Abstract
BACKGROUND:
Diffuse coronary artery disease affects the safety and efficacy of percutaneous coronary intervention (PCI). Pathophysiologic coronary artery disease patterns can be quantified using fractional flow reserve (FFR) pullbacks incorporating the pullback pressure gradient (PPG) calculation. This study aimed to establish the capacity of PPG to predict optimal revascularization and procedural outcomes.
METHODS:
This prospective, investigator-initiated, single-arm, multicenter study enrolled patients with at least one epicardial lesion with an FFR ≤0.80 scheduled for PCI. Manual FFR pullbacks were used to calculate PPG. The primary outcome of optimal revascularization was defined as an FFR ≥0.88 after PCI.
RESULTS:
A total of 993 patients with 1044 vessels were included. The mean FFR was 0.68±0.12, PPG 0.62±0.17, and the post-PCI FFR was 0.87±0.07. PPG was significantly correlated with the change in FFR after PCI (r=0.65 [95% CI, 0.61–0.69]; P<0.001) and demonstrated excellent predictive capacity for optimal revascularization (area under the receiver operating characteristic curve, 0.82 [95% CI, 0.79–0.84]; P<0.001). FFR alone did not predict revascularization outcomes (area under the receiver operating characteristic curve, 0.54 [95% CI, 0.50–0.57]). PPG influenced treatment decisions in 14% of patients, redirecting them from PCI to alternative treatment modalities. Periprocedural myocardial infarction occurred more frequently in patients with low PPG (<0.62) compared with those with focal disease (odds ratio, 1.71 [95% CI, 1.00–2.97]).
CONCLUSIONS:
Pathophysiologic coronary artery disease patterns distinctly affect the safety and effectiveness of PCI. PPG showed an excellent predictive capacity for optimal revascularization and demonstrated added value compared with an FFR measurement.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04789317.
Keywords: coronary artery disease, hemodynamics, percutaneous coronary intervention
Clinical Perspective.
What Is New?
The pullback pressure gradient (PPG) standardizes the assessment of pathophysiologic coronary artery disease patterns.
PPG helps guide revascularization decisions by assessing the presence and severity of diffuse disease.
The PPG value, derived after a fractional flow reserve pullback, provides additional information to fractional flow reserve forecasting revascularization results.
What Are the Clinical Implications?
PPG identifies patients more likely to benefit from percutaneous coronary intervention.
Patients with focal coronary artery disease experience greater fractional flow reserve improvement and lower rates of periprocedural myocardial infarction compared with those with diffuse coronary artery disease.
A randomized clinical trial is warranted to assess the benefit of a PPG-guided percutaneous coronary intervention strategy.
Editorial, see p 598
In stable patients with obstructive coronary artery disease (CAD), the primary goal of revascularization is to improve myocardial blood flow. However, a sizable proportion of patients remains with a low fractional flow reserve (FFR) despite angiographically successful percutaneous coronary intervention (PCI). A low FFR after PCI is associated with a worse prognosis.1 Furthermore, the magnitude of FFR improvement after PCI tracks directly with angina relief.2 Therefore, the ability to predict the potential benefits of PCI in terms of final vessel physiology carries substantial diagnostic and therapeutic implications.
FFR measurement captures total pressure loss along the coronary artery. An adjunct pullback maneuver spatially localizes pressure gradients and allows recognition of diffuse atherosclerotic patterns. The pullback pressure gradient (PPG) has emerged as an objective metric for characterizing pressure loss patterns on a continuous scale ranging from 0=diffuse to 1=focal.3 PPG may allow for the prediction of improvement in blood flow with PCI before intervention. Initial studies indicated that PCI might be more effective in patients with focal disease.4,5
We assessed the potential of PPG to predict optimal revascularization (defined as a post-PCI FFR ≥0.88) and investigated its influence on treatment decisions and procedural outcomes. The overarching hypothesis was that PCI would be more effective in vessels with high PPG, indicative of focal disease.
METHODS
PPG Global (Pullback Pressure Gradient Global Registry; URL: https://www.clinicaltrials.gov; Unique identifier: NCT04789317) was a prospective, investigator-initiated, multicenter, international, single-arm study. Its design and rationale have been published previously.6 Patients ≥18 years of age who had stable CAD or had experienced an acute coronary syndrome (ACS) with nonculprit lesions were candidates for inclusion in the study. Eligible patients had epicardial angiographic stenosis intended to be treated with PCI. For inclusion, lesions had to be defined as hemodynamically significant on the basis of an FFR ≤0.80. Patients with acute myocardial infarction (MI), ejection fraction <30%, estimated glomerular filtration rate <30 mL/min/1.73 m2, aorto-ostial lesions, severe vessel tortuosity, or planned 2-stent bifurcation PCI were excluded. Every participant gave written informed consent, and every site received approval from its local institutional review board. Tables S1 and S2 detail the study leadership and committee composition and participating sites. An independent clinical events committee adjudicated adverse events, blinded to the invasive data. An external core laboratory centralized data collection and analyzed imaging and physiologic data. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Invasive Physiology Procedure
Coronary angiography was acquired in 2 views of ≥30 degrees apart after injection of 100 to 200 μg of intracoronary nitroglycerin. A coronary wire equipped with a distal pressure sensor (PressureWire X; Abbott Vascular) was introduced into the target vessel after pressure equalization at the tip of the guiding catheter. The pressure wire was positioned in the distal coronary artery in a segment ≥2 mm and ≥15 mm beyond the most distal stenosis by visual estimation. Wire position was recorded during a contrast injection. A standardized physiologic assessment was performed, including measurements of nonhyperemic pressure ratios, distal FFR, and a manual pullback during hyperemia induced by a pharmacologic agent that ensured sufficient hyperemic plateau (Table S3). During study initiation, operators received training on the pullback maneuver, which involved manual withdrawal of the pressure wire at a constant speed over 20 to 30 seconds. When the pressure sensor reached the catheter tip, the pullback recording was stopped, and PPG was calculated onsite using CoroFlow v3.5.1 software (Coroventis Research AB). Calculation of PPG involves the integration of 2 measures derived from the FFR pullback curves: the maximal pressure gradient observed >20% of the pullback duration and the extent of functional disease. This integration of these measures results in a numeric value ranging from 0 to 1. PPG values nearing 1.0 are indicative of focal disease, whereas values approaching 0 signify diffuse CAD.3 FFR pullbacks after PCI were analyzed to derive the residual PPG, defined as the maximal focal pressure gradients in FFR units in 20% of the pullback duration.7
Before performing the PPG assessment, operators answered a dedicated questionnaire about the anticipated PCI strategy to assess the effect of the PPG on decision-making. On the basis of the PPG value, operators could opt for medical therapy or coronary artery bypass graft (CABG) surgery instead of PCI. The change in decision-making to CABG or medical therapy after a comprehensive physiologic assessment with FFR and PPG was left to the operator’s discretion. In PCI cases, the procedure was performed according to the operator’s discretion, with use of everolimus-eluting stents encouraged (Xience drug-eluting stent; Abbott Vascular). After PCI, nonhyperemic pressure ratio and FFR were measured at the same anatomic location as before PCI, and a post-PCI FFR pullback was repeated with visual coregistration of the stent position on the pressure tracing. Physicians were allowed to optimize PCI on the basis of post-PCI physiology. Additional measurements of coronary flow reserve before and after PCI were encouraged.
Core Laboratory Analysis
All angiographic and physiologic data underwent centralized, independent review at the CoreAalst core laboratory. Quantitative coronary angiography was performed through 2 views using 3-dimensional quantitative coronary angiography with CAAS 8.2 software (Pie Medical Imaging). Offline evaluation of physiology tracings was conducted using CoroFlow software (Coroventis Research AB). The physiology core laboratory assessed each recording for quality following predefined criteria, including an examination of the aortic and coronary pressure tracings for any signs of waveform distortion or loss, aortic pressure ventricularization, and the presence of limiting arrhythmias. A binary decision was made for each tracing, determining its suitability for inclusion.
Hyperemic pullback curves were scrutinized for artifacts and the extent of pressure drift, and any drift <0.05 was considered acceptable and algorithmically corrected. All tracings were reviewed by an experienced physician specializing in physiology measurements. During the initial cases conducted at each site, prompt feedback was provided to ensure high quality of the physiologic data. In addition, weekly case review meetings were conducted throughout the execution of the study for continued education.
Symptoms and Clinical Outcomes
Symptoms before PCI were collected using the 7-item Seattle Angina Questionnaire (SAQ-7) containing 3 domains: angina frequency, physical limitation, and quality of life. Higher scores indicate better health status.8 A score of 100 in the angina frequency domain denoted freedom from angina, and scores <60 defined severe angina. The SAQ-7 questionnaire will be re-administered after 1 year.
Target vessel failure (TVF) was defined as cardiac death, target-vessel MI, or ischemia-driven target vessel revascularization. Periprocedural MI was defined according to the Fourth Universal Definition of Myocardial Infarction.9 Troponin measurements were collected from 4 to 24 hours after PCI. Results are reported here as a normalized ratio between the value and its established normal threshold specific to each local troponin assay, expressed as multiples beyond the upper limit of normal (ULN) and specifically categorized as ≥5×ULN, ≥35×ULN, and ≥70×ULN. In this article, we present in-hospital clinical outcomes; clinical follow-up will be performed for up to 3 years. An independent clinical events committee blinded to the invasive data adjudicated adverse events.
Objectives
The primary objective was to determine the predictive capacity of PPG for optimal functional revascularization, defined as a post-PCI FFR ≥0.88.10 The key secondary end points addressed the influence of PPG on clinical decision-making in patients intended to be treated with PCI and assessed the effect of PPG on clinical outcomes.
Statistical Analysis
Analyses were performed using R version 4.3.1 (R Foundation for Statistical Computing) using standard statistical techniques; applicable tests were 2-tailed, and P<0.05 was considered statistically significant.
The median PPG value of 0.62 was used for the main analysis to categorize vessels as predominantly focal and diffuse disease. For the primary objective (ie, to evaluate the predictive capability of PPG to achieve a post-PCI FFR of ≥0.88), this cutoff was predefined on the basis of previous randomized clinical trials assessing the prognostic capacity of post-PCI FFR for clinical outcomes.10 Sensitivity analyses with different post-PCI FFR cutoffs were performed. The area under the receiver operating characteristic curve (AUC) method adjusted for epicardial vessel and baseline FFR was used to assess the predictive capacity of PPG to predict post-PCI FFR. The optimal PPG cutoff was derived from the Youden index. In addition, PPG cutoffs were explored using positive and negative likelihood ratios (LRs). We also report the results after dividing the cohort according to the PPG cutoff at a LR+ of 5 and a LR− of 0.40 for achieving optimal revascularization.11 For group comparisons, we used a univariate mixed-effects logistic regression model, where the dependent variable is PPG dichotomized as focal (PPG ≥0.62) or diffuse (PPG <0.62), and the independent variable is the angiographic, physiologic, or procedural characteristic. We used a mixed-effects model with a random intercept at the patient level to account for clustering of vessels within patients. In addition, to determine the capability of PPG to predict the post-PCI FFR value (on a continuous scale), linear regression models were built using PPG, epicardial vessel, and pre-PCI FFR as variables. For the development of the prediction model for post-PCI FFR, calibration of the predicted post-PCI FFR from PPG was internally trained in a derivation cohort (n=524 [60%]), and then evaluated in a validation cohort (n=367 [40%]); cohorts were selected using random sampling.
RESULTS
Demographic and Procedural Data
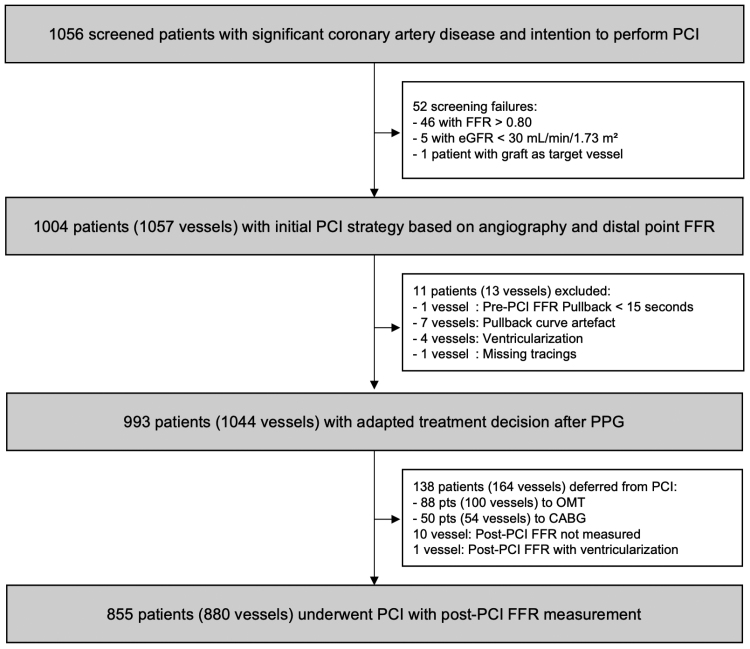
Between December 2020 and September 2023, 1004 patients (1057 vessels) were enrolled. Invasive physiologic assessments were performed in 1057 of 1057 (100%) and 880 of 890 (99%) vessels before and after PCI, respectively (Figure 1). Table 1 shows baseline clinical characteristics in the overall population stratified by CAD pattern. Mean age was 68±10 years, 24% of the patients were women, and 29% of the patients had diabetes. Clinical presentation was predominantly stable angina (89%). Patients with focal disease reported greater physical limitation, experienced angina more frequently, and reported a lower quality of life compared with patients with diffuse disease (Table S4). Twenty-four percent of patients reported severe angina, without difference between focal and diffuse patterns (25% versus 22%; P=0.359).
Figure 1.
Study flow chart. CABG indicates coronary artery bypass graft; eGFR, estimated glomerular filtration rate; FFR, fractional flow reserve; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; and PPG, pullback pressure gradient.
Table 1.
Baseline and Clinical Characteristics Stratified by PPG
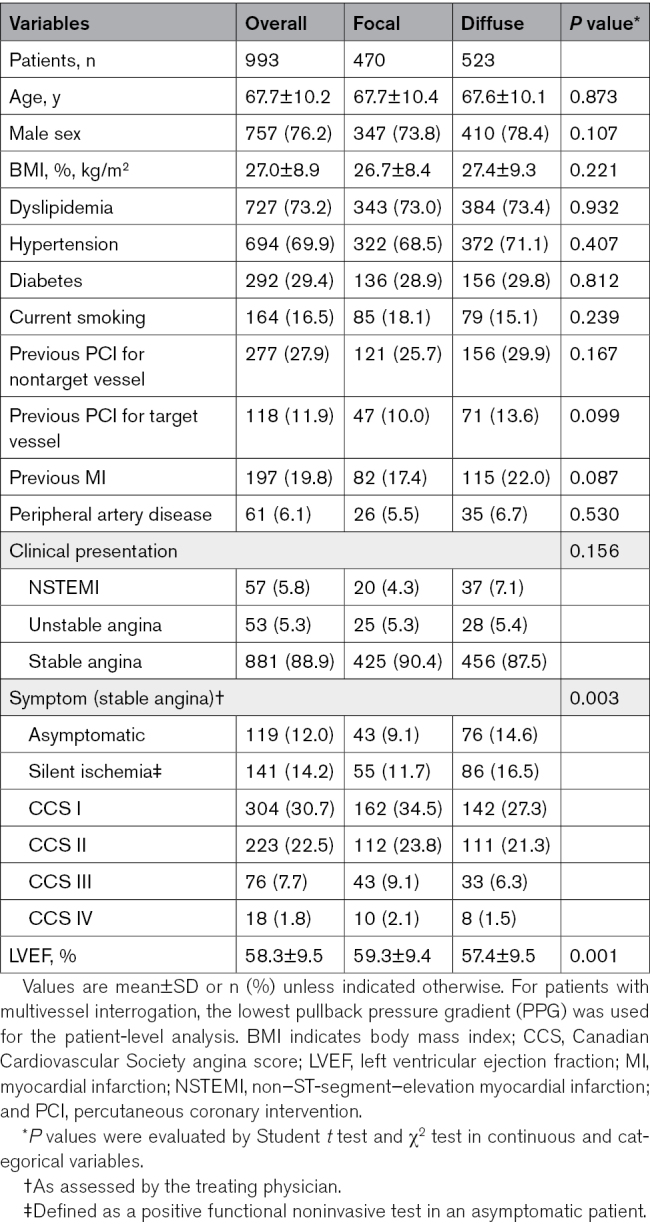
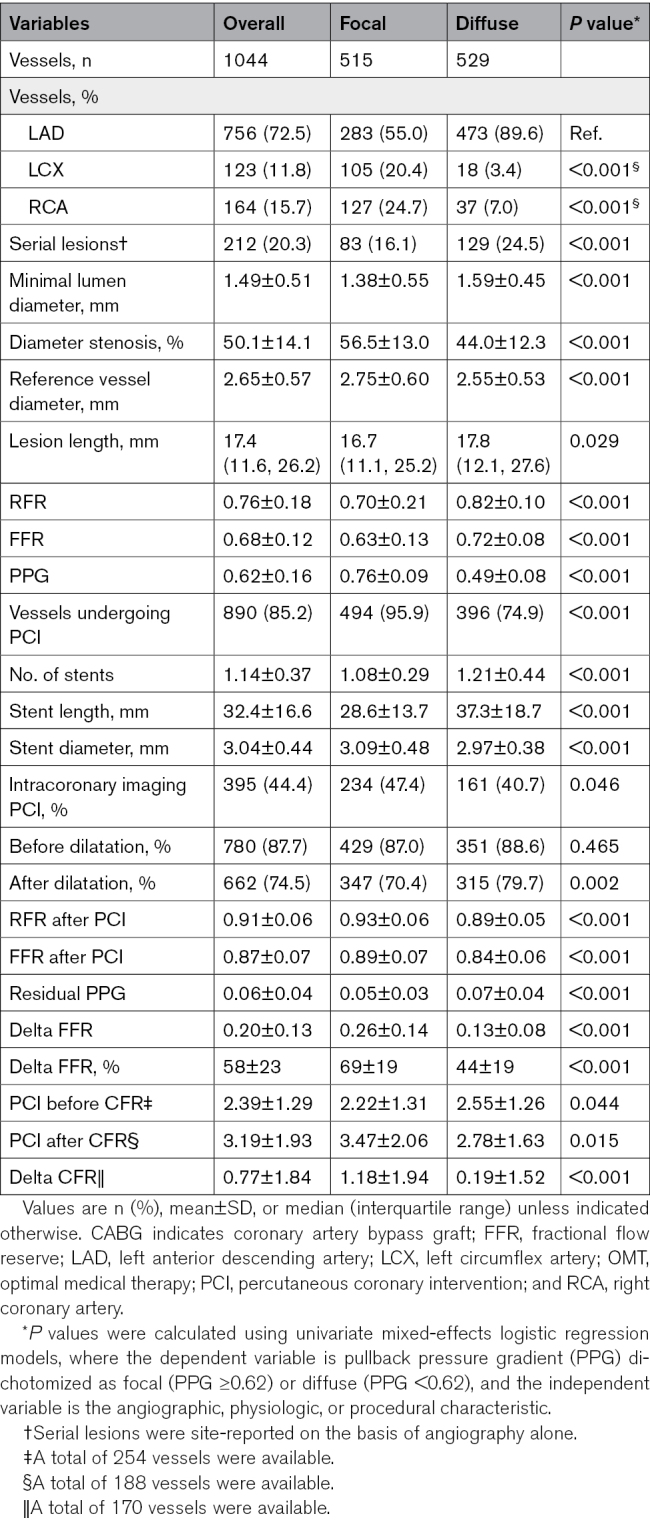
Table 2 shows angiographic, physiologic, and procedural characteristics. The left anterior descending artery was the most frequently assessed vessel (73%). In the overall population, mean diameter stenosis and reference vessel diameter were 50±14% and 2.7±0.6 mm; vessels with focal disease had more severe stenosis and larger reference size compared with diffuse disease. Vessels with diffuse disease were treated with more, smaller-diameter, and longer stents than vessels with focal CAD (P<0.001 for all).
Table 2.
Angiographic, Physiologic, and Procedural Characteristics Stratified by PPG
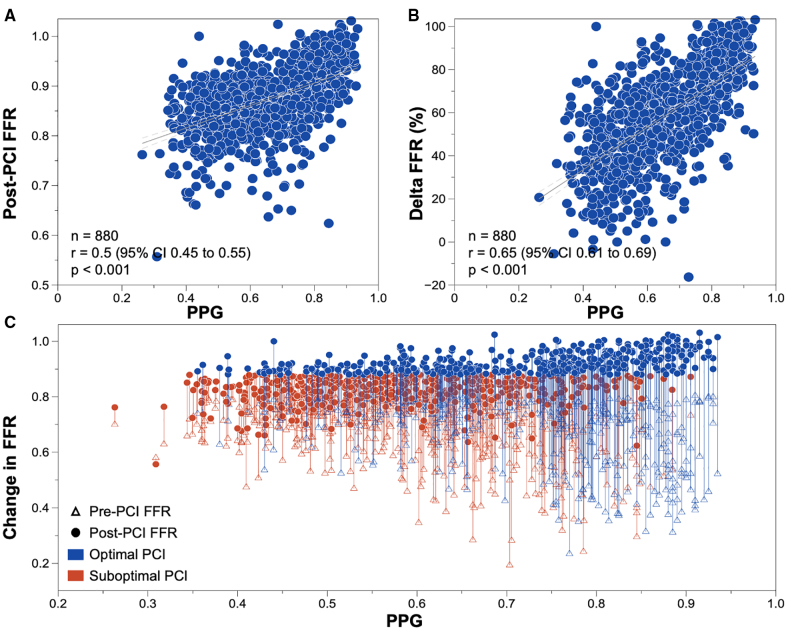
Baseline FFR was lower in vessels with focal disease. Overall, FFR increased from 0.68±0.12 to 0.87±0.07. PPG exhibited significant and moderate correlations with both post-PCI FFR and the change in FFR (Figure 2). PPG showed a weak correlation with pre-PCI FFR (Figure S1). Figure S2 shows the correlations between PPG and angiographic measures. PCI of vessels with focal disease achieved greater FFR improvements compared with diffusely diseased vessels (Δ=0.26±0.14 versus Δ=0.13±0.08; P<0.001) and a higher final FFR (0.89±0.07 versus 0.84±0.06; P<0.001). PPG also correlated with changes in coronary flow reserve after PCI, with a larger improvement in coronary flow reserve observed in focal disease (Figures S3 and S4).
Figure 2.
Correlation between pullback pressure gradient and fractional flow reserve before and after percutaneous coronary intervention. A, Relationship between pullback pressure gradient (PPG) and fractional flow reserve (FFR) after percutaneous coronary intervention (PCI). B, Relationship between PPG and delta FFR (%): ([post-PCI FFR – pre-PCI FFR]/[1 – pre-PCI FFR]). C, Relationship between PPG and change in FFR after PCI; the triangles indicate the FFR at baseline, and the circles the post-PCI FFR. The length of the lines displays the change in FFR. The blue lines indicate PCI, where FFR after PCI was ≥0.88, and the red lines where post-PCI FFR was <0.88. P values were derived from Pearson correlation.
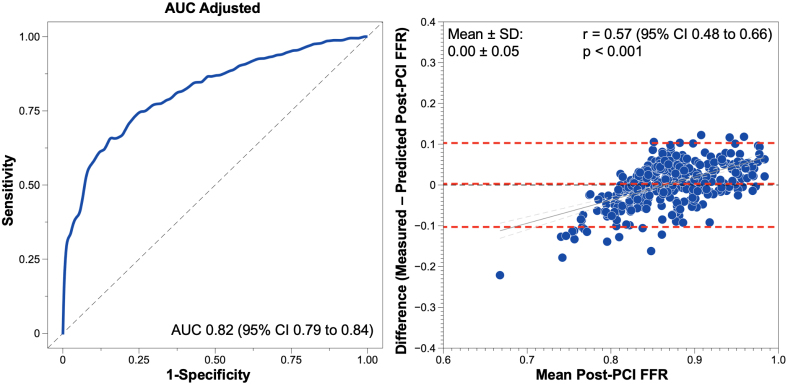
PPG showed an excellent capacity to predict post-PCI FFR ≥0.88 with an AUC of 0.82 (95% CI, 0.79 to 0.84); an optimal PPG cutoff was 0.73 (Figure 3). Figure 4 shows case examples of focal and diffuse disease before and after PCI. The predictive capacity stratified by different post-PCI FFR cutoffs is shown in Table S5. PPG cutoffs at an LR+ of 5 and an LR− of 0.40 were 0.73 and 0.50, respectively (Table S6). Post-PCI FFR and changes in FFR stratified by these cutoffs are shown in Figure S5. FFR alone did not predict revascularization outcomes (AUC, 0.54 [95% CI, 0.50–0.57]). Suboptimal FFR (<0.88) after an angiographically successful PCI occurred in 471 vessels (53.5%) and was significantly higher in patients with diffuse disease (37.1% versus 74.0%; P<0.001; Figure S6). In the post-PCI FFR pullback evaluation, the mean residual PPG was 0.06±0.03 and was lower after PCI in vessels with focal disease (0.05±0.03 FFR units in focal disease versus 0.07±0.04 FFR units in diffuse disease; P<0.001; Figure S7). Residual PPG was not associated with adverse events (Table S7).
Figure 3.
Predictive capacity of pullback pressure gradient for fractional flow reserve after percutaneous coronary intervention. Left, The predictive capacity of pullback pressure gradient for predicting a fractional flow reserve (FFR) after percutaneous coronary intervention (PCI) ≥0.88. Right, the mean difference between the prediction of FFR in the validation cohort derived from the pullback pressure gradient regression analysis and measured post-PCI FFR. AUC indicates area under the receiver operating characteristic curve.
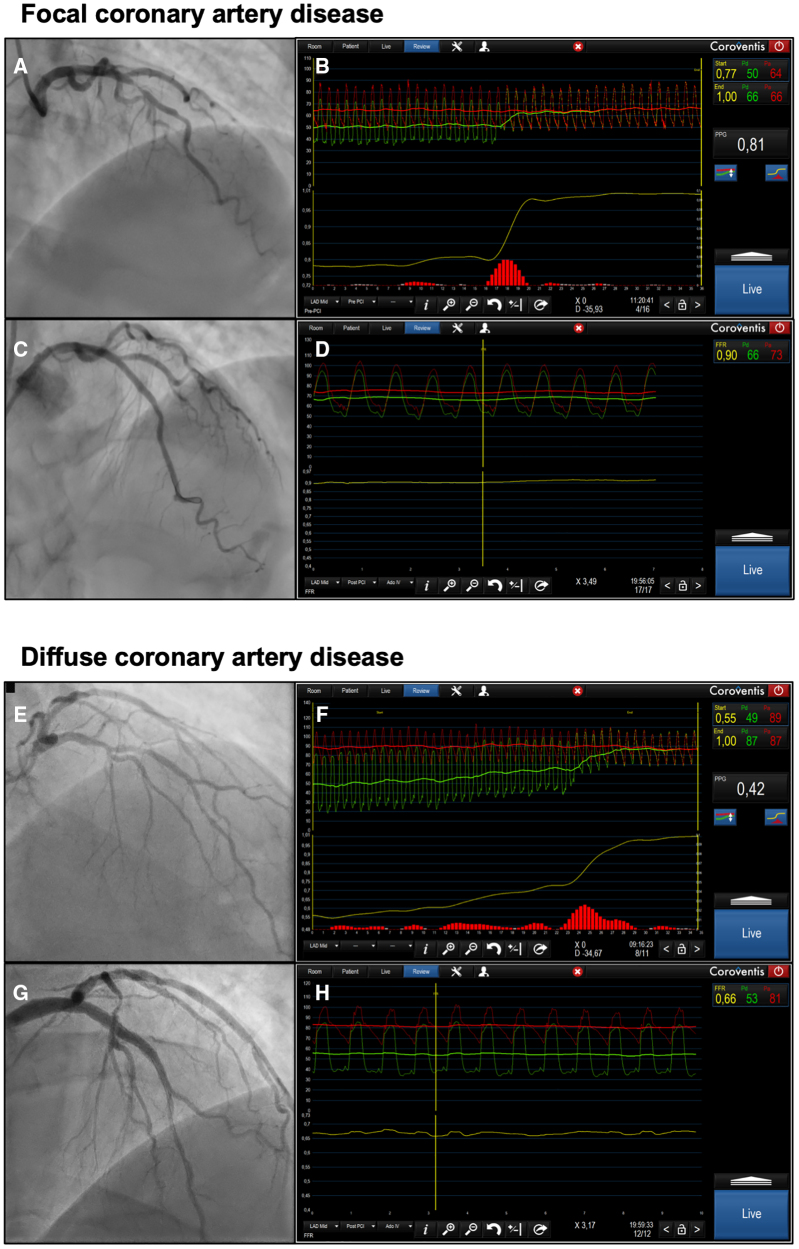
Figure 4.
Case examples of focal and diffuse coronary artery disease. A, Angiogram of a left anterior descending artery with a lesion in the mid segment. B, Fractional flow reserve (FFR) with a pullback before percutaneous coronary intervention (PCI). This case had an FFR of 0.77 with a pullback pressure gradient of 0.81. C, Angiogram after PCI. D, Post-PCI FFR of 0.90. E, Angiogram of a left anterior descending artery with a lesion in the mid segment. F, FFR of 0.55 with a pullback pressure gradient of 0.42, pointing at diffuse coronary artery disease. G, Post-PCI angiogram. H, Post-PCI FFR of 0.66.
The model based on PPG, vessel type, and baseline FFR to predict the absolute post-PCI FFR value showed a mean difference of 0±0.05 FFR units compared with invasive post-PCI FFR (Figure 3). Clinical, angiographic, and functional characteristics were well balanced between the training and validation cohorts (Table S8). Prediction of post-PCI FFR remained unchanged in different clinical presentations (stable CAD versus ACS) and in the presence of serial lesions (Figure S9).
Clinical Decision-Making
In the overall cohort of patients intended to be treated by PCI, PPG altered treatment decisions in 138 patients (13.9%), leading to CABG referral in 50 (5.0%) and medical management in 88 (8.9%). Changes in treatment decisions occurred more frequently after detection of diffuse disease with PPG (4% in focal versus 25% in diffuse disease; P<0.001). PPG was 0.65±0.15 in the PCI cohort versus 0.51±0.13 in patients referred to CABG versus 0.48±0.11 in patients managed medically (P<0.001; Figure S9). Whereas PPG was similar in patients referred to CABG or deferred to medical therapy (P=0.139), FFR was lower in patients referred to surgical revascularization (P<0.001).
In-Hospital Clinical Outcomes After PCI
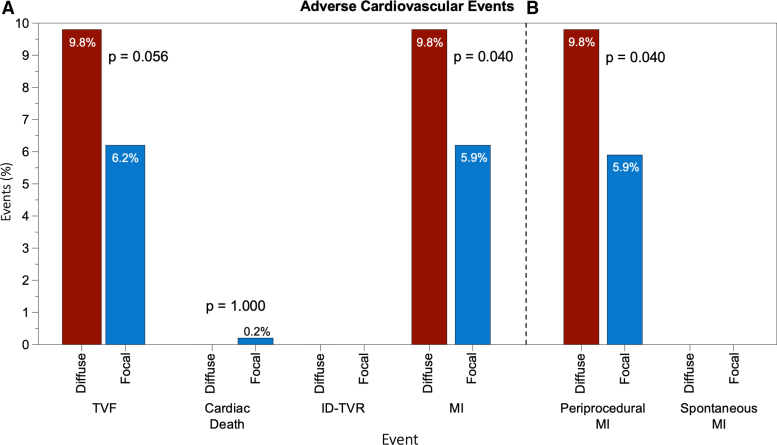
A total of 855 patients (455 with focal and 400 with diffuse disease) underwent PCI. The clinical characteristics stratified by disease pattern are shown in Table S9. The rate of in-hospital TVF was similar between patients with focal versus diffuse disease (6.2% versus 9.8%; P=0.056; Figure 5). Target vessel MI was significantly higher in patients with diffuse disease (odds ratio, 1.71 [95% CI, 1.00–2.97]); this was driven entirely by a higher incidence of periprocedural MI (5.9% versus 9.8%; P=0.040). Table S10 shows the rate of each component of TVF stratified by PPG. Baseline characteristics and procedural and clinical outcomes stratified by PPG tertiles are shown in Tables S11 and S12.
Figure 5.
In-hospital clinical outcomes after percutaneous coronary intervention in patients with predominantly focal or diffuse disease on the basis of pullback pressure gradient. Patients were stratified on the basis of the median pullback pressure gradient value of 0.62 into predominantly focal or diffuse disease. A, Incidence of target vessel failure (TVF) and its components. B, Rate of target vessel myocardial infarction (MI) stratified by periprocedural or spontaneous MI. The incidence of periprocedural MI was significantly higher in patients with diffuse disease. P value was calculated using the Fisher exact test. ID-TVR indicates ischemia-driven target vessel revascularization.
DISCUSSION
This prospective, large-scale, multicenter study of PPG offers several insights into the clinical relevance of applying coronary physiology in a novel way to differentiate focal from diffuse disease. First, PPG discriminates patients who will have optimal functional revascularization from those who will have a suboptimal FFR after PCI, and FFR alone did not predict revascularization outcomes. Second, patients with predominantly focal disease defined by a PPG >0.62 achieved higher final FFR values after PCI compared with those with diffuse disease. Third, PPG before intervention predicted post-PCI FFR accurately. Fourth, the measurement of PPG in patients already planned for PCI changed the revascularization decision in 1 out of 7 patients. Fifth, PCI of vessels with focal disease was associated with a lower rate of periprocedural MI than with diffuse disease.
Pressure-Derived Epicardial Physiology
Coronary physiology has been mainly used to define hemodynamic lesion severity. Measurement of one distal FFR value provides information on the perfusion of the underlying myocardial territory expressed on a scale from 0 to 1. A pullback maneuver complements this evaluation by adding the spatial distribution of abnormal epicardial resistance, which PPG quantifies, and it is also expressed on a scale from 0 to 1. Hence, FFR assesses the severity of epicardial resistance, whereas PPG portrays its spatial distribution. The 2 indices, both derived from intracoronary pressure measurements, are therefore highly complementary. PPG, in addition to FFR, adds a second dimension to epicardial coronary physiology. FFR helps determine the need for revascularization, whereas PPG offers insight into the potential outcomes of PCI.
From a practical standpoint, obtaining PPG can be seamlessly integrated into the same measurement procedure as FFR by performing a manual pullback. The extra time required to gather this additional information is ≈30 seconds, and the results have been shown to be highly reproducible.12 In the current study, PPG values spanned from 0.25 to 0.95, with a median of 0.62, which was used to distinguish focal from diffuse CAD in this analysis. In addition, we used LRs to derive additional PPG cutoffs and demonstrated that physiologic and clinical outcomes were progressively improved in patients with PPG >0.73 and worse in patients with PPG <0.50. Nonetheless, despite the AUC and LR analyses suggesting that PPG thresholds were associated with procedural outcomes, PPG should be interpreted as a continuous metric, with lower values associated with lower PCI clinical success rates and higher values associated with higher blood flow improvement and related to nearly complete resolution of angina.3,5 The long-term follow-up of this cohort with the collection of clinical and patient-reported outcomes will further inform about PPG cutoffs for clinical decision-making.
Clinical Use of PPG
For patients with hemodynamically significant stenoses, PPG identified the subset of patients for whom PCI will yield its most favorable outcomes. Vessels with high PPG achieved higher post-PCI FFR and larger delta FFR. The pattern of CAD, as quantified by PPG, significantly influenced the change in FFR after PCI (R2=0.42). In other words, the improvement in blood flow after PCI was partly determined by the baseline pathophysiologic disease pattern. This holds prognostic significance because a low post-PCI FFR independently predicts clinical prognosis. In the FAME studies (Fractional Flow Reserve versus Angiography for Multivessel Evaluation), patients with post-PCI FFR <0.88 had significantly higher rates of adverse events compared with those with higher post-PCI FFR.10,13 Nevertheless, post-PCI FFR remains a surrogate marker, necessitating clinical follow-up to establish its association with an increased risk of adverse events. The current findings are in line with previous studies in which PCI of hemodynamic focal disease resulted in larger FFR improvement, higher FFR, reduced ischemia, and less angina compared with vessels with diffuse disease.4,5,14 In the longer term, studies using angiography-derived FFR have shown that the risk of TVF after angiographically successful PCI is determined by the physiologic distribution of coronary atherosclerosis before PCI. In the study by Shin et al,15 patients with low angiography-derived PPG, indicative of diffuse disease, had a significantly higher risk of TVF compared with those with predominant focal disease. This segregation of CAD phenotypes using physiology before intervention may facilitate better patient selection and improve outcomes with revascularization.1,16
After PCI, the residual PPG was higher in vessels with baseline diffuse disease, confirming previous observations and suggesting the potential for further improvement in epicardial vessel conductance after PCI in diffuse disease.7 The use of coronary physiology targeting residual pressure gradients for PCI optimization is being investigated in randomized clinical trials (INSIGHTFUL-FFR [Pressure Microcatheter vs Pressure Wire for Clinical Decision Making and PCI Optimization; URL: https://www.clinicaltrials.gov; Unique identifier: NCT05437900] and DEFINE GPS [Distal Evaluation of Functional Performance With Intravascular Sensors to Assess the Narrowing Effect: Guided Physiologic Stenting; URL: https://www.clinicaltrials.gov; Unique identifier: NCT04451044]).
Forecasting PCI Results and Patient Selection
The expansion of coronary physiology toward predicting PCI results will likely influence the contemporary management of CAD. The current study demonstrated an excellent predictive capacity of PPG for optimal post-PCI physiology, with a PPG cutoff of 0.73. PPG cutoffs predictive of symptom improvement and clinical outcomes will be derived from long-term data collection. Post-PCI FFR can be forecast using several tools (eg, the PCI planner derived from coronary CT angiography, angiography-derived software, and invasively with an instantaneous wave-free ratio system).17–19 All these approaches are based on the potential physiologic effects of stent implantation.20 In the current study, PPG predicted the absolute post-PCI FFR value without bias and with an acceptable precision (mean difference of 0 with SD of 0.05 FFR units compared with invasively measured FFR); however, there was a trend to higher differences between predicted and measured in lower post-PCI FFR values. Vessel-level prediction of outcomes may avoid unnecessary procedures when the expected benefit of the intervention is low, and may also be useful in the consenting and shared decision-making process. In this study, we observed that in patients with hemodynamically significant disease intended to be treated by PCI, knowledge of PPG changed the initial clinical decision in 1 out of 7 patients. Patients referred for CABG or managed medically had a significantly lower PPG than those treated with PCI. Patients referred to surgery had lower FFR and higher symptom burden than those managed medically, even with comparable PPG values. In patients with diffuse disease, van Beek and colleagues21 found no discernible difference in clinical outcomes between those treated with CABG and those managed medically over a 2-year follow-up. Another study indicated investigating outcomes of CABG showed a significantly lower patency rate of the internal mammary artery in cases with baseline diffuse functional disease.22 Plaque composition in patients with diffuse disease appears to be more stable, atherosclerosis being primarily of a calcific nature.23 The current study also demonstrates that at baseline, patients with diffuse disease had less angina burden and a better quality of life compared with those with focal disease. These findings collectively suggest that medical therapy may be an acceptable initial strategy for managing patients with diffuse disease, reserving revascularization for individuals with persistent symptoms despite medical therapy. However, determining the optimal approach for treating diffuse CAD requires further investigation. The introduction of PPG holds promise for standardizing its diagnosis and facilitating future trials in this domain.
Considering clinical translation, the results of this study indicate that in patients with CAD scheduled for invasive management, PPG guidance may optimize revascularization decisions, improve the benefit–risk ratio for periprocedural MI, and improve clinical outcomes. Therefore, a randomized clinical trial is warranted to compare the safety and effectiveness of a PPG-guided PCI approach.
Limitations
The study has several limitations. First, the inclusion criteria were based on the decision of the operator to perform PCI; therefore, these results do not apply to patients with extensive, diffuse multivessel disease. Second, the study was not powered to detect differences in clinical outcomes, and thus these findings should be interpreted as hypothesis-generating. Moreover, the differences in clinical outcomes were sensitive to the PPG cutoff used. Third, no PPG threshold was offered at the start of the study to guide clinical decision-making. A PPG cutoff should be derived from patient-reported or clinical outcomes; follow-up of this cohort is planned for up to 3 years to address this question. Fourth, we used FFR after PCI as a metric of optimal revascularization. Although this definition is supported by many studies, it does not address other morphologic aspects of PCI that can also be used to define optimal PCI, such as stent expansion, also linked to prognosis.24 Fifth, operators were trained to perform manual pullbacks with the recommendation of manual wire withdrawal at a constant speed for 20 to 30 seconds; however, variable pullback speed may influence the pullback morphology and PPG value. Sixth, an extended follow-up period is essential to assess the benefit of the alterations in patient management prompted by the PPG.
Conclusions
Pathophysiologic CAD patterns (ie, focal or diffuse) distinctly affect the safety and effectiveness of PCI. Intervention in focal disease, characterized by high PPG values, was associated with improved hemodynamic outcomes and reduced MI compared with vessels with low PPG values. In cases with hemodynamically significant lesions, quantifying the PPG index before intervention makes it possible to predict which patients will achieve optimal revascularization on the basis of coronary physiology. Further investigation through a randomized trial is warranted to explore the potential advantages of a PPG-guided PCI strategy.
ARTICLE INFORMATION
Sources of Funding
This study was sponsored by the Cardiovascular Research Institute Aalst with an unrestricted grant from Abbott Vascular.
Disclosures
Dr Collet reports receiving research grants from Biosensors, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, and Abbott Vascular; consultancy fees from HeartFlow, OpSens Medical, Abbott Vascular, and Philips Volcano; and has patents pending on diagnostic methods for coronary artery disease. Dr Munhoz reports a research grant provided by the CardioPath PhD programme and speaker fees from Abbott Vascular. Dr Mizukami reports receiving research grants from Boston Scientific and speaker fees from Abbott Vascular, CathWorks, and Boston Scientific. Dr Matsuo has received consulting fees from Kaneka and Zeon and speaker’s fees from Abbott Medical Japan, Boston Scientific, Philips, and Amgen. Dr Ko has received consulting fees from Canon Medical, Abbott, and Medtronic. Dr Biscaglia received research grants provided by Sahajanand Medical Technologies, Medis Medical Imaging, Eukon Srl, Siemens Healthineers, General Electric Healthcare, and Insight Lifetech. Dr Engstrøm reports speaker and advisory board fees from Abbott, Boston Scientific, and Novo Nordisk. Dr Leone reports receiving consultancy fees from Abbott and honoraria for sponsored symposia from Abbott, Medtronic, and Abiomed. Dr Fearon receives institutional research support from Abbott, Boston Scientific, and Medtronic and has consulting relationships with CathWorks and Siemens and stock options from HeartFlow. Dr Christiansen has received consulting fees from Abbott Medical Denmark A/S. Dr Yong has received minor honoraria from Abbott Vascular and research grants from Abbott Vascular and Philips. Dr Escaned is supported by the Intensification of Research Activity project INT22/00088 from the Spanish Instituto de Salud Carlos III and received speaker and advisory board member fees from Abbott and Philips. Dr Storozhenko reports a grant provided by the EAPCI Fellowship Programme. Dr West is an employee of Abbott Vascular. Dr De Potter is a paid consultant for Biosense Webster and receives grant support (institutional) and consultancy fees (institutional) from Abbott. Dr Berry receives research funding from the British Heart Foundation (grants RE/18/6134217, BHF/FS/17/26/32744, and PG/19/28/34310) and is employed by the University of Glasgow, which holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Coroventis Research, GlaxoSmithKline, HeartFlow, Menarini, Novartis, Servier, Siemens Healthcare, and Valo Health. Dr Collison has received consulting fees from Abbott. Dr Johnson has received consultancy or speaker fees from Abbott Vascular, Boston Scientific, Medtronic, Shockwave, and Terumo, and research grants from Abbott Vascular. Dr Amano reports receiving lecture fees from Astellas Pharma, Astra Zeneca, Bayer, Daiichi Sankyo, and Bristol Myers Squibb. Dr Perera has received research grant support from Abbott Vascular, HeartFlow, and Philips. Dr Jeremias has received consulting fees from Canon Medical, Artrya Medical, and Boston Scientific. Dr Ali reports institutional grant support from Abbott, Abiomed, Acist, Amgen, Boston Scientific, CathWorks, Canon Medical, Conavi, HeartFlow, Inari, Medtronic, the US National Institutes of Health, Nipro, OpSens Medical, Medis, Philips, Shockwave, Siemens, SpectraWAVE, and Teleflex; consulting fees from Abiomed, Astra Zeneca, Boston Scientific, CathWorks, OpSens Medical, Philips, and Shockwave; and equity in Elucid, Lifelink, SpectraWAVE, Shockwave, and VitalConnect. Dr Pijls has received research grants from Abbott and Hexacath; consultancy fees from Abbott, GE, Philips, and HeartFlow; and has equity in General Electric, Philips, and HeartFlow. Dr De Bruyne reports receiving consultancy fees from Boston Scientific and Abbott and research grants from Coroventis Research, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, and Abbott Vascular. Dr Johnson received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis; has received significant institutional research support from St Jude Medical (CONTRAST [Can Contrast Injection Better Approximate FFR Compared to Pure Resting Physiology?; URL: https://www.clinicaltrials.gov; Unique identifier: NCT02184117]) and Philips Volcano (DEFINE-FLOW [Combined Pressure and Flow Measurements to Guide Treatment of Coronary Stenoses; URL: https://www.clinicaltrials.gov; Unique identifier: NCT02328820]) for other studies using intracoronary pressure and flow sensors; has an institutional licensing agreement with Boston Scientific for the smart-minimum FFR algorithm (now commercialized under 510[k] K191008); and has patents pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology and on methods to correct pressure tracings from fluid-filled catheters.
Supplemental Material
Tables S1–S12
Figures S1–S9
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ACS
- acute coronary syndrome
- AUC
- area under the receiver operating characteristic curve
- CABG
- coronary artery bypass graft
- CAD
- coronary artery disease
- DEFINE GPS
- Distal Evaluation of Functional Performance With Intravascular Sensors to Assess the Narrowing Effect: Guided Physiologic Stenting
- FAME
- Fractional Flow Reserve versus Angiography for Multivessel Evaluation
- FFR
- fractional flow reserve
- INSIGHTFUL-
- Pressure Microcatheter vs FFR Pressure Wire for Clinical Decision Making and PCI Optimization
- LR
- likelihood ratio
- MI
- myocardial infarction
- PCI
- percutaneous coronary intervention
- PPG
- pullback pressure gradient
- PPG Global
- Pullback Pressure Gradient Global Registry
- SAQ-7
- 7-item Seattle Angina Questionnaire
- TVF
- target vessel failure
- ULN
- upper limit of normal
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.124.069450.
This work was presented as an abstract at EuroPCR, Paris, France, May 14–17, 2024.
For Sources of Funding and Disclosures, see page 596.
Circulation is available at www.ahajournals.org/journal/circ
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Contributor Information
Daniel Munhoz, Email: dbmunhoz@gmail.com.
Takuya Mizukami, Email: mizukami.tky@gmail.com.
Jeroen Sonck, Email: jeroensonck@icloud.com.
Toshiro Shinke, Email: shinke@med.showa-u.ac.jp.
Hirohiko Ando, Email: andou.hirohiko.903@mail.aichi-med-u.ac.jp.
Brian Ko, Email: brianshiuhangko@gmail.com.
Simone Biscaglia, Email: simone.biscaglia@gmail.com.
Fernando Rivero, Email: feriver@gmail.com.
Thomas Engstrøm, Email: thomas.engstroem@regionh.dk.
Ketina Arslani, Email: ketina.arslani@regionh.dk.
Antonio Maria Leone, Email: antoniomarialeone@gmail.com.
William F. Fearon, Email: wfearon@stanford.edu.
Stephane Fournier, Email: stephane.fournier@chuv.ch.
Liyew Desta, Email: ldesta517@gmail.com.
Andy Yong, Email: andysc.yong@gmail.com.
Julien Adjedj, Email: juliendjdj@gmail.com.
Javier Escaned, Email: escaned@secardiologia.es.
Masafumi Nakayama, Email: masafumi331@gmail.com.
Ashkan Eftekhari, Email: asef@rn.dk.
Frederik M. Zimmermann, Email: frederik.zimmermann@cze.nl.
Koshiro Sakai, Email: koshiro-s@med.showa-u.ac.jp.
Tatyana Storozhenko, Email: dr.tstorozhenko@gmail.com.
Gianluca Campo, Email: cmpglc@unife.it.
Tom De Potter, Email: tom.de.potter@olvz-aalst.be.
Ward Heggermont, Email: ward.heggermont@gmail.com.
Dimitri Buytaert, Email: dimitri.buytaert@gmail.com.
Jozef Bartunek, Email: Jozef.Bartunek@olvz-aalst.be.
Colin Berry, Email: colin.berry@glasgow.ac.uk.
Damien Collison, Email: dgcollison@gmail.com.
Thomas Johnson, Email: Nils.Johnson@uth.tmc.edu.
Tetsuya Amano, Email: amanotaha@yahoo.co.jp.
Divaka Perera, Email: divaka.perera@kcl.ac.uk.
Allen Jeremias, Email: ajeremias@gmail.com.
Ziad Ali, Email: zali@crf.org.
Nico H.J. Pijls, Email: nico.pijls@xs4all.nl.
Bernard De Bruyne, Email: bernard.de.bruyne@olvz-aalst.be.
REFERENCES
- 1.Hwang D, Koo BK, Zhang J, Park J, Yang S, Kim M, Yun JP, Lee JM, Nam CW, Shin ES, et al. Prognostic implications of fractional flow reserve after coronary stenting: a systematic review and meta-analysis. JAMA Netw Open. 2022;5:e2232842. doi: 10.1001/jamanetworkopen.2022.32842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collison D, Copt S, Mizukami T, Collet C, McLaren R, Didagelos M, Aetesam-Ur-Rahman M, McCartney P, Ford TJ, Lindsay M, et al. Angina after percutaneous coronary intervention: patient and procedural predictors. Circ Cardiovasc Interv. 2023;16:e012511. doi: 10.1161/CIRCINTERVENTIONS.122.012511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collet C, Sonck J, Vandeloo B, Mizukami T, Roosens B, Lochy S, Argacha J-F, Schoors D, Colaiori I, Di Gioia G, et al. Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J Am Coll Cardiol. 2019;74:1772–1784. doi: 10.1016/j.jacc.2019.07.072 [DOI] [PubMed] [Google Scholar]
- 4.Mizukami T, Sonck J, Sakai K, Ko B, Maeng M, Otake H, Koo BK, Nagumo S, Nørgaard BL, Leipsic J, et al. Procedural outcomes after percutaneous coronary interventions in focal and diffuse coronary artery disease. J Am Heart Assoc. 2022;11:e026960. doi: 10.1161/JAHA.122.026960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collet C, Collison D, Mizukami T, McCartney P, Sonck J, Ford T, Munhoz D, Berry C, De Bruyne B, Oldroyd K. Differential improvement in angina and health-related quality of life after PCI in focal and diffuse coronary artery disease. JACC Cardiovasc Interv. 2022;15:2506–2518. doi: 10.1016/j.jcin.2022.09.048 [DOI] [PubMed] [Google Scholar]
- 6.Munhoz D, Collet C, Mizukami T, Yong A, Leone AM, Eftekhari A, Ko B, da Costa BR, Berry C, Collison D, et al. Rationale and design of the pullback pressure gradient (PPG) global registry. Am Heart J. 2023;265:170–179. doi: 10.1016/j.ahj.2023.07.016 [DOI] [PubMed] [Google Scholar]
- 7.Ohashi H, Mizukami T, Sonck J, Boussiet F, Ko B, Nørgaard BL, Mæng M, Jensen JM, Sakai K, Ando H, et al. Intravascular imaging findings after PCI in patients with focal and diffuse coronary artery disease. J Am Heart Assoc. 2024;13:e032605. doi: 10.1161/JAHA.123.032605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9 [DOI] [PubMed] [Google Scholar]
- 9.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 10.Piroth Z, Otsuki H, Zimmermann FM, Ferenci T, Keulards DCJ, Yeung AC, Pijls NHJ, De Bruyne B, Fearon WF. Prognostic value of measuring fractional flow reserve after percutaneous coronary intervention in patients with complex coronary artery disease: insights from the FAME 3 trial. Circ Cardiovasc Interv. 2022;15:884–891. doi: 10.1161/CIRCINTERVENTIONS.122.012542 [DOI] [PubMed] [Google Scholar]
- 11.Dujardin B, Van den Ende J, Van Gompel A, Unger JP, Van der Stuyft P. Likelihood ratios: a real improvement for clinical decision making? Eur J Epidemiol. 1994;10:29–36. doi: 10.1007/BF01717448 [DOI] [PubMed] [Google Scholar]
- 12.Sonck J, Mizukami T, Johnson NP, Nagumo S, Gallinoro E, Candreva A, Mileva N, Munhoz D, Shinke T, Svanerud J, et al. Development, validation, and reproducibility of the pullback pressure gradient (PPG) derived from manual fractional flow reserve pullbacks. Catheter Cardiovasc Interv. 2022;99:1518–1525. doi: 10.1002/ccd.30064 [DOI] [PubMed] [Google Scholar]
- 13.Piroth Z, Toth GG, Tonino PAL, Barbato E, Aghlmandi S, Curzen N, Rioufol G, Pijls NHJ, Fearon WF, Juni P, et al. Prognostic value of fractional flow reserve measured immediately after drug-eluting stent implantation. Circ Cardiovasc Interv. 2017;10:e005973. doi: 10.1161/CIRCINTERVENTIONS.117.005973 [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar CA, Shun-Shin M, Seligman H, Ahmad Y, Warisawa T, Cook CM, Howard JP, Ganesananthan S, Amarin L, Khan C, et al. Placebo-controlled efficacy of percutaneous coronary intervention for focal and diffuse patterns of stable coronary artery disease. Circ Cardiovasc Interv. 2021;14:e009891. doi: 10.1161/CIRCINTERVENTIONS.120.009891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin D, Dai N, Lee SH, Choi KH, Lefieux A, Molony D, Hwang D, Kim HK, Jeon KH, Lee HJ, et al. Physiological distribution and local severity of coronary artery disease and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14:1771–1785. doi: 10.1016/j.jcin.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 16.Collison D, Didagelos M, Aetesam-Ur-Rahman M, Copt S, McDade R, McCartney P, Ford TJ, McClure J, Lindsay M, Shaukat A, et al. Post-stenting fractional flow reserve vs coronary angiography for optimisation of percutaneous coronary intervention: TARGET-FFR trial. Eur Heart J. 2021;42:4656–4668. doi: 10.1093/eurheartj/ehab449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonck J, Nagumo S, Norgaard BL, Otake H, Ko B, Zhang J, Mizukami T, Maeng M, Andreini D, Takahashi Y, et al. Clinical validation of a virtual planner for coronary interventions based on coronary CT angiography. JACC Cardiovasc Imaging. 2022;15:1242–1255. doi: 10.1016/j.jcmg.2022.02.003 [DOI] [PubMed] [Google Scholar]
- 18.Nijjer SS, Sen S, Petraco R, Escaned J, Echavarria-Pinto M, Broyd C, Al-Lamee R, Foin N, Foale RA, Malik IS, et al. Pre-angioplasty instantaneous wave-free ratio pullback provides virtual intervention and predicts hemodynamic outcome for serial lesions and diffuse coronary artery disease. JACC Cardiovasc Interv. 2014;7:1386–1396. doi: 10.1016/j.jcin.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 19.van Diemen PA, de Winter RW, Schumacher SP, Bom MJ, Driessen RS, Everaars H, Jukema RA, Somsen YB, Popelkova L, van de Ven PM, et al. Residual quantitative flow ratio to estimate post-percutaneous coronary intervention fractional flow reserve. J Interv Cardiol. 2021;2021:4339451. doi: 10.1155/2021/4339451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escaned J, Berry C, De Bruyne B, Shabbir A, Collet C, Lee JM, Appelman Y, Barbato E, Biscaglia S, Buszman PP, et al. Applied coronary physiology for planning and guidance of percutaneous coronary interventions: a clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the European Society of Cardiology. EuroIntervention. 2023;19:464–481. doi: 10.4244/EIJ-D-23-00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Beek KAJ, van Steenbergen GJ, Vervaat FE, Mulders B, van Straten BH, van Nunen LX, Wijnbergen IF. Single center experience in the treatment of hemodynamically significant diffuse coronary artery disease of the left anterior descending. Int J Cardiol. 2022;352:40–44. doi: 10.1016/j.ijcard.2022.01.048 [DOI] [PubMed] [Google Scholar]
- 22.Shiono Y, Kubo T, Honda K, Katayama Y, Aoki H, Satogami K, Kashiyama K, Taruya A, Nishiguchi T, Kuroi A, et al. Impact of functional focal versus diffuse coronary artery disease on bypass graft patency. Int J Cardiol. 2016;222:16–21. doi: 10.1016/j.ijcard.2016.07.052 [DOI] [PubMed] [Google Scholar]
- 23.Sakai K, Mizukami T, Leipsic J, Belmonte M, Sonck J, Nørgaard BL, Otake H, Ko B, Koo BK, Maeng M, et al. Coronary atherosclerosis phenotypes in focal and diffuse disease. JACC Cardiovasc Imaging. 2023;16:1452–1464. doi: 10.1016/j.jcmg.2023.05.018 [DOI] [PubMed] [Google Scholar]
- 24.Soeda T, Uemura S, Park SJ, Jang Y, Lee S, Cho JM, Kim SJ, Vergallo R, Minami Y, Ong DS, et al. Incidence and clinical significance of poststent optical coherence tomography findings: one-year follow-up study from a multicenter registry. Circulation. 2015;132:1020–1029. doi: 10.1161/CIRCULATIONAHA.114.014704 [DOI] [PubMed] [Google Scholar]