic and Biotic Stress Management in Rice, p.163.
- Alnaass N.S., Agil H.K., Alyaseer N.A., Abubaira M., Ibrahim H.K. The effect of biofertilization on plant growth and its role in reducing soil pollution problems with chemical fertilizers. Afr. J. Adv. Pure Appl. Sci. (AJAPAS) 2023:387–400. [Google Scholar]
- Alvarado-Herrejón M., Larsen J., Gavito M.E., Jaramillo-López P.F., Vestberg M., Martínez-Trujillo M., Carreón-Abud Y. Relation between arbuscular mycorrhizal fungi, root-lesion nematodes and soil characteristics in maize agroecosystems. Appl. Soil Ecol. 2019;135:1–8. [Google Scholar]
- Angom J., Viswanathan P.K. The Palgrave Handbook of Socio-ecological Resilience in the Face of Climate Change: Contexts from a Developing Country. Springer Nature Singapore; Singapore: 2023. Climate-smart agricultural practices and technologies in India and South Africa: implications for climate change adaption and sustainable livelihoods; pp. 161–195. [Google Scholar]
- Bandyopadhyay P., Yadav B.G., Kumar S.G., Kumar R., Kogel K.H., Kumar S. Piriformospora indica and Azotobacter chroococcum consortium facilitates higher acquisition of N, P with improved carbon allocation and enhanced plant growth in Oryza sativa. J. Fungi. 2022;8(5):453. doi: 10.3390/jof8050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X., Zou J., Zhang B., Wu L., Yang T., Huang Q. Arbuscular mycorrhizal fungi and microbes interaction in rice mycorrhizosphere. Agronomy. 2022;12(6):1277. [Google Scholar]
- Barman S., Das S., Bhattacharya S.S. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; 2019. The prospects of bio-fertilizer technology for productive and sustainable agricultural growth; pp. 233–253. [Google Scholar]
- Barragán-Ocaña A., del Carmen del-Valle-Rivera M. Rural development and environmental protection through the use of biofertilizers in agriculture: an alternative for underdeveloped countries? Technol. Soc. 2016;46:90–99. [Google Scholar]
- Basu A., Prasad P., Das S.N., Kalam S., Sayyed R.Z., Reddy M.S., El Enshasy H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability. 2021;13(3):1140. [Google Scholar]
- Belimov A.A., Shaposhnikov A.I., Azarova T.S., Makarova N.M., Safronova V.I., Litvinskiy V.A., Nosikov V.V., Zavalin A.A., Tikhonovich I.A. Microbial consortium of PGPR, rhizobia and arbuscular mycorrhizal fungus makes pea mutant SGECdt comparable with Indian mustard in cadmium tolerance and accumulation. Plants. 2020;9(8):975. doi: 10.3390/plants9080975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berruti A., Bianciotto V., Lumini E. Seasonal variation in winter wheat field soil arbuscular mycorrhizal fungus communities after non-mycorrhizal crop cultivation. Mycorrhiza. 2018;28(5):535–548. doi: 10.1007/s00572-018-0845-9. [DOI] [PubMed] [Google Scholar]
- Bharathi V., Sudhakar R., Parimala K., Reddy V.A. Evaluation of bioagents and biofertilizers for the managament of seed and seedling diseases of Sesamum indicum (Sesame) Int. J. Phytopathol. 2013;2(3):179–186. [Google Scholar]
- Bhardwaj D., Ansari M.W., Sahoo R.K., Tuteja N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014;13:1–10. doi: 10.1186/1475-2859-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat T.A., Ahmad L., Ganai M.A., Khan O.A. Nitrogen fixing biofertilizers; mechanism and growth promotion: a review. J. Pure Appl. Microbiol. 2015;9(2):1675–1690. [Google Scholar]
- Bhattacharjee R., Dey U. Biofertilizer, a way towards organic agriculture: a review. Afr. J. Microbiol. Res. 2014;8(24):2332–2343. [Google Scholar]
- Bhattacharjee R.B., Singh A., Mukhopadhyay S.N. Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: prospects and challenges. Appl. Microbiol. Biotechnol. 2008;80(2):199–209. doi: 10.1007/s00253-008-1567-2. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya P.N., Jha D.K. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J. Microbiol. Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- Binodh A.K., Thankappan S., Ravichandran A., Mitra D., Alagarsamy S., Panneerselvam P., Senapati A., Sami R., Al-Mushhin A.A., Aljahani A.H., Alyamani A. Synergistic modulation of seed metabolites and enzymatic antioxidants tweaks moisture stress tolerance in non-cultivated traditional rice genotypes during germination. Plants. 2022;11(6):775. doi: 10.3390/plants11060775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas I., Mitra D., Mitra D., Chattaraj S., Senapati A., Chakraborty A., Basak G., Das Mohapatra P.K. Application of egg shell with fortified vermicompost in Capsicum cultivation: a strategy in waste management. Int. J. Recycl. Org. Waste Agric. 2022;11(4):451–461. [Google Scholar]
- Biswas I., Mitra D., Senapati A., Mitra D., Chattaraj S., Ali M., Basak G., Panneerselvam P., Das Mohapatra P.K. Valorization of vermicompost with bacterial fermented chicken feather hydrolysate for the yield improvement of tomato plant: a novel organic combination. Int. J. Recycl. Org. Waste Agric. 2021;10(1):29–42. [Google Scholar]
- Boswell E.P., Koide R.T., Shumway D.L., Addy H.D. Winter wheat cover cropping, VA mycorrhizal fungi and maize growth and yield. Agric. Ecosyst. Environ. 1998;67(1):55–65. [Google Scholar]
- Bothe H., Schmitz O., Yates M.G., Newton W.E. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol. Mol. Biol. Rev. 2010;74(4):529–551. doi: 10.1128/MMBR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruto M., Prigent-Combaret C., Muller D., Moënne-Loccoz Y. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci. Rep. 2014;4(1):6261. doi: 10.1038/srep06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhat S., Imran A., Javaid S., Shahid M., Majeed A., Naqqash T. Communication of plants with microbial world: exploring the regulatory networks for PGPR mediated defensesignaling. Microbiol. Res. 2020;238 doi: 10.1016/j.micres.2020.126486. [DOI] [PubMed] [Google Scholar]
- Carvajal-Muñoz J.S., Carmona-Garcia C.E. Benefits and limitations of biofertilization in agricultural practices. Livest. Res. Rural Dev. 2012;24(3):1–8. [Google Scholar]
- Celebi S.Z., Demir S., Celebi R., Durak E.D., Yilmaz I.H. The effect of Arbuscular Mycorrhizal Fungi (AMF) applications on the silage maize (Zea mays L.) yield in different irrigation regimes. Eur. J. Soil Biol. 2010;46(5):302–305. [Google Scholar]
- Chabot R., Antoun H., Cescas M.P. Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar. phaseoli. Plant Soil. 1996;184:311–321. [Google Scholar]
- Chakraborty T., Akhtar N. Biofertilizers: Study and Impact. Wiley; 2021. Biofertilizers: prospects and challenges for future; pp. 575–590. [Google Scholar]
- Chareesri A., De Deyn G.B., Sergeeva L., Polthanee A., Kuyper T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza. 2020;30(2):315–328. doi: 10.1007/s00572-020-00953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartres C.J., Noble A. Sustainable intensification: overcoming land and water constraints on food production. Food Secur. 2015;7:235–245. [Google Scholar]
- Chattaraj S., Behera B.K., Das Mohapatra P.K. Effective valorization of Chlorella biomass and Brewers spent grain substituting fish meal and soybean meal in the diet of herbivorous fish Cirrhinus reba for higher growth, digestibility and sustainable cultivation. J. Appl. Phycol. 2024;36(1):217–232. [Google Scholar]
- Chattaraj S., Das Mohapatra P.K. Immunostimulatory and antagonistic potential of the methanolic extract of Oedogonium intermedium SCB in Cirrhinus reba challenged with Aeromonas hydrophila. Aquacult. Int. 2023:1–26. [Google Scholar]
- Chattaraj S., Ganguly A., Mandal A., Das Mohapatra P.K. A review of the role of probiotics for the control of viral diseases in aquaculture. Aquac. Int. 2022;30(5):2513–2539. [Google Scholar]
- Chattaraj S., Ganguly A., Mitra D., Mitra D., Das Mohapatra P.K. Study of intestinal bacteria of Cirrhinus reba and characterization of a new probiotic bacteria: an initiative to save the threatened species of Cirrhinus. Appl. Biochem. Microbiol. 2023;59(6):833–849. [Google Scholar]
- Chattaraj S., Mitra D., Chattaraj A., Chattaraj M., Kundu M., Ganguly A., Mohapatra P.K.D. Antioncogenic potential of probiotics: challenges and future prospective. Indian J. Microbiol. Res. 2023;10(1):1–10. [Google Scholar]
- Chattaraj S., Mitra D., Ganguly A., Thatoi H., Mohapatra P.K.D. A critical review on the biotechnological potential of Brewers’ waste: Challenges and future alternatives. Current Research in Microbial Sciences. 2024:100228. doi: 10.1016/j.crmicr.2024.100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. Beneficial effects of viruses for horticultural plants. Hortic. Rev. 1981;3:394–411. [Google Scholar]
- Cong R.G., Thomsen M. Review of ecosystem services in a bio-based circular economy and governance mechanisms. Ecosyst. Serv. 2021;50 [Google Scholar]
- Contreras-Soto M.B., Tovar-Pedraza J.M. Viruses of plant-pathogenic fungi: a promising biocontrol strategy for Sclerotinia sclerotiorum. Arch. Microbiol. 2024;206(1):38. doi: 10.1007/s00203-023-03774-8. [DOI] [PubMed] [Google Scholar]
- Cozzolino V., Di Meo V., Piccolo A. Impact of arbuscular mycorrhizal fungi applications on maize production and soil phosphorus availability. J. Geochem. Explor. 2013;129:40–44. [Google Scholar]
- Das H.K. Azotobacters as biofertilizer. Adv. Appl. Microbiol. 2019;108:1–43. doi: 10.1016/bs.aambs.2019.07.001. [DOI] [PubMed] [Google Scholar]
- David O.M., Olawusi A.C., Oluwole O.A., Adeola P.O., Odeyemi A.T. Isolation, molecular characterization and application of Aspergillus niger and Penicillium chrysogenum with biofertilizer potentials to enhance rice growth. Trop. J. Nat. Prod. Res. 2023;7(4) [Google Scholar]
- de Faria S.M., de Carvalho Balieiro F., Paula R.R., Santos F.M., Zilli J.E. Mixed Plantations of Eucalyptus and Leguminous Trees: Soil, Microbiology and Ecosystem Services. Springer; 2020. Biological nitrogen fixation (BNF) in mixed-forest plantations; pp. 103–135. [Google Scholar]
- de Novais C.B., Sbrana C., da Conceição Jesus E., Rouws L.F.M., Giovannetti M., Avio L., Siqueira J.O., Saggin Junior O.J., da Silva E.M.R., de Faria S.M. Mycorrhizal networks facilitate the colonization of legume roots by a symbiotic nitrogen-fixing bacterium. Mycorrhiza. 2020;30(2):389–396. doi: 10.1007/s00572-020-00948-w. [DOI] [PubMed] [Google Scholar]
- Deilamirad M., Sarikhani M.R., Oustan S. Effect of potassium releasing pseudomonads on growth and k uptake of tomato in two soils with different amount of available K. Water Soil. 2017;31(4):1159–1170. [Google Scholar]
- Dong Y., Zhang J., Xu X., Dong Q., Zhang A., Xiong Z. Symbiotic nitrogen fixation enhanced crop production and mitigated nitrous oxide emissions from paddy crops. Field Crops Res. 2024;307 [Google Scholar]
- Douds D.D., Jr, Nagahashi G., Reider C., Hepperly P.R. Inoculation with arbuscular mycorrhizal fungi increases the yield of potatoes in a high P soil. Biol. Agric. Hortic. 2007;25(1):67–78. [Google Scholar]
- Etesami H. Nutrient Dynamics for Sustainable Crop Production. Springer; 2020. Enhanced phosphorus fertilizer use efficiency with microorganisms; pp. 215–245. [Google Scholar]
- Fatima P., Mishra A., Om H., Saha B., Kumar P. Biofertilizers and Biopesticides in Sustainable Agriculture. Apple Academic Press; 2019. Free living nitrogen fixation and their response to agricultural crops; pp. 173–200. [Google Scholar]
- Figueiredo G.G.O., Lopes V.R., Romano T., Camara M.C. Beneficial Microbes in Agro-Ecology. Academic Press; 2020. Clostridium; pp. 477–491. [Google Scholar]
- Gabor J., et al. Mycorrhizal fungi effects on nutrient composition and yield of soybean seeds. J. Plant Nutr. 1997;20:4–5. [Google Scholar]
- Galván G.A., Parádi I., Burger K., Baar J., Kuyper T.W., Scholten O.E., Kik C. Molecular diversity of arbuscular mycorrhizal fungi in onion roots from organic and conventional farming systems in the Netherlands. Mycorrhiza. 2009;19:317–328. doi: 10.1007/s00572-009-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Chattaraj S., Ganguly M., Chattaraj M., Banerjee A., Mandal A., Ali Khan M., Sen S.K., Das Mohapatra P.K. Effect of three probiotic Bacillus strains supplemented feeds on growth, carcass composition and blood parameters of Clarias magur (Hamilton) J. Appl. Aquac. 2024:1–26. [Google Scholar]
- Ganugi P., Masoni A., Pietramellara G., Benedettelli S. A review of studies from the last twenty years on plant–arbuscular mycorrhizal fungi associations and their uses for wheat crops. Agronomy. 2019;9(12):840. [Google Scholar]
- García de Salamone I.E., Esquivel-Cote R., Hernández-Melchor D.J., Alarcón A. Microbial Interventions in Agriculture and Environment: Volume 2: Rhizosphere, Microbiome and Agro-ecology. Springer; 2019. Manufacturing and quality control of inoculants from the paradigm of circular agriculture; pp. 37–74. [Google Scholar]
- Garg N., Pandove G., Kalia A., Pandey V., Mahala P. Differential effects of plant growth-promoting rhizobacteria used as soil application vis-à-vis root dip of seedlings on the performance of onion (Allium cepa L.) In three distinct agro-climatic zones of Indian Punjab. Commun. Soil Sci. Plant Anal. 2022;53(18):2509–2528. [Google Scholar]
- Garrity D.P., Akinnifesi F.K., Ajayi O.C., Weldesemayat S.G., Mowo J.G., Kalinganire A., Larwanou M., Bayala J. Evergreen Agriculture: a robust approach to sustainable food security in Africa. Food Secur. 2010;2:197–214. [Google Scholar]
- Gautam K., Rajvanshi M., Chugh N., Dixit R.B., Kumar G.R.K., Kumar C., Sagaram U.S., Dasgupta S. Microalgal applications toward agricultural sustainability: recent trends and future prospects. Microalgae. 2021:339–379. [Google Scholar]
- Gautam S.S., Gondwal M., Soni R., Gautam B.P.S. Trends of Applied Microbiology for Sustainable Economy. Academic Press; 2022. Potash biofertilizers: current development, formulation, and applications; pp. 481–500. [Google Scholar]
- Genre A., Lanfranco L., Perotto S., Bonfante P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020;18(11):649–660. doi: 10.1038/s41579-020-0402-3. [DOI] [PubMed] [Google Scholar]
- Gii research, 2024. Accessed from: https://www.giiresearch.com/report/moi1431276-india-biofertilizer-market-share-analysis-industry.html (Last accessed on 22.03.2024).
- Giles J., Grosjean G., Le Coq J.F., Huber B., Bui V.L., Läderach P. Barriers to implementing climate policies in agriculture: a case study from Viet Nam. Front. Sustain. Food Syst. 2021;5 [Google Scholar]
- Goswami D., Thakker J.N., Dhandhukia P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): a review. Cogent Food Agric. 2016;2(1) [Google Scholar]
- Gough E.C., Owen K.J., Zwart R.S., Thompson J.P. Arbuscular mycorrhizal fungi acted synergistically with Bradyrhizobium sp. to improve nodulation, nitrogen fixation, plant growth and seed yield of mung bean (Vigna radiata) but increased the population density of the root-lesion nematode Pratylenchusthornei. Plant Soil. 2021;465(1):431–452. [Google Scholar]
- Gupta V., Ratha S.K., Sood A., Chaudhary V., Prasanna R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)–prospects and challenges. Algal Res. 2013;2(2):79–97. [Google Scholar]
- Habibzadeh Y., Jalilian J., Zardashti M.R., Pirzad A., Eini O. Some morpho-physiological characteristics of mung bean mycorrhizal plants under different irrigation regimes in field condition. J. Plant Nutr. 2015;38(11):1754–1767. [Google Scholar]
- Hajiboland R., Ahammed G.J. Arbuscular Mycorrhizal Fungi and Higher Plants: Fundamentals and Applications. Springer Nature Singapore; Singapore: 2024. Signaling events during the establishment of symbiosis between arbuscular mycorrhizal fungi and plant roots; pp. 67–97. [Google Scholar]
- Haroun M., Xie S., Awadelkareem W., Wang J., Qian X. Influence of biofertilizer on heavy metal bioremediation and enzyme activities in the soil to revealing the potential for sustainable soil restoration. Sci. Rep. 2023;13(1):20684. doi: 10.1038/s41598-023-44986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanpour B., Keshavarz K., Chakeralhosseini M.R. Economic study of bio-fertilizer effectiveness on yield and profitability in corn fields. Agric. Econ. 2021;15(4):23–44. [Google Scholar]
- Hata S., Tsuda R., Kojima S., Tanaka A., Kouchi H. Both incompatible and compatible rhizobia inhabit the intercellular spaces of leguminous root nodules. Plant Signal. Behav. 2023;18(1) doi: 10.1080/15592324.2023.2245995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iquebal M.A., Jagannadham J., Jaiswal S., Prabha R., Rai A., Kumar D. Potential use of microbial community genomes in various dimensions of agriculture productivity and its management: a review. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.708335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa A.A., Abd-Alla M.H., Ohyama T. Vol. 2. IntechOpen; 2014. Nitrogen fixing cyanobacteria: future prospect; pp. 24–48. (Advances in Biology and Ecology of Nitrogen Fixation). [Google Scholar]
- Jabbar A.K., Al-Ziyadi D.Q. Proceedings of the IOP Conference Series: Earth and Environmental Science. Vol. 923. IOP Publishing; 2021. November. Effect of sulfur-oxidizing bacteria thiobacillus thioparus and different levels of agricultural sulfur on wheat yield (Triticum aestivium L.) [Google Scholar]
- Jami N., Rahimi A., Naghizadeh M., Sedaghati E. Investigating the use of different levels of Mycorrhiza and Vermicompost on quantitative and qualitative yield of saffron (Crocus sativus L.) Sci. Hortic. 2020;262 [Google Scholar]
- Jefwa J.M., Sinclair R., Maghembe J.A. Diversity of glomale mycorrhizal fungi in maize/sesbania intercrops and maize monocrop systems in southern Malawi. Agrofor. Syst. 2006;67:107–114. [Google Scholar]
- Jenkins N.E., Grzywacz D. Quality control of fungal and viral biocontrol agents-assurance of product performance. Biocontrol Sci. Technol. 2000;10(6):753–777. [Google Scholar]
- Joshi H., Shourie A., Singh A. Advances in Cyanobacterial Biology. Academic Press; 2020. Cyanobacteria as a source of biofertilizers for sustainable agriculture; pp. 385–396. [Google Scholar]
- Joshi S.K., Gauraha A.K. Global biofertilizer market: emerging trends and opportunities. Trends Appl. Microbiol. Sustain. Econ. 2022:689–697. [Google Scholar]
- Kalayu G. Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int. J. Agron. 2019;2019:1–7. [Google Scholar]
- Kalita N., Bhuyan S., Maibangsa S., Saud R.K. Effect of Biofertilizer seed treatment on Growth, yield and economics of toria (Brassica campestris L.) under rainfed condition in hill zone of Assam. Curr. Agric. Res. J. 2019;7(3):332. [Google Scholar]
- Kapoor R., Evelin H., Mathur P., Giri B. Plant Acclimation to Environmental Stress. Springer New York; New York, NY: 2012. Arbuscular mycorrhiza: approaches for abiotic stress tolerance in crop plants for sustainable agriculture; pp. 359–401. [Google Scholar]
- Kaur P, Purewal S.S. Biofertilizers and their role in sustainable agriculture. Biofertil. Sustain. Agric. Environ. 2019:285–300. [Google Scholar]
- KCa B., Pandeya B., Chanda H., Bhusala P., Pandita S., Khanalb S. Promotion of organic and sustainable agriculture through the use of bio-fertilizers. J. Wastes Biomass Manag. (JWBM) 2021;3(1):01–08. [Google Scholar]
- Kenawy A., Dailin D.J., Abo-Zaid G.A., Abd Malek R., Ambehabati K.K., Zakaria K.H.N., Sayyed R.Z., El Enshasy H.A. Plant Growth Promoting Rhizobacteria for Sustainable Stress Management: Volume 2: Rhizobacteria in Biotic Stress Management. Springer; 2019. Biosynthesis of antibiotics by PGPR and their roles in biocontrol of plant diseases; pp. 1–35. [Google Scholar]
- Khoshru B., Mitra D., Joshi K., Adhikari P., Rion M.S.I., Fadiji A.E., Alizadeh M., Priyadarshini A., Senapati A., Sarikhani M.R., Panneerselvam P. Decrypting the multi-functional biological activators and inducers of defense responses against biotic stresses in plants. Heliyon. 2023;9(3) doi: 10.1016/j.heliyon.2023.e13825. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Khoshru B., Mitra D., Nosratabad A.F., Reyhanitabar A., Mandal L., Farda B., Djebaili R., Pellegrini M., Guerra-Sierra B.E., Senapati A., Panneerselvam P. Enhancing manganese availability for plants through microbial potential: a sustainable approach for improving soil health and food security. Bacteria. 2023;2(3):129–141. [Google Scholar]
- Khoshru B., Nosratabad A.F., Mitra D., Chaithra M., Danesh Y.R., Boyno G., Chattaraj S., Priyadarshini A., Anđelković S., Pellegrini M., Guerra-Sierra B.E. Rock phosphate solubilizing potential of soil microorganisms: advances in sustainable crop production. Bacteria. 2023;2(2):98–115. [Google Scholar]
- Koch A.M., Antunes P.M., Kathryn Barto E., Cipollini D., Mummey D.L., Klironomos J.N. The effects of arbuscular mycorrhizal (AM) fungal and garlic mustard introductions on native AM fungal diversity. Biol. Invasions. 2011;13:1627–1639. [Google Scholar]
- Koirala A., Brözel V.S. Phylogeny of nitrogenase structural and assembly components reveals new insights into the origin and distribution of nitrogen fixation across bacteria and archaea. Microorganisms. 2021;9(8):1662. doi: 10.3390/microorganisms9081662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kour D., Rana K.L., Yadav A.N., Yadav N., Kumar M., Kumar V., Vyas P., Dhaliwal H.S., Saxena A.K. Microbial biofertilizers: bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020;23 [Google Scholar]
- Kumar A., Meena V.S., Roy P., Vandana, Kumari R. Plant Growth Promoting Rhizobacteria for Agricultural Sustainability: From Theory to Practices. Springer; 2019. Role of rhizobia for sustainable agriculture: lab to land; pp. 129–149. [Google Scholar]
- Kumar N., Srivastava P., Vishwakarma K., Kumar R., Kuppala H., Maheshwari S.K., Vats S. The rhizobium–plant symbiosis: state of the art. Plant Microbe Symbiosis. 2020:1–20. [Google Scholar]
- Kumar P.R., Adhipathi P., Nakkeeran S. Antimicrobial peptide genes of PGPR for the management of Fusarium wilt of carnation under protected cultivation. J. Mycol. Plant Pathol. 2014;44:54. [Google Scholar]
- Kumar S., Sindhu S.S., Kumar R. Biofertilizers: an ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022;3 doi: 10.1016/j.crmicr.2021.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumawat K.C., Nagpal S., Sharma P. Biofertilizers. Woodhead Publishing; 2021. Present scenario of bio-fertilizer production and marketing around the globe; pp. 389–413. [Google Scholar]
- Latif S.A.A., Mustafa H.A. Effect of biofertilizers and carbolizer on growth of gerbera plant (Gerbera jamesonii) Plant Arch. 2019;19(1):1733–1754. [Google Scholar]
- Lei Z., Qun L., Zhang Y.Q., Cui Q.Y., Liang Y.C. Effect of acid phosphatase produced by Trichoderma asperellum Q1 on growth of Arabidopsis under salt stress. J. Integr. Agric. 2017;16(6):1341–1346. [Google Scholar]
- Lendzemo V.W., Kuyper T.W., Matusova R., Bouwmeester H.J., Ast A.V. Colonization by arbuscular mycorrhizal fungi of sorghum leads to reduced germination and subsequent attachment and emergence of Striga hermonthica. Plant Signal. Behav. 2007;2(1):58–62. doi: 10.4161/psb.2.1.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Hu Z., Tan G., Zhi Q., Yin H. Enhancing plant growth in biofertilizer-amended soil through nitrogen-transforming microbial communities. Front. Plant Sci. 2023;14 doi: 10.3389/fpls.2023.1259853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pang Z., Zhou Y., Fallah N., Hu C., Lin W., Yuan Z. Metagenomic analysis exploring taxonomic and functional diversity of soil microbial communities in sugarcane fields applied with organic fertilizer. Biomed. Res. Int. 2020 doi: 10.1155/2020/9381506. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Song C., Yi Y., Kuipers O.P. Characterization of plant growth-promoting rhizobacteria from perennial ryegrass and genome mining of novel antimicrobial gene clusters. BMC Genom. 2020;21:1–11. doi: 10.1186/s12864-020-6563-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpens E., Bisseling T. Nod factor signal transduction in the Rhizobium–legume symbiosis. Root Hairs. 2009:249–276. [Google Scholar]
- Lohosha R., Mazur K., Alieksieieva O., Babyna O., Babyn I., Belkin I., Germaniuk N., Gontaruk Y., Harbar Z., Kubai O., Koval O. International Science Group; 2023. Peculiarities of Marketing Activities of Agrarian Enterprises in the Conditions of Martial Law. [Google Scholar]
- Lutz S., Thuerig B., Oberhaensli T., Mayerhofer J., Fuchs J.G., Widmer F., Freimoser F.M., Ahrens C.H. Harnessing the microbiomes of suppressive composts for plant protection: from metagenomes to beneficial microorganisms and reliable diagnostics. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.01810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari M., Abulreesh H.H., Khan M.S., Ahmad I., Pichtel J. Agriculturally Important Microbes for Sustainable Agriculture: Volume I: Plant-Soil-Microbe Nexus. Springer; 2017. Horizontal gene transfer in soil and the rhizosphere: impact on ecological fitness of bacteria; pp. 111–130. [Google Scholar]
- Malusà E., Pinzari F., Canfora L. Microbial Inoculants in Sustainable Agricultural Productivity: Vol. 2: Functional Applications. Springer; 2016. Efficacy of biofertilizers: challenges to improve crop production; pp. 17–40. [Google Scholar]
- Malviya D., Varma A., Singh U.B., Singh S., Singh H.V., Saxena A.K. Sulfur-oxidizing bacteria from coal mine enhance sulfur nutrition in Pigeonpea (Cajanus cajan L.) Front. Environ. Sci. 2022;10 doi: 10.3389/fmicb.2022.927702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawar R., Manjunatha B.L., Kumar S. Commercialization, diffusion and adoption of bioformulations for sustainable disease management in Indian arid agriculture: prospects and challenges. Circ. Econ. Sustain. 2021;1(4):1367–1385. doi: 10.1007/s43615-021-00089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehboob I., Naveed M., Zahir Z.A., Sessitsch A. Plant Microbe Symbiosis: Fundamentals and Advances. Springer; 2013. Potential of rhizosphere bacteria for improving Rhizobium-legume symbiosis; pp. 305–349. [Google Scholar]
- Meyer J.B., Frapolli M., Keel C., Maurhofer M. Pyrroloquinoline quinone biosynthesis gene pqqC, a novel molecular marker for studying the phylogeny and diversity of phosphate-solubilizing pseudomonads. Appl. Environ. Microbiol. 2011;77(20):7345–7354. doi: 10.1128/AEM.05434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mia M.B., Shamsuddin Z.H. Rhizobium as a crop enhancer and biofertilizer for increased cereal production. Afr. J. Biotechnol. 2010;9(37):6001–6009. [Google Scholar]
- Michelson H., Gourlay S., Lybbert T., Wollburg P. Purchased agricultural input quality and small farms. Food Policy. 2023;116 [Google Scholar]
- Mitra D., BE G.S., Khoshru B., De Los Santos Villalobos S., Belz C., Chaudhary P., Shahri F.N., Djebaili R., Adeyemi N.O., El-Ballat E.M., El-Esawi M.A. Impacts of arbuscular mycorrhizal fungi on rice growth, development, and stress management with a particular emphasis on strigolactone effects on root development. Commun. Soil Sci. Plant Anal. 2021;52(14):1591–1621. [Google Scholar]
- Mitra D., Dam P., Mondal R., Mahakur B., Al-Tawaha A.R.M., Sangeetha J., Thangadurai D., Chippalakatti P. Mycorrhizal Technology. Apple Academic Press; 2023. Application of arbuscular mycorrhiza fungi in agricultural and horticultural crops; pp. 55–68. [Google Scholar]
- Mitra D., De Los, Santos-Villalobos S., Parra-Cota F.I., Montelongo A.M.G., Blanco E.L., Olatunbosun A.N., Khoshru B., Mondal R., Chidambaranathan P., Panneerselvam P., Mohapatra P.K.D. Rice (Oryza sativa L.) plant protection using dual biological control and plant growth-promoting agents: current scenarios and future prospects. Pedosphere. 2023;33(2):268–286. [Google Scholar]
- Mitra D., Mondal A.K., Mondol M., Mukhopadhyay A. Plant growth promoting traits and prospects of using phosphate solubilizing Pseudomonas sp. Isolated from laterite soil. Ann. Food Sci. Technol. 2014;15(2):309–314. [Google Scholar]
- Mitra D., Mondal R., Khoshru B., Senapati A., Radha T.K., Mahakur B., Uniyal N., Myo E.M., Boutaj H., Sierra B.E.G., Panneerselvam P. Actinobacteria-enhanced plant growth, nutrient acquisition, and crop protection: advances in soil, plant, and microbial multifactorial interactions. Pedosphere. 2022;32(1):149–170. [Google Scholar]
- Mitra D., Mondal R., Khoshru B., Shadangi S., Mohapatra P.K.D., Panneerselvam P. Rhizobacteria mediated seed bio-priming triggers the resistance and plant growth for sustainable crop production. Curr. Res. Microb. Sci. 2021;2 doi: 10.1016/j.crmicr.2021.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra D., Panneerselvam P., Chidambaranathan P., Nayak A.K., Priyadarshini A., Senapati A., Mohapatra P.K.D. Strigolactone GR24-mediated mitigation of phosphorus deficiency through mycorrhization in aerobic rice. Curr. Res. Microb. Sci. 2024 doi: 10.1016/j.crmicr.2024.100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra D., Panneerselvam P., Senapati A., Chidambaranathan P., Nayak A.K., Mohapatra P.K.D. Arbuscular mycorrhizal fungi response on soil phosphorus utilization and enzymes activities in aerobic rice under phosphorus-deficient conditions. Life. 2023;13(5):1118. doi: 10.3390/life13051118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra D., Pellegrini M., Olatunbosun A.N., Mondal R., Del Gallo M., Chattaraj S., Chakroborty D., Priyadarshini A., Khoshru B., Sierra B.G., de los Santos-Villalobos S. Microbial Inoculants. Academic Press; 2023. Seed priming with microbial inoculants for enhanced crop yield; pp. 99–123. [Google Scholar]
- Mitra D., Rodriguez A.M.D., Cota F.I.P., Khoshru B., Panneerselvam P., Moradi S., Sagarika M.S., Anđelković S., de los Santos-Villalobos S., Mohapatra P.K.D. Amelioration of thermal stress in crops by plant growth-promoting rhizobacteria. Physiol. Mol. Plant Pathol. 2021;115 [Google Scholar]
- Mohan A., Kumar B. Cyanobacterial diversity in agriculturally fertile soil of patna and their population density. Biotechnol. Appl. Biochem. 2019;5(1):15–32. [Google Scholar]
- Mohan J., Reddy R. A review on empower food, nutritional security and improve soil health for zero budget natural farming viable for small farmers. J. Eng. Sci. 2020;11(2):535–550. [Google Scholar]
- Mushtaq Z., Faizan S., Hussain A. Microbiota and Biofertilizers: A Sustainable Continuum for Plant and Soil Health. Springer; 2021. Role of microorganisms as biofertilizers; pp. 83–98. [Google Scholar]
- Nad S., Konar U., Chattaraj S., Ganguly A. Agro-Waste to Microbe Assisted Value Added Product: Challenges and Future Prospects. Environmental Science and Engineering. Springer; Cham: 2024. Valorization of feather waste by microbial enzymatic activity: bioconversion, production and application. In: Saha, S.P., Mazumdar, D., Roy, S., Mathur, P. (eds) [DOI] [Google Scholar]
- Nett R.S., Bender K.S., Peters R.J. Production of the plant hormone gibberellin by rhizobia increases host legume nodule size. ISME J. 2022;16(7):1809–1817. doi: 10.1038/s41396-022-01236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njeru E.M., Avio L., Sbrana C., Turrini A., Bocci G., Bàrberi P., Giovannetti M. First evidence for a major cover crop effect on arbuscular mycorrhizal fungi and organic maize growth. Agron. Sustain. Dev. 2014;34:841–848. [Google Scholar]
- Nosheen S., Ajmal I., Song Y. Microbes as biofertilizers, a potential approach for sustainable crop production. Sustainability. 2021;13(4):1868. [Google Scholar]
- Nurkhaida R., Hamdani A., Suriadi A., Heryani N. Proceedings of the E3S Web of Conferences. Vol. 306. EDP Sciences; 2021. Increasing rice productivity and profitability through irrigation water management and bio-fertilizer in West Nusa Tenggara; p. 04012. [Google Scholar]
- Okpala C.O.R., Korzeniowska M. Understanding the relevance of quality management in agro-food product industry: from ethical considerations to assuring food hygiene quality safety standards and its associated processes. Food Rev. Int. 2023;39(4):1879–1952. [Google Scholar]
- Ortega-Urquieta M.E., Valenzuela-Ruíz V., Mitra D., Hyder S., Elsheery N.I., Kumar Das Mohapatra P., Parra-Cota F.I., de Los Santos-Villalobos S. Draft genome sequence of Priestia sp. strain TSO9, a plant growth-promoting bacterium associated with wheat (Triticum turgidum subsp. durum) in the Yaqui Valley, Mexico. Plants. 2022;11(17):2231. doi: 10.3390/plants11172231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z., Dong F., Liu Q., Lin W., Hu C., Yuan Z. Soil metagenomics reveals effects of continuous sugarcane cropping on the structure and functional pathway of rhizospheric microbial community. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.627569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankievicz V.C., Irving T.B., Maia L.G., Ané J.M. Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC Biol. 2019;17(1):99. doi: 10.1186/s12915-019-0710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam P., Mohapatra P.K.D., Nayak A.K., Mitra D., Velmourougane K., De Los Santos-Villalobos S. CRC Press; 2023. Arbuscular Mycorrhizal Fungi: For Nutrient, Abiotic and Biotic Stress Management in Rice. [Google Scholar]
- Panneerselvam P., Selvakumar G., Ganeshamurthy A.N., Mitra D., Senapati A. Enhancing pomegranate (Punica granatum L.) plant health through the intervention of a Streptomyces consortium. Biocontrol Sci. Technol. 2021;31(4):430–442. [Google Scholar]
- Panneerselvam P., Senapati A., Kumar U., Sharma L., Lepcha P., Prabhukarthikeyan S.R., Jahan A., Parameshwaran C., Govindharaj G.P.P., Lenka S., Nayak P.K. Antagonistic and plant-growth promoting novel Bacillus species from long-term organic farming soils from Sikkim, India. 3 Biotech. 2019;9:1–12. doi: 10.1007/s13205-019-1938-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam P., Senapati A., Sharma L., Nayak A.K., Kumar A., Kumar U., Prabhukarthikeyan S.R., Mitra D., Sagarika M.S. Understanding rice growth-promoting potential of Enterobacter spp. isolated from long-term organic farming soil in India through a supervised learning approach. Curr. Res. Microb. Sci. 2021;2 doi: 10.1016/j.crmicr.2021.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida A. Sustaining and enhancing small farm productivity in an era of emerging challenges. Family Farming. 2016 [Google Scholar]
- Pastor N., Rosas S., Luna V., Rovera M. Inoculation with Pseudomonas putida PCI2, a phosphate solubilizing rhizobacterium, stimulates the growth of tomato plants. Symbiosis. 2014;62:157–167. [Google Scholar]
- Pathak A., Chakraborti S.K. Impact of bio-fertilizer seed treatment on seed and seedling parameters of maize (Zea mays L.) Bioscan. 2014;9(1):133–135. [Google Scholar]
- Paul S., Rakshit A. Effect of seed bio-priming with Trichoderma viride strain BHU-2953 for enhancing soil phosphorus solubilization and uptake in soybean (Glycine max) J. Soil Sci. Plant Nutr. 2021;21(2):1041–1052. [Google Scholar]
- Pedrosa F.D.O., Yates M.G. Physiology, biochemistry, and genetics of azospirillum and other root-associated nitrogen-fixing bacteria. CRC Crit. Rev. Plant Sci. 1988;6(4):345–384. [Google Scholar]
- Powell C.L., Clark G.E., Verberne N.L. Growth response of four onion cultivars to several isolates of VA mycorrhizal fungi. NZ J. Agric. Res. 1982;25(3):465–470. [Google Scholar]
- Poza-Carrión C., Echavarri-Erasun C., Rubio L.M. Regulation of nif gene expression in Azotobacter vinelandii. Biol. Nitrogen Fixat. 2015:99–108. [Google Scholar]
- Prabhu R., Dhyani S.K., Nayak D., Rizvi J. Indian Agriculture Towards 2030. Springer; 2022. Transformative agroecology-based alternatives for a sustainable and biodiverse future; p. 183. [Google Scholar]
- Pramanik B., Sar P., Bharti R., Gupta R., Purkayastha S., Sinha S., Chattaraj S., Mitra D. Multifactorial role of nanoparticles in alleviating environmental stresses for sustainable crop production and protection. Plant Physiol. Biochem. 2023 doi: 10.1016/j.plaphy.2023.107831. [DOI] [PubMed] [Google Scholar]
- Precedence research, 2023. Accessed from: https://www.precedenceresearch.com/biofertilizers-market (Last accessed on 22.03.2024).
- Priyadarshini A., Behera S., Mitra D., Senapati A., Shubhadarshi S., Satapathy S., Pattanayak S., Panneerselvam P. Arbuscular Mycorrhizal Fungi: For Nutrient, Abiotic and Biotic Stress Management in Rice. CRC Press; 2023. 5 Arbuscular mycorrhizal fungi and their role in plant growth promotion in rice; pp. 35–40. [Google Scholar]
- Puglia D., Pezzolla D., Gigliotti G., Torre L., Bartucca M.L., Del Buono D. The opportunity of valorizing agricultural waste, through its conversion into biostimulants, biofertilizers, and biopolymers. Sustainability. 2021;13(5):2710. [Google Scholar]
- Rabari A., Ruparelia J., Jha C.K., Sayyed R.Z., Mitra D., Priyadarshini A., Senapati A., Panneerselvam P., Mohapatra P.K.D. Articulating beneficial rhizobacteria-mediated plant defenses through induced systemic resistance: a review. Pedosphere. 2023;33(4):556–566. [Google Scholar]
- Radhakrishnan G.V., Keller J., Rich M.K., Vernié T., MbadingaMbadinga D.L., Vigneron N., Cottret L., Clemente H.S., Libourel C., Cheema J., Linde A.M. An ancestral signalling pathway is conserved in intracellular symbioses-forming plant lineages. Nat. Plants. 2020;6(3):280–289. doi: 10.1038/s41477-020-0613-7. [DOI] [PubMed] [Google Scholar]
- Raghuwanshi R., Prasad J.K. Perspectives of rhizobacteria with ACC deaminase activity in plant growth under abiotic stress. Root Biol. 2018:303–321. [Google Scholar]
- Rahimzadeh S., Sohrabi Y., Heidari G., Pirzad A., Golezani K.G. Effect of bio-fertilizers on the essential oil yield and components isolated from Dracocephalummoldavica L. using nanoscale injection method. J. Essent.Oil Bear. Plants. 2016;19(3):529–541. [Google Scholar]
- Rai M.K. Vol. 10. Haworth Press, Inc; 2006. (Microbial Biofertilizers). 13904-1580. [Google Scholar]
- Raimi A., Adeleke R., Roopnarain A. Soil fertility challenges and Biofertiliseras a viable alternative for increasing smallholder farmer crop productivity in sub-Saharan Africa. Cogent Food Agric. 2017;3(1) [Google Scholar]
- Raimi A., Roopnarain A., Adeleke R. Biofertilizer production in Africa: current status, factors impeding adoption and strategies for success. Sci. Afr. 2021;11:e00694. [Google Scholar]
- Raju P.S., Clark R.B., Ellis J.R., Maranville J.W. Effects of species of VA-mycorrhizal fungi on growth and mineral uptake of sorghum at different temperatures. Plant Soil. 1990;121:165–170. [Google Scholar]
- Ranadev P., Revanna A., Bagyaraj D.J., Shinde A.H. Sulfur oxidizing Bacteria in Agro ecosystem and its role in Plant Productivity-A Review. J. Appl. Microbiol. 2023:lxad161. doi: 10.1093/jambio/lxad161. [DOI] [PubMed] [Google Scholar]
- Rathnathilaka T., Premarathna M., Madawala S., Pathirana A., Karunaratne K., Seneviratne G. Biofilm biofertilizer application rapidly increases soil quality and grain yield in large scale conventional rice cultivation: a case study. J. Plant Nutr. 2023;46(7):1220–1230. [Google Scholar]
- Rawat P., Das S., Shankhdhar D., Shankhdhar S.C. Phosphate-solubilizing microorganisms: mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021;21(1):49–68. [Google Scholar]
- Ray A., Kundu S., Mohapatra S.S., Sinha S., Khoshru B., Keswani C., Mitra D. An insight into the role of phenolics in abiotic stress tolerance in plants: current perspective for sustainable environment. J. Pure Appl. Microbiol. 2024;18(1) [Google Scholar]
- Ray P., Lakshmanan V., Labbé J.L., Craven K.D. Microbe to microbiome: a paradigm shift in the application of microorganisms for sustainable agriculture. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.622926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S.C., Cleveland C.C., Townsend A.R. Relationships among phosphorus, molybdenum and free-living nitrogen fixation in tropical rain forests: results from observational and experimental analyses. Biogeochemistry. 2013;114:135–147. [Google Scholar]
- Riaz U., Mehdi S.M., Iqbal S., Khalid H.I., Qadir A.A., Anum W., Ahmad M., Murtaza G. Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation. Springer; 2020. Bio-fertilizers: eco-friendly approach for plant and soil environment; pp. 189–213. [Google Scholar]
- Rigby D., Woodhouse P., Young T., Burton M. Constructing a farm level indicator of sustainable agricultural practice. Ecol. Econ. 2001;39(3):463–478. [Google Scholar]
- Roossinck M.J. The good viruses: viral mutualistic symbioses. Nat. Rev. Microbiol. 2011;9(2):99–108. doi: 10.1038/nrmicro2491. [DOI] [PubMed] [Google Scholar]
- Ruparelia J., Rabari A., Mitra D., Panneerselvam P., Das-Mohapatra P.K., Jha C.K. Efficient applications of bacterial secondary metabolites for management of biotic stress in plants. Plant Stress. 2022;6 [Google Scholar]
- Sadhukhan J., Martinez-Hernandez E., Murphy R.J., Ng D.K., Hassim M.H., Ng K.S., Kin W.Y., Jaye I.F.M., Hang M.Y.L.P., Andiappan V. Role of bioenergy, biorefinery and bioeconomy in sustainable development: strategic pathways for Malaysia. Renew. Sustain. Energy Rev. 2018;81:1966–1987. [Google Scholar]
- Sadras V., Guirao M., Moreno A., Fereres A. Inter-virus relationships in mixed infections and virus-drought relationships in plants: a quantitative review. Plant J. 2024;117(6):1786–1799. doi: 10.1111/tpj.16516. [DOI] [PubMed] [Google Scholar]
- Saiyad S.A., Jhala Y.K., Vyas R.V. Comparative efficiency of five potash and phosphate solubilizing bacteria and their key enzymes useful for enhancing and improvement of soil fertility. Int. J. Sci. Res. Publ. 2015;5(2):1–6. [Google Scholar]
- Sarkar A., Chattaraj S., Chattaraj M., Maity S., Ganguly A., Nandi B., Pahari P.R., Das Mohapatra P.K. Ichthyodiversity and water quality parameters of Uttar Dinajpur, West Bengal, India. North-West. J. Zool. 2022;18(2) [Google Scholar]
- Sarker A., Mitra D., Das Mohapatra P.K., Ansary M.W.R., Islam T. Biostimulants for Crop Production and Sustainable Agriculture. CABI; GB: 2022. Plant growth-promoting rhizobacteria as biostimulants in sustainable crop production; pp. 455–483. [Google Scholar]
- Sarker P.K., Paul A.S., Karmoker D. Mitigating climate change and pandemic impacts on global food security: dual sustainable agriculture approach (2S approach) Planta. 2023;258(6):104. doi: 10.1007/s00425-023-04257-2. [DOI] [PubMed] [Google Scholar]
- Schwember A.R., Schulze J., Del Pozo, Cabeza R.A. Regulation of symbiotic nitrogen fixation in legume root nodules. Plants. 2019;8(9):333. doi: 10.3390/plants8090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seenivasagan R., Babalola O.O. Utilization of microbial consortia as biofertilizers and biopesticides for the production of feasible agricultural product. Biology. 2021;10(11):1111. doi: 10.3390/biology10111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S.K., Raut S., Chattaraj S., Mohapatra P.K.D. Application of novel hot spring bacterial consortium in Arsenic (III) bioremediation: a model based approach for clean environment. Sustain. Chem. Pharm. 2023;36 [Google Scholar]
- Sharma M.P., Reddy U.G., Adholeya A. Response of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) grown conventionally and on beds in a sandy loam soil. Indian J. Microbiol. 2011;51:384–389. doi: 10.1007/s12088-011-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Bano A., Singh S.P., Tong Y.W. Microbial inoculants: recent progress in formulations and methods of application. Microb. Inoculants. 2023:1–28. [Google Scholar]
- Shi Z., Zhang J., Lu S., Li Y., Wang F. Arbuscular mycorrhizal fungi improve the performance of sweet sorghum grown in a mo-contaminated soil. J. Fungi. 2020;6(2):44. doi: 10.3390/jof6020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B.K. Creating new business, economic growth and regional prosperity through microbiome-based products in the agriculture industry. Microb. Biotechnol. 2017;10(2):224. doi: 10.1111/1751-7915.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. Isolation and characterization of insoluble inorganic phosphate solubilizer rice rhizosphere strain Enterobacter cloacae BAU3. J. Appl. Nat. Sci. 2018;10(4):1204–1209. [Google Scholar]
- Singh M., Dotaniya M.L., Mishra A., Dotaniya C.K., Regar K.L., Lata M. Conservation Agriculture: An Approach to Combat Climate Change in Indian Himalaya. Springer; 2016. Role of biofertilizers in conservation agriculture; pp. 113–134. [Google Scholar]
- Singh N. Microbial Products. CRC Press; 2022. Application and impact of biofertilizers in sustainable agriculture; pp. 205–234. [Google Scholar]
- Singhalage I.D., Seneviratne G., Madawala H.M.S.P., Wijepala P.C. Profitability of strawberry (Fragaria ananassa) production with biofilmed biofertilizer application. Sci. Hortic. 2019;243(3):411–413. [Google Scholar]
- Solaiman M.Z., Hirata H. Effects of indigenous arbuscular mycorrhizal fungi in paddy fields on rice growth and N, P, K nutrition under different water regimes. Soil Sci. Plant Nutr. 1995;41(3):505–514. [Google Scholar]
- Stephens J.H.G., Rask H.M. Inoculant production and formulation. Field Crops Res. 2000;65(2–3):249–258. [Google Scholar]
- Sudheer S., Bai R.G., Usmani Z., Sharma M. Insights on engineered microbes in sustainable agriculture: biotechnological developments and future prospects. Curr. Genom. 2020;21(5):321–333. doi: 10.2174/1389202921999200603165934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhag M. Potential of biofertilizers to replace chemical fertilizers. Int. Adv. Res. J. Sci. Eng. Technol. 2016;3(5):163–167. [Google Scholar]
- Sultan S., El–Qassem A. Future prospects for sustainable agricultural development. Int. J. Mod. Agric. Environ. 2021;1(2):54–82. [Google Scholar]
- Swaminathan M.S. An evergreen revolution. Crop Sci. 2006;46(5):2293–2303. [Google Scholar]
- Szopa D., Mielczarek M., Skrzypczak D., Izydorczyk G., Mikula K., Chojnacka K., Witek-Krowiak A. Encapsulation efficiency and survival of plant growth-promoting microorganisms in an alginate-based matrix–A systematic review and protocol for a practical approach. Ind. Crops Prod. 2022;181 [Google Scholar]
- Thomas L., Singh I. Biofertilizers for Sustainable Agriculture and Environment. Springer; 2019. Microbial biofertilizers: types and applications; pp. 1–19. [Google Scholar]
- Tiwari P., Adholeya A., Prakash A., Arora D.K. Vol. 21. Routledge; 2004. Commercialization of arbuscular mycorrhizal biofertilizer; pp. 195–203. (Fungal Biotechnology in Agricultural, Food, and Environmental Applications). In: Arora, D.K. (Ed.) [Google Scholar]
- Trivedi P., Mattupalli C., Eversole K., Leach J.E. Enabling sustainable agriculture through understanding and enhancement of microbiomes. New Phytol. 2021;230(6):2129–2147. doi: 10.1111/nph.17319. [DOI] [PubMed] [Google Scholar]
- Valverde R.A., Sabanadzovic S., Hammond J. Viruses that enhance the aesthetics of some ornamental plants: beauty or beast? Plant Dis. 2012;96(5):600–611. doi: 10.1094/PDIS-11-11-0928-FE. [DOI] [PubMed] [Google Scholar]
- Vishwakarma K., Kumar N., Shandilya C., Mohapatra S., Bhayana S., Varma A. Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: a review. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.560406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahane M.R., Salvi V.G., Dodake S.B., Khobragade N.H. Effect of phosphorus, vesicular arbuscular mycorrhizae (VAM) and phosphate solubilizing bacteria (PSB) on yield and nutrient content of groundnut and soil physical properties of Alfisols. Agric. Res. J. 2022;59(3) [Google Scholar]
- Wang B., Wang X., Wang Z., Zhu K., Wu W. Comparative metagenomic analysis reveals rhizosphere microbial community composition and functions help protect grapevines against salt stress. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1102547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li T., Li Y., Björn L.O., Rosendahl S., Olsson P.A., Li S., Fu X. Community dynamics of arbuscular mycorrhizal fungi in high-input and intensively irrigated rice cultivation systems. Appl. Environ. Microbiol. 2015;81(8):2958–2965. doi: 10.1128/AEM.03769-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani S.A., Chand S., Wani M.A., Ramzan M., Hakeem K.R. Soil Science: Agricultural and Environmental Prospectives. Springer; 2016. Azotobacter chroococcum–a potential biofertilizer in agriculture: an overview; pp. 333–348. [Google Scholar]
- Waqeel J., Khan S.T. Microbial Biofertilizers and Micronutrient Availability: The Role of Zinc in Agriculture and Human Health. Springer; 2022. Microbial biofertilizers and micronutrients bioavailability: approaches to deal with zinc deficiencies; pp. 239–297. [Google Scholar]
- Wei X., Xie B., Wan C., Song R., Zhong W., Xin S., Song K. Enhancing soil health and plant growth through microbial fertilizers: mechanisms, benefits, and sustainable agricultural practices. Agronomy. 2024;14(3):609. [Google Scholar]
- Wu L., Jiang Y., Zhao F., He X., Liu H., Yu K. Increased organic fertilizer application and reduced chemical fertilizer application affect the soil properties and bacterial communities of grape rhizosphere soil. Sci. Rep. 2020;10(1):9568. doi: 10.1038/s41598-020-66648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier Martins W.F., Rodrigues B.F. Identification of dominant arbuscular mycorrhizal fungi in different rice ecosystems. Agric. Res. 2020;9:46–55. [Google Scholar]
- Yadav A., Yadav K. Challenges and opportunities in biofertilizer commercialization. SVOA Microbiol. 2024;5(1):01–14. [Google Scholar]
- Yadav A.N., Kour D., Kaur T., Devi R., Yadav A., Dikilitas M., Abdel-Azeem A.M., Ahluwalia A.S., Saxena A.K. Biodiversity, and biotechnological contribution of beneficial soil microbiomes for nutrient cycling, plant growth improvement and nutrient uptake. Biocatal. Agric. Biotechnol. 2021;33 [Google Scholar]
- Yadav, K.K. and Smritikana Sarkar, S.S., 2019. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture.
- Yang L.Y., Lin C.S., Huang X.R., Neilson R., Yang X.R. Effects of biofertilizer on soil microbial diversity and antibiotic resistance genes. Sci. Total Environ. 2022;820 doi: 10.1016/j.scitotenv.2022.153170. [DOI] [PubMed] [Google Scholar]
- Yin Z., Shi F., Jiang H., Roberts D.P., Chen S., Fan B. Phosphate solubilization and promotion of maize growth by Penicillium oxalicum P4 and Aspergillus niger P85 in a calcareous soil. Can. J. Microbiol. 2015;61(12):913–923. doi: 10.1139/cjm-2015-0358. [DOI] [PubMed] [Google Scholar]
- Youssif B.D., Hosna M.A.F., Mervat A.A.T. Effect of sulphur and sulphur oxidizing bacteria on growth and production of garlic (Allium sativum, L.) under saline conditions. Middle East J. Agric. Res. 2015;4(3):446–459. [Google Scholar]
- Yu Y.Y., Li S.M., Qiu J.P., Li J.G., Luo Y.M., Guo J.H. Combination of agricultural waste compost and biofertilizer improves yield and enhances the sustainability of a pepper field. J. Plant Nutr. Soil Sci. 2019;182(4):560–569. [Google Scholar]
- Zainuddin N., Keni M.F., Ibrahim S.A.S., Masri M.M.M. Effect of integrated biofertilizers with chemical fertilizers on the oil palm growth and soil microbial diversity. Biocatal. Agric. Biotechnol. 2022;39 [Google Scholar]
- Zewail R.M.Y., Ahmed H.S.A. Effect of some biofertilizers (pgpr, biosoal and compost tea) on growth, yield, fiber quality and yarn properties of egyptian cotton. (promising hybrid 10229xg86) Ann. Agric. Sci. Moshtohor. 2015;53(2):199–210. [Google Scholar]
- Zhou L., Song C., Li Z., Kuipers O.P. Antimicrobial activity screening of rhizosphere soil bacteria from tomato and genome-based analysis of their antimicrobial biosynthetic potential. BMC Genom. 2021;22:1–14. doi: 10.1186/s12864-020-07346-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkefli N.S., Hwang S.J. Heterocyst development and diazotrophic growth of Anabaena variabilis under different nitrogen availability. Life. 2020;10(11):279. doi: 10.3390/life10110279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.