Abstract
Objectives:
Heart donation after circulatory death was recently reintroduced in the United States with hopes of increasing donor heart availability. We examined its national use and outcomes.
Methods:
The United Network for Organ Sharing database was used to identify validated adult patients undergoing heart transplantation using donation after circulatory death donors (n = 266) and donation after brain death donors (n = 5998) between December 1, 2019, and December 31, 2021, after excluding heart-lung transplants. Propensity score matching was used to create more balanced groups for comparison.
Results:
The monthly percentage of donation after circulatory death heart transplant increased from 2.5% in December 2019 to 6.8% in December 2021 (P < .001). Twenty-two centers performed donation after circulatory death heart transplants, ranging from 1 to 75 transplants per center. Four centers performed 70% of the national volume. Recipients of donation after circulatory death hearts were more likely to be clinically stable (80.4% vs 41.1% in status 3–6, P < .001), to have type O blood (58.3% vs 39.9%, P < .001), and to wait longer after listing (55, interquartile range, 15–180 days vs 32, interquartile range, 9–160 days, P = .003). Six-month survival was 92.1% (95% confidence interval, 91.3–92.8) after donation after brain death heart transplants and 92.6% (95% confidence interval, 88.1–95.4) after donation after circulatory death heart transplants (hazard ratio, 0.94, 95% confidence interval, 0.57–1.54, P = .79). Outcomes in propensity-matched patients were similar except for higher rates of treated acute rejection in donation after circulatory death transplants before discharge (14.4% vs 8.8%, P = .01). In donation after circulatory death heart recipients, outcomes did not differ based on the procurement technique (normothermic regional perfusion vs direct procurement and perfusion).
Conclusions:
Heart transplantation with donation after circulatory death donors has short-term survival comparable to donation after brain death transplants. Broader implementation could substantially increase donor organ availability.
Keywords: direct procurement and perfusion, donation after circulatory death, heart transplantation, normothermic regional perfusion, organ donation
Graphical Abstract
Six-month survival after heart transplantation with DCD versus DBD donors.
Heart transplantation is an effective treatment for selected patients with end-stage heart failure; however, use is constrained by a widening deficit of donor hearts, with waitlist mortality more than 30% at 1 year.1–3 Hearts are predominantly procured from donors with intact circulatory function after brain death (donation after brain death [DBD]). In contrast, heart transplantation with donation after circulatory death (DCD) involves donors on circulatory and respiratory support with irreversible brain injury who do not meet the full criteria for brain death, in whom the decision to withdraw support is made independently of organ donation potential. In DCD donors, death occurs when respiration and circulation spontaneously cease and will not spontaneously resume: The observation period required to confirm this varies according to governing agencies.4,5
In 2019, DCD organs represented 23% of all deceased donor kidney, liver, pancreas, and lung donation recorded in the United States. However, broader implementation in heart transplantation has been limited in contemporary US practice because of ethical and technical constraints.6 Currently, there are little data to characterize clinical outcomes of DCD heart transplants in the United States, although a randomized trial comparing DCD and DBD heart transplantation has recently completed enrollment (NCT03831048). Additionally, there is no consensus regarding the best technique for DCD donor heart recovery, and little comparative data exist on the use of normothermic regional perfusion (NRP) versus direct procurement and perfusion (DPP). Our study was designed to evaluate trends and outcomes of DCD heart transplantation in the United States using a national transplant registry.
MATERIALS AND METHODS
Data Source
This analysis was performed using the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research file as of December 31, 2021, which included data for organ donations, transplants, and new listings occurring through December 31, 2021. From the thoracic organ transplant recipient file, we identified 6264 adult recipients (aged ≥18 years) undergoing DCD (n = 266) or DBD (n = 5998) heart transplant between December 1, 2019, and December 31, 2021, after excluding heart-lung transplants and patients with unvalidated records (Figure 1). From the deceased donor file, we identified 355 DCD donors whose hearts were recovered during the same period; 36 hearts were discarded, and 319 hearts were successfully transplanted. Among the 319 hearts transplanted, 51 had unvalidated recipient records, 1 was used for heart and lung transplant, and 1 was transplanted in a pediatric recipient. These 53 patients were included in the analysis of practice trends but excluded from the analysis of primary and secondary outcomes (Figure E1).
FIGURE 1.
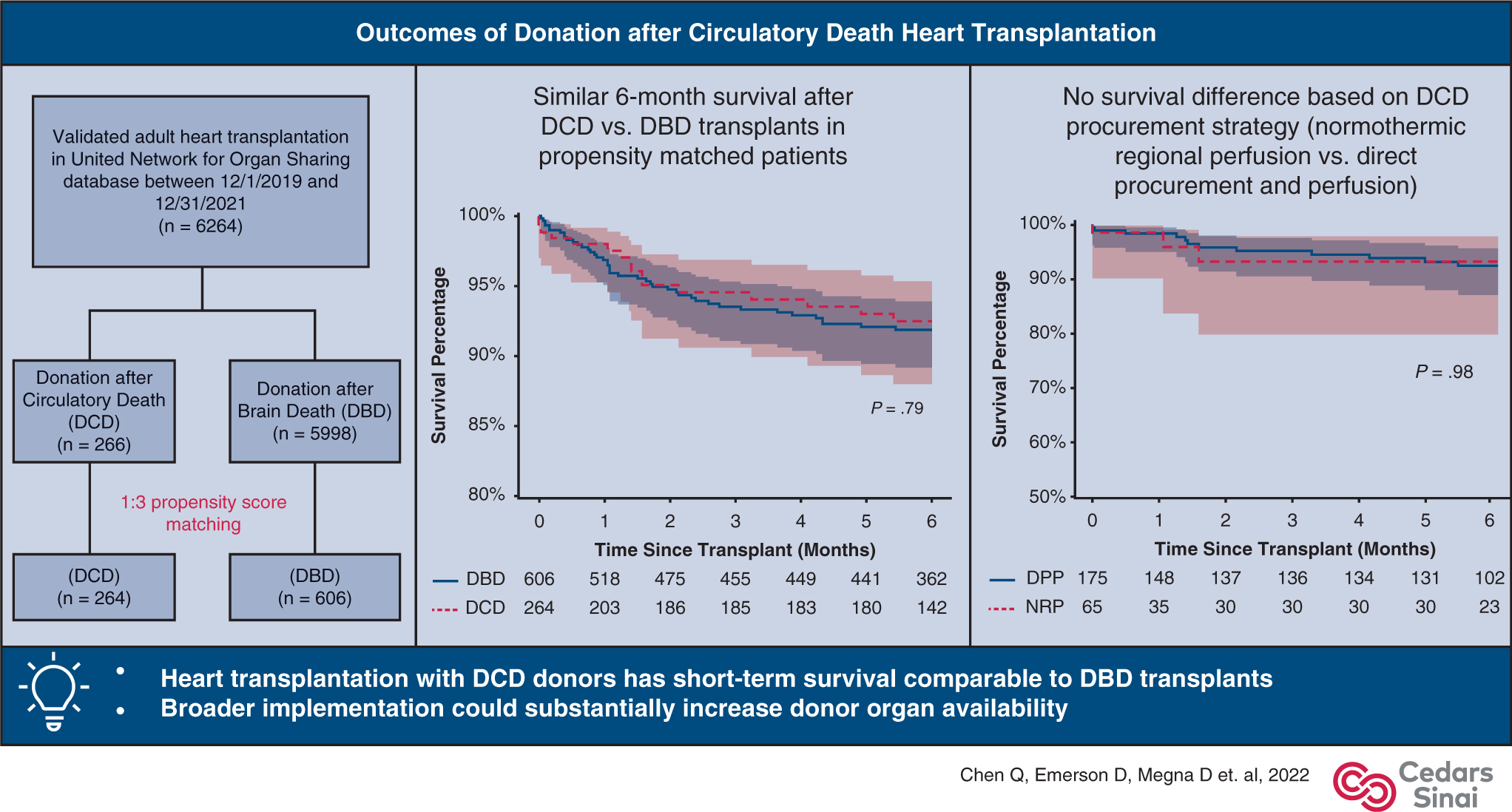
Outcomes of DCD heart transplantation. DCD, Donation after circulatory death; DBD, donation after brain death; DPP, direct procurement and perfusion; NRP, normothermic regional perfusion.
FIGURE E1.
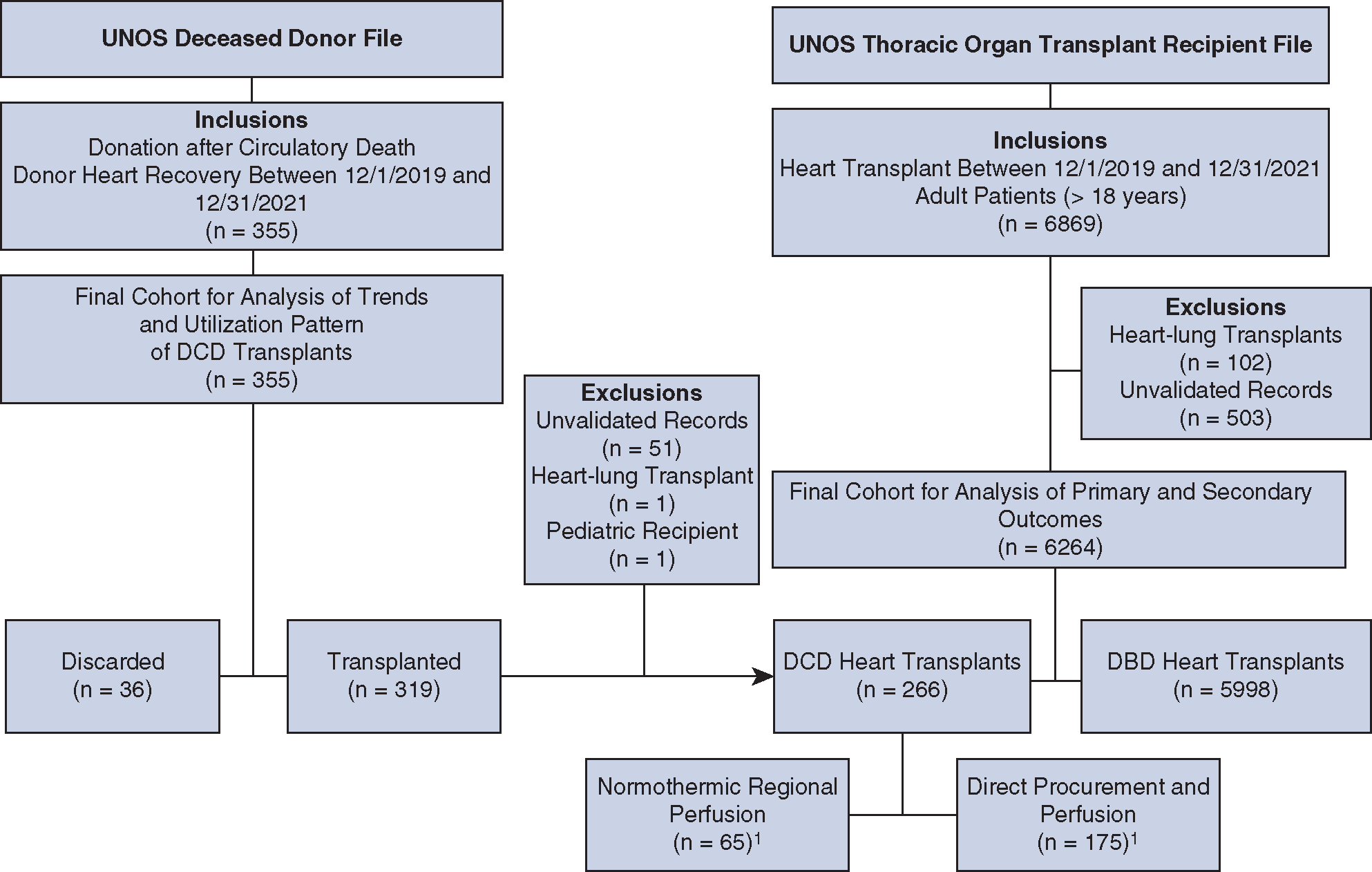
Cohort identification from the UNOS database. 1The use of DPP versus NRP during DCD heart recovery was identified using the interval between reported time of death and time of aortic crossclamping during procurement (available in 240/266 DCD donors included in the outcomes analysis). UNOS, United Network for Organ Sharing; DCD, donation after circulatory death; DBD, donation after brain death.
Recipient/donor characteristics and patient outcomes were defined according to the standard UNOS definitions. Total graft preservation time was defined as the time from aortic crossclamp during procurement to in situ reperfusion during implantation. This was the same as the total ischemic time in DBD donors. Patients with a history of previous cardiac surgery or previous heart transplants were considered to have had a prior sternotomy. Patients designated UNOS status 1 or 2 (unable to be discharged from hospital and requiring mechanical circulatory support or with life-threatening ventricular arrhythmias) at the time of transplantation were considered urgent. Donor to recipient predicated heart mass ratio was calculated with a previously developed formula and used as a surrogate for donor-recipient size match.7–9 Recipient functional status was classified using the Karnofsky Performance Scale Index (Table E1). This study was approved by the Institutional Review Board at Cedars-Sinai Medical Center, with a waiver of informed consent (STUDY00001188, approved on February 19, 2021).
TABLE E1.
Karnofsky performance scale index
| Index description | General category |
|---|---|
|
| |
| 10%-Moribund, fatal processes progressing rapidly | Severe limitation: unable to care for self; requires equivalent of institutional or hospital care; disease may be progressing rapidly (severe limitation) |
| 20%-Very sick, hospitalization necessary: active treatment necessary | |
| 30%-Severely disabled: hospitalization is indicated, death not imminent | |
| 40%-Disabled: requires special care and assistance | |
| 50%-Requires considerable assistance and frequent medical care | Moderate limitation: unable to work; able to live at home and care for most personal needs; varying amount of assistance needed (moderate limitation) |
| 60%-Requires occasional assistance but is able to care for needs | |
| 70%-Cares for self: unable to carry on normal activity or active work | |
| 80%-Normal activity with effort: some symptoms of disease | Mild limitation: able to carry on normal activity and to work; no special care needed (mild limitation) |
| 90%-Able to carry on normal activity: minor symptoms of disease | |
| 100%-Normal, no complaints, no evidence of disease | |
Donation After Circulatory Death Process
After consent is obtained, the DCD process starts with controlled withdrawal of life support. As circulatory function declines, the donor enters an agonal phase, which begins when systolic blood pressure is less than 80 mm Hg or oxygen saturation is less than 80% (according to UNOS definitions). Progression to circulatory arrest may ensue, in which case after a legally required stand-off period of variable duration to confirm the absence of autoresuscitation, death is declared. In contemporary practice, the subsequent procurement of DCD donor hearts has used 1 of 2 techniques: DPP or NRP. In DPP, expeditious sternotomy and aortic crossclamping are performed to minimize warm ischemia. Once the heart is explanted, it is placed on an ex situ organ perfusion machine for further assessment and resuscitation. In contrast, when NRP is used, the donor is placed on extracorporeal oxygenation and circulatory support after death, with occlusion of the head and neck vessels to prevent cerebral blood flow. The donor heart is then reanimated in situ, assessed, and retrieved in a similar fashion to DBD heart donation if deemed suitable.
To identify the procurement technique used, we used reported time of death and time of aortic crossclamping during DCD procurement (available in 240/266 DCD transplants included). Because DPP requires expeditious sternotomy and crossclamping of the donor aorta after death to minimize warm ischemia, an interval of less than 15 minutes between time of death and aortic crossclamping was considered to involve its use. Conversely, a greater than 15-minute interval was considered to indicate the use of NRP, because it typically involves a prolonged period of in situ reanimation and functional assessment of the donor heart.
Primary and Secondary Outcomes
The primary outcome was survival 6 months after transplantation. Comparisons were made between patients who received DBD and DCD donor hearts in both the overall cohort and the propensity-matched cohort. Median follow-up was 6.6 (interquartile range [IQR], 2.0–12.0) months for the entire cohort. The proportion of patients who have reached 6-month follow-up was 60.2% (160/266) in the DCD group and 64.5% (3866/5998) in the DBD group (P = .15). Secondary outcomes included in-hospital adverse events (treated acute rejections, post-transplant dialysis, stroke, and permanent pacemaker implant) and post-transplant hospital length of stay.
Subgroup analyses were performed among recipients of DCD hearts to evaluate the impact of total graft preservation time, recipient age, pretransplant ventricular assist device or artificial heart use, and total DCD heart transplant volume per center on 6-month survival. For total graft preservation time and recipient age, patients were stratified into quartiles. The total volume of DCD heart transplantation during the study period at each center was investigated as a categorical variable that included 4 groups: 10 cases or less, 11 to 30 cases, 31 to 50 cases, and greater than 50 cases. Additionally, in DCD heart recipients, unadjusted post-transplant survival stratified by procurement techniques was evaluated.
Statistical Analyses
Baseline patient characteristics were reported as mean ± standard deviation or median with IQR for continuous variables and proportions for categorical variables. Between-group comparisons were performed using Student t test or Wilcoxon signed-rank test for continuous variables depending on variable distribution. Pearson’s chi-square test was performed for categorical variables. Temporal trends in DCD heart transplants were analyzed with the Cochran-Armitage trend test. Categorical variables with missing data included recipient diabetes (0.1% missing), pretransplant dialysis (0.2%), transfusions after listing (0.7%), cerebrovascular disease (0.7%), functional status at transplant (5.7%), donor diabetes (1.3%), donor hypertension (1.4%), post-transplant dialysis (0.1%), and post-transplant permanent pacemaker implant (0.2%). These were marked as unknown in the analysis. For continuous variables, missing values of baseline creatinine (0.1%) and total bilirubin (0.2%) were imputed to the median value of the overall cohort. For procurement time intervals, only complete cases were reported in the descriptive analysis.
We used 1:3 propensity score matching to create more balanced groups of DCD and DBD transplants. A greedy nearest neighbor algorithm was used with a caliper of 0.3 of the logit of the propensity score without replacement. Covariates were selected a priori based on clinical experience and previous literature. Baseline recipient variables entered into the propensity score model included age, gender, race, body mass index, blood type, diabetes, ischemic cardiomyopathy, status at transplant, waitlist time, prior sternotomy, multi-organ transplant, dialysis after listing, blood transfusions after listing, creatinine, total bilirubin, mechanical circulatory support use (including extracorporeal membrane oxygenation, intra-aortic balloon pump, ventricular assist device, and total artificial heart), mechanical ventilation, inotropic use, pretransplant location, pretransplant functional status, and the total number of heart transplants performed per center during the study period. Donor variables included age, gender, hypertension, diabetes, left ventricular ejection fraction, cause of death, donor-recipient predicted heart mass ratio, gender mismatch, and total graft preservation time. The area under the receiver operating curve for the propensity score model was 0.92. In propensity-matched patients, a standardized difference of 10% or less was deemed to be the ideal balance, and a standardized difference of 20% or less was deemed to be an acceptable balance. Baseline characteristics and in-hospital/short-term outcomes in matched patients were compared using the paired t test for continuous variables and the McNemar’s test for categorical variables.
For the primary end point, survival curves were derived using the Kaplan–Meier method. For survival analysis in the matched cohort, marginal Cox proportional hazards regression models with robust sandwich variance estimators were fitted with only donor type (DCD vs DBD) entered as a covariate to control for dependence due to matching. Otherwise, the difference in 6-month overall survival was compared using the Cox model. Right censoring was performed at 6-month follow-up, and patients who did not reach 6-month follow-up were censored on the last follow-up date. To address informative censoring bias due to our use of recent UNOS data, we performed a sensitivity analysis using only patients who received transplants before April 30, 2021, to allow at least 6-month follow-up plus a 2-month lag time in data collection to better estimate unadjusted survival in DCD and DBD transplants (Figure E2).
FIGURE E2.
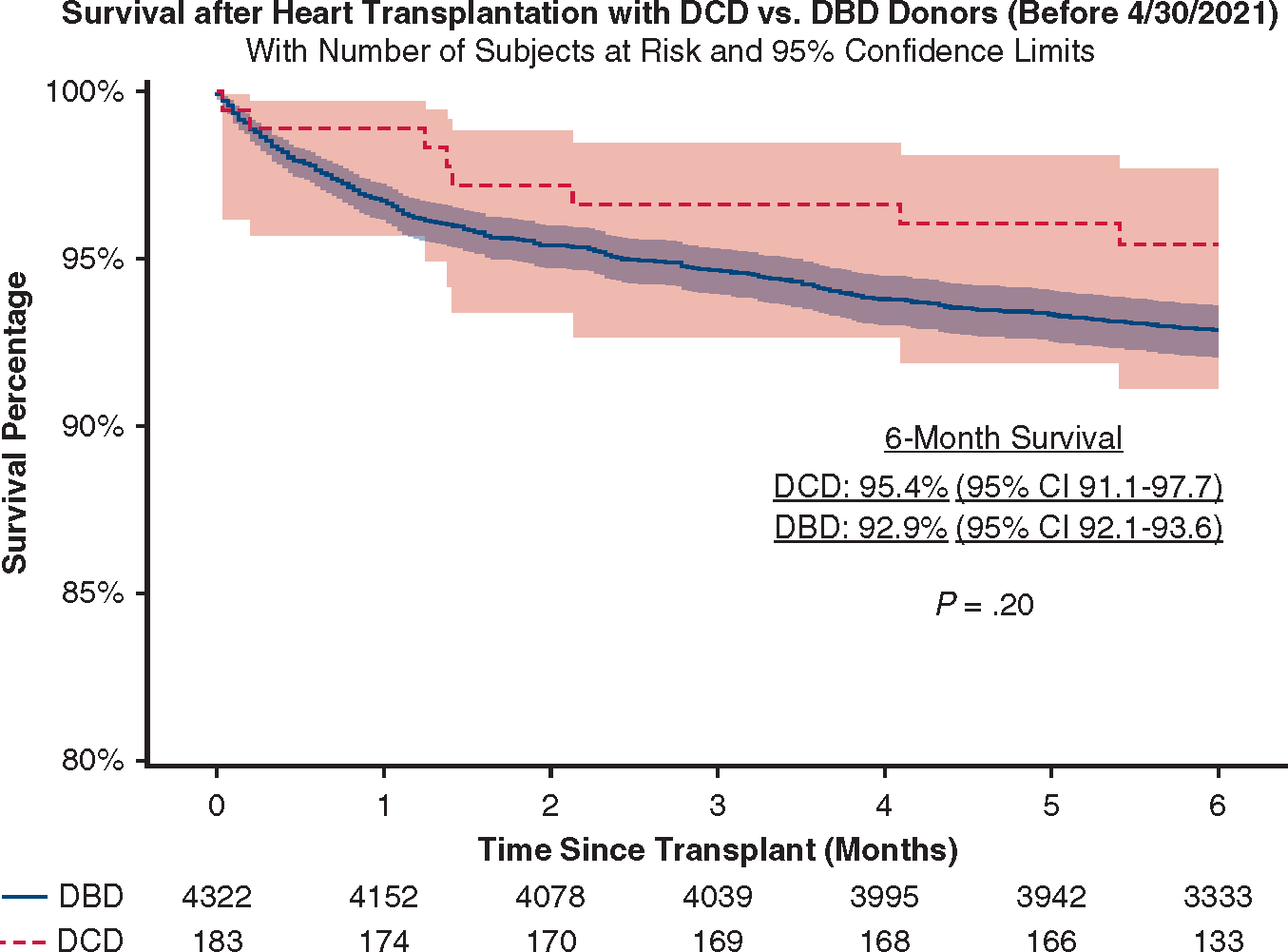
Sensitivity analysis of survival after DCD and DBD transplants in patients undergoing transplantation before April 30, 2021. We included only patients undergoing transplantation before April 30, 2021, to allow at least 6-month follow-up plus a 2-month lag time in data collection to minimize informative censoring bias. DCD, Donation after circulatory death; DBD, donation after brain death; CI, confidence interval.
All tests were 2-tailed with an alpha level of 0.05. All statistical analyses were performed using SAS 9.4.
RESULTS
Trends in Donation After Circulatory Death Heart Transplantation
Between December 1, 2019, and December 31, 2021, a total of 319 DCD heart transplants were performed by 22 centers. The monthly percentage of DCD heart transplants increased from 2.5% (n = 7) in December 2019 to 6.8% (n = 18) in December 2021 (P < .001 for trend, Figure 2). Total volume per center ranged from 1 to 75. Four centers performed 70% of the national volume during the study period, which accounted for 21% to 49% of their total heart transplant volume and resulted in a 27% to 96% increase in their overall heart transplant activity (Figure E3).
FIGURE 2.
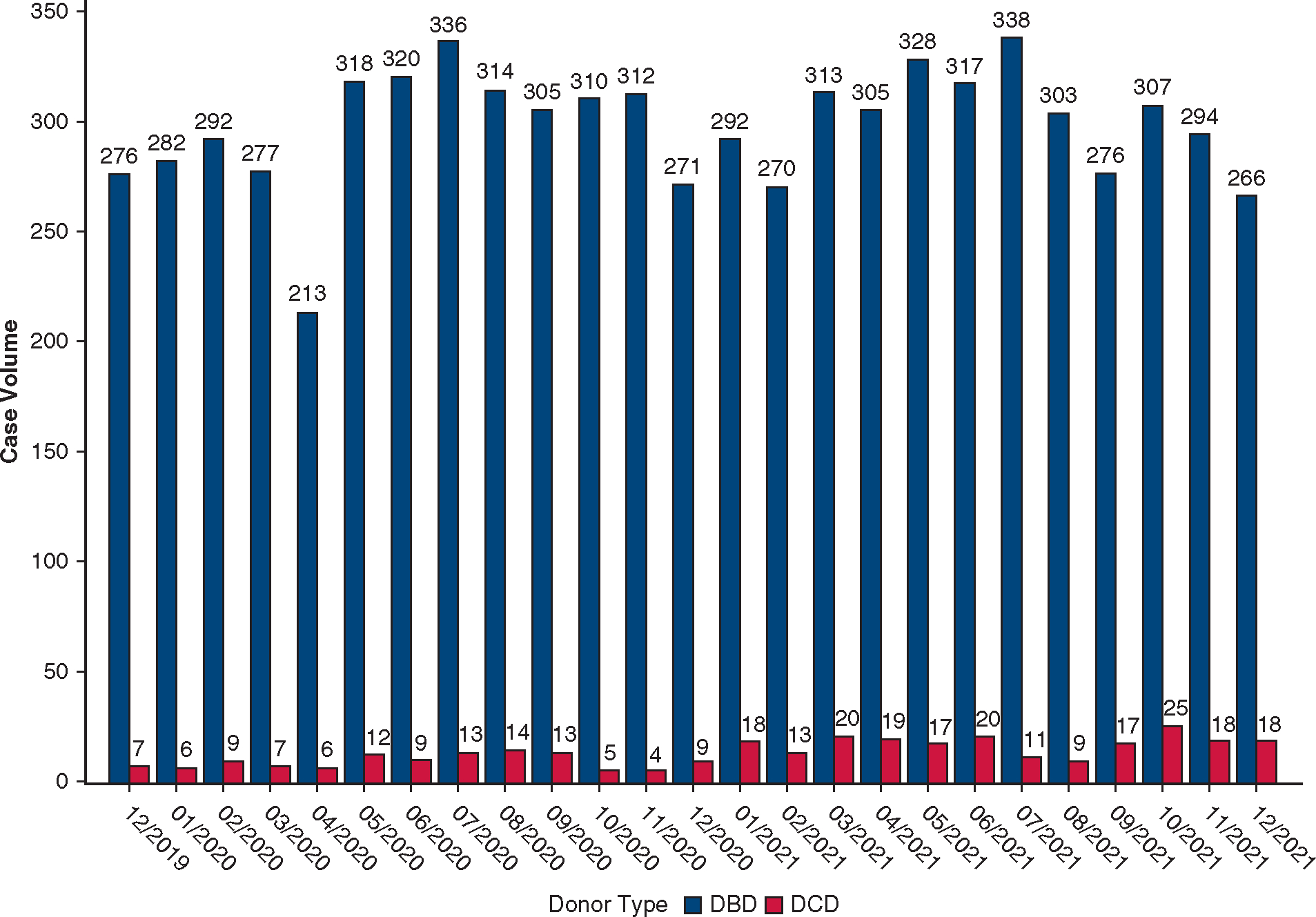
Monthly volume of DCD heart transplantation in the United States between December 1, 2019, and December 31, 2021. The monthly volume of DBD heart transplantation is shown in comparison. DCD, Donation after cardiac death; DBD, donation after brain death.
FIGURE E3.
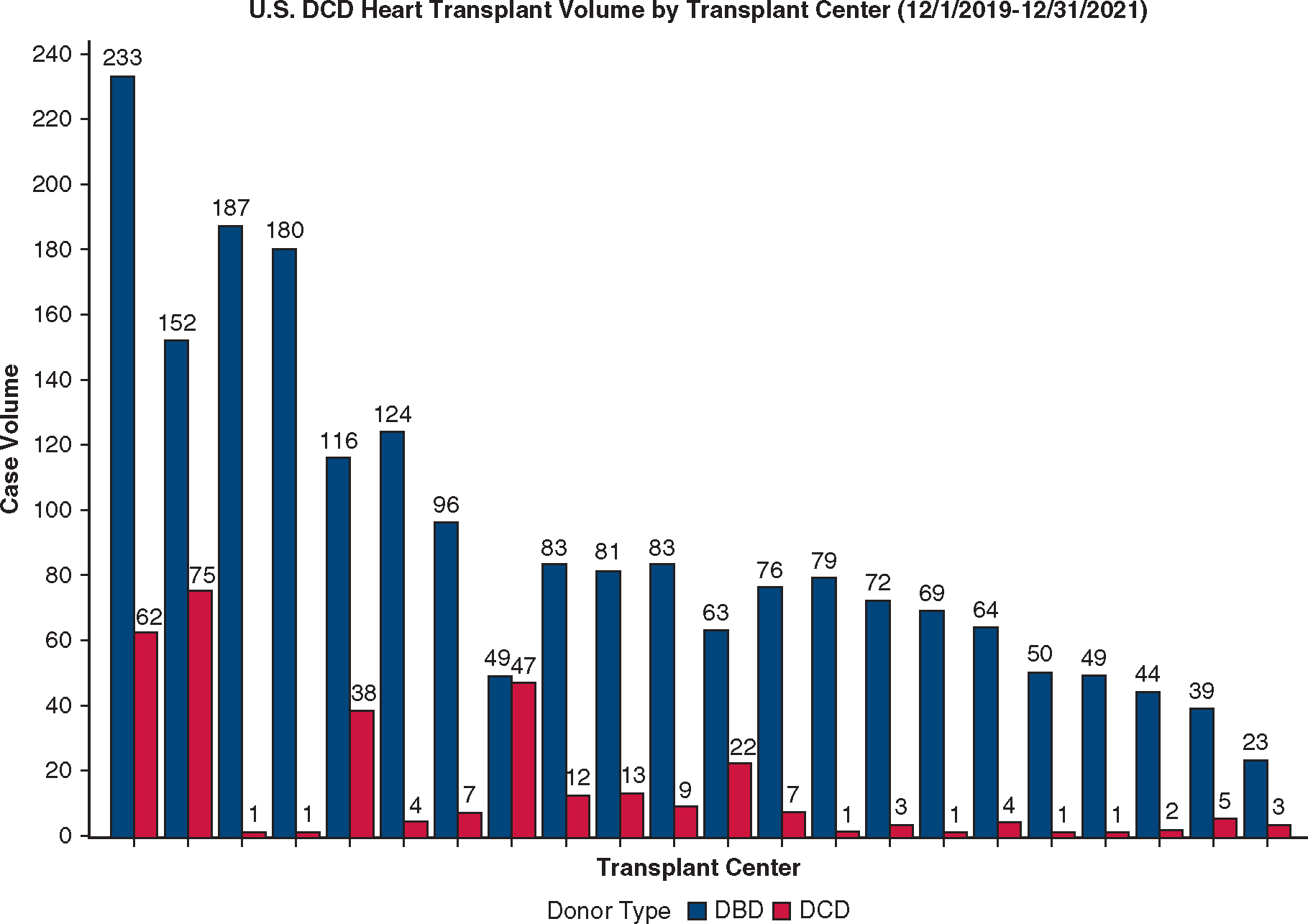
Total volume of DCD heart transplantation in the United States (US) between December 1, 2019, and December 31, 2021, by transplant center. Only transplant centers that have performed at least 1 DCD heart transplant are included in the figure. The volume of DBD heart transplants at each center during the same period is shown. DCD, Donation after circulatory death; DBD, donation after brain death.
Recovery of Donation After Circulatory Death Donor Hearts
A total of 355 hearts were recovered from DCD donors. The median time between controlled withdrawal of life support and the beginning of agonal phase was 2 (IQR, 1–4 range, 1–40) minutes (available in 346/355 patients), and median time between the beginning of agonal phase and confirmation of death was 15 (IQR, 11–20, range, 3–91) minutes (available in 312/355 patients). Among them, 319 (90.0%) successfully underwent transplantation, and 36 hearts were discarded.
Using time of death and aortic crossclamping (available in 321/355 DCD donors with hearts recovered), we identified 101 donor hearts recovered using NRP and 220 donor hearts recovered using DPP. Median time between death confirmation and aortic crossclamp was 6 (IQR, 4–8, range, 1–13) minutes when DPP was used and 70 (IQR, 57–117, range, 45–264) minutes when NRP was used (P < .001). The percentage of recovered hearts that were successfully used for transplantation was 94.1% (95/101) with NRP and 89.1% (196/220) with DPP (P = .16). Among the 22 centers where DCD heart transplants were performed, 7 have used NRP during DCD heart recovery.
Recipient and Donor Characteristics
A total of 266 DCD heart transplants and 5998 DBD heart transplants were included in the outcome analysis. Recipients of DCD hearts were more likely to be clinically stable (80.4% vs 41.1% in status 3–6, P < .001), to be taller (175.3, IQR, 170.1–182.9 vs 175.3, IQR, 167.6–180.3 cm, P = .05), to have type O blood (58.3% vs 39.9%, P < .001), and to wait longer after listing (55, IQR, 15–180 vs 32, IQR, 9–160 days, P = .003) compared with DBD heart recipients. Hearts from DCD donors had longer total graft preservation time (5.4, IQR, 3.8–6.5 vs 3.4, IQR, 2.9–3.9 hours, P < .001) and were further from the recipient hospital (361, IQR, 168–582 vs 221, IQR, 95–388 nautical miles, P < .001) than DBD hearts. These DCD donors were also younger (29 years, IQR, 23–35 vs 32 years, IQR, 24–40, P < .001) with better left ventricular ejection fraction (62%, IQR, 60–66 vs 60%, IQR, 56–65). Other differences in baseline recipient and donor characteristics are outlined in Table 1. Propensity score matching (1:3) resulted in 264 DCD heart transplants and 606 DBD heart transplants (Table 1).
TABLE 1.
Baseline recipient and donor characteristics in donation after circulatory death and donation after brain death heart transplants
| All patients |
Propensity-matched patients |
||||||
|---|---|---|---|---|---|---|---|
| DBD (n = 5998) | DCD (n = 266) | P value | DBD (n = 606) | DCD (n = 264) | P value | Standardized difference | |
|
| |||||||
| Recipient characteristics | |||||||
| Age (y) | 57 (46–63) | 57 (45–64) | .91 | 58 (48–64) | 57 (45–64) | .43 | −5.2% |
| BMI (kg/m2) | 27.4 (24.1–31.2) | 29.4 (26.2–32.8) | <.001 | 28.7 (25.7–32.4) | 29.4 (26.1–32.9) | .34 | 10.4% |
| Height (cm) | 175.3 (167.6–180.3) | 175.3 (170.1–182.9) | .05 | 175.0 (165.5–180.3) | 175.3 (170.2–182.9) | .07 | 14.2% |
| Race: white | 59.8 (3584) | 68.1 (181) | .007 | 64.7 (392) | 68.2(180) | .53 | −7.3% |
| Gender: male | 73.6 (4416) | 77.1 (205) | .21 | 75.7 (459) | 77.3 (204) | .78 | −3.5% |
| Diabetes (0.1% missing) | 29.4 (1762) | 30.8 (82) | .61 | 29.4 (178) | 31.1 (82) | .91 | −3.7% |
| Cerebrovascular disease (0.7% missing) | 7.8 (465) | 5.6 (15) | .32 | 8.9 (54) | 5.7 (15) | .17 | −8.4% |
| Ischemic cardiomyopathy | 29.8 (1790) | 28.2 (75) | .57 | 30.9 (187) | 28.4 (75) | .55 | 5.4% |
| Blood type O | 39.9 (2393) | 58.3 (155) | <.001 | 49.8 (302) | 58.0 (153) | .24 | −16.5% |
| Urgent status* | 58.9 (3532) | 19.6 (52) | <.001 | 25.6 (155) | 19.7 (52) | .74 | 13.2% |
| Waitlist time (d) | 32 (9–160) | 55 (15–180) | .003 | 54 (13–270) | 55 (15–179) | .62 | −17.6% |
| Creatinine (mg/dL) (0.1% missing) | 1.20 (0.95–1.50) | 1.20 (0.98–1.49) | .83 | 1.20 (1.00–1.51) | 1.20 (0.98–1.50) | .70 | 2.4% |
| Total bilirubin (mg/dL) (0.2% missing) | 0.7 (0.5–1.1) | 0.7 (0.5–1.0) | .96 | 0.6 (0.4–1.0) | 0.7 (0.5–1.0) | .21 | −0.7% |
| Pretransplant MCS | |||||||
| ECMO | 6.0 (360) | 0.4 (1) | <.001 | 2.2 (13) | 0.4 (1) | .11 | −9.1% |
| IABP | 28.5 (1709) | 10.2 (27) | <.001 | 11.4 (69) | 10.2 (27) | .70 | −3.0% |
| VAD or TAH | 33.8 (2026) | 43.2 (115) | .003 | 41.4 (251) | 43.2 (114) | .83 | 3.6% |
| Mechanical ventilation | 2.3 (137) | 0.0 (0) | .01 | 0 (0) | 0 (0) | .99 | 0.0% |
| Inotropic support | 40.1 (2403) | 23.7 (63) | <.001 | 25.7 (156) | 23.5 (62) | .89 | 4.9% |
| Transfusions after listing (0.7% missing) | 14.5 (870) | 6.0 (16) | <.001 | 7.1 (43) | 6.1 (16) | .90 | 3.5% |
| Dialysis after listing (0.2% missing) | 5.7 (340) | 1.1 (3) | .005 | 1.0 (6) | 1.1 (3) | .76 | −0.8% |
| Prior sternotomy | 39.3 (2274) | 44.5 (118) | .09 | 43.7 (265) | 44.7 (118) | .94 | −2.0% |
| Heart retransplantation | 2.9 (176) | 1.9 (5) | .32 | 3.5 (21) | 1.9 (5) | .10 | −10.0% |
| Multi-organ transplant | 11.1 (666) | 4.9 (13) | .001 | 5.1 (31) | 4.9 (13) | .81 | 0.7% |
| Location before transplant | <.001 | ||||||
| ICU | 55.2 (3309) | 19.9 (53) | 24.8 (150) | 20.1 (53) | .91 | 10.3% | |
| Hospitalized (non-ICU) | 13.6 (814) | 14.7 (39) | 14.5 (88) | 14.8 (39) | .85 | −0.7% | |
| Not hospitalized | 31.3 (1874) | 65.4 (174) | 60.7 (368) | 65.2 (172) | .97 | −9.4% | |
| Functional status at transplant Mild limitation | 6.4 (384) | 10.2 (27) | <.001 | 11.1 (67) | 10.2 (27) | .45 | −2.1% |
| Moderate limitation | 23.4 (1406) | 41.7 (111) | 41.1 (249) | 41.7 (110) | .90 | −1.2% | |
| Severe limitation | 64.3 (3856) | 46.6 (124) | 45.5 (276) | 46.6 (123) | .50 | 3.0% | |
| Unknown | 5.9 (352) | 1.5 (4) | 2.3 (14) | 1.5 (4) | .65 | −1.5% | |
| Total center volume† | 73 (46–110) | 147 (84–199) | <.001 | 112 (78–199) | 147 (84–199) | .28 | 14.9% |
| Donor characteristics | |||||||
| Age, y | 32 (24–40) | 29 (23–35) | <.001 | 29 (22–36) | 30 (23–35) | .36 | 0.1% |
| Gender: male | 71.7 (4302) | 86.8 (231) | <.001 | 81.5 (494) | 86.7 (229) | .35 | 10.6% |
| Body mass index (kg/m2) | 26.9 (23.7–31.3) | 26.8 (24.3–30.8) | .83 | 26.7 (23.3–31.1) | 26.8 (24.4–30.7) | .55 | −3.7% |
| Diabetes (1.3% missing) | 5.5 (328) | 2.3 (6) | .02 | 3.3 (20) | 2.3 (6) | .60 | 5.4% |
| Hypertension (1.4% missing) | 14.9 (891) | 11.7 (31) | .11 | 12.7 (77) | 11.7 (31) | .88 | 2.8% |
| Cause of death | .001 | ||||||
| Anoxia | 45.3 (2716) | 46.2 (123) | 48.5 (294) | 46.6 (123) | .43 | −3.9% | |
| Cerebrovascular/stroke | 12.7 (761) | 4.5 (12) | 5.8 (35) | 4.6 (12) | .65 | 4.4% | |
| Head trauma | 39.3 (2357) | 45.9 (122) | 43.1 (261) | 45.5 (120) | .43 | 4.8% | |
| Others | 2.7 (164) | 3.4 (9) | 0 (0) | 0 (0) | .99 | 0.0% | |
| Left ventricular ejection fraction (%) | 60 (56–65) | 62 (60–66) | .005 | 61 (58–65) | 62 (60–66) | .46 | 4.5% |
| Gender mismatch | 21.4 (1284) | 18.1 (48) | .19 | 17.7 (107) | 17.8 (47) | .93 | 0.4% |
| Donor/recipient predicted heart mass ratio | 1.01 (0.92–1.13) | 1.02 (0.92–1.13) | .63 | 1.01 (0.92–1.13) | 1.02 (0.92–1.13) | .40 | 5.9% |
| Graft preservation time >4 h | 22.3 (1338) | 72.2 (192) | <.001 | 63.9 (387) | 72.0 (190) | .72 | −18.8% |
All values are in % (n) or median (IQR). DBD, Donation after brain death; DCD, donation after circulatory death; BMI, body mass index; MCS, mechanical circulatory support; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; VAD, ventricular assist device; TAH, total artificial heart; ICU, intensive care unit.
Patients with UNOS status 1 or 2 (unable to be discharged from hospital and requiring mechanical circulatory support or with life-threatening ventricular arrhythmias) at the time of transplant were considered to have urgent status.
Total center volume refers to the total number of heart transplants (including both DCD and DBD cases) per center during the study period.
When DCD heart transplants with available time of death and aortic crossclamping during donor organ recovery (n = 240) were stratified by procurement method, recipients of DCD donor hearts recovered using NRP (n = 65) were older (60 years, IQR, 53–66 vs 55 years, IQR, 42–64, P = .003), more clinically stable (92.3% vs 76.6% in status 3–6), and less likely to be hospitalized before transplant (20.0% vs 38.9%, P = .007) compared with those who received a donor heart recovered with DPP (n = 175). Donor hearts recovered using NRP were located geographically closer to their recipients and had significantly shorter total graft preservation time (Table 2).
TABLE 2.
Baseline recipient and donor characteristics stratified by donation after circulatory death heart procurement technique
| Direct procurement and perfusion (n = 175) | Normothermic regional perfusion (n = 65) | P value | |
|---|---|---|---|
|
| |||
| Recipient characteristics | |||
| Age (y) | 55 (42–64) | 60 (53–66) | .003 |
| Gender: male | 76.0 (133) | 78.5 (51) | .69 |
| Body mass index (kg/m2) | 29.4 (26.1–32.7) | 29.1 (26.7–32.7) | .76 |
| Race: white | 67.4 (118) | 67.7 (44) | .97 |
| Diabetes | 29.1 (51) | 29.2 (19) | .99 |
| Cerebrovascular disease | 6.3 (11) | 1.5(1) | .33 |
| Ischemic cardiomyopathy | 25.7 (45) | 33.9 (22) | .21 |
| Dialysis after listing | 1.1 (2) | 1.5 (1) | 1.00 |
| Creatinine (mg/dL) | 1.16 (0.95–1.46) | 1.24 (1.01–1.50) | .13 |
| Total bilirubin (mg/dL) | 0.7 (0.5–1.1) | 0.7 (0.5–0.8) | .09 |
| Prior sternotomy | 42.5 (74) | 49.2 (32) | .35 |
| Heart retransplantation | 2.3 (4) | 1.5 (1) | 1.00 |
| Transfusion after listing | 4.6 (8) | 7.7 (5) | .35 |
| Blood type O | 58.9 (103) | 50.8 (33) | .61 |
| Multiorgan transplant | 2.9 (5) | 9.2 (6) | .07 |
| Waitlist time (d) | 53 (15–175) | 67 (19–212) | .52 |
| Urgent status* | 23.4 (41) | 7.7 (5) | .006 |
| Pretransplant inotrope | 27.4 (48) | 15.4 (10) | .06 |
| Pretransplant MCS | |||
| ECMO | 0.6 (1) | 0 (0) | 1.00 |
| IABP | 12.0 (21) | 3.1 (2) | .05 |
| LVAD | 44.0 (77) | 40.0 (26) | .75 |
| Pretransplant location | .007 | ||
| Hospitalized: ICU | 22.9 (40) | 6.2 (4) | |
| Hospitalized: non-ICU | 16.0 (28) | 13.9 (9) | |
| Not hospitalized | 61.1 (107) | 80.0 (52) | |
| Functional status | .001 | ||
| Mild limitation | 9.7 (17) | 13.9 (9) | |
| Moderate limitation | 34.9 (61) | 58.5 (38) | |
| Severe limitation | 53.1 (93) | 27.7 (18) | |
| Unknown | 2.3 (4) | 0 (0) | |
| Donor characteristics | |||
| Age, y | 30 (23–35) | 28 (23–35) | .63 |
| Gender: male | 86.9 (152) | 86.2 (56) | .89 |
| Body mass index (kg/m2) | 26.6 (24.0–30.8) | 27.4 (24.8–30.1) | .36 |
| Race: white | 79.4 (139) | 81.5 (53) | .72 |
| Diabetes | 2.9 (5) | 1.5 (1) | 1.00 |
| Hypertension | 13.1 (23) | 9.2 (6) | .19 |
| Left ventricular ejection fraction (%) | 63 (60–65) | 61 (60–68) | .46 |
| Gender mismatch | 18.9 (33) | 16.9 (11) | .73 |
| Predicted heart mass ratio | 1.00 (0.90–1.13) | 0.98 (0.93–1.06) | .52 |
| Cause of death | .94 | ||
| Anoxia | 45.7 (80) | 44.6 (29) | |
| Cerebrovascular/stroke | 4.0 (7) | 4.6 (3) | |
| Head trauma | 46.9 (82) | 46.2 (30) | |
| Others | 3.4 (6) | 4.6 (3) | |
| Total graft preservation time (h) | 6.1 (5.1–6.9) | 3.3 (2.2–3.8) | <.001 |
| Distance from transplant center (nautical miles) | 410 (229–612) | 228 (16–381) | <.001 |
All values are in % (n) or median (IQR). MCS, Mechanical circulatory support; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; ICU, intensive care unit.
Patients with UNOS status 1 or 2 (unable to be discharged from hospital and requiring mechanical circulatory support or with life-threatening ventricular arrhythmias) at the time of transplant were considered to have urgent status.
Outcomes of Donation After Circulatory Death and Donation After Brain Death Transplants
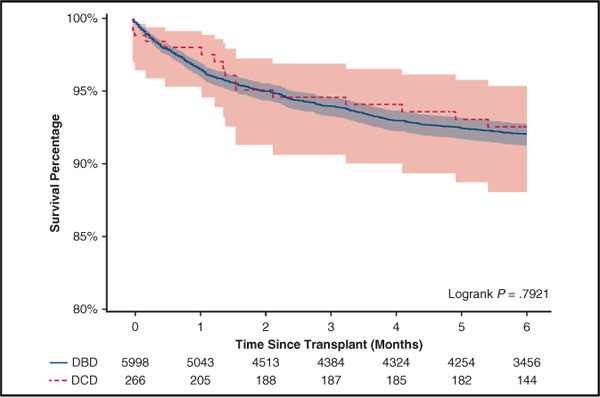
In the unmatched cohort, recipients of DCD hearts had higher rates of treated acute rejection before discharge (14.3% vs 8.8%, P = .002). Rates of post-transplant dialysis, permanent pacemaker implant, stroke, length of stay, 30-day mortality, and 90-day mortality were similar (Table 3). Six-month survival was 92.1% (95% confidence interval [CI], 91.3–92.8) after DBD heart transplants and 92.6% (95% CI, 88.1–95.4) after DCD heart transplants (hazard ratio, 0.94, 95% CI, 0.57–1.54, P = .79) (Figure 3). There were 16 mortalities in the DCD group. Causes of death included bacteremia/infection (n = 6), respiratory failure (n = 2), primary graft dysfunction (n = 3), anoxic brain injury (n = 1), postoperative hemorrhage (n = 2), multiorgan failure (n = 1), and unknown (n = 1). Among the 2 DCD patients who were unmatched, both were alive during follow-up.
TABLE 3.
In-hospital and short-term outcomes in donation after circulatory death and donation after brain death heart transplants
| All patients |
Propensity-matched patients |
|||||
|---|---|---|---|---|---|---|
| DBD (n = 5998) | DCD (n = 266) | P value | DBD (n = 606) | DCD (n = 264) | P value | |
|
| ||||||
| In-hospital outcomes | ||||||
| Treated acute rejection | 8.8 (525) | 14.3 (38) | .002 | 8.8 (53) | 14.4 (38) | .01 |
| Postoperative dialysis (0.1% missing) | 15.9 (952) | 18.1 (48) | .55 | 14.7 (89) | 18.2 (48) | .18 |
| Permanent pacemaker implant (0.2% missing) | 1.7 (100) | 0.4 (1) | .22 | 1.8 (11) | 0.4 (1) | .16 |
| Stroke | 3.7 (223) | 3.8 (10) | .70 | 3.8 (23) | 3.8 (10) | .91 |
| Hospital length of stay | 17 (12–25) | 16 (12–23) | .10 | 16 (11–24) | 16 (12–23) | .69 |
| Short-term mortality | ||||||
| 30-d mortality | 3.2 (192) | 1.9 (5) | .23 | 3.0 (18) | 1.9 (5) | .39 |
| 90-d mortality | 5.4 (321) | 4.5 (12) | .55 | 5.8 (35) | 4.6 (12) | .58 |
All values are in % (n) or median (IQR). DBD, Donation after brain death; DCD, donation after circulatory death.
FIGURE 3.
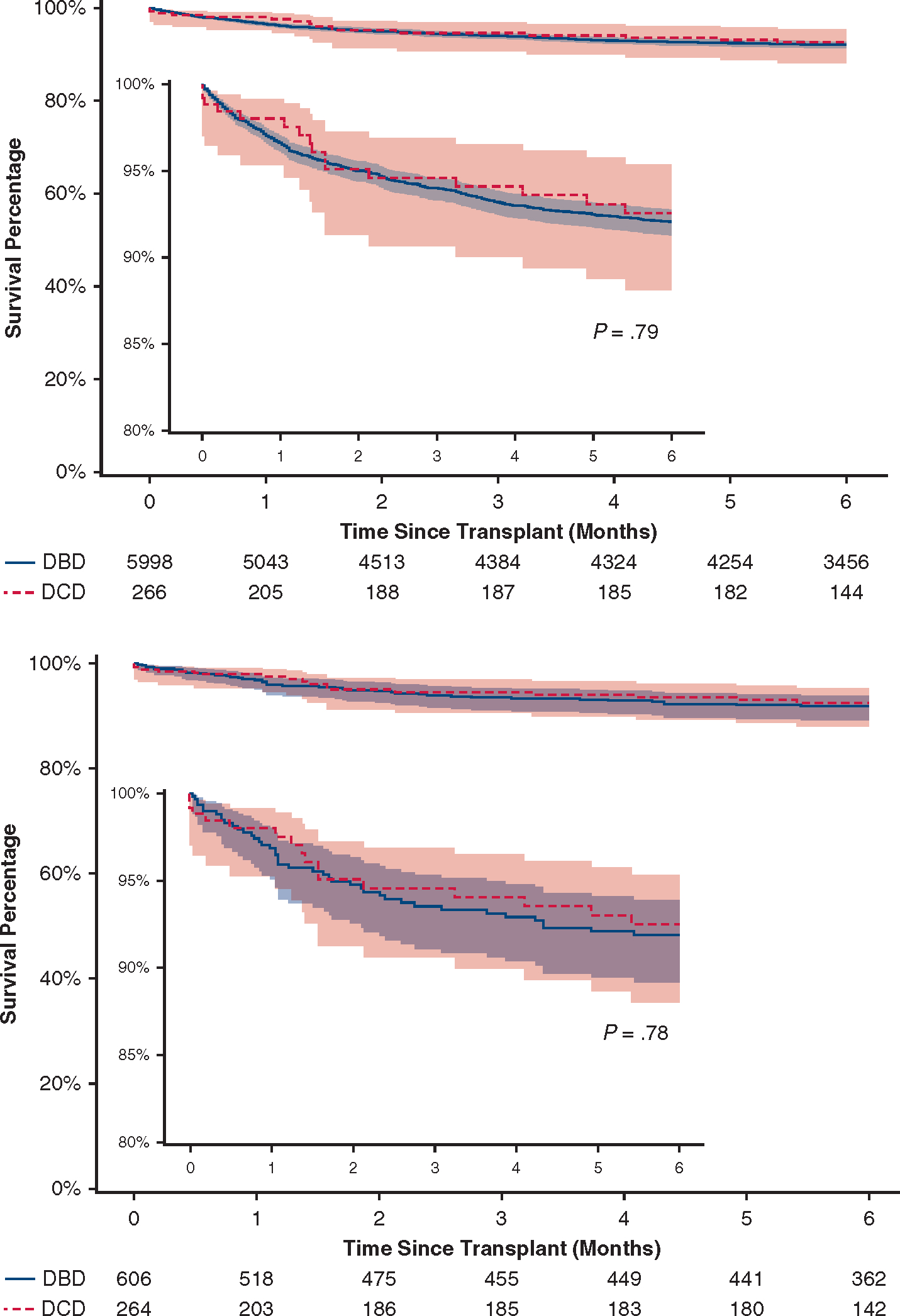
Six-month survival after heart transplantation using DCD and DBD donors. Six-month survival after heart transplantation using DCD donors was comparable to patients receiving hearts from DBD donors during the same period in both the overall cohort (top) and the propensity-matched cohort (bottom). The expanded vertical axis (80%-100%) (insert) shows a detailed comparison of both survival curves. The 95% confidence bands are shown. DBD, Donation after brain death; DCD, donation after cardiac death.
In 1:3 propensity-matched patients, in-hospital adverse events and short-term mortalities were similar except for higher rates of treated acute rejections in DCD heart transplants (Table 3). Six-month survival was 91.9% (95% CI, 89.2–93.9) after DBD heart transplants and 92.5% (95% CI, 88.0–95.3) after DCD heart transplants (hazard ratio, 0.92, 95% CI, 0.52–1.64, P = .79) (Figure 3).
Subgroup Analyses of Donation After Circulatory Death Heart Recipients
In recipients of DCD donor hearts, the use of NRP and DPP resulted in similar in-hospital and short-term outcomes (Table 4). Six-month survival was 92.0% (95% CI, 86.4–95.4) with DPP and 92.8% (95% CI, 78.7–97.8) with NRP (P = .98, Figure 4).
TABLE 4.
In-hospital and short-term outcomes stratified by donation after circulatory death heart procurement technique
| Direct procurement and perfusion (n = 175) | Normothermic regional perfusion (n = 65) | P value | |
|---|---|---|---|
|
| |||
| In-hospital outcomes | |||
| Treated acute rejection | 16.0 (28) | 12.3 (8) | .48 |
| Postoperative dialysis | 16.0 (28) | 23.1 (15) | .20 |
| Permanent pacemaker implant | 0.6 (1) | 0.0 (0) | 1.00 |
| Stroke | 4.0 (7) | 4.6 (3) | .83 |
| Hospital length of stay (d) | 15 (12–23) | 16 (12–24) | .73 |
| Short-term mortality | |||
| 30-d mortality | 1.7 (3) | 1.5 (1) | 1.00 |
| 90-d mortality | 4.6 (8) | 4.6 (3) | 1.00 |
All values are in % (n) or median (IQR).
FIGURE 4.
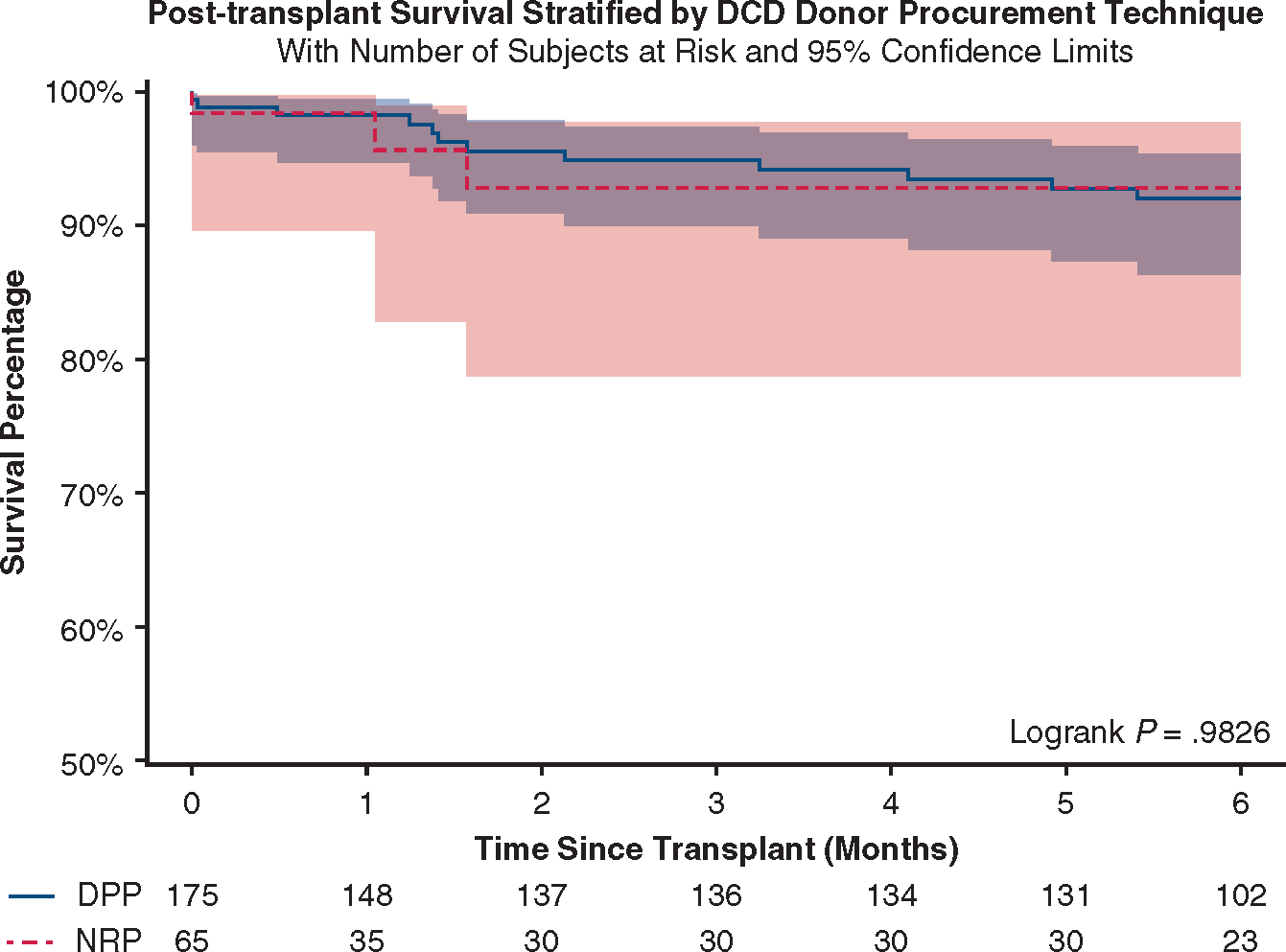
Six-month survival after DCD heart transplantation stratified by the procurement technique. The 95% confidence bands are shown. DCD, Donation after cardiac death; DPP, direct procurement and perfusion; NRP, normothermic regional perfusion.
Additionally, 6-month post-transplant survival did not differ on total organ preservation time quartiles, recipient age quartiles, pretransplant durable mechanical circulatory support, or total center volume of DCD heart transplants (Figure E4).
FIGURE E4.
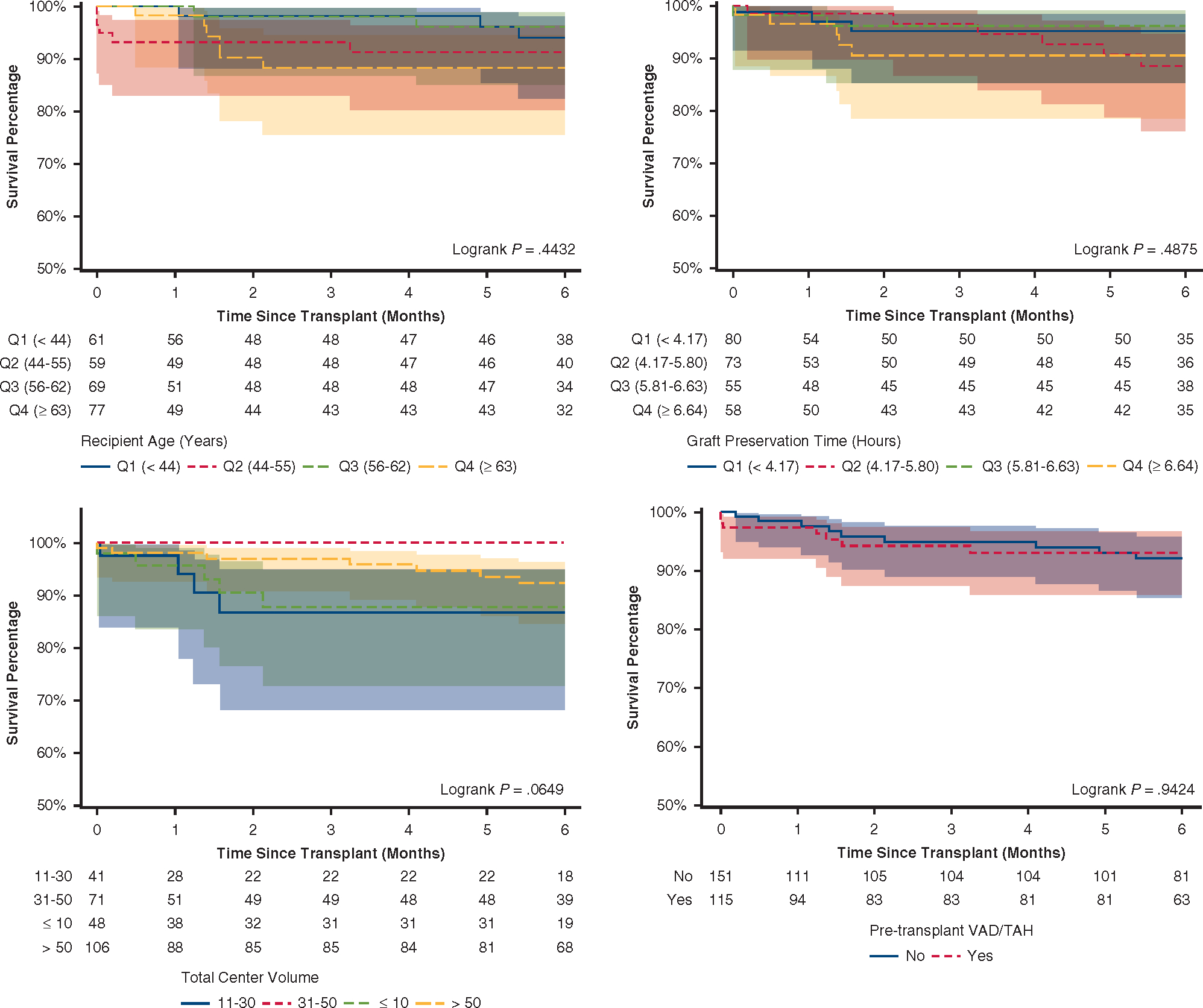
Six-month survival of DCD heart recipients stratified by selected risk factors. Unadjusted 6-month survival after heart transplant among recipients of DCD hearts as stratified by (A) total graft preservation time quartiles, (B) recipient age quartiles, (C) pretransplant ventricular assist device or total artificial heart use, and (D) total volume of DCD heart transplantation during study period at each center. VAD, Ventricular assist device; TAH, total artificial heart.
DISCUSSION
Heart transplantation using DCD donors has been recently reintroduced into clinical practice and may potentially increase donor heart availability. However, comparative clinical outcome data are scarce. Enrollment of approximately 180 participants was recently completed in a multicenter, randomized trial comparing post-transplant outcomes between DCD heart transplants using direct procurement and ex vivo machine perfusion and DBD heart transplants using standard cold storage for allograft preservation (NCT03831048).10 In this context, our analysis of a national transplant registry represents an all-inclusive cohort of patients who were included in the US DCD trial and patients who underwent transplantation outside of the trial. This study also included patients who received DCD donor hearts retrieved using NRP, which was not assessed in the trial.
Our findings suggests that DBD and DCD heart transplants are associated with similar 6-month survival and selected in-hospital adverse events. Early single-center reports from Britain and Australia have shown similar short and mid-term survival between DCD and DBD heart transplants, with 91% to 95% 6-month survival after DCD heart transplantation11,12 In an initial analysis of the UNOS registry, short-term outcomes of 127 DCD heart transplants were also similar to matched DBD recipients.13 Patient selection may have played a role in these outcomes. We observed that recipients selected for DCD heart transplant in contemporary national practice had lower overall baseline risk, with less likelihood of being hospitalized or supported with extracorporeal membrane oxygenation or intra-aortic balloon pump, less inotrope dependence, and better functional status at transplant. The DCD donors were also younger with better ventricular function. After matching, DBD and DCD transplants were associated with similar short-term survival. This finding may support the view that the period of warm ischemia experienced by DCD donor hearts before procurement does not significantly affect post-transplant outcomes with careful donor and recipient selection. However, we observed a higher rate of treated acute rejection before discharge in DCD heart transplants, a finding that was not previously reported.11,12 Because we were unable to control for pretransplant sensitization status due to significant missing data in the UNOS registry, randomized data and more granular reports from individual centers may shed light on this.
Consequently, heart transplantation using DCD donors may represent a significant opportunity to increase the donor pool and reduce waitlist mortality.13–16 A key finding of our study was that a large proportion of DCD heart recipients were patients who traditionally wait the longest for a suitable donor, including patients assigned to status 3 to 6, taller patients, and those with type O blood. These patients currently represent more than one-third of those waitlisted for heart transplant, waiting on average more than 12 months for transplant with pretransplant mortality of approximately 15 deaths per 100 patient-years of wait time.3 Increased availability of DCD transplant represents a particularly important advance in care for this patient population.
The optimal method to recover DCD donor hearts remains unknown. Little comparative data exist in literature, and the US DCD heart trial only included the use of DPP with the TransMedics Organ Care System (OCS). Early single-center experiences with NRP in DCD heart procurement showed 100% short-term survival and a 12% to 60% incidence of primary graft dysfunction.17,18 In the UK experience, recipients of DCD heart recovered using NRP had 100% 1-year survival, compared with 91% in the DPP group (P = .50).11 In the OCS Heart EXPAND trial, 30-day and 6-month survivals were 94.7% and 88%, respectively, when OCS was used to resuscitate, preserve, and assess marginal DBD donor hearts.19
Compared with DPP, NRP has the theoretical advantages of rapid heart reperfusion and in situ functional assessment, potentially leading to improved mitigation of warm ischemia and a higher acceptance rate of donor hearts (Table E2). Additionally, NRP allows improved reperfusion of transplantable abdominal organs and can significantly reduce cost when static cold storage is used after organ recovery.20,21 We observed that the percentage of recovered DCD hearts that were discarded was numerically lower with NRP compared with DPP (5.9% vs 10.9%, P =.16). Post-transplant outcomes were similar regardless of the procurement technique, although the sample size was small, the follow-up was short, and the comparison was not risk adjusted. Significant ethical concerns also surround the artificial reinstitution of circulation in NRP, which may contravene the declaration of death based on the irreversible cessation of circulatory and respiratory function.22 Consensus guidelines from major stakeholders are anticipated.
TABLE E2.
Comparison of normothermic regional perfusion and direct procurement and perfusion for donation after circulatory death heart procurement
| Normothermic regional perfusion | Direct procurement and perfusion | |
|---|---|---|
|
| ||
| Advantages | • Earlier reperfusion • In situ hemodynamic and functional assessment of the donor heart • Minimized warm ischemia for transplantable abdominal organs • Lower cost if used with static cold storage |
• Less personnel needed • No ethical controversy |
| Disadvantages | • Increased technical complexity • Additional equipment and personnel • Ethical constraints |
• Delayed reperfusion • Dependence on surrogate biomarker for assessment of donor heart function • Longer warm ischemia for transplantable abdominal organs • Increased cost associated with the ex vivo perfusion console |
There are several key considerations in the wider use of DCD heart transplant. For example, identifying favorable DCD donor and recipient characteristics may become increasingly important as DCD transplant is applied to a wider donor and recipient population. DCD heart transplantation may in practice be limited to centers with the resources able to support the additional personnel needed during procurement, which often includes an extra surgeon and up to 2 perfusionists.11,12 Furthermore, the incremental cost associated with perfusion equipment for ex situ reanimation of the donor heart and transportation may be a further barrier to wider adoption. To minimize disparities in access to DCD heart transplantation, further work will be needed regarding practical implementation of various procurement techniques and optimization of resource use.
Study Limitations
This national report of early outcomes of DCD heart transplantation has several limitations. First, residual confounding and selection bias may influence the results, and the follow-up was too short to make meaningful conclusions about longerterm outcomes. Second, DCD donors who were assessed but did not progress to circulatory death were not captured, and there was insufficient information on donor organs that were retrieved but declined for transplant. Third, during DCD donor heart recovery, the time from asystole to reperfusion was not recorded, a potential key factor in determining postoperative outcomes. Accurate total ischemic time for DCD donor hearts also could not be determined, because many these organs were perfused on an ex situ perfusion platform during transport and were not subjected to true ischemia. Fourth, because the technique of DCD heart procurement was not captured in the UNOS registry, the use of NRP and DPP was identified using the available time interval between time of death and aortic crossclamping during organ recovery. Using 15 minutes as the cutoff was not previously validated and could result in erroneous classification. Fifth, the use of postoperative mechanical circulatory support was not reported, and primary graft dysfunction could not be reliably identified from the UNOS registry. Last, informative censoring bias resulting from using recent UNOS data could have underestimated survival. We performed a sensitivity analysis using only patients who have reached at least 6-month follow-up to provide more accurate survival estimates.
CONCLUSIONS
In this analysis of a national transplant registry, 6-month survival after DCD heart transplants was comparable to DBD heart transplants, and post-transplant outcomes did not differ on procurement technique in DCD donors. Broader implementation of DCD heart transplantation may represent an opportunity to increase the donor pool and reduce waitlist mortality.
PERSPECTIVE.
DCD heart transplants have short-term survival comparable to DBD heart transplants. It is increasingly adopted in the United States but remains limited to a few large centers. Although no consensus exists on the optimal DCD heart recovery method, NRP and DPP resulted in similar short-term outcomes. Broader implementation of DCD heart transplant could substantially increase donor organ availability.
Funding:
Q.C. and A.R. are supported by grants from the National Institutes of Health for advanced heart disease research (T32HL116273).
This work was supported in part by Health Resources and Services Administration Contract 234–2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
This study was approved by the Institutional Review Board at Cedars-Sinai Medical Center, with a waiver of informed consent (STUDY00001188, approved on 2/19/2021)
Abbreviations and Acronyms
- CI
confidence interval
- DBD
donation after brain death
- DCD
donation after circulatory death
- DPP
direct procurement and perfusion
- IQR
interquartile range
- NRP
normothermic regional perfusion
- OCS
Organ Care System
- UNOS
United Network for Organ Sharing
Biographies
Dr Ranjit John (Minneapolis, Minn). Thank you for giving me an option to discuss this paper, and congratulations on an outstanding presentation.

Dr Dominick Megna (Los Angeles, Calif). Thank you.

Footnotes
Conflict of Interest Statement
The authors reported no conflicts of interest.
Read at the 102nd Annual Meeting of The American Association for Thoracic Surgery, Boston, Massachusetts, May 14–17, 2022.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/1322.
CENTRAL MESSAGE
Heart transplantation using DCD donors has similar short-term survival to DBD transplants. Short-term outcomes did not differ between recipients of DCD hearts recovered using NRP versus DPP.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Discussion
Presenter: Dr Dominick Megna
Dr John. We know that DCD has been around since the inception of cardiac transplantation. However, advances in technology have allowed this option to potentially become mainstream. What is exciting for the field is that it has the potential to increase the number of heart transplants, which have been fairly stagnant over the past few decades by as much as 40% and as low as 25%. I have the following few questions for you. Currently, the logistics to prepare DBD hearts, whether it is by DPP/OCS or NRP is certainly more complex in terms of logistics than the traditional donor procurement techniques. Do you anticipate this becoming easier to further widen its applicability? The second question is more of a philosophical one. Are hearts procured by DCD versus the traditional method equivalent in terms of quality? In other words, and your presentation did shed some light on it, are the recipients you choose moving forward going to be different for these 2 kinds of procurement techniques? In your institution, how have you solved some of the ethical issues?
Dr Megna. So, to answer your first question, in terms of logistics, I think that it’s going to be center-dependent. There are some centers that still like ours have been going through the Institutional Review Board and ethical constraints surrounding NRP. We have colleagues to the south of us who have exploded their use of NRP, and I think that comes down to cost as well. Cost is a big concern for all of us. The OCS device, as we know, comes with cost. We know the OCS device has been studied now. We don’t have the granular data on NRP to make this direct comparison. We tried to make a comparison here, but we still don’t have granular data to let us decide if NRP is superior to DPP or not, and that will play a part. If we can show results that NRP may have some superiority to the DPP, then the question involves ethics. I think there’s more of a push toward using NRP. But as it stands now, I think OCS, if the costs aren’t a constraint, is probably logistically easier for a lot of programs. With that being said, the cost of using a perfusionist versus not using a perfusionist and the additional personnel really are center-dependent. And your second question?
Dr John. Was the one on quality. Are hearts procured by either of the 2 techniques similar? Is it apples to apples?
Dr Megna. I think looking at these data, the quality of hearts was good, especially those that were included in the trial. They were not marginal donors. But I know that since the trial stopped enrolling, there have been hearts that we would consider extended criteria. There have been some long ischemic times. There have been older donors who were not included in the trial. So, I think what we’re going to see is a wide variety of uses for both DCD and DBD that will be comparable in terms of what kind of donors we use and what kind of recipients we use as well.
Dr John. And the last one was the ethical issues. I know we could have a whole day on that.
Dr Megna. Again, that’s going to be center-dependent. Like I said, for our institution, we’ve been going through iterations of this and multiple meetings with our ethics committee, and we actually have an Institutional Review Board that’s still in process to get approval for the use of NRP. So, I think that it would be nice as a society and group to decide on the approach and have the same streamlined approach because I think that’s where we get our granular data if we’re doing things the same way and we can really see which approach is superior.
References
- 1.Bakhtiyar SS, Godfrey EL, Ahmed S, Lamba H, Morgan J, Loor G, et al. Survival on the heart transplant waiting list. JAMA Cardiol. 2020;5:1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharmavaram N, Hess T, Jaeger H, Smith J, Hermsen J, Murray D, et al. National trends in heart donor usage rates: are we efficiently transplanting more hearts? J Am Heart Assoc. 2021;10:e019655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colvin M, Smith JM, Ahn Y, Skeans MA, Messick E, Goff R, et al. OPTN/SRTR 2019 annual data report: heart. Am J Transplant. 2021;21(Suppl 2):356–440. [DOI] [PubMed] [Google Scholar]
- 4.Steinbrook R Organ donation after cardiac death. N Engl J Med. 2007;357:209–13. [DOI] [PubMed] [Google Scholar]
- 5.White CW, Messer SJ, Large SR, Conway J, Kim DH, Kutsogiannis DJ, et al. Transplantation of hearts donated after circulatory death. Front Cardiovasc Med. 2018;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National data–OPTN. Accessed November 15, 2021. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/
- 7.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, et al. The relation-ship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008; 52:2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kransdorf EP, Kittleson MM, Benck LR, Patel JK, Chung JS, Esmailian F, et al. Predicted heart mass is the optimal metric for size match in heart transplantation. J Heart Lung Transplant. 2019;38:156–65. [DOI] [PubMed] [Google Scholar]
- 10.Shudo Y, Benjamin-Addy R, Koyano TK, Hiesinger W, MacArthur JW, Woo YJ. Donors after circulatory death heart trial. Future Cardiol. 2021;17:11–7. [DOI] [PubMed] [Google Scholar]
- 11.Messer S, Cernic S, Page A, Berman M, Kaul P, Colah S, et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant. 2020;39:1463–75. [DOI] [PubMed] [Google Scholar]
- 12.Chew HC, Iyer A, Connellan M, Scheuer S, Villanueva J, Gao L, et al. Outcomes of donation after circulatory death heart transplantation in Australia. J Am Coll Cardiol. 2019;73:1447–59. [DOI] [PubMed] [Google Scholar]
- 13.Madan S, Saeed O, Forest SJ, Goldstein DJ, Jorde UP, Patel SR. Feasibility and potential impact of heart transplantation from adult donors after circulatory death. J Am Coll Cardiol. 2022;79:148–62. [DOI] [PubMed] [Google Scholar]
- 14.Jawitz OK, Raman V, DeVore AD, Mentz RJ, Patel CB, Rogers J, et al. Increasing the United States heart transplant donor pool with donation after circulatory death. J Thorac Cardiovasc Surg. 2020;159:e307–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messer S, Page A, Rushton S, Berman M, Tsui S, Catarino P, et al. The potential of heart transplantation from donation after circulatory death donors within the United Kingdom. J Heart Lung Transplant. 2019;38:872–4. [DOI] [PubMed] [Google Scholar]
- 16.Osaki S, Anderson JE, Johnson MR, Edwards NM, Kohmoto T. The potential of cardiac allografts from donors after cardiac death at the University of Wisconsin Organ Procurement Organization. Eur J Cardiothorac Surg. 2010;37:74–9. [DOI] [PubMed] [Google Scholar]
- 17.Smith DE, Kon ZN, Carillo JA, Chen S, Gidea CG, Piper GL, et al. Early experience with donation after circulatory death heart transplantation using normothermic regional perfusion in the United States. J Thorac Cardiovasc Surg. 2022;164:557–68.e1. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman JRH, McMaster WG, Rali AS, Rahaman Z, Balsara K, Absi T, et al. Early US experience with cardiac donation after circulatory death (DCD) using normothermic regional perfusion. J Heart Lung Transplant. 2021;40:1408–18. [DOI] [PubMed] [Google Scholar]
- 19.Schroder JN, D’Alessandro D, Esmailian F, Boeve T, Tang P, Liao K, et al. Successful utilization of extended criteria donor (ECD) hearts for transplantation–results of the OCS™ heart EXPAND trial to evaluate the effectiveness and safety of the OCS heart System to preserve and assess ECD hearts for transplantation. J Heart Lung Transplant. 2019;38:S42. [Google Scholar]
- 20.Oniscu GC, Randle LV, Muiesan P, Butler AJ, Currie IS, Perera MT, et al. In situ normothermic regional perfusion for controlled donation after circulatory death–the United Kingdom experience. Am J Transplant. 2014;14:2846–54. [DOI] [PubMed] [Google Scholar]
- 21.Miñambres E, Suberviola B, Dominguez-Gil B, Rodrigo E, Ruiz-San Millan JC, Rodríguez-San Juan JC, et al. Improving the outcomes of organs obtained from controlled donation after circulatory death donors using abdominal normothermic regional perfusion. Am J Transplant. 2017;17:2165–72. [DOI] [PubMed] [Google Scholar]
- 22.Ethics, determination of death, and organ transplantation in normothermic regional perfusion (NRP) with controlled donation after circulatory determination of death (cDCD): statement of concern. American College of Physicians. Published 2021. Accessed January 12, 2022. https://www-acponline-org.mlprox.csmc.edu/acp_policy/policies/ethics_determi [Google Scholar]


