Abstract
Background:
HER2 mutations are targetable alterations in patients with hormone receptor-positive (HR+) metastatic breast cancer (MBC). In the SUMMIT basket study, patients with HER2-mutant MBC received neratinib monotherapy, neratinib + fulvestrant, or neratinib + fulvestrant + trastuzumab (N + F + T). We report results from 71 patients with HR+, HER2-mutant MBC, including 21 (seven in each arm) from a randomized substudy of fulvestrant versus fulvestrant + trastuzumab (F + T) versus N + F + T.
Patients and methods:
Patients with HR+ HER2-negative MBC with activating HER2 mutation(s) and prior cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) therapy received N + F + T (oral neratinib 240 mg/day with loperamide prophylaxis, intramuscular fulvestrant 500 mg on days 1, 15, and 29 of cycle 1 then q4w, intravenous trastuzumab 8 mg/kg then 6 mg/kg q3w) or F + T or fulvestrant alone. Those whose disease progressed on F + T or fulvestrant could cross-over to N + F + T. Efficacy endpoints included investigator-assessed objective response rate (ORR), clinical benefit rate (RECIST v1.1), duration of response, and progression-free survival (PFS). Plasma and/or formalin-fixed paraffin-embedded tissue samples were collected at baseline; plasma was collected during and at end of treatment. Extracted DNA was analyzed by next-generation sequencing.
Results:
ORR for 57 N + F + T-treated patients was 39% [95% confidence interval (CI) 26% to 52%); median PFS was 8.3 months (95% CI 6.0–15.1 months). No responses occurred in fulvestrant- or F + T-treated patients; responses in patients crossing over to N + F + T supported the requirement for neratinib in the triplet. Responses were observed in patients with ductal and lobular histology, 1 or ≥1 HER2 mutations, and co-occurring HER3 mutations. Longitudinal circulating tumor DNA sequencing revealed acquisition of additional HER2 alterations, and mutations in genes including PIK3CA, enabling further precision targeting and possible re-response.
Conclusions:
The benefit of N + F + T for HR+ HER2-mutant MBC after progression on CDK4/6is is clinically meaningful and, based on this study, N + F + T has been included in the National Comprehensive Cancer Network treatment guidelines. SUMMIT has improved our understanding of the translational implications of targeting HER2 mutations with neratinib-based therapy.
Keywords: metastatic breast cancer, HER2-mutant, ERBB2, neratinib, hormone receptor-positive
INTRODUCTION
Somatic activating mutations in the ERBB2 (HER2) gene in the absence of gene amplification or overexpression are present in ~2% of primary breast cancers,1–4 in 3%−5% of patients with hormone receptor-positive (HR+) metastatic breast cancer (MBC), and further enriched (5%−8%) in patients with lobular histology.3,5,6 A subset of HER2 mutations, including the most common hotspot mutations, have been confirmed to be oncogenic and associated with poor prognosis.1,5–13 Single-nucleotide variants of HER2 are predominantly localized to the extracellular, transmembrane, and kinase domains, whereas small insertions are most commonly found in exon 20.4,9,14 In MBC, HER2 mutations confer resistance to endocrine therapies due to cross-talk between HER2 and estrogen receptor (ER) signaling pathways.8,11
Neratinib is an oral, irreversible, pan-HER tyrosine kinase inhibitor (TKI) with preclinical and clinical activity in patients with HER2-mutant tumors.7,10,12,15–18 Treatment with neratinib overcomes endocrine resistance in HR+ HER2-mutant breast cancer cell lines and xenografts.8,11 The efficacy of neratinib-based therapy in patients with HR+ HER2-mutant MBC was evaluated in the SUMMIT trial (NCT01953926); SUMMIT was hypothesis generating and its basket design enabled evolution of MBC cohorts in response to clinical results and biomarker studies from preceding cohorts. Initially, 18 patients with HR+ HER2-mutant MBC received single-agent neratinib with a confirmed objective response rate (ORR) of 17% and progression-free survival (PFS) of 3.6 months.9 Based on preclinical data suggesting synergy between neratinib and endocrine therapies,8,11 a subsequent cohort tested neratinib and fulvestrant (N + F; N = 39 RECIST-evaluable patients). Combination therapy resulted in an ORR of 30% and prolongation of PFS to 5.4 months.12 Notably, the independent MutHER clinical trial (NCT01670877) of N + F for HR+ HER2-mutant MBC reported similar results.16
In the SUMMIT and MutHER trials, genomic analysis of circulating tumor DNA (ctDNA) at baseline and progression revealed secondary HER2 mutations and/or amplification as a putative mechanism of neratinib resistance.12,16 No other genetic event was consistently observed, suggesting that a subset of HER2-mutant MBCs remained dependent on HER2 signaling upon disease progression.19 Additionally, detection of HER2 copy number amplification (CNA), or more than one HER2 mutation at baseline, was associated with lack of clinical benefit in SUMMIT.12 Enhancement of HER2 signaling associated with additional HER2-activating alterations suggested a need for combinations of HER2-targeted therapies to more completely inhibit HER2 signaling and maximize treatment response. Indeed, in MutHER, addition of trastuzumab in five patients at progression on N + F resulted in three responses and one long-term stable disease (SD).16 Furthermore, neratinib plus trastuzumab in HER2-mutant cancer models led to greater inhibition of tumor growth and HER2 signaling than either agent alone.15,20 We hypothesized that upfront treatment with triplet neratinib, fulvestrant, and trastuzumab (N + F + T) would prolong clinical benefit in patients with HR+ HER2-mutant MBC.
To test this hypothesis, a new SUMMIT cohort (N = 78) was opened in patients with HR+ HER2-negative HER2-mutant MBC. Prior cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) therapy was incorporated as an inclusion criterion for this study arm as CDK4/6i therapy had emerged as a standard of care for HR+ MBC. Under the advisement of regulatory authorities, a small, randomized sub-cohort (N = 21) comparing N + F + T versus F + T versus fulvestrant was included to isolate the contribution of neratinib. Genomic determinants of response, HER2 expression level, and mechanisms of acquired resistance were explored retrospectively via next-generation sequencing (NGS) of DNA and RNA from tissue and/or plasma samples, immunohistochemistry (IHC), and fluorescence in situ hybridization (FISH).
PATIENTS AND METHODS
Study design and treatment
The open-label, single-arm, multicohort, multi-tumor, phase II SUMMIT trial was conducted at 23 centers internationally, 15 of which enrolled ≥1 patient with breast cancer. The SUMMIT study has previously been described in detail.9,12 Patients eligible for inclusion in this cohort were aged ≥18 years with Eastern Cooperative Oncology Group performance status 0–2, histologically confirmed HR+ HER2-negative (institutionally reported HER2 IHC 0 or 1+, or IHC 2+/FISH non-amplified), advanced breast cancer with activating HER2 mutation(s) assessed by local/institutional testing. All patients had received prior treatment with CDK4/6is. Eligibility for the study required a documented somatic activating HER2 mutation, detected either from formalin-fixed paraffin-embedded (FFPE) tumor tissue or ctDNA by local testing carried out in a Clinical Laboratory Improvements Act or equivalent regionally certified laboratory. Central confirmation of HER2 mutation was carried out retrospectively and concordance was evaluated. Key exclusion criteria were prior therapy with HER TKIs, cumulative epirubicin dose >900 mg/m2 or cumulative doxorubicin dose >450 mg/m2, and unstable brain metastases (treated and/or asymptomatic brain metastases were allowed).
Patients received N + F + T (oral neratinib 240 mg/day with mandatory loperamide prophylaxis for the first two cycles and as needed thereafter, intramuscular fulvestrant 500 mg on days 1, 15, and 29 of cycle 1 then every 4 weeks thereafter, and intravenous trastuzumab 8 mg/kg initially then 6 mg/kg every 3 weeks) or F + T (intramuscular fulvestrant 500 mg on days 1, 15, and 29 of cycle 1 then every 4 weeks thereafter, and intravenous trastuzumab 8 mg/kg initially then 6 mg/kg every 3 weeks) or fulvestrant (500 mg intramuscularly on days 1, 15, and 29, then once every 4 weeks thereafter). Patients were treated until disease progression, unacceptable toxicity, or withdrawal of consent. The protocol was approved by institutional review boards at all participating institutions; written informed consent was obtained for all patients before carrying out study-related procedures.
In a substudy, patients were randomized 1:1:1 to single-agent fulvestrant, F + T, or N + F + T, stratified by lines of prior therapy for metastatic disease (≤2 versus >2 lines) and prior fulvestrant therapy (yes versus no). Patients who initially received F + T or fulvestrant alone could cross over to the triplet upon clinical and/or radiologic progression (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2023.08.003).
Tumor assessment
Tumor response was assessed locally according to RECIST (version 1.1) every 8 weeks by computed tomography or magnetic resonance imaging.12 Adverse events (AEs) were classified according to Common Terminology Criteria for AEs (version 4.0) from consent until day 28 after discontinuation of study treatment.
Biomarker analysis
Central NGS.
Retrospective tissue NGS was conducted using either the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) or Tempus xT assays. Retrospective ctDNA sequencing of baseline, on-treatment, and end-of-treatment samples was conducted using either the Memorial Sloan Kettering-Analysis of Circulating cell-free DNA to Examine Somatic Status (MSK-ACCESS) or Tempus xF+ (Tempus Labs, Chicago, IL) cell-free DNA assays. Further information on these assays, Tempus mRNA analysis, and the Tempus deidentified multimodal database is provided in the Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2023.08.003.
Central IHC and FISH.
Retrospective central HER2, ER, and progesterone receptor (PgR) IHC, and HER2 FISH were carried out on samples with sufficient FFPE tissue remaining after NGS. HER2, ER, and PgR IHC and HER2 FISH scores were determined according to manufacturer specifications [HercepTest™ and IQFISH pharmDx (Agilent Dako, Santa Clara, CA)]. HER2 IHC was further evaluated for H-score from original IHC images (Discovery Life Sciences GmbH, Kassel, Germany).
Statistical considerations
Description of the statistical analyses can be found in the Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2023.08.003.
Preclinical experiments
Description of the HER2-mutant cell lines and immunoassays can be found in the Supplementary Methods, available at https://doi.org/10.1016/j.annonc.2023.08.003.
RESULTS
Patient characteristics
In total, 71 patients with HR+ HER2-mutant MBC with prior CDK4/6i therapy were enrolled between 27 February 2018 and 2 April 2022, and were assessable for efficacy. Twenty-one of the 71 patients comprised a small randomized, three-arm design intended to demonstrate the requirement for neratinib within the triplet; 7 patients received N + F + T, 7 received F + T, and 7 received fulvestrant alone. Efficacy of the triplet was evaluated in the 57 patients who received N + F + T upfront, i.e. 50 from the non-randomized cohort and 7 from the randomized cohort (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2023.08.003).
Patients were extensively pretreated, with a median of 3 (range 1–10) prior lines of systemic therapy in the locally advanced/metastatic setting (Table 1). Twenty-seven patients (47%) had lobular breast cancer, higher than the previously reported incidence of 10%−15% for MBC and consistent with the enriched occurrence of HER2 mutations in lobular versus ductal cancer.1 In the randomized subset, 1/7 (14%), 2/7 (29%), and 5/7 (71%) patients had lobular cancer in the fulvestrant alone, F + T, and N + F + T groups, respectively.
Table 1.
Baseline demographics and characteristics
| Characteristics | Non-randomized + randomized HR+, prior CDK4/6i (N + F + T, N = 57) | Randomized HR+, prior CDK4/6i (F + T, n = 7) | Randomized HR+, prior CDK4/6i (F, n = 7) | Randomized HR+, prior CDK4/6i (N + F + T, n = 7) |
|---|---|---|---|---|
|
| ||||
| Median age, years (range) | 59.0 (25–83) | 65.0 (37–72) | 55.0 (46–80) | 57.0 (44–77) |
| Sex, n (%) | ||||
| Female | 56 (98) | 7 (100) | 7 (100) | 7 (100) |
| Male | 1 (2) | 0 | 0 | 0 |
| Menopausal status, n (%) | ||||
| Post-menopausal | 49 (86) | 7 (100) | 7 (100) | 7 (100) |
| Pre-menopausal | 7 (12) | 0 | 0 | 0 |
| NA | 1 (2) | 0 | 0 | 0 |
| ECOG performance status, n (%) | ||||
| 0 | 27 (47) | 4 (57) | 5 (71) | 3 (43) |
| 1 | 29 (51) | 3 (43) | 2 (29) | 4 (57) |
| 2 | 1 (2) | 0 | 0 | 0 |
| Histological type, n (%) | ||||
| Ductal | 23 (40) | 5 (71) | 5 (71) | 2 (29) |
| Lobular | 27 (47) | 2 (29) | 1 (14) | 5 (71) |
| Mixed ductal and lobular | 1 (2) | 0 | 0 | 0 |
| Other | 6 (11) | 0 | 1 (14) | 0 |
| Location of disease at time of enrollment, n (%) | ||||
| Visceral | 51 (89) | 6 (86) | 7 (100) | 7 (100) |
| Non-visceral only | 5 (9) | 1 (14) | 0 | 0 |
| Missing | 1 (2) | 0 | 0 | 0 |
| Median time from first metastasis to enrollment, years (range) | 2.3 (0–15) | 1.0 (0–4) | 1.6 (0–4) | 2.1 (1–7) |
| Prior treatment for locally advanced/metastatic disease, n (%) | 56 (98)a | 7 (100) | 7 (100) | 7 (100) |
| Median no. of prior anticancer regimens (range) | 3 (1–10) | 2 (1–10) | 2 (1–6) | 2 (1–6) |
| Prior endocrine therapy, n (%) | 56 (98) | 6 (86) | 7 (100) | 7 (100) |
| Aromatase inhibitor | 33 (58) | 5 (71) | 5 (71) | 5 (71) |
| Fulvestrant | 44 (77) | 3 (43) | 4 (57) | 5 (71) |
| Tamoxifen | 7 (12) | 1 (14) | 0 | 0 |
| Prior chemotherapy, n (%) | 34 (60) | 2 (29) | 4 (57) | 3 (43) |
| Prior HER2 antibody-directed therapy, n (%) | 5 (9) | 1 (14) | 1 (14) | 1 (14) |
| Prior CDK4/6i, n (%) | 54 (95) | 7 (100) | 7 (100) | 7 (100) |
| Prior PIK3CAi, n (%) | 7 (12) | 1 (14) | 1 (14) | 1 (14) |
| Prior mTORi, n (%) | 16 (28) | 0 | 1 (14) | 1 (14) |
CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; ECOG, Eastern Cooperative Oncology Group; F, fulvestrant; HR+, hormone receptor-positive; mTORi, mTOR inhibitor; N, neratinib; NA, not applicable; PIK3CAi, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha inhibitor; T, trastuzumab.
Missing information for one patient.
The spectrum of HER2 mutations (Supplementary Table S1 and Figure S2A, available at https://doi.org/10.1016/j.annonc.2023.08.003) was consistent with the expected mutation distribution in patients with breast cancer21 and with prior MBC cohorts in SUMMIT.12 The distribution of HER2 mutations was also similar for ductal and lobular histologies (Supplementary Figure S2B, available at https://doi.org/10.1016/j.annonc.2023.08.003).
Efficacy
Among the 57 patients who received N + F + T, the investigator-assessed ORR [confirmed complete response (CR) or partial response (PR)] was 39% [95% confidence interval (CI) 26% to 52%], including 1 CR and 21 PRs (Figure 1A; Supplementary Table S2 and Figure S3, available at https://doi.org/10.1016/j.annonc.2023.08.003). The clinical benefit rate (CBR; confirmed CR or PR, or SD ≥24 weeks) was 54% (N = 31/57). The median duration of response (DOR) was 14.4 months (95% CI 6.4–21.7 months) and the median PFS was 8.3 months (95% CI 6.0–15.1 months).
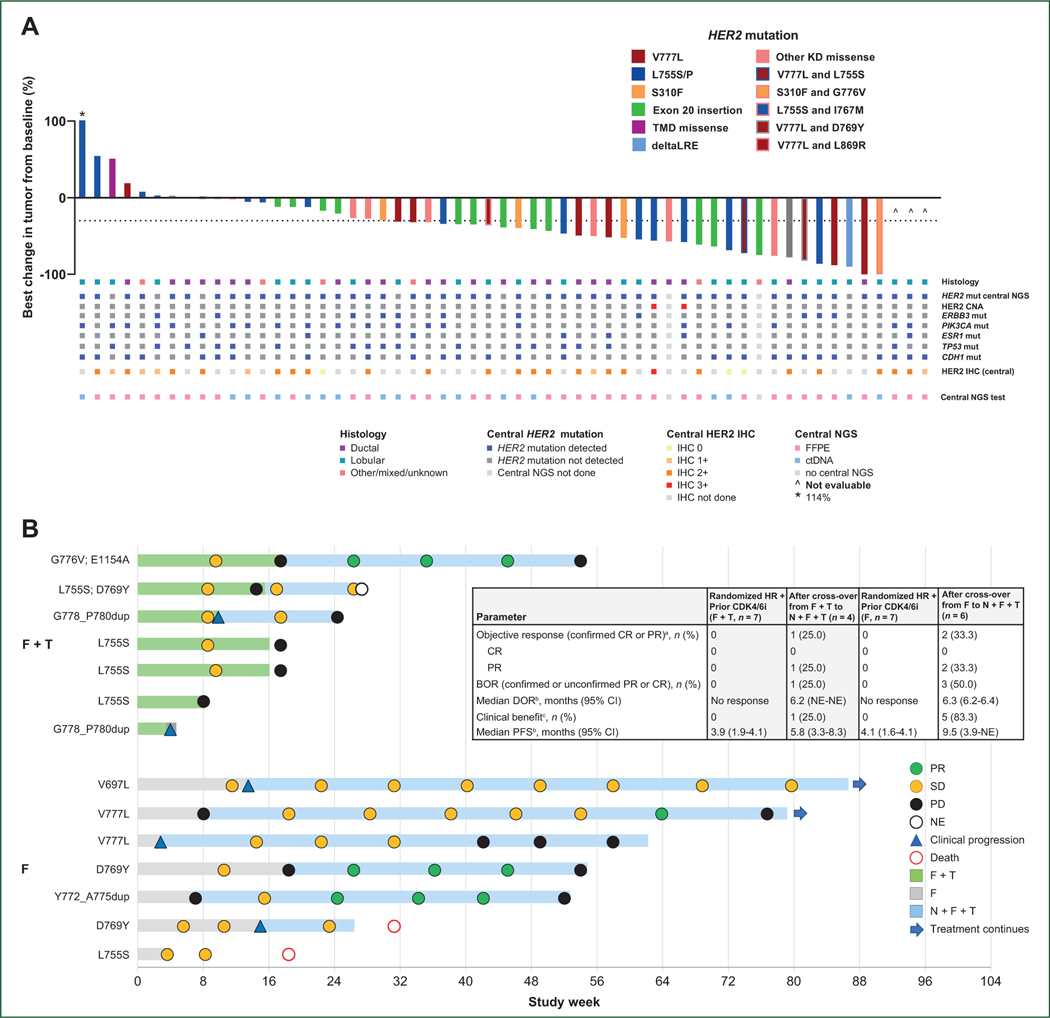
Figure 1. (A) Best change in tumor from baseline and corresponding histology and central biomarker analysis. (B) Duration of treatment and best response in patients randomized to F + T or F, before and after cross-over to N + F + T. (C) HER2 expression in patients treated with N + F + T. (D) HER2 H-score, FISH ratio, or mRNA expression, and response to N + F + T.
The solid horizontal line represents the median.
BOR, best overall response; CEN-17, centromere of chromosome 17; CI, confidence interval; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; CNA, copy number amplification; CR, complete response; ctDNA, circulating tumor DNA; dup, duplication; F, fulvestrant; FFPE, formalin-fixed paraffin-embedded; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; KD, kinase domain; mut, mutation; N, neratinib; NE, not evaluable; NGS, next-generation sequencing; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; T, trastuzumab; TMD, transmembrane domain.
aObjective response defined as either a CR or PR that is confirmed no less than 4 weeks after the criteria for response are initially met.
bKaplan–Meier analysis. For cross-over patients, calculated from time of cross-over to N + F + T.
cClinical benefit is defined as confirmed CR or PR or SD for ≥24 weeks (within a ±7-day visit window).
In the subset of seven patients randomized to N + F + T, the ORR was 29% (N = 2/7). No CRs or PRs were observed in either the fulvestrant monotherapy or F + T arms (Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2023.08.003). Four patients with disease progression on F + T crossed over to N + F + T; one of these patients subsequently had a confirmed PR (25%). Two of six patients (33%) with progression on fulvestrant and crossing over to N + F + T had a confirmed PR (Figure 1B).
Patients with ductal and lobular MBC experienced similar benefit from treatment with N + F + T (Table 2). Patients with lobular MBC had an ORR of 41% (95% CI 22% to 61%; N = 11/27), median DOR of 14.4 months (95% CI 5.0–21.7 months), CBR of 52% (95% CI 32% to 71%; N = 14/27), and median PFS of 8.3 months (95% CI 4.2–18.0 months). Those with ductal MBC had an ORR of 39% (95% CI 20% to 62%; N = 9/23), DOR of 14.3 months (95% CI 4.1 months-not estimable), CBR of 61% (95% CI 39% to 80%; N = 14/23), and median PFS of 8.3 months (95% CI 4.3–18.6 months).
Table 2.
Efficacy by histology or enrollment HER2 mutation in patients treated with neratinib + fulvestrant + trastuzumab
| Outcome | Histology | Enrollment HER2 mutation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Lobular (n = 27) | Ductal (n = 23) | Other/mixed/unknown (n = 7) | L755S (n = 17) | Exon 20 insertion (n = 12) | Other KD missense (n = 9) | V777L (n = 8) | S310F (n = 3) | TMD missense (n = 2) | Multiple mutationsa (n = 5) | Exon 19 deletion (n = 1) | |
|
| |||||||||||
| Objective responseb, n (%) | 11 (41) | 9 (39) | 2 (29) | 4 (24) | 5 (42) | 3 (33) | 5 (63) | 1 (33) | 0 | 4 (80) | 0 |
| CR | 1 (4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (20) | 0 |
| PR | 10 (37) | 9 (39) | 2 (29) | 4 (24) | 5 (42) | 3 (33) | 5 (63) | 1 (33) | 0 | 3 (60) | 0 |
| Best overall response (confirmed or unconfirmed PR or CR), n (%) | 14 (52) | 13 (57) | 2 (29) | 5 (29) | 7 (58) | 5 (56) | 6 (75) | 1 (33) | 0 | 4 (80) | 1 (100) |
| Median DORC, months (95% Cl) | 14.4 (5.0–21.7) | 14.3 (4.1-NE) | NE | 14.3 (11.1–21.7) | NE | 6.4 (5.0–18.6) | NE | 8.2 (NE-NE) | NE | 16.0 (6.0–16.0) | NE |
| Clinical benefitd, n (%) | 14 (52) | 14 (61) | 3 (43) | 9 (53) | 6 (50) | 4 (44) | 5 (63) | 1 (33) | 0 | 5 (100) | 1 (100) |
| Median PFSC, months (95% Cl) | 8.3 (4.2–18.0) | 8.3 (4.3–18.6) | NE (NE-NE) | 15.1 (2.6–25.7) | 10.2 (4.3-NE) | 7.0 (2.0–8.5) | 6.1 (1.9-NE) | 3.4 (1.9–10.2) | 1.8 (NE-NE) | 18.0 (8.3–18.0) | 12.7 (NE-NE) |
Data cut-off: 21 October 2022. Tumor response based on investigator tumor assessments (RECIST version 1.1).
CI, confidence interval; CR, complete response; DOR, duration of response; KD, kinase domain; NE, not estimable; PFS, progression-free survival; PR, partial response; TMD, transmembrane domain.
S310F/G776V, V777L/D769Y, G776S/H878Y, L755S/V777L, L755S/I767M/L786V.
Objective response defined as either a CR or PR that is confirmed ≥4 weeks after the criteria for response are initially met.
Kaplan–Meier analysis.
Clinical benefit is defined as confirmed CR or PR, or stable disease for ≥24 weeks (within a ±7-day visit window).
ORRs were 63% (N = 5/8) for patients with V777L HER2 kinase domain mutations, 24% (N = 4/17) for L755S, and 33% (N = 3/9) for those with other kinase domain missense mutations (Table 2). The ORR was 42% (N = 5/12) for patients with exon 20 insertion mutations and 33% (N = 1/3) for those with the extracellular domain S310F mutation, which stabilizes HER2-containing dimers and is the only HER2 mutation reported to respond to HER2 antibody therapy.22 Interestingly, despite patients whose tumors harbored L755S having a lower response rate, their median PFS was 15.1 months. Finally, 80% (N = 4/5) of patients with dual activating HER2 mutations had a confirmed response.
Patients with prior CDK4/6i therapy and their outcomes according to prior CDK4/6i duration are described in the Supplementary Results, Supplementary Figure S4, and Table S4, available at https://doi.org/10.1016/j.annonc.2023.08.003.
Biomarkers
To standardize biomarker evaluation, pretreatment biopsies were centrally assessed retrospectively. The biomarker workflow is shown in Supplementary Figure S5, available at https://doi.org/10.1016/j.annonc.2023.08.003.
Central HER2 mutation confirmation
In total, 47 of 71 patients had sufficient tissue available for central NGS: 30 were fresh biopsies (taken <50 days before cycle 1, day 1) and 17 were archival. HER2 mutation was centrally detected in 27/30 fresh biopsies (90%) and 16/17 archival tissues (94%). Central NGS was carried out on ctDNA for patients who did not have sufficient tissue (N = 20); of those, HER2 mutation was detected in 8/10 patients (80%) enrolled on tissue biopsy and 8/10 (80%) enrolled on liquid biopsy (Supplementary Table S5, available at https://doi.org/10.1016/j.annonc.2023.08.003). Four patients did not have sufficient tissue or ctDNA for central NGS.
HER2 mutation was centrally detected in 48 of 57 patients who received N + F + T, in whom the ORR was 42% (20/48) (Table 3). An additional six patients had sufficient sample for central NGS but HER2 mutation was not detected: none of those patients experienced response. HER2 CNA was not detected in any tumor; low-level copy number ‘gain’ was detected in two patients (Supplemental Figure S6, available at https://doi.org/10.1016/j.annonc.2023.08.003). The remaining 3/57 patients did not have sufficient sample for central NGS.
Table 3.
Efficacy by centrally assessed exploratory biomarker in patients treated with neratinib + fulvestrant + trastuzumab
| Centrally assessed exploratory biomarker | No. of patients | ORR, n (%) | CBR, n (%) | Median PFS, months (95% CI) |
|---|---|---|---|---|
|
| ||||
| NGS mutation | ||||
| HER2 | ||||
| Yes | 48 | 20 (42) | 28 (58) | 10.2 (6.1–18.6) |
| No | 6 | 0 | 0 | 4.2 (1.8–6.2) |
| Insufficient sample | 3 | 2 (67) | 3 (100) | 12.7 (8.2–12.7) |
| HER2 and ERBB3 | 10 | 4 (40) | 6 (60) | 25.7 (1.0-NE) |
| HER2 and ESR1 | 6 | 3 (50) | 4 (67) | 8.3 (1.9–18.6) |
| HER2 and CDH1 | 27 | 11 (41) | 15 (56) | 15.1 (2.4–18.6) |
| HER2 and TP53 | 13 | 3 (23) | 5 (38) | 6.0 (1.9–8.3) |
| HER2 and PIK3CA | 19 | 4 (21) | 10 (53) | 7.8 (2.2–10.2) |
| HER2 and none of above | 7 | 4 (57) | 4 (57) | NE (3.9-NE) |
| IHC category | ||||
| 0/1+ | 10 | 2 (20) | 4 (40) | 7.0 (1.8-NE) |
| 2+ | 21 | 9 (43) | 12 (57) | 8.3 (2.6–18.0) |
| 3+ | 1 | 0 | 0 | 3.9 (NE-NE) |
| Insufficient tissue | 25 | 11 (44) | 15 (60) | 10.2 (4.3–18.6) |
| FISH category | ||||
| Amplified | 10 | 3 (30) | 5 (50) | 6.1 (1.0-NE) |
| Non-amplified | 20 | 8 (40) | 11 (55) | 8.5 (4.1–18.0) |
| Insufficient tissue | 27 | 11 (41) | 15 (56) | 8.3 (4.2–18.6) |
| Overall HER2 status (IHC 3+ or FISH amplified) | ||||
| Positive | 10 | 3 (30) | 5 (50) | 6.1 (1.0-NE) |
| Negative | 20 | 8 (40) | 11 (55) | 8.5 (4.1–18.0) |
| Insufficient tissue | 27 | 11 (41) | 15 (56) | 8.3 (4.2–18.6) |
| Molecular subtype | ||||
| Luminal A | 4 | 0 | 1 (25) | 3.1 (1.0-NE) |
| Luminal B | 4 | 2 (50) | 2 (50) | 14.0 (2.0–25.7) |
| HER2 enriched | 11 | 5 (45) | 8 (73) | 8.3 (1.9–10.2) |
| Insufficient tissue | 38 | 15 (39) | 20 (53) | 8.3 (4.7–18.0) |
CBR, clinical benefit rate; CI, confidence interval; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NE, not estimable; NGS, next-generation sequencing; ORR, objective response rate; PFS, progression-free survival.
Co-occurring genomic alterations
Co-mutations in ERBB3 and/or PIK3CA have been associated with resistance to neratinib or N + F in patients with HER2-mutant MBC.9,12,16,22 In the present study, the ORR for patients with co-mutation in ERBB3 by central NGS was 40% (N = 4/10), with a median PFS of 25.7 months. Those with co-occurring PIK3CA mutation had a 21% ORR (N = 4/19) and median PFS of 7.8 months (Table 3). The ORR of patients with ESR1 co-mutation was 50% (N = 3/6) and the median PFS was 8.3 months. CDH1 mutation, a hallmark of lobular cancer, was detected in 27 patients; these patients had an ORR of 41% (11/27) and median PFS of 15.1 months. Patients with co-occurring TP53 mutation, a general hallmark of poor prognosis,23,24 had an ORR of 23% (3/13) and median PFS of 6.0 months. Mutation trends in other genes were not apparent (Supplementary Figure S6, available at https://doi.org/10.1016/j.annonc.2023.08.003).
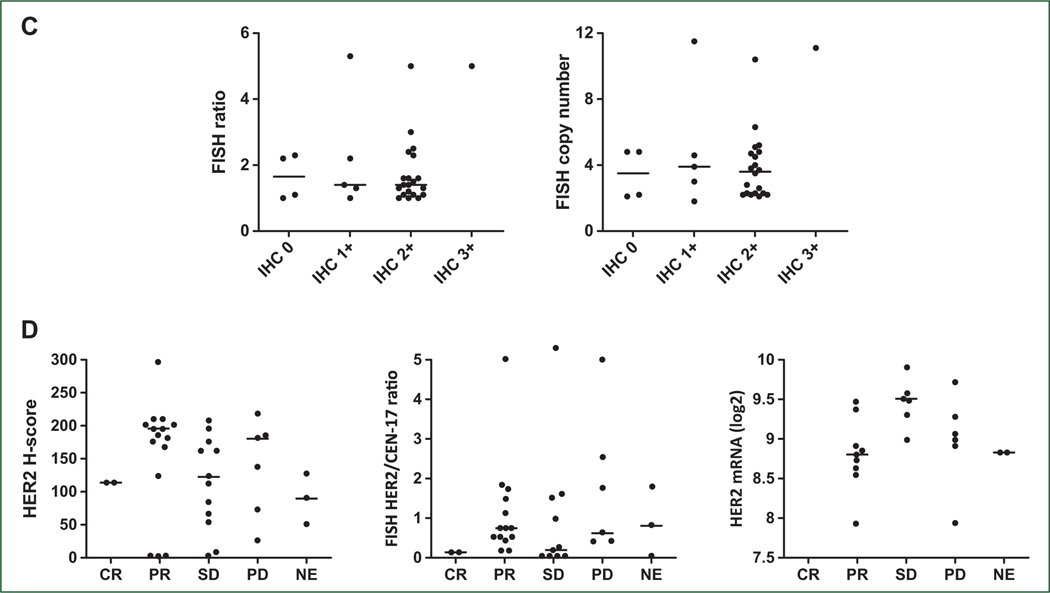
HER2 expression
Post-enrollment, retrospective, central HER2 IHC and HER2 FISH were carried out on 32 and 30 patients, respectively, treated with N + F + T and with sufficient material for testing. Four patients had tissue centrally scored as IHC 0, 6 were IHC 1+, 21 were IHC 2+, and 1 was IHC 3+. Of those who were IHC 2+, the median FISH copy number was 3.6 (range 2.1–10.4) and the median central FISH ratio was 1.4 (range 1.0–5.0; Figure 1C). Central HER2 IHC or FISH status did not appear to be associated with response to N + F + T: patients with IHC 0/1+, 2+, or 3+ had ORRs of 20% (N = 2/10), 43% (N = 9/21), and 0% (N = 0/1), respectively. The ORR in patients whose breast cancer was FISH non-amplified was 40% (8/20) versus 30% (3/10) in those with central FISH-amplified disease (Figure 1D; Table 3). Patients with central FISH-amplified disease had to have been deemed HER2-negative at their local institutions to meet eligibility criteria. Analysis of centrally assessed HER2 mRNA expression is described in the Supplementary Results, and Supplementary Figure S7 available at https://doi.org/10.1016/j.annonc.2023.08.003.
Mechanisms of acquired resistance
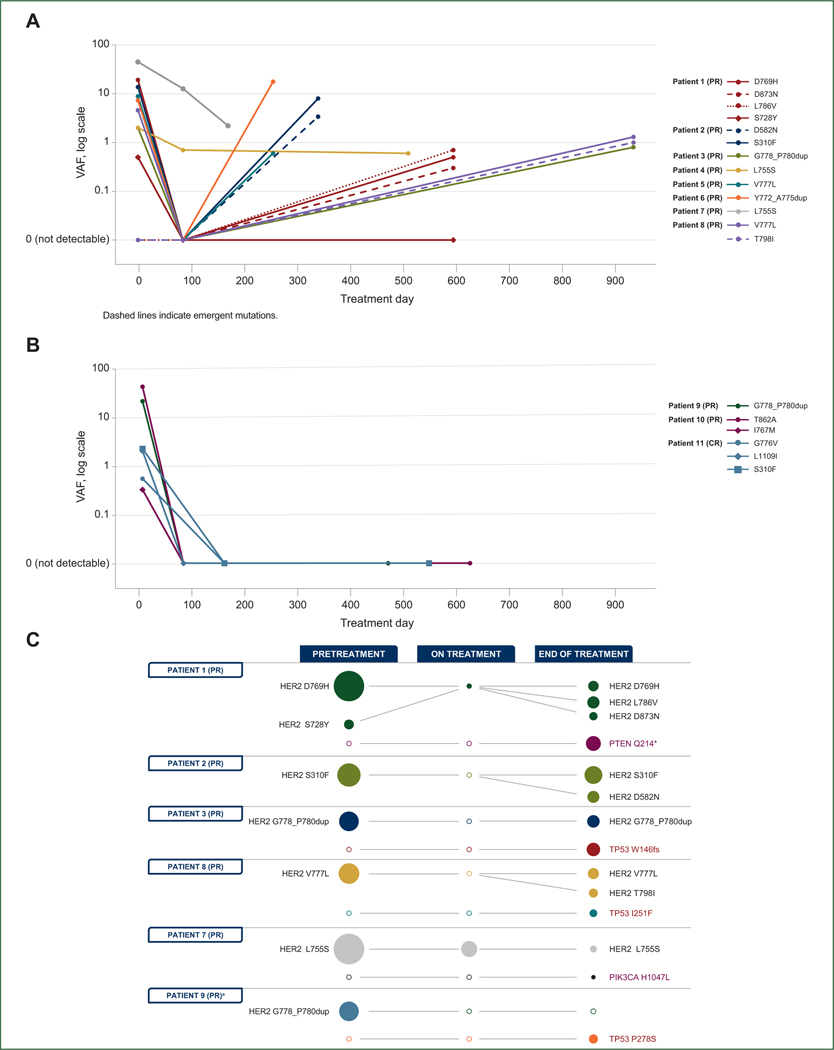
Detection of additional HER2 mutations and/or HER2 CNA12 upon progression on neratinib or N + F prompted us to test combination therapy with trastuzumab as an additional arm of SUMMIT. Thus, we carried out longitudinal ctDNA sequencing to evaluate whether the same or alternative mechanisms of acquired resistance were observed in patients progressing on dual HER2 targeting. Eight patients with a PR had evaluable ctDNA from liquid biopsies at enrollment (i.e. before treatment), while on treatment, and at progression; an additional three patients (two PR and one CR) remained on treatment at the time of the last sequenced blood draw. In all cases, the variant allele frequency (VAF) of the enrollment HER2 mutation, along with secondary HER2 mutations in three cases, decreased upon treatment with N + F + T (Figure 2A and B). Notably, the enrollment HER2 mutation became undetectable following treatment in six of eight patients and remained undetectable at the last blood draw in the three patients still on treatment (Figure 2A and B). Both patients whose enrollment HER2 mutations did not become undetectable during treatment had L755S mutations (Figure 2A). Upon progression, re-emergence of the enrollment HER2 mutation was observed, coincident with apparent acquisition of additional HER2 mutations in three patients, including the gatekeeper T798I mutation, and several variants of unknown significance (L786V, D873N, and D582N; Figure 2A and C). Two patients acquired PTEN Q214* and PIK3CA H1047L mutations and three acquired TP53 mutations following N + F + T treatment (Figure 2C).
Figure 2. Longitudinal HER2 mutation VAFs in patients with response to N + F + T.
Blood draw and ctDNA sequencing: (A) before treatment, on treatment, and at the end of treatment in patients who progressed; and (B) before treatment and in two on-treatment samples for patients who had not yet progressed. Solid and dotted lines represent original and emergent mutations, respectively. (C) Prevalence of notable mutations throughout the course of treatment with N + F + T; size of the bubble corresponds with VAF. Empty circle indicates mutation not detectable.
CR, complete response; ctDNA, circulating tumor DNA; dup, duplication; F, fulvestrant; N, neratinib; PR, partial response; T, trastuzumab; VAF, variant allele frequency.
aPatient remained on treatment as of data cut-off.
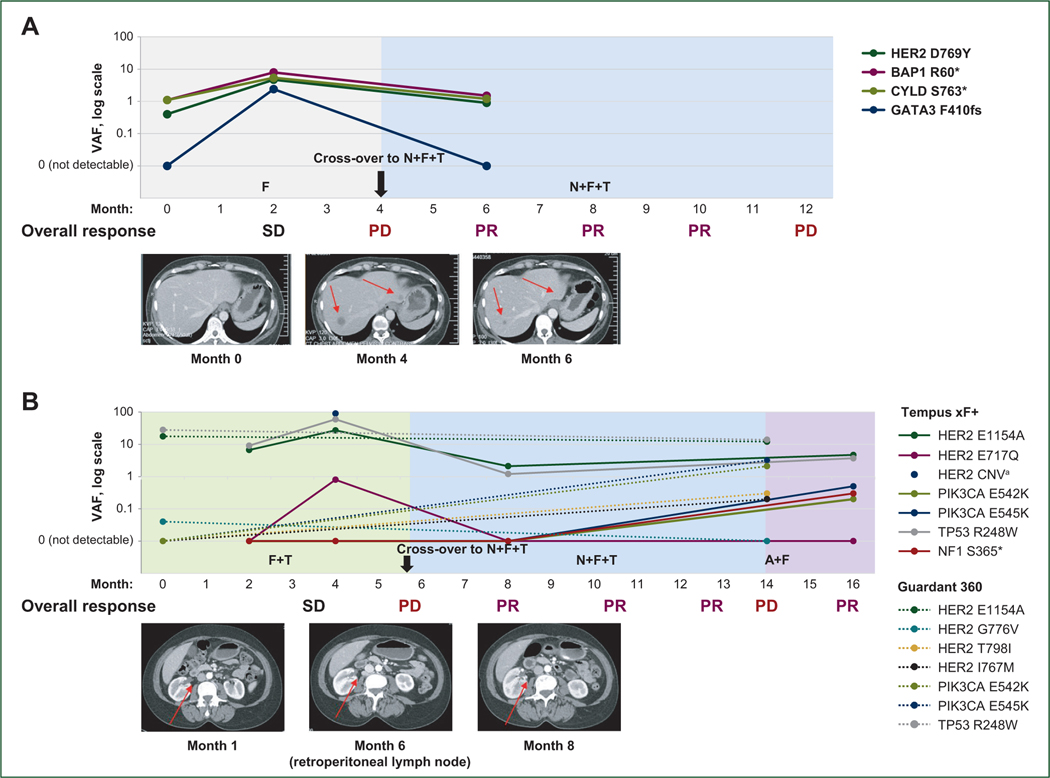
Longitudinal ctDNA analysis was also carried out for two patients who experienced PR upon cross-over to N + F + T after progression on fulvestrant (Figure 3A) and F + T (Figure 3B). In both cases, decreases in VAFs for ctDNA mutations were observed after cross-over, coinciding with imaging response. The patient illustrated in Figure 3B enrolled in SUMMIT based on detection of HER2 G776V (0.04%) by ctDNA analysis following disease progression after 10 months on letrozole and palbociclib. On central ctDNA testing, HER2 G776V was not detected in the baseline sample, possibly because of its low VAF. On treatment with F + T, this patient experienced SD, with 4 months’ PFS. After switching to N + F + T, she experienced PR, with a PFS of 8 months. Upon progression on N + F + T, she acquired HER2 T798I (0.3% VAF) and several PIK3CA mutations including E545K (3%), E542K (2%), and E545A (0.06%), which were not detected before enrollment. Based on ctDNA results at progression, she was subsequently treated with fulvestrant plus the phosphoinositide 3-kinase (PI3Ka) inhibitor alpelisib, on which she achieved PR (Figure 3B).
Figure 3. Cross-over case studies.
(A) patient crossed over from F to N + F + T. The patient was enrolled on tissue-based NGS (FoundationOne CDx), which reported HER2 D769Y. The patient was initially randomized to F alone; upon progression, she crossed over to receive N + F + T. HER2 D769Y VAF increased upon initial treatment with F then decreased upon cross-over. (B) Patient crossed over from F + T to N + F + T. The patient was enrolled on ctDNA (Guardant 360), which detected HER2 G776V at 0.04% VAF. HER2 E1145 and E717Q, but not G776V, were centrally detected in pretreatment ctDNA. The patient was initially randomized to F + T; upon progression she crossed over to receive N + F + T. HER2 VAFs of both mutations increased upon initial treatment with F + T, then decreased upon addition of N. HER2 copy number gain was also detected upon initial treatment, but not after the patient crossed over to receive the triplet. Upon progression to N + F + T, the HER2 T798I gatekeeper mutation emerged, along with PIK3CA-activating mutations E542K and E545K. After progression on N + F + T, the patient was treated with A + F and experienced a PR.
A, alpelisib; CNV, copy number variation; ctDNA, circulating tumor DNA; F, fulvestrant; N, neratinib; NGS, next-generation sequencing; PD, progressive disease; PR, partial response; SD, stable disease; T, trastuzumab; VAF, variant allele frequency.
aHER2 CNV only detected at second timepoint. Solid lines indicate Tempus xF+ results; dotted lines indicate Guardant360 results.
Preclinical rationale for dual HER2 targeting
Mutant HER2 exhibits enhanced dimerization with the HER3 co-receptor, with enhanced PI3K/AKT/mTOR signaling.8 Addition of trastuzumab to neratinib is predicted to block this heterodimerization25 and potentially enhance inhibition of PI3K/AKT/mTOR signaling and antitumor activity. We explored this possibility using MCF7 cells engineered to express HER2 V777L via knock-in of the mutant allele at the endogenous locus. Treatment of MCF7 HER2 V777L cells with 100 nM neratinib resulted in strong and sustained inhibition of phosphorylated HER2 for up to 72 h. Phosphorylated HER3, phosphorylated AKT, and phosphorylated S6 levels were also markedly inhibited in HER2-mutant cells, although they began to recover after 24 h. Co-treatment with trastuzumab abrogated recovery of HER3 and AKT phosphorylation (Supplementary Figure S8, available at https://doi.org/10.1016/j.annonc.2023.08.003), providing a rationale for enhanced activity with the combination. These data, combined with acquisition of HER2 gene amplifications following treatment of HER2-mutant tumors with N + F,12 support adding trastuzumab to the combination.
Safety
The most common treatment-emergent AEs are summarized in Supplementary Table S6, available at https://doi.org/10.1016/j.annonc.2023.08.003. Diarrhea of any grade occurred in 93% (N = 53/57) of patients who received N + F + T, in 29% (N = 2/7) of those who received F + T, and in none of those who received fulvestrant alone. Grade 3 diarrhea occurred in 53% (N = 30/57) of patients in the N + F + T group and was not observed in the F + T or fulvestrant monotherapy groups. For N + F + T, grade 3 diarrhea typically occurred early [median time to grade 3 diarrhea was 8 days; interquartile range (IQR) 4–32 days] after the start of treatment and was transient (median cumulative duration 4 days; IQR: 2–10 days). Among patients with diarrhea, 27 and 13 were managed by dose interruptions and dose reductions, respectively; only 2 patients withdrew from treatment.
DISCUSSION
The SUMMIT trial has provided a clinical platform for evaluating HER2 TKI-containing therapies for patients with MBC whose tumors harbor HER2 mutations. We speculated that dual HER2 targeting, through addition of trastuzumab to N + F, might prevent or delay emergence of additional HER2 genomic alterations and lengthen responses observed with the doublet. This was further supported by preclinical studies using breast cancer cells expressing HER2 V777L, suggesting that addition of trastuzumab to neratinib monotherapy prolonged suppression of HER3 phosphorylation. Indeed, DOR, CBR, and PFS were all superior in patients treated with N + F + T versus N + F. Of note, all patients treated with N + F + T had progressed on prior CDK4/6i. Neratinib appeared to be a critical component of the combination, as demonstrated by lack of response in the small cohorts of patients treated with fulvestrant alone or F + T, and subsequent response in patients who crossed over to N + F + T after progression on fulvestrant alone or F + T.
Although we recognize that cross-trial and cross-cohort comparisons must be interpreted with caution, treatment with N + F + T appeared to be superior to N + F in patients with HR+ HER2-mutant MBC. Firstly, responses to N + F + T were observed in patients with ductal and lobular histology, as opposed to the previously reported association of lobular histology with response to N + F.16 Notably, the incidence of lobular breast cancer was higher in our cohort than previously reported for MBC, but consistent with enrichment for HER2 mutations in patients with lobular versus ductal breast cancers.1 Secondly, four of five patients with tumors harboring more than one activating HER2 mutation experienced a confirmed response, in contrast to previous association of this characteristic with lack of clinical benefit to N + F.12 Thirdly, co-occurrence of HER2 and ERBB3 mutations did not preclude response to N + F + T, in contrast to neratinib alone or N + F.12 Additionally, >20% of patients with co-occurring PIK3CA mutation responded to treatment, suggesting that dual HER2 targeting may block the enhanced PI3K signaling in these tumors. Finally, the low response rate and high median PFS in patients whose tumors harbored L755S were consistent with both the indolence and reduced sensitivity to neratinib of L755S compared with other activating HER2 mutations in preclinical models.7,22,26–28
The finding that most HER2-mutant MBC samples were IHC 2+ is consistent with a recent genomic description of HER2-low breast cancers, in which HER2-mutant samples were exclusive to the HER2 IHC 2+/copy number-equivocal (HLBC-2E) group and in fact comprised 14% of that group. Indeed, HER2 mRNA levels in HER2-mutant tumors from SUMMIT patients with available tissue and those in a clinicogenomic/transcriptomic database of >4000 patients with HR+ MBC29 were midway between HER2-positive and HER2-negative disease. We thus speculate that HR+ HER2-mutant tumors may fall broadly into the newly described ‘HER2-low’ classification.30,31 At the time of writing, data were not available for patients with HER2 mutations from the DESTINY-Breast04 clinical trial (ClinicalTrials.gov Identifier: NCT03734029), which measured the efficacy of trastuzumab deruxtecan (T-DXd) in patients with HER2-low MBC. If results are positive, this may enable exploration of sequencing of antibody–drug conjugates (ADCs) and TKIs targeting HER2. In preclinical experiments, neratinib induced HER2 receptor ubiquitination and endocytosis;32 thus, combining neratinib with a HER2 ADC may increase ADC internalization. Further, combining neratinib with trastuzumab emtansine or with T-DXd (T-DM1) in HER2-mutant breast and lung cancer patient-derived xenografts resulted in synergistic growth inhibition in preclinical models.26,33 A phase II study of T-DXd in HER2-mutant solid tumors has recently completed enrollment (ClinicalTrials.gov identifier: NCT04639219), and a phase I clinical trial exploring the safety and tolerability of neratinib + T-DXd in HER2-altered breast cancer is currently underway (ClinicalTrials.gov identifier: NCT05372614).
Serial ctDNA sequencing enables an individualized approach to cancer treatment. We observed that HER2 mutation VAFs decreased to below the level of detection upon treatment in most responders with emergence of additional HER2 mutations upon progression, TP53 mutations, and/or mutations in downstream effectors of HER2 signaling (PIK3CA, PTEN), consistent with prior cohorts.9,12,34 Supporting the requirement of neratinib in the combination, one patient initially randomized to F + T had a concomitant increase in VAFs and emergence of HER2 CNA. Upon addition of neratinib, HER2 amplification became undetectable, and the patient responded for 6.2 months. Furthermore, ctDNA sequencing upon progression revealed acquisition of an activating PIK3CA mutation, which responded to alpelisib. These cases highlight that N + F + T can be effective for patients who acquire HER2 mutations at disease progression on a CDK4/6i, and that PIK3CA mutations could represent acquired resistance mechanisms to N + F + T that could potentially be further treated with PI3K inhibitors. These cases also highlight the potential utility of serial ctDNA in sequentially tailoring cancer treatment in response to individual tumor evolution.
The overall safety profile of neratinib for patients with HR+, HER2-mutant MBC was consistent with prior studies, although the rate of grade 3 diarrhea (53%) in patients who received the triplet combination of N + F + T was higher than that observed in other SUMMIT breast cohorts of either neratinib monotherapy (26.5%) or N + F (23.4%),12 in the N + F cohort of the MutHER clinical trial (25%),16 and also higher than that in a phase I/II study of N + T (15.6%)35 or a phase I study of N + T + paclitaxel in HER2-positive MBC (0%).36 Studies of other neratinib combinations with recommended or mandatory antidiarrheal prophylaxis in MBC have also reported lower rates of grade 3 diarrhea: 24% with neratinib plus capecitabine in the phase III NALA trial,37 23% with neratinib + T-DM1 in the TBCRC 022 trial,38 and 30% with neratinib plus paclitaxel in the NEfERT-T trial.39 The reason for increased grade 3 diarrhea within this cohort remains unclear; it is possible that the N + F + T triplet increases diarrhea, although no mechanism for such an effect has been reported. Importantly, over half of the patients in this cohort were enrolled during the peak of the COVID-19 pandemic, and we speculate that close monitoring for compliance with loperamide prophylaxis may have been compromised during that time. Of note, 2-week dose escalation of neratinib with loperamide as needed is now recognized as an optimal diarrhea management strategy and included in the US prescribing information and National Comprehensive Cancer Network (NCCN) guidelines based on results from the phase II CONTROL trial, which demonstrated a grade 3 diarrhea rate of 13.3%.40 Dose escalation was not used in the present study but is now recommended for use in the clinical setting.
In conclusion, these data represent completion of the hypothesis-generating SUMMIT basket trial, which supports a model of the Nimble trial adaptation in response to translational findings. N + F + T showed encouraging clinical efficacy in heavily pretreated patients with HR+ breast cancer harboring HER2-activating mutations. In line with this result, neratinib-based combinations have recently been endorsed for this molecularly defined population of patients by NCCN guidelines. Furthermore, 16 patients enrolled in this trial exhibited a HER2 mutation in DNA retrieved from archival biopsy, confirming these pathogenic alterations are present much earlier in tumor evolution. This implies that with future increased early utilization of NGS, and thus increased detection of HER2 mutations at the time of breast cancer diagnosis or at endocrine resistance,10 the results presented herein support future testing of neratinib-based combinations in patients with HR+ HER2-mutant early breast cancer.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank patients who participated in this study and their families, Bethann Hromatka (Puma Biotechnology) for publications support, Bo Zhang for statistical support, Jane Liang and Fauzia Ihsanullah for programming support, Tonya Novara-Demgen for sample handling and clinical operations, and members of the MSKCC Kravis Center for Molecular Oncology for assistance with biomarker studies. Editorial support, not including writing, was provided by Lee Miller and Deirdre Carman (Miller Medical Communications Ltd.).
FUNDING
The SUMMIT study and the provision of editorial support by Miller Medical Communications Ltd. were supported by Puma Biotechnology, Inc. Funding support was provided to the Memorial Sloan Kettering Cancer Center under the NCI Cancer Center support [grant number P30-CA008748]. SL is supported by the National Breast Cancer Foundation of Australia Endowed Chair and the Breast Cancer Research Foundation, New York, NY, USA. DBS and correlative science studies were supported by grant number R01-CA234361. MEB: Carbone Cancer Center, University of Wisconsin-Madison [grant number P30CA014520 UW]. CLA and ABH: grant number NCI R01CA224899.
DISCLOSURE
KJ: Consultant/Advisory Board: Novartis, AstraZeneca, Pfizer, BMS, Jounce Therapeutics, Taiho Oncology, Genentech/Roche, Lilly Pharmaceuticals/Loxo Oncology, AbbVie, Eisai, Blueprint Medicines, Seattle Genetics, Daiichi Sankyo, Gilead, Olema Pharmaceuticals, Sun Pharma Advanced Research Company Ltd., Menarini/Stemline, and Scorpion Therapeutics. Research Funding: Novartis, Genentech/Roche, AstraZeneca, Debio Pharmaceuticals, Pfizer, Lilly Pharmaceuticals/Loxo Oncology, Zymeworks, Gilead, Puma Biotechnology, Merck Pharmaceuticals, and Scorpion Therapeutics. HW: His institution received financial compensation on his behalf for advisory boards, lecture fees and/or consultancy fees from Daiichi Sankyo, Gilead, Lilly, Augustine Therapeutics, AstraZeneca, Immutep Pty, MSD, and Roche. He received travel support from Gilead, Daiichi Sankyo, and Pfizer. SAH: Contracted research paid to institution +/− editorial support for authorship: Ambrx, Arvinas, AstraZeneca, Bayer, Celcuity, Cytomx, Daiichi Sankyo, Dantari, Dignitana, Genentech/Roche, G1-Therapeutics, Gilead, Greenwich Life Sciences Inc., GSK, Immunomedics, Eli Lilly, Loxo, Macrogenics, Novartis, OBI Pharma, Orinove, Orum, Pfizer, Phoenix Molecular Designs, Ltd., Pieris, PUMA, Radius, Sanofi, Seattle Genetics/Seagen, and Zymeworks. Speaking: Daiichi Sankyo (2021). AG-Z: Advisory/Consultancy: AstraZeneca, Novartis, MSD, Pierre-Fabre, and Exact Science. Speaker Bureau/Expert testimony: Roche, AstraZeneca, Novartis, MSD, Pfizer, Lilly, and Pierre-Fabre. Research grant/Funding (institution): Pfizer. Travel/Accommodation/Expenses: Roche, Novartis, and Pfizer. NU: Consultant/Advisory Board: Novartis, Eli Lilly, BioTheranostics, Gilead. AB: Consultant: AstraZeneca, Pfizer, Novartis, Lilly, Genentech/Roche, Seagen, Daiichi Sankyo, Merck, Agendia, Sanofi, Puma, Myriad, and Gilead; research support: Agendia and AstraZeneca. HP: Research grants to institution: Adlai Nortye USA, Ambrx, Aprea Therapeutics AB, Array BioPharma, AstraZeneca, BJ Bioscience, Bristol-Myers Squibb, Daiichi Pharmaceutical, Elicio Therapeutics, Exelixis, Fate Therapeutics, Genentech, GlaxoSmithKline, Gossamer Bio, Hutchison MediPharma, ImmuneOncia Therapeutics, ImmunoGen, Mabspace Biosciences, MacroGenics, Merck, Mirati Therapeutics, Novartis Pharmaceuticals, Oncologie, PsiOxus Therapeutics, RePare Therapeutics, Seattle Genetics, Synermore Biologics, TopAlliance Biosciences, Turning Point Therapeutics, Vedanta Biosciences, Xencor. ESY: Research funding: Puma Biotechnology; Advisory boards: Bayer, Clovis; Consultant: AstraZeneca. IS: Institutional Research Funding: Roche/Genentech, Puma Biotechnology, MSD, Merck, AstraZeneca, Incyte, Orion, Genmab, Bristol-Myers Squibb, Bayer/Loxo Oncology, Lilly Pharmaceuticals/Loxo Oncology, Novartis, Pfizer, Amgen, Repare Therapeutics. Honoraria: AstraZeneca. Support for travel and meeting attendance: Roche, Novartis, Merck/Pfizer, Incyte, and AstraZeneca. SR: Consultant/Advisory board: Novartis, AstraZeneca, Gilead, and Daiichi Sankyo. MBh: Consulting/Advisory Board: Daiichi Sankyo, Merck, Pfizer, and AstraZeneca. SV: Funding from Pfizer directly to institution for conducting clinical trial. MA: Consulting or advisory role: Pfizer, AstraZeneca, Menarini, Novartis, Daiichi Sankyo, and Gilead. Speaker’s bureau: Pfizer, Novartis, and Gilead. Research funding: AstraZeneca. F-CB: Consulting or advisory role: Pfizer; AstraZeneca; Lilly; Novartis; Menarini; Sanofi; GSK; Rain Oncology; Caris Life Sciences; GE Healthcare; Exact Sciences; Gilead. Speaker’s bureau: Pfizer; Novartis; AstraZeneca; Roche; Lilly; Rain Oncology; Daiichi Sankyo; and Menarini-Stemline. Research Funding: Novartis; Pfizer; Menarini Silicon Biosystems; Prolynx; Merck KGaA; and GE Healthcare. SL: Receives research funding to her institution from Novartis, Bristol Myers Squibb, Merck, Puma Biotechnology, Eli Lilly, Nektar Therapeutics, AstraZeneca, and Seattle Genetics. She has acted as consultant (not compensated) to Seattle Genetics, Novartis, Bristol Myers Squibb, Merck, AstraZeneca, Eli Lilly, Pfizer, Gilead Therapeutics, and Roche-Genentech. She has acted as consultant (paid to her institution) to Aduro Biotech, Novartis, GlaxoSmithKline, Roche-Genentech, AstraZeneca, Silverback Therapeutics, G1 Therapeutics, Puma Biotechnology, Inc., Pfizer, Gilead Therapeutics, Seattle Genetics, Daiichi Sankyo, Merck, Amunix, Tallac Therapeutics, Eli Lilly, and Bristol Myers Squibb. JC: Has taken part in advisory boards for Puma Biotechnology. His unit is the recipient of a peer-reviewed grant from Science Foundation Ireland (an Irish government organization) which is part-funded by Puma Biotechnology Inc. Neither he nor his spouse own Puma Biotechnology stock. MEB: Medical advisory board of Strata Oncology; Research funding from AbbVie, Arcus, Apollomics, Elevation Oncology, Endeavor, Genetech, Puma, Loxo Oncology, and Seagen. SAP-P: Clinical trial research support/grant funding through the institution from AbbVie, Inc., ABM Therapeutics, Inc., Acepodia, Inc., Alkermes, Aminex Therapeutics, Amphivena Therapeutics, Inc., BioMarin Pharmaceutical, Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Cerulean Pharma, Inc., Chugai Pharmaceutical Co., Ltd., Curis, Inc., Cyclacel Pharmaceuticals, Daiichi Sankyo, Eli Lilly, ENB Therapeutics, Epigenetix Inc., Five Prime Therapeutics, F-Star Beta Limited, F-Star Therapeutics, Gene Quantum, Genmab A/S, Gilead Sciences, Inc., GlaxoSmithKline, Helix BioPharma Corp., Hengrui Pharmaceuticals, Co., Ltd., HiberCell, Inc., Immorna Biotherapeutics, Inc., Immunomedics, Inc., Incyte Corp., Jacobio Pharmaceuticals Co., Ltd., Jiangsu Simcere Pharmaceutical Co., Ltd., Lytix Biopharma AS, Medimmune, LLC., Medivation, Inc., Merck Sharp and Dohme Corp., Nectin Therapeutics, Ltd., Novartis Pharmaceuticals, Pieris Pharmaceuticals, Inc., Pfizer, Phanes Therapeutics, Principia Biopharma, Inc., Puma Biotechnology, Inc., Purinomia Biotech, Inc., Rapt Therapeutics, Inc., Replimune, Seattle Genetics, Silverback Therapeutics, Synlogic Therapeutics, Taiho Oncology, Tesaro, Inc., TransThera Bio, ZielBio, Inc., NCI/NIH, P30CA016672—Core Grant (CCSG Shared Resources); and consulting fees from CRC Oncology. JMS: Research grant support: AstraZeneca and Strata Oncology. SC: Advisory/Consultancy: Novartis, F. Hoffmann-La Roche, Pfizer, Eli Lilly, AstraZeneca, Amgen, Gilead, Merck, and Exact Sciences. Research funding to the institution: Novartis, F. Hoffmann-La Roche, Pfizer, Genomic Health/Exact Sciences, AstraZeneca, Genentech, Celgene, Amgen, BMS, Merck, Sanofi, Puma, and Gilead. CS: Consulting, advisory role or travel grants from: AstraZeneca, AX’Consulting, Byondis B.V, Daiichi Sankyo, Eisai, Exact Sciences, Exeter Pharma, F. Hoffmann-La Roche Ltd., Lilly, MediTech, Merck Sharp & Dohme, Novartis, Pfizer, Philips, Pierre-Fabre, PintPharma, Puma Biotechnology, Seagen, and Zymeworks. JAG-S: Consulting or advisory role: Novartis Pharmaceuticals Corporation, AstraZeneca, Lilly, Seagen, Daiichi Sankyo, Gilead Sciences, Exact Sciences, and Stemline Menarini; speakers bureau: Novartis Pharmaceuticals Corporation, Lilly, AstraZeneca, and Stemline Menarini; travel, accommodations, expenses: Daiichi Sankyo, Gilead Sciences, and Exact Sciences. VG: Advisory Board: Boehringer. Research Funding: Bayer, Boehringer, Roche. Institutional Funding: Genentech, Merck Serono, Roche, Beigene, Bayer, Servier, Lilly, Novartis, Takeda, Astellas, Fibrogen, Amcure, Natera, Sierra Oncology, AstraZeneca, Medimmune, BMS, and MSD. ENG-Y: Honoraria from Novartis, Pfizer, Eli Lilly, MSD, and AstraZeneca. CM: Has received research grants from Puma Biotechnology and Pfizer. She received consulting fees from AstraZeneca, Olaris, Novartis, and Sanofi. ABH: Reports stock and other ownership interests in Pfizer (immediate family member) and research funding from Takeda. JWG: has received institutional research funding from Puma Biotechnology Inc. RB: Receives research grant from Puma; has performed consulting for Genentech. JSKB: Employee (and stockholder) of Tempus Labs. LDE, DD, AF, and AW: Employees and stockholders of Puma Biotechnology. CLA: Has received research grants from Pfizer, Lilly, and Takeda. He serves or has served as scientific advisor to Novartis, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Immunomedics, OrigiMed, Sanofi, TAIHO Oncology, and Puma Biotechnology. DBS: Has served as a consultant for/received honorarium from Pfizer, Vividion Therapeutics, Scorpion Therapeutics, FORE Therapeutics, Fog Pharma, Elsie Biotechnologies, Fog Pharma, Rain Oncology, Function Oncology, and BridgeBio.
Footnotes
All other authors have declared no conflicts of interest.
DATA SHARING
The authors declare that the data supporting the findings of this study are available within the article. Qualified researchers and study participants may submit requests for other study documentation and clinical trial data to clinicaltrials@pumabiotechnology.com for consideration.
REFERENCES
- 1.Kurozumi S, Alsaleem M, Monteiro CJ, et al. Targetable ERBB2 mutation status is an independent marker of adverse prognosis in estrogen receptor positive, ERBB2 non-amplified primary lobular breast carcinoma: a retrospective in silico analysis of public datasets. Breast Cancer Res. 2020;22:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocco E, Javier Carmona F, Razavi P, et al. Neratinib is effective in breast tumors bearing both amplification and mutation of ERBB2 (HER2). Sci Signal. 2018;11(551):eaat9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deniziaut G, Tille JC, Bidard FC, et al. ERBB2 mutations associated with solid variant of high-grade invasive lobular breast carcinomas. Oncotarget. 2016;7(45):73337–73346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schram A, Won HH, Andre F, et al. Abstract LB-103: landscape of somatic ERBB2 mutations: findings from AACR GENIE and comparison to ongoing ERBB2 mutant basket study. Cancer Res. 2017;77(suppl 13): LB103. [Google Scholar]
- 5.Desmedt C, Zoppoli G, Gundem G, et al. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol. 2016;34(16):1872–1881. [DOI] [PubMed] [Google Scholar]
- 6.Razavi P, Chang MT, Xu G, et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34(3):427–438.e426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croessmann S, Formisano L, Kinch LN, et al. Combined blockade of activating ERBB2 mutations and ER results in synthetic lethality of ER+/HER2 mutant breast cancer. Clin Cancer Res. 2019;25(1):277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554(7691):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma CX, Bose R, Gao F, et al. Neratinib efficacy and circulating tumor DNA detection of HER2 mutations in HER2 nonamplified metastatic breast cancer. Clin Cancer Res. 2017;23(19):5687–5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nayar U, Cohen O, Kapstad C, et al. Acquired HER2 mutations in ER(+) metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat Genet. 2019;51(2):207–216. [DOI] [PubMed] [Google Scholar]
- 12.Smyth LM, Piha-Paul SA, Won HH, et al. Efficacy and determinants of response to HER kinase inhibition in HER2-mutant metastatic breast cancer. Cancer Discov. 2020;10(2):198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T, Xu Y, Sheng S, et al. HER2 somatic mutations are associated with poor survival in HER2-negative breast cancers. Cancer Sci. 2017;108(4):671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connell CM, Doherty GJ. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open. 2017;2(5):e000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5(8):832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma CX, Luo J, Freedman RA, et al. The phase II MutHER study of neratinib alone and in combination with fulvestrant in HER2-mutated, non-amplified metastatic breast cancer. Clin Cancer Res. 2022;28:1258–1267. [DOI] [PubMed] [Google Scholar]
- 17.Rabindran SK, Discafani CM, Rosfjord EC, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64(11):3958–3965. [DOI] [PubMed] [Google Scholar]
- 18.Turner NC, Kingston B, Kilburn LS, et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21(10):1296–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eli L, Kavuri S. Mechanisms of neratinib resistance in HER2-mutant metastatic breast cancer. Cancer Drug Resist. 2022;5(4):873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanova E, Kuraguchi M, Xu M, et al. Use of ex vivo patient-derived tumor organotypic spheroids to identify combination therapies for HER2 mutant non-small cell lung cancer. Clin Cancer Res. 2020;26(10):2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeNoue-Newton ML, Chen S-C, Stricker T, et al. Natural history and characteristics of ERBB2-mutated hormone receptor–positive metastatic breast cancer: a multi-institutional retrospective case–control study from AACR Project GENIE. Clin Cancer Res. 2022;28(10):2118–2130. [DOI] [PubMed] [Google Scholar]
- 22.Hanker AB, Brown BP, Meiler J, et al. Co-occurring gain-of-function mutations in HER2 and HER3 modulate HER2/HER3 activation, oncogenesis, and HER2 inhibitor sensitivity. Cancer Cell. 2021;39(8):1099–1114.e1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overgaard J, Yilmaz M, Guldberg P, et al. TP53 mutation is an independent prognostic marker for poor outcome in both node-negative and node-positive breast cancer. Acta Oncol. 2000;39(3):327–333. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Hao R, Guo Q, et al. TP53 mutation infers a poor prognosis and is correlated to immunocytes infiltration in breast cancer. Front Cell Dev Biol. 2021;9:759154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–440. [DOI] [PubMed] [Google Scholar]
- 26.Li BT, Michelini F, Misale S, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 2020;10(5):674–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalra R, Chen CH, Wang J, et al. Poziotinib inhibits HER2-mutant-driven therapeutic resistance and multiorgan metastasis in breast cancer. Cancer Res. 2022;82(16):2928–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagano M, Kohsaka S, Ueno T, et al. High-throughput functional evaluation of variants of unknown significance in ERBB2. Clin Cancer Res. 2018;24(20):5112–5122. [DOI] [PubMed] [Google Scholar]
- 29.Beaubier N, Tell R, Lau D, et al. Clinical validation of the tempus xT next-generation targeted oncology sequencing assay. Oncotarget. 2019;10(24):2384–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denkert C, Seither F, Schneeweiss A, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. [DOI] [PubMed] [Google Scholar]
- 31.Venetis K, Crimini E, Sajjadi E, et al. HER2 low, ultra-low, and novel complementary biomarkers: expanding the spectrum of HER2 positivity in breast cancer. Front Mol Biosci. 2022;9:834651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Zhang J, Liu C, et al. Neratinib induces ErbB2 ubiquitylation and endocytic degradation via HSP90 dissociation in breast cancer cells. Cancer Lett. 2016;382(2):176–185. [DOI] [PubMed] [Google Scholar]
- 33.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudhan DR, Guerrero-Zotano A, Won H, et al. Hyperactivation of TORC1 drives resistance to the pan-HER tyrosine kinase inhibitor neratinib in HER2-mutant cancers. Cancer Cell. 2020;37(2):183–199.e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackwell KL, Zaman K, Qin S, et al. Neratinib in combination with trastuzumab for the treatment of patients with advanced HER2-positive breast cancer: a phase I/II study. Clin Breast Cancer. 2019;19(2):97–104.e104. [DOI] [PubMed] [Google Scholar]
- 36.Jankowitz RC, Abraham J, Tan AR, et al. Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2-positive breast cancer: an NSABP Foundation Research Program phase I study. Cancer Chemother Pharmacol. 2013;72(6):1205–1212. [DOI] [PubMed] [Google Scholar]
- 37.Saura C, Oliveira M, Feng YH, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with >/= 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freedman RA, Ren S, Tayob N, et al. Neratinib and ado-trastuzumabemtansine (T-DM1) for HER2+ breast cancer brain metastases (BCBM): Translational Breast Cancer Research Consortium (TBCRC) Trial 022. Cancer Res. 2023;83(5_Supplement):PD7–03. [Google Scholar]
- 39.Awada A, Colomer R, Inoue K, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2(12):1557–1564. [DOI] [PubMed] [Google Scholar]
- 40.Chan A, Ruiz-Borrego M, Marx G, et al. Final findings from the CONTROL trial: strategies to reduce the incidence and severity of neratinib-associated diarrhea in patients with HER2-positive early-stage breast cancer. Breast. 2023;67:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article. Qualified researchers and study participants may submit requests for other study documentation and clinical trial data to clinicaltrials@pumabiotechnology.com for consideration.