Abstract
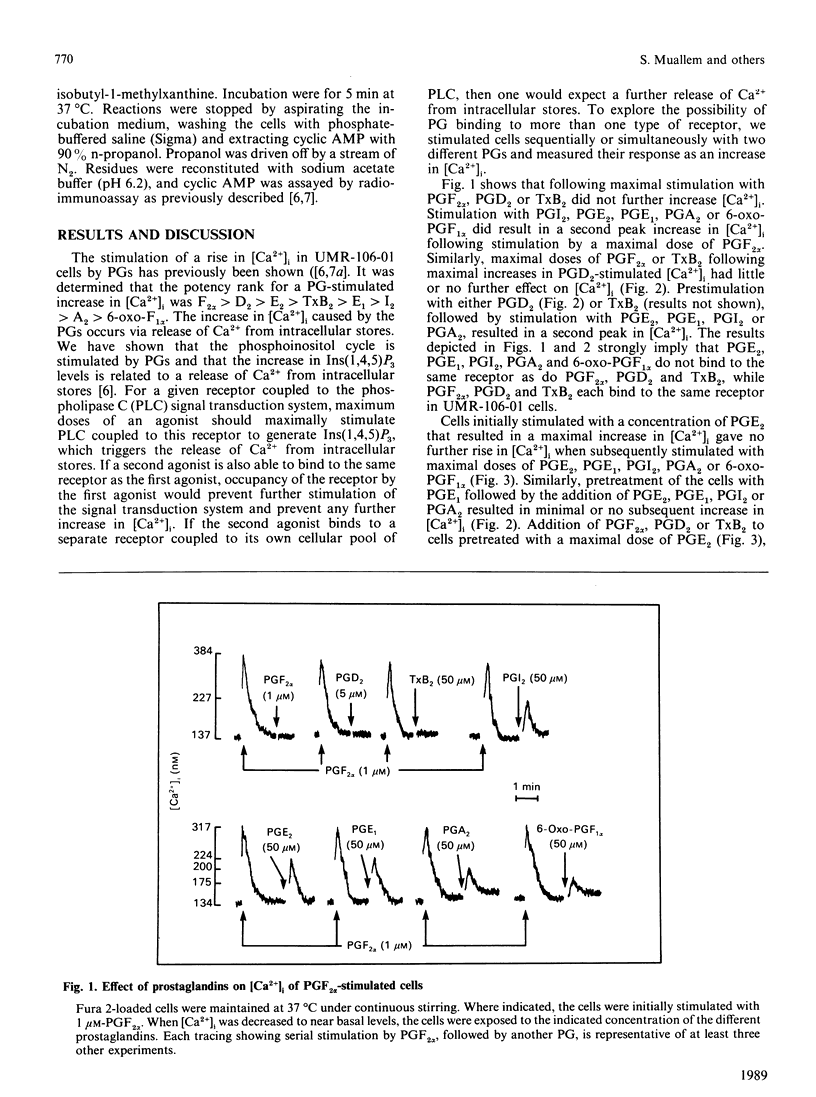
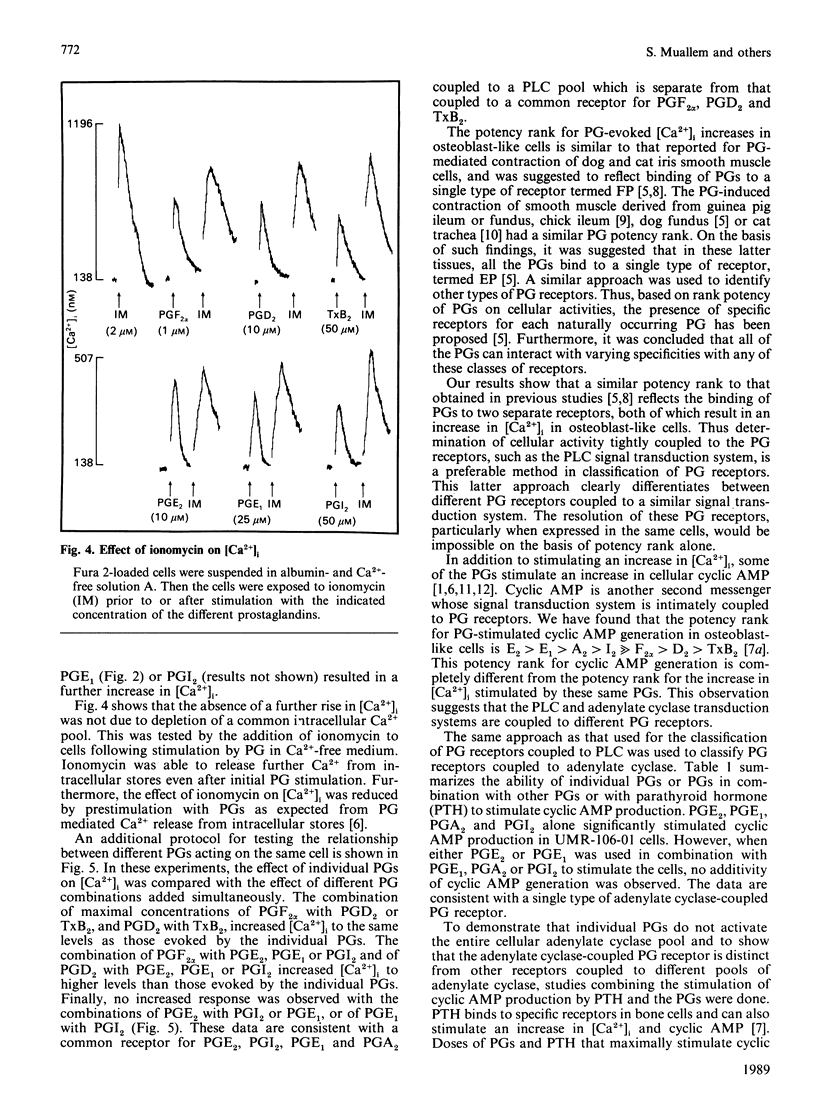
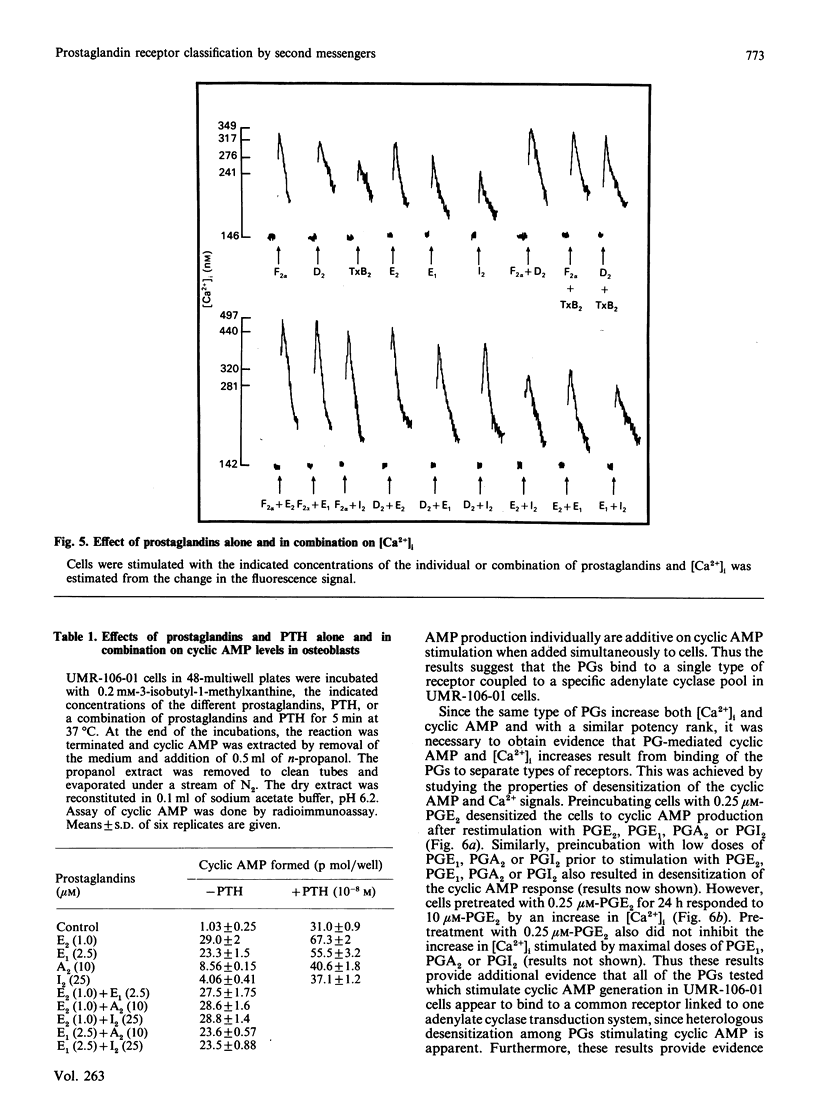
A wide spectrum of prostaglandins (PG) stimulate both the production of cyclic AMP and an increase in free cytosolic Ca2+ concentration [( Ca2+]i) in the osteogenic osteosarcoma cell line, UMR-106-01, which has characteristics compatible with osteoblasts. Using PG-stimulated determinations of the second messengers cyclic AMP and [Ca2+]i, a method for classification of PG receptors is presented. UMR-106-01 cells demonstrate three subclasses of PG receptors. One receptor interacts with PGF2 alpha, PGD2, and thromboxane B2 (TxB2) to increase [Ca2+]i. A second receptor binds PGE2, PGE1, PGI2, PGA2 and 6-oxo-PGF1 alpha to increase [Ca2+]i by stimulation of a second separate phospholipase C pool. A third receptor accepts PGE2, PGE1, PGA2, PGI2 and to a lesser extent PGF2 alpha, PGD2 and TxB2 to increase cyclic AMP. Such a classification system may be applicable to other cells responding to multiple PGs by inducing changes in cellular second messengers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apperley G. H., Coleman R. A., Kennedy I., Levy G. P. The cat isolated trachea, a useful preparation for the study of the smooth muscle relaxant action of prostaglandins [proceedings]. Br J Pharmacol. 1979 Nov;67(3):412P–413P. [PMC free article] [PubMed] [Google Scholar]
- Brunton L. L., Wiklund R. A., Van Arsdale P. M., Gilman A. G. Binding of (3H)prostaglandin E1 to putative receptors linked to adenylate cyclase of cultured cell clones. J Biol Chem. 1976 May 25;251(10):3037–3044. [PubMed] [Google Scholar]
- Coleman R. A., Humphrey P. P., Kennedy I., Levy G. P., Lumley P. Comparison of the actions of U-46619, a prostaglandin H2-analogue, with those of prostaglandin H2 and thromboxane A2 on some isolated smooth muscle preparations. Br J Pharmacol. 1981 Jul;73(3):773–778. doi: 10.1111/j.1476-5381.1981.tb16814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper B., Ahern D. Characterization of the platelet prostaglandin D2 receptor. Loss of prostaglandin D2 receptors in platelets of patients with myeloproliferative disorders. J Clin Invest. 1979 Aug;64(2):586–590. doi: 10.1172/JCI109497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziak R. M., Hurd D., Miyasaki K. T., Brown M., Weinfeld N., Hausmann E. Prostaglandin E2 binding and cyclic AMP production in isolated bone cells. Calcif Tissue Int. 1983;35(2):243–249. doi: 10.1007/BF02405038. [DOI] [PubMed] [Google Scholar]
- Kennedy I., Coleman R. A., Humphrey P. P., Levy G. P., Lumley P. Studies on the characterisation of prostanoid receptors: a proposed classification. Prostaglandins. 1982 Nov;24(5):667–689. doi: 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Stuart R. K. Interaction of prostaglandins E1 and E2 in regulation of cyclic-AMP and aggregation in human platelets: evidence for a common prostaglandin receptor. J Lab Clin Med. 1974 Jul;84(1):111–121. [PubMed] [Google Scholar]
- Siegl A. M., Smith J. B., Silver M. J., Nicolaou K. C., Ahern D. Selective binding site for [3H]prostacyclin on platelets. J Clin Invest. 1979 Feb;63(2):215–220. doi: 10.1172/JCI109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateson J. E., Moncada S., Vane J. R. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977 Mar;13(3):389–397. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi D. T., Hahn T. J., Beeker T. G., Kleeman C. R., Muallem S. Relationship of cAMP and calcium messenger systems in prostaglandin-stimulated UMR-106 cells. J Biol Chem. 1988 Aug 5;263(22):10745–10753. [PubMed] [Google Scholar]
- Yamaguchi D. T., Hahn T. J., Iida-Klein A., Kleeman C. R., Muallem S. Parathyroid hormone-activated calcium channels in an osteoblast-like clonal osteosarcoma cell line. cAMP-dependent and cAMP-independent calcium channels. J Biol Chem. 1987 Jun 5;262(16):7711–7718. [PubMed] [Google Scholar]
- van Alphen G. W., Angel M. A. Activity of prostaglandin E, F, A and B on sphincter, dilator and ciliary muscle preparations of the cat eye. Prostaglandins. 1975 Feb;9(2):157–166. doi: 10.1016/0090-6980(75)90020-9. [DOI] [PubMed] [Google Scholar]


