Abstract
Equine gastric ulcer syndrome (EGUS) is a multifactorial disorder and one of the most common diseases in horses. The objective of this research was to detect one of the potential risk factors of equine squamous gastric disease (ESGD), the Helicobacter pylori specific gene, and tracing the presence of the duodenal ulcer-promoting gene (dupA) as a possible virulence marker. Gastric fluid together with faecal samples were collected from twenty rural horses from around Tabriz, Iran. Throughout the endoscopic examinations, the type, numbers, severity, and the location of the lesions were documented. Nine of twenty horses exhibited macroscopic lesions in the squamous mucosa that were later classified into grades 1, 2, 3, and 4. Only three of these horses exhibited H. pylori in their gastric fluid samples, whereas all faecal samples were H. pylori-negative. All the H. pylori-positive cases manifested severe forms of ESGD (grades 3–4). The age and sex were both unrelated to the lesion severity and ESGD status in this study. Research is required to further discuss the virulence aspects of dupA regarding ESGD.
Keywords: dupA, EGUS, H. pylori
Equine gastric ulcer syndrome (EGUS) is a broad term, commonly used to characterise erosive and ulcerative stomach diseases in horses. The non-glandular stomach (squamous mucosa), glandular stomach, terminal oesophagus, and proximal duodenum are relatively the most prevalent anatomical sites in which gastric disease may occur (Andrews et al. 1999; Andrews et al. 2005; Sykes et al. 2015; Camacho-Luna et al. 2018).
Primarily, EGUS is divided into equine glandular gastric disease (EGGD) and equine squamous gastric disease (ESGD) to better elucidate and specify the anatomical attributes of this disorder (Sykes et al. 2015). The lesion severity is usually assessed by assigning a grading system describing the stomach lining’s mucosal and anatomical integrity.
Recent findings in identifying the link between the risk factors for ESGD and EGGD suggest no connection (Scheidegger et al. 2017; Banse and Andrews 2019; Hallowell 2018). Given the current data, it is best to contemplate the risk factors for ESGD and EGGD in a distinctive manner, given that they have different pathogeneses (McGovern 2017).
EGGD is widely recognised by a disruption in the glandular mucosa, which is likely to have an inflammatory origin (Hallowell 2018). Factors, such as diet and performance, are related to the ESGD occurrence (Banse et al. 2018). Evidence on the role of age and sex as ESGD risk factors remains unclear. The focus of this research is on ESGD; thus, discussing EGGD any further in a detailed manner is not in the scope of this study.
Animals with an otherwise healthy gastric tract usually exhibit primary lesions rather than secondary lesions in the non-glandular region. On the contrary, delayed gastric emptying, caused by pyloric stenosis conditions, induces secondary lesions. Up to 93% of active Thoroughbred racehorses manifested ESGD lesions in contrast to those withdrawn from the racecourse (37%) (Sykes et al. 2015). Performance horses are far more susceptible to ESGD than those of non-performance horses (around 48% and 11%, respectively) (Sykes et al. 2015). Recently, it has been established that high-speed treadmill training contributes to increased abdomen pressure and reduced stomach volume in horses (Furr et al. 1994). The daily performance gives rise to higher gastrin levels and increases acid flow to the non-glandular region, which might account for the presence of lesions in performance horses (Furr et al. 1994). However, the exact role of gastrin in ESGD is poorly understood due to the limited number of studies (Furr et al. 1994). Diet is a substantial risk factor for ESGD. Volatile fatty acids (VFAs) induce acid damage in lower pH levels (≤ 4) in the non-glandular region (Nadeau et al. 2003).
Moreover, receiving a diet with high amounts of calcium and protein increases the gastric pH levels and might be associated with a decreased severity of lesions as opposed to a diet with low amounts of these materials. Consequently, grazing on alfalfa has a protective effect on non-glandular regions (Buchanan and Andrews 2003). Stall confinement is considered yet another risk factor. Horses who were kept in a pasture manifested a lower occurrence of gastric ulcer syndrome (Feige et al. 2002). The cause of this phenomenon can be multidimensional given the scheduled diet and not being in contact with other horses (Feige et al. 2002). It appears that constant gastric acid secretion (hydrochloric acid) is one of the elemental factors due to its damaging effect on the non-glandular region in horses. Hydrochloric acid, VFAs, and bile acids are known as damaging acids (Berschneider et al. 1999). Hydrochloric acid is responsible for inhibiting cellular sodium and cell swelling and it ultimately induces ulcers in the non-secretory region in pH ≤ 4. The combination of VFA and hydrochloric acid can cause a miscellaneous number of lesions in a variety of severe forms (Nadeau et al. 2003). Bile acids present in the stomach originate from duodenal reflux. These acids provoke sensitivity to hydrogen ions in the non-secretory cells and, as a result, give rise to ulcers (Berschneider et al. 1999). Pepsinogen turns into pepsin in pH levels below four and has a major role in the incidence of gastric ulcer syndrome. This enzyme has a synergistic capacity combined with hydrochloric acid in proteolytic functions.
Helicobacter pylori
Despite the ongoing evaluation of bacterial factors involved in the aetiology of EGUS, their role in either the initiation or contribution to the severity of EGUS remains unclear.
Helicobacter pylori is a well-adapted resident of the human gastric mucosa and can contribute to diverse and persistent forms of diseases ranging from gastric ulcers to gastric cancer in people. Similarly, Helicobacter spp. may colonise in non-human primates, dogs, and pigs (Haesebroucket al. 2009; Casagrande Proietti et al. 2010; Hong et al. 2015). The ability to withstand and neutralise the acidic environment, given the urease enzyme’s role, enables a more convenient mechanism for acid survival in the Helicobacter genus (Krakowka 2007).
Helicobacter pylori-specific DNA has been isolated from the equine stomach in both normal and abnormal gastric mucosa in glandular and non-glandular regions (Scott et al. 2001; Bezdekova and Futas 2009). The presence of Helicobacter-resembling microorganisms in the gastric mucosa was also confirmed in eight out of twelve affected horses in histological studies (Morales et al. 2010). Regardless, no studies link the presence of Helicobacter pylori to EGUS.
The dupA gene
The duodenal ulcer promoting gene (dupA) of Helicobacter pylori is associated with an increased risk of duodenal ulcers (DUs) in humans and, thus, is considered a possible virulent marker in this regard. However, research shows controversial results about different populations from different geographical regions. Limitations in polymerase chain reaction (PCR) techniques could be one possible rationale leading to inconsistent results regarding the link between dupA and DU in different geographical regions (Abadi and Perez-Perez 2016; Rani 2020). More research is required to shed some light on its function, especially on animal models, such as horses, which are susceptible to gastrointestinal diseases. To the best of our knowledge, this is the first study considering dupA as a possible virulent factor in equine species.
MATERIAL AND METHODS
Twenty privately owned rural horses in the Tabriz western suburban regions were selected in a cluster random sampling method; their age, sex and body condition score (BCS) were recorded (Table 1). The haircoat condition, diet, colic history, deworming history, and living environment were also documented. A 12-hour fasting period was executed before the process, and the horses were under sedation with 0.04 mg/kg detomidine (Detomo Vet®; Ceva, Girona, Spain) prior to the endoscopic examination (Olympus Vet 3M; Olympus, Tokyo, Japan). In all the animals, the squamous mucosa and the visible portion of the glandular body from the cardia were examined clearly for the ulcer evaluation. However, we did not check the pylorus in any of the selected subjects. Furthermore, data concerning the presence of lesions, the number of lesions, and their severity were documented, and the lesion grading was undertaken based on MacAllister et al.’s (1997) table of lesions (Tables 2 and 3).
Table 1. Summary of the clinical findings and results of the PCR examination.
| Sample | Age (years) | Sex | BCS (1–10) | Lesion presence | H. pylori | dupA | |||
| gastric fluid PCR result | faecal sample PCR result | gastric fluid PCR result | faecal sample PCR result | ||||||
| 1 | 11 | M | 5 | P | NE | NE | NT | NT | |
| 2 | 10 | S | 6 | P | NE | NE | NT | NT | |
| 3 | 12 | M | 6 | P | NE | NE | NT | NT | |
| 4 | 6 | S | 6 | P | PO | NE | NE | NT | |
| 5 | 5 | S | 5 | A | NE | NE | NT | NT | |
| 6 | 5 | S | 5 | A | NE | NE | NT | NT | |
| 7 | 15 | M | 6 | A | NE | NE | NT | NT | |
| 8 | 2 | S | 4 | P | PO | NE | NE | NT | |
| 9 | 9 | S | 5 | P | NE | NE | NT | NT | |
| 10 | 8 | M | 5 | A | NE | NE | NT | NT | |
| 11 | 2 | S | 4 | A | NE | NE | NT | NT | |
| 12 | 11 | S | 5 | P | PO | NE | NE | NT | |
| 13 | 4 | S | 6 | P | NE | NE | NT | NT | |
| 14 | 3 | M | 4 | P | NE | NE | NT | NT | |
| 15 | 5 | S | 5 | A | NE | NE | NT | NT | |
| 16 | 5 | S | 5 | A | NE | NE | NT | NT | |
| 17 | 12 | M | 4.5 | A | NE | NE | NT | NT | |
| 18 | 12 | M | 3.5 | A | NE | NE | NT | NT | |
| 19 | 8 | S | 5 | A | NE | NE | NT | NT | |
| 20 | 5 | S | 5 | A | NE | NE | NT | NT | |
A = absent; BCS = body condition score; M = mare; NE = negative; NT = not tracked; P = present; PCR = polymerase chain reaction; PO = positive; S = stallion
Table 2. The gastric lesion grading system (MacAllister et al. 1997).
| Lesion severity score | Description |
| Grade 0 | no lesion |
| Grade 1 | appears superficial (only mucosa missing) |
| Grade 2 | deeper structures involved (greater depth than No. 1) |
| Grade 3 | multiple lesions and variable severity (1, 2 and/or 4) |
| Grade 4 | same as 2 and has an active appearance |
| Grade 5 | same as 4 plus active haemorrhage or adherent blood clot |
Table 3. Summary of the findings regarding the lesions.
| Sample | Grade | Number of lesions | Type | Region |
| 1 | 2 (mild) | 3 | ESGD | greater curvature |
| 2 | 1 (mild) | 1 | ESGD | greater curvature |
| 3 | 2 (mild) | 3 | ESGD | lesser curvature |
| 4 | 3 (severe) | 4 | ESGD | greater curvature |
| 8 | 4 (severe) | 3 | ESGD | greater curvature |
| 9 | 2 (mild) | 1 | ESGD | lesser curvature |
| 12 | 4 (severe) | 4 | ESGD | greater and lesser curvature |
| 13 | 1 (mild) | 1 | ESGD | lesser curvature |
| 14 | 1 (mild) | 1 | ESGD | lesser curvature |
ESGD = equine squamous gastric disease
Following the examination, 50 cc of gastric fluid was collected from each horse. A rectal examination was performed thereafter, and 20 g of a faecal sample were collected from each horse in sterile tubes. The samples were then kept at –21 °C in a freezer for further analysis. The owners’ approval was obtained before starting the examination, and each step was conducted carefully in a way to avoid any unnecessary discomfort to the animals.
Sample analysis
The bacterial DNA was extracted according to the manufacturer’s instructions from the faecal and gastric lavage samples by a Mini Stool Kit (Qiagene, Hilden, Germany and Sinagene, Tehran, Iran). Furthermore, samples were examined via a PCR assay to detect Helicobacter using HP (16S rRNA, 145 bp) and dupA (197 bp) primers [Table 4 (Scholte et al. 1997; Gomes et al. 2008)]. To set up our samples, a PCR assay was performed on a control positive sample of H. pylori as a marker to validate the positivity or negativity of the samples (Ghotaslou et al. 2017). The products were loaded on a 1.5% electrophoresis gel (Benchtop UV Transilluminator; BioDoc, Hannover, Germany).
Table 4. Primers used in the PCR.
| Primers | Sequence (5'→3') | Size (bp) | Reference |
| HP (16S rRNA region) | F: CTGGAGARACTAAGYCCTCC | 145 | Scholte et al. (1997) |
| R: GAGGAATACTCATTGCGAAGGCGA | |||
| dupA | F: CGTGATCAATATGGATGCTT | 197 | Gomes et al. (2008) |
| R: TCTTTCTAGCTTGAGCGA |
Statistical analysis
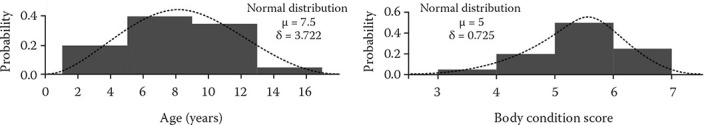
The data analysis was performed using SPSS software v26 (IBM, USA), and the Pearson chi-squared test along with a one-way analysis of variance (ANOVA) test was carried out according to the type of variables with a 95% confidence interval considered as significant (Table 5). The mean, standard error, standard deviation together with the frequency were also conducted in terms of the variable types (Table 6). The data were also assessed for normality prior to the test implementation (Figure 1).
Table 5. Statistical data analysis.
| Correlation | Method | P-value | Significance |
| H. pylori presence and lesion presence | Pearson chi-squared test | 0.037 8 | yes |
| H. pylori presence and lesion severity | Pearson chi-squared test | 0.002 7 | yes |
| H. pylori presence and lesion number | one-way ANOVA | 0.018 | yes |
| H. pylori presence ESGD status | Pearson chi-squared test | 0.037 8 | yes |
| Age and ESGD status | one-way ANOVA | 0.955 | no |
| Age and lesion severity | one-way ANOVA | 0.464 | no |
| Sex and ESGD status | Pearson chi-squared test | 0.887 5 | no |
| Sex and lesion severity | Pearson chi-squared test | 0.133 6 | no |
ESGD = equine squamous gastric disease
Table 6. Statistical data analysis.
| Features | Mean | 95% confidence interval for mean | Std. deviation | Frequency | Percentage (%) | Confidence interval summary (95% CI) one-sample binomial success rate (Clopper-Pearson) | ||
| statistics | std. error | |||||||
| Age | 7.5 | 0.853 | 4.6–10.5 | 3.818 | – | – | – | |
| Sex | mare | – | – | – | – | 7 | 35 | 0.35 (0.154–0.592) |
| stallion | 13 | 65 | 0.65 (0.408–0.846) | |||||
| BCS (1-10) | 5.22 | 0.277 | 4.58–5.86 | 0.833 | – | – | – | |
| Lesion presence | present | – | – | – | – | 9 | 45 | 0.45 (0.231–0.685) |
| absent | 11 | 55 | 0.55 (0.315–0.769) | |||||
| Lesion severity | mild | 2.22 | 0.4 | 1.29–3.14 | 1.201 | 6 | 66.6 | 0.66 (0.15–1.40) |
| severe | 3 | 33.3 | 0.33 (0.031–1.08) | |||||
| Number of sessions | 2.33 | 0.44 | 1.31–3.35 | 1.322 | – | – | – | |
| ESGD status | positive | – | – | – | – | 9 | 45 | 0.45 (0.231–0.685) |
| negative | 11 | 55 | 0.55 (0.315–0.769) | |||||
| H. pylori status | positive | – | – | – | – | 3 | 15 | 0.15 (0.032–0.379) |
| negative | 17 | 85 | 0.85 (0.621–0.968) | |||||
BSC = body condition score; ESGD = equine squamous gastric disease
Figure 1. Normal distribution.
RESULTS
All the horses were kept in stalls and were in good haircoat condition, and their main diet consisted of alfalfa, hay, and barley in the amounts that the animals needed. According to the owners, they had no history of colic and had not received any deworming treatment before.
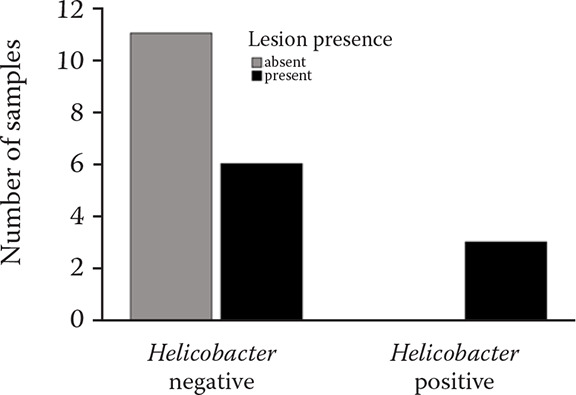
The gastroscopy results revealed nine horses (45%) with lesions present (Tables 3 and 6, Figures 2–4) in either mild or severe forms. All the lesions were present in the non-glandular region. Three of the twenty gastric fluid samples (samples 4, 8, and 12) were H. pylori positive in the PCR results, covering 15% of the demographic (Table 6). However, the findings concerning the dupA gene were negative in all of the H. pylori positive cases. The faecal samples were H. pylori negative according to the PCR results. Thus, the dupA gene was not tracked. The positive samples had severe type lesions comprising grades 3 and 4, respectively, and were all stallions. Six of the negative samples exhibited mild lesions (35.29%, 1–2 grades). The horses were divided into two groups with mild lesions (either no lesions or grade 2 lesions) and severe lesions (grade 3 and 4). It appears that the mild lesions were more prevalent in this demographic (Figure 5). A significant correlation existed between the H. pylori specific gene presence and the four factors in this study, including the lesion presence, lesion severity, lesion number, and ESGD status in horses (Table 6, Figure 6). The age and sex both turned out to be unrelated to the ESGD status and lesion severity in this study.
Figure 2. Grade 2 lesion in the non-glandular greater curvature region in an 11-year-old mare. Hyperkeratosis lesion in a 3-year-old mare in the lesser curvature region and non-glandular stomach (left to right, respectively).
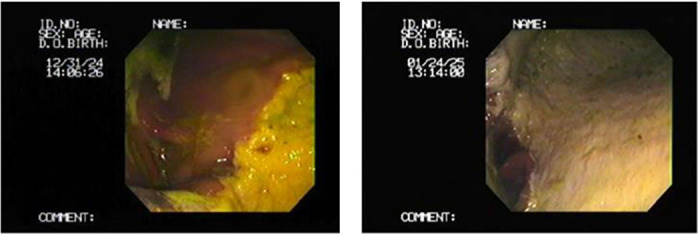
Figure 3. 6-year-old stallion with a number of grade 3 lesions in the non-glandular stomach and greater curvature region.
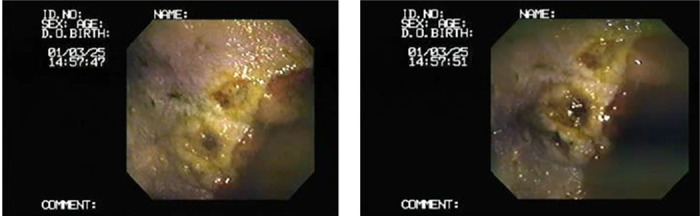
Figure 4. 11-year-old stallion with a grade 4 lesion in the greater curvature of the stomach.

Figure 5. Lesion distribution according to the severity in association with the Helicobacter presence.
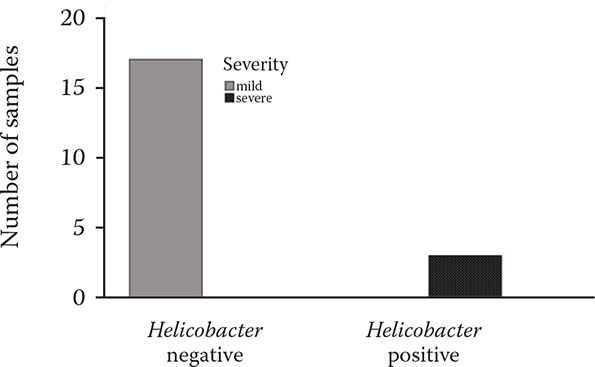
Mild = either no lesion, grade 1 or grade 2 lesions; severe = grade 3 and 4 lesions
Figure 6. Lesion presence in the Helicobacter positive and Helicobacter negative horses.
Additionally, due to similar haircoat conditions, diet, maintenance, colic history, and deworming administration, performing a statistical analysis and comparing these factors was not possible.
DISCUSSION AND CONCLUSION
Equine gastric syndrome has been identified as an erosive disease that shares similar characteristics with those of the human peptic ulcer disease (Andrews et al. 2005).
EGUS has different prevalence rates and various potential risk factors in horses. H. pylori is one of the risk factors and is the primary cause in the aetiology of gastric ulcers in humans and also has a significant role in inducing gastric cancer (Feldman et al. 2016). However, there is not enough evidence to conclude the same can be said for horses since H. pylori has not been cultured in either stomach regions of the equine gastric mucosa (Camacho-Luna and Andrews 2015). In a histological study in Spain, the presence of H. pylori, gastric mucosal inflammatory response, and abrasive lesions were manifestation of the further irritation of the gastric mucosa by the bacteria and, thereby, confirming a correlation with EGUS (Morales et al. 2010). To date, nineteen species of Helicobacter have been colonised from the gastric tract of animals, most of which replicate in humans as well (Fox 2002). Helicobacter sp. was detected in both the glandular and non-glandular regions of Thoroughbred horses (Contreras et al. 2007). In a study in 2001, positive reactions to PCR by Helicobacter sp. in three horses with only non-glandular regions were detected (Scott et al. 2001). In a study undertaken by Bezdekova and Futas (2009), 100% of the horses with lesions revealed non-glandular lesions on the gastric mucosa in a gastroscopy examination. Prolonged exposure to hydrochloric acid (HCl) and organic acids such as volatile fatty acids (VFAs) are the primary cause of ulcers in the non-glandular mucosa (Berschneider et al. 1999).
In the present study, nine out of twenty horses had carried macroscopic gastric ulcers. The abundance of gastric ulcers in the rural horse demographic was 45%, and among those, three were detected as H. pylori positive within the gastric fluid samples via the PCR (15%). However, none of the faecal samples were H. pylori positive. Needless to say, these samples all exhibited grade 3 or 4 lesions in the non-glandular region. Finally, the H. pylori positive samples all tested dupA gene-negative, which may account for the lack of correlation between the gastric ulcer disease and the dupA gene. To our knowledge, this is the first study investigating the relationship between dupA and EGUS. Meanwhile, epidemiology research should be extended with wider sample sizes and other populations.
In conclusion, the correlation between the presence of the H. pylori-specific gene and lesion presence, lesion severity, lesion number, and ESGD status can be considered as one of the risk factors in EGUS. In addition, lesions were only found in the non-glandular region. Further studies are required due to the sample size limitations to clarify the role of the dupA gene in gastric disorders.
Acknowledgement
We thank to the owners of horses for their collaboration in this study.
Funding Statement
Supported by vice-chancellor of research of the University of Tabriz, Iran.
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- Abadi ATB, Perez-Perez G. Role of dupA in virulence of Helicobacter pylori. World J Gastroenterol. 2016 Dec 14;22(46):10118-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews F, Bernard W, Byars D, Cohen N, Divers T, MacAllister C, McGladdery A, Merritt A, Murray M, Orsini J et al. Recommendations for the diagnosis and treatment of equine gastric ulcer syndrome (EGUS). Equine Vet Educ. 1999;11(5):262-72. [Google Scholar]
- Andrews FM, Buchanan B, Elliot SB, Clariday NA, Edwards LH. Gastric ulcers in horses. J Anim Sci. 2005 Jun 1;83(Suppl_13):E18-21. [Google Scholar]
- Banse HE, Andrews FM. Equine glandular gastric disease: Prevalence, impact and management strategies. Vet Med (Auckl). 2019 Jul 16;10:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banse HE, MacLeod H, Crosby C, Windeyer MC. Prevalence of and risk factors for equine glandular and squamous gastric disease in polo horses. Can Vet J. 2018 Aug;59(8):880-4. [PMC free article] [PubMed] [Google Scholar]
- Berschneider HM, Blikslager AT, Roberts MC. Role of duodenal reflux in nonglandular gastric ulcer disease of the mature horse. Equine Vet J Suppl. 1999 Apr;(29):24-9. [DOI] [PubMed] [Google Scholar]
- Bezdekova B, Futas J. Helicobacter species and gastric ulceration in horses: A clinical study. Vet Med-Czech. 2009 Dec;54(12):577-82. [Google Scholar]
- Buchanan BR, Andrews FM. Treatment and prevention of equine gastric ulcer syndrome. Vet Clin North Am Equine Pract. 2003 Dec;19(3):575-97. [DOI] [PubMed] [Google Scholar]
- Camacho-Luna P, Andrews FM. Equine gastric ulcer syndrome. Sprayberry KA, Robinson NE, editors. Robinson’s current therapy in equine medicine. 7th ed. St. Louis: Elsevier Inc.; 2015. 280 p. [Google Scholar]
- Camacho-Luna P, Buchanan B, Andrews FM. Advances in diagnostics and treatments in horses and foals with gastric and duodenal ulcers. Vet Clin North Am Equine Pract. 2018 Apr;34(1):97-111. [DOI] [PubMed] [Google Scholar]
- Casagrande Proietti P, Bietta A, Brachelente C, Lepri E, Davidson I, Franciosini MP. Detection of Helicobacter spp. in gastric, fecal and saliva samples from swine affected by gastric ulceration. J Vet Sci. 2010 Sep;11(3):221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Morales A, Garcia-Amado MA, De Vera M, Bermudez V, Gueneau P. Detection of Helicobacter-like DNA in the gastric mucosa of Thoroughbred horses. Lett Appl Microbiol. 2007 Nov;45(5):553-7. [DOI] [PubMed] [Google Scholar]
- Feige K, Furst A, Eser MW. Auswirkungen von Haltung, Futterung und Nutzung auf die Pferdegesundheit unter besonderer Berucksichtigung respiratorischer und gastrointestinaler Krankheiten [Effects of housing, feeding and use on equine health with emphasis on respiratory and gastrointestinal diseases]. Schweiz Arch Tierheilkd. 2002 Jul;144(7):348-55. German. [DOI] [PubMed] [Google Scholar]
- Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s gastrointestinal and liver disease: Pathophysiology, diagnosis, management. Philadelphia, Pennsylvania: Saunders/Elsevier, 2016; p. 1098. [Google Scholar]
- Fox JG. The non-H pylori helicobacters: Their expanding role in gastrointestinal and systemic diseases. Gut. 2002 Feb;50(2):273-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr M, Taylor L, Kronfeld D. The effects of exercise training on serum gastrin responses in the horse. Cornell Vet. 1994 Jan;84(1):41-5. [PubMed] [Google Scholar]
- Ghotaslou R, Leylabadlo HE, Akhi MT, Sadeghi J, Yousefi L, Bialvaei AZ et al. The importance of Helicobacter pylori tnpA, tnpB, and cagA genes in various gastrointestinal diseases. Mol Genet Microbiol Virol. 2017 Jul;32(1):62-5. [Google Scholar]
- Gomes LI, Rocha GA, Rocha AM, Soares TF, Oliveira CA, Bittencourt PF, Queiroz DM. Lack of association between Helicobacter pylori infection with dupA-positive strains and gastroduodenal diseases in Brazilian patients. Int J Med Microbiol. 2008 Apr;298(3-4):223-30. [DOI] [PubMed] [Google Scholar]
- Haesebrouck F, Pasmans F, Flahou B, Chiers K, Baele M, Meyns T, Decostere A, Ducatelle R. Gastric helicobacters in domestic animals and nonhuman primates and their significance for human health. Clin Microbiol Rev. 2009 Apr;22(2):202-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallowell G. Pathogenesis of equine squamous and glandular gastric disease. UK-Vet Equine. 2018 May 2;2(3):70-5. [Google Scholar]
- Hong S, Chung Y, Kang WG, Choi YS, Kim O. Comparison of three diagnostic assays for the identification of Helicobacter spp. in laboratory dogs. Lab Anim Res. 2015 Jun;31(2):86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowka S. Comparative aspects of bacterial gastritis in domestic animals. In: Proceeding of the ACVP/ASVCP concurrent annual meetings; 2007 Nov 10-14; Savannah, Georgia; 2007. p. 1-5. [Google Scholar]
- MacAllister CG, Andrews FM, Deegan E, Ruoff W, Olovson SG. A scoring system for gastric ulcers in the horse. Equine Vet J. 1997 Nov;29(6):430-3. [DOI] [PubMed] [Google Scholar]
- McGovern K. Updates on gastric ulceration in adult horses. Livestock. 2017 Sep 2;22(5):272-7. [Google Scholar]
- Morales A, Garcia F, Bermudez V. Detection of Helicobacter-like organisms in Thoroughbred horses from Venezuela. Braz J Vet Path. 2010;3(1):52-5. [Google Scholar]
- Nadeau JA, Andrews FM, Patton CS, Argenzio RA, Mathew AG, Saxton AM. Effects of hydrochloric, acetic, butyric, and propionic acids on pathogenesis of ulcers in the nonglandular portion of the stomach of horses. Am J Vet Res. 2003 Apr;64(4):404-12. [DOI] [PubMed] [Google Scholar]
- Rani S. Study of genetic diversity, dupA gene, and association with previous illness, and smoking in peptic ulcer disease. J Xi'an Univ Archit & Technol. 2020;XII(VIII):629-32. [Google Scholar]
- Scheidegger MD, Gerber V, Bruckmaier RM, van der Kolk JH, Burger D, Ramseyer A. Increased adrenocortical response to adrenocorticotropic hormone (ACTH) in sport horses with equine glandular gastric disease (EGGD). Vet J. 2017 Oct;228:7-12. [DOI] [PubMed] [Google Scholar]
- Scholte GH, van Doorn LJ, Quint WG, Lindeman J. Polymerase chain reaction for the detection of Helicobacter pylori in formaldehyde-sublimate fixed, paraffin-embedded gastric biopsies. Diagn Mol Pathol. 1997 Aug;6(4):238-43. [DOI] [PubMed] [Google Scholar]
- Scott DR, Marcus EA, Shirazi-Beechey SSP, Murray M. Evidence of Helicobacter infection in the horse. In: Proceedings of the American Society of Microbiologists; 2001 May; Washington, DC; 2001, p. 287. [Google Scholar]
- Sykes BW, Hewetson M, Hepburn RJ, Luthersson N, Tamzali Y. European College of Equine Internal Medicine Consensus Statement – Equine gastric ulcer syndrome in adult horses. J Vet Intern Med. 2015 Sep-Oct;29(5):1288-99. [DOI] [PMC free article] [PubMed] [Google Scholar]


