Abstract
From 1998 to 2008, 1229 foodborne outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus were reported in the United States; 39% were reported with a confirmed etiology. Vomiting was commonly reported in B. cereus (median, 75% of cases) and S. aureus outbreaks (median, 87%), but rarely in C. perfringens outbreaks (median, 9%). Meat or poultry dishes were commonly implicated in C. perfringens (63%) and S. aureus (55%) outbreaks, and rice dishes were commonly implicated in B. cereus outbreaks (50%). Errors in food processing and preparation were commonly reported (93%), regardless of etiology; contamination by a food worker was only common in S. aureus outbreaks (55%). Public health interventions should focus on these commonly reported errors to reduce the occurrence of outbreaks caused by B. cereus, C. perfringens, and S. aureus in the United States.
Keywords: disease outbreaks, bacterial toxins, Bacillus cereus, Clostridium perfringens, Staphylococcus aureus
An estimated 9.4 million foodborne illnesses caused by a known pathogen occur annually in the United States; 1.3 million (14%) are caused by Bacillus cereus, Clostridium perfringens, or Staphylococcus aureus [1]. These 3 pathogens cause illness through preformed toxin production in improperly handled foods (B. cereus and S. aureus) or in vivo toxin production within the gastrointestinal tract after consumption of a contaminated food (C. perfringens and hypothesized for B. cereus diarrheal syndrome) [2–5]. Estimated costs range from US$166 per illness caused by B. cereus to US$539 per illness caused by S. aureus [6]; total annual costs of illnesses caused by these pathogens are estimated at US $523 million [6].
Determining the etiology of an illness or outbreak caused by one of these pathogens classically involves evaluating a combination of findings that include symptoms, incubation period, duration of illness, and suspected contaminated food. However, for these pathogens, these findings often overlap, and determining the etiology of a single illness or in the setting of a foodborne outbreak can be challenging without laboratory confirmation in clinical samples or food specimens. Laboratory confirmation can be complicated because persons affected often do not seek medical attention [7], clinical stool specimens are not routinely collected or tested for these pathogens or their enterotoxins [8, 9], and implicated foods may no longer be available for testing at the time of outbreak investigation. A better understanding of the distinguishing epidemiologic and clinical characteristics of outbreaks caused by these pathogens will help investigators determine which one pathogen was the likely cause.
In the United States, sporadic illnesses caused by B. cereus, C. perfringens, and S. aureus are not reportable. Thus, estimates of the annual number of illnesses and descriptive epidemiology rely on illnesses that occur in outbreaks. Data reported to a national outbreak surveillance system provides a unique opportunity to examine the epidemiologic and clinical characteristics of outbreaks caused by these pathogens.
METHODS
A foodborne disease outbreak is defined as the occurrence of ≥2 similar illnesses resulting from the ingestion of a common food. State, local, territorial, and tribal health departments voluntarily submit outbreak investigation reports to the Centers for Disease Control and Prevention’s (CDC) Foodborne Disease Outbreak Surveillance System. Data collected for each outbreak include the number of illnesses, case demographics, incubation period, symptoms, duration of illness, etiology, implicated foods, setting of food preparation and consumption, methods of food preparation, and factors contributing to food contamination.
We reviewed foodborne disease outbreaks caused by B. cereus, C. perfringens, and S. aureus that occurred during 1998–2008. Outbreaks were included if a single confirmed or suspected etiology was reported. A confirmed outbreak was one in which the etiology met predefined laboratory confirmation criteria for clinical or food specimens (Table 1) [10].
Table 1.
Guidelines for the Confirmation of Foodborne Disease Outbreaks Caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus, United States, 1998–2008
| Etiologic Agent | Confirmationa |
|---|---|
| Bacillus cereus | Isolation of organism from stool of 2 or more ill persons and not from stool of control patients |
| OR | |
| Isolation of 105 organisms per gram from epidemiologically implicated foodb, provided specimen is properly handled | |
| Clostridium perfringens | Isolation of 106 organisms per gram from stool of 2 or more ill persons, provided specimen is properly handled |
| OR | |
| Demonstration of enterotoxin in the stool of 2 or more ill persons | |
| OR | |
| Isolation of 105 organisms per gram from epidemiologically implicated food, provided specimen is properly handled | |
| Staphylococcus aureus | Isolation of organism of same phage type from stool or vomitus of 2 or more ill persons |
| OR | |
| Detection of enterotoxin in epidemiologically implicated food | |
| OR | |
| Isolation of 105 organisms per gram from epidemiologically implicated food, provided specimen is properly handled |
Source: Centers for Disease Control and Prevention[10].
Laboratory findings that meet or exceed the listed confirmation criteria are reported as confirmed etiology; laboratory findings below these criteria are reported as suspect etiology. In the absence of laboratory data, the etiologic agent may be reported as a suspect etiology if the reporting agency has a reasonable suspicion, based on other data available (eg, incubation period, clinical syndromes, implicated foods), that one of these pathogens was the causal agent.
Although not part of confirmation guidelines during the study period, testing for B. cereus enterotoxins in implicated foods is encouraged.
Implicated foods were manually reviewed and sorted into mutually exclusive food groups (eg, meat or poultry dish) after considering both the food reported and the usual manner of food preparation. For example, steak was assigned to the meat or poultry group, and further classified as beef, whereas beef stew was assigned to soups and stews. Outbreaks for which a single implicated food could not be identified were excluded from the food group analysis. Implicated foods were also categorized by ethnicity (eg, Mexican-style, Asian). Methods of food preparation were analyzed for all foods included in the food group analysis. Methods of food preparation were not mutually exclusive and were based on predefined categories of food preparation [11]. In brief, a “solid mass of potentially hazardous food” refers to foods containing 1 (eg, rice) or more (eg, casserole) ingredients that are cooked and then held at warm temperatures for extended periods of time before service. “Cook-and-serve foods” are those that are completely cooked, typically in <30 minutes, and served immediately, usually within 1 hour of preparation. “Roasted meat or poultry” refers to pieces of meat or poultry >3 inches thick that are roasted or baked for >30 minutes. Finally, “liquid or semisolid mixtures of potentially hazardous food” includes foods (eg, sauces, gravies) that are cooked, then either served immediately or held at warm temperatures before service.
Location of food preparation and consumption were analyzed for outbreaks reporting a single location. Reported contributing factors to food contamination, proliferation, and amplification of the pathogen and toxin production [12] were analyzed for all outbreaks with data available; factors were grouped according to whether they represented environmental contamination of raw ingredients or foods, contamination during food preparation and processing (eg, inadequate time or temperature during cooking), direct contamination of food by an ill food worker or carrier of the pathogen, cross-contamination in the food preparation environment, and other contamination factors.
This outbreak surveillance system is dynamic; data were downloaded on 10 March 2011 and are subject to change. All analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
From 1998 through 2008, there were 13 405 foodborne disease outbreaks reported, and 1229 outbreaks were caused by 1 of the following 3 pathogens, either confirmed or suspected: C. perfringens (44%, n = 536 outbreaks) was the most common, followed by S. aureus (37%, n = 458) and B. cereus (19%, n = 235; Table 2). Together, these outbreaks resulted in 29 835 illnesses, and at least 3486 healthcare visits, 438 hospitalizations, and 11 deaths. The number of reported outbreaks increased from 121 in 1998 to 152 in 2002, and then declined to 66 outbreaks in 2008. Outbreaks with a suspected etiology contributed disproportionately to this observed decline, especially those caused by S. aureus, which decreased from a maximum of 43 outbreaks in 2002 to 5 outbreaks in 2008 (Figure 1). Outbreaks were reported from all 50 states, the District of Columbia, Guam, Puerto Rico, and the Republic of Palau. No outbreaks reported exposures that occurred in multiple states, and only 1% of outbreaks reported exposures that occurred in multiple counties.
Table 2.
Foodborne Disease Outbreaks Caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus, United States, 1998–2008
| Characteristic |
Bacillus cereus
|
Clostridium perfringens
|
Staphylococcus aureus
|
All Outbreaks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Confirmed | Suspected | Total | Confirmed | Suspected | Total | Confirmed | Suspected | Total | ||
| Outbreaks | 56 (24) | 179 (76) | 235 | 253 (47) | 283 (53) | 536 | 167 (36) | 291 (64) | 458 | 1229 |
|
| ||||||||||
| Illnesses | 881 | 1169 | 2050 | 13 182 | 7862 | 21 044 | 4818 | 1923 | 6741 | 29 835 |
|
| ||||||||||
| Median per outbreak (range) | 8 (2–140) | 3 (2–100) | 4 (2–140) | 24 (2–950) | 10 (2–741) | 16 (2–950) | 17 (2–225) | 3 (2–218) | 4 (2–225) | 7 (2–950) |
|
| ||||||||||
| Healthcare visitsa | 52 | 48 | 100 | 1719 | 111 | 1830 | 1245 | 311 | 1556 | 3486 |
|
| ||||||||||
| Hospitalizationsa | 7 | 10 | 17 | 74 | 14 | 88 | 296 | 37 | 333 | 438 |
|
| ||||||||||
| Deathsa | 0 | 0 | 0 | 5 | 3 | 8 | 3 | 0 | 3 | 11 |
|
| ||||||||||
| Ageb | ||||||||||
|
| ||||||||||
| <5 y | 9 (2) | 19 (3) | 28 (3) | 55 (1) | 101 (2) | 156 (1) | 185 (6) | 46 (4) | 231 (5) | 415 |
|
| ||||||||||
| 5–19 y | 49 (14) | 108 (14) | 157 (14) | 1071 (12) | 686 (14) | 1757 (13) | 553 (17) | 250 (23) | 803 (19) | 2717 |
|
| ||||||||||
| 20–49 y | 197 (54) | 389 (53) | 586 (53) | 5559 (61) | 2643 (55) | 8202 (59) | 1451 (46) | 572 (54) | 2023 (48) | 10 811 |
|
| ||||||||||
| >50 y | 107 (30) | 221 (30) | 328 (30) | 2375 (26) | 1343 (28) | 3718 (27) | 1003 (31) | 201 (19) | 1204 (28) | 5250 |
|
| ||||||||||
| Sexb | ||||||||||
|
| ||||||||||
| Males | 240 (49) | 395 (50) | 635 (50) | 6866 (66) | 3815 (58) | 10 681 (63) | 1586 (48) | 539 (49) | 2125 (48) | 13 441 |
|
| ||||||||||
| Females | 250 (51) | 397 (50) | 647 (50) | 3565 (34) | 2799 (42) | 6364 (37) | 1733 (52) | 572 (51) | 2305 (52) | 9316 |
Data are presented as No. (%) unless otherwise specified.
Healthcare visits reported for 59% of outbreaks; hospitalizations and deaths reported for 70% of outbreaks.
Age information available for 64% of cases; sex information available for 76% of cases.
Figure 1.
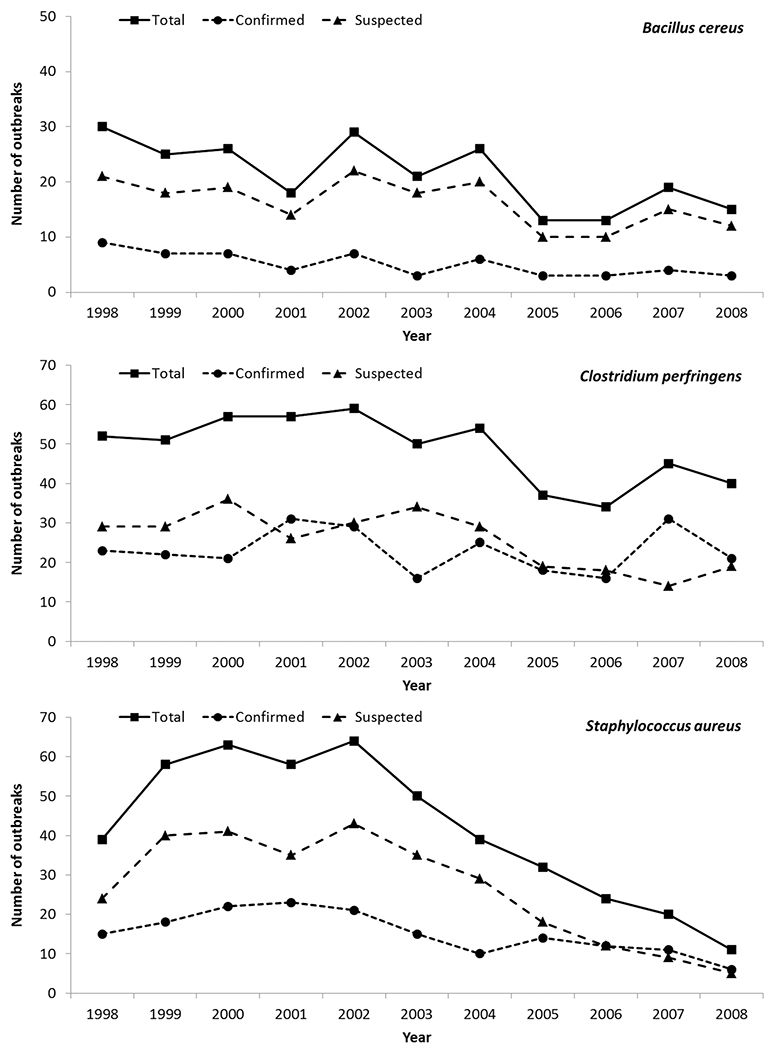
Number of foodborne disease outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus, United States, 1998–2008.
Only 39% of outbreaks had a confirmed etiology; further analyses include only these outbreaks. Forty-seven percent of C. perfringens outbreaks were confirmed compared with 36% and 24% of S. aureus and B. cereus outbreaks, respectively (Table 2). Outbreaks caused by C. perfringens were larger (median, 24 [range, 2–950] illnesses) than outbreaks caused by S. aureus (median, 17 [range, 2–225] illnesses) or B. cereus (median, 8 [range, 2–140 illnesses]). Illnesses most often occurred in persons ≥20 years of age (85%; Figure 2). Males were more commonly affected than females in C. perfringens outbreaks (66% males); however, males and females were equally affected in outbreaks caused by B. cereus and S. aureus (Table 2).
Figure 2.
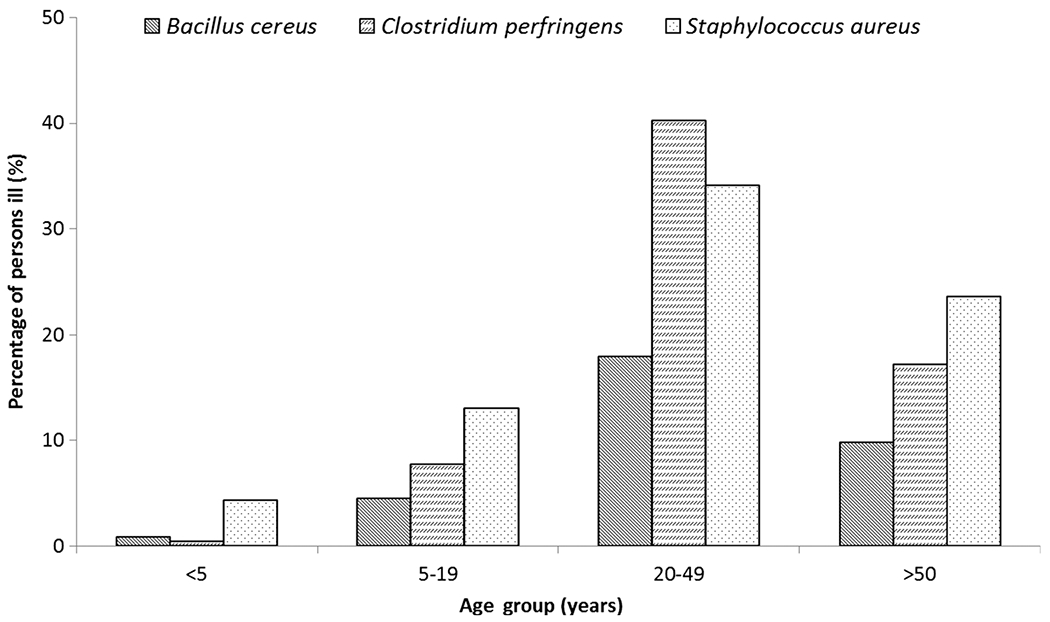
Proportion of cases reported in confirmed outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus by age group, United States, 1998–2008.
The median incubation period was 4 hours (interquartile range [IQR], 3–4 hours) for S. aureus outbreaks, 5 hours (IQR, 2–12 hours) for B. cereus outbreaks, and 11 hours (IQR, 9–12 hours) for C. perfringens outbreaks (Table 3). Diarrhea was commonly reported (86%–100% of cases), and abdominal cramps were reported by 61%–95% of cases. Vomiting was reported by a median of 75% of cases in B. cereus outbreaks and 87% of cases in S. aureus outbreaks, but only 9% of cases in C. perfringens outbreaks. Fever was rarely reported; however, it was most commonly reported in S. aureus outbreaks (9% of cases). The median duration of illness was shortest for S. aureus outbreaks (15 hours [IQR, 7–24 hours]); median duration of illness was 21 hours (IQR, 12–24 hours) for B. cereus and 24 hours (IQR, 16–25 hours) for C. perfringens outbreaks.
Table 3.
Clinical and Epidemiologic Features of Foodborne Disease Outbreaks Caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus, United States, 1998–2008
| Feature |
Bacillus cereus
|
Clostridium perfringens
|
Staphylococcus aureus
|
All Outbreaks | |||
|---|---|---|---|---|---|---|---|
| Confirmed | Suspected | Confirmed | Suspected | Confirmed | Suspected | ||
| Incubation perioda,b | |||||||
|
| |||||||
| median, h (IQR) | 5 (2–12) | 5 (2–9) | 11 (9–12) | 11 (9–13) | 4 (3–4) | 3 (2–5) | 7 (3–11) |
|
| |||||||
| Symptomsa, median % cases (IQR) | |||||||
|
| |||||||
| Diarrhea | 97 (69–100) | 100 (83–100) | 100 (96–100) | 100 (97–100) | 89 (71–100) | 100 (73–100) | 100 (87–100) |
|
| |||||||
| Abdominal cramps | 78 (58–100) | 87 (50–100) | 80 (67–96) | 86 (67–100) | 72 (50–87) | 100 (55–100) | 83 (62–100) |
|
| |||||||
| Vomiting | 75 (40–100) | 61 (33–100 | 9 (0–20) | 6 (0–17) | 87 (74–100) | 100 (50–100) | 50 (8–100) |
|
| |||||||
| Fever | 0 (0–12) | 0 (0–25) | 5 (0–13) | 0 (0–11) | 9 (0–28) | 0 (0–23) | 5 (0–15) |
|
| |||||||
| Duration of illnessa,b | |||||||
|
| |||||||
| Median, h (IQR) | 21 (12–24) | 18 (12–24) | 24 (16–25) | 24 (14–27) | 15 (7–24) | 24 (12–25) | 23 (12–24) |
|
| |||||||
| Location of preparationc | n = 51 | n = 168 | n = 223 | n = 251 | n = 144 | n = 277 | n = 1114 |
|
| |||||||
| Restaurant or deli | 34 (67) | 155 (92) | 100 (45) | 167 (67) | 64 (44) | 240 (87) | 760 (68) |
|
| |||||||
| Caterer | 5 (10) | 2 (1) | 40 (18) | 36 (14) | 17 (12) | 4 (1) | 104 (9) |
|
| |||||||
| Home or private residence | 1 (2) | 4 (2) | 20 (9) | 19 (8) | 24 (17) | 6 (2) | 74 (7) |
|
| |||||||
| Correctional facility | 2 (4) | 1 (1) | 27 (12) | 4 (2) | 5 (3) | 2 (1) | 41 (4) |
|
| |||||||
| School | 2 (4) | 2 (1) | 9 (4) | 5 (2) | 5 (3) | 6 (2) | 29 (3) |
|
| |||||||
| Grocery store | 1 (2) | 0 (0) | 3 (1) | 4 (2) | 3 (2) | 9 (3) | 20 (2) |
|
| |||||||
| Otherd | 6 (12) | 4 (2) | 24 (11) | 16 (6) | 26 (18) | 10 (4) | 86 (8) |
|
| |||||||
| Location of consumptionc | n = 51 | n = 164 | n = 233 | n = 267 | n = 140 | n = 275 | n = 1130 |
|
| |||||||
| Restaurant or deli | 20 (39) | 117 (71) | 60 (26) | 125 (47) | 33 (24) | 185 (67) | 540 (48) |
|
| |||||||
| Home or private residence | 11 (22) | 31 (19) | 27 (12) | 34 (13) | 20 (14) | 48 (17) | 171 (15) |
|
| |||||||
| Workplace, not cafeteria | 3 (6) | 4 (2) | 16 (7) | 16 (6) | 12 (9) | 5 (2) | 56 (5) |
|
| |||||||
| School | 2 (4) | 2 (1) | 18 (8) | 12 (4) | 10 (7) | 8 (3) | 52 (5) |
|
| |||||||
| Church or other religious facility | 1 (2) | 1 (1) | 16 (7) | 8 (3) | 13 (9) | 4 (1) | 43 (4) |
|
| |||||||
| Correctional facility | 2 (4) | 1 (1) | 26 (11) | 4 (1) | 6 (4) | 2 (1) | 41 (4) |
|
| |||||||
| Othere | 12 (24) | 8 (5) | 70 (30) | 68 (25) | 46 (33) | 23 (8) | 227 (20) |
Data are presented as No. (%) unless otherwise specified.
Abbreviation: IQR, interquartile range.
Proportion of outbreaks with complete reporting: incubation period (87%), diarrhea (92%), abdominal cramps (86%), vomiting (87%), fever (68%), and duration of illness (71%).
5th–95th percentiles (confirmed etiology): incubation period, B. cereus: 2–28 hours, C. perfringens: 7–15 hours, S. aureus: 2–7 hours; duration of illness, B. cereus: 6–48 hours, C. perfringens: 10–48 hours, S. aureus: 4–60 hours.
Restricted to outbreaks reporting a single setting of preparation or consumption.
Other includes church or other religious setting (18 outbreaks), camp (7), hospital or nursing home (6), fairs and festivals (4), workplace, not cafeteria (4), banquet facility (3), picnic (3), daycare facility (2), workplace cafeteria (2), other (37).
Other includes picnic (18 outbreaks), fairs and festivals (14), hospital or nursing home (11), office (11), banquet facility (10), camp (10), daycare facility (3), workplace cafeteria (3), wedding (2), other (145).
Among the 431 (91%) confirmed etiology outbreaks with reported foods, at least 1 ethnic food was reported in 18%. Mexican-style and Asian foods were reported in 10% and 7% of outbreaks, respectively. Mexican-style foods were commonly reported in C. perfringens outbreaks (15%), whereas Asian foods were commonly reported in B. cereus outbreaks (31%; Table 4).
Table 4.
Foods Implicated in Outbreaks Caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus, United States, 1998–2008
| Food Type |
Bacillus cereus
|
Clostridium perfringens
|
Staphylococcus aureus
|
All Outbreaks | |||
|---|---|---|---|---|---|---|---|
| Confirmed | Suspected | Confirmed | Suspected | Confirmed | Suspected | ||
| Outbreak food Ethnicitya | n = 52 | n = 155 | n = 229 | n = 246 | n = 150 | n = 242 | n= 1074 |
|
| |||||||
| Any ethnic | 19 (37) | 60 (39) | 41 (18) | 69 (28) | 18 (12) | 34 (14) | 241 (22) |
|
| |||||||
| Mexican-style | 2 (4) | 19 (12) | 34 (15) | 57 (23) | 7 (5) | 13 (5) | 132 (12) |
|
| |||||||
| Asian | 16 (31) | 36 (23) | 4 (2) | 6 (2) | 9 (6) | 10 (4) | 81 (8) |
|
| |||||||
| Single food groupingsb | n = 38 | n = 115 | n = 169 | n = 193 | n = 106 | n = 199 | n = 820 |
|
| |||||||
| Meat or poultryc | 9 (24) | 16 (14) | 106 (63) | 94 (49) | 58 (55) | 53 (27) | 336 (41) |
|
| |||||||
| Beef | 2 (22) | 4 (25) | 52 (49) | 44 (47) | 6 (10) | 8 (15) | 116 (35) |
|
| |||||||
| Poultryd | 5 (56) | 11 (69) | 34 (32) | 31 (33) | 8 (14) | 26 (49) | 115 (34) |
|
| |||||||
| Pork | 2 (22) | 1 (6) | 19 (18) | 17 (18) | 39 (67) | 14 (26) | 92 (27) |
|
| |||||||
| Unspecified origin | 0 (0) | 0 (0) | 1 (1) | 2 (2) | 4 (7) | 4 (8) | 11 (3) |
|
| |||||||
| Other | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (2) | 2 (1) |
|
| |||||||
| Rice dishes | 19 (50) | 52 (45) | 0 (0) | 4 (2) | 5 (5) | 5 (3) | 85 (10) |
|
| |||||||
| Sandwiches | 2 (5) | 3 (3) | 3 (2) | 7 (4) | 8 (8) | 45 (23) | 68 (8) |
|
| |||||||
| Pasta dishes | 0 (0) | 9 (8) | 6 (4) | 9 (5) | 9 (8) | 10 (5) | 43 (5) |
|
| |||||||
| Burritos and tacos | 1 (3) | 3 (3) | 9 (5) | 17 (9) | 4 (4) | 5 (3) | 39 (5) |
|
| |||||||
| Soups and stews | 0 (0) | 2 (2) | 15 (9) | 13 (7) | 3 (3) | 3 (2) | 36 (4) |
|
| |||||||
| Bean dishes | 0 (0) | 7 (6) | 9 (5) | 16 (8) | 0 (0) | 0 (0) | 32 (4) |
|
| |||||||
| Sauces | 1 (3) | 9 (8) | 8 (5) | 8 (4) | 2 (2) | 4 (2) | 32 (4) |
|
| |||||||
| Salads | 2 (5) | 2 (2) | 4 (2) | 5 (3) | 4 (4) | 14 (7) | 31 (4) |
|
| |||||||
| Pizza | 1 (3) | 1 (1) | 0 (0) | 2 (1) | 1 (1) | 25 (13) | 30 (4) |
|
| |||||||
| Seafood | 2 (5) | 3 (3) | 1 (1) | 1 (1) | 3 (3) | 11 (6) | 21 (3) |
|
| |||||||
| Other produce | 1 (3) | 3 (3) | 2 (1) | 6 (3) | 1 (1) | 6 (3) | 19 (2) |
|
| |||||||
| Pocket foodse | 0 (0) | 2 (2) | 4 (2) | 7 (4) | 2 (2) | 1 (1) | 16 (2) |
|
| |||||||
| Dairy | 0 (0) | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 5 (3) | 9 (1) |
|
| |||||||
| Baked goods | 0 (0) | 0 (0) | 1 (1) | 2 (1) | 2 (2) | 3 (2) | 8 (1) |
|
| |||||||
| Other foods | 0 (0) | 2 (2) | 0 (0) | 1 (1) | 3 (3) | 9 (5) | 15 (2) |
Data are presented as No. (%) unless otherwise specified.
At least 1 dish reported as ethnic.
Analysis restricted to outbreaks with a single likely implicated dish.
The denominator for the subgroups beef, poultry, pork, unspecified origin, and other is the total number of meat or poultry.
Includes turkey (30/115, 26%).
Precooked ingredients assembled into a pocket or roll and then cooked again (eg, egg roll or enchilada).
Meat or poultry dishes were the most common foods reported in C. perfringens and S. aureus outbreaks (Table 4). A meat or poultry dish was reported in 63% of C. perfringens outbreaks (beef [49%], poultry [32%]). Roasting or baking meat or poultry was the most common method of preparation reported (48%); preparation as part of a large, solid mass of food (29%) was also common. A meat or poultry dish was reported in 55% of S. aureus outbreaks; these dishes most commonly contained pork (67%), and 41% of pork dishes were ham. Implicated meat or poultry dishes were most often cooked and served immediately (41%), roasted (26%), or cooked as large, solid masses (15%). In contrast with C. perfringens and S. aureus, B. cereus outbreaks were most often attributed to rice dishes (50%); fried rice was the most common type of rice dish (68%). Rice dishes were most commonly cooked and served immediately (42%) or were part of large, solid masses of food (33%). Twenty-four percent of B. cereus outbreaks were associated with meat or poultry dishes (poultry [56%], beef [22%], pork [22%]). Meat or poultry dishes were cooked and served immediately (50%), roasted (33%), or part of liquid or semisolid mixtures (17%).
Foods implicated were most often prepared in a restaurant or deli (47%). Preparation in a restaurant or deli was more commonly reported for B. cereus outbreaks (67%) than C. perfringens (45%) or S. aureus (44%) outbreaks (Table 3). At least 1 contributing factor was reported for 79% of outbreaks. Regardless of the pathogen, the most common factors contributing to the occurrence of outbreaks were errors in food processing and preparation (93%); 45% of these errors occurred in restaurants or delis and 16% in homes or private residences. Reported errors included allowing foods to remain at room or outdoor temperature for several hours (58%), insufficient time or temperature during reheating (57%), slow cooling of prepared foods (44%), insufficient time or temperature during the initial cooking process (40%), preparing foods more than one-half day in advance of serving (33%), insufficient time or temperature during hot holding (33%), and inadequate cold holding temperatures (22%). Contamination by a food worker was reported in 55% of S. aureus outbreaks, but rarely in B. cereus (12%) and C. perfringens (6%) outbreaks. Contamination of a raw product or ingredient was reported more commonly in C. perfringens (21%) and B. cereus (17%) outbreaks compared with S. aureus outbreaks (2%). Cross-contamination in the food processing and preparation environment occurred in outbreaks caused by all 3 pathogens (13%); the most common errors reported were inadequate cleaning of processing equipment or utensils (67%) and storage in a contaminated environment (39%).
DISCUSSION
Almost 1 in 10 foodborne disease outbreaks reported to the Foodborne Disease Outbreak Surveillance System during 1998–2008 was caused by B. cereus, C. perfringens, or S. aureus. This study provided valuable information that might help to discriminate between these pathogens in the outbreak setting (Table 5).Vomiting was the most useful characteristic for discriminating B. cereus and S. aureus outbreaks from C. perfringens outbreaks; vomiting was infrequent among cases in C. perfringens outbreaks. Implicating a rice dish was almost always associated with B. cereus outbreaks, although other foods, including meat or poultry, were also implicated. S. aureus outbreaks usually reported the shortest median incubation periods (<5 hours).
Table 5.
Summary of Characteristics Associated With Outbreaks of Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus, United States
| Characteristic | Incubation (<5 h) | Vomiting (≥30% Cases) | Diarrhea (≥90% Cases) | Rice Implicateda | Meat or Poultry Implicateda | If Meat or Poultry, Most Common Typea |
|---|---|---|---|---|---|---|
| Bacillus cereus | Sometimesb | Usual | Sometimes | Sometimes | Infrequent | Poultry |
| Clostridium perfringens | Infrequent | Almost never | Usual | Never | Sometimes | Beef |
| Staphylococcus aureus | Usual | Usual | Sometimes | Almost never | Sometimes | Pork |
Among outbreaks with a single food implicated.
Scale uses proportion of confirmed outbreaks: never = 0% of confirmed outbreaks; almost never = 1%–10%; infrequent = 11%–30%; sometimes = 31%–69%; usual = 70%–89%; almost always = 90%–99%; always = 100%.
Other factors, including implicated foods, methods of food preparation, and contributing factors might provide additional clues to the etiology of outbreaks. C. perfringens and S. aureus outbreaks were more likely than B. cereus outbreaks to be associated with meat or poultry. In particular, roasted meats prepared in large, solid masses and Mexican-style foods were commonly implicated in C. perfringens outbreaks. Meats, poultry, and Mexican-style foods have been previously associated with outbreaks caused by C. perfringens [5, 13]. In S. aureus outbreaks, pork, specifically ham, was commonly implicated. Foods previously associated with S. aureus outbreaks include meat, especially ham, poultry, egg products, casseroles, bakery products, and milk or other dairy products [5]. Few outbreaks in this analysis were found to be associated with eggs, bakery products, or dairy, consistent with observations that foods implicated in S. aureus outbreaks might differ between countries because of differences in food consumption patterns [14]. For example, in France during 1999–2000, milk and cheese products were commonly implicated in S. aureus outbreaks, possibly because of the popularity of consuming raw milk cheeses in France [14, 15].
In contrast to C. perfringens and S. aureus, B. cereus outbreaks were most commonly attributed to rice or fried rice dishes, which is consistent with previous reviews of intoxications caused by the emetic toxin of B. cereus [3]. Meat dishes were the second most commonly implicated foods in B. cereus outbreaks, and the diarrheal syndrome of B. cereus has previously been associated with meat and meat products, sauces and vegetables, puddings, and milk products [3]. These data could not be further used to discriminate between outbreaks caused by the emetic and diarrheal toxins of B. cereus. This discrimination is important because features of the emetic syndrome are classically more similar to outbreaks caused by S. aureus, whereas features of the diarrheal syndrome are more similar to outbreaks caused by C. perfringens. The inability to discriminate between these 2 syndromes might explain the overlapping incubation periods, durations of illness, and symptoms observed.
Contamination of foods can occur at the source of food production and during food processing and preparation, transport, and storage. The initial contamination of foods implicated in B. cereus and C. perfringens outbreaks might have occurred in the environment as B. cereus and C. perfringens are ubiquitous in soil, in intestinal tracts of animals, and in a variety of foods and ingredients [3, 4]. Temperature and time abuse of foods during the initial cooking, cooling, storage, and reheating processes commonly contributed to outbreaks caused by all 3 of these pathogens, regardless of where food was prepared. The spores of B. cereus and C. perfringens are resistant to cooking, and improper preparation practices allow these pathogens to replicate and, in the case of B. cereus and S. aureus, produce toxins that are heat stable and can withstand additional cooking [16]. Food handling by a food worker was particularly problematic in S. aureus outbreaks, and although our data did not indicate the timing of contamination, food contamination likely occurred after food preparation as S. aureus is usually eliminated by cooking and pasteurization [5]. Foods such as raw meat and dairy products can become contaminated because of animal staphylococcal carriage or infections, for example, mastitis leading to contamination of raw milk [14]; if these foods are consumed raw or after insufficient cooking, illness might result from staphylococcal proliferation and toxin production. Devising measures that reduce contamination at the source of food production, during food processing, preparation, and handling, through cross-contamination in the food processing and preparation environment, and during transportation from the location of food preparation to the location of consumption could reduce the occurrence of B. cereus, C. perfringens, and S. aureus outbreaks. Although prevention messages on food handling, proper heating and cooling of foods, and food storage are available to the public (www.foodsafety.gov), wider dissemination of this knowledge may be needed.
For unknown reasons, the number of outbreaks caused by these pathogens, especially S. aureus, has decreased since 2002. One hypothesis for this observation is a decrease in the number of outbreak investigations because of resource constraints. Furthermore, the decrease in outbreak occurrence was not uniformly observed for all 3 pathogens, suggesting mechanisms unique to S. aureus outbreaks. A hypothesis more specific to the occurrence of S. aureus outbreaks may be improved control measures in commercial food preparation settings to reduce contact with foods by infected carriers and increased awareness of proper food handling practices.
Our analysis was subject to some limitations. First, only a small proportion of foodborne illnesses reported each year are identified and associated with outbreaks; therefore, foodborne outbreaks may be underreported. Outbreaks caused by B. cereus, C. perfringens, and S. aureus might suffer from greater underreporting because the mild illnesses typically caused by these pathogens result in few ill persons seeking medical care [7] and nondiagnosis because of the lack of routine clinical specimen testing for these pathogens or their toxins [8, 9]. Except in outbreak settings, none of these illnesses are reportable to public health agencies; therefore, outbreaks may go undetected. If an outbreak is detected, investigating agencies may lack the resources necessary to complete the investigation and report it to surveillance. Second, because there is no system for surveillance of sporadic illnesses caused by these pathogens, there is no method for determining whether the epidemiologic and clinical features determined in this analysis are representative of all illnesses caused by these pathogens. A third limitation is the incomplete reporting of variables of interest, including demographics, symptoms, incubation period, duration of illness, implicated foods, methods of preparing foods, and contributing factors. Finally, although reported contributing factors should represent the causal events preceding the onset of the outbreak, some reported factors may represent general deficiencies noted at the food preparation site at the time of the investigation. Data reported were also insufficient to determine whether bacterial contamination of implicated foods was due to pathogens of animal or human origin; this type of information might improve our understanding of where foods become contaminated and the roles that reported contributing factors have in propagating outbreaks.
Data reported to the CDC’s Foodborne Disease Outbreak Surveillance System are the only comprehensive source available to evaluate the clinical and epidemiologic characteristics of outbreaks caused by B. cereus, C. perfringens, and S. aureus in the United States. A better understanding of these discriminating characteristics might assist future outbreak investigations and guide laboratory testing. Outbreaks caused by these pathogens occur frequently and serious illnesses resulting in hospitalization or death have been reported. Although the number of outbreaks caused by these pathogens has decreased, reporting these outbreaks remains essential for understanding current factors associated with outbreak occurrence and to determine future public health interventions. Current public health interventions should focus on reducing contamination at the source of food production and cross-contamination in the food preparation environment and educating the general public, especially food handlers, about the importance of preventing temperature abuse and improved food handling practices.
Acknowledgments.
The findings in this study are based in part on contributions by state, local, tribal, and territorial health departments. We thank Patricia Griffin, Deborah Talkington, and Gerry Gomez for their insightful comments on the manuscript.
Financial support.
This work was supported by the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 2011; 17:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afghani B, Stutman H. Toxin-related diarrheas. Pediatr Ann 1994; 10:553–55. [DOI] [PubMed] [Google Scholar]
- 3.Stenfors Arnesen LP, Fagerlund A, Granum PE. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 2008; 32:579–606. [DOI] [PubMed] [Google Scholar]
- 4.Brynestad S, Granum PE. Clostridium perfringens and foodborne infections. Int J Food Microbiol 2002; 74:195–202. [DOI] [PubMed] [Google Scholar]
- 5.Stewart GC. Staphylococcus aureus. In: Fratamico P, Bhunia A, Smith J, eds. Foodborne pathogens: microbiology and molecular biology. Norfolk, United Kingdom: Caister Academic Press; 2005:273–84. [Google Scholar]
- 6.Scharff RL. Economic burden from health losses due to foodborne illness in the United States. J Food Prot 2012; 75:123–31. [DOI] [PubMed] [Google Scholar]
- 7.Scallan E, Jones TF, Cronquist A, et al. Factors associated with seeking medical care and submitting a stool sample in estimating the burden of foodborne illness. Foodborne Pathog Dis 2006; 3:432–8. [DOI] [PubMed] [Google Scholar]
- 8.Guerrant RL, Van Gilder T, Steiner TS, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 2001; 32:331. [DOI] [PubMed] [Google Scholar]
- 9.Thielman NM, Guerrant RL. Acute infectious diarrhea. N Engl J Med 2004; 350:38–47. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Guide to confirming a diagnosis in foodborne disease. Available at: http://www.cdc.gov/outbreaknet/references_resources/guide_confirming_diagnosis.html. Accessed 7 March 2013.
- 11.Weingold SE, Guzewich JJ, Fudala JK. Use of foodborne disease data for HACCP risk assessment. J Food Prot 1994; 57:820–30. [DOI] [PubMed] [Google Scholar]
- 12.Bryan FL, Guzewich JJ, Todd EC. Surveillance of foodborne disease III. Summary and presentation of data on vehicles and contributory factors; their value and limitations. J Food Prot 1997; 60:701–14. [DOI] [PubMed] [Google Scholar]
- 13.Grass JE, Gould LH, Mahon BE. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog Dis 2013; 10:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genet Mol Res 2003; 2:63–76. [PubMed] [Google Scholar]
- 15.Haeghebaert S, Le Querrec F, Gallay A, Bouvet P, Gomez M, Vaillant V. Food poisonings in France, 1999–2000 [in French]. BEH 2002; 23:105–9. [Google Scholar]
- 16.Brown K. Control of bacterial spores. Br Med Bull 2000; 56:158–71. [DOI] [PubMed] [Google Scholar]


