Abstract
Background.
Standard-of-care biomarkers for renal allograft rejection are lagging indicators, signaling existing organ injury. This precludes early intervention, when immunological cascades leading to rejection are most susceptible. Donor-derived cell-free DNA (dd-cfDNA) shows promise as an early indicator of rejection, allowing earlier and possibly more effective treatment. This analysis was designed to assess this promise using real-world dd-cfDNA testing evidence.
Methods.
This retrospective analysis of the prospective, observational ProActive registry study (NCT04091984) assessed dd-cfDNA and serum creatinine levels before biopsy in 424 patients with ≥1 dd-cfDNA test (n = 1013) in the 6 mo before biopsy.
Results.
Of 4667 enrolled patients, 1631 patients had ≥18 mo of follow-up data, of which 424 had a biopsy and were included in this analysis. Twenty-six biopsies showed antibody-mediated rejection (ABMR), 62 showed T cell–mediated rejection, and 336 showed nonrejection; each from a unique patient. dd-cfDNA fractions were significantly elevated 5 mo before ABMR biopsies, and 2 mo before T cell–mediated rejection biopsies, compared with nonrejection biopsies. In contrast, serum creatinine did not discriminate between rejection and nonrejection in advance, or concurrent with biopsy. Among patients with nonrejection biopsies, estimated glomerular filtration rate was significantly lower in cases with ≥2 increased dd-cfDNA results (≥1%), compared with those with 0 or 1 increased dd-cfDNA result.
Conclusions.
These data indicate that dd-cfDNA is an early indicator of biopsy-proven rejection, especially ABMR, suggesting a greater role for dd-cfDNA in surveillance to identify patients at high risk of ongoing or future rejection, thus requiring closer monitoring, biopsy, or other management changes.
INTRODUCTION
Allograft rejection remains a major contributor to long-term kidney allograft dysfunction and graft loss.1 Despite a decrease in active rejection (AR) episodes because of more potent immunosuppressive therapies and improved HLA matching, long-term kidney allograft survival has shown minimal improvement.2 A major contributing factor to this discrepancy is the limited ability to detect impending AR episodes in a timely manner.3 This hinders physicians from initiating appropriate therapies before substantial allograft injury has occurred. Additionally, this has impeded a better understanding of the different pathophysiological trajectories of the 2 distinct types of AR, that is, antibody-mediated acute rejection (ABMR) and T cell–mediated rejection (TCMR), which are each mediated through their own distinct immunological mechanisms.4,5 Thus, both directly and indirectly, early, accurate identification of rejection is critical in posttransplant monitoring to improve long-term allograft survival.
Traditional biomarkers used in posttransplant surveillance for AR are limited in their prognostic values. Serum creatinine and proteinuria, common measures of kidney function, are influenced by several factors unrelated to the immunological status of the allograft, leading to a lack of sensitivity and specificity.6 Moreover, as lagging indicators, they only raise alarm once substantial injury has already occurred and impacted renal function.3,7,8 Donor-specific antibodies (DSAs) and immunosuppression drug-level monitoring may help indicate risk of future rejection but do not identify the onset of AR itself, preventing their use in early detection of rejection.9 Additionally, the prognostic ability of DSAs may be less valuable than previously assumed, as illustrated by a recent prospective multicenter trial showing >50% of the ABMR cases were not associated with known DSA.10,11
In an effort to uncover subclinical rejection earlier in the arc of rejection, some centers have adopted surveillance biopsies. However, there are challenges to the use of invasive biopsies in routine clinical monitoring such as potential morbidity, patient discomfort, logistical burdens, costs, interobserver variability, and sampling error of histological findings.12,13 As such, there is potential for the utility of novel noninvasive diagnostic tests in the surveillance setting.14,15
Donor-derived cell-free DNA (dd-cfDNA) has been extensively evaluated as a diagnostic biomarker in solid organ transplant recipients, showing high sensitivity and specificity in the detection of both ABMR and TCMR in kidney, heart, and lung transplantation.10,16–19 Additionally, several studies also suggest that dd-cfDNA may be an early indicator of rejection.20–23 As a result, the promise of dd-cfDNA to detect rejection earlier and more accurately, enabling an improvement in management, has been recognized.24
The ProActive registry, a multicenter, observational, longitudinal study, was designed to evaluate the benefit of using longitudinal dd-cfDNA testing to help manage kidney transplant recipients in a real-world setting. In this analysis, a predefined objective of the study, we evaluated the ability of dd-cfDNA to detect acute rejection earlier than serum creatinine. Specifically, we sought to determine whether dd-cfDNA was elevated in advance of a diagnosis of rejection in patients with a biopsy showing ABMR or TCMR.
MATERIALS AND METHODS
ProActive Study Design and Study Population
The ProActive registry is an ongoing, longitudinal, multicenter study that enrolled 4902 kidney transplant recipients from 54 participating transplant centers (ClinicalTrials.gov Identifier: NCT04091984) to observe the clinical utility of dd-cfDNA (The Prospera test, Natera, Inc, Austin, TX) in the management of kidney transplant recipients. The patients were monitored and provided care in accordance with the local standard of care by each participating site, including providing results of clinically ordered dd-cfDNA testing. All patients who met the eligibility criteria were consented according to local or central institutional review board-approved protocol. The institutional review board details of the participating sites in the current analysis cohort are listed in Table S1 (SDC, http://links.lww.com/TP/D22). The study has been performed in full adherence to the Declaration of Helsinki. The clinical and research activities reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.25
Patients were considered eligible for enrollment in the ProActive registry if they met the following inclusion criteria: (1) age ≥18 y at the time of consent; (2) transplanted up to 2 y before informed consent; (3) transplant from a genetically different donor; (4) monitored with Prospera testing by healthcare provider; (5) able to read, understand, and provide written consent; and (6) willing and able to comply with study requirements. Exclusion criteria for the ProActive registry study were (1) patients who were pregnant; (2) patients with a history of any organ transplant other than a kidney (prior kidney transplants permissible); (3) patients with a serious medical condition that may have adversely impacted their ability to participate in the study, such as active cancer; and (4) patients who were already managed with a dd-cfDNA test other than the Prospera test. dd-cfDNA testing was performed in accordance with each site’s standard of care. Patient management decisions, including those informed by the dd-cfDNA test result, were made based on the individual judgment of the healthcare providers participating in this study.
Study Cohort
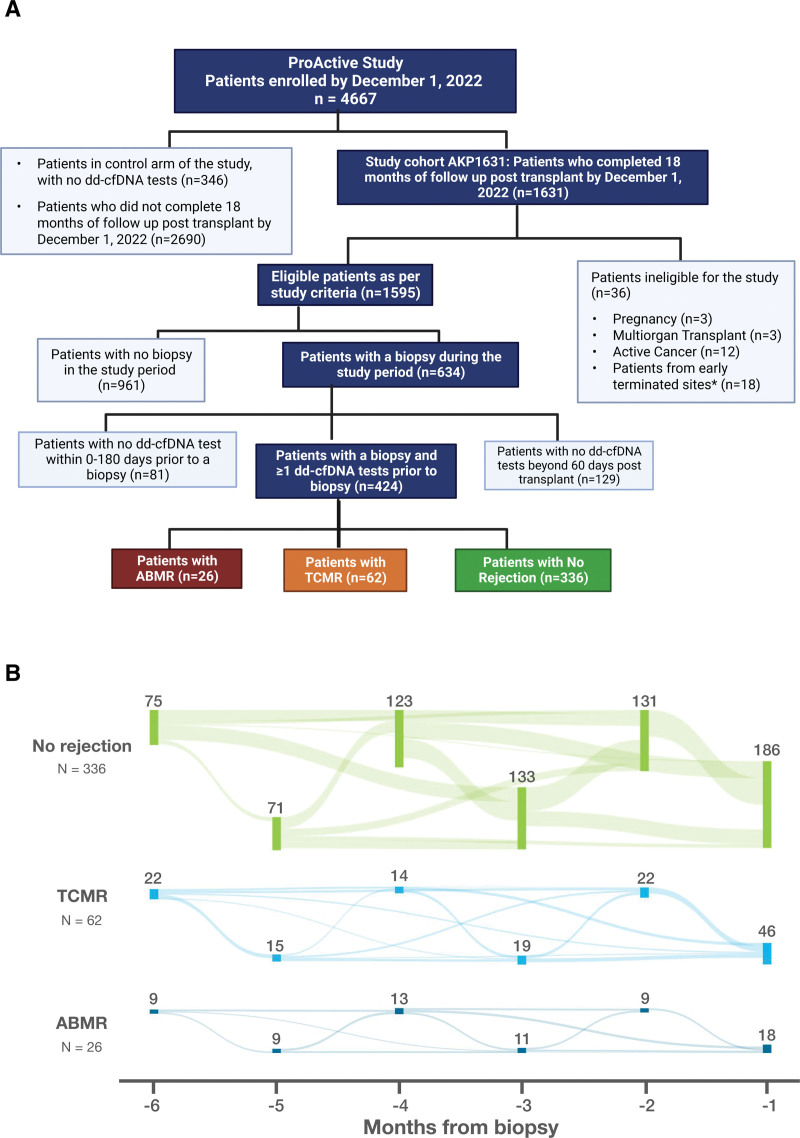
The cohort was designed with the goal of addressing a predefined study objective, namely to evaluate whether dd-cfDNA can detect biopsy-proven acute rejection (BPAR) earlier than serum creatinine. Patients were selected for inclusion into this analysis if they had (1) received a kidney transplant and were enrolled in the study by June 1, 2021; (2) at least 18 mo of posttransplantation follow-up data; (3) at least 1 kidney biopsy in the data collection window; (4) at least 1 dd-cfDNA test performed within 6 mo before kidney biopsy; and (5) at a site still active in the study (Figure 1). For each patient, clinical, laboratory, and histologic data were available from the time of transplant until December 31, 2022. If a patient had >1 biopsy, only a single biopsy (“index biopsy”) from each patient was included in the primary analysis. The index biopsy was chosen as follows: (1) if the patient had >1 biopsy indicating AR, or biopsies indicating both AR and nonrejection, only the first biopsy indicating rejection was included in the analysis and (2) if the patient had >1 biopsy and none indicated rejection, only the latest biopsy in the data collection window was used. All dd-cfDNA tests within 6 mo before the selected biopsy were included in the analysis, provided they were drawn ≥60 d after transplant.
FIGURE 1.
Analysis cohort patient flow diagrams. A, Overview and patient flow of the ProActive study and analysis cohorts (n = 424). B, Visit months are annotated on the x-axis, and each node, corresponding to the month, represents the number of patients in each category with a visit in that stipulated period. The weighted networks connecting different nodes represent the flow of patients visits from 6 to 1 mo before the index biopsy. ABMR, antibody-mediated rejection; dd-cfDNA, donor-derived cell-free DNA; TCMR, T cell–mediated rejection.
The clinical details including types of biopsy and the clinical diagnosis for each biopsy were recorded in the study database. Biopsies were performed, diagnosed, and treated according to local standard of care which may have included incorporation of dd-cfDNA testing in biopsy decision-making. Biopsies with a rejection diagnosis were recorded as either ABMR or TCMR in the study database; all other biopsy results, including borderline rejection, were considered “nonrejection.” Clinicians recorded whether or not dd-cfDNA testing influenced their biopsy decision. Estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease epidemiology Collaboration 2021 equation without race.26
dd-cfDNA Testing
All blood samples collected for dd-cfDNA testing using the Prospera test (Natera, Inc) were drawn in two 10 mL Streck Cell-Free DNA BCT tubes and shipped to the processing laboratory. cfDNA was amplified using massively-multiplexed PCR targeting 13 926 single nucleotide polymorphisms selected to maximize the number of informative single nucleotide polymorphisms across ethnicities, followed by next-generation sequencing of the resultant amplicons on the Illumina NextSeq 500 on rapid run with a minimum of 8 million reads per sample.27 The samples were processed according to standard operating protocol used in the Clinical Laboratory Improvement Amendments laboratory responsible for running the Prospera test. For all samples, the dd-cfDNA fraction, analyzed as the percentage of total cfDNA, was reported to the treating physician for use in routine clinical care. Samples with ≥1% dd-cfDNA were considered at increased risk for rejection.17
Statistical Analysis
Descriptive statistics are presented as mean, median, SD, and interquartile range (IQR), as appropriate. Differences between groups were assessed using the Wilcoxon rank-sum test. The false discovery rate method was employed to adjust for multiple comparisons when a group of hypothesis tests could be considered related.28 Statistical significance was defined as P <0.05 after false discovery rate correction.
Bayesian multilevel regression models were used to estimate posterior means to elucidate longitudinal trends in dd-cfDNA fraction and serum creatinine levels, after log10 transforming the values to reduce skewness. Fixed effects included a second-degree polynomial of time from biopsy, biopsy findings, and their interactions. A random intercept was included at the patient level. The intrapatient SD was regressed as a linear function of time and biopsy outcome. A robust Student t likelihood29 was used with weakly informative priors including a lasso prior for fixed effects.30 Model diagnostics ensured proper mixing.31 Model parameters were estimated using Hamiltonian Markov Chain Monte Carlo and 2 chains of 25 000 posterior samples via the brms package32 in R 4.2.3. Goodness of fit of the models was assessed by comparing the density of the posterior predictive distribution to the empirical density of the raw data. All analyses were performed in R 4.2.3 and Python 3.10.9.33
RESULTS
Cohort Demographics
As of December 1, 2022, 4667 patients were enrolled into the ProActive study, including 1631 patients from 40 sites with ≥18 mo of follow-up data (ProActive Study Cohort AKP1631). Four hundred twenty-four of these met the inclusion criteria for this analysis, all of whom had ≥18 mo of follow-up data (range: 18–36 mo), ≥1 biopsy during the data collection window, and ≥1 dd-cfDNA test performed within 6 mo (0–180 d) before the index biopsy (1 test: 96; 2 tests: 161; 3 tests: 105; 4 tests: 39; 5 tests: 17; and 6 or more tests: 6), for a total of 1013 dd-cfDNA tests. Of 1013, 958 (94.5%) dd-cfDNA tests had a matched serum creatinine test performed at the same visit. Of the 424 patients, 6.1% (26) and 14.6% (62) had a biopsy indicating ABMR and TCMR, respectively; the remaining 336 patient biopsies were considered “nonrejection” (Figure 1A). Of the nonrejection biopsies, 53 showed borderline rejection. The flow of patients’ visits in the 6 mo before biopsy, stratified by biopsy result, is shown in Figure 1B. Physician questionnaire for each patient visit indicated that dd-cfDNA results influenced the decision to biopsy in 43.3% (110/254) of for cause biopsies.
Among the 424 patients in this analysis, 59.9% were male (n = 254) and 40.1% were female (n = 170), the median body mass index was 28.0 kg/m2 (range: 24.7–33.1 kg/m2) and the median age was 52.0 y (range: 41.0–62.3 y). Patients were reported to be 26.9% African American, 52.1% White, and 19.1% of Hispanic ethnicity (Table 1). The majority of patients received kidneys from deceased donors (76.4%; n = 324) and the median kidney donor profile index was 51.0 (range: 30.8–71.0). The most common causes of kidney failure, type 2 diabetes (n = 96), and hypertension (n = 80) together accounted for 41.4% of cases; the full list of indications for transplant are listed in Table 1. The 424 patient subcohort matched with the original cohort (AKP1631) across 55 covariates as indicated by the propensity score distributions illustrated in Figure S1 (SDC, http://links.lww.com/TP/D22).34 The covariates of this subcohort were also comparable to the publicly available national cohort’s covariates reported in the Scientific Registry of Transplant Recipient’s annual report.35
TABLE 1.
Cohort demographics and clinical features
| Patients with ABMR (N = 26) | Patients with TCMR (N = 62) | Patients with Nonrejection (N = 336) | All patientsa (N = 424) | |
|---|---|---|---|---|
| Age (median [IQR]), y | 46 (33.5–62.3) | 50 (33.5–59.4) | 53 (42.3–63.0) | 52 (41–62.3) |
| BMI (median [IQR]) (kg/m2) | 27 (22.9–31.2) | 30 (25.2–33.4) | 28 (24.7–33.1) | 28 (24.7–33.1) |
| Sex | ||||
| Male | 14 (53.8%) | 35 (56.5%) | 205 (61.0%) | 254 (59.9%) |
| Female | 12 (46.2%) | 27 (43.5%) | 131 (39.0%) | 170 (40.1%) |
| Race | ||||
| African American | 8 (30.8%) | 22 (35.6%) | 84 (25.0%) | 114 (26.9%) |
| White | 12 (46.2%) | 27 (43.5%) | 182 (54.2%) | 221 (52.1%) |
| Asian | 2 (7.7%) | 3 (4.8%) | 15 (4.5%) | 20 (4.7%) |
| Other | 1 (3.8%) | 5 (8.1%) | 30 (8.9%) | 36 (8.5%) |
| Unknown | 1 (3.8%) | 2 (3.2%) | 9 (2.7%) | 12 (2.8%) |
| Not entered | 2 (7.7%) | 3 (4.8%) | 16 (4.7%) | 21 (5.0%) |
| Ethnicity | ||||
| Hispanic | 7 (26.9%) | 11 (17.7%) | 63 (18.7%) | 81 (19.1%) |
| Non-Hispanic | 18 (69.2%) | 50 (80.7%) | 268 (79.8%) | 336 (79.2%) |
| Not entered | 1 (3.8%) | 1 (1.6%) | 5 (1.5%) | 7 (1.7%) |
| Days from transplant to biopsy (median [range]) | 294 (186–425) | 246 (129–407) | 224(149–376) | 238 (148–378) |
| No. dd-cfDNA tests before biopsy (per patient) (mean [range]) | 3 (2–4)b | 2 (1–3) | 2 (2–3) | 2 (2–3) |
| Biopsy type | ||||
| For cause | 22 (84.6%)c | 578(93.5%)d | 175 (52.1%) | 255 (60.1%) |
| For protocol | 4 (15.4%)c | 4 (6.5%)d | 161 (47.9%) | 169 (39.9%) |
| Donor type | ||||
| Living | 3 (11.5%) | 13 (21.0%) | 84 (25.0%) | 100 (23.6%) |
| Related | 0 (0.0%) | 6 (9.7%) | 23 (6.8%) | 29 (6.8%) |
| Unrelated | 26 (100%) | 53 (85.5%) | 290 (86.4%) | 369 (87.1%) |
| Unknown | 0 (0%) | 3 (4.8%) | 23 (6.8%) | 26 (6.1%) |
| Deceased | 23 (88.5%) | 49 (79.0%) | 252 (75.0%) | 324 (76.4%) |
| Donation after brain death | 21 (80.8%)c | 30 (48.4%) | 148 (44.0%) | 199 (46.9%) |
| Donation after circulatory death | 2 (7.7%)c | 18 (29.0%) | 101 (30.1%) | 121 (28.5%) |
| Unknown | 3 (11.5%)c | 14 (22.6%) | 87 (25.9%) | 104 (24.5%) |
| KDPI (median [IQR]) | 39 (17.5–49.5)a | 44 (24.5–68.7) | 53 (36.0–72.0) | 51 (30.8–71.0) |
| Sensitization status | ||||
| cPRA > 80 | 8 (30.8%)d | 8 (12.9%)d | 34 (10.1%) | 50 (11.8%) |
| cPRA ≤ 80 | 11 (42.3%)d | 25 (40.3%)d | 249 (74.1%) | 285 (67.2%) |
| Unavailable | 7 (26.9%)d | 29 (46.8%)d | 53 (15.8%) | 89 (21.0%) |
| DSA status | ||||
| DSA-positive | 15 (57.7%)d | 11 (17.7%) | 55 (16.4%) | 81 (19.1%) |
| DSA-negative | 5 (19.2%)d | 22 (35.5%) | 147 (43.7%) | 174 (41.0%) |
| Not donee | 6 (23.1%)d | 29 (46.8%) | 134 (39.9%) | 169 (39.9%) |
| Transplant indication | ||||
| Type 2 diabetes | 5 (19.2%) | 7 (11.2%) | 84 (25%) | 96 (22.6%) |
| Hypertension | 8 (30.7%) | 18 (29%) | 54 (16.0%) | 80 (18.8 %) |
| Autoimmune diseases | 2 (7.7%) | 5 (8.0%) | 35 (10.4%) | 42 (9.9%.) |
| Polycystic kidney Disease | 1 (3.8%) | 5 (8.0%) | 32 (9.5%) | 38 (8.9%) |
| Glomerulonephritis | 4 (15.4%) | 5 (8.0%) | 21 (6.2%) | 30 (7.1%) |
| FSGS | 0 (0%) | 5 (8.0%) | 22 (6.5%) | 27 (6.6%) |
| IgA nephropathy | 1 (3.8%) | 6 (9.7%) | 21 (6.2%) | 28 (6.6%) |
| Others | 5 (19.2%) | 11 (17.7%) | 67 (19.9%) | 83 (19.6%) |
| Retransplant | ||||
| Yes | 3 (11.5%) | 3 (4.8%) | 27 (8.0%) | 33 (7.8%) |
| No | 23 (88.5%) | 59 (95.2%) | 304 (90.5%) | 386 (91.0%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 5 (1.5%) | 5 (1.2%) |
| Graft status | ||||
| Functioning | 25 (96.2%) | 56 (90.3%) | 323 (96.1%) | 404 (95.3%) |
| Loss | 1 (3.8%) | 6 (9.7%) | 13 (3.9%) | 20 (4.7%) |
| Recipient status | ||||
| Alive | 24 (92.3%) | 61 (98.4%) | 320 (95.2%) | 405 (95.5%) |
| Dead | 2 (7.7%) | 1 (1.6%) | 16 (4.8%) | 19 (4.5%) |
Wilcoxon rank-sum test or Fisher exact test with FDR correction for multiple testing comparing to No Rejection.
P < 0.05.
P < 0.01.
P < 0.001.
No DSA test was done within 90 d of biopsy.
ABMR, antibody-mediated rejection; BMI, body mass index; cPRA, calculated panel-reactive antibody; DSA, donor-specific antibody; FSGS, focal segmental glomerulosclerosis; KDPI, kidney donor profile index; TCMR, T cell–mediated rejection.
The median time-from-transplant to index biopsy was 238 d (range: 148–378 d), and 59.9% (254) of biopsies were performed for cause, whereas 40.1% (170) were surveillance biopsies. The number of for cause biopsies was significantly higher in patients with AR (ABMR P = 0.006 or TCMR, P < 0.001) compared with patients with nonrejection. Patients with ABMR had significantly more dd-cfDNA tests performed compared with patients with nonrejection (mean 3.08 versus 2.32; P = 0.016); this was not the case for serum creatinine tests (mean 2.62 versus 2.22; P = 0.512). When normalized for observation period, these differences were not significant for either dd-cfDNA or serum creatinine. At the time of biopsy, 19.1% (81/424) of patients were DSA-positive (Table 1).
dd-cfDNA Is Elevated Before BPAR, Whereas Serum Creatinine Is Not
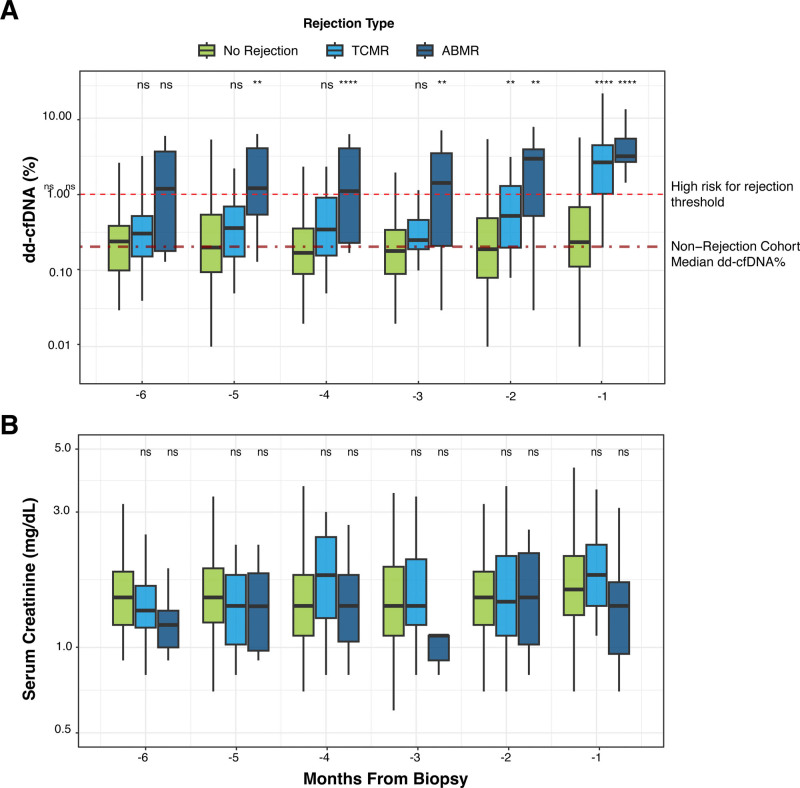
To assess the timeframe in which dd-cfDNA levels were first elevated before BPAR compared with nonrejection, dd-cfDNA tests were stratified by time, in months before biopsy (1-30d, 31-60d, 61d-90d, etc). In patients with ABMR biopsies, the median dd-cfDNA fraction was significantly elevated 1–5 mo before biopsy compared with the nonrejection group (P values for months 1–6: <0.001, 0.003, 0.007, <0.001, 0.002, 0.053, respectively), with a median dd-cfDNA above 1% across all 6 mo. In patients with TCMR biopsies, the median dd-cfDNA level was significantly elevated compared with the nonrejection group both 1 and 2 mo before biopsy (P values for months 1–6: <0.001, 0.004, 0.083, 0.064, 0.175, 0.238, respectively; Figure 2A). In contrast, there were no significant elevations in serum creatinine levels in either the ABMR or TCMR groups compared with nonrejection at any of the timeframes before biopsy (Figure 2B). Four patients had a nonrejection biopsy in the 8 mo before the index biopsy diagnosing ABMR, with a median of 102 d (range: 78–210 d) between nonrejection and ABMR biopsies (Figure S2, SDC, http://links.lww.com/TP/D22). These 4 patients had a total of 9 dd-cfDNA tests performed within 2 mo of the nonrejection biopsy (range: 26 d before to 30 d after biopsy), of which 55.5% (n = 5) had dd-cfDNA ≥1.0% (median 1.04% [IQR: 0.45%–1.18%]), which was significantly elevated compared dd-cfDNA fractions across all blood draws with matched nonrejection biopsies (n = 187; median: 0.24% [IQR: 0.12%–0.69%]; P value 0.022).
FIGURE 2.
dd-cfDNA and serum creatinine levels before BPAR. Box plots with median and interquartile range of dd-cfDNA% (A) and serum creatinine (B), shown for each biopsy diagnosis group (nonrejection, TCMR, ABMR) stratified by number of months before biopsy. A, The 2 horizontal red lines, from top to bottom, correspond to 1.0% dd-cfDNA (CLIA-validated threshold indicating high risk for rejection), and 0.23% dd-cfDNA (the median dd-cfDNA value for all samples in the nonrejection cohort). Wilcoxon rank-sum test with FDR correction for multiple testing: ****P < 1e-04, ***P < 0.001, **P < 0.01, *P < 0.05, and ns: P > 0.05. ABMR, antibody-mediated rejection; CLIA, Clinical Laboratory Improvement Amendments; dd-cfDNA, donor-derived cell-free DNA; TCMR, T cell–mediated rejection.
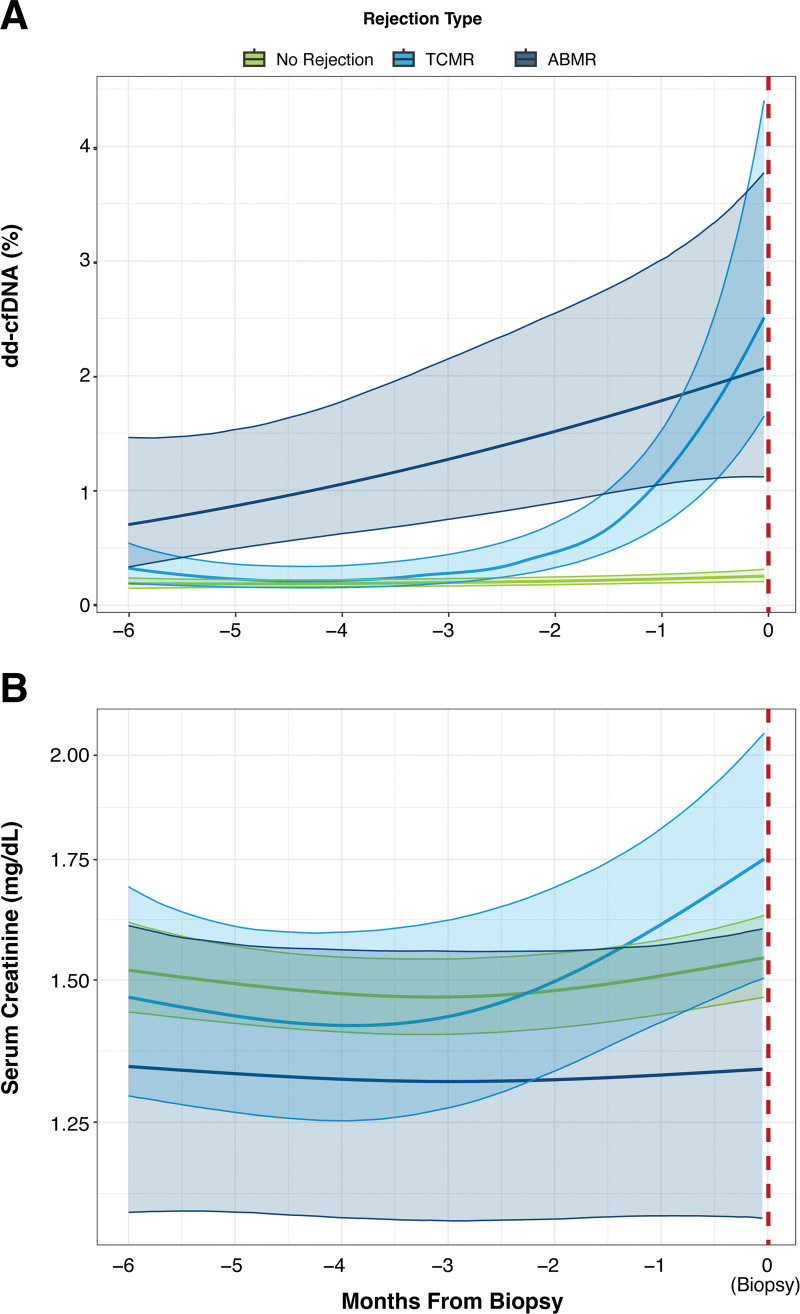
To elucidate the longitudinal trends for both dd-cfDNA and serum creatinine, Bayesian multilevel regression modeling was performed using time as a continuous variable and accounting for repeated sampling from patients. Before ABMR biopsies, dd-cfDNA levels consistently increased, trending upward as the biopsy time approached (Figure 3A). A stark increase in dd-cfDNA levels was observed beginning approximately 2 mo before TCMR biopsies. However, before nonrejecting biopsies, no significant trends in dd-cfDNA were observed. The adjusted P values for dd-cfDNA regression modeling were <0.0001 for ABMR and <0.001 for TCMR. In contrast to the trends in dd-cfDNA levels, a rise in serum creatinine levels was observed only shortly before TCMR biopsies but not before ABMR biopsies. This elevation was not significant as indicated by log pointwise predictive density values and P values of 0.49 for ABMR and 0.99 for TCMR for the serum creatinine model (Figure 3B).
FIGURE 3.
dd-cfDNA and serum creatinine trends before biopsy. Estimated population means for (A) dd-cfDNA levels and (B) serum creatinine stratified by biopsy result (no rejection, TCMR, or ABMR). Thick lines represent the regression fit, and shaded regions indicate 95% confidence intervals. ABMR, antibody-mediated rejection; dd-cfDNA, donor-derived cell-free DNA; TCMR, T cell–mediated rejection.
Decreased eGFR Is Associated With Prior Increased dd-cfDNA in Nonrejecting Patients
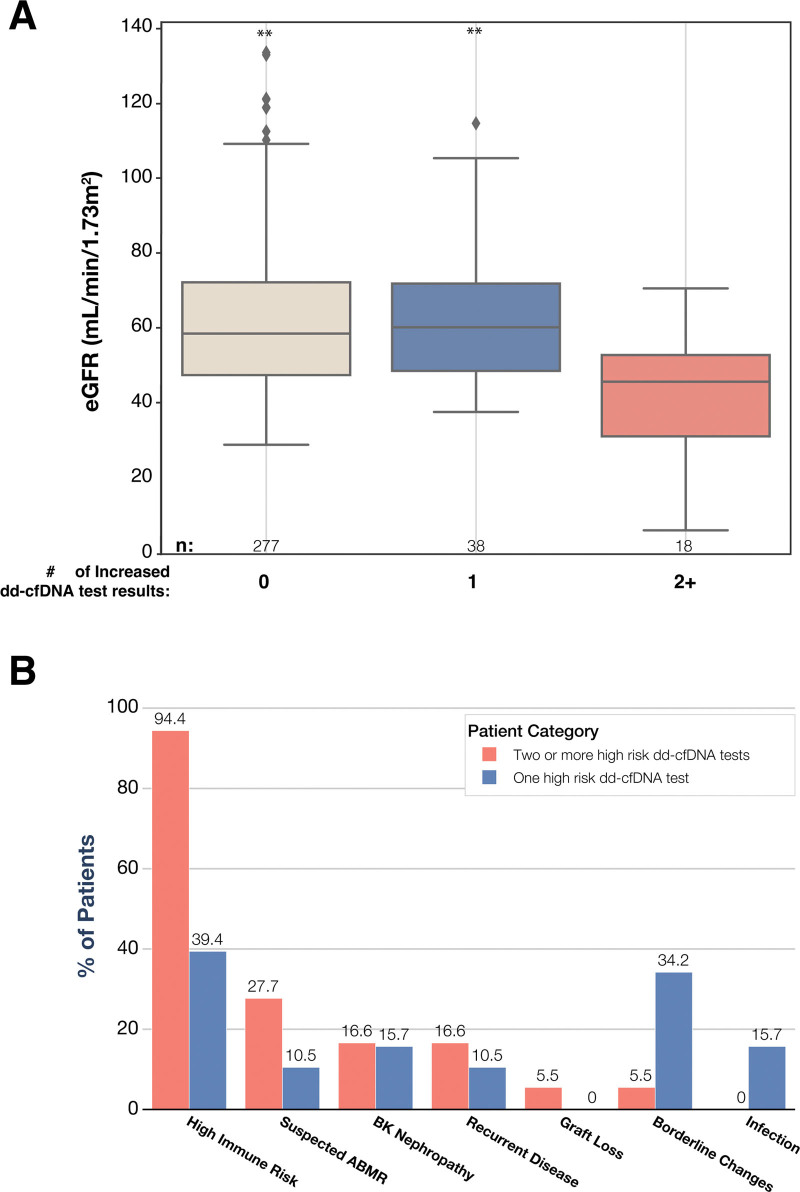
Of the 336 patients with a nonrejection biopsy, 11.3% (n = 38) had 1 increased dd-cfDNA test result (defined as ≥1%), and 5.3% (n = 18) had >2 increased dd-cfDNA test results during the 6-mo period before biopsy. At the time of the nonrejection biopsy, the median eGFR was significantly lower in patients with ≥2 prior increased dd-cfDNA test results (45.4 [30.5–52.6]) compared with patients with either 0 (58.5 [47.2–72.4], P = 0.00018), or 1 prior increased dd-cfDNA test result (60.2 [48.3–72.0], P = 0.0006; Figure 4A). We further characterized the clinical features of the nonrejecting patients with increased dd-cfDNA test results for insight into why eGFR may decrease in patients with increased dd-cfDNA results. Of the 56 nonrejecting patients with >1 increased dd-cfDNA results, 55 of 56 (98.2%) had ≥1 significant finding, including de novo DSA, retransplantation, suspected ABMR on histology, borderline changes on histology, C4d positivity on histology, BK nephropathy, pyelonephritis, recurrent disease, or graft loss (Figure 4B). We combined the presence of de novo DSA, retransplant, and/or C4d positivity into the “high immune risk” composite variable; 94.4% (17/18) of the nonrejection patients with ≥2 increased dd-cfDNA test results qualified as “high immune risk,” along with 39.4% (15/38) of the nonrejection patients with 1 increased dd-cfDNA result. The next most commonly observed phenotypes among nonrejection patients with increased dd-cfDNA results were “suspected ABMR” (5/18) among the patients with 2 or more increased dd-cfDNA test results, and “borderline changes” (13/38) among patients with 1 increased risk result.
FIGURE 4.
Key histological and laboratory findings among patients with nonrejection biopsy and increased dd-cfDNA results. A, Box plots show the median eGFR and interquartile range among 333 patients with a nonrejection biopsy, stratified by the number of dd-cfDNA tests with an increased result (≥1%): 0 (n = 277), 1 (n = 38), or 2 or more (n = 18). Wilcoxon rank-sum test with FDR correction for multiple testing was used to compare eGFR among patients with 0 and 1 increased dd-cfDNA test result (P = 0.0006) or patients with 2 increased dd-cfDNA test results (P = 0.00018), ***P < 0.001. B, Key histological and laboratory findings among patients with 1 (n = 38) and ≥2 (n = 18) increased (≥1%) dd-cfDNA results. High immune risk category includes patients with at least one of the following: de novo DSA, retransplant, C4d positivity; recurrent disease category includes IgA nephropathy and lupus glomerulonephritis; infection category includes pyelonephritis, urinary tract infection, parvovirus, CMV and EBV. Suspected ABMR was noted as such on the histology report by the pathologist. ABMR, antibody-mediated rejection; dd-cfDNA, donor-derived cell-free DNA; EBV, Epstein-Barr virus; eGFR, estimated glomerular filtration rate; FDR, false discovery rate; TCMR, T cell–mediated rejection.
Real-world Performance of dd-cfDNA to Detect Rejection
To assess the performance of this assay in a real-world setting across many sites, which is important because of the heterogeneity of real-world clinical practice, we evaluated the ability of dd-cfDNA to detect AR in the subset of 249 patients with matched biopsy (dd-cfDNA tested 0–14 d before biopsy). The median dd-cfDNA level was significantly elevated in both TCMR and ABMR patients compared with stable patients (both P < 0.001; Figure S3, SDC, http://links.lww.com/TP/D22). Positive and negative predictive values for all rejection were 32.6% (95% confidence interval [CI]: 20.0%-47.3%) and 97.9% (95% CI: 94.8%-99.4%); and for ABMR alone was 36.6% (95% CI: 22.7%-52.3%) and 99.4% (95% CI: 96.8%-100%). Real-world performance data can be found in Table S2 (SDC, http://links.lww.com/TP/D22). The area under the receiver operating curve curve to discriminate AR from nonrejection was 0.88 (Figure S4, SDC, http://links.lww.com/TP/D22). Similar elevations in dd-cfDNA levels were seen when stratified by biopsy indication for the first month before biopsy (Table S3, SDC, http://links.lww.com/TP/D22).
DISCUSSION
The ProActive study, a large, multicenter observational registry study, was designed to demonstrate real-world utility across the heterogeneous management scenarios found in the United States. This analysis, focusing on a predefined study objective, showed that dd-cfDNA fraction was elevated several months in advance of BPAR, supporting the hypothesis that dd-cfDNA is an early indicator of AR. We observed that dd-cfDNA was significantly elevated 5 mo before biopsy-proven ABMR and 2 mo before biopsy-proven TCMR, compared with patients whose biopsy did not show rejection, suggesting that elevated dd-cfDNA is associated with ABMR before the manifestation of clinical signs sufficient to prompt biopsy. In contrast, serum creatinine levels were not significantly elevated at any time point before TCMR or ABMR biopsies, compared with nonrejection, illustrating the limitations of serum creatinine as a rejection biomarker. Interestingly, in an exploratory analysis, dd-cfDNA was elevated before histologic diagnosis of ABMR in a subset of patients, at a time when histology indicated nonrejection. Moreover, in the cohort of patients with a biopsy showing nonrejection, the presence of multiple increased dd-cfDNA results was associated with lower eGFR, high immunological risk factors, and suspected ABMR. Altogether, these data support dd-cfDNA as an early indicator of BPAR, providing important prognostic information with potential to optimize the management of kidney allograft recipients and improve overall outcomes.
The majority of kidney allograft loss is caused by cumulative allograft injuries that are inadequately treated in large part because of late detection.2,6,36–38 It is becoming increasingly evident that there is an early phase in AR, involving initial stimulation of lymphocytes following antigen recognition, clonal expansion of T and B cells by persistent antigen stimulation, antibody production, recruitment of innate immune cells, and natural killer cell activation.6,39–41 Cellular and mouse studies indicate that interventions during this early phase are more likely to interrupt the progression to AR.42–45 Therefore, the ability to easily identify patients as early as possible in the arc of the rejection process is a key part of investigating and implementing new interventions. A recent analysis revealed subtle, ABMR-like changes in gene expression in many biopsies that had been classified as nonrejection.23,46 These molecular “ABMR-like” signatures were associated with elevated dd-cfDNA levels and impaired graft survival,47 supporting the idea of a “subthreshold” rejection phase that challenges the current boundaries between nonrejection and rejection.23,48,49 As such, elevations in dd-cfDNA before BPAR may be detecting an early phase of the ABMR rejection process that may otherwise not be clinically apparent.
These subthreshold events are potentially reversible with the implementation of appropriate immunosuppressive therapies based on more timely biopsies.24 Indeed, several studies have demonstrated that the early treatment of AR has led to improved outcomes.50–54 A recent study found that only 54.3% of patients with ABMR stabilized or improved after 6 mo of treatment with standard therapies including plasmapheresis, intravenous immunoglobulin, corticosteroids and rituximab. Importantly, patients with late ABMR responded more poorly to treatment, showing more persistent inflammation on follow-up biopsy compared with early ABMR (24% versus 63%, respectively).36 It is hypothesized that persistent low-grade inflammation contributes to the development of chronic humoral damage leading to transplant glomerulopathy, a lesion associated with poor graft prognosis.2,38,55 Importantly, Parajuli et al50,56 have shown that earlier detection of subclinical ABMR led to timely treatments and improved outcomes compared with patients with similarly treated clinical ABMR. The authors highlight that earlier detection and treatment may improve “structural and functional” outcomes, ultimately improving graft survival.
These data further demonstrate that the current most commonly used diagnostic test, serum creatinine, is unable to detect these upstream immunological processes. In this study, levels of serum creatinine did not rise significantly before BPAR, as compared with nonrejection biopsies, highlighting this limitation. Likewise, a prior study showed that DSA was only elevated in approximately 50% of patients with ABMR and has been found to be inferior in the detection of ABMR compared with dd-cfDNA.57 By detecting ABMR and TCMR several months before histological findings, dd-cfDNA testing provides a window of opportunity where appropriate therapies may be more effective in improving outcomes.
The fact that dd-cfDNA was significantly elevated up to 5 mo before biopsy-proven ABMR indicates that patients with increased dd-cfDNA should be considered at high risk for ongoing or future rejection and monitored more closely using the panoply of tools available to clinicians. Additional evidence from this study supports this conclusion: first, 3 of the 4 patients with nonrejection biopsies before biopsy-proven ABMR had increased dd-cfDNA (≥1%) results at the time of the nonrejection biopsy, warning of the impending rejection. Several recent reports similarly found that transcriptional analysis of biopsies can detect inflammation and damage not apparent by light microscopy.23,49,58 Second, 5 of 6 of the nonrejection biopsies noted as suspected rejection had >1 increased dd-cfDNA results (≥1%). Third, among patients with a nonrejection biopsy, having ≥2 increased dd-cfDNA results was associated with lower eGFR and rejection-related clinical features. Moreover, these conclusions align with the conclusions of the ADMIRAL (Assessing Donor-derived cell-free DNA Monitoring Insights of kidney Allografts with Longitudinal surveillance) study, which found that elevated dd-cfDNA predicted eGFR progression and de novo DSA over a 3-y period, and a composite endpoint including allograft rejection measured within a month of dd-cfDNA results.59 Building on these results, this study showed that dd-cfDNA is associated with ABMR considerably further in advance of biopsy than had been shown previously. Taken together, these data suggest that heightened monitoring in patients with elevated dd-cfDNA is warranted, including more frequent clinic visits, laboratory testing, and/or further evaluation with other diagnostic testing including DSA, and in some cases, a kidney transplant biopsy.3,60 This may be especially valuable after the first year after transplant when ABMR is the most predominant form of rejection.61–63 We note that dd-cfDNA is nonspecific to type or grade of injury/rejection, and the decision to change immunosuppression management should be predicated on biopsy results. As the evidence grows supporting the status of dd-cfDNA as an early indicator of AR, dd-cfDNA testing in combination with other noninvasive clinical testing may provide information that supports management changes, including earlier biopsy and modification of immunosuppressive therapies.3,64
Interestingly, our study showed different patterns of dd-cfDNA elevation in patients with impending ABMR and TCMR, in line with the different pathophysiological mechanisms of immune-mediated injury. Before biopsy-proven ABMR, a slow steady rise in dd-cfDNA levels was observed; in contrast, an acute steep rise in dd-cfDNA levels was observed before biopsy-proven TCMR. These trends are consistent with previous literature describing ABMR pathophysiology as a smoldering process where sustained low-grade damage results in persistent glomerular inflammation and ultimately transplant glomerulopathy, whereas TCMR has been described as an acute process of tubulitis and cellular injury resulting in more acute and severe damage.65–67 This is further supported by studies demonstrating differences in the molecular pathways between TCMR and ABMR.57,68 In these studies, the authors state that although both are associated with injury to the allograft, TCMR induces acute nephron injury and accelerates atrophy-fibrosis, whereas ABMR induces microcirculation and glomerular damage that slowly leads to nephron failure and atrophy-fibrosis.
This study has a few limitations that need to be considered. First and foremost, the study was observational, meaning the study did not mandate predetermined protocols in the management of these patients, including standardized intervals or frequencies of diagnostic testing or treatment, or requiring a biopsy after an increased dd-cfDNA result. However, given the heterogeneous clinical practice patterns in postkidney transplant care, capturing the utility of dd-cfDNA testing in a real-world clinical setting was best accomplished with an observational registry design.69 For example, in a controlled trial with a biopsy mandated after increased dd-cfDNA results, it would not have been possible to show that dd-cfDNA is an early indicator of biopsy-proven rejection. Interestingly, if the study had involved blinded dd-cfDNA results, a number of ABMR biopsies likely would have been performed later, suggesting that the lead time of dd-cfDNA elevation in relation to biopsy-proven ABMR reported in this study may in fact be an underestimate. The fact that dd-cfDNA was shown to be prognostic of biopsy-proven ABMR across a heterogeneous set of management protocols indicates that the test should perform well in a variety of clinical settings. Additionally, the fact that only a subset of patients had a biopsy when the dd-cfDNA first rose above 1% means that it is not possible to conclude whether dd-cfDNA was elevated in advance of the histological onset of rejection, or elevated because of ongoing subclinical rejection. Finally, the study was not designed to be a diagnostic clinical validation, and the performance estimates represent those expected in real-world practice.
In conclusion, data from this large, prospective, multicenter study show that dd-cfDNA is elevated 5 mo in advance of biopsy-proven ABMR and 2 mo in advance of biopsy-proved TCMR, compared with patients whose biopsy did not show rejection. This indicates that dd-cfDNA is an early indicator of biopsy-proven rejection, suggesting a greater role for dd-cfDNA in identifying patients who require closer monitoring, and possibly informing management changes. Future analysis from the ProActive study further investigating the value of dd-cfDNA surveillance are planned.
ACKNOWLEDGMENTS
See Table S1 (SDC, http://links.lww.com/TP/D22). The authors thank D. Giovanni Biagini and Yen-An Chen for help with data engineering and analysis and for help with figure development.
The authors thank the ProActive principal investigators for enrolling patients and collecting samples and clinical data. The ProActive principal investigators are: Tarek Alhamad, Anup Patel, Liise K Kayler, Nadiesda Costa, David Wojciechowski, Yasir Qazi, Christine Du, Ali Zarrinpar, Thuy Le, Richard Fatica, Henry Randall, Muna Alnimri, Anil Chandraker, Lewis Teperman, Sanjeev Akkina, Layla Kamal, Thomas Diflo, Neerja Agrawal, David Leeser, Fuad Shihab, Edward Walshe, Martin Aldana, Silas Norman, Robert Stratta, Nicolae Leca, Ayoola Adekile, Ekamol Tantisattamo, Obi Ekwenna, Arman Faravardeh, James Sondheimer, Shikha Mehta, Joseph Tremaglio, Venkatesh Ariyamuthu, Scott Westphal, and Mallika Gupta.
Supplementary Material
Footnotes
A full list of the ProActive investigators is included under Acknowledgments and in Table S1 (SDC, http://links.lww.com/TP/D22).
The PROspera Kidney Transplant ACTIVE Rejection Assessment Registry (ProActive). ClinicalTrials.gov Identifier: NCT04091984.
J.S.B., P.G., A.P., Z.D., H.T., and , M.C. were involved in conceptualization. P.G., A.P., Z.D., H.T., and N.K. were involved in methodology. J.S.B., M.S.-P., Su.B., and , M.C. were involved in data collection. A.P. was involved in validation. M.A.-C., K.M., A.P., and N.K. were involved in formal analysis. J.S.B., Su.B., E.S., M.S.-P., S.A., and M.C. were involved in resources. M.A.-C. and A.P. were involved in data curation. Z.D., M.S.B., P.G., A.P., N.K., and Sa.B. were involved in writing original draft. P.G., A.P., Sa.B., J.B., and M.C. were involved in supervision. All authors reviewed and approved of the final article.
J.S.B. has received research grants from Natera, Eurofins, and CareDx. Su.B. received research funding from AlloVir, CareDX, CSL Behring, Eurofins, Merck, Natera, OneLegacy, and Sanofi; speaking fees or member of speaker’s bureau for CareDX, Eurofins, Natera, Sanofi, Takeda, and Veloxis; and consultant for CareDX, Eurofins, Natera, Nephrosant, Sanofi, and Veloxis. M.C. is a consultant for Natera Inc. M.S.-P. has financial relationships with Natera, Tutivia, and CSL Behring. S.A. is a speaker for Alexion Pharmaceuticals and CareDx. E.S. is a speaker and advisory board honorarium from CareDx. P.G., Z.D., A.P., M.A.-C., K.M., N.K., M.S.B., H.T., and Sa.B. are employees of Natera Inc, with stocks or options to buy stocks in the company.
This study was funded by Natera Inc.
Patients’ aggregated data are summarized in Table 1. Sharing of patient-level data is not allowed as per this registry study’s institutional review board.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
Contributor Information
Collaborators: Tarek Alhamad, Anup Patel, Liise K Kayler, Nadiesda Costa, David Wojciechowski, Yasir Qazi, Christine Du, Ali Zarrinpar, Thuy Le, Richard Fatica, Henry Randall, Muna Alnimri, Anil Chandraker, Lewis Teperman, Sanjeev Akkina, Layla Kamal, Thomas Diflo, Neerja Agrawal, David Leeser, Fuad Shihab, Edward Walshe, Martin Aldana, Silas Norman, Robert Stratta, Nicolae Leca, Ayoola Adekile, Ekamol Tantisattamo, Obi Ekwenna, Arman Faravardeh, James Sondheimer, Shikha Mehta, Joseph Tremaglio, Venkatesh Ariyamuthu, Scott Westphal, and Mallika Gupta.
REFERENCES
- 1.Rodrigo E, Chedid MF, Segundo DS, et al. Acute rejection following kidney transplantation: state-of-the-art and future perspectives. Curr Pharm Des. 2020;26:3468–3496. [DOI] [PubMed] [Google Scholar]
- 2.Lai X, Zheng X, Mathew JM, et al. Tackling chronic kidney transplant rejection: challenges and promises. Front Immunol. 2021;12:661643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oellerich M, Budde K, Osmanodja B, et al. Donor-derived cell-free DNA as a diagnostic tool in transplantation. Front Genet. 2022;13:1031894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong AS. Mechanisms of organ transplant injury mediated by B cells and antibodies: implications for antibody-mediated rejection. Am J Transplant. 2020;20(Suppl 4):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oellerich M, Sherwood K, Keown P, et al. Liquid biopsies: donor-derived cell-free DNA for the detection of kidney allograft injury. Nat Rev Nephrol. 2021;17:591–603. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H, Hewitt SM, Yuen PS, et al. Acute kidney injury biomarkers—needs, present status, and future promise. Nephrol Self Assess Program. 2006;5:63–71. [PMC free article] [PubMed] [Google Scholar]
- 8.American Society of N. American Society of Nephrology Renal Research Report. J Am Soc Nephrol. 2005;16:1886–1903. [DOI] [PubMed] [Google Scholar]
- 9.van den Broek DAJ, Meziyerh S, Budde K, et al. ; ESOT Working Group Subclinical DSA Monitoring. The clinical utility of post-transplant monitoring of donor-specific antibodies in stable renal transplant recipients: a consensus report with guideline statements for clinical practice. Transpl Int. 2023;36:11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halloran PF, Reeve J, Madill-Thomsen KS, et al. ; Trifecta Investigators*. Combining donor-derived cell-free DNA fraction and quantity to detect kidney transplant rejection using molecular diagnoses and histology as confirmation. Transplantation. 2022;106:2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halloran PF, Reeve J, Madill-Thomsen KS, et al. Antibody-mediated rejection without detectable donor-specific antibody releases donor-derived cell-free DNA: results from the Trifecta study. Transplantation. 2023;107:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad I. Biopsy of the transplanted kidney. Semin Intervent Radiol. 2004;21:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finderup J, Peschardt L, Sander MR, et al. How do patients experience a kidney biopsy? J Ren Care. 2016;42:137–143. [DOI] [PubMed] [Google Scholar]
- 14.Oellerich M, Shipkova M, Asendorf T, et al. Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: results from a prospective observational study. Am J Transplant. 2019;19:3087–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight SR, Thorne A, Lo Faro ML. Donor-specific cell-free DNA as a biomarker in solid organ transplantation. A systematic review. Transplantation. 2019;103:273–283. [DOI] [PubMed] [Google Scholar]
- 16.Bloom RD, Bromberg JS, Poggio ED, et al. ; Circulating Donor-Derived Cell-Free DNA in Blood for Diagnosing Active Rejection in Kidney Transplant Recipients (DART) Study Investigators. Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol. 2017;28:2221–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigdel TK, Archila FA, Constantin T, et al. Optimizing detection of kidney transplant injury by assessment of donor-derived cell-free DNA via massively multiplex PCR. J Clin Med. 2018;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim PJ, Olymbios M, Siu A, et al. A novel donor-derived cell-free DNA assay for the detection of acute rejection in heart transplantation. J Heart Lung Transplant. 2022;41:919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenheck JP, Ross DJ, Botros M, et al. Clinical validation of a plasma donor-derived cell-free DNA assay to detect allograft rejection and injury in lung transplant. Transplant Direct. 2022;8:e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agbor-Enoh S, Wang Y, Tunc I, et al. Donor-derived cell-free DNA predicts allograft failure and mortality after lung transplantation. EBioMedicine. 2019;40:541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang E, Haas M, Gillespie M, et al. An assessment of the value of donor-derived cell-free DNA surveillance in patients with preserved kidney allograft function. Transplantation. 2023;107:274–282. [DOI] [PubMed] [Google Scholar]
- 22.De Vlaminck I, Valantine HA, Snyder TM, et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6:241ra277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauthier PT, Madill-Thomsen KS, Demko Z, et al. ; Trifecta-Kidney Investigators. Distinct molecular processes mediate donor-derived cell-free DNA release from kidney transplants in different disease states. Transplantation. 2024;108:898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer KA, Budde K, Jilma B, et al. Emerging drugs for antibody-mediated rejection after kidney transplantation: a focus on phase II & III trials. Expert Opin Emerg Drugs. 2022;27:151–167. [DOI] [PubMed] [Google Scholar]
- 25.International Summit on Transplant T, Organ T. The declaration of Istanbul on organ trafficking and transplant tourism. Clin J Am Soc Nephrol. 2008;3:1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inker LA, Eneanya ND, Coresh J, et al. ; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altug Y, Liang N, Ram R, et al. Analytical validation of a single-nucleotide polymorphism-based donor-derived cell-free DNA assay for detecting rejection in kidney transplant patients. Transplantation. 2019;103:2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57:289–300. [Google Scholar]
- 29.Lange KL, Little RJA, Taylor JMG. Robust statistical modeling using the t distribution. J Am Stat Assoc. 1989;84:881–896. [Google Scholar]
- 30.Carvalho CM, Polson NG, Scott JG. The horseshoe estimator for sparse signals. Biometrika. 2010;97:465–480. [Google Scholar]
- 31.Vehtari A, Gelman A, Gabry J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput. 2016;27:1433–1433. [Google Scholar]
- 32.Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. J Stat Software. 2017;80:1–28. [Google Scholar]
- 33.R Core Team. R: A Language and Environment for Statistical Computing [Computer Program]. R Foundation for Statistical Computing; 2023. [Google Scholar]
- 34.Kline A, Luo YP. A package for retrospective cohort matching in Python. Annu Int Conf IEEE Eng Med Biol Soc. 2022;2022:1354–1357. [DOI] [PubMed] [Google Scholar]
- 35.Lentine KL, Smith JM, Miller JM, et al. OPTN/SRTR 2021 annual data report: kidney. Am J Transplant. 2023;23(2 Suppl 1):S21–S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pineiro GJ, Montagud-Marrahi E, Rios J, et al. Influence of persistent inflammation in follow-up biopsies after antibody-mediated rejection in kidney transplantation. Front Med (Lausanne). 2021;8:761919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. [DOI] [PubMed] [Google Scholar]
- 38.Lefaucheur C, Loupy A. Antibody-mediated rejection of solid-organ allografts. N Engl J Med. 2018;379:2580–2582. [DOI] [PubMed] [Google Scholar]
- 39.Moreau A, Varey E, Anegon I, et al. Effector mechanisms of rejection. Cold Spring Harb Perspect Med. 2013;3:a015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornell LD, Smith RN, Colvin RB. Kidney transplantation: mechanisms of rejection and acceptance. Annu Rev Pathol. 2008;3:189–220. [DOI] [PubMed] [Google Scholar]
- 41.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol. 2012;12:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuda H, Dvorina N, Keslar KS, et al. Molecular signature of antibody-mediated chronic vasculopathy in heart allografts in a novel mouse model. Am J Pathol. 2022;192:1053–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shim YJ, Khedraki R, Dhar J, et al. Early T cell infiltration is modulated by programed cell death-1 protein and its ligand (PD-1/PD-L1) interactions in murine kidney transplants. Kidney Int. 2020;98:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayin I, Chong AS. Beyond adaptive alloreactivity: contribution of innate B cells to allograft inflammation and rejection. Transplantation. 2023;107:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexander KL, Ford ML. The entangled world of memory T cells and implications in transplantation. Transplantation. 2024;108:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madill-Thornsen K, Bohmig GA, Bromberg J, et al. Donor-specific antibody is associated with increased expression of rejection transcripts in renal transplant biopsies classified as no rejection. J Am Soc Nephrol. 2021;32:2743–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madill-Thomsen KS, Bohmig GA, Bromberg J, et al. ; INTERCOMEX Investigators. Donor-specific antibody is associated with increased expression of rejection transcripts in renal transplant biopsies classified as no rejection. J Am Soc Nephrol. 2021;32:2743–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaulet T, Divard G, Thaunat O, et al. Data-driven derivation and validation of novel phenotypes for acute kidney transplant rejection using semi-supervised clustering. J Am Soc Nephrol. 2021;32:1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halloran PF, Madill-Thomsen KS, Reeve J. The molecular phenotype of kidney transplants: insights from the MMDx project. Transplantation. 2024;108:45–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parajuli S, Joachim E, Alagusundaramoorthy S, et al. Subclinical antibody-mediated rejection after kidney transplantation: treatment outcomes. Transplantation. 2019;103:1722–1729. [DOI] [PubMed] [Google Scholar]
- 51.Orandi BJ, Chow EH, Hsu A, et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant. 2015;15:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer KA, Doberer K, Tillgren A, et al. Diagnostic value of donor-derived cell-free DNA to predict antibody-mediated rejection in donor-specific antibody-positive renal allograft recipients. Transpl Int. 2021;34:1689–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. J Am Soc Nephrol. 2015;26:1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chittka D. Early diagnosis and treatment of subclinical amr is vital for improving clinical outcomes. Transplantation. 2019;103:1542–1543. [DOI] [PubMed] [Google Scholar]
- 55.Vazquez Martul E. The pathology of renal transplants. Rev Esp Patol. 2018;51:110–123. [DOI] [PubMed] [Google Scholar]
- 56.Aziz F, Parajuli S, Jorgenson M, et al. Chronic active antibody-mediated rejection in kidney transplant recipients: treatment response rates and value of early surveillance biopsies. Transplant Direct. 2022;8:e1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halloran PF, Reeve J, Madill-Thomsen KS, et al. ; Trifecta Investigators. The Trifecta study: comparing plasma levels of donor-derived cell-free DNA with the molecular phenotype of kidney transplant biopsies. J Am Soc Nephrol. 2022;33:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halloran PF, Reeve J, Akalin E, et al. Real time central assessment of kidney transplant indication biopsies by microarrays: the INTERCOMEX study. Am J Transplant. 2017;17:2851–2862. [DOI] [PubMed] [Google Scholar]
- 59.Bu L, Gupta G, Pai A, et al. Clinical outcomes from the assessing donor-derived cell-free DNA monitoring insights of kidney allografts with longitudinal surveillance (ADMIRAL) study. Kidney Int. 2022;101:793–803. [DOI] [PubMed] [Google Scholar]
- 60.Martuszewski A, Paluszkiewicz P, Krol M, et al. Donor-derived cell-free DNA in kidney transplantation as a potential rejection biomarker: a systematic literature review. J Clin Med. 2021;10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. [DOI] [PubMed] [Google Scholar]
- 62.Halloran PF, Chang J, Famulski K, et al. Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol. 2015;26:1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Betjes MGH, Roelen DL, van Agteren M, et al. Causes of kidney graft failure in a cohort of recipients with a very long-time follow-up after transplantation. Front Med (Lausanne). 2022;9:842419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oellerich M, Budde K, Osmanodja B, et al. Donor-derived cell-free DNA for personalized immunosuppression in renal transplantation. Ther Drug Monit. 2023;45:20–25. [DOI] [PubMed] [Google Scholar]
- 65.Madill-Thomsen KS, Bohmig GA, Bromberg J, et al. ; the INTERCOMEX Investigators. Relating molecular T cell-mediated rejection activity in kidney transplant biopsies to time and to histologic tubulitis and atrophy-fibrosis. Transplantation. 2023;107:1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuji T, Yanai M, Itami H, et al. Microvascular inflammation in early protocol biopsies of renal allografts in cases of chronic active antibody-mediated rejection. Nephrology (Carlton). 2015;20(Suppl 2):26–30. [DOI] [PubMed] [Google Scholar]
- 67.Gupta A, Broin PO, Bao Y, et al. Clinical and molecular significance of microvascular inflammation in transplant kidney biopsies. Kidney Int. 2016;89:217–225. [DOI] [PubMed] [Google Scholar]
- 68.Halloran PF, Reeve JP, Pereira AB, et al. Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: new insights from the Genome Canada studies of kidney transplant biopsies. Kidney Int. 2014;85:258–264. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe JH, Simon GE, Horberg M, et al. When are treatment blinding and treatment standardization necessary in real-world clinical trials? Clin Pharmacol Ther. 2022;111:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.