Abstract
BACKGROUND:
The present study evaluated the effects of aerobic training with variable intensities on apoptotic indices of cardiac tissue in fatty diabetic rats.
METHODS:
Twenty-four male Wistar rats were randomly divided into non-diabetic (ND, n=8), trained diabetic (TD, n=8), and control diabetic (CD, n=8) groups. Following a high-fat dietary regimen, type 2 diabetes was induced by streptozotocin, with blood glucose levels above 300 mg/dL considered indicative of diabetes. The TD group underwent aerobic exercise five times a week for six weeks. Subsequently, measurements were taken for left ventricular end-diastolic (LVEDV) and end-systolic volumes (LVESV), ejection fraction (EF%), catalase, caspase-9, P53, glucose, insulin, and HOMA-IR.
RESULTS:
Aerobic training led to a significant decrease in blood glucose levels (P < 0.01), caspase-9 (P < 0.05), HOMA-IR (P < 0.05), and P53 expression (P < 0.001) compared with the CD group. LVEDV and LVESV decreased significantly (P < 0.05 for both), while LVEF increased significantly (P < 0.05). Catalase activation showed an insignificant increase in the TD group pre- to post-training compared to CD.
CONCLUSION:
Incremental aerobic exercise training (6 weeks) may exert a cardioprotective effect in diabetic rats by reducing apoptosis and oxidative stress indices, while simultaneously increasing aerobic fitness and reducing body weight.
Key Words: Physical Activity, Diabetes, Apoptosis, Oxygen Consumption, Rat
Introduction
Diabetes mellitus is a metabolic disorder that is rapidly growing and emerging as a public health issue due to its high prevalence rate and contribution to morbidity and mortality. Diabetes mellitus induces risk factors for cardiovascular disease that eventually lead to heart failure1. Diabetic Cardiomyopathy (DC) is a primary pathological factor contributing to heart disease, characterized by left ventricular (LV) diastolic and systolic dysfunction in the absence of hypertension2. Various molecular and metabolic factors, such as insulin resistance, reactive oxidative species, fatty acids, and inflammation, have been identified as contributing factors in DC3-5.
In reviewing the literature related to DC, hyperglycemia caused by insulin resistance has been reported to be the main cause of cardiac dysfunction6,7. Endothelial cells, unable to limit glucose transport, are more vulnerable to the toxic effects of hyperglycemia1. Following hyperglycemia, there is an elevation in reactive oxygen species in the diabetic heart, which seems to be counterbalanced by the promotion of antioxidant defense through enhanced activation of enzymes such as Catalase, Glutathione Peroxidase, and Superoxide Dismutase1,8-10. Recent studies have demonstrated that antioxidant defense is also induced by voluntary physical training1,9, 11. However, the deleterious effects of metabolic dysfunctions following diabetes stimulate both extrinsic and intrinsic apoptotic pathways3.
Apoptosis is a programmed cell death process necessary for the physiological balance of cells12. In the extrinsic apoptotic pathway, inflammation following hyperglycemia leads to the binding of tumor necrosis factor-α and FAS Cell Surface Death Receptor (FAS) to their respective receptors to trigger cell death13. Consequently, cardiomyocyte-induced Caspase 9 activates the apoptotic pathways leading to rapid and irreversible cell death12, which decreases the contractile function of the heart and ultimately promotes cardiac remodeling14.
During the intrinsic pathway, hyperglycemia leads to the release of a group of mitochondrial proteins such as cytochrome C (Cyto C), Bcl-2 Associated X-protein (Bax), FAS, and P53 into the cytosol, forming a complex that serves as a platform to activate Caspase-9 and Caspase-3 as downstream factors of apoptosis15. The rate of activation of downstream Caspases (Caspase-9 and Caspase-3), and the inclusion of proteins such as B-cell lymphoma 2 (Bcl-2) and BclXL (B-cell lymphoma-extra-large) that inhibit apoptosis, depend on changes in the mediators related to gene and protein expressions of the aforementioned factors3,4,12,16,17.
Among these factors, P53 plays an essential regulatory role in mitochondria and metabolism in diabetic cardiomyopathy in cells under pathological conditions18,19. A growing body of evidence has demonstrated that inhibition of P53 prevents cardiac apoptosis during the initial stages of diabetes, attenuates cell degradation induced by diabetes, and improves both angiogenic and glycolytic defects20. Reports also show that P53 is a regulatory factor responsible for cardiovascular adaptation to aerobic training19. However, the potential mechanisms underlying these effects remain largely elusive.
In addition to its positive effects on insulin sensitivity, glycemic control, glucose uptake, cardiac structure, and function, aerobic training is considered a potential positive intervention recommended for diabetic patients21-26. In this context, the molecular processes that might be positively or negatively affected by aerobic training in diabetic cardiomyopathy are increasingly considered. Although several aerobic training protocols have been introduced as effective approaches in healthy individuals27-31 and people with medical conditions, there is significant diversity among the utilized aerobic training schedules, and the adaptation of oxidative stress depends on the intensity, duration, and type of recruited activity.
Therefore, the purpose of the present study was to address the probable effects of aerobic training on aerobic capacity, insulin resistance, and LV catalase, caspase-9, and P53 activity as regulators of diabetic cardiomyopathy in type 2 diabetic fatty rats. We hypothesized that aerobic training might reduce cardiac apoptosis in trained rats with type 2 diabetes via the induction of efficient enzyme and protein production.
Materials and Methods
Figure 1 presents a schematic of the testing sequence and protocols. Twenty-four male rats (10 weeks old; 170-190 g) were used in this experiment. The rats were housed in standard rodent cages with dimensions of 25×27×42 cm within a clean and well-ventilated room at the University of Tehran’s animal laboratory. The environment was maintained at a temperature of 22.1°C and adhered to a 12:12 light/dark cycle. The animals had unrestricted access to a standard diet and water. The experimental research was approved by the Animal Ethics Committee of Shahid Rajaee University of Tehran, with the reference number IR.SRTTU.SSF, 2020.129. Furthermore, all experiments were conducted in accordance with the guidelines prescribed by the Institutional Animal Care and Use Committee.
Figure 1.
The timeline and schematic protocol of the experiment
To ensure unbiased allocation, the rats were randomized and divided into three groups (each of 8): healthy non-diabetic (ND), high-fat diet groups including control diabetic (CD), and trained diabetic (TD). In the healthy control group, rodent-specific normal food was freely provided; but in the high-fat diet group, rats were given a high-fat diet for 4 weeks, containing 55% of total fat energy (derived from animal fat oil), 10% protein, and 35% carbohydrate (freely available). After 4 weeks on a high-fat diet, type 2 diabetes was induced by injecting streptozotocin (STZ) dissolved in sodium citrate buffer with pH = 4.2, at a dose of 40 mg/kg intraperitoneally1. Three days after STZ injection, blood glucose levels were evaluated, and values above 300 mg/dL were considered indicative of diabetes.
Rat height and weight were measured after a period of receiving a high-fat diet, and rats recognized as obese were matched based on body weight and fasting glucose. They were then randomly divided into two groups: diabetic aerobic exercise training and control group. For the remaining days of the study, the high-fat diet was switched to a normal diet.
Familiarization and Speed in VO 2max Test
After STZ-induced type 2 diabetes, the rats in the exercise group engaged in aerobic activity for 6 weeks. Seven days before the start of the experiment, the rats in the TD group participated in a familiarization training session on a horizontal treadmill (BIOSEB, Vitrolles, France), which included 10 minutes of running at a speed of 10 m/min. Before the beginning of the training period, running speed at maximal oxygen consumption (VO2max) was evaluated by an incremental test. The test began with a 10-minute warm-up at a speed of 5 m/min. Then, the running speed increased by 1.8 m/min every 2 minutes until the animals could no longer continue running despite an increase in speed. The correlation between running velocity and VO2max was previously investigated and validated by Hoydal et al. (2007), and the highest running speed was recorded as the speed at VO2max 32. Subsequently, the animals participated in a running program for 6 weeks, with 5 sessions per week.
Training protocol
The treadmill running program began at 65% of running speed in VO2max on first week and progressively increased to 85% of running speed in vVO2max at week 6 (65,70,70,75,75,85% running speed in VO2max, from 1st to 6th week, respectively) 5 sessions per week. Also, the training duration progressively increased by 1 min/session. During each training session, following a 3-minute warm-up at a speed of 7 m/min, the speed was increased by 1.8 m/min every minute until the desired speed for that session was achieved. After finishing the exercise, the speed was continuously reduced to reach the initial speed. Subjects repeated the incremental test at the end of 3rd week to set running speed in VO2max according to new VO2max capability. Running speed in VO2max and body weight was also measured again at the end of the workout.
Echocardiography
Rats underwent echocardiography 24 hours after the final training session, following the protocol outlined in our previous study21. To summarize the procedure briefly, rats were anesthetized using a Ketamine/Xylazine combination (1:10 ratio) and positioned in a left lateral decubitus position. M-mode and two-dimensional echocardiography (ECO) images were acquired using a 10-MHz probe from a GE-Vingmed Ultrasound system based in the USA. Left Ventricular End Diastolic Volume (LVEDV), Left Ventricular End Systolic Volume (LVESV), and the percentage of Ejection Fraction (EF %) were measured. The procedure was conducted by an operator who was blinded to the genotype, using specialized software (EchoPac v113; GE Healthcare).
Surgical Preparation and Metabolic Assessment
The animals received intraperitoneal anesthesia with pentobarbital sodium (65 mg/kg) 48 hours after the completion of the last training session. Subsequently, 2 ml of blood was collected from the left ventricle, and the heart was excised. The LV myocardium was separated from the heart, rapidly frozen in liquid nitrogen, and then stored at −80°C for later analysis of protein expression changes. To separate plasma, blood samples were centrifuged for 15 minutes at 3,000 g, and plasma samples were stored in 2 ml microtubes at −80°C for the measurement of fasting insulin and glucose levels. The insulin resistance index was calculated using the following equation:
HOMA-IR = (insulin * glucose) / 22.5
Plasma glucose levels were determined using the glucose oxidase method, and levels of insulin, Catalase, and Caspase-9 were measured using ELISA kits (Roche AS, Indianapolis, IN)33,34.
P53 Measurement and Western Blotting
Total protein was extracted from lysates obtained from the left ventricular myocardium after centrifugation at 14,000 g for 10 minutes at 4°C. The protein was separated by SDS-PAGE (12 percent) and transferred onto a nitrocellulose membrane. The membrane was then blocked with 5% dry milk and incubated overnight in a solution containing primary antibodies. In this study, Anti-P53 antibody and Anti-Beta actinin antibody (rabbit/rat; polyclonal; 1:1,000 dilution; #2772; Cell Signaling Technology, Inc., Danvers, MA, USA) were used as primary antibodies. The following day, the membrane was incubated with the secondary antibody (goat/rabbit; polyclonal; 1:1,000; A0208; Beyotime Institute of Biotechnology) for 30 minutes. Blots were imaged using a Bio-Rad gel imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Subsequently, band densities were quantified using Quantity One software, version 4.5 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analyses were performed using SPSS software version 14.0 (IBM Corp., Chicago, IL). Descriptive data are presented as mean ± SD. A two-factor mixed analysis of variance (ANOVA) with the between-factor “group” (ND, TD, and CD) and repeated factor “trial” (baseline, week 3, and post-test) was conducted to determine if changes in measured variables over time differed by intervention. The Shapiro-Wilk test was used to examine the normality of distribution, and Levene’s test assessed the homogeneity of variances. Mauchly’s W test confirmed sphericity, indicating that the sphericity assumptions for repeated measures ANOVA were met. The alpha level was set at 0.05.
Results
Table 1 presents the main effects and time-regimen interactions for the measured variables. At baseline, weight, glucose, and HOMA-IR levels were significantly higher in the TD and CD groups compared to the ND group. Over time, a significant increase in weight was observed in the ND and CD groups. However, no significant time-regimen interaction was detected for this variable.
Table 1.
Body weight, Glucose, Insulin level, and insulin resistance index (HOMA-IR) in untrained Non-Diabetic (ND), Control Diabetic (CD) and Trained Diabetic (TD) rats in pre-test, at the end of 3th week and post-test (Mean ± SD).
| Variables | Group | ||
|---|---|---|---|
| ND | TD | CD | |
| Weight (g) | |||
|
Pre
Week 3 p Post p |
227 ± 23 240 ± 19 P = 0.00 245 ± 27* P = 0.00 |
256 ± 28‡ 249 ± 30 P = 0.29 243 ± 32 P = 0.12 |
254 ± 39‡ 251 ± 29 P = 0.48 257 ± 34* P = 0.66 |
| vV O 2peak (m/min) | |||
|
Pre
Week 3 p Post p |
21.23 ± 4.9† 20.92 ± 6.1 P = 0.69 20.69 ± 4.7 P = 0.63 |
20.16 ± 5.8† 23.8 ± 3.7*’⁑’†’‡ P = 0.04 27.6 ± 5.1*’† P = 0.00 |
18.87 ± 6.6 19.13 ± 5.1 P = 0.74 19.41 ± 5.4 P = 0.61 |
| Glucose (mg/dL) | |||
|
Pre
Week 3 p Post p |
97 ± 8 105 ± 5 P = 0.46 107 ± 9 P = 0.27 |
449 ± 54‡ 432 ± 41‡’⁑ P = 0.37 367 ± 36*'†’‡ P = 0.00 |
463 ± 81‡ 459 ± 69‡ P = 0.82 468 ± 6‡ P = 0.66 |
| Insulin (μIU/ml ) | |||
|
Pre
Week 3 p Post p |
2.4 ± 0.5 2.2 ± 0.7 P = 0.31 2.0 ± 0.3† P = 0.07 |
2.5 ± 0.3 2.6 ± 0.6‡ P = 0.57 2.8 ± 0.3*'†’‡ P = 0.04 |
2.4 ± 0.4 2.3 ± 0.6 P = 0.63 2.3 ± 0.3 P = 0.53 |
| HOMA-IR | |||
|
Pre
Week 3 p Post p |
0.3 ± 0.08 0.4 ± 0.7 P = 0.13 0.6 ± 0.04* P = 0.01 |
2.3 ± 0.1‡ 1.7 ± 0.2*’⁑’†’‡ P = 0.04 1.1 ± 0.1*’†’‡ P = 0.00 |
1.8 ± 0.3‡ 2.6 ± 0.3*’‡ P = 0.03 2.7 ± 0.1*’‡ P = 0.00 |
Data are presented as means ± SD
* Significant difference compared to pre-test (P ≤ 0.05), ⁑ Significant difference compared to post-test (P≤0.05), † Significant difference compared to CD (P≤0.05), ‡ Significant difference compared to ND (P ≤ 0.05).
At baseline, a significant between-group difference was observed in vVO2peak, with the ND and TD groups showing higher values compared to the CD group. A significant time effect and time-regimen interaction (p < 0.05) were noted for this variable. Specifically, TD led to a significant change in vVO2peak from pre-training to week 3 and from week 3 to post-test (Table 1). Moreover, the increase in this variable over the first three weeks was significantly greater in the TD group compared to the ND (P = 0.02) and CD (P = 0.00) groups. Additionally, the TD group experienced a significantly greater change in vVO2peak from pre- to post-training compared to the CD group (P = 0.01).
At baseline, week 3, and post-test, both TD and CD groups exhibited notably higher glucose levels compared to the ND group (Table 1). A significant time effect and time-regimen interaction (P < 0.05) were observed in glucose levels. Specifically, the TD group experienced a significant reduction in glucose levels from pre- to post-training, with the magnitude of this change significantly greater in TD than in the CD (P = 0.66) and ND (P = 0.27) groups.
No notable between-group differences were observed for insulin levels at baseline. However, a significant time effect and time-regimen interaction (p < 0.05) were detected in insulin levels. Specifically, the TD group showed a significant change in insulin levels from pre- to post-training. Additionally, the change in this variable over the initial three weeks was significantly greater in the TD group compared to the ND group (P = 0.03). Moreover, the TD group experienced a greater change in insulin levels from pre- to post-training (P = 0.04) compared to both the CD (P = 0.53) and ND (P = 0.07) groups.
HOMA-IR levels in the TD and CD groups were significantly higher than those in the ND group at all three time points (P < 0.05). A significant time effect and time-regimen interaction were observed for this variable. Both TD and CD groups exhibited significant changes in HOMA-IR levels from pre-training to week 3, and from week 3 to post-test (Table 1). The magnitude of change in this factor from week 3 to post-test was significantly greater in the TD group compared to the CD group (P = 0.01).
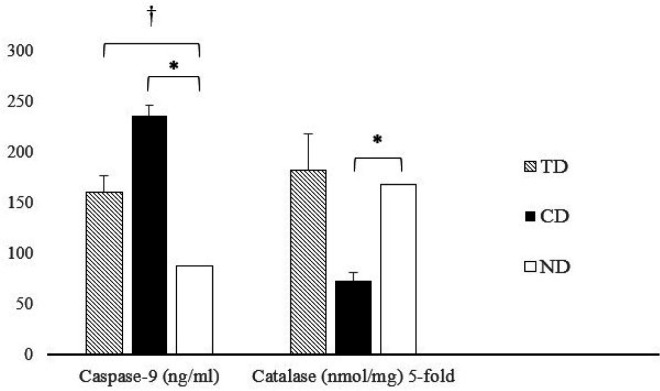
As depicted in Figure 2, Catalase activation was significantly lower in diabetic rats than in the ND group (P = 0.00). Aerobic training increased Catalase activation in diabetic rats, with no significant between-group differences. Additionally, Caspase-9 levels were significantly higher in both diabetic groups compared to ND rats (P = 0.00 for both). Furthermore, Caspase-9 levels in response to TD were significantly lower than those in the CD group (P = 0.00).
Figure 2.
The level of Caspase-9 (nanograms/milliliters) and 5-fold Catalase (nanomole/milligrams) enzymes in diabetic aerobic trained (TD), diabetic control (CD) and non-diabetic healthy rats. † significant difference compared to TD (P≤0.05); * significant difference compared to CD (P≤0.05)
Cardiac Functional Changes
Echocardiography results indicated that left ventricular end-diastolic volume (LVEDV) signifi-cantly decreased in the TD group (0.394 ± 0.12 ml) compared to the CD group (0.431 ± 0.15 ml, P = 0.02) over time. Additionally, this change was significantly lower in the ND group (0.388 ± 0.08 ml) compared to the CD group (P = 0.02).
Regarding left ventricular end-systolic volume (LVESV), findings showed a significantly greater reduction in the TD group (0.156 ± 0.18 ml) compared to the CD group (0.183 ± 0.16 ml, P = 0.02). The ND group (0.147 ± 0.11 ml) also exhibited significantly greater changes compared to the CD group (P = 0.01). Left ventricular ejection fraction (%) demonstrated significantly greater improvement in the TD group (73.8 ± 1.3%, P = 0.03) and the ND group (75.1 ± 0.8%, P = 0.04) compared to the CD group (61.7 ± 0.9%) over time.
Western Blot
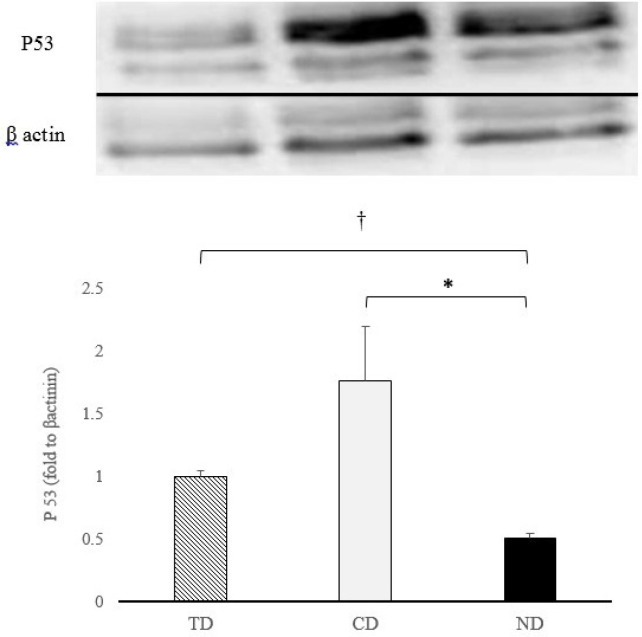
Results of western blotting test indicate that both diabetic groups resulted in significantly greater expression of P53 compared to ND group (P = 0.02 and P = 0.03 respectively). Also, the decrease in the expression of P53 in TD was significantly greater compared to CD group (P = 0.001) Figure 3.
Figure 3.
P53 expression (fold changes from β-actinin) in diabetic aerobic trained (TD), diabetic control (CD) and non-diabetic healthy rats. † significant difference compared to TD (P≤0.05); * significant difference compared to CD (P≤0.05)
Discussion
This study investigated the potential effects of aerobic training with variable intensities on apoptotic indices in the cardiac tissue of diabetic and obese rats. The findings reveal significant reductions in plasma glucose levels, insulin resistance, and the expression of Caspase-9 and P53 proteins following a 6-week aerobic training program with variable intensities. Furthermore, it is well-established that a high-fat diet and STZ-induced diabetes lead to insulin resistance and hyperglycemia.
This study is the first to examine changes in apoptotic indices resulting from aerobic training with variable intensities (ranging from 65% to 85% of running speed at VO2max) in diabetic rats. Consistent with previous research, a negligible reduction in body weight was observed despite improvements in aerobic capacity among male rats21,35 following aerobic training; the body weight of the trained diabetic rats remained statistically unchanged from pre- to post-training. However, their aerobic capacity was significantly enhanced. Conversely, several studies have shown that consistent endurance training effectively promotes weight loss36 and improves cardiorespiratory indicators in overweight individuals with type 2 diabetes 26,37.
In line with prior studies that investigated the impact of aerobic exercise training on performance and cardiorespiratory function in healthy individuals38-40 and obese diabetic subjects1,41, this study also demonstrated a notable improvement in aerobic capacity after a 6-week training program. Although the aerobic capacity did not significantly change in untrained healthy rats over time, it was markedly lower in untrained diabetic rats compared to the healthy group. The decline in cardiac function due to diabetes seems to contribute to the reduced endurance capacity observed in untrained diabetic rats compared to their healthy counterparts.
Despite hyperglycemia in both diabetic groups, trained diabetic rats exhibited significantly lower blood glucose levels and insulin resistance than untrained diabetic rats after 6 weeks of training. Additionally, insulin resistance decreased significantly after 3 weeks of training, suggesting that 3 weeks of training can initiate protective mechanisms against insulin resistance, but 6 weeks of training is more effective. This corroborates the findings of Lavie et al. (2014) and Pieri et al. (2014), which reported that aerobic endurance exercise improves glucose and insulin metabolism, upregulates Glucose Transporter-4 (GLUT-4) expression—a critical intracellular protein for glucose uptake and utilization—and reduces cardiovascular disease risks in diabetic patients16,23,42,43.
It is widely recognized that moderate endurance training regulates blood glucose in patients with type 2 diabetes by compensating for impaired energy metabolism in an insulin-deficient state (which protects pancreatic β-cells by increasing insulin-sensitive adenosine monophosphate-activated protein kinase [AMPK] expression), enhancing insulin-mediated glucose transport by increasing protein kinase C expression, promoting insulin secretion and activation, and improving insulin signaling pathway and its downstream protein expressions in the myocardium to ultimately protect cardiomyocytes43,44.
In accordance with studies by Kwak et al. (2006), Peterson et al. (2008), and Cheng et al. (2013), the current endurance training regimen led to a significant decrease in Caspase-9 and P53. However, the activation of Catalase did not differ significantly between trained and untrained diabetic groups following the training protocol44. While French et al. (2008) reported insignificant changes in Catalase in the left ventricle after endurance training, aligning with our findings9, this contrasts with reports by Kanter et al. (2017) and Naderi et al. (2015), who observed increases in the activity of antioxidant enzymes such as Catalase and reductions in oxidative stress in the hearts of streptozotocin-induced diabetic rats following endurance training1,11. The discrepancy may be due to differences in training protocols (voluntary low-intensity aerobic training vs. incremental forced aerobic training), suggesting that aerobic training plays a crucial role in stimulating antioxidant defense through the activation of anti-apoptotic antioxidants. Analogous to these protective changes, and in line with reports from Peterson et al (2009), present study revealed decreases in rate of cardiomyocytes apoptosis following endurance training by decreasing Caspase-940. Instead, Ho et al (2012) reported apoptotic effects of endurance exercise evidenced by increased Caspase-943. The differences in training protocol and the age of participants might be the reason of this controversy. It seems that aging is a potential cause of apoptosis which may negatively triggers apoptotic factors following endurance exercise.
As increasing evidence has demonstrated the role of Caspase-9 on apoptosis, this factor notably triggers the apoptotic pathways following Bax/Bcl-2 ratio regulation immediately after activation in cardiomyocytes12,16. Hyperglycemia can promote this activation by cytochrome C release to cytoplasm and triggering cascade activation of caspase-3, which lead to apoptosis of cardiomyocytes. Releasing cytochrome C together with Bax, FAS and P53 into the cytosol forms a complex serving as a platform for Caspase-9 activation as the downstream factor of apoptosis15. Therefore, Long-term hyperglycemia in diabetic patients is a strong potent to induce heart failure via increases in Caspase in diabetic patients6. The mentioned changes ultimately lead to cardiac function deterioration3,14. We assume that such a decrease in this apoptotic factor (Caspase-9) following aerobic training alleviates the vulnerable effects of hyperglycemia on cardiac tissue.
In agreement with previous studies by Cai & Kang (2003) and Gu et al. (2018), Western blot analysis showed a significant increase in P53 protein expression in both diabetic groups, while aerobic training reduced this apoptotic factor in trained rats compared to untrained ones20,43. As suggested by Gu et al. (2018), the prevention of functional and pathological heart anomalies in diabetic mice can be achieved through P53 inhibition. This suppression of P53 hinders early apoptotic cell death and prevents subsequent pathogenic effects, including increased cell senescence, impaired glycolysis, and reduced angiogenesis20.
Schwartzenberg et al. (2004) reported that the upregulation of P53 in diabetic rats may be linked to its role in impeding glucose import via the pentose phosphate pathway and disrupting glycolysis by altering the balance between glycolysis and oxidative phosphorylation. This could account for the observed increase in P53 in diabetic rats and its reduction in trained diabetic rats compared to untrained ones45.
Furthermore, due to P53’s inhibitory effects on the transcriptional activity of GLUT4 (the primary tissue-specific and insulin-sensitive glucose transporter), the suppression of P53 following endurance training may explain the lower insulin resistance index observed in trained diabetic rats compared to untrained diabetic rats.
According to findings related to cardiac function, significant decreases in LVEDV and LVESV, together with significant increases in LVEF approved the improvement in LV function in diabetic rats following aerobic training. These findings are in line with Rodriguez et al (2018) who reported cardiac functional increases in diabetic rats pertained to aerobic training46. But, reports from Gharaat et al (2019) which showed significant increase in LVEDV and LVESV after aerobic training is not in agreement with present findings21. The difference of cardiac structure following diabetes might be the reason of this contrary. It seems that aerobic training empowers cardiac tissue and promote the function of heart with increasing the ability of contractility due to more EF % in TD.
These observations support the hypothesis that aerobic training programs are as effective as non-pharmacological interventions for improving cardiac function, reducing apoptotic indices, and enhancing antioxidant capacity in diabetic individuals. The current results indicate that incorporating aerobic training into the regimen of diabetic rats may improve fundamental aerobic fitness, boost antioxidant defense mechanisms, thereby reducing cardiac tissue apoptosis, and diminishing the detrimental effects of diabetes on the heart.
Acknowledgements
We are thankful of Professor. Majid Kashef and Dr. Fereshteh Golab for those professional guidelines in various parts of the study.
Conflict of Interest
The authors declare that they have no financial associations or conflicts of interest pertaining to the material discussed in the manuscript.
Funding
The authors declare that they have no financial associations pertaining to the material discussed in the manuscript.
Author’s Contributions
MAG and HC designed the study and performed the experiments and collected data; MS contributed to the data analysis. HC done the literature search. MAG, HC and MS carefully read and approved the final version of the manuscript. MAG edited the final version of the manuscript. MAG and MS re-edited the revised version of the manuscript and prepared it for submission.
References
- 1.Kanter M, Aksu F, Takir M, Kostek O, Kanter B, Oymagil A. Effects of Low Intensity Exercise Against Apoptosis and Oxidative Stress in Streptozotocin-induced Diabetic Rat Heart. Exp Clin Endocrinol Diabetes. 2017 ;125(9):583–91. doi: 10.1055/s-0035-1569332. [DOI] [PubMed] [Google Scholar]
- 2.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972 ;30(6):595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Hua Y, Li X, Arslan IM, Zhang W, Meng G. Distinct Types of Cell Death and the Implication in Diabetic Cardiomyopathy. Front Pharmacol. 2020 ;11 doi: 10.3389/fphar.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang GG, Li W, Lu XH, Zhao X, Xu L. Taurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic rats. Croat Med J. 2013 ;54(2):171–9. doi: 10.3325/cmj.2013.54.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li GX, Jiao XH, Cheng XB. Correlations between blood uric acid and the incidence and progression of type 2 diabetes nephropathy. Eur Rev Med Pharmacol Sci. 2018 ;22(2):506–11. doi: 10.26355/eurrev_201801_14202. [DOI] [PubMed] [Google Scholar]
- 6.Joubert M, Manrique A, Cariou B, Prieur X. Diabetes-related cardiomyopathy: The sweet story of glucose overload from epidemiology to cellular pathways. Diabetes Metab. 2019 ;45(3):238–47. doi: 10.1016/j.diabet.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Rosa CM, Xavier NP, Henrique Campos D, Fernandes AA, Cezar MD, Martinez PF, et al. Diabetes mellitus activates fetal gene program and intensifies cardiac remodeling and oxidative stress in aged spontaneously hypertensive rats. Cardiovasc Diabetol. 2013 ;12:152 . doi: 10.1186/1475-2840-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrne NJ, Rajasekaran NS, Abel ED, Bugger H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radic Biol Med. 2021 ;169:317–42. doi: 10.1016/j.freeradbiomed.2021.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. FASEB J. 2008 ;22(8):2862–71. doi: 10.1096/fj.07-102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wohaieb SA, Godin DV. Alterations in free radical tissue-defense mechanisms in streptozocin-induced diabetes in rat Effects of insulin treatment. Diabetes. 1987 ;36(9):1014–8. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 11.Rasouli Mojez M, Ali Gaeini A, Choobineh S, Sheykhlouvand M. Hippocampal Oxidative Stress Induced by Radiofrequency Electromagnetic Radiation and the Neuroprotective Effects of Aerobic Exercise in Rats: A Randomized Control Trial. J Phys Act Health. 2021 ;18(12):1532–38. doi: 10.1123/jpah.2021-0213. [DOI] [PubMed] [Google Scholar]
- 12.Huang ML, Chiang S, Kalinowski DS, Bae DH, Sahni S, Richardson DR. The Role of the Antioxidant Response in Mitochondrial Dysfunction in Degenerative Diseases: Cross-Talk between Antioxidant Defense, Autophagy, and Apoptosis. Oxid Med Cell Longev. 2019 ;2019:6392763. doi: 10.1155/2019/6392763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phaneuf S, Leeuwenburgh C. Apoptosis and exercise. Med Sci Sports Exerc. 2001 ;33(3):393–6. doi: 10.1097/00005768-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Jousilahti P, Barengo NC, Qiao Q, Lakka TA, Tuomilehto J. Physical activity, cardiovascular risk factors, and mortality among Finnish adults with diabetes. Diabetes Care. 2005 ;28(4):799–805. doi: 10.2337/diacare.28.4.799. [DOI] [PubMed] [Google Scholar]
- 15.Zhou YY, Li Y, Jiang WQ, Zhou LF. MAPK/JNK signalling: a potential autophagy regulation pathway. Biosci Rep. 2015 ;35(3):e00199. doi: 10.1042/BSR20140141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo WW, Chung LC, Liu CT, Wu SP, Kuo CH, Tsai FJ, et al. Effects of insulin replacement on cardiac apoptotic and survival pathways in streptozotocin-induced diabetic rats. Cell Biochem Funct. 2009 ;27(7):479–87. doi: 10.1002/cbf.1601. [DOI] [PubMed] [Google Scholar]
- 17.Sayevand Z, Nazem F, Nazari A, Sheykhlouvand M, Forbes SC. Cardioprotective effects of exercise and curcumin supplementation against myocardial ischemia–reperfusion injury. Sport Sci Health. 2022;18(3):1011–19. [Google Scholar]
- 18.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006 ;126(1):107–20. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Kim B, Hart P, Kang M, Roth S, Brown MD, Hagberg JM, et al. Functional Study of Tumor Suppressor p53 Gene Variation: Effect on Cardiovascular Adaptation to Exercise Training. Exp Biol. 2012;26(S1):1138–45. [Google Scholar]
- 20.Gu J, Wang S, Guo H, Tan Y, Liang Y, Feng A, et al. Inhibition of p53 prevents diabetic cardiomyopathy by preventing early-stage apoptosis and cell senescence, reduced glycolysis, and impaired angiogenesis. Cell Death Dis. 2018 ;9(2):82 . doi: 10.1038/s41419-017-0093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gharaat MA, Kashef M, Jameie B, Rajabi H. Regulation of PI3K and Hand2 gene on physiological hypertrophy of heart following high-intensity interval, and endurance training. J Res Med Sci. 2019 ;24:32. doi: 10.4103/jrms.JRMS_292_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheykhlouvand M, Gharaat M, Khalili E, Agha-Alinejad H, Rahmaninia F, Arazi H. Low-Volume High-Intensity Interval Versus Continuous Endurance Training: Effects on Hematological and Cardiorespiratory System Adaptations in Professional Canoe Polo Athletes. J Strength Cond Res. 2018 ;32(7):1852–60. doi: 10.1519/JSC.0000000000002112. [DOI] [PubMed] [Google Scholar]
- 23.Lavie CJ, Johannsen N, Swift D, Sénéchal M, Earnest C, Church T, et al. Exercise is Medicine - The Importance of Physical Activity, Exercise Training, Cardiorespiratory Fitness and Obesity in the Prevention and Treatment of Type 2 Diabetes. Eur Endocrinol. 2014 ;10(1):18–22. doi: 10.17925/EE.2014.10.01.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009 ;119(25):3244–62. doi: 10.1161/CIRCULATIONAHA.109.192521. [DOI] [PubMed] [Google Scholar]
- 25.Veeranki S, Givvimani S, Kundu S, Metreveli N, Pushpakumar S, Tyagi SC. Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J Mol Cell Cardiol. 2016 ;92:163–73. doi: 10.1016/j.yjmcc.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derouich M, Boutayeb A. The effect of physical exercise on the dynamics of glucose and insulin. J Biomech. 2002 ;35(7):911–7. doi: 10.1016/s0021-9290(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Sheykhlouvand M, Khalili E, Gharaat M, Arazi H, Khalafi M, Tarverdizadeh B. Practical Model of Low-Volume Paddling-Based Sprint Interval Training Improves Aerobic and Anaerobic Performances in Professional Female Canoe Polo Athletes. J Strength Cond Res. 2018 ;32(8):2375–82. doi: 10.1519/JSC.0000000000002152. [DOI] [PubMed] [Google Scholar]
- 28.Fereshtian S, Sheykhlouvand M, Forbes S, Agha-Alinejad H, Gharaat M. Physiological and performance responses to high-intensity interval training in female inline speed skaters Apunts. Med. l’Esport . 2017;52:131–8. [Google Scholar]
- 29.Sheykhlouvand M, Gharaat M. Optimal homeostatic stress to maximize the homogeneity of adaptations to interval interventions in soccer players. Front Physiol. 2024;15:1377552. doi: 10.3389/fphys.2024.1377552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheykhlouvand M, Gharaat M, Khalili E, Agha-Alinejad H. The effect of high-intensity interval training on ventilatory threshold and aerobic power in well-trained canoe polo athletes. Sci Sports. 2016;31:283–89. [Google Scholar]
- 31.Sheykhlouvand M, Arazi H, Astorino TA, Suzuki K. Effects of a New Form of Resistance-Type High-Intensity Interval Training on Cardiac Structure, Hemodynamics, and Physiological and Performance Adaptations in Well-Trained Kayak Sprint Athletes. Front Physiol. 2022 ;13:850768. doi: 10.3389/fphys.2022.850768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Høydal MA, Wisløff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007 ;14(6):753–60. doi: 10.1097/HJR.0b013e3281eacef1. [DOI] [PubMed] [Google Scholar]
- 33.Gharaat MA, Sheykhlouvand M, Eidi LA. Performance and recovery: effects of caffeine on a 2000-m rowing ergometer. Sport Sci Health. 2020;16:531–42. [Google Scholar]
- 34.Barzegar H, Arazi H, Mohebbi H, Sheykhlouvand M, Forbes SC. Caffeine coingested with carbohydrate on performance recovery in national-level paddlers: a randomized, double-blind, crossover, placebo-controlled trial. J Sports Med Phys Fitness. 2022 ;62(3):337–342. doi: 10.23736/S0022-4707.21.12125-5. [DOI] [PubMed] [Google Scholar]
- 35.Song W, Kwak HB, Lawler JM. Exercise training attenuates age-induced changes in apoptotic signaling in rat skeletal muscle. Antioxid Redox Signal. 2006 ;8(3-4):517–28. doi: 10.1089/ars.2006.8.517. [DOI] [PubMed] [Google Scholar]
- 36.Gharaat MA, Kashef M, Jameie SB, Rajabi H. Effect of endurance and high intensity interval swimming training on cardiac hypertrophy of male rats. J Shahid Sadoughi Uni Med Sci. 2018;26(4):306–18. [Google Scholar]
- 37.Jakicic JM, Jaramillo SA, Balasubramanyam A, Banc-roft B, Curtis JM, Mathews A, et al. Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes (Lond) 2009 ;33(3):305–16. doi: 10.1038/ijo.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheykhlouvand M, Forbes SC. Aerobic capacities, anaerobic power, and anthropometric characteristics of elite female canoe polo players based on playing position. Sport Sci Health. 2017;14:19–24. [Google Scholar]
- 39.Sheykhlouvand M, Gharaat M, Bishop P, Khalili E, Karami E, Fereshtian S. Anthropometric, physiological, and performance characteristics of elite canoe polo players. Psychol Neurosci. 2015;8(2):257–66. [Google Scholar]
- 40.Samadi M, Nazem F, Gharaat MA. Designing the simulation training of taekwondo competition according to heart rate, blood lactate and rating of perceived exertion. Med dello Sport. 2014;67(4):581–92. [Google Scholar]
- 41.Peterson JM, Bryner RW, Sindler A, Frisbee JC, Alway SE. Mitochondrial apoptotic signaling is elevated in cardiac but not skeletal muscle in the obese Zucker rat and is reduced with aerobic exercise. J Appl Physiol. 2000;105(6):1934–43. doi: 10.1152/japplphysiol.00037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pieri BL, Souza DR, Luciano TF, Marques SO, Pauli JR, Silva AS, et al. Effects of physical exercise on the P38MAPK/REDD1/14-3-3 pathways in the myocardium of diet-induced obesity rats. Horm Metab Res. 2014 ;46(9):621–7. doi: 10.1055/s-0034-1371824. [DOI] [PubMed] [Google Scholar]
- 43.Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol. 2003;3(3):219–28. doi: 10.1385/ct:3:3:219. [DOI] [PubMed] [Google Scholar]
- 44.Ho TJ, Huang CC, Huang CY, Lin WT. Fasudil, a Rho-kinase inhibitor, protects against excessive endurance exercise training-induced cardiac hypertrophy, apoptosis and fibrosis in rats. Eur J Appl Physiol. 2012 ;112(8):2943–55. doi: 10.1007/s00421-011-2270-z. [DOI] [PubMed] [Google Scholar]
- 45.Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004 ;64(7):2627–33. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues B, Jorge L, Mostarda CT, Rosa KT, Medeiros A, Malfitano C, et al. Aerobic exercise training delays cardiac dysfunction and improves autonomic control of circulation in diabetic rats undergoing myocardial infarction. J Card Fail. 2012 ;18(9):734–44. doi: 10.1016/j.cardfail.2012.07.006. [DOI] [PubMed] [Google Scholar]