Abstract
BK polyomavirus (BKPyV) remains a significant challenge after kidney transplantation. International experts reviewed current evidence and updated recommendations according to Grading of Recommendations, Assessment, Development, and Evaluations (GRADE). Risk factors for BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy include recipient older age, male sex, donor BKPyV-viruria, BKPyV-seropositive donor/-seronegative recipient, tacrolimus, acute rejection, and higher steroid exposure. To facilitate early intervention with limited allograft damage, all kidney transplant recipients should be screened monthly for plasma BKPyV-DNAemia loads until month 9, then every 3 mo until 2 y posttransplant (3 y for children). In resource-limited settings, urine cytology screening at similar time points can exclude BKPyV-nephropathy, and testing for plasma BKPyV-DNAemia when decoy cells are detectable. For patients with BKPyV-DNAemia loads persisting >1000 copies/mL, or exceeding 10 000 copies/mL (or equivalent), or with biopsy-proven BKPyV-nephropathy, immunosuppression should be reduced according to predefined steps targeting antiproliferative drugs, calcineurin inhibitors, or both. In adults without graft dysfunction, kidney allograft biopsy is not required unless the immunological risk is high. For children with persisting BKPyV-DNAemia, allograft biopsy may be considered even without graft dysfunction. Allograft biopsies should be interpreted in the context of all clinical and laboratory findings, including plasma BKPyV-DNAemia. Immunohistochemistry is preferred for diagnosing biopsy-proven BKPyV-nephropathy. Routine screening using the proposed strategies is cost-effective, improves clinical outcomes and quality of life. Kidney retransplantation subsequent to BKPyV-nephropathy is feasible in otherwise eligible recipients if BKPyV-DNAemia is undetectable; routine graft nephrectomy is not recommended. Current studies do not support the usage of leflunomide, cidofovir, quinolones, or IVIGs. Patients considered for experimental treatments (antivirals, vaccines, neutralizing antibodies, and adoptive T cells) should be enrolled in clinical trials.
INTRODUCTION
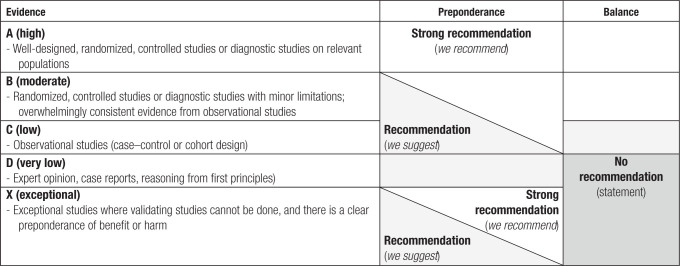
BK polyomavirus (BKPyV) nephropathy complicates kidney transplantation by directly and indirectly causing premature kidney allograft failure. Although there are no vaccines or effective antivirals currently established for clinical use,1 significant advances related to the pathophysiology, diagnosis, and treatment have provided important opportunities for optimizing the management of BKPyV replication and nephropathy in kidney transplant patients. These developments necessitated a thorough review of the current state of the art and an update of The Transplantation Society (TTS) guidelines on BKPyV published in 20052 to improve global kidney transplant outcomes. Since 2005, BKPyV has evolved from a rare emerging opportunist in some transplant centers to a consistently identified complication in kidney transplantation programs around the world. At the same time, evidence-based approaches to clinical risk assessment and immunosuppression have become common practice. Molecular diagnostics and pathology techniques are more widely available and partly standardized. Scholarly reports from different centers in all 6 TTS regions largely support the importance of more frequent screening than previously proposed in 20052 and are now supported by new and broader cost–benefit analyses. We have added pharmacokinetic (PK) and pharmacodynamic (PD) considerations and explicitly reviewed pediatric aspects. Importantly, the updated TTS guidelines include more international representation, including low-income regions and a broader array of specialists, and now use Grading of Recommendations, Assessment, Development, and Evaluations (GRADE; Table 1), which has shown utility in other clinical guidelines, such as those on cytomegalovirus (CMV) in solid organ transplantation (SOT).3
TABLE 1.
Quality of evidence, preponderance, and balance considerations for developing recommendations according to Grading of Recommendations, Assessment, Development, and Evaluations (GRADE)
To describe the natural continuum in kidney transplant patients consisting of no/low-level BKPyV-viruria, high-level BKPyV-viruria, new-onset BKPyV-DNAemia, and biopsy-proven BKPyV-nephropathy without and then with impaired baseline allograft function,4-6 we used the following definitions:
Possible BKPyV-nephropathy: high-level urine BKPyV loads defined as BKPyV-DNAuria >10 million copies/mL (c/mL; or equivalent) or decoy cells or PyV virions by electron microscopy, but undetectable plasma BKPyV-DNAemia.
Probable BKPyV-nephropathy: plasma BKPyV-DNAemia >1000 c/mL (or equivalent) sustained for >2 wk.
Presumptive BKPyV-nephropathy: plasma BKPyV-DNAemia >10 000 c/mL (or equivalent).
Biopsy-proven BKPyV-nephropathy: detection of compatible cytopathic effects plus immunohistochemistry and a specific diagnostic test identifying BKPyV as opposed to JC polyomavirus (JCPyV).
Thus, BKPyV-DNAemia and plasma BKPyV-DNA loads replaced the widely used term BKPyV viremia introduced 2 decades ago6 as outlined recently.4,5 A list of specific working definitions that complement current nomenclature recommendations7 is provided in Table S1 (SDC, http://links.lww.com/TP/D9).
EPIDEMIOLOGY AND RISK FACTORS
This update focuses on new-onset BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy. Overall, the number of risk factors and the evidence level associated with BKPyV-DNAemia were higher than those with biopsy-proven BKPyV-nephropathy (Table 2). As discussed in the respective sections, this perhaps reflects the higher testing and event rates improving the statistical power of BKPyV-DNAemia compared with biopsy-proven BKPyV-nephropathy, which is limited by invasiveness, contraindications, and missed diagnoses because of the focality of viral replication sites in the renal allograft.
TABLE 2.
Risk factors of BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy in kidney transplantation
| BKPyV-DNAemiaa | Biopsy-proven BKPyV-nephropathyb | ||
|---|---|---|---|
| Risk factor | Evidence levelc | Risk factor | Evidence levelc |
| Donor factors | Donor factors | ||
| Urinary BKPyV shedding | Low, C | Urinary BKPyV shedding | Low, C |
| BKPyV genotypes and subgenotypes | Very low, D | BKPyV genotypes and subgenotypes | Very low, D |
| BKPyV-seropositive antibodyd status (D+) if antibody levels are very high in living donors | Low, C | BKPyV genotypes different from the recipient (mismatching) | Very low, D |
| BKPyV genotypes different from the recipient (mismatching) | Very low, D | ||
| LVGR polymorphisms | Very low, D | ||
| Recipient factors | Recipient factors | ||
| Older recipient age | Moderate, B | Older recipient age | Low, C |
| Male recipient sex | Moderate, B | Male recipient sex | Low, C |
| BKPyV-seronegative recipient antibody status (R–) if the donor is BKPyV-seropositive D+ | Moderate, B | ||
| Low recipient neutralizing antibodye levels against the donor BKPyV serotype | Very low, D | Low recipient neutralizing antibody levelse against the donor BKPyV serotype | Very low, D |
| Previous kidney transplantation | Low, C | ||
| HLA class I (absence of A2, B7, B8, B51, B44, B51, B13, CW7) | Very low, D | ||
| HLA class II (DR15) | Very low, D | HLA-E*01:03 vs protective HLA-E*01:01 | Very low, D |
| Interferon-γ gene rs2435061 | Very low, D | ||
| Younger pediatric recipient age | Very low, D | ||
| Obstructive uropathy as primary renal disease of pediatric recipients | Very low, D | ||
| Transplantation factors | Transplantation factors | ||
| Tacrolimus (compared with cyclosporine A) | High, A | Tacrolimus (compared with cyclosporine A) | High, A |
| Lymphocyte-depleting agents | Low, C | Lymphocyte-depleting agents | Low, C |
| Acute rejection | Low, C | Acute rejection | Low, C |
| Corticosteroids (higher maintenance; cumulative, rejection therapy) | Moderate, B | Corticosteroids (higher maintenance; cumulative, rejection therapy) | Moderate, B |
| mTOR inhibitors (decrease risk) | Low, C | mTOR inhibitors (decrease risk) | Low, C |
| Ureteric stents | Low, C | Ureteric stents | Low, C |
| BKPyV genome rearranged NCCR | Low, C | ||
| ABOi kidney transplantation | Low, C | ||
LVGR encodes agnoprotein and capsid proteins Vp1, Vp2, and Vp3. NCCR harbors the origin of viral DNA replication and transcription promoter/enhancer elements.
Defined as >1000 c/mL (or equivalent) for >2wk (probable BKPyV-nephropathy) or increasing >10 000 c/mL or equivalent (presumptive BKPyV-nephropathy).
Defined as biopsy-proven BKPyV-nephropathy using histological evidence and demonstrating BKPyV-specific involvement.4
Based on a literature review using the GRADE classification.
Measured using ELISA with coated antigens of the major capsid protein Vp1 or the Vp1-derived virus-like particles.
Measured using infectious BKPyV or pseudovirion preparations.
ABOi, ABO-incompatible; BKPyV, BK polyomavirus; GRADE, Grading of Recommendations, Assessment, Development, and Evaluations; LVGR, late viral gene region; mTOR, mammalian target of rapamycin; NCCR, noncoding control region.
The risk factors are graded according to the quality of the evidence without making recommendations as to their relevance for interventions (Table 2). Although some of the testing is widely available, many of these tests are available in research settings only, and routine use is not expected without further clinical validation studies.
Donor factors: they are associated with an increased risk of recipient BKPyV-DNAemia, including donor urinary BKPyV shedding, very high donor antibody levels against BKPyV major capsid protein Vp1, certain donor BKPyV genotypes, and BKPyV genotypes different from the recipient (mismatching). Donor factors associated with an increased risk of biopsy-proven BKPyV-nephropathy were urinary BKPyV shedding and BKPyV genotype mismatching.
Recipient factors: they are associated with an increased risk of BKPyV-DNAemia, including older recipient age, male patients, a seronegative BKPyV-Vp1 antibody status, previous kidney transplantation, and the absence of potentially protective HLA types or their combination (such as A2, A24, B7, B8, B13, B44, B51, Cw7, and DR15). Several of these factors also increase the risk for biopsy-proven BKPyV-nephropathy. Pediatric-specific risk factors are younger recipient age and obstructive uropathy as primary renal disease.8
Transplantation factors: they are associated with an increased risk of BKPyV-DNAemia, including use of tacrolimus compared with cyclosporine, T cell–depleting agents, acute rejection episodes, higher corticosteroid exposure, AB0-incompatible transplants, and ureteric stents. Most of these factors also increase the risk for biopsy-proven BKPyV-nephropathy.
The donor factors primarily related to the transplanted kidneys, such as the replicative activity, tissue load, and serotype/genotype of BKPyV, on the one hand, and on the other hand, the immunosuppression, needed to reduce the immunological risk and the strength of the BKPyV-specific immunity in the recipient. The viral parameters can be either directly assessed in the case of urinary shedding of the donor or indirectly by the immune response, such as the type and level of antibodies. Several studies reported an increased risk of BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy when the donor is BKPyV-seropositive or the donor antibody levels are high and the recipient is BKPyV-seronegative or the antibody levels are low.9-18 Among living donor transplants, the risk of posttransplant BKPyV-DNAemia was strongly increased when BKPyV-specific Vp1-IgG levels of the donors were in the highest quartile.19 Pairing of donors with the highest quartile BKPyV antibody levels with low- or nonreactive recipients was associated with a 10-fold increased risk of BKPyV-DNAemia (hazard ratio [HR] 10.1; 95% confidence interval [CI], 3.5-29.0; P < 0.001). In multivariate analysis, donor BKPyV-specific antibody levels were the strongest pretransplantation factor associated with BKPyV-DNAemia (P < 0.001) and biopsy-proven BKPyV-nephropathy (P = 0.007).19 However, no routine testing of donor and recipient anti-BKPyV serologies is currently approved or performed available outside of research settings.
Recipient factors relate to effective BKPyV-specific immunity and the ability to respond to the tissue BKPyV load, serotype/genotype, and replicative activity with sufficient humoral and cellular effector functions. Thus, in addition to low and undetectable antibody levels, low recipient neutralizing antibody titers of <10 000, that is, the serum or plasma dilution yielding 50% inhibitory concentration of pseudovirion infectivity before transplantation, were associated with a higher risk of developing BKPyV-DNAemia posttransplant.20 Furthermore, male sex and older recipient age were associated with an increased risk of BKPyV-DNAemia.21-25 The relative effect (HR or odds ratio [OR]) reported for male recipients ranged from 1.04,26 2.4923 to 3.47.27 Older recipient age was also associated with the occurrence of presumptive and biopsy-proven BKPyV-nephropathy in some studies.24-26,28 Other recipient factors have been associated with posttransplant BKPyV-DNAemia, such as the pretransplant hemodialysis, compared with peritoneal dialysis or preemptive transplantation, and longer duration of dialysis29-31 or duration of diabetes in simultaneous pancreas and kidney (SPK) transplantation.32 Ureteric stents have been associated with an increased risk of BKPyV-DNAemia in several prospective and retrospective studies with relative effects ranging from HR of 1.36 (95% CI, 1.05-1.76; P = 0.024),33 adjusted OR of 1.55 (P = 0.04),34 and OR of 3.17 (P = 0.02)35 to HR of 4.3 (P = 0.044).36 Moreover, stent placement for >3 wk was associated with an increased risk of BKPyV-DNAemia with an OR of 1.92 (95% CI, 1.04-3.74; P = 0.044), whereas a stent for <3 wk was no longer significant compared with no stent group (OR 1.31; 95% CI, 0.672-2.61; P = 0.438).37 The use of a stent has also been associated with biopsy-proven BKPyV-nephropathy with an OR of 5.63 (P = 0.004) in a univariate analysis and remained significant in multivariate models (adjusted OR 4.71; P = 0.03).38
Class I HLA molecules have been shown to exert a protective effect against BKPyV-DNAemia, such as HLA-A2, HLA-B44,39 HLA-B*13,40 HLA-Cw7,41 or HLA-B51.42 A case–control study of 141 BKPyV-DNAemia-positive and 294 BKPyV-DNAemia-negative kidney transplant recipients from the Swiss Transplant Cohort Study found no impact of HLA-B51 alone, but BKPyV-DNAemia events were significantly reduced among recipients having HLA-B51, -B7, or -B8,43 which allow the presentation of the immunodominant 9mer epitope LPLMRKAYL to CD8 T cells.44 The risk of BKPyV-DNAemia has also been shown to be lower in kidney transplant recipients harboring some HLA class II (eg, HLA-DR15).39 Nonclassical major histocompatibility complex class Ib molecules, such as homozygous HLA-E*01:01, state of recipients has been associated with a lower rate of biopsy-proven BKPyV-nephropathy (OR 0.09; 95% CI, 0.83-4.89), but higher rates for HLA-E*01:03.45 Of note, reducing immunosuppression is also needed to release the CD8 T-cell effector functions for those HLA types that appear to be protective by facilitating antiviral immune control.43 Single nucleotide polymorphisms (SNPs) in genes orchestrating innate and adaptive immune responses have pointed to a higher incidence of BKPyV-DNAemia among homozygous carriers of the C allele of the rs12369470 SNP in the interferon-gamma (IFN-γ) gene.46 The TT genotype of the IFN-γ +874 (A > T) rs2435061 SNP had a protective role, and a combination of rs2435061–rs2406918–rs2870953 suggested that the A-G-T haplotype was associated with a significantly reduced risk for BKV infection (OR 0.43; 95% CI, 0.25-0.73; P = 0.001).
Associations between the degree of HLA mismatch or the absolute amount of panel-reactive antibodies and the risk of BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy are not clearly established.6,19,39,47-52 The general use of lymphocyte-depleting agents,47,53 including alemtuzumab,49 thymoglobulin/ATG/ATGAM/rATG,52,54-58 and the B cell–depleting agent rituximab59 has been associated with higher rates of BKPyV replication in some but not all studies. Tacrolimus, compared with cyclosporine maintenance immunosuppression, has been associated with higher rates of BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy.52,56,58,60-63 Two64,65 of 3 studies48 found associations between tacrolimus trough levels and the diagnosis of biopsy-proven BKPyV-nephropathy. Mammalian target of rapamycin (mTOR) inhibitors were associated with fewer BKPyV events in 2 analyses of registries scoring the treatment of BKPyV events.53,56 Conversely, steroids were found to be a significant risk factor for increasing BKPyV replication,55 BKPyV-DNAemia,23,49 and the need for intervention.56 Multiple studies reported a significant association between BKPyV replication and rejection episodes.19,28,29,47,48,50,51,53,56,58,66,67 This accounts for all BKPyV-related outcomes, including BKPyV-DNAemia,6,29,48,51,62,66,67 biopsy-proven BKPyV-nephropathy,19,28,47,58 and BKPyV events requiring treatment.53,56 It is likely that rejection is not a risk factor but is confounded by antirejection treatment and increased immunosuppression.6,68 The hypothesis that the overall intensity of immunosuppression increases the risk of BKPyV replication is indirectly supported by recent studies reporting higher viral load levels of Torque teno virus proposed as a surrogate of profound immunosuppression.69,70 A recent study found higher numbers of biopsy-proven BKPyV-nephropathy among patients with incompatible living donor grafts and a correlation with the intensity of desensitization.71 An increased incidence of BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy has been associated with AB0-incompatible procedures in some studies,72-74 particularly among high-titer (anti-A/B isoagglutinin titer ≥1:256) recipients.75 Lymphocyte-depleting agents used in desensitization protocols and more intense immunosuppression may contribute to impaired BKPyV-specific cell-mediated immunity (CMI) in AB0-incompatible KT recipients.66
In a multivariate analysis of CMV replication events after alemtuzumab induction, a higher rate of biopsy-proven BKPyV-nephropathy was reported with an HR of 2.72 (95% CI, 1.19-6.24; P = 0.018).28 However, other studies have failed to show that previous CMV events impact the risk of BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy.6,76 Overall, the potential interaction between CMV and BKPyV replication yielded conflicting results,77-79 which may reflect the confounding role of reducing immunosuppression and the use of valganciclovir prophylaxis.80
Retransplantation of recipients has been associated with an increased risk of BKPyV-DNAemia in some29,46 but not all studies.26 Retransplantation after allograft failure because of biopsy-proven BKPyV-nephropathy with undetectable BKPyV-DNAemia has been successfully performed, with a 93% 3-y graft survival,81-83 but selection bias for this procedure cannot be excluded. Transplant nephrectomy of the failed allograft has been performed in about half of the cases but did not protect against recurrent BKPyV-DNAemia or BKPyV-nephropathy.83 In a recent analysis of the United States Organ Procurement and Transplantation Network/United Network for Organ Sharing database evaluating 341 patients who lost a first graft because of biopsy-proven BKPyV-nephropathy and underwent retransplantation, there was no difference in death-censored graft survival, acute rejection, or patient survival compared with 13 260 retransplants who lost their first graft because of causes other than biopsy-proven BKPyV-nephropathy with a median follow-up of 4.7 y after retransplant.84
Replication of BKPyV genotype IV, as well as the presence of multiple BKPyV genotypes, has been linked to a higher risk of BKPyV-nephropathy, early onset of viral replication in the first year after transplantation, higher plasma BKPyV loads, and an increased risk of nephropathy.14,85 Polymorphisms within the VP1 gene sequence may affect cellular entry tropism and replication rates. For example, subtype I isolates have been reported to replicate more efficiently in human renal epithelial cells than in subtype IV isolates.86 Urine BKPyV loads were higher for BKPyV genotype Ia compared with Ib.87 However, comprehensive analyses of BKPyV genome variations that compare the impact of these determinants on BKPyV-DNAemia and biopsy-proven nephropathy are lacking. The BKPyV-encoded micro-RNA (miRNA)-5p and miRNA-3p may play a role in immune evasion by reducing the expression of viral large tumor antigen (LTag)88 and by targeting the stress-induced protein ULBP3 to reduce killing by natural killer cells,89 whereas the viral agnoprotein has been reported to promote innate and adaptive immune escape.90,91
Several viral genetic changes appear to emerge in kidney transplant recipients after the onset of BKPyV replication. These include rearrangements of the noncoding control region (NCCR)92 in patients with longer duration and higher peak levels of plasma BKPyV-DNAemia,93 and which correlate with progression to BKPyV-nephropathy to more tissue damage and inflammatory infiltrates (polyomavirus-associated nephropathy-B).93 Consistent with the high number of partly redundant transcription factor binding sites,92 no specific NCCR mutations have been identified as risk factors for BKPyV-nephropathy. However, some SNPs have been detected in archetypes and rearranged NCCR that may facilitate disease progression.94-96 A recent study reported that persistent high-level BKPyV-DNAuria in kidney transplant recipients was associated with the accumulation of VP1 mutations in the BC loop of the capsid protein Vp1 that might escape antibody neutralization.97 Taken together, this update on the epidemiology and risk factors of BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy may help to optimize current diagnostics and treatment approaches and to define targets for future studies (Table 3) to mitigate the impact of BKPyV in kidney transplant outcomes.
TABLE 3.
Future directions in epidemiology and risk factors
| ➢ Define rate and factors increasing the risk of BKPyV-DNAemia/-nephropathy in ABO-incompatible living donor kidney transplant recipients |
| ➢ Define the effect of mTOR inhibitors on endpoints in randomized clinical trials regarding rates and course of BKPyV-DNAemia/-nephropathy |
| ➢ Evaluate the role of high Torque teno virus loads as a risk factor of BKPyV-DNAemia/-nephropathy |
| ➢ Assess whether optimizing immunosuppression reduces BKPyV-DNAemia/-nephropathy rates in recipients with low neutralizing antibodies against the donor BKPyV serotype |
| ➢ Evaluate the role of BKPyV-DNAuria for recurrent BKPyV-DNAemia/-nephropathy after retransplantation of patients with a failed transplant from BKPyV-nephropathy |
BKPyV, BK polyomavirus; mTOR, mammalian target of rapamycin.
PATHOLOGY
Renal allograft biopsies provide important information for treatment decisions that are pivotal for ensuring renal allograft function and survival. Indeed, renal allograft biopsies were key when identifying BKPyV-nephropathy as a newly emerging complication jeopardizing kidney transplant outcomes during the last 2 decades.68,98-100 Since then, it has become evident that renal allograft biopsies fail to detect intragraft replication foci in 10% to 30% of the cases with BKPyV-DNAemia during early onset or when biopsy-proven BKPyV-nephropathy is resolving.4,101-105 Moreover, interstitial inflammatory infiltrates are difficult to interpret in patients with ongoing BKPyV replication (Consensus recommendations and future directions, see Table 4). Therefore, we recommend interpreting renal allograft biopsies in the context of all clinical and laboratory data and specifically validated plasma BKPyV loads. However, BKPyV-DNAemia per se is not an indication for renal allograft biopsy in a kidney transplant patient with baseline renal function and standard immunological risk. Similarly, an allograft biopsy is unnecessary during BKPyV-DNAemia monitoring unless there is a concern for rejection, and its detection will alter management. This also applies to new-onset urinary decoy cells or high-level BKPyV-DNAuria of >10 million c/mL. The general indications for a renal allograft biopsy remain in the case of altered renal function or signs of pathology (eg, a significant rise in serum creatinine of >15% from baseline, hematuria, or proteinuria). Conversely, if protocol biopsies are taken at predefined time points as per the standard of care of the transplant center, testing of plasma for BKPyV-DNAemia is recommended to assist the expert pathologist’s approach and interpretation.
TABLE 4.
Consensus recommendations: pathology
| • We recommend that in the context of detectable BKPyV-DNAemia, a kidney biopsy be performed as clinically indicated (eg, rise in serum creatinine, proteinuria, hematuria; strong, A) |
| • We suggest that in the context of detectable BKPyV-DNAemia and stable renal function, a kidney biopsy should be considered for patients at high immunological risk or high virologic risk (weak, D) |
| • We suggest that kidney transplant biopsies be interpreted in the context of clinical, laboratory, and virologic data and prior biopsy findings (weak, C) |
| • We recommend reporting the semiquantitative PyVL score to enable the classification into the Banff Working Group proposal (strong, C) |
| • We recommend the parallel reporting of the classification of the American Society of Transplantation (AST-PyVAN) using the 5 strata of PyVAN-A, -B1, -B2, -B3, and -C to accommodate inflammation and tubulitis (strong, C) |
| • We recommend that antibody-mediated rejection be diagnosed in a patient with detectable BKPyV-DNAemia if Banff diagnostic criteria are met (strong, C) |
| • We recommend that concomitant interstitial TCMR (Banff grade IA/B) is not diagnosed on the basis of inflammation and tubulitis; instead, an explanatory diagnostic comment incorporating interdisciplinary discussion should be used (strong, B) |
| • We recommend immunohistochemistry (clone PAb 416 against SV40 large T-antigen) for confirming the diagnosis of biopsy-proven PyVAN (strong, A) |
| • We recommend routine SV40 (LTag) immunohistology in patients with detectable BKPyV-DNAemia (strong, B) |
| • We suggest to use SV40 (LTag) immunohistology in patients with unknown BKPyV-DNAemia status with inflammatory changes in the biopsy (weak, D) |
| • We suggest to not use routine SV40 (LTag) immunohistology staining in patients with undetectable BKPyV-DNAemia (weak, C) |
| • We suggest to not perform an allograft biopsy during the course or resolution of BKPyV-DNAemia/-nephropathy unless rejection or another renal disease is a matter of concern and its detection will alter management (weak, D) |
| Future directions |
| ➢ Standardize immunohistochemistry protocols that can distinguish between different polyomaviruses, such as BKPyV, JCPyV, and other PyVs, including SV40 |
| ➢ Compare Banff PyVL and AST-PyVAN staging for capturing concurrent kidney allograft failure and predicting treatment response and allograft survival |
| ➢ Define clinically actionable thresholds of molecular tests of allograft biopsy viral loads that justify reduction in immunosuppression |
| ➢ Investigate how to best combine results from BKPyV-specific cell-mediated immunity with BKPyV-DNAemia and biopsy findings to optimize adjusting immunosuppression |
| ➢ Develop noninvasive assays that provide information equivalent to a kidney biopsy for staging BKPyV-nephropathy and forms of acute or chronic active rejection |
AST-PyVAN, American Society of Transplantation-polyomavirus-associated nephropathy; BKPyV, BK polyomavirus; JCPyV, JC polyomavirus; LTag, large tumor antigen; PyAN, polyomavirus-associated nephropathy; PyV, polyomavirus; PyVL, polyoma tissue viral load; SV40, simian virus 40; TCMR, T cell–mediated rejection.
Histopathology of BKPyV-nephropathy
The biopsy findings of BKPyV-nephropathy range from minor, often focal signs of viral replication to severe tubular damage, interstitial inflammation, and tubulitis, and pronounced interstitial fibrosis. The leading features may change during the natural course of the disease and may vary as a result of other factors, including but not limited to lowering, increasing, or switching immunosuppressive drugs, administrating IVIGs, or other known or presumed antiviral or immunological therapies. Thus, ancillary diagnostic methods, including immunohistochemistry, for detecting BKPyV-LTag with the use of a cross-reacting antibody to SV40-LTag (referred to as LTag below) are necessary to assess the etiologic and pathogenetic contribution of BKPyV replication. The expert interpretation aims to integrate clinical and laboratory data as well as earlier biopsy findings.
BKPyV-nephropathy can be classified by 2 approaches. The first one, adopted by the American Society of Transplantation, is based on the 5 strata of A, B1, B2, B3, and C4 and aims at providing a semiquantitative assessment of the extent of viral replication,101,106,107 interstitial inflammation and tubulitis,108-112 and interstitial fibrosis and tubular atrophy.4,101,108 The second classification, proposed by the Banff Working Group on polyomavirus nephropathy, relies on a semiquantitative score of the extent of BKPyV replicating cells in 3 strata of tissue involvement termed polyomavirus load (PyVL) 1, 2, and 3 and the Banff ci-score.111 Both classifications have been correlated with the risk of graft loss. Validation studies reported mixed results, with some studies supporting the initial findings,112-115 but large-scale side-by-side comparative studies are scarce.25 The interstitial infiltrates are not necessarily adjacent to virally affected tubules.99,106,116,117 When tubulitis is seen with intratubular inflammatory infiltrates, it may appear disproportionately mild compared with the density of the interstitial infiltrates. Viral replication and associated cytopathic changes may affect the renal cortex and extend to the parietal epithelium of Bowman’s capsule and rarely podocytes.118-122
There are challenging cases in which immunohistochemistry fails to detect LTag expression in the renal allograft; however, there is evidence of high-level BKPyV replication as defined by decoy cell shedding or urine viral loads >10 million c/mL or even detectable BKPyV-DNAemia.103,123,124 The biopsies are characterized by an influx of inflammatory cells and increased intraepithelial lymphocytes. Peaking of serum creatinine concentration can be observed in approximately 50% of the patients. Inflammation and tubulitis can persist for a prolonged duration in roughly 25% of patients having cleared plasma BKPyV-DNA loads, that is, being below the limit of detection (LOD).103-105 Conversely, in patients with new-onset plasma BKPyV-DNA loads persisting at >1000 c/mL or increasing >10 000 c/mL or equivalent, LTag may not (yet) be detectable. Some of these cases may show inflammation and tubulitis, but the extent and severity are less pronounced compared with biopsy-proven BKPyV-nephropathy with pattern B. Also, inflammatory tissue infiltrates can be encountered in kidney transplant patients with high-level viruria who never develop detectable BKPyV-DNAemia.125,126 As outlined below, these biopsy findings cannot be interpreted with confidence as borderline rejection, T cell–mediated rejection (TCMR), or antibody-mediated rejection (AMR) in kidney transplant patients with ongoing high-level BKPyV replication.
Differential Diagnosis of BKPyV-nephropathy and Renal Allograft Rejection
Allograft dysfunction is the most frequent indication for a diagnostic biopsy in the context of BKPyV-DNAemia. Pathologists are confronted with the question as to whether they can diagnose polyomavirus nephropathy, rejection, or both with certainty. A combination of the 2 poses a dilemma because, currently, both conditions cannot be treated at the same time. The current Banff 2019 classification recognizes 3 types of rejection according to their pathogenesis: AMR, TCMR, and a mixed type.127
Active and chronic active AMR can be diagnosed with certainty if all 3 criteria or their surrogates are met (tissue injury, complement deposition, and donor-specific antibodies [DSAs]).127 Active AMR triggers microvascular inflammation in the glomeruli (glomerulitis) and peritubular capillaries (peritubular capillaritis) and may involve arteries (“intimal arteritis” with fibrinoid necrosis in the most severe cases). It associates with DSAs and complement C4d deposition in the peritubular capillaries. Active AMR can occur before, concomitant to, or after reducing immunosuppression for BKPyV-DNAemia/nephropathy.104,128-132 Except for peritubular capillaritis, the pattern of active AMR does not overlap with the key features of biopsy-proven BKPyV-nephropathy.127
TCMR confined to the tubulointerstitium (Banff grades IA/B) without the so-called vascular Banff type II/III rejection characterized by intimal arteritis cannot be reliably diagnosed in cases of biopsy-proven BKPyV-nephropathy because no morphological, immunohistochemical, or molecular features distinguish both entities with certainty.98,99,103,108,117,118,133-141 The presence of a virus-mediated cytopathic effect ultimately lysing LTag-positive cells argues for BKPyV-associated inflammation and tubulitis. However, failure to identify LTag-positive cells in a focus of interstitial inflammation cannot reliably exclude a role of BKPyV in kidney transplant patients with markers of high-level BKPyV replication (eg, BKPyV-DNAuria >10 million c/mL or BKPyV-DNAemia). The Banff rules to diagnose TCMR grade IA/B127,142 should not be applied to kidney transplant patients with BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy. Instead, descriptive terminology avoiding the term “rejection” and an explanatory comment is recommended for the report. Chronic active TCMR grade IA/B presents with the same problem of overlapping histological features and, therefore, cannot be diagnosed with certainty. However, intimal arteritis,143 as well as chronic allograft arteriopathy seen in both AMR and TCMR, should be reported as possible concomitant rejection.
Differential Diagnosis of BKPyV-nephropathy and Other Viral Nephropathies
Renal allograft pathology can be caused by other viral agents, such as JCPyV, human adenovirus (HAdV), CMV, or herpes simplex virus (HSV). The diagnosis of JCPyV-nephropathy should be suspected in biopsies detecting LTag expression using the cross-reacting SV40-LTag antibody in a kidney transplant recipient without detectable BKPyV-DNAemia or high-level BKPyV-DNuria. Morphologically, BKPyV- and JCPyV-nephropathy are indistinguishable.102,144 The specific diagnosis of JCPyV-nephropathy requires immunohistochemistry staining using JCPyV-specific antibodies, such as those raised against JCPyV major capsid Vp1 protein or in situ hybridization with JCPyV-specific probes. Another approach is the detection of JCPyV-DNA tissue viral loads in biopsy material by (semi-) quantitative molecular testing, whereby BKPyV-DNA should not be detectable. Kidney transplant patients with JCPyV-nephropathy are characterized by high-level urine JCPyV loads of >10 million c/mL (or equivalent), whereas urine BKPyV loads are low or undetectable. Unlike for BKPyV screening, plasma JCPyV loads are not a reliable marker for screening, diagnosing, or monitoring JCPyV-nephropathy because these are usually undetectable or low.102,145-147
HAdV, CMV, and HSV are rare causes of renal allograft pathologies.148-151 HAdV cytopathic change closely resembles PyV, but the nephritis is often associated with more extensive tubular necrosis.148,152 Cystitis is a frequent HAdV complication in hematopoietic cell transplantation that is exceptional in BKPyV-nephropathy, and cases with systemic multiorgan disease have been described.153 CMV nephritis has become extremely rare since the adoption of antiviral prophylactic and surveillance protocols. CMV manifestations are characterized by the presence of typical viral cytopathic effects with intranuclear inclusions (owl’s eye) affecting predominantly nontubular cells, such as endothelial cells of the glomerulus and the peritubular capillaries with associated interstitial inflammation. HSV may cause cytopathic changes, tubular necrosis, and interstitial inflammation. Thus, viral nephropathies require confirmation by an ancillary technique, such as virus-specific antibodies for immunohistochemistry or molecular genome detection by in situ hybridization or quantitative nucleic acid testing (QNAT) loads in tissue, urine, or blood.
Ancillary Techniques
Ancillary techniques are necessary to detect and confirm BKPyV-nephropathy in kidney transplant biopsies, immunohistochemistry being the most frequently used method. Ancillary techniques should be used in all renal allograft biopsies from patients with BKPyV-DNAemia, those with viral cytopathic effect by light microscopy, and in the context of interstitial inflammation and tubulitis in patients without information about BKPyV replication.
The cross-reactive clone PAb416 raised against the monkey PyV SV40-LTag is the most widely used antibody for formalin-fixed paraffin-embedded samples in clinical diagnostics. Optimized staining protocols have been validated in a multicenter setting.154 This antibody cannot discriminate between BKPyV subtypes and certain other species of polyomaviruses; hence, specific molecular assays or QNATs are needed to distinguish BKPyV from JCPyV. Accordingly, without specific assays indicating BKPyV replication, the generic term PyV-nephropathy should be used, even if BKPyV-nephropathy is the most likely because it is the most frequent entity. In situ hybridization can be used to detect BKPyV-DNA but has no advantage over the immunohistochemical detection of BKPyV proteins. Rarely do tissue samples processed for transmission electron microscopy detect PyV particles. Electron microscopy cannot distinguish between the 14 different human polyomavirus species. Measuring the virion diameter of PyVs of 40 to 45 nm requires optimal fixation and high-quality resolution and may then allow to distinguish between other agents, such as parvovirus (18–26 nm), papillomaviruses (only marginally larger at 52–55 nm), and adenoviruses (70 nm) or herpesviruses (100 nm).
There is a substantial overlap in pathogenetic mechanisms between polyomavirus-associated nephropathy and rejection-associated allograft injury.155 Currently, available molecular tests cannot reliably quantify the relative contributions of viral and rejection-associated injury in biopsies with BKPyV-nephropathy because the TCMR classifier could not distinguish between cognate T-cell responses to donor-specific versus virus-specific antigens.156 It may be possible to use these as ancillary tools after additional refinement. This would require studying large numbers of patients with serial follow-up and careful documentation of therapies administered and, ultimately, clinical outcomes. Importantly, no biopsy-based machine learning or gene expression profiling test is currently available to confidently rule in or rule out the clinical diagnosis of probable and presumptive BKPyV-nephropathy.
DIAGNOSTICS
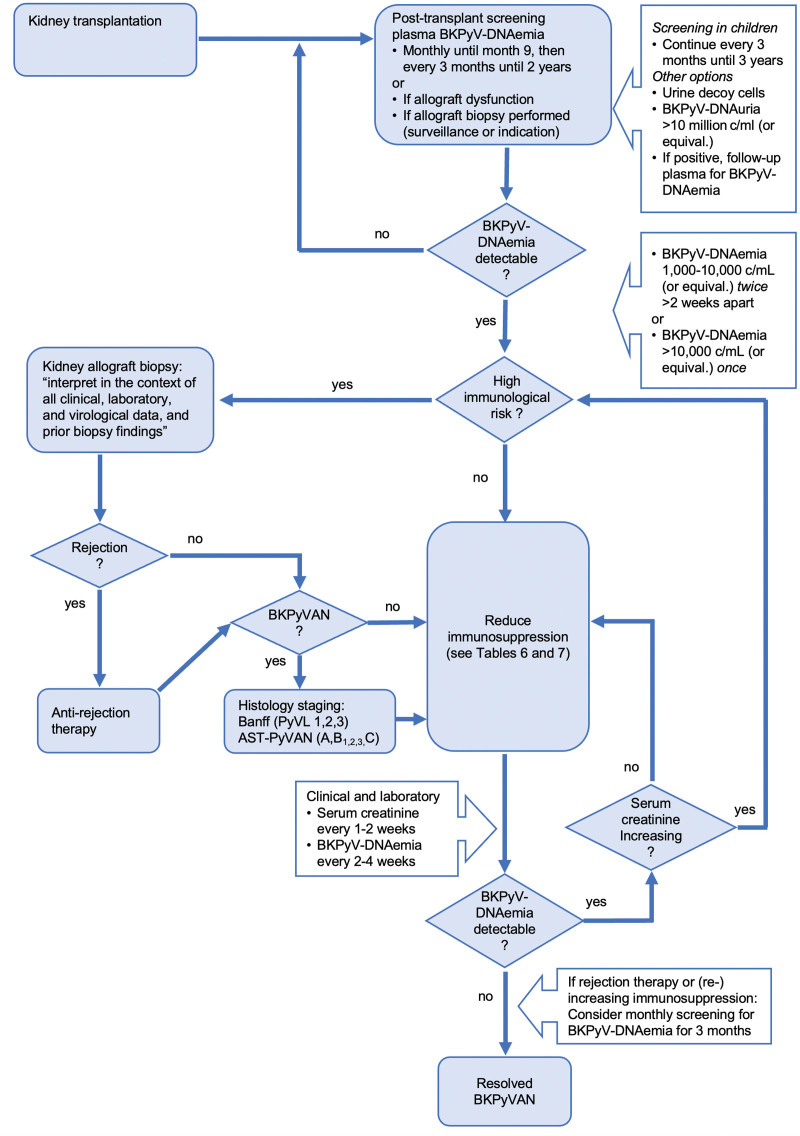
A number of laboratory methods have become key to identify kidney transplant patients at risk of developing BKPyV-nephropathy and to complement the histology of the renal allograft biopsies, thereby assisting decisions regarding clinical management (Consensus recommendations and future directions in diagnostics, see Table 5 and Figure 1).
TABLE 5.
Consensus recommendations: diagnostics
| Screening |
| • We recommend regular screening of kidney transplant recipients for BKPyV replication to identify patients for treatment of probable/presumptive/biopsy-proven BKPyV-nephropathy (strong, A) |
| • We recommend screening kidney transplant recipients for plasma BKPyV loads monthly until mo 9, then every 3 mo until 2 y posttransplantation (strong, B; Figure 1) |
| • If plasma BKPyV-DNA loads are 1000–10 000 c/mL (or equivalent), we suggest confirmatory testing within 2–3 wk (weak, B) |
| • In kidney transplant recipients with sustained plasma BKPyV-DNA loads >1000 c/mL (or equivalent), we suggest monitoring BKPyV-DNAemia every 2–4 wk to assess dynamics and response to the intervention (weak, D) |
| • In kidney transplant recipients requiring increased immunosuppression or antirejection therapy, we suggest resuming monthly screening for BKPyV-DNAemia for the next 3 mo (weak, D) |
| • In resource-limited settings, we recommend using urine cytology for decoy cells as the minimal screening approach (strong, B) at similar time points to the above (weak, D) |
| • If blood sampling is not available or considered inappropriate for screening, we suggest measuring urine BKPyV-DNA loads by QNAT at similar time points as recommended above (weak, D) |
| • If urine decoy cells or urine BKPyV-DNA loads of >10 million copies/mL (or equivalent) are detected, we recommend measuring plasma BKPyV-DNA loads to guide clinical management (strong, B) |
| • For combined kidney/solid organ transplants, including pancreas, we suggest extending screening for BKPyV-DNAemia every 3 mo up to 36 mo posttransplant (weak, C) |
| • For non-kidney solid organ transplant recipients, we recommend to not routinely screen for BKPyV-DNAemia (strong, B) |
| • For non-kidney solid organ transplant recipients presenting with declining renal function, in the absence of other reasons for the renal compromise, we suggest testing for BKPyV-DNAemia and looking for BKPyV-nephropathy if a renal biopsy is performed (weak, C) |
| Laboratory testing |
| • We recommend that the same specimen type and assay be used in the same diagnostic laboratory to avoid uncertainty because of assay variability when monitoring the dynamics of BKPyV-DNAemia (strong, B) |
| • We recommend using QNAT assays that target conserved BKPyV genome sequences to permit the detection of all genotypes and variants (strong, C) |
| • We recommend using QNAT assays with a short amplicon size of <150 bp to avoid significant underquantification (strong, C) |
| • We recommend that clinical virology laboratories serving transplantation programs participate in external quality assurance programs for quantitative BKPyV-DNA load testing (strong, C) |
| Statements |
| • Further data are needed: |
| - before pretransplant BKPyV serology of donor or recipient can be recommended for risk stratifying kidney transplant recipients for posttransplant BKPyV-DNAemia/-nephropathy |
| - before pretransplant BKPyV-specific CMI measurement can be recommended for routine clinical use to predict posttransplant BKPyV-DNAemia/-nephropathy |
| - before posttransplant BKPyV serology can be recommended for routine clinical use to predict the course of BKPyV-DNAemia/-nephropathy |
| - before posttransplant BKPyV-specific CMI can be recommended for routine clinical use to predict the course of posttransplant BKPyV-DNAemia/-nephropathy |
| - before posttransplant BKPyV-specific CMI can be used to safely guide changes in immunosuppression |
| - before recommendations can be made as to how best to screen for BKPyV-associated urothelial carcinoma in kidney transplant recipients with ongoing BKPyV-DNAemia/-nephropathy |
| Future directions |
| ➢ Develop commutable international standards for BKPyV-DNA loads (plasma, whole blood, urine, and tissue) based on defined molecular sequences and copy numbers of early and late viral gene regions |
| ➢ Better define optimal intervals for screening and monitoring using relevant assays, minimizing additional diagnostics without compromising outcomes |
| ➢ Evaluate the utility of donor and recipient BKPyV serostatus, serotype, and neutralizing antibody pretransplantation and posttransplant |
| ➢ Evaluate the role of BKPyV serotype/genotypes and mutants in increasing the rate, severity, and duration of BKPyV-DNAemia/-nephropathy |
| ➢ Identify BKPyV-specific CMI assays and thresholds pretransplant and posttransplant to predict protection from BKPyV-DNAemia/-nephropathy posttransplant |
BKPyV, BK polyomavirus; CMI, cell-mediated immunity; QNAT, quantitative nucleic acid testing.
FIGURE 1.
Flowchart integrating screening, diagnosis, and management of BKPyV replication in kidney transplant recipients. For details, see consensus statements and recommendations, including Tables 6 and 7, which describe the principal approaches to reducing immunosuppression. AST, American Society of Transplantation; BK polyomavirus nephropathy; BKPyV, BK polyomavirus; BKPyVAN, PyVL, polyomavirus-tissue load.
Virologic Methods
The natural course and relative rates of BKPyV events after kidney transplantation have been confirmed in multiple studies, and they provide the rationale for recommending universal screening to identify patients with new-onset high-level BKPyV replication during the first 2 y posttransplant and to monitor their course.4,157 Both the choice of the analyte and the characteristics of the specific assay are essential for the validity and robustness of the results and their clinical interpretation. Thus, quantitative detection of BKPyV-DNA by molecular tests and the determination of viral DNA loads in urine and blood by QNAT expressed as c/mL (or equivalent) have demonstrated broad utility and are the key diagnostic tools in clinical virology laboratories in North America and Europe (Table S2, SDC, http://links.lww.com/TP/D9). In contrast, qualitative NAT is not considered to be sufficiently informative because a positive result in urine cannot distinguish kidney transplant patients with high-level viruria from those with low-level viruria, which occurs even in immunocompetent populations, including healthy blood donors.158 Although the detection of BKPyV-DNA in plasma by qualitative NAT uncovered its principal diagnostic value for BKPyV-nephropathy,133 quantification of BKPyV-DNA loads by QNAT is required to capture the dynamics of onset, peak, levels, and clearance of BKPyV-DNAemia and resolving BKPyV-nephropathy. In an international multicenter study of >600 de novo kidney transplant recipients, 19 (5.0%) of 378 patients with residual urine production had low-level BKPyV-DNAuria before kidney transplantation.23 Importantly, the levels were similar to healthy blood donors (ie, <100 000 c/mL; 5 log10)158 and none of these patients developed high-level BKPyV-DNAuria or BKPyV-DNAemia posttransplant.23 Thus, new-onset high-level viruria defined by urine BKPyV loads of >10 million c/mL or the presence of “decoy cells” is seen in approximately 20% to 40% of patient posttransplant, followed by new-onset plasma BKPyV-DNAemia in 10% to 20%, and biopsy-proven BKPyV-nephropathy in 8% (range, 1%–15%) of patients in the first 12 mo after kidney transplantation.6,23,102,159,160 Subsequently, BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy are seen in another 10% to 20% for the next 24 mo and another 1% to 10% for the next 3 to 5 y.8,67,102,161,162
BKPyV-DNAemia loads have a higher positive predictive value for biopsy-proven BKPyV-nephropathy than high-level urine BKPyV loads or “decoy cells.” The positive predictive value of urine and blood tests increases with increasing order of magnitude and months duration after the first positive screening event.102 Indeed, high-level viruria precedes plasma BKPyV-DNAemia on average by approximately 6 wk (range, 1–12 wk).6,36,102,163,164 Independent studies are lacking to examine the risks and benefits of using high-level viruria for guiding immunosuppression reduction in patients with undetectable BKPyV-DNAemia. Urine viral loads are associated with higher variability and may be outside the linear range of the assays, which, together with the physiological changes in urine composition, impairs reliable decision-making regarding the impact of immunosuppression reduction. If urine samples are used for screening kidney transplant patients, plasma testing should be considered when urine BKPyV-DNAuria exceeds 10 million c/mL.165,166 If urine QNAT is not used or unavailable for screening, smeared or cytocentrifuged urine could be examined for “decoy cells” with enlarged nuclei and intranuclear viral inclusions, either directly or after Papanicolaou staining or immunohistochemistry for viral antigens (eg, LTag). Decoy cell screening followed by plasma BKPyV-DNAemia has been shown to be successful in larger clinical studies.102,162,167 However, “decoy cells” are not specific for BKPyV and can also reflect high-level JCPyV replication.102 Cases of biopsy-proven JCPyV-nephropathy are often positive for urine “decoy cells” and BKPyV-DNAemia negative, whereas JCPyV-DNAemia is mostly low or undetectable. In such cases, urine JCPyV-DNA loads are commonly >10 million c/mL, and JCPyV-nephropathy can be demonstrated (see the Pathology section).
Critical issues regarding QNAT include underquantification or false-negative results because of high viral target sequence variability and primer–probe mismatch,168,169 and false-positive results because of cross-detection of sequences conserved across other polyomavirus genomes (eg, JCPyV). The insufficient assay coverage of patient variants cannot be corrected by calibration to international standards. Differences in specimen handling, nucleic acid extraction, assay performance, and standard curve calibration may contribute to the variability of BKPyV-DNAemia loads and impair intra- and interlaboratory commutability of the results.170 Although plasma BKPyV-DNAemia levels significantly associated with biopsy-proven BKPyV-nephropathy have been listed in several guidelines as >10 000 c/mL in a single sample or as >1000 c/mL persisting for >2 wk,4 some single-center studies have reported lower168,171 or higher viral loads.172,173 Most but not all of these thresholds were within the 0.5 log10 c/mL range accepted as not significantly different in amplification-based QNATs. Resolving these issues includes targeting highly conserved sequences in the early viral gene region (EVGR) encoding sTag or LTag or in the late viral gene region (LVGR) encoding the capsid proteins Vp1, Vp2, or Vp3.5,174 To avoid detection failure, regular review of available sequences has been recommended to allow optimization of targets, primers and probes, and adapted use of degenerated primers and probes5,174 and by developing dual genome target assays.175 Sequence variation is well known in the serotype-defining domain of VP1 and possibly also occurs as a result of viral mutations and immune escape.97,176-178 Limited data suggest a lower sensitivity of <90% for assays based on a modified VP1 probe.179 Notably, plasma BKPyV-DNAemia mostly derives from nonencapsidated fragmented DNA of <150 bp.5,178 Extended transport, storage, freezing, and thawing can significantly reduce plasma BKPyV-DNAemia loads and should be avoided.5 Moreover, the use of QNATs with larger amplicons of >150 bp will lead to significant underquantification of BKPyV-DNAemia.5 The use of internationally approved calibrators was expected to improve the commutability of BKPyV-DNA load results. However, recent next-generation sequencing analysis of the World Health Organization–approved international standard produced by the National Institute for Biological Controls (a national UK agency) has identified large deletions in the BKPyV-EVGR in approximately 80% to 90% of the viral genome coverage, which is not seen in the sequence from the National Institute of Standards and Technology (US government).180 Although these deletions in the calibrator may only marginally affect LVGR- or dual-target assays including LVGR-targets, EVGR QNATs targeting the deleted region will underquantify the calibrator standards and yield 5- to 10-fold higher BKPyV-DNA loads after conversion into international units. Therefore, some centers have proposed using the National Institute of Standards and Technology (instead of the World Health Organization standard) or calibrators containing complete genomes or plasmids containing all available target sequences in equimolar concentrations.170 Thus, the copy number of the calibrators in international units should not rely on averaging variability across different assays and procedures from different arbitrarily selected laboratories but rather determine precise copy numbers by limiting dilution replicas or digital-droplet NAT to permit calibration of the different QNAT targets of the BKPyV genome.5,170 External quality assurance programs play an important role in this process and allow for documenting diagnostic proficiency when assessing QNAT performance and variability across different laboratories and assays.170
Besides QNAT using plasma and urine samples to capture BKPyV replication as the standard of care in kidney transplant patients, a number of studies explored methodological adaptations (Table S2, SDC, http://links.lww.com/TP/D9), including the nucleic acid extraction from EDTA-anticoagulated whole blood rather than plasma for parallel processing together with other transplant-relevant viruses, such as Epstein-Barr virus; the use of digital-droplet NAT for a more precise enumeration of the true genome viral load; the use of point-of-care/near patient testing for outpatient management; the measurement of viral transcripts, such as VP1 mRNA or viral miRNA by reverse transcription-QNAT; the development of CRISPR-cas9 and similar technologies; the direct testing of native urine without extraction; and the processing of resuspended native urine, or urine supernatants or of urine pellets after centrifugation, as well as electron microscopy for PyV virion particles and virion aggregates (hauffen). Although these approaches have plausible rationales, the available clinical data and the pro-and-cons as to their utility in guiding diagnosis and management are limited. Nevertheless, they speak to the fact that there is considerable innovation potential for improving the current diagnostic status in the near future, pending appropriately designed comparative clinical studies. This also applies to BKPyV genome sequencing using Sanger technology to assess the viral serotype and genotype present in donors and recipients or to identify rearrangements of the viral NCCR as a marker of advanced stages of BKPyV-nephropathy, the use of next-generation sequencing to capture the genome variability and minority variants more in-depth, as well as transcriptomic approaches to identify biomarkers of BKPyV pathology and the differential diagnoses including innate and alloimmune responses in the setting of transplantation.
BKPyV genotyping is based on the sequence heterogeneity in the viral genome, whereby a specific region of the VP1 gene has been reported to define 4 major serotypes that correlate with the target of serotype-specific neutralizing antibodies.20,181 BKPyV serotype I is most common worldwide (70%–80%) followed by serotype IV (10%–20%), whereas serotypes II and III are less frequently identified (1%–10%).182 Comprehensive genotyping requires the combined assessment of the BKPyV serotype sequence VP1 and parts of the LTAG gene to identify 10 subtypes of genotype I and IV (ie, genotype Ia, Ib-1, Ib-2, Ic; genotype IV into IVa-1, IVa-2, IVb-1, IVb-2, IVc-1, IVc-2).182-184 In addition to the serotype-specific neutralizing antibodies, recent work has identified genotype-specific differences in cytotoxic T-cell responses and genotype-independent escape.178,184
Immunological Methods
BKPyV-specific Antibodies
BKPyV-specific antibodies are most commonly analyzed in plasma or serum using ELISA format providing antigens coated to the solid phase. Total Ig, IgG, IgM, or IgA can be distinguished depending on the choice of the secondary antibody. Although some studies explored the LTag or agnoprotein,90,185 the most widely used antigen is the major capsid viral protein Vp1. The Vp1 antigen can be present as 3-dimensional virion-like virus-like particles (VLPs) or as Vp1 monomers or pentamers. Whereas purified VLPs mostly present the unique outer surface for antibody binding, Vp1-monomers and pentamers also allow access to the less specific, cross-reactive internal capsid surfaces, especially when coupled to other recombinant units, such as glutathione S-transferase or streptavidin. Without further characterization, Vp1 ELISAs do not distinguish whether the binding antibodies have different functional activities, such as opsonizing or neutralizing activities. Other assays include hemagglutination inhibition, preventing the VLP or virion binding to sugar residues on red blood cells, and neutralization of infectious BKPyV preparations or pseudoviruses (for review, see Kaur et al17 and Table S3, SDC, http://links.lww.com/TP/D9). Technically, preadsorption assays may help to estimate the cross-reactivity between different subtypes and other human polyomaviruses, especially JCPyV.186 In the general population, BKPyV seroprevalence increases to >90% during childhood, followed by declining rates and titers among adults 50 y of age.158,186-189 As outlined in the Epidemiology and Risk Factors section, the detection of BKPyV-specific antibodies in donors has been associated with an increased risk of BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy in kidney transplantation,190,191 particularly when donor BKVPyV IgG levels are high19,190 or when they are undetectable or low in the recipient.12,192 Notably, pretransplant seropositivity in the recipient does not confer protection from high-level viruria or BKPyV-DNAemia.6,13,18 However, high pseudovirus-neutralizing antibody titers of >10 000 (4 log10) 50% inhibitory concentration against the specific donor serotype BKPyV have been correlated with reduced BKPyV-DNAemia events posttransplant.20 BKPyV-specific antibody levels, as measured by ELISA using Vp1-based antigens, have been shown to significantly increase in response to BKPyV events, such as new-onset viruria, DNAemia, and nephropathy, and include the appearance of IgM.44,90,185,189,193-197 However, no correlation with clearance of BKPyV-DNAemia has been observed unless paralleled by increasing BKPyV-specific T-cell responses.44,195 Similarly, neutralizing antibody titers have been reported to increase in patients clearing BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy,20,198 but the potential contributory role of serotype-specific T-cell responses has not been assessed.178,184 A barrier to routine clinical use of BKPyV-specific IgG is the lack of standardization of such assays as well as limited commercial availability with only 1 ELISA currently available, which is not food and drug administration-approved for organ donor or recipient testing and does not allow for distinguishing between the different serotypes. Thus, prospective randomized interventional studies using validated serological assays are needed before recommendations can be made regarding the use of serological assays for risk stratification, organ allocation, adapted screening, or treatment modalities.
BKPyV-specific CMI
BKPyV-specific CMI is most commonly measured in peripheral blood without or with prior preparation and enrichment of peripheral blood mononuclear cells (PBMCs) through buffy coats or Ficoll density gradients. Other rarely described sources include kidney biopsy samples.199 CMI assays measure a variety of cellular differentiation and activation markers as well as effector functions, as summarized recently.17,200 Most clinical data refer to BKPyV-specific T-cell activities directly ex vivo or after prestimulation and short-term expansion in vitro (Table S3, SDC, http://links.lww.com/TP/D9). CMI read-outs are typically based on T-cell receptor binding and activation when interacting with viral peptides presented by HLA class I or class II molecules on the surface of nucleated cells or by recombinant multimers (HLA-tetramers, -pentamers, -dextramers, -streptamers). The HLA-presented BKPyV peptides used for the assay are chemically synthesized and correspond to amino acid sequences of viral proteins, such as LTag, sTag, Vp1, Vp2, Vp3 or agnoprotein, or come from the respective recombinant viral proteins or from BKPyV infected cell cultures preparations processed and presented by antigen-presenting cells (Table S3, SDC, http://links.lww.com/TP/D9). IFN-γ is the most frequently used functional read-out and can be extended to other cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-2, thereby defining so-called polyfunctional responses. Other read-outs are markers of activation, proliferation, degranulation, cell proliferation or the cytotoxic killing of pulsed phythemagglutinin (PHA) stimulated PHA-blasts, antigen-presenting cells, or other cells.44,189,195,201,202 Cytokine-release assays measure the secreted activity in cell culture supernatants by ELISA or enumerate the number of cytokine-secreting PBMCs by enzyme-linked immune absorbent spot (ELISpot) assays.200
Flow cytometry can be combined with intracellular cytokine staining (ICS) to identify T-cell responses expressing IFN-γ, TNF-α, or IL-2 and is typically used together with differentiation and secretion markers. When using fluorescently labeled HLA-multimers presenting viral peptides, BKPyV-specific T-cell receptors can be identified directly without or with stimulation/activation.44,202-206 Other functional assays include the antigen-dependent cell proliferation measured by H3-thymidine incorporation, fluorescent dye dilution (eg, carboxyfluorescein diacetate succinimidyl ester), or cytotoxic killing of peptide-pulsed autologous PHA-blast or other antigen-presenting cells.44,202-206 Combining the results of several CMI assays with different read-outs is usually considered to strengthen the interpretation and evidence level.
Overall, the clinical utility of direct testing for BKPyV-specific CMI is challenged by the often very low or undetectable number of BKPyV-specific T cells in peripheral blood. PBMC preparations have been expanded by BKPyV-specific stimulation in specialized laboratory settings to increase sensitivity.44,195,201,204 Studies exploring BKPyV-specific CMI in recipients before transplantation have reported somewhat conflicting results with respect to predicting BKPyV-DNAemia posttransplant. For example, no association was seen between pretransplant CMI measured via ELISpot and new-onset BKPyV-DNAemia posttransplant.207 Another study using ICS reported an association between posttransplant BKPyV-DNAemia and possibly senescent CD8 T cells, although a definition was lacking.208 Loss of pretransplant detectable BKPyV-specific CMI after kidney transplantation was associated with new-onset BKPyV-DNAemia.66 In several studies, subsequent mounting of BKPyV-specific CMI has been correlated with a >2 log10 c/mL decline or clearance of plasma BKPyV-DNAemia44,195,201,204,209,210 (Table S3, SDC, http://links.lww.com/TP/D9). BKPyV-specific CMI correlated inversely with the levels of maintenance immunosuppression in kidney transplant recipients in vivo, and increasing calcineurin inhibitor (CNI) concentrations also impaired polyfunctional CMI in vitro.211 Conversely, reducing immunosuppression was associated with increasing BKPyV-specific CMI and eventual clearance of BKPyV-DNAemia.195,201,212 Polyfunctional CMI and higher frequencies of CD4 and CD8 BKPyV-specific T cells correlated with a shorter duration and clearance of BKPyV-DNAemia.205,213-215 Detailed characterization of the T cells suggested a functional role of terminally differentiated effector memory phenotype in the clearance of BKPyV-DNAemia.216 Similarly, cytotoxic CD8 T cells responding to LTag-derived immunodominant 9mer-specific appeared to correlate better with clearance of BKPyV-DNAemia than CD4 T cells responding to 15mers.44 Although the overall results appear in line with current immunology concepts, the clinical translation of the evidence is hampered by differences in methodology, viral antigens used for stimulation, detection assays (ELISpot, ICS, or major histocompatibility complex-multimers), sensitivity and specificity, and time points posttransplant and relative to new-onset BKPyV-DNAemia. Identifying the most informative approach and working toward standardization of definitions, time points, and techniques may be key to identifying robust and commutable benchmarks that define the risk and show utility in guiding clinical management. Accordingly, clinically validated commercial CMI assays currently remain an unmet need in the field. Such assays are expected to guide decisions regarding immunosuppression and the potential clinical application of adoptive transfer of virus-specific T cells for the prevention and treatment of BKPyV events in kidney transplant patients.
Other Laboratory Assays
A variety of other markers have been studied to predict BKPyV-DNAemia (Table S4, SDC, http://links.lww.com/TP/D9). These include lymphocyte counts,66 nonspecific IgG levels, donor-derived cell-free DNA,217-219 measuring other cytokines, chemokines and their combination,220,221 Torque teno virus loads in plasma, as well as determining HLA or KIR polymorphisms.42,222,223 These markers need further development and validation before they can be used for guiding clinical decisions.
Considerations for Screening and Monitoring of Kidney Transplant Recipients
Given the absence of effective prophylaxis, kidney transplant patients should be tested regularly for plasma BKPyV-DNAemia by sensitive and specific plasma QNAT to identify BKPyV-nephropathy early at the stage of limited viral allograft involvement. When BKPyV-DNAemia is detected, testing should be repeated within 2 to 3 wk to confirm whether BKPyV-DNAemia has spontaneously resolved or is persistent, hence justifying intervention. In contrast, a threshold BKPyV-DNAemia of >10 000 c/mL (or equivalent) identifies presumptive BKPyV-nephropathy necessitating prompt reduction of immunosuppression if laboratory errors are unlikely. Persistent BKPyV-DNAemia at 1000 to 10 000 c/mL (or equivalent) identifies kidney transplant patients with probable BKPyV-nephropathy in cases without biopsy-proven BKPyV-nephropathy. Notably, persistent BKPyV-DNAemia at levels <10 000 c/mL has been reported in patients eventually diagnosed with biopsy-proven BKPyV-nephropathy.6,224,225 A positive screening result should trigger a clinical review of the immunosuppression and the monitoring of plasma BKPyV-DNAemia every 2 to 4 wk. Although higher plasma BKPyV loads have been linked to longer time to clearance,162,226,227 routine monitoring every 2 to 4 wk is still recommended because the use of intervals beyond every 4 wk may fail detection of significant BKPyV-DNAemia dynamics.224 After clearance of BKPyV-DNAemia, the optimal duration of monitoring is unknown, and it should be individualized, guided by viral kinetics, immunological risk, renal function, and cautious evaluation of (re-)increasing immunosuppression to prevent rejection and possible DSA formation. BKPyV recurrence may arise late after transplantation following treatment for rejection, with the risk depending on the type of antirejection treatment and other factors. Enhanced screening rates of high-risk patients (eg, needing re-increased immunosuppression) for BKPyV-DNAemia may allow early identification of recurrence.
Other Diagnostic Considerations
Resource-limited Settings
Although molecular testing for viruses has become more widely available because of the SARS-CoV-2/COVID-19 pandemic, screening and monitoring kidney transplant recipients for BKPyV by QNAT may represent a significant burden for transplant programs in resource-limited settings. Transplant programs in resource-limited settings may consider screening for urine decoy cells at similar time points as recommended for plasma BKPyV-DNAemia. Due to the high negative predictive value, undetectable decoy cells on properly processed urine cytology analyzed by trained personal can virtually exclude BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy.102,162 Conversely, the detection of urinary decoy cells should be followed up by measuring plasma BKPyV-DNAemia. Similar considerations apply to screening urine for BKPyV-DNA loads, whereby high-level loads of >10 million c/mL should be followed up by testing for BKPyV-DNAemia.164,228
Multiorgan Transplant Recipients, Including Kidney
A later onset of BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy has been reported in SPK transplant recipients compared with kidney transplant recipients.54,161,229 Some centers extend the duration of screening for BKPyV-DNAemia in SPK transplant recipients. However, there are insufficient data from cohort or prospective studies to define optimal duration and benefits regarding outcome. Even less data are available regarding screening other multiorgan transplants, including kidneys.230-233
Non-kidney SOT Recipients
BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy are rarely reported in non-kidney SOTs.234,235 Although some studies have suggested an association of chronic kidney failure and BKPyV-DNAemia in these patients,236-239 this association has not been found in cross-sectional studies.240,241 A series of 74 pancreas-only transplants reported a higher risk of pancreas allograft failure with BKPyV-DNAemia (associated with older age and lymphopenia), but no kidney biopsies or end-stage kidney disease were reported242; the impact of reduction of immunosuppression on the pancreas allograft in the setting of BKPyV-DNAemia needs further study. A recent retrospective study of lung transplant patients identified BKPyV-DNAemia in approximately 4% of patients, with biopsy-proven BKPyV-nephropathy in 1%.239
BKPyV-associated Urothelial Carcinoma
Prolonged high-level BKPyV replication with high BKPyV loads and biopsy-proven BKPyV-nephropathy may be associated with rearrangements of the NCCR in the viral genome93 and also increase the risk of chromosomal integration of the BKPyV genome and subsequent urothelial cancer.243-247 Increased incidence of urothelial cancers (adjusted IRR 2.2; 95% CI, 0.9-5.4) has been reported among kidney transplant recipients subsequent to prolonged BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy, supporting an association between BKPyV and urothelial carcinogenesis in this population.248 The diagnosis of BKPyV-associated urothelial carcinoma is not different from other forms of bladder cancer and requires histology of cancer obtained by cystoscopy and surgical removal. There is no specific screening modality for BKPyV-associated urothelial cancer compared with non-BKPyV-associated cancer. However, BKPyV-DNAemia is detectable in such patients and has been used for monitoring treatment outcomes, especially when metastatic disease is present.
MANAGEMENT
Given that there are no effective antivirals to prevent or treat BKPyV replication, the primary therapeutic intervention for sustained BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy in kidney transplant patients aims at reducing maintenance immunosuppression to allow for sufficient BKPyV-specific immune control, without precipitating rejection episodes (Figure 1).4,17
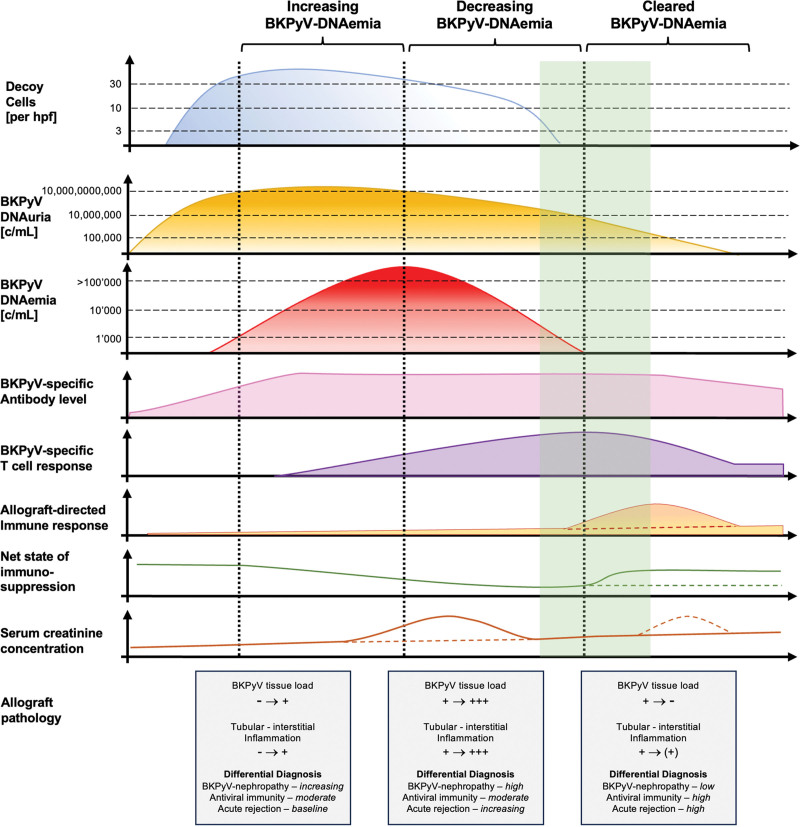
The primary outcome of the therapeutic intervention is monitored by following BKPyV-DNAemia loads because the significant decline and eventual clearance of BKPyV-DNAemia (ie, defined as decline below the LOD) correlate with the sustained disappearance of BKPyV replication foci from the renal allograft, a prerequisite for regenerating the tubular lining.4,162,164 A schematic diagram summarizes the timing of key events in the screening, intervention, and follow-up monitoring of kidney transplant recipients (Figure 2). The optimal strategy to reduce immunosuppression has not been defined; it varies among transplant centers and is often individualized because there are no randomized controlled trials (RCTs) directly comparing different protocols.4 The time point of diagnosis, early or late after transplantation, the magnitude of BKPyV-DNAemia, and whether routine screening or rather graft dysfunction led to diagnosing BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy may all influence clinical decision-making. Other important factors are the immunosuppressive regimen (eg, standard triple-drug regimen, CNI-sparing, steroid-sparing) and the dosing and target range as per institutional protocol; the known or perceived immunological risk; the presence of DSAs; the presence of other SOTs (eg, pancreas, liver, heart, lung); the history of rejection and antirejection treatment; and failure of a previous kidney transplant (Consensus recommendations and future directions, see Tables 6 and 7).
FIGURE 2.
Timelines of BKPyV replication and related laboratory and clinical events after kidney transplantation. Urinary cytology data mostly describe the onset of decoy cell shedding. Low-level BKPyV-DNAuria in native urine is detected in <10% before transplantation and increases to high-level BKPyV-viruria defined by decoy cells or BKPyV-DNAuria of >10 million copies/mL of urine. BKPyV-DNAemia loads are identified in plasma by QNAT approximately 2 to 6 wk after high-level BKPyV-DNAuria. BKPyV-specific antibody levels increase before immunosuppression is reduced. As the net state of immunosuppression decreases, rising BKPyV-specific T cell activity is detectable. The colored dashed lines attempt to capture different scenarios of marker and disease evolution. Serum creatinine concentration may increase when allograft BKPyV loads and the associated interstitial inflammation become more extensive. Reducing immunosuppression facilitates antiviral immunity (immune reconstitution), clearance of intragraft replication foci, and clearance of BKPyV-DNAemia loads below the limit of detection while increasing the risk of antidonor immunity and allograft rejection. Increase in serum creatinine may arise because of antiviral immune reconstitution or acute rejection, whereby the former may be transient, unlike the latter. The shaded green area marks the window of opportunity for reincreasing maintenance immunosuppression to prevent acute T cell–mediated rejection. Potentially accelerated generation of donor-specific antibodies and antibody-mediated rejection are not depicted. BKPyV, BK polyomavirus; c/mL, copies/mL; hpf, high-power field; QNAT, quantitative nucleic acid testing.
TABLE 6.
Practice guidance suggestions for reducing immunosuppression
| General management approach |
| • We suggest first confirming that all immunosuppressive drug doses and concentrations are within the institutional target range (weak, C) |
| • We recommend that BKPyV-DNAemia should be monitored every 2–4 wk until clearance (strong, B) or stabilizing at plasma viral loads <1000 c/mL (or equivalent) (weak, C) |
| • For rare patients on the lowest acceptable immunosuppression with detectable BKPyV-DNAemia below <1000 c/mL, we suggest follow-up of BKPyV-DNAemia and serum creatinine concentration every 3 mo (weak, D) |
| Strategy 1: Antimetabolite is reduced first |
| I. Reduction of the dose of antimetabolite by at least 50% |
| • We suggest further immunosuppression reduction if BKPyV-DNAemia does not decrease by 10-fold at 4 wk or does not clear below lower limit of detection (weak, C), as follows: |
| II. Discontinuation of the antimetabolite and tapering of corticosteroid dose to 5–10 mg/d of prednisone or equivalent, if applicable |
| • We suggest adding prednisone (or equivalent) 5–10 mg/d for patients who are not on corticosteroids to avoid CNI monotherapy (weak, C) |
| III. If further decrease in immunosuppression is necessary, we suggest a stepwise reduction of the CNI dose (tacrolimus trough target 5 ng/mL; cyclosporine trough target 100 ng/mL; weak, C) |
| • The target concentrations for further reduction are not well defined and need to be individualized. Expert opinion and case reports discuss tacrolimus target trough concentrations of 3 ng/mL and cyclosporine target trough concentrations 75 ng/mL followed by tacrolimus target trough of 1.5 ng/mL; cyclosporine target trough of 50 ng/mL (no recommendation—statement only) |
| Strategy 2: CNI is reduced first |
| I. Reduction of the dose of CNI by 25%–50% in 1 or 2 steps to target trough concentrations of tacrolimus of 3–5 ng/mL and cyclosporine trough concentrations of 75–125 ng/mL) |
| • We suggest further immunosuppression reduction if BKPyV-DNAemia does not decrease by 10-fold at 4 wk or does not clear below the lower limit of detection (weak, C), as follows: |
| II. Reduction of the antimetabolite by 50% and tapering of corticosteroid dose to 5–10 mg/d of prednisone or equivalent, if applicable |
| III. Discontinuation of the antimetabolite |
| • We suggest adding prednisone (or equivalent) 5–10 mg/d for patients who are not on corticosteroids to avoid CNI monotherapy (weak, C) |
| • The target concentrations of further reduction are not well defined and need to be individualized. Expert opinion and case reports discuss tacrolimus target trough concentrations of 3 ng/mL and cyclosporine target trough concentrations of 75 ng/mL followed by tacrolimus target trough of 1.5 ng/mL; cyclosporine target trough of 50 ng/mL (no recommendation—statement only) |
BKPyV, BK polyomavirus; CNI, calcineurin inhibitor.
TABLE 7.
Consensus recommendations: management
| Reduction of immunosuppression (see Table 6 for detailed guidance) |
| • We recommend reducing maintenance immunosuppression as the primary treatment of sustained BKPyV-DNAemia/-nephropathy in kidney transplant patients without high immunologic risk or concurrent acute rejection (strong, B) |
| • We suggest reducing immunosuppression when BKPyV-DNAemia is between 1000–10 000 copies/mL (or equivalent) on 2 measurements within 2–3 wk (weak, B) |
| • We recommend reducing immunosuppression based on 1 measurement BKPyV-DNAemia >10 000 copies/mL (or equivalent) or if biopsy-proven BKPyV-nephropathy (strong, B) |
| • We recommend reducing immunosuppression for biopsy-proven BKPyV-nephropathy even if plasma BKPyV-DNA load results needed to confirm the diagnosis are still pending (strong, B) |
| • We suggest each transplant center to develop an institutional algorithm and standard operating procedure of how to reduce immunosuppression in patients with BKPyV-DNAemia (weak, D) |
| • There is insufficient data to evaluate the efficacy of switching to mTOR inhibitors for treating BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy (no recommendation—statement only) |
| • We suggest to judiciously reincrease maintenance immunosuppression based on the individual immunologic risk after confirmed BKPyV-DNAemia clearance, with appropriate screening for BKPyV-DNAemia (weak, D) |
| • We suggest testing patients with persistent BKPyV-DNAemia despite the lowest acceptable immunosuppression for de novo DSA if there is evidence of renal dysfunction to assist decisions regarding kidney transplant biopsy (weak, D) |
| • For multiorgan transplant recipients, including kidney or non-kidney solid organ transplant recipients with BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy, we suggest a careful reduction of immunosuppression as per above, with close clinical and laboratory monitoring, weighing the risks and benefits of rejection and graft loss (weak, D) |
|
Statement • In the absence of data defining the best treatment of acute rejection in patients with ongoing BKPyV-DNAemia/-nephropathy, most experts apply high-dose steroid therapy followed by resuming close monitoring of renal allograft function and at least monthly monitoring of BKPyV-DNAemia for the next 3 to 6 mo (expert opinion) • Depending on the clinical course, some experts consider a judicious increment of maintenance immunosuppression, whereas others consider decreasing immunosuppression as a second step, especially in cases experiencing a significant rise in BKPyV-DNAemia loads (expert opinion) |
|
mTOR inhibitor regimens • For kidney transplant recipients developing BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy while receiving a combination of mTOR inhibitors and calcineurin inhibitors, there is insufficient data to guide the reduction of immunosuppression. |
| Possible approaches include |
| - to first reduce the dose of calcineurin inhibitor followed by a reduction of the dose of mTOR inhibitor if needed (expert opinion) |
| - to first switch to low-dose cyclosporine followed by a reduction of the dose of mTOR inhibitor if needed (expert opinion) |
|
Belatacept regimens • For kidney transplant recipients developing BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy while receiving a belatacept-based regimen, there is insufficient data to guide the reduction of immunosuppression. |
| Possible approaches include |
| -to first reduce or discontinue the antimetabolite (expert opinion) |
| -to increase the interval of belatacept administration to every 6–8 wk (expert opinion) |
| -to switch to a low-level calcineurin-based or mTOR inhibitor–based immunosuppressive regimen (expert opinion) |
| Adjunctive therapies |
| • We suggest consideration of intravenous immunoglobulin administration as adjuvant therapy in kidney transplant recipients with insufficient response to reduced immunosuppression to facilitate viral clearance (weak, D) |
| • We suggest consideration of IVIG administration as adjuvant therapy to prevent acute rejection in recipients with high immunological risk when immunosuppression reduction is necessary to facilitate viral clearance (weak, D) |
| • We recommend to not use cidofovir to treat BKPyV-DNAemia/-nephropathy in kidney transplant recipients (strong, B) |
| • We recommend to not use leflunomide to treat BKPyV-DNAemia/-nephropathy (strong, B) |
| • We recommend to not use fluoroquinolones to prevent or treat BKPyV-DNAemia or BKPyV-nephropathy in kidney transplant recipients (strong, A) |
| • We recommend to not use statins to prevent or treat BKPyV-DNAemia or BKPyV-nephropathy in kidney transplant recipients (strong, A) |
| Future directions |
| ➢ Randomized controlled trials to evaluate the administration of IVIG to prevent or treat BKPyV-DNAemia/-nephropathy |
| ➢ Randomized controlled trials to evaluate the administration of BKPyV-neutralizing monoclonal antibodies to prevent or treat BKPyV-DNAemia/-nephropathy |
| ➢ Randomized controlled trials to evaluate the administration of adoptive virus-specific T cells to prevent or treat BKPyV-DNAemia/-nephropathy |
| ➢ Development of BKPyV vaccines to prevent or improve treatment responses of BKPyV-DNAemia/-nephropathy |
| ➢ Development of effective and safe antiviral therapies to prevent or treat BKPyV-DNAemia/-nephropathy |
BKPyV, BK polyomavirus; DSA, donor-specific antibody; mTOR, mammalian target of rapamycin.
Kidney Transplant Recipients Receiving Standard Combination Immunosuppression
Reducing maintenance immunosuppression consisting of standard triple therapy with CNIs, such as tacrolimus or cyclosporine, antiproliferative agents, such as mycophenolate or azathioprine, and corticosteroids led to successful clearance of BKPyV-DNAemia below the LOD in 80% to 100% of cases according to a meta-analysis249 and several prospective observational studies.36,67,162,167,250 Treatment of biopsy-proven BKPyV-nephropathy tended to require more interventional steps, a longer time to clearance, and a higher risk of allograft failure.67,162 Rapid immunosuppression tapering was associated with a shorter duration of BKPyV-DNAemia and better allograft function in patients with biopsy-proven BKPyV-nephropathy.251 However, acute cellular rejection36,162,195,252,253 and subsequent de novo DSAs have been reported in around 10% to 30% of the patients during or after clearance of BKPyV-DNAemia in some studies.251,254-256 Overall, earlier intervention at lower levels of BKPyV-DNAemia required fewer intervention steps and resulted in fewer rejection episodes. As pointed out in the diagnostic section, assay sensitivity, the respective LOD, and the lower limit of quantification may differ between the various commercial and laboratory-developed assays.257 However, most experts agree to use a cautious watch-and-wait strategy once BKPyV-DNAemia loads have declined to <1000 c/mL (or equivalent) after reducing immunosuppression. Follow-up testing is not well defined, but most agree that this would correspond to monthly measurements for 3 mo of follow-up.
Notably, recurrent BKPyV-DNAemia has been observed in 10% to 20% of patients in the context of reincreasing immunosuppression for maintenance or actual treatment of rejection.162,164 There are 2 major strategies for reducing immunosuppression, which differ in the initial steps of either first reducing the antiproliferative drugs (signal-3) to facilitate the expansion of virus-specific lymphocytes or first reducing the CNIs (signal-1) to facilitate T-cell receptor activation and effector functions (Table 6). For both strategies, we suggest starting by reviewing and promptly adjusting the doses and trough concentrations of immunosuppressive drugs if found to be in excess of the respective predefined institutional target range.
Evidence for first reducing the antimetabolite mostly concerns the use of mycophenolate mofetil (MMF) or mycophenolic acid (MPA) and consists of initial dose reduction or discontinuation (Table S5, SDC, http://links.lww.com/TP/D9). MMF discontinuation for kidney transplant patients with sustained BKPyV-DNAemia (no allograft biopsy performed) is safe and effective for clearance of BKPyV-DNAemia.36 Notably, these patients underwent lymphocyte-depleting induction therapy, and in 8 of 23 (35%) patients, the CNI was reduced in a second step to achieve clearance.36 The risk of acute rejection was not elevated as long as CNI trough concentrations were within the therapeutic range, for example, tacrolimus (3–5 ng/mL) or cyclosporine (50–100 ng/mL) along with low-dose corticosteroids.36 This approach was safe and effective when assessed at 5 and 10 y of follow-up.250,254 Not all studies support the reduction/discontinuation of antimetabolites for those with sustained BKPyV-DNAemia. For example, in patients receiving alemtuzumab induction and MMF 1000 mg twice per day, reduction of the MMF dose may clear low-grade BKPyV-DNAemia without the need for discontinuation.258 Other studies reported that stopping mycophenolate was associated with a higher chance and faster clearance of the BKPyV-DNAemia with fewer graft losses than those in whom the mycophenolate was reduced but not discontinued.259 The role of mycophenolate discontinuation permitting de novo antibody formation has been a concern with respect to DSAs162 and has also been demonstrated for antibody formation in response to SARS-CoV-2 vaccines.260,261 Some studies reported an association of persistent BKPyV-DNAemia with de novo DSA formation following MPA discontinuation and tacrolimus reduction.255 Because the underlying mechanisms need further study, testing for de novo DSAs is performed according to center practice and available resources and is typically considered in the case of renal allograft dysfunction.
Evidence for first reducing the dose of the CNIs comes from 2 approaches to achieve declining and clearing BKPyV-DNAemia and resolving BKPyV-nephropathy. The first approach is to reduce the CNI dose by 25% to 50% in 1 or 2 steps, followed by a 50% reduction and subsequent discontinuation of the antiproliferative drug. The second is to simultaneously reduce both the CNI and the antimetabolite (Table S6, SDC, http://links.lww.com/TP/D9). In a prospective study of 293 patients, stepwise reduction of tacrolimus dose resulted in 92% clearance of BKPyV-DNAemia and 8.6% incidence of subsequent clinical rejection occurrence.167 Comparable findings were observed for pediatric patients, without increasing the rate of acute rejection or graft dysfunction.195 In a large retrospective study of 644 consecutive kidney transplants, clearance of BKPyV-DNAemia was seen in 96% of 105 patients, 39% of them after stepwise tacrolimus dose reduction and 43% after additional reduction of mycophenolate.162 Over a follow-up of 6 y, rates of rejection, graft survival, and patient survival were similar between kidney transplant recipients with and without BKPyV-DNAemia.162 A prospective study of reducing CNIs together with antimetabolites has been reported to clear BKPyV-DNAemia in all 28 patients with plasma viral loads >10 000 c/mL within 6 mo with ensuing acute cellular rejection in 14%, which responded to steroid treatment.67 A retrospective study found that >20% reduction in CNI concentration 1 mo after the initial BKPyV-DNAemia was associated with an increased rate of acute rejection262 but needed to be distinguished from virus-specific immune reconstitution.134
The treatment of acute rejection in patients with ongoing BKPyV-DNAemia/-nephropathy is as challenging as its diagnosis (see the Pathology section). In the absence of data defining the best approaches in such cases, most experts apply high-dose steroid therapy followed by resuming close monitoring of renal allograft function and at least monthly monitoring of BKPyV-DNAemia for the next 3 to 6 mo. Depending on the further course, some experts consider a judicious increment of maintenance immunosuppression, whereas others consider decreasing immunosuppression as a second step, especially in cases experiencing a significant rise in BKPyV-DNAemia loads. These approaches and additional interventions are not well supported by published data and represent expert case-by-case management integrating the clinical course, histopathological and virological findings, and potential novel biomarkers.263,264
mTOR Inhibitor–based and Belatacept-based Regimens
Two large multicenter trials in kidney transplant recipients reported a lower incidence of BKPyV-DNAemia and BKPyV-nephropathy in patients receiving tacrolimus, mTOR inhibitors, and steroids compared with those receiving tacrolimus, mycophenolate, and steroids.265,266 Potential mechanisms include lower CNI exposure, absence of mycophenolate, and specific antiviral mechanisms of mTOR inhibitors.
There are insufficient data to evaluate the efficacy of switching to mTOR inhibitors for treating BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy (Table S7, SDC, http://links.lww.com/TP/D9).85,267-269 Two case series reported improved viral clearance and superior graft function after switching from mycophenolate to everolimus together with reduced tacrolimus exposure.85,269 In a larger trial, switching patients from tacrolimus, mycophenolate, and steroids to either everolimus and reduced CNI or reduced mycophenolate and reduced CNI reported no significant difference in the combined endpoint of clearance of BKPyV-DNAemia or 50% reduction of viruria.267
The use of belatacept, mycophenolate, and steroids together has not been associated with a higher incidence of BKPyV replication.270,271 There are no data to guide the reduction of immunosuppression in this specific setting.
Multiorgan Transplant Recipients
A larger case series of BKPyV-DNAemia and nephropathy events in multiorgan transplants are available from SPK transplant recipients.32,229,272-275 In some studies, the rate of BKPyV-DNAemia tends to be higher, and the outcome of biopsy-proven BKPyV-nephropathy is inferior in SPK transplant recipients compared with kidney-only transplantation229,272 but not in other studies.32,273,275 Reduction of immunosuppression has been effective and reported a low risk of pancreas allograft loss.32,273-275 Only a few case reports are available from other multiorgan transplant recipients.231 Also, there is not sufficient evidence to guide immunosuppression reduction for treating other multiorgan transplant recipients, including kidney or non-kidney SOT recipients with BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy.
Adjuvant Therapies: Update and Rating
Very limited clinical data suggest benefits of currently available adjuvant therapies in addition to reducing maintenance immunosuppression for clearing BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy, with most data from noncontrolled case series, while results from randomized controlled studies are currently lacking.276
IVIG preparations contain high neutralizing antibody titers against BKPyV.277,278 Although one study suggested a lower incidence of BKPyV-DNAemia associated with the preventive administration of IVIG,279 there are only a few studies reporting a trend of improved clearance of BKPyV-DNAemia in patients receiving adjunctive IVIG compared with historical controls treated solely with immunosuppression reduction.109,280,281 Given the limitations in study design and sample size (Table S8, SDC, http://links.lww.com/TP/D9), adjunctive IVIG may potentially reduce or prevent rejection following immunosuppression reduction. IVIG is generally well tolerated, and the risk for complications, such as fever, nausea, or rarely thrombosis, is low, making it an option to consider. There is little evidence regarding which IVIG dosing is most appropriate or effective. Several scenarios have been considered: low dose of 0.1 to 0.3 g/kg body weight once every 2 to 4 wk as used to supply neutralizing antibodies; high-dose of 0.5 to 2 g/kg body weight for corticosteroid-sparing immunomodulation 1 to 3 times per week as used for treating immune thrombocytopenia; adjusted dosing as needed for correcting hypogammaglobulinemia.
Cidofovir has documented in vitro activity against BKPyV,282 but there are conflicting data regarding the in vivo activity of cidofovir against BKPyV.109,276,283 No RCTs provide evidence that cidofovir is beneficial for adjunctive treatment of biopsy-proven BKPyV-nephropathy. A 2010 meta-analysis of 1 cohort study and 11 case series (each with ≤25 patients) found no benefit of cidofovir.164,249 If it is considered for use, the lack of demonstrated benefit needs to be balanced against the risk of nephrotoxicity and ophthalmologic toxicity (Table S9, SDC, http://links.lww.com/TP/D9).
Leflunomide is an antiproliferative immunosuppressive agent originally approved for treating rheumatoid arthritis. The active metabolite teriflunomide inhibits T- and B-lymphocyte proliferation and may inhibit BKPyV replication in cell culture unless uridine is added in physiological concentrations.17,284 The clinical data on leflunomide use for BKPyV-DNAemia and nephropathy are conflicting (Table S10, SDC, http://links.lww.com/TP/D9).249,281,285-291 There are no RCTs of leflunomide; a phase II trial with FK778 (a derivative of leflunomide) failed to show any benefit for resolving BKPyV-nephropathy.292 A recent case series suggests that combining leflunomide with mTOR inhibitors, such as everolimus, may increase efficacy.293 Leflunomide has a long half-life and has been associated with hepatotoxicity.
Fluoroquinolones are bacterial gyrase/topoisomerase-inhibiting antibiotics that have modest inhibitory activity on BKPyV replication in vitro. Clinical data have reported mixed results (Table S11, SDC, http://links.lww.com/TP/D9). A retrospective study reported that a 1-mo treatment with fluoroquinolones led to reduced BKPyV-DNAemia rates compared with placebo.294 Another retrospective study reported that 30 d of ciprofloxacin resulted in fewer BKPyV replication events at 3 mo but not 12 mo.295 In a systematic review, fluoroquinolones were not associated with reduced rates of BKPyV-DNAemia, biopsy-proven BKPyV-nephropathy, or graft loss.296 A prospective RCT found that 3 mo of levofloxacin prophylaxis did not prevent BKPyV-DNAemia rates compared with placebo.297 Another RCT reported that 3 mo of ciprofloxacin did not reduce BKPyV-DNAemia rates but increased the rate of fluoroquinolone-resistant infections.298
3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) can decrease the expression of caveolin-1 required for BKPyV entry into cells. An in vitro study reported that pravastatin reduced the percentage of BKPyV-infected cells.299 However, in kidney transplant recipients, administration of statins at maximal cholesterol-lowering dose did not prevent the progression of BKPyV-DNAemia to BKPyV-nephropathy300 (Table S12, SDC, http://links.lww.com/TP/D9).
PEDIATRIC CONSIDERATIONS
Seroepidemiology studies indicate that the majority of primary BKPyV exposure occurs during childhood. Accordingly, the proportion of BKPyV-naive patients who lack BKPyV-specific cellular and humoral immunity is higher in the pediatric transplant population.189,301 According to a large multicenter study of the Cooperative European Paediatric Renal Transplant Initiative (CERTAIN) registry, the prevalence of BKPyV-DNAemia in pediatric kidney transplant recipients is highest (33.4%) in the first year posttransplant.8 Presumptive BKPyV-nephropathy defined by high-level plasma BKPyV-DNAemia loads of >10 000 c/mL and biopsy-proven BKPyV-nephropathy were reported in approximately 16% and 5% of patients, respectively,8 and slightly higher than in adult studies. Graft loss rates are variable and were reported in 24% at a mean of 24 mo after diagnosis in the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) cohort.302 Unlike in adults, a significant proportion of high-level BKPyV-DNAemia (12.5%) and biopsy-proven BKPyV-nephropathy (21.4%) in pediatric kidney transplant recipients occurs more than after 24 mo posttransplant,8 and would be missed by current screening interval recommendations for adult kidney transplant recipients (Consensus recommendations and future directions, see Table 8).
TABLE 8.
Consensus recommendations: pediatric kidney transplantation
| • For pediatric kidney transplant recipients, we recommend monthly screening for plasma BKPyV-DNAemia until mo 9, then every 3 mo until mo 24 posttransplant (strong, B), and we suggest further screening every 3 mo until mo 36 posttransplant (weak, C) |
| • We recommend reducing maintenance immunosuppression as the primary intervention of sustained BKPyV-DNAemia, presumptive, or biopsy-proven BKPyV-nephropathy in pediatric kidney transplant patients without concurrent acute rejection (strong, B) |
| • For pediatric kidney transplant recipients with BKPyV-DNAemia, we recommend performing a kidney biopsy as clinically indicated (eg, rise in serum creatinine, new-onset proteinuria, hematuria; strong, A) |
| • For pediatric patients with stable kidney transplant function and persistent BKPyV-DNAemia >10 000 c/mL (or equivalent) despite reducing immunosuppression, we suggest performing a renal allograft biopsy because serum creatinine rise may be delayed in children with significant renal injury including rejection (weak, B) |
| • For pediatric kidney transplant patients, we suggest to not use adjunctive therapies, including leflunomide, cidofovir, or fluoroquinolones, because of the lack of well-designed studies, poorly documented efficacy, and confounders arising from concomitant reduction in immunosuppression (weak, D) |
| Future directions |
| ➢ Evaluate the role of pretransplant BKPyV serology (qualitative and quantitative) in donor and pediatric recipient pairs to predict the risk of BKPyV-DNAemia/-nephropathy |
| ➢ Evaluate the role of pretransplant and posttransplant BKPyV-specific CMI (qualitative and quantitative) to predict BKPyV-DNAemia/-nephropathy and to guide reducing immunosuppression |
| ➢ Evaluate the role of antibody preparations in targeting and neutralizing BKPyV (sub-)types for preventing or treating BKPyV-DNAemia/-nephropathy |
| ➢ Evaluate the role of adoptive T-cell therapy for preventing or treating BKPyV-DNAemia/-nephropathy |
BKPyV, BK polyomavirus; CMI, cell-mediated immunity.
Risk factors for BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy are common to both adult and pediatric kidney transplant recipients (see above). Obstructive uropathy as the primary cause of kidney disease appeared as an independent risk factor for these outcomes.8,303 The underlying mechanism as to how obstructive uropathy predisposes to BKPyV-nephropathy is unclear8 and might derive from vesicoureteral reflux.164 Because congenital anomalies, including obstructive uropathy, are the most common cause of end-stage kidney disease in children,304 the association between obstructive uropathy and BKPyV events should be further examined. Other risk factors for BKPyV-nephropathy identified in the NAPRTCS cohort are depleting lymphocyte induction therapy and zero HLA-DR mismatch.302 According to the CERTAIN registry, younger recipient age at kidney transplantation (OR, 1.1 per year younger; P < 0.001) was an independent risk factor associated with new-onset BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy.8 Although the risk factor underlying younger age is unknown, it likely includes an increased rate of BKPyV-naive children. Calculations made from the available data suggest significantly elevated ORs for BKPyV-nephropathy ranging from 4.9301 to 13.75.305 Comparing BKPyV antibody levels in adult donor and pediatric recipient sera, only 17% of recipients younger than 6 y had high titers compared with 73% of older recipients (P < 0.002).10 The combination of having high antibody levels in the donor and low antibody levels in the recipients was associated with BKPyV-DNAemia of 57% (4/7), as compared with 0% (0/3) and 4% (1/26) for donor–recipient combinations being low–low or low–high, respectively (P = 0.004).10 For donor–recipient pairs being high–low, the OR for BKPyV-DNAemia was 5.16.10
Laboratory Testing in Pediatric Kidney Transplant Recipients
No specific data exist for identifying clinically relevant BKPyV-DNAemia loads in pediatric kidney transplant recipients; most reports use those proposed for adults.8,195 Analysis of a patient’s adaptive immune response toward BKPyV may be considered a useful tool to evaluate the individual capacity for effective BKPyV control. Measuring BKPyV-specific CMI in children indicated that clearance of BKPyV-DNAemia was associated with increasing BKPyV-specific T cells in peripheral blood.195 Measuring BKPyV-specific CD4 and CD8 T cells at the time of new-onset BKPyV-DNAemia allowed us to identify patients with transient, self-limiting BKPyV-DNAemia and those in need of preemptive treatment.306 BKPyV-specific CMI correlated with the duration of plasma BKPyV-DNAemia but not with the peak viral load. The detection of BKPyV-specific CD4 T cells (≥0.5 cells/μL) and CD8 T cells (≥0.1 cells/μL) revealed a positive predictive value of 1.0 and a negative predictive value of 0.86 for self-limiting DNAemia.306 Clearance of BKPyV-DNAemia and BKPyV-specific CMI responses may differ between standard immunosuppressive regimens and mTOR inhibitor–based therapy.306 As with adults, the measurement of BKPyV-specific CMI in children requires further validation in randomized clinical trials to ascertain its role in individualized decision-making, which may include unnecessary reduction of immunosuppression in patients with self-limiting BKPyV-DNAemia, thereby reducing the risk of posttreatment rejection events.
Indications for Allograft Biopsy in Pediatric Kidney Transplant Recipients
The studies guiding the indication for kidney allograft biopsy in children with BKPyV-DNAemia and baseline renal function are limited by their heterogeneity regarding study design, length of follow-up, screening protocols, and immunosuppressive regimens. This is captured in a survey of 90 pediatric providers where 31% of respondents said they performed a biopsy before adjusting medication in children with stable kidney function and high-level BKPyV-viruria, and 50% of respondents stated that biopsy was indicated in patients with stable kidney function and significant BKPyV-DNAemia.307 In studies including pediatric kidney transplant recipients with stable renal function undergoing surveillance biopsies, biopsy-proven BKPyV-nephropathy was identified in 1.3% to 4.9% within 3 to 24 mo after transplant; most but not all of these patients had documented BKPyV-DNAemia at the time of protocol biopsy.308-311 Biopsy-proven BKPyV-nephropathy was diagnosed in 2.2% to 7.7% of children with persistent BKPyV-DNAemia or high-level viruria undergoing for-cause biopsies.8,215,301,305,306,312-316
Management of BKPyV-DNAemia and BKPyV-nephropathy in Children
There is no evidence supporting any specific treatment for BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy in pediatric kidney transplant recipients other than reduction of immunosuppression. Most studies in children are retrospective, single-center analyses with findings confounded by patients receiving multiple interventions, typically in combination with reduced immunosuppression. In the absence of specific randomized clinical trials, timely decreasing of immunosuppression is the current treatment option for BKPyV-DNAemia/-nephropathy in pediatric kidney transplant recipients. In a prospective study of 62 children with a follow-up of 2 y, 13 developed BKPyV-DNAemia. Treatment was initiated without allograft biopsy and consisted of stepwise reduction of the CNI in 15% and of mycophenolate in 50%, followed by mycophenolate discontinuation if BKPyV-DNAemia persisted. All patients cleared BKPyV-DNAemia by a median of 2 mo after reducing immunosuppression, and none developed rejection.195 However, other studies reported lesser efficacy in reducing immunosuppression, especially for biopsy-proven BKPyV-nephropathy. Among 20 patients followed prospectively, 4 developed biopsy-proven BKPyV-nephropathy, with only 2 clearing BKPyV-DNAemia after reducing immunosuppression.313 Retrospective studies reported mixed results, including targeting lower tacrolimus trough levels (3–7 ng/mL) and switching from tacrolimus to cyclosporine or sirolimus.215,305,306,311,312,315,317-324
Three multicenter registries report variability in treatment approaches for BKPyV events. Among 313 children followed in the European CERTAIN registry, 64 developed BKPyV-DNAemia or biopsy-proven BKPyV-nephropathy, 45 (70.3%) were treated with reducing CNI, and 23 (35.9%) with reducing mycophenolate.8 In a North American registry of 542 patients, 25 developed BKPyV-nephropathy with 13/25 clearing BKPyV-DNAemia at a median of 5 mo after reducing immunosuppression (CNI reduction [n = 11] and mycophenolate reduction [n = 18]).305 Finally, a survey of European pediatric programs indicated treatment by reducing mycophenolate in 40%, CNI in 29%, and both in 31% of patients.307 The use of adjunctive therapies included cidofovir in 32%, leflunomide in 25%, fluoroquinolones in 20%, and IVIG in 22%.307 However, no trials in children systematically assessed the efficacy of adjunctive therapies, including leflunomide, cidofovir, or IVIG. Small studies have described patients who were switched from mycophenolate to leflunomide, with variable response rates and even prolonged times to clear BKPyV-DNAemia ranging from 10 to 27 mo.311,319,324-328 Cidofovir has been administered in doses of 0.25 to 1 mg/kg every 1 to 3 wk, typically without probenecid. Even with reduced immunosuppression, permanent decreases in kidney function and graft loss have been reported in patients receiving cidofovir, and not all patients have cleared BKPyV-DNAemia.312,314,318,320,322,325,326,329,330 Finally, IVIG in doses up to 2 g/kg body weight has been administered to children who did not respond to immunosuppression reduction, with mixed results that do not create enough evidence to recommend IVIG treatment in children.317,318,324,326,327,330,331
PK AND PD ASPECTS
Aspects of PK and PD in kidney transplant recipients with BKPyV-DNAemia and BKPyV-nephropathy include the following: (1) kinetics and dynamics of BKPyV replication; (2) PK and PD of immunosuppressive drugs directly promoting or inhibiting BKPyV replication; (3) PK and PD of immunosuppressive drugs and drug–drug interactions that may affect the kinetics and dynamics of BKPyV replication; (4) PK and PD of ancillary medications such as those that have been used as an adjunct therapy for prevention or treatment of BKPyV replication; and (5) pediatric aspects of immunosuppressive therapy (Consensus recommendations and future directions, see Table 9).
TABLE 9.
Consensus recommendations: PK and PD aspects
| • We recommend interpreting dosing and trough concentrations of immunosuppressive drugs in the context of PK/PD, drug–drug interactions (including all other medications, any alternative and complimentary medicines, and over-the-counter medications), as well as liver and kidney function when managing patients with BKPyV-DNAemia/-nephropathy (strong, B) |
| • We suggest carefully considering and monitoring the interactive PK/PD role(s) of immunosuppression on promoting or inhibiting viral replication, kinetics, and dynamics of BKPyV-DNAemia (weak, C) |
| • We recommend providing ongoing medication education to patients and caregivers regarding medication adherence and routinely assessing adherence (strong, B) |
| Future directions |
| ➢ Evaluate the role of immunosuppressive drugs in different compartments, such as unbound (free), total, or intracellular concentration, to guide the management of BKPyV-DNAemia/-nephropathy |
| ➢ Evaluate the PK/PD of immunosuppressive drugs for optimizing prevention and treatment of BKPyV-DNAemia/-nephropathy in pediatric and adult kidney transplant recipients |
| ➢ Include PK/PD analyses of novel agents in both pediatric and adult transplant populations, focusing on the prevention/treatment of BKPyV-DNAemia/-nephropathy |
BKPyV, BK polyomavirus; PD, pharmacodynamics; PK, pharmacokinetics.
The kinetics and dynamics of BKPyV replication in kidney transplant patients have been most directly approximated by measuring plasma BKPyV-DNAemia following transplant nephrectomy.332 In 3 patients undergoing allograft nephrectomy, the half-life [t(1/2)] of BKPyV-DNAemia ranged from 1 to 2 h up to 20 to 38 h and was independent of continued immunosuppressive treatment, whereas in 12 patients without allograft nephrectomy, lowering immunosuppression was associated with slower BKPyV-DNAemia clearance with t(1/2) of 6 h to 17 d, reflecting differences in sampling density and efficacy from reconstituting immunity.332 Assuming average half-lives of 2 h, >99% of plasma BKPyV-DNAemia are turned over daily in steady-state patients with stable plasma viral loads.332 Assuming urine BKPyV-DNAuria loads are 3 to 5 orders of magnitude higher in kidney transplant patients with than without detectable BKPyV-DNAemia.36,101,163,164 In patients with low-level BKPyV-viruria of <5.5 log10 c/mL, large increases in BKPyV-DNAuria were associated with only moderate increases in BKPyV-DNAemia, whereas in patients with very high urine BKPyV-viruria of >9.5 log10 c/mL, BKPyV-DNAemia loads increased more steeply. Mathematical modeling supported the notion that BKPyV replication starts in the kidney and is amplified by partial reflux from the urothelial compartment (renal pelvis, ureter, and bladder) into the allograft. Cytopathic loss was estimated to be >70 million renal tubular epithelial cells daily.164 This process drives the progression of BKPyV-nephropathy through inflammation, fibrosis, and tubular atrophy. Monitoring plasma BKPyV-DNAemia loads helps to ensure that the efficacy of a given intervention results in >80% curtailing of BKPyV replication needed for clearance.36,164
In patients without allograft nephrectomy, the time to clearance of BKPyV-DNAemia will be related to the peak level and the rate of ongoing replication in the kidney allograft and its representation in the blood of patients. In one RCT that used a strategy of monitoring and preemptive withdrawal of the antimetabolite on detection of BKPyV-DNAemia, the median time to onset of BKPyV-DNAuria was 40 d and the median time to onset of BKPyV-DNAemia was 60 d.36 The mean time to clearance of BKPyV-DNAemia was 54 d (range, 7–213 d). Only 5 of 23 (21%) patients with BKPyV-DNAemia cleared their urine during the >12-mo observation period. Twenty percent of patients underwent for-cause biopsy, but BKPyV-nephropathy was not detected on histology. Thus, this study describes the wide range of clearance of BKPyV-DNAemia in the absence of biopsy-proven BKPyV-nephropathy.
In another study of patients receiving tacrolimus, MMF, and prednisone, BKPyV-DNAemia was diagnosed at 44 to 986 d (mean 377) posttransplantation.253 At the time of diagnosis, the mean plasma BKPyV loads ranged from 10 205 to 1 920 691 c/mL (mean 460 409 c/mL) and serum creatinine ranged from 1.2 to 2.8 mg/dL (mean 1.8 mg/dL), consistent with presumptive BKPyV-nephropathy in all patients. Following the simultaneous lowering of both tacrolimus and MMF but not the discontinuation of either, a gradual decline in plasma BKPyV-DNAemia was noticed within 2 to 4 wk postintervention. BKPyV-DNAemia cleared after a mean period of 5.8 mo (range, 1–9.5). This study describes the wide range of clearance of BKPyV-DNAemia in the presence of biopsy-proven BKPyV-nephropathy.
Discerning the PK and PD of immunosuppressive drugs on BKPyV replication is difficult because some immunosuppressive drugs, such as cyclosporine,333,334 MPA, and mTOR inhibitors,335 have been reported to directly inhibit BKPyV replication in cell culture, whereas others such as tacrolimus and corticosteroids have been shown to promote BKPyV replication.17,93,335-337 By contrast, MPA, the active metabolite of leflunomide A771726, or the mTOR inhibitor sirolimus did not inhibit BKPyV-specific CMI as measured by IFN-γ, TNF-α, and IL-2 production, but interfered with antigen-specific T-cell expansion.211 Several clinical studies have associated the combined use of tacrolimus and MMF with an increased risk of BKPyV replication compared with cyclosporine, MMF, azathioprine, or other immunosuppressants, such as mTOR inhibitors and respective combinations (see above).
Another PK/PD effect of interest in kidney transplant patients is the reduced renal excretion of the metabolite mycophenolate acid glucuronide (MPAG) in patients with renal impairment.338 This is in part because uremia decreases MPA binding to albumin, leading to higher free fractions of MPA.339 MPAG is normally cleared by the kidney. In patients with a glomerular filtration rate (GFR) <25 mL/min, MPAG accumulates 3- to 6-fold, and worsening uremia leads to progressive 3- to 5-fold increases in the MPA-free fraction. These PK/PD considerations, including altered MPA/MPAG exposure in plasma and urine than expected on the basis of dosing, may contribute to the onset of BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy after transplant.
In pediatric kidney transplant patients, there are age-related PK and PD differences for many drugs compared with adult kidney transplant recipients.340 Some of the reasons are differences in gastric emptying time, intestinal transit time, plasma protein binding, liver enzyme metabolic capacity, and GFRs.341 Children typically need higher doses of many immunosuppressants (calculated according to body surface area) than those used in adults. Also, special preparations of the drugs, that is, as liquids or suspensions, are needed to facilitate and secure administration. PK monitoring based on trough levels or abbreviated area-under-curve measures can be important in children to reach the therapeutic window of the immunosuppressants.
Immunosuppressive protocols in pediatric kidney transplantation have substantially changed in the last decade. Many programs now rely on tacrolimus and MMF with early steroid withdrawal, frequently combined with antibody induction therapy. The higher treatment efficacy of this approach is accompanied by higher rates of viral complications, especially when children undergo primary viral infections. Summarizing the few randomized controlled pediatric trials, tacrolimus seems to have advantages over cyclosporine A (CsA) in pediatric kidney transplantation,342 steroid-free protocols are feasible in low immunological risk, and in White patients, if there is no overimmunosuppression,343 and induction therapy with IL-2 receptor antagonists might not be advantageous in a standard-risk population.344 However, RCTs of pediatric patients are lacking to compare all possible combinations of CsA, tacrolimus, MMF, or steroids. Nonrandomized trials have shown that additional therapy with MMF can reduce the number of acute rejections345 and can reverse the loss of GFR in children.346 Concerning the use of the mTOR inhibitor everolimus, retrospective studies and registry analyses reported fewer BKPyV events compared with controls; the rates of elevated cholesterol and arterial hypertension were increased.8,347,348 In a prospective randomized controlled study, early conversion to the mTOR inhibitor everolimus has shown to be equally effective in children despite a higher discontinuation rate than standard immunosuppression.349,350 However, there were no significant differences in the cumulative incidence of BKPyV-DNAemia during 1 and 3 y posttransplant.349,350 Finally, combining mTOR inhibitor with a low-dose CNI might be an option in children at high risk of BKPyV-DNAemia/-nephropathy, but sufficient data are lacking. In summary, the mainstay of immunosuppression in children after kidney transplantation is a combination of tacrolimus and MMF with or without induction therapy in combination with early steroid cessation in the case of a low immunological risk. Individualized approaches using mTOR inhibitors and biomarker-based personalizing of immunosuppression are expected in the near future.
COST–BENEFIT CONSIDERATIONS
Precise estimates of the cost–benefit of routine screening for BKPyV replication are difficult to determine as implementation varies widely. Programs differ in transplant donor and recipient populations, underlying diseases, immunological risk, immunosuppression protocols used, diagnostic testing, their performance, specimen type, frequency, and duration. Moreover, the clinical approaches to positive screening have been different. Here, we examine the long-term patient benefits and costs of a recommended screening strategy based on published test characteristics, including all relevant clinical and resource parameters as well as reduction in immunosuppression potentially contributing to rejection and developing donor-specific alloimmunity (Consensus recommendations and future directions see Table 10).
TABLE 10.
Consensus recommendations: cost–benefit considerations
| • We recommend routine screening for BKPyV-DNAemia using the strategies proposed in this current guideline because it is associated with an improvement in clinical outcomes and is cost-effective in kidney transplant recipients in robust models up to the seventh decade of life (strong, B) |
| • We suggest not decreasing the frequency of screening because it may reduce the efficacy of intervention by reducing immunosuppression, thereby increasing the overall direct healthcare costs (weak, B) |
| Future directions |
| ➢ Evaluate different screening strategies (modality and frequency) on cost-effectiveness, particularly because they relate to different geographic areas, ethnicities, and limited access to laboratory testing |
| ➢ Determine the cost-effectiveness ratio of duration and frequency of monitoring of patients not clearing BKPyV-DNAemia at the lowest possible level of immunosuppression (persistent low plasma viral loads with or without biopsy-proven BKPyV-nephropathy) |
| ➢ Evaluate the cost-effectiveness ratio of BKPyV-specific immunity, such as serotype-specific antibodies, neutralizing antibodies, or CMI, to shorten or extend screening for BKPyV-DNAemia |
| ➢ Evaluate the cost-effectiveness ratio of novel interventions such as neutralizing antibodies, novel antivirals, or adoptive T-cell therapies for prevention and therapy of BKPyV-DNAemia/-nephropathy |
| ➢ Assess the benefits of customized screening strategies based on immunosuppression exposure, including choice of induction agent and need for additional therapy for desensitization |
BKPyV, BK polyomavirus; CMI, cell-mediated immunity.
A first medical decision analysis model demonstrated in 2005 that routine screening for BKPyV replication after kidney transplantation would improve outcomes and reduce the total cost of care compared with a no-screening strategy.351,352 Key model parameters included the rate of biopsy-proven BKPyV-nephropathy presenting with declining renal function and rate of graft loss. In addition, the reduction of immunosuppression was modeled because the risk of acute rejection may be greater in this population. Modeled screening parameters reflected the proposed practice in 2005 with 6 urine cytology samples during 2 y and testing for BKPyV-DNAemia in positive screens. This testing was assumed to reduce the incidence of advanced biopsy-proven BKPyV-nephropathy by 80%. The model included estimates of the risk of a “false-positive” screen leading to inappropriate reduction of immunosuppression, increasing the risk of subclinical alloimmune injury and acute rejection. Cost estimates were drawn from US Medicare payments. In this model, screening for BKPyV replication provided a small net benefit of 0.02 quality-adjusted life years (QALYs) and cost savings of $1912. Importantly, if the rate of BKPyV-nephropathy in the unscreened group was <2.1%, the benefits of screening were lost and potentially harmful.
A second contemporary model of plasma screening for BKPyV-DNAemia monthly for 6 mo, then 3 monthly for up to 1 y, considered the greater use of lymphocyte-depleting induction and increased risk of BKPyV-nephropathy.353 This model estimated that biopsy-proven BKPyV-nephropathy developed in 10% of unscreened recipients. In the screened arm, 4.6% of patients were assumed to have a higher BKPyV-DNAemia load with a predicted probability of 87% of biopsy-proven BKPyV-nephropathy. An additional 13.5% had a lower BKPyV-DNAemia, and the estimated risk of biopsy-proven BKPyV-nephropathy was assumed to be 31%. The model included the possibility of retransplantation and had a longer time horizon than the 2005 study.352 Furthermore, at baseline, 10% of patients in the new study were assumed to require adjunctive therapies beyond the reduction in immunosuppression. Costs were reported in Australian dollars (AUD; 1 AUD = 0.7 USD = 0.6 EUR), with an estimated screening cost of $762 AUD per patient. The study demonstrated the benefit of screening, which resulted in a gain of 0.236 QALYs and savings of $6833 AUD. In a sensitivity analysis, the benefits of screening were stable across ages and rates of BKPyV-DNAemia in the screened arm. This study strongly supported the use and cost savings of routine screening for BKPyV-DNAemia (Figure 3).
FIGURE 3.
One-way sensitivity analyses: tornado diagram showing the influential variables on the incremental cost-effectiveness ratio in the base case model. The tornado diagram indicates the extent of the variability associated with these important variables on the incremental health benefits and costs. For example, if the age of transplantation is decreased from 70 y (shades of black) to 18 y (shades of gray), the incremental benefits of screening would increase from 0.20 to 0.24 QALYs. However, the total savings will be reduced from $7884 to $6844, as younger recipients would incur greater resources used over their lifetime compared with their older counterparts because of their longer expected posttransplant survival. EV, expected value; HD, hemodialysis; ICER, incremental cost-effectiveness ratio; PyVAN, polyomavirus-associated nephropathy; QALY, quality-adjusted life year. Adapted from Wong et al.353
A third analysis considered a more frequent screening strategy with BKPyV-DNAemia testing monthly for 9 and then every 3 mo through 2 y, leading to a reduction in immunosuppression in screening-positive patients (Bryce Kiberd, MD, unpublished data). The baseline incidence of biopsy-proven BKPyV-nephropathy developed in 8% of unscreened patients. BKPyV-DNAemia was detected in 18% of the screened arm, and treatment reduced the rate of biopsy-proven BKPyV-nephropathy by 90%. The model included the harms of screening and reducing immunosuppression, such as developing acute rejection with and without de novo DSAs. Patients with a failed transplant could be retransplanted. The time horizon was truncated at 95 y of age. Costs were from the perspective of the US federal government. In the baseline case analysis, the screening strategy added 0.189 more QALYs, saving $5497 USD. These results were most sensitive to the estimated rate of BKPyV-nephropathy with impaired allograft function, the rate of alloimmunity following immunosuppression reduction, and the economic benefits of transplantation. The benefit of screening was clearly greater if rates of BKPyV-nephropathy were higher and diminished if screening did not completely eliminate the development of BKPyV-nephropathy. Thus, frequent screening may benefit patients exposed to more intense immunosuppression, such as those undergoing desensitization regimens.74,354 Screening appeared beneficial if the rate of de novo DSA formation and acute rejection did not exceed 20% after immunosuppression reduction. Cost savings were sensitive to patient age and the cumulative incidence of biopsy-proven BKPyV-nephropathy in the unscreened arm. Screening of patients older than 70 y was associated with about a 50% reduction in savings. The largest driver of savings was the difference between the annual maintenance costs of dialysis and that of a functioning transplant. There were no savings from screening if the annual cost difference between dialysis and transplant was <$35 000.
There are significant limitations to the econometric analyses. Without a randomized control trial examining the clinical and economic benefits of screening, the risks and benefits of screening are extrapolated from published literature, without directly comparing the screened and unscreened groups, and may be subjected to a range of biases, including selection, confounding, and indication biases. Because historic outcomes of BKPyV-nephropathy were poor, in part because the screening was not in place and the disease was diagnosed late at a stage of allograft failure, it is possible that the assumed rates of graft loss from BKPyV-nephropathy are overestimated. The models may also underestimate the harm of screening by favoring unnecessary reduction of immunosuppression and the risk of subsequent rejection.251 Conversely, additional benefits of reducing immunosuppression, such as a lower risk of infectious complications and cancer, may not be accurately captured. Moreover, besides differences between pediatric and adult patients, it is often unclear, with few exceptions,6,23,28 whether episodes of rejection preceded or followed the detection of BKPyV-DNAemia in the cohort studies used to populate the models.156 Therefore, robust sensitivity analyses over a range of plausible estimates were performed, demonstrating that screening remained clinically and economically beneficial under all reasonable variations in these estimates.
Despite these limitations and differences in tree structures, assumptions, and perspectives, the key findings of the 3 cost-utility models are remarkably consistent. Screening results in net survival benefits and is cost-effective for kidney transplant recipients. It is important to note that these studies did not consider screening in other SOTs. SPK transplants may have higher rates of BKPyV-nephropathy and include patients with later onset.161,229 Screening duration and frequency may need to be adapted to this population to reduce missing cases but may affect the risk–benefit relationship. A report of biopsy-proven BKPyV-nephropathy has been described in a recipient of a simultaneous liver-kidney recipient.231 Further research is needed to determine the risks and benefits of screening in SLK recipients (ClinicalTrials.gov NCT05224583) as well as in other multi-SOTs, including kidneys.
RETRANSPLANTATION
Analyzing transplants performed between 2004 and 2008, retransplantation after graft loss from BKPyV-nephropathy was associated with a greater risk of a subsequent graft loss from BKPyV-nephropathy (15.2% versus 2.2% in second transplants without a history of BKPyV-nephropathy). However, death-censored graft survival was found to be equivalent (adjusted HR 0.78; 95% CI, 0.58-1.06; P = 0.11).84 To date, several retrospective analyses from the United States confirmed that retransplantation outcomes in patients with a failed first kidney transplant from BKPyV-nephropathy were acceptable.81,355 Patients retransplanted after a failed first transplant following BKPyV-nephropathy (n = 341) had slightly better 5-y death-censored graft survival (90.6%) than those whose transplant (n = 13 260) failed from other causes (83.9%). In an adjusted analysis, the outcomes were not statistically different (adjusted HR 0.78; 95% CI, 0.58-1.06; P = 0.11). Overall patient survival and risk of acute rejection were also equivalent between the 2 groups. Using Organ Procurement and Transplantation Network data between 2005 and 2016, including 495 kidney retransplantation procedures after allograft loss following BKPyV-nephropathy,355 the retransplanted kidney allografts had longer survival times than the original transplants that had failed because of BKPyV-nephropathy (10.44 versus 3.70 y, P < 0.0001). Only 10 of 495 retransplants had a subsequent graft loss from BKPyV-nephropathy. Overall 5-y death-censored graft survival (90.6%) was better in this population than those whose transplant failed from other causes (83.9%).355 Similarly, earlier literature review supported the feasibility of retransplantation because recurrent BKPyV-nephropathy occurred in only 2.3% of retransplant recipients, with the reported rate of graft loss from BKPyV-nephropathy being <1%83,356 (Consensus recommendations and future directions, see Table 11).
TABLE 11.
Consensus recommendations: retransplantation
| • We recommend retransplantation in otherwise eligible patients who lost their prior allograft from BKPyV-nephropathy (strong, B) |
| • We suggest that BKPyV-DNAemia be resolved before retransplantation (weak, C) |
| • We suggest not routinely performing graft nephrectomy before retransplantation in those patients with allograft failure subsequent to BKPyV-nephropathy having undetectable BKPyV-DNAemia (weak, C) |
| • There is insufficient information to make recommendations on the choice of immunosuppression for a subsequent kidney transplant after a prior kidney transplant failed because of BKPyV-nephropathy (no recommendation—statement only) |
| Future directions |
| ➢ Evaluate the need for BKPyV-DNAemia clearance before retransplantation in patients with a failed kidney transplant from BKPyV-nephropathy |
| ➢ Evaluate thresholds of BKPyV-specific immunity, such as neutralizing antibody titers and CMI, predicting increased risk or protection from recurrent BKPyV-DNAemia/-nephropathy |
| ➢ Evaluate the need for significant BKPyV-DNAuria decrease or clearance before retransplantation in patients with a failed kidney transplant from BKPyV-nephropathy |
| ➢ Evaluate the need for nephrectomy in patients with a failed transplant from BKPyV-nephropathy and persistent BKPyV-DNAemia before retransplantation |
| ➢ Evaluate the role of nephrectomy before retransplantation in patients with a previous multiorgan transplant and a failed kidney transplant from BKPyV-nephropathy |
BKPyV, BK polyomavirus; CMI, cell-mediated immunity.
Despite observational data indicating the principal feasibility, the rates of recurrent BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy may be higher, which raises questions about predictors of successful retransplantation after graft loss from biopsy-proven BKPyV-nephropathy. For patients without other non-kidney SOT, sustained clearance of BKPyV-DNAemia for 3 to 6 mo after reducing or discontinuing immunosuppression has been used as an indirect marker of sufficient BKPyV-specific immunity before retransplantation. For patients having persistent plasma BKPyV-DNAemia or being evaluated for preemptive retransplantation, a significant decrease of >2 log10 c/mL following reduced immunosuppression201 has been proposed as a marker for sufficient BKPyV-specific immunity in patients considered for preemptive retransplantation.83 Although graft nephrectomy has been demonstrated to instantaneously clear BKPyV-DNAemia and thereby reduce the risk of this source for reinfection of the subsequent renal allograft, there is no evidence that routine nephrectomy of the failed allograft lowers the risk of BKPyV-DNAemia and biopsy-proven BKPyV-nephropathy originating from and manifesting in the subsequent kidney transplant.164,357,358 Allograft nephrectomy cannot be assumed to increase the BKPyV-specific immunity. Moreover, the characteristics of the new donor organ, including tissue viral load, serotype mismatching, and the immunosuppression needed to accommodate the allograft, remain important factors unaffected by prior allograft nephrectomy. Evidence supporting the reduction of immunosuppression in patients with other organ transplants is limited and may otherwise compromise the function of vital organs. The current evidence is inconclusive regarding the need for allograft nephrectomy before retransplantation in patients with persistent BKPyV-DNAemia. Moreover, the native kidneys also remain as a potential reservoir of BKPyV infection, from where reactivation and superinfection of the second kidney transplant can occur particularly in cases with residual urine production and ureteral stenting. Together with the increased risk of surgical complications, routine nephrectomy of the failed kidney allograft cannot be endorsed either before or at the time of retransplantation.
CONCLUSIONS
Although long recognized for its impact on kidney transplant outcomes, BKPyV-nephropathy continues to, directly and indirectly, contribute to premature kidney allograft failure. In these guidelines, we have comprehensively reviewed and highlighted significant advances in the field and identified areas where we were able to reach a robust international consensus to provide both updated recommendations and future directions addressing unmet clinical needs in epidemiology and risk factors, pathology, diagnostics, management, pediatric and pharmacologic considerations, cost–benefit, and retransplantation. Clearly, proactive, careful screening and monitoring after kidney transplantation, integrated histopathology evaluation, and the importance of a timely reduction of immunosuppression for BKPyV-DNAemia/-nephropathy after careful consideration of the individual risks are the cornerstones of mitigating the impact of BKPyV in kidney transplantation. As highlighted in each section, much additional research is needed to advance this field, whereby randomized clinical trials play a key role regarding evidence and future recommendations.
ACKNOWLEDGMENTS
The authors thank Veronique Malo and Nataniel Barrera of The Transplantation Society and Elizaveta Skomorokhova, BA, of the University of Basel for administrative and technical support.
Supplementary Material
APPENDIX: The Transplantation Society International BK Consensus Groupa/The TTS International Consensus/Guideline Meeting on BK Polyomavirus in Kidney Transplantation
Leaders: Camille N. Kotton (United States) and Hans H. Hirsch (Switzerland)
– Epidemiology and Risk Factors: Nassim Kamar (France), David Wojciechowski (United States), Sophie Caillard (France), Michael Eder (Austria), Mario Fernandez-Ruiz (Spain), Rainer Oberbauer (Austria), and Ligia Pierrotti (Brazil).
– Pathology: Parmjeet Randhawa (United States), Helmut Hopfer (Switzerland), Cinthia Drachenberg (United States), Yael Heher (United States), Gang Huang (China), Michael Mengel (Canada), Luis Morales Buenrostro (Mexico), and Brian Nankivell (Australia).
– Diagnostics: Martina Sester (Germany), Patrizia Comoli (Italy), Laurie Bertels (South Africa), Anantharaman Vathsala (Singapore), Franck Halary (France), Albert Heim (Germany), Deepali Kumar (Canada), Karoline Leuzinger (Switzerland), Christine Hanssen Rinaldo (Norway), Joanna Schaenman (United States), and Monica Slavin (Australia).
– Management: Helio Tedesco-Silva (Brazil), Greg Knoll (Canada), Stefan Schaub (Switzerland), Ilies Benotmane (France), Nicole Theodoropoulos (United States), Ilkka Helantera (Finland), Oliver Witzke (Germany), Steven Chadban (Australia), Soumita Bagchi (India), and Daniel C. Brennan (United States).
– Pediatrics and PK/PD: Lars Pape (Germany), Jennifer Trofe-Clark (United States), Nissreen Elfadawy (United States), Vikas R. Dharnidharka (United States), Motoshi Hattori (Japan), Raman Venkataramanan (United States), Burkhard Tönshoff (Germany), and Benjamin Laskin (United States).
– Cost–benefit and retransplant: David Axelrod (United States), Bryce Kiberd (Canada), Germaine Wong (Australia), Daniel Abramowicz (Belgium), Marcelo Cantarovich (Canada), Marta Crespo (Spain), Sanjay Kumar Agarwal (India), Nicolas Mueller (Switzerland), and Ya-Chung Tian (Taiwan).
Consensus contributors (leaders underlined, then in alphabetical order).
C.N.K. and H.H.H. contributed equally to developing this guideline.
A full list of participants of The Transplantation Society International BK Polyomavirus Consensus Group is included under Appendix.
All authors participated in the consensus meeting, review and summary of available data, and in writing the article.
C.N.K. received grants from Biohope and funding for serving on scientific advisory boards for Roche Diagnostics. N.K. received consulting fees, honoraria, and travel support from Astellas, AstraZeneca, Biotest, CSL Behring, Chiesi, Gilead, Hansa, Merck, Sharp and Dohme, Glasgow Smith Kline, Neovii, Novartis Pharma, Roche, Sanofi, Sandoz, and Takeda. P.R. received consulting fees from Allovir. M.S. received consulting fees from Merck, Sharp and Dohme, Moderna, and Biotest and honoraria from Biotest, Novartis, Merck, Sharp and Dohme, Takeda, and Qiagen. P.C. received honoraria from Atara Bio and Pierre Fabre Pharma. H.T.S. received grants from Biohope, Merck Sharp and Dohme, Natera, Novartis, and Takeda; honoraria from Alexion, CareDx, EMS Pharmaceuticals, Natera, and Takeda; and consulting fees and travel support from Takeda. D.C.B. received grants from CareDx and VeraTherapeutics and consulting fees from CareDx, Medeor Therapeutics, Natera, Sanofi, and Vera Therapeutics. L.P. received grants from Alexion, Chiesi, and Novartis; consulting fees from Alnylam and Chiesi; and travel support from Alexion. H.H.H. received consulting fees from AICuris, Allovir, Moderna, VeraTX, and Roche and honoraria from VeraTX, Takeda, Biotest, and Gilead. The other authors declare no conflicts of interest.
The BK Polyomavirus Consensus Conference was organized by The Transplantation Society. An independent, nonrestricted grant from Roche Diagnostics to The Transplantation Society made this conference possible.
At no time did the funding sources influence the list of attendees, discussion, or content.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
Contributor Information
Collaborators: Sophie Caillard, Mario Fernandez-Ruiz, Rainer Oberbauer, Ligia Pierrotti, Cinthia Drachenberg, Yael Heher, Gang Huang, Michael Mengel, Luis Morales Buenrostro, Brian Nankivell, Laurie Bertels, Anantharaman Vathsala, Franck Halary, Albert Heim, Deepali Kumar, Karoline Leuzinger, Christine Hanssen Rinaldo, Joanna Schaenman, Monica Slavin, Stefan Schaub, Ilies Benotmane, Nicole Theodoropoulos, Ilkka Helantera, Oliver Witzke, Steven Chadban, Soumita Bagchi, Nissreen Elfadawy, Vikas R. Dharnidharka, Motoshi Hattori, Raman Venkataramanan, Burkhard Tönshoff, Benjamin Laskin, Daniel Abramowicz, Marcelo Cantarovich, Marta Crespo, Sanjay Kumar Agarwal, Nicolas Mueller, and Ya-Chung Tian
REFERENCES
- 1.Wu Z, Graf FE, Hirsch HH. Antivirals against human polyomaviruses: leaving no stone unturned. Rev Med Virol. 2021;31:e2220. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79:1277–1286. [DOI] [PubMed] [Google Scholar]
- 3.Kotton CN, Kumar D, Caliendo AM, et al. ; The Transplantation Society International CMV Consensus Group. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102:900–931. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch HH, Randhawa PS; AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation—guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13528. [DOI] [PubMed] [Google Scholar]
- 5.Leuzinger K, Naegele K, Schaub S, et al. Quantification of plasma BK polyomavirus loads is affected by sequence variability, amplicon length, and non-encapsidated viral DNA genome fragments. J Clin Virol. 2019;121:104210. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–496. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Eckardt KU, Dorman NM, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97:1117–1129. [DOI] [PubMed] [Google Scholar]
- 8.Höcker B, Schneble L, Murer L, et al. Epidemiology of and risk factors for BK polyomavirus replication and nephropathy in pediatric renal transplant recipients: an international CERTAIN Registry study. Transplantation. 2019;103:1224–1233. [DOI] [PubMed] [Google Scholar]
- 9.Andrews CA, Shah KV, Daniel RW, et al. A serological investigation of BK virus and JC virus infections in recipients of renal allografts. J Infect Dis. 1988;158:176–181. [DOI] [PubMed] [Google Scholar]
- 10.Ali AM, Gibson IW, Birk P, et al. Pretransplant serologic testing to identify the risk of polyoma BK viremia in pediatric kidney transplant recipients. Pediatr Transplant. 2011;15:827–834. [DOI] [PubMed] [Google Scholar]
- 11.Grellier J, Hirsch HH, Mengelle C, et al. Impact of donor BK polyomavirus replication on recipient infections in living donor transplantation. Transpl Infect Dis. 2018;20:e12917. [DOI] [PubMed] [Google Scholar]
- 12.Sood P, Senanayake S, Sujeet K, et al. Donor and recipient BKV-specific IgG antibody and posttransplantation BKV infection: a prospective single-center study. Transplantation. 2013;95:896–902. [DOI] [PubMed] [Google Scholar]
- 13.Bijol V, Cimic A, Viscidi RP, et al. Pretransplant IgG antibodies to polyoma BK virus in pediatric renal transplants. Pediatr Transplant. 2010;14:224–227. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz A, Linnenweber-Held S, Heim A, et al. Viral origin, clinical course, and renal outcomes in patients with BK virus infection after living-donor renal transplantation. Transplantation. 2016;100:844–853. [DOI] [PubMed] [Google Scholar]
- 15.Verghese PS, Schmeling DO, Filtz EA, et al. The impact of recipient BKV shedding before transplant on BKV viruria, DNAemia, and nephropathy post-transplant: a prospective study. Pediatr Transplant. 2017;21:e12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verghese PS, Schmeling DO, Knight JA, et al. The impact of donor viral replication at transplant on recipient infections posttransplant: a prospective study. Transplantation. 2015;99:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur A, Wilhelm M, Wilk S, et al. BK polyomavirus-specific antibody and T-cell responses in kidney transplantation: update. Curr Opin Infect Dis. 2019;32:575–583. [DOI] [PubMed] [Google Scholar]
- 18.Bohl DL, Brennan DC, Ryschkewitsch C, et al. BK virus antibody titers and intensity of infections after renal transplantation. J Clin Virol. 2008;43:184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wunderink HF, van der Meijden E, van der Blij-de Brouwer CS, et al. Pretransplantation donor-recipient pair seroreactivity against BK polyomavirus predicts viremia and nephropathy after kidney transplantation. Am J Transplant. 2017;17:161–172. [DOI] [PubMed] [Google Scholar]
- 20.Solis M, Velay A, Porcher R, et al. Neutralizing antibody-mediated response and risk of BK virus-associated nephropathy. J Am Soc Nephrol. 2018;29:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demey B, Tinez C, François C, et al. Risk factors for BK virus viremia and nephropathy after kidney transplantation: a systematic review. J Clin Virol. 2018;109:6–12. [DOI] [PubMed] [Google Scholar]
- 22.Pourkazemi A, Shenagari M, Monfared A, et al. Analysis of risk factors influencing the BK polyomavirus replication in patients with ESRD waiting for kidney transplantation. Microb Pathog. 2020;149:104558. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch HH, Vincenti F, Friman S, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramos E, Drachenberg CB, Portocarrero M, et al. BK virus nephropathy diagnosis and treatment: experience at the University of Maryland Renal Transplant Program. Clin Transpl. 2002:143–153. [PubMed] [Google Scholar]
- 25.Cleenders E, Koshy P, Van Loon E, et al. An observational cohort study of histological screening for BK polyomavirus nephropathy following viral replication in plasma. Kidney Int. 2023;104:1018–1034. [DOI] [PubMed] [Google Scholar]
- 26.Brochot E, Descamps V, Handala L, et al. BK polyomavirus in the urine for follow-up of kidney transplant recipients. Clin Microbiol Infect. 2019;25:112.e1–112.e5. [DOI] [PubMed] [Google Scholar]
- 27.Dogan SE, Celebi ZK, Akturk S, et al. Prevalence and risk factors of BK viremia in patients with kidney transplantation: a single-center experience from Turkey. Transplant Proc. 2017;49:532–536. [DOI] [PubMed] [Google Scholar]
- 28.Theodoropoulos N, Wang E, Penugonda S, et al. BK virus replication and nephropathy after alemtuzumab-induced kidney transplantation. Am J Transplant. 2013;13:197–206. [DOI] [PubMed] [Google Scholar]
- 29.Jacobi J, Prignitz A, Büttner M, et al. BK viremia and polyomavirus nephropathy in 352 kidney transplants; risk factors and potential role of mTOR inhibition. BMC Nephrol. 2013;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shenagari M, Monfared A, Eghtedari H, et al. BK virus replication in renal transplant recipients: analysis of potential risk factors may contribute in reactivation. J Clin Virol. 2017;96:7–11. [DOI] [PubMed] [Google Scholar]
- 31.Mitterhofer AP, Umbro I, Pietropaolo V, et al. Polyomavirus BK infection in end-stage renal disease: analysis of viral replication in patients on hemodialysis or peritoneal dialysis. Transplant Proc. 2012;44:1869–1872. [DOI] [PubMed] [Google Scholar]
- 32.Mindlova M, Boucek P, Saudek F, et al. Prevalence and risk factors of polyomavirus BK replication in simultaneous pancreas/kidney transplant recipients from a single transplant center. Clin Transplant. 2012;26:267–274. [DOI] [PubMed] [Google Scholar]
- 33.Maliakkal JG, Brennan DC, Goss C, et al. Ureteral stent placement and immediate graft function are associated with increased risk of BK viremia in the first year after kidney transplantation. Transpl Int. 2017;30:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashim F, Rehman S, Gregg JA, et al. Ureteral stent placement increases the risk for developing BK viremia after kidney transplantation. J Transplant. 2014;2014:459747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siparsky NF, Kushnir LF, Gallichio MH, et al. Ureteral stents: a risk factor for polyomavirus BK viremia in kidney transplant recipients undergoing protocol screening. Transplant Proc. 2011;43:2641–2644. [DOI] [PubMed] [Google Scholar]
- 36.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–594. [DOI] [PubMed] [Google Scholar]
- 37.Wingate JT, Brandenberger J, Weiss A, et al. Ureteral stent duration and the risk of BK polyomavirus viremia or bacteriuria after kidney transplantation. Transpl Infect Dis. 2017;19:e12644. [DOI] [PubMed] [Google Scholar]
- 38.Thomas A, Dropulic LK, Rahman MH, et al. Ureteral stents: a novel risk factor for polyomavirus nephropathy. Transplantation. 2007;84:433–436. [DOI] [PubMed] [Google Scholar]
- 39.Masutani K, Ninomiya T, Randhawa P. HLA-A2, HLA-B44 and HLA-DR15 are associated with lower risk of BK viremia. Nephrol Dial Transplant. 2013;28:3119–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kavuzlu M, Baştürk B, Ataç FB, et al. Investigation of the relationship between BK virus and human leukocyte antigens in kidney transplant recipients. Exp Clin Transplant. 2020;18(Suppl 1):51–54. [DOI] [PubMed] [Google Scholar]
- 41.Gheith O, Al-Otaibi T, Zakaria Z, et al. Human leukocyte antigen Cw7-mediated protection against polyoma BK virus in renal transplant recipients who received grafts from antigen-positive donors. Exp Clin Transplant. 2015;13(Suppl 1):383–387. [PubMed] [Google Scholar]
- 42.Wunderink HF, De Brouwer CS, Gard L, et al. Source and relevance of the BK polyomavirus genotype for infection after kidney transplantation. Open Forum Infect Dis. 2019;6:ofz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willhelm M, Wilk S, Kaur A, et al. Can HLA-B51 protect against BKPyV-DNAemia? Transplantation. 2019;103:e384–e385. [DOI] [PubMed] [Google Scholar]
- 44.Leboeuf C, Wilk S, Achermann R, et al. ; Swiss Transplant Cohort Study. BK polyomavirus-specific 9mer CD8 T cell responses correlate with clearance of BK viremia in kidney transplant recipients: first report from the Swiss Transplant Cohort Study. Am J Transplant. 2017;17:2591–2600. [DOI] [PubMed] [Google Scholar]
- 45.Rohn H, Michita RT, Schramm S, et al. HLA-E polymorphism determines susceptibility to BK virus nephropathy after living-donor kidney transplant. Cells. 2019;8:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vu D, Sakharkar P, Shah T, et al. Association of interferon gamma gene polymorphisms with BK virus infection among Hispanic renal allograft recipients. Transplantation. 2014;97:660–667. [DOI] [PubMed] [Google Scholar]
- 47.Awadalla Y, Randhawa P, Ruppert K, et al. HLA mismatching increases the risk of BK virus nephropathy in renal transplant recipients. Am J Transplant. 2004;4:1691–1696. [DOI] [PubMed] [Google Scholar]
- 48.Borni-Duval C, Caillard S, Olagne J, et al. Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation. 2013;95:1498–1505. [DOI] [PubMed] [Google Scholar]
- 49.El-Husseini A, Hassan W, Yaseen M, et al. Impact of human leukocyte antigen and calculated panel reactive antibody on BK viremia in kidney transplant recipients: a single-center experience and literature review. Transpl Infect Dis. 2019;21:e13071. [DOI] [PubMed] [Google Scholar]
- 50.Favi E, Puliatti C, Sivaprakasam R, et al. Incidence, risk factors, and outcome of BK polyomavirus infection after kidney transplantation. World J Clin Cases. 2019;7:270–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hässig A, Roos M, Etter A, et al. Association of BK viremia with human leukocyte antigen mismatches and acute rejection, but not with type of calcineurin inhibitor. Transpl Infect Dis. 2014;16:44–54. [DOI] [PubMed] [Google Scholar]
- 52.Schold JD, Rehman S, Kayle LK, et al. Treatment for BK virus: incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl Int. 2009;22:626–634. [DOI] [PubMed] [Google Scholar]
- 53.Thangaraju S, Gill J, Wright A, et al. Risk factors for BK polyoma virus treatment and association of treatment with kidney transplant failure: insights from a paired kidney analysis. Transplantation. 2016;100:854–861. [DOI] [PubMed] [Google Scholar]
- 54.Benavides CA, Pollard VB, Mauiyyedi S, et al. BK virus-associated nephropathy in sirolimus-treated renal transplant patients: incidence, course, and clinical outcomes. Transplantation. 2007;84:83–88. [DOI] [PubMed] [Google Scholar]
- 55.Dadhania D, Snopkowski C, Ding R, et al. Epidemiology of BK virus in renal allograft recipients: independent risk factors for BK virus replication. Transplantation. 2008;86:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019–1026. [DOI] [PubMed] [Google Scholar]
- 57.Kim SJ, Rhu J, Yoo H, et al. Outcome comparison between low-dose rabbit anti-thymocyte globulin and basiliximab in low-risk living donor kidney transplantation. J Clin Med. 2020;9:1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prince O, Savic S, Dickenmann M, et al. Risk factors for polyoma virus nephropathy. Nephrol Dial Transplant. 2009;24:1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barbosa D, Kahwaji J, Puliyanda D, et al. Polyomavirus BK viremia in kidney transplant recipients after desensitization with IVIG and rituximab. Transplantation. 2014;97:755–761. [DOI] [PubMed] [Google Scholar]
- 60.Hasegawa M, Ito T, Saigo K, et al. Association of DNA amplification with progress of BK polyomavirus infection and nephropathy in renal transplant recipients. Transplant Proc. 2014;46:556–559. [DOI] [PubMed] [Google Scholar]
- 61.Hirsch HH, Randhawa P; AST Infectious Diseases Community of Practice. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):179–188. [DOI] [PubMed] [Google Scholar]
- 62.Huang G, Zhang L, Liang X, et al. Risk factors for BK virus infection and BK virus-associated nephropathy under the impact of intensive monitoring and pre-emptive immunosuppression reduction. Transplant Proc. 2014;46:3448–3454. [DOI] [PubMed] [Google Scholar]
- 63.Mengel M, Marwedel M, Radermacher J, et al. Incidence of polyomavirus-nephropathy in renal allografts: influence of modern immunosuppressive drugs. Nephrol Dial Transplant. 2003;18:1190–1196. [DOI] [PubMed] [Google Scholar]
- 64.Manitpisitkul W, Drachenberg C, Ramos E, et al. Maintenance immunosuppressive agents as risk factors for BK virus nephropathy: a case-control study. Transplantation. 2009;88:83–88. [DOI] [PubMed] [Google Scholar]
- 65.Cosio FG, Amer H, Grande JP, et al. Comparison of low versus high tacrolimus levels in kidney transplantation: assessment of efficacy by protocol biopsies. Transplantation. 2007;83:411–416. [DOI] [PubMed] [Google Scholar]
- 66.Schachtner T, Stein M, Babel N, et al. The loss of BKV-specific immunity from pretransplantation to posttransplantation identifies kidney transplant recipients at increased risk of BKV replication. Am J Transplant. 2015;15:2159–2169. [DOI] [PubMed] [Google Scholar]
- 67.Sood P, Senanayake S, Sujeet K, et al. Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation. 2012;94:814–821. [DOI] [PubMed] [Google Scholar]
- 68.Binet I, Nickeleit V, Hirsch HH, et al. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation. 1999;67:918–922. [DOI] [PubMed] [Google Scholar]
- 69.Doberer K, Schiemann M, Strassl R, et al. Torque teno virus for risk stratification of graft rejection and infection in kidney transplant recipients—a prospective observational trial. Am J Transplant. 2020;20:2081–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernández-Ruiz M, Albert E, Giménez E, et al. Early kinetics of Torque teno virus DNA load and BK polyomavirus viremia after kidney transplantation. Transpl Infect Dis. 2020;22:e13240. [DOI] [PubMed] [Google Scholar]
- 71.Avery RK, Motter JD, Jackson KR, et al. Quantifying infection risks in incompatible living donor kidney transplant recipients. Am J Transplant. 2021;21:1564–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharif A, Alachkar N, Bagnasco S, et al. Incidence and outcomes of BK virus allograft nephropathy among ABO- and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bentall A, Neil D, Sharif A, et al. ABO-incompatible kidney transplantation is a novel risk factor for BK nephropathy. Transplantation. 2015;99:e8–e9. [DOI] [PubMed] [Google Scholar]
- 74.Kauke T, Klimaschewski S, Schoenermarck U, et al. Outcome after desensitization in HLA or ABO-incompatible kidney transplant recipients: a single center experience. PLoS One. 2016;11:e0146075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Speer C, Kälble F, Nusshag C, et al. Outcomes and complications following ABO-incompatible kidney transplantation performed after desensitization by semi-selective immunoadsorption—a retrospective study. Transpl Int. 2019;32:1286–1296. [DOI] [PubMed] [Google Scholar]
- 76.Rodríguez-Goncer I, Ruiz-Ruigómez M, López-Medrano F, et al. CMV infection, valganciclovir exposure, and the risk of BK viremia and associated nephropathy after kidney transplantation: is there a link? Transpl Infect Dis. 2021;23:e13597. [DOI] [PubMed] [Google Scholar]
- 77.Blazquez-Navarro A, Dang-Heine C, Wittenbrink N, et al. BKV, CMV, and EBV interactions and their effect on graft function one year post-renal transplantation: results from a large multi-centre study. EBioMedicine. 2018;34:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elfadawy N, Flechner SM, Liu X, et al. CMV viremia is associated with a decreased incidence of BKV reactivation after kidney and kidney-pancreas transplantation. Transplantation. 2013;96:1097–1103. [DOI] [PubMed] [Google Scholar]
- 79.Toyoda M, Puliyanda DP, Amet N, et al. Co-infection of polyomavirus-BK and cytomegalovirus in renal transplant recipients. Transplantation. 2005;80:198–205. [DOI] [PubMed] [Google Scholar]
- 80.Reischig T, Kacer M, Hes O, et al. Cytomegalovirus prevention strategies and the risk of BK polyomavirus viremia and nephropathy. Am J Transplant. 2019;19:2457–2467. [DOI] [PubMed] [Google Scholar]
- 81.Dharnidharka VR, Cherikh WS, Neff R, et al. Retransplantation after BK virus nephropathy in prior kidney transplant: an OPTN database analysis. Am J Transplant. 2010;10:1312–1315. [DOI] [PubMed] [Google Scholar]
- 82.Geetha D, Sozio SM, Ghanta M, et al. Results of repeat renal transplantation after graft loss from BK virus nephropathy. Transplantation. 2011;92:781–786. [DOI] [PubMed] [Google Scholar]
- 83.Hirsch HH, Ramos E. Retransplantation after polyomavirus-associated nephropathy: just do it? Am J Transplant. 2006;6:7–9. [DOI] [PubMed] [Google Scholar]
- 84.Leeaphorn N, Thongprayoon C, Chon WJ, et al. Outcomes of kidney retransplantation after graft loss as a result of BK virus nephropathy in the era of newer immunosuppressant agents. Am J Transplant. 2020;20:1334–1340. [DOI] [PubMed] [Google Scholar]
- 85.Muñoz-Gallego I, Díaz-Madridano N, Moral N, et al. Detection of BK polyomavirus genotypes to predict the development of BK polyomavirus-associated complications in kidney transplant recipients: a retrospective analysis. Transpl Infect Dis. 2021;23:e13615. [DOI] [PubMed] [Google Scholar]
- 86.Nukuzuma S, Takasaka T, Zheng HY, et al. Subtype I BK polyomavirus strains grow more efficiently in human renal epithelial cells than subtype IV strains. J Gen Virol. 2006;87(Pt 7):1893–1901. [DOI] [PubMed] [Google Scholar]
- 87.Varella RB, Zalona ACJ, Diaz NC, et al. BK polyomavirus genotypes Ia and Ib1 exhibit different biological properties in renal transplant recipients. Virus Res. 2018;243:65–68. [DOI] [PubMed] [Google Scholar]
- 88.Virtanen E, Seppälä H, Helanterä I, et al. BK polyomavirus microRNA expression and sequence variation in polyomavirus-associated nephropathy. J Clin Virol. 2018;102:70–76. [DOI] [PubMed] [Google Scholar]
- 89.Bauman Y, Nachmani D, Vitenshtein A, et al. An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe. 2011;9:93–102. [DOI] [PubMed] [Google Scholar]
- 90.Leuenberger D, Andresen PA, Gosert R, et al. Human polyomavirus type 1 (BK virus) agnoprotein is abundantly expressed but immunologically ignored. Clin Vaccine Immunol. 2007;14:959–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manzetti J, Weissbach FH, Graf FE, et al. BK polyomavirus evades innate immune sensing by disrupting the mitochondrial network and promotes mitophagy. iScience. 2020;23:101257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bethge T, Hachemi HA, Manzetti J, et al. Sp1 sites in the noncoding control region of BK polyomavirus are key regulators of bidirectional viral early and late gene expression. J Virol. 2015;89:3396–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gosert R, Rinaldo CH, Funk GA, et al. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J Exp Med. 2008;205:841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perets TT, Silberstein I, Rubinov J, et al. High frequency and diversity of rearrangements in polyomavirus BK noncoding regulatory regions cloned from urine and plasma of Israeli renal transplant patients and evidence for a new genetic subtype. J Clin Microbiol. 2009;47:1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boldorini R, Allegrini S, Miglio U, et al. Genomic mutations of viral protein 1 and BK virus nephropathy in kidney transplant recipients. J Med Virol. 2009;81:1385–1393. [DOI] [PubMed] [Google Scholar]
- 96.Sharma PM, Gupta G, Vats A, et al. Polyomavirus BK non-coding control region rearrangements in health and disease. J Med Virol. 2007;79:1199–1207. [DOI] [PubMed] [Google Scholar]
- 97.McIlroy D, Hönemann M, Nguyen NK, et al. Persistent BK polyomavirus viruria is associated with accumulation of VP1 mutations and neutralization escape. Viruses. 2020;12:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Purighalla R, Shapiro R, McCauley J, et al. BK virus infection in a kidney allograft diagnosed by needle biopsy. Am J Kidney Dis. 1995;26:671–673. [DOI] [PubMed] [Google Scholar]
- 99.Randhawa PS, Finkelstein S, Scantlebury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67:103–109. [DOI] [PubMed] [Google Scholar]
- 100.Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2:25–30. [DOI] [PubMed] [Google Scholar]
- 101.Drachenberg CB, Papadimitriou JC, Hirsch HH, et al. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. Am J Transplant. 2004;4:2082–2092. [DOI] [PubMed] [Google Scholar]
- 102.Drachenberg CB, Hirsch HH, Papadimitriou JC, et al. Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: a prospective evaluation. Transplantation. 2007;84:323–330. [DOI] [PubMed] [Google Scholar]
- 103.Menter T, Mayr M, Schaub S, et al. Pathology of resolving polyomavirus-associated nephropathy. Am J Transplant. 2013;13:1474–1483. [DOI] [PubMed] [Google Scholar]
- 104.Drachenberg CB, Papadimitriou JC, Chaudhry MR, et al. Histological evolution of BK virus-associated nephropathy: importance of integrating clinical and pathological findings. Am J Transplant. 2017;17:2078–2091. [DOI] [PubMed] [Google Scholar]
- 105.Kable K, Davies CD, O’Connell PJ, et al. Clearance of BK virus nephropathy by combination antiviral therapy with intravenous immunoglobulin. Transplant Direct. 2017;3:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Drachenberg CB, Beskow CO, Cangro CB, et al. Human polyoma virus in renal allograft biopsies: morphological findings and correlation with urine cytology. Hum Pathol. 1999;30:970–977. [DOI] [PubMed] [Google Scholar]
- 107.Meehan SM, Kraus MD, Kadambi PV, et al. Nephron segment localization of polyoma virus large T antigen in renal allografts. Hum Pathol. 2006;37:1400–1406. [DOI] [PubMed] [Google Scholar]
- 108.Drachenberg RC, Drachenberg CB, Papadimitriou JC, et al. Morphological spectrum of polyoma virus disease in renal allografts: diagnostic accuracy of urine cytology. Am J Transplant. 2001;1:373–381. [PubMed] [Google Scholar]
- 109.Wadei HM, Rule AD, Lewin M, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN). Am J Transplant. 2006;6(5 Pt 1):1025–1032. [DOI] [PubMed] [Google Scholar]
- 110.Masutani K, Shapiro R, Basu A, et al. The Banff 2009 Working Proposal for polyomavirus nephropathy: a critical evaluation of its utility as a determinant of clinical outcome. Am J Transplant. 2012;12:907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nickeleit V, Singh HK, Randhawa P, et al. ; Banff Working Group on Polyomavirus Nephropathy. The Banff Working Group classification of definitive polyomavirus nephropathy: morphologic definitions and clinical correlations. J Am Soc Nephrol. 2018;29:680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kowalewska J, El Moudden I, Perkowska-Ptasinska A, et al. Assessment of the Banff Working Group classification of definitive BK polyomavirus nephropathy. Transpl Int. 2021;34:2286–2296. [DOI] [PubMed] [Google Scholar]
- 113.Bouatou Y, Nguyen TQ, Roelofs J, et al. A multicenter application of the 2018 Banff Classification for BK polyomavirus-associated nephropathy in renal transplantation. Transplantation. 2019;103:2692–2700. [DOI] [PubMed] [Google Scholar]
- 114.Nickeleit V, Singh HK, Dadhania D, et al. The 2018 Banff Working Group classification of definitive polyomavirus nephropathy: a multicenter validation study in the modern era. Am J Transplant. 2021;21:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang M, Zhou Q, Wang H, et al. An application of the 2018 Banff Classification for BK polyomavirus-associated nephropathy in renal transplantation. Transpl Infect Dis. 2021;23:e13557. [DOI] [PubMed] [Google Scholar]
- 116.Howell DN, Smith SR, Butterly DW, et al. Diagnosis and management of BK polyomavirus interstitial nephritis in renal transplant recipients. Transplantation. 1999;68:1279–1288. [DOI] [PubMed] [Google Scholar]
- 117.Nickeleit V, Hirsch HH, Binet IF, et al. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol. 1999;10:1080–1089. [DOI] [PubMed] [Google Scholar]
- 118.Jeong HJ, Hong SW, Sung SH, et al. Polyomavirus nephropathy in renal transplantation: a clinico-pathological study. Transpl Int. 2003;16:671–675. [DOI] [PubMed] [Google Scholar]
- 119.Celik B, Randhawa PS. Glomerular changes in BK virus nephropathy. Hum Pathol. 2004;35:367–370. [DOI] [PubMed] [Google Scholar]
- 120.Chen XT, Deng RH, Yang SC, et al. Pathological characteristics of BK polyomavirus-associated nephropathy with glomerular involvement. Ann Transl Med. 2020;8:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brealey JK. Ultrastructural observations in a case of BK virus nephropathy with viruses in glomerular subepithelial humps. Ultrastruct Pathol. 2007;31:1–7. [DOI] [PubMed] [Google Scholar]
- 122.Ravindran A, Kashtan CE, Sethi S. BK nephropathy with glomerular involvement. Kidney Int. 2018;94:432. [DOI] [PubMed] [Google Scholar]
- 123.Nankivell BJ, Renthawa J, Shingde M, et al. The importance of kidney medullary tissue for the accurate diagnosis of BK virus allograft nephropathy. Clin J Am Soc Nephrol. 2020;15:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nankivell BJ, Renthawa J, Sharma RN, et al. BK virus nephropathy: histological evolution by sequential pathology. Am J Transplant. 2017;17:2065–2077. [DOI] [PubMed] [Google Scholar]
- 125.Batal I, Franco ZM, Shapiro R, et al. Clinicopathologic analysis of patients with BK viruria and rejection-like graft dysfunction. Hum Pathol. 2009;40:1312–1319. [DOI] [PubMed] [Google Scholar]
- 126.Masutani K, Shapiro R, Basu A, et al. Putative episodes of T-cell-mediated rejection in patients with sustained BK viruria but no viremia. Transplantation. 2012;94:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Womer KL, Guerra G, Dibadj K, et al. Immunosuppression reduction for BK virus nephropathy: a case for caution. Transpl Infect Dis. 2007;9:244–248. [DOI] [PubMed] [Google Scholar]
- 129.Atsumi H, Asaka M, Kimura S, et al. A case of second renal transplantation with acute antibody-mediated rejection complicated with BK virus nephropathy. Clin Transplant. 2010;24(Suppl 22):35–38. [DOI] [PubMed] [Google Scholar]
- 130.Mainra R, Xu Q, Chibbar R, et al. Severe antibody-mediated rejection following IVIG infusion in a kidney transplant recipient with BK-virus nephropathy. Transpl Immunol. 2013;28:145–147. [DOI] [PubMed] [Google Scholar]
- 131.Batal I, Zainah H, Stockhausen S, et al. The significance of renal C4d staining in patients with BK viruria, viremia, and nephropathy. Mod Pathol. 2009;22:1468–1476. [DOI] [PubMed] [Google Scholar]
- 132.Mohamed M, Parajuli S, Muth B, et al. In kidney transplant recipients with BK polyomavirus infection, early BK nephropathy, microvascular inflammation, and serum creatinine are risk factors for graft loss. Transpl Infect Dis. 2016;18:361–371. [DOI] [PubMed] [Google Scholar]
- 133.Nickeleit V, Hirsch HH, Zeiler M, et al. BK-virus nephropathy in renal transplants-tubular necrosis, MHC-class II expression and rejection in a puzzling game. Nephrol Dial Transplant. 2000;15:324–332. [DOI] [PubMed] [Google Scholar]
- 134.Schaub S, Mayr M, Egli A, et al. Transient allograft dysfunction from immune reconstitution in a patient with polyoma BK-virus-associated nephropathy. Nephrol Dial Transplant. 2007;22:2386–2390. [DOI] [PubMed] [Google Scholar]
- 135.Dziubianau M, Hecht J, Kuchenbecker L, et al. TCR repertoire analysis by next generation sequencing allows complex differential diagnosis of T cell-related pathology. Am J Transplant. 2013;13:2842–2854. [DOI] [PubMed] [Google Scholar]
- 136.Zeng G, Huang Y, Huang Y, et al. Antigen-specificity of T cell infiltrates in biopsies with T cell-mediated rejection and BK polyomavirus viremia: analysis by next generation sequencing. Am J Transplant. 2016;16:3131–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Adam BA, Kikic Z, Wagner S, et al. Intragraft gene expression in native kidney BK virus nephropathy versus T cell-mediated rejection: prospects for molecular diagnosis and risk prediction. Am J Transplant. 2020;20:3486–3501. [DOI] [PubMed] [Google Scholar]
- 138.Meehan SM, Kadambi PV, Manaligod JR, et al. Polyoma virus infection of renal allografts: relationships of the distribution of viral infection, tubulointerstitial inflammation, and fibrosis suggesting viral interstitial nephritis in untreated disease. Hum Pathol. 2005;36:1256–1264. [DOI] [PubMed] [Google Scholar]
- 139.Mannon RB, Hoffmann SC, Kampen RL, et al. Molecular evaluation of BK polyomavirus nephropathy. Am J Transplant. 2005;5:2883–2893. [DOI] [PubMed] [Google Scholar]
- 140.Rogers NM, Russ GR, Cooper J, et al. Immunophenotyping of interstitial infiltrate does not distinguish between BK virus nephropathy and acute cellular rejection. Nephrology (Carlton). 2009;14:118–122. [DOI] [PubMed] [Google Scholar]
- 141.Buettner M, Xu H, Böhme R, et al. Predominance of TH2 cells and plasma cells in polyoma virus nephropathy: a role for humoral immunity? Hum Pathol. 2012;43:1453–1462. [DOI] [PubMed] [Google Scholar]
- 142.Solez K, Axelsen RA, Benediktsson H, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993;44:411–422. [DOI] [PubMed] [Google Scholar]
- 143.McGregor SM, Chon WJ, Kim L, et al. Clinical and pathological features of kidney transplant patients with concurrent polyomavirus nephropathy and rejection-associated endarteritis. World J Transplant. 2015;5:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wiegley N, Walavalkar V, Aujla H, et al. Clinicopathologic characteristics of JC virus nephropathy in kidney transplant recipients. Transplantation. 2021;105:1069–1076. [DOI] [PubMed] [Google Scholar]
- 145.Lautenschlager I, Jahnukainen T, Kardas P, et al. A case of primary JC polyomavirus infection-associated nephropathy. Am J Transplant. 2014;14:2887–2892. [DOI] [PubMed] [Google Scholar]
- 146.Höcker B, Tabatabai J, Schneble L, et al. JC polyomavirus replication and associated disease in pediatric renal transplantation: an international CERTAIN Registry study. Pediatr Nephrol. 2018;33:2343–2352. [DOI] [PubMed] [Google Scholar]
- 147.Aguilar J, Chang DH, Lin M, et al. JC virus-associated nephropathy in a post-heart and -kidney transplantation patient. Transpl Infect Dis. 2020;22:e13288. [DOI] [PubMed] [Google Scholar]
- 148.Asim M, Chong-Lopez A, Nickeleit V. Adenovirus infection of a renal allograft. Am J Kidney Dis. 2003;41:696–701. [DOI] [PubMed] [Google Scholar]
- 149.Kozlowski T, Nickeleit V, Andreoni K. Donor-transmitted adenovirus infection causing kidney allograft nephritis and graft loss. Transpl Infect Dis. 2011;13:168–173. [DOI] [PubMed] [Google Scholar]
- 150.Nanmoku K, Ishikawa N, Kurosawa A, et al. Clinical characteristics and outcomes of adenovirus infection of the urinary tract after renal transplantation. Transpl Infect Dis. 2016;18:234–239. [DOI] [PubMed] [Google Scholar]
- 151.Hemmersbach-Miller M, Duronville J, Sethi S, et al. Hemorrhagic herpes simplex virus type 1 nephritis: an unusual cause of acute allograft dysfunction. Am J Transplant. 2017;17:287–291. [DOI] [PubMed] [Google Scholar]
- 152.Hensley JL, Sifri CD, Cathro HP, et al. Adenoviral graft-nephritis: case report and review of the literature. Transpl Int. 2009;22:672–677. [DOI] [PubMed] [Google Scholar]
- 153.Lee Y, Kim YJ, Cho H. BK virus nephropathy and multiorgan involvement in a child with heart transplantation. Clin Nephrol. 2019;91:107–113. [DOI] [PubMed] [Google Scholar]
- 154.Adam B, Randhawa P, Chan S, et al. Banff Initiative for Quality Assurance in Transplantation (BIFQUIT): reproducibility of polyomavirus immunohistochemistry in kidney allografts. Am J Transplant. 2014;14:2137–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pan L, Lyu Z, Adam B, et al. Polyomavirus BK nephropathy-associated transcriptomic signatures: a critical reevaluation. Transplant Direct. 2018;4:e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Halloran PF, Madill-Thomsen KS, Böhmig GA, et al. ; INTERCOMEX Investigators. A 2-fold approach to polyoma virus (BK) nephropathy in kidney transplants: distinguishing direct virus effects from cognate T cell-mediated inflammation. Transplantation. 2021;105:2374–2384. [DOI] [PubMed] [Google Scholar]
- 157.Ramos E, Drachenberg CB, Wali R, et al. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87:621–630. [DOI] [PubMed] [Google Scholar]
- 158.Egli A, Dumoulin A, Köhli S, et al. Polyomavirus BK after kidney transplantation—role of molecular and immunological markers. Trends Transpl. 2009;3:85–102. [Google Scholar]
- 159.Ahlenstiel-Grunow T, Sester M, Sester U, et al. BK polyomavirus-specific T cells as a diagnostic and prognostic marker for BK polyomavirus infections after pediatric kidney transplantation. Transplantation. 2020;104:2393–2402. [DOI] [PubMed] [Google Scholar]
- 160.David-Neto E, Agena F, Silva Ribeiro David D, et al. Effect of polyoma viremia on 3-year allograft kidney function. Transpl Infect Dis. 2019;21:e13056. [DOI] [PubMed] [Google Scholar]
- 161.Imlay H, Whitaker K, Fisher CE, et al. Clinical characteristics and outcomes of late-onset BK virus nephropathy in kidney and kidney-pancreas transplant recipients. Transpl Infect Dis. 2018;20:e12928. [DOI] [PubMed] [Google Scholar]
- 162.Bischof N, Hirsch HH, Wehmeier C, et al. Reducing calcineurin inhibitor first for treating BK polyomavirus replication after kidney transplantation: long-term outcomes. Nephrol Dial Transplant. 2019;34:1240–1250. [DOI] [PubMed] [Google Scholar]
- 163.Randhawa P, Ho A, Shapiro R, et al. Correlates of quantitative measurement of BK polyomavirus (BKV) DNA with clinical course of BKV infection in renal transplant patients. J Clin Microbiol. 2004;42:1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Funk GA, Gosert R, Comoli P, et al. Polyomavirus BK replication dynamics in vivo and in silico to predict cytopathology and viral clearance in kidney transplants. Am J Transplant. 2008;8:2368–2377. [DOI] [PubMed] [Google Scholar]
- 165.Kim GJ, Lee JH, Lee DH. Clinical and prognostic significance of Merkel cell polyomavirus in nonsmall cell lung cancer. Medicine (Baltimore). 2017;96:e5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ramanan P, Timmerman EA, Fidler ME, et al. A kidney transplant recipient with renal medullary viral cytopathic changes. Transpl Infect Dis. 2017;19:e12646. [DOI] [PubMed] [Google Scholar]
- 167.Schaub S, Hirsch HH, Dickenmann M, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10:2615–2623. [DOI] [PubMed] [Google Scholar]
- 168.Hassan S, Mittal C, Amer S, et al. Currently recommended BK virus (BKV) plasma viral load cutoff of ≥4 log10/mL underestimates the diagnosis of BKV-associated nephropathy: a single transplant center experience. Transpl Infect Dis. 2014;16:55–60. [DOI] [PubMed] [Google Scholar]
- 169.Agrawal N, Echenique IA, Meehan SM, et al. Variability in assessing for BK viremia: whole blood is not reliable and plasma is not above reproach—a retrospective analysis. Transpl Int. 2017;30:670–678. [DOI] [PubMed] [Google Scholar]
- 170.Leuzinger K, Hirsch HH. Human polyomaviruses. Man Clin Microbiol. 2023;2:2093–2130. [Google Scholar]
- 171.Kudose S, Dong J. Clinical validation study of quantitative real-time PCR assay for detection and monitoring of BK virus nephropathy. Ann Clin Lab Sci. 2014;44:455–460. [PubMed] [Google Scholar]
- 172.Bicalho CS, Oliveira RDR, David DR, et al. Determination of viremia cut-off for risk to develop BKPyV-associated nephropathy among kidney transplant recipients. Transpl Infect Dis. 2018;20:e12969. [DOI] [PubMed] [Google Scholar]
- 173.Costa C, Bergallo M, Astegiano S, et al. Monitoring of BK virus replication in the first year following renal transplantation. Nephrol Dial Transplant. 2008;23:3333–3336. [DOI] [PubMed] [Google Scholar]
- 174.Dumoulin A, Hirsch HH. Reevaluating and optimizing polyomavirus BK and JC real-time PCR assays to detect rare sequence polymorphisms. J Clin Microbiol. 2011;49:1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hoffman NG, Cook L, Atienza EE, et al. Marked variability of BK virus load measurement using quantitative real-time PCR among commonly used assays. J Clin Microbiol. 2008;46:2671–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Solis M, Meddeb M, Sueur C, et al. ; French BKV Study Group. Sequence variation in amplification target genes and standards influences interlaboratory comparison of BK virus DNA load measurement. J Clin Microbiol. 2015;53:3842–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Randhawa PS, Khaleel-Ur-Rehman K, Swalsky PA, et al. DNA sequencing of viral capsid protein VP-1 region in patients with BK virus interstitial nephritis. Transplantation. 2002;73:1090–1094. [DOI] [PubMed] [Google Scholar]
- 178.Leuzinger K, Kaur A, Wilhelm M, et al. Molecular characterization of BK polyomavirus replication in allogeneic hematopoietic cell transplantation patients. J Infect Dis. 2023;227:888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hasan MR, Tan R, Al-Rawahi G, et al. Comparative evaluation of laboratory developed real-time PCR assays and RealStar(®) BKV PCR kit for quantitative detection of BK polyomavirus. J Virol Methods. 2016;234:80–86. [DOI] [PubMed] [Google Scholar]
- 180.Bateman AC, Greninger AL, Atienza EE, et al. Quantification of BK virus standards by quantitative real-time PCR and droplet digital PCR is confounded by multiple virus populations in the WHO BKV international standard. Clin Chem. 2017;63:761–769. [DOI] [PubMed] [Google Scholar]
- 181.Jin L. Molecular methods for identification and genotyping of BK virus. Methods Mol Biol. 2001;165:33–48. [DOI] [PubMed] [Google Scholar]
- 182.Torres C. Evolution and molecular epidemiology of polyomaviruses. Infect Genet Evol. 2020;79:104150. [DOI] [PubMed] [Google Scholar]
- 183.Luo C, Bueno M, Kant J, et al. Genotyping schemes for polyomavirus BK, using gene-specific phylogenetic trees and single nucleotide polymorphism analysis. J Virol. 2009;83:2285–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Leuzinger K, Kaur A, Wilhelm M, et al. Variations in BK polyomavirus immunodominant large tumor antigen-specific 9mer CD8 T-cell epitopes predict altered HLA-presentation and immune failure. Viruses. 2020;12:1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bodaghi S, Comoli P, Bösch R, et al. Antibody responses to recombinant polyomavirus BK large T and VP1 proteins in young kidney transplant patients. J Clin Microbiol. 2009;47:2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kardas P, Leboeuf C, Hirsch HH. Optimizing JC and BK polyomavirus IgG testing for seroepidemiology and patient counseling. J Clin Virol. 2015;71:28–33. [DOI] [PubMed] [Google Scholar]
- 187.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–123. [DOI] [PubMed] [Google Scholar]
- 188.Kean JM, Rao S, Wang M, et al. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schmidt T, Adam C, Hirsch HH, et al. BK polyomavirus-specific cellular immune responses are age-dependent and strongly correlate with phases of virus replication. Am J Transplant. 2014;14:1334–1345. [DOI] [PubMed] [Google Scholar]
- 190.Abend JR, Changala M, Sathe A, et al. Correlation of BK virus neutralizing serostatus with the incidence of BK viremia in kidney transplant recipients. Transplantation. 2017;101:1495–1505. [DOI] [PubMed] [Google Scholar]
- 191.Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5:2213–2221. [DOI] [PubMed] [Google Scholar]
- 192.Smith JM, McDonald RA, Finn LS, et al. Polyomavirus nephropathy in pediatric kidney transplant recipients. Am J Transplant. 2004;4:2109–2117. [DOI] [PubMed] [Google Scholar]
- 193.Hariharan S, Cohen EP, Vasudev B, et al. BK virus-specific antibodies and BKV DNA in renal transplant recipients with BKV nephritis. Am J Transplant. 2005;5:2719–2724. [DOI] [PubMed] [Google Scholar]
- 194.Randhawa PS, Gupta G, Vats A, et al. Immunoglobulin G, A, and M responses to BK virus in renal transplantation. Clin Vaccine Immunol. 2006;13:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ginevri F, Azzi A, Hirsch HH, et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant. 2007;7:2727–2735. [DOI] [PubMed] [Google Scholar]
- 196.Randhawa P, Zemlicka J, Sauerbrei A, et al. Anti-BK virus activity of nucleoside analogs. Antimicrob Agents Chemother. 2008;52:1519–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wunderink HF, van der Meijden E, van der Blij-de Brouwer CS, et al. Stability of BK polyomavirus IgG seroreactivity and its correlation with preceding viremia. J Clin Virol. 2017;90:46–51. [DOI] [PubMed] [Google Scholar]
- 198.Lorentzen EM, Henriksen S, Kaur A, et al. Early fulminant BK polyomavirus-associated nephropathy in two kidney transplant patients with low neutralizing antibody titers receiving allografts from the same donor. Virol J. 2020;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hammer MH, Brestrich G, Andree H, et al. HLA type-independent method to monitor polyoma BK virus-specific CD4 and CD8 T-cell immunity. Am J Transplant. 2006;6:625–631. [DOI] [PubMed] [Google Scholar]
- 200.Sester M, Leboeuf C, Schmidt T, et al. The “ABC” of virus-specific T cell immunity in solid organ transplantation. Am J Transplant. 2016;16:1697–1706. [DOI] [PubMed] [Google Scholar]
- 201.Binggeli S, Egli A, Schaub S, et al. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;7:1131–1139. [DOI] [PubMed] [Google Scholar]
- 202.Wilhelm M, Kaur A, Wernli M, et al. BK polyomavirus-specific CD8 T-cell expansion in vitro using 27mer peptide antigens for developing adoptive T-cell transfer and vaccination. J Infect Dis. 2021;223:1410–1422. [DOI] [PubMed] [Google Scholar]
- 203.Krymskaya L, Sharma MC, Martinez J, et al. Cross-reactivity of T lymphocytes recognizing a human cytotoxic T-lymphocyte epitope within BK and JC virus VP1 polypeptides. J Virol. 2005;79:11170–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen Y, Trofe J, Gordon J, et al. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol. 2006;80:3495–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Weist BJ, Wehler P, El Ahmad L, et al. A revised strategy for monitoring BKV-specific cellular immunity in kidney transplant patients. Kidney Int. 2015;88:1293–1303. [DOI] [PubMed] [Google Scholar]
- 206.Cioni M, Leboeuf C, Comoli P, et al. Characterization of immunodominant BK polyomavirus 9mer epitope T cell responses. Am J Transplant. 2016;16:1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mutlu E, Köksoy S, Mutlu D, et al. Quantitative analysis of BKV-specific CD4+ T cells before and after kidney transplantation. Transpl Immunol. 2015;33:20–26. [DOI] [PubMed] [Google Scholar]
- 208.DeWolfe D, Gandhi J, Mackenzie MR, et al. Pre-transplant immune factors may be associated with BK polyomavirus reactivation in kidney transplant recipients. PLoS One. 2017;12:e0177339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lepore M, Crespo E, Melilli E, et al. Functional immune monitoring of BK virus and donor-specific T-cell effector immune responses to guide treatment decision-making after kidney transplantation; an illustrative case report and literature review. Transpl Infect Dis. 2021;23:e13495. [DOI] [PubMed] [Google Scholar]
- 210.Udomkarnjananun S, Kerr SJ, Francke MI, et al. A systematic review and meta-analysis of enzyme-linked immunosorbent spot (ELISpot) assay for BK polyomavirus immune response monitoring after kidney transplantation. J Clin Virol. 2021;140:104848. [DOI] [PubMed] [Google Scholar]
- 211.Egli A, Köhli S, Dickenmann M, et al. Inhibition of polyomavirus BK-specific T-cell responses by immunosuppressive drugs. Transplantation. 2009;88:1161–1168. [DOI] [PubMed] [Google Scholar]
- 212.Schachtner T, Müller K, Stein M, et al. BK virus-specific immunity kinetics: a predictor of recovery from polyomavirus BK-associated nephropathy. Am J Transplant. 2011;11:2443–2452. [DOI] [PubMed] [Google Scholar]
- 213.Trydzenskaya H, Sattler A, Müller K, et al. Novel approach for improved assessment of phenotypic and functional characteristics of BKV-specific T-cell immunity. Transplantation. 2011;92:1269–1277. [DOI] [PubMed] [Google Scholar]
- 214.Schaenman JM, Korin Y, Sidwell T, et al. Increased frequency of BK virus-specific polyfunctional CD8+ T cells predict successful control of BK viremia after kidney transplantation. Transplantation. 2017;101:1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ahlenstiel-Grunow T, Pape L. Immunosuppression, BK polyomavirus infections, and BK polyomavirus-specific T cells after pediatric kidney transplantation. Pediatr Nephrol. 2020;35:625–631. [DOI] [PubMed] [Google Scholar]
- 216.van Aalderen MC, Remmerswaal EB, Heutinck KM, et al. Clinically relevant reactivation of polyomavirus BK (BKPyV) in HLA-A02-positive renal transplant recipients is associated with impaired effector-memory differentiation of BKPyV-specific CD8+ T cells. PLoS Pathog. 2016;12:e1005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sigdel TK, Vitalone MJ, Tran TQ, et al. A rapid noninvasive assay for the detection of renal transplant injury. Transplantation. 2013;96:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Beck J, Bierau S, Balzer S, et al. Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clin Chem. 2013;59:1732–1741. [DOI] [PubMed] [Google Scholar]
- 219.Chen XT, Chen WF, Hou XT, et al. Non-invasive urinary sediment double-immunostaining predicts BK polyomavirus associated-nephropathy in kidney transplant recipients. Ann Transl Med. 2020;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jackson JA, Kim EJ, Begley B, et al. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am J Transplant. 2011;11:2228–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hu H, Aizenstein BD, Puchalski A, et al. Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am J Transplant. 2004;4:432–437. [DOI] [PubMed] [Google Scholar]
- 222.Willhelm M, Wilk S, Kaur A, et al. ; Swiss Transplant Cohort Study. Can HLA-B51 protect against BKPyV-DNAemia? Transplantation. 2019;103:e384–e385. [DOI] [PubMed] [Google Scholar]
- 223.Burek Kamenaric M, Ivkovic V, Kovacevic Vojtusek I, et al. The role of HLA and KIR immunogenetics in BK virus infection after kidney transplantation. Viruses. 2020;12:1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Azar MM, Assi R, Valika AK, et al. Graft loss among renal-transplant recipients with early reduction of immunosuppression for BK viremia. World J Transplant. 2017;7:269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sharma R, Zachariah M. BK virus nephropathy: prevalence, impact and management strategies. Int J Nephrol Renovasc Dis. 2020;13:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Reischig T, Kacer M, Hes O, et al. Viral load and duration of BK polyomavirus viraemia determine renal graft fibrosis progression: histologic evaluation of late protocol biopsies. Nephrol Dial Transplant. 2019;34:1970–1978. [DOI] [PubMed] [Google Scholar]
- 227.Lee S, Lee KW, Kim SJ, et al. Clinical characteristic and outcomes of BK virus infection in kidney transplant recipients managed using a systematic surveillance and treatment strategy. Transplant Proc. 2020;52:1749–1756. [DOI] [PubMed] [Google Scholar]
- 228.McGann K, DeWolfe D, Jacobs M, et al. Comparing urine and blood screening methods to detect BK virus after renal transplant. Exp Clin Transplant. 2021;19:104–109. [DOI] [PubMed] [Google Scholar]
- 229.Schachtner T, Zaks M, Kahl A, et al. Simultaneous pancreas/kidney transplant recipients present with late-onset BK polyomavirus-associated nephropathy. Nephrol Dial Transplant. 2016;31:1174–1182. [DOI] [PubMed] [Google Scholar]
- 230.Serrano OK, Kutzler HL, Rochon C, et al. Incidental COVID-19 in a heart-kidney transplant recipient with malnutrition and recurrent infections: implications for the SARS-CoV-2 immune response. Transpl Infect Dis. 2020;22:e13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ujire MP, Curry MP, Stillman IE, et al. A simultaneous liver-kidney transplant recipient with IgA nephropathy limited to native kidneys and BK virus nephropathy limited to the transplant kidney. Am J Kidney Dis. 2013;62:331–334. [DOI] [PubMed] [Google Scholar]
- 232.Puliyanda DP, Amet N, Dhawan A, et al. Isolated heart and liver transplant recipients are at low risk for polyomavirus BKV nephropathy. Clin Transplant. 2006;20:289–294. [DOI] [PubMed] [Google Scholar]
- 233.Razonable RR, Brown RA, Humar A, et al. ; PV16000 Study Group. A longitudinal molecular surveillance study of human polyomavirus viremia in heart, kidney, liver, and pancreas transplant patients. J Infect Dis. 2005;192:1349–1354. [DOI] [PubMed] [Google Scholar]
- 234.Viswesh V, Yost SE, Kaplan B. The prevalence and implications of BK virus replication in non-renal solid organ transplant recipients: a systematic review. Transplant Rev (Orlando). 2015;29:175–180. [DOI] [PubMed] [Google Scholar]
- 235.Egli A, Helmersen DS, Taub K, et al. Renal failure five years after lung transplantation due to polyomavirus BK-associated nephropathy. Am J Transplant. 2010;10:2324–2330. [DOI] [PubMed] [Google Scholar]
- 236.Mallavarapu RK, Sanoff SL, Howell DN, et al. BK virus nephropathy in non-renal solid organ transplant recipients: are we looking hard enough? Clin Transplant. 2021;35:e14265. [DOI] [PubMed] [Google Scholar]
- 237.Loeches B, Valerio M, Palomo J, et al. BK virus in heart transplant recipients: a prospective study. J Heart Lung Transplant. 2011;30:109–111. [DOI] [PubMed] [Google Scholar]
- 238.Loeches B, Valerio M, Pérez M, et al. ; BKV Study Group of the Gregorio Marañón Hospital. BK virus in liver transplant recipients: a prospective study. Transplant Proc. 2009;41:1033–1037. [DOI] [PubMed] [Google Scholar]
- 239.Munker D, Veit T, Schönermarck U, et al. Polyomavirus exerts detrimental effects on renal function in patients after lung transplantation. J Clin Virol. 2021;145:105029. [DOI] [PubMed] [Google Scholar]
- 240.Barton TD, Blumberg EA, Doyle A, et al. A prospective cross-sectional study of BK virus infection in non-renal solid organ transplant recipients with chronic renal dysfunction. Transpl Infect Dis. 2006;8:102–107. [DOI] [PubMed] [Google Scholar]
- 241.Herrmann A, Sandmann L, Adams O, et al. Role of BK polyomavirus (BKV) and Torque teno virus (TTV) in liver transplant recipients with renal impairment. J Med Microbiol. 2018;67:1496–1508. [DOI] [PubMed] [Google Scholar]
- 242.Yetmar ZA, Kudva YC, Seville MT, et al. BK polyomavirus DNAemia in pancreas transplant recipients compared to pancreas-kidney recipients. Clin Transplant. 2023;37:e15135. [DOI] [PubMed] [Google Scholar]
- 243.Chikeka IO, Paulk A, Haririan A, et al. Concurrent cytomegalovirus glomerulitis and BK polyomavirus-associated nephropathy in a kidney allograft biopsy. Transpl Infect Dis. 2016;18:247–250. [DOI] [PubMed] [Google Scholar]
- 244.Kenan DJ, Mieczkowski PA, Burger-Calderon R, et al. The oncogenic potential of BK-polyomavirus is linked to viral integration into the human genome. J Pathol. 2015;237:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Müller DC, Rämö M, Naegele K, et al. Donor-derived, metastatic urothelial cancer after kidney transplantation associated with a potentially oncogenic BK polyomavirus. J Pathol. 2018;244:265–270. [DOI] [PubMed] [Google Scholar]
- 246.Cotiguala L, Masood A, Park JM, et al. Increasing net immunosuppression after BK polyoma virus infection. Transpl Infect Dis. 2021;23:e13472. [DOI] [PubMed] [Google Scholar]
- 247.Querido S, Fernandes I, Weigert A, et al. High-grade urothelial carcinoma in a kidney transplant recipient after JC virus nephropathy: the first evidence of JC virus as a potential oncovirus in bladder cancer. Am J Transplant. 2020;20:1188–1191. [DOI] [PubMed] [Google Scholar]
- 248.Gupta G, Kuppachi S, Kalil RS, et al. Treatment for presumed BK polyomavirus nephropathy and risk of urinary tract cancers among kidney transplant recipients in the United States. Am J Transplant. 2018;18:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Johnston O, Jaswal D, Gill JS, et al. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89:1057–1070. [DOI] [PubMed] [Google Scholar]
- 250.Hardinger KL, Koch MJ, Bohl DJ, et al. BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant. 2010;10:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Devresse A, Tinel C, Vermorel A, et al. No clinical benefit of rapid versus gradual tapering of immunosuppression to treat sustained BK virus viremia after kidney transplantation: a single-center experience. Transpl Int. 2019;32:481–492. [DOI] [PubMed] [Google Scholar]
- 252.Alméras C, Foulongne V, Garrigue V, et al. Does reduction in immunosuppression in viremic patients prevent BK virus nephropathy in de novo renal transplant recipients? A prospective study. Transplantation. 2008;85:1099–1104. [DOI] [PubMed] [Google Scholar]
- 253.Saad ER, Bresnahan BA, Cohen EP, et al. Successful treatment of BK viremia using reduction in immunosuppression without antiviral therapy. Transplantation. 2008;85:850–854. [DOI] [PubMed] [Google Scholar]
- 254.Seifert ME, Gunasekaran M, Horwedel TA, et al. Polyomavirus reactivation and immune responses to kidney-specific self-antigens in transplantation. J Am Soc Nephrol. 2017;28:1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sawinski D, Forde KA, Trofe-Clark J, et al. Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor-specific antibodies. J Am Soc Nephrol. 2015;26:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Cheungpasitporn W, Kremers WK, Lorenz E, et al. De novo donor-specific antibody following BK nephropathy: the incidence and association with antibody-mediated rejection. Clin Transplant. 2018;32:e13194. [DOI] [PubMed] [Google Scholar]
- 257.Leuzinger K, Hirsch HH. Human polyomaviruses. In: Carroll KC, Pfaller MA, eds. Manual of Clinical Microbiology. Vol 2. 12th ed; ASM Press; 2023. [Google Scholar]
- 258.Kharel A, Djamali A, Jorgenson MR, et al. Risk factors for progression from low level BK DNAemia to unfavorable outcomes after BK management via immunosuppressive reduction. Transpl Infect Dis. 2021;23:e13561. [DOI] [PubMed] [Google Scholar]
- 259.Steubl D, Baumann M, Schuster T, et al. Risk factors and interventional strategies for BK polyomavirus infection after renal transplantation. Scand J Urol Nephrol. 2012;46:466–474. [DOI] [PubMed] [Google Scholar]
- 260.Kantauskaite M, Muller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22:634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Meziyerh S, Bouwmans P, van Gelder T, et al. ; RECOVAC Collaborators. Mycophenolic acid exposure determines antibody formation following SARS-CoV-2 vaccination in kidney transplant recipients: a nested cohort study. Clin Pharmacol Ther. 2023;114:118–126. [DOI] [PubMed] [Google Scholar]
- 262.Baek CH, Kim H, Yu H, et al. Risk factors of acute rejection in patients with BK nephropathy after reduction of immunosuppression. Ann Transplant. 2018;23:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kant S, Bromberg J, Haas M, et al. Donor-derived cell-free DNA and the prediction of BK virus-associated nephropathy. Transplant Direct. 2020;6:e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bestard O, Augustine J, Wee A, et al. Prospective observational study to validate a next-generation sequencing blood RNA signature to predict early kidney transplant rejection. Am J Transplant. 2024;24:436–447. [DOI] [PubMed] [Google Scholar]
- 265.Tedesco-Silva H, Pascual J, Viklicky O, et al. ; TRANSFORM Investigators. Safety of everolimus with reduced calcineurin inhibitor exposure in de novo kidney transplants: an analysis from the randomized TRANSFORM study. Transplantation. 2019;103:1953–1963. [DOI] [PubMed] [Google Scholar]
- 266.Sommerer C, Suwelack B, Dragun D, et al. ; Athena Study Group. An open-label, randomized trial indicates that everolimus with tacrolimus or cyclosporine is comparable to standard immunosuppression in de novo kidney transplant patients. Kidney Int. 2019;96:231–244. [DOI] [PubMed] [Google Scholar]
- 267.Wojciechowski D, Chandran S, Webber A, et al. Mycophenolate mofetil withdrawal with conversion to everolimus to treat BK virus infection in kidney transplant recipients. Transplant Proc. 2017;49:1773–1778. [DOI] [PubMed] [Google Scholar]
- 268.Bussalino E, Marsano L, Parodi A, et al. Everolimus for BKV nephropathy in kidney transplant recipients: a prospective, controlled study. J Nephrol. 2021;34:531–538. [DOI] [PubMed] [Google Scholar]
- 269.Belliere J, Kamar N, Mengelle C, et al. Pilot conversion trial from mycophenolic acid to everolimus in ABO-incompatible kidney-transplant recipients with BK viruria and/or viremia. Transpl Int. 2016;29:315–322. [DOI] [PubMed] [Google Scholar]
- 270.Adams AB, Goldstein J, Garrett C, et al. Belatacept combined with transient calcineurin inhibitor therapy prevents rejection and promotes improved long-term renal allograft function. Am J Transplant. 2017;17:2922–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Karadkhele G, Hogan J, Magua W, et al. CMV high-risk status and posttransplant outcomes in kidney transplant recipients treated with belatacept. Am J Transplant. 2021;21:208–221. [DOI] [PubMed] [Google Scholar]
- 272.Westphal SG, Lyden ER, Langewisch ED, et al. BK viremia surveillance and outcomes in simultaneous pancreas-kidney transplant recipients. Clin Transplant. 2017;31:e13010. [DOI] [PubMed] [Google Scholar]
- 273.Mujtaba M, Fridell J, Sharfuddin A, et al. BK virus nephropathy in simultaneous pancreas kidney transplant: a potentially preventable cause of kidney allograft loss. Clin Transplant. 2012;26:E87–E93. [DOI] [PubMed] [Google Scholar]
- 274.Akpinar E, Ciancio G, Sageshima J, et al. BK virus nephropathy after simultaneous pancreas-kidney transplantation. Clin Transplant. 2010;24:801–806. [DOI] [PubMed] [Google Scholar]
- 275.Gupta G, Shapiro R, Thai N, et al. Low incidence of BK virus nephropathy after simultaneous kidney pancreas transplantation. Transplantation. 2006;82:382–388. [DOI] [PubMed] [Google Scholar]
- 276.Kuten SA, Patel SJ, Knight RJ, et al. Observations on the use of cidofovir for BK virus infection in renal transplantation. Transpl Infect Dis. 2014;16:975–983. [DOI] [PubMed] [Google Scholar]
- 277.Velay A, Solis M, Benotmane I, et al. Intravenous immunoglobulin administration significantly increases BKPyV genotype-specific neutralizing antibody titers in kidney transplant recipients. Antimicrob Agents Chemother. 2019;63:e00393–e00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Randhawa P, Pastrana DV, Zeng G, et al. Commercially available immunoglobulins contain virus neutralizing antibodies against all major genotypes of polyomavirus BK. Am J Transplant. 2015;15:1014–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Benotmane I, Solis M, Velay A, et al. Intravenous immunoglobulin as a preventive strategy against BK virus viremia and BKV-associated nephropathy in kidney transplant recipients—results from a proof-of-concept study. Am J Transplant. 2021;21:329–337. [DOI] [PubMed] [Google Scholar]
- 280.Moon J, Chang Y, Shah T, et al. Effects of intravenous immunoglobulin therapy and Fc gamma receptor polymorphisms on BK virus nephropathy in kidney transplant recipients. Transpl Infect Dis. 2020;22:e13300. [DOI] [PubMed] [Google Scholar]
- 281.Nesselhauf N, Strutt J, Bastani B. Evaluation of leflunomide for the treatment of BK viremia and biopsy proven BK nephropathy; a single center experience. J Nephropathol. 2016;5:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Bernhoff E, Gutteberg TJ, Sandvik K, et al. Cidofovir inhibits polyomavirus BK replication in human renal tubular cells downstream of viral early gene expression. Am J Transplant. 2008;8:1413–1422. [DOI] [PubMed] [Google Scholar]
- 283.Burgos D, López V, Cabello M, et al. Polyomavirus BK nephropathy: the effect of an early diagnosis on renal function or graft loss. Transplant Proc. 2006;38:2409–2411. [DOI] [PubMed] [Google Scholar]
- 284.Bernhoff E, Tylden GD, Kjerpeseth LJ, et al. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol. 2010;84:2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Keller N, Duquennoy S, Conrad A, et al. Clinical utility of leflunomide for BK polyomavirus associated nephropathy in kidney transplant recipients: a multicenter retrospective study. Transpl Infect Dis. 2019;21:e13058. [DOI] [PubMed] [Google Scholar]
- 286.Cuellar-Rodriguez J, Stephany B, Poggio E, et al. Contrasting patterns of viral load response in transplant recipients with BK polyomavirus DNAemia on leflunomide therapy. Clin Transplant. 2013;27:E230–E236. [DOI] [PubMed] [Google Scholar]
- 287.Krisl JC, Taber DJ, Pilch N, et al. Leflunomide efficacy and pharmacodynamics for the treatment of BK viral infection. Clin J Am Soc Nephrol. 2012;7:1003–1009. [DOI] [PubMed] [Google Scholar]
- 288.Teschner S, Gerke P, Geyer M, et al. Leflunomide therapy for polyomavirus-induced allograft nephropathy: efficient BK virus elimination without increased risk of rejection. Transplant Proc. 2009;41:2533–2538. [DOI] [PubMed] [Google Scholar]
- 289.Leca N, Muczynski KA, Jefferson JA, et al. Higher levels of leflunomide are associated with hemolysis and are not superior to lower levels for BK virus clearance in renal transplant patients. Clin J Am Soc Nephrol. 2008;3:829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Faguer S, Hirsch HH, Kamar N, et al. Leflunomide treatment for polyomavirus BK-associated nephropathy after kidney transplantation. Transpl Int. 2007;20:962–969. [DOI] [PubMed] [Google Scholar]
- 291.Josephson MA, Gillen D, Javaid B, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81:704–710. [DOI] [PubMed] [Google Scholar]
- 292.Guasch A, Roy-Chaudhury P, Woodle ES, et al. ; FK778 BK Nephropathy Study Group. Assessment of efficacy and safety of FK778 in comparison with standard care in renal transplant recipients with untreated BK nephropathy. Transplantation. 2010;90:891–897. [DOI] [PubMed] [Google Scholar]
- 293.Yamazaki T, Shirai H, Tojimbara T. Use of leflunomide as an antiviral agent with everolimus for BK virus nephropathy patients after kidney transplantation: a case series. Am J Case Rep. 2020;21:e927367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gabardi S, Waikar SS, Martin S, et al. Evaluation of fluoroquinolones for the prevention of BK viremia after renal transplantation. Clin J Am Soc Nephrol. 2010;5:1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wojciechowski D, Chanda R, Chandran S, et al. Ciprofloxacin prophylaxis in kidney transplant recipients reduces BK virus infection at 3 months but not at 1 year. Transplantation. 2012;94:1117–1123. [DOI] [PubMed] [Google Scholar]
- 296.Song TR, Rao ZS, Qiu Y, et al. Fluoroquinolone prophylaxis in preventing BK polyomavirus infection after renal transplant: a systematic review and meta-analysis. Kaohsiung J Med Sci. 2016;32:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Knoll GA, Humar A, Fergusson D, et al. Levofloxacin for BK virus prophylaxis following kidney transplantation: a randomized clinical trial. JAMA. 2014;312:2106–2114. [DOI] [PubMed] [Google Scholar]
- 298.Patel SJ, Knight RJ, Kuten SA, et al. Ciprofloxacin for BK viremia prophylaxis in kidney transplant recipients: results of a prospective, double-blind, randomized, placebo-controlled trial. Am J Transplant. 2019;19:1831–1837. [DOI] [PubMed] [Google Scholar]
- 299.Moriyama T, Sorokin A. Repression of BK virus infection of human renal proximal tubular epithelial cells by pravastatin. Transplantation. 2008;85:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gabardi S, Ramasamy S, Kim M, et al. Impact of HMG-CoA reductase inhibitors on the incidence of polyomavirus-associated nephropathy in renal transplant recipients with human BK polyomavirus viremia. Transpl Infect Dis. 2015;17:536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ginevri F, De Santis R, Comoli P, et al. Polyomavirus BK infection in pediatric kidney-allograft recipients: a single-center analysis of incidence, risk factors, and novel therapeutic approaches. Transplantation. 2003;75:1266–1270. [DOI] [PubMed] [Google Scholar]
- 302.Bracamonte E, Leca N, Smith KD, et al. Tubular basement membrane immune deposits in association with BK polyomavirus nephropathy. Am J Transplant. 2007;7:1552–1560. [DOI] [PubMed] [Google Scholar]
- 303.Matossian D, Langman CB, Cohn RA, et al. Obstructive uropathy is associated with polyomavirus viremia in pediatric kidney transplantation. Pediatr Transplant. 2012;16:729–734. [DOI] [PubMed] [Google Scholar]
- 304.Harada R, Hamasaki Y, Okuda Y, et al. Epidemiology of pediatric chronic kidney disease/kidney failure: learning from registries and cohort studies. Pediatr Nephrol. 2022;37:1215–1229. [DOI] [PubMed] [Google Scholar]
- 305.Smith JM, Dharnidharka VR, Talley L, et al. BK virus nephropathy in pediatric renal transplant recipients: an analysis of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry. Clin J Am Soc Nephrol. 2007;2:1037–1042. [DOI] [PubMed] [Google Scholar]
- 306.Ahlenstiel-Grunow T, Liu X, Schild R, et al. Steering transplant immunosuppression by measuring virus-specific T cell levels: the randomized, controlled IVIST trial. J Am Soc Nephrol. 2021;32:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Pape L, Tönshoff B, Hirsch HH; Members of the Working Group ‘Transplantation’ of the European Society for Paediatric Nephrology. Perception, diagnosis and management of BK polyomavirus replication and disease in paediatric kidney transplant recipients in Europe. Nephrol Dial Transplant. 2016;31:842–847. [DOI] [PubMed] [Google Scholar]
- 308.Dharnidharka VR, Vyas N, Gaut JP, et al. The utility of surveillance biopsies in pediatric kidney transplantation. Pediatr Nephrol. 2018;33:889–895. [DOI] [PubMed] [Google Scholar]
- 309.Kanzelmeyer NK, Ahlenstiel T, Drube J, et al. Protocol biopsy-driven interventions after pediatric renal transplantation. Pediatr Transplant. 2010;14:1012–1018. [DOI] [PubMed] [Google Scholar]
- 310.Odum JD, Kats A, VanSickle JS, et al. Characterizing the frequency of modifiable histological changes observed on surveillance biopsies in pediatric kidney allograft recipients. Pediatr Nephrol. 2020;35:2173–2182. [DOI] [PubMed] [Google Scholar]
- 311.Zaman RA, Ettenger RB, Cheam H, et al. A novel treatment regimen for BK viremia. Transplantation. 2014;97:1166–1171. [DOI] [PubMed] [Google Scholar]
- 312.Alexander RT, Langlois V, Tellier R, et al. The prevalence of BK viremia and urinary viral shedding in a pediatric renal transplant population: a single-center retrospective analysis. Pediatr Transplant. 2006;10:586–592. [DOI] [PubMed] [Google Scholar]
- 313.Hamasaki Y, Dolan NM, Cubitt D, et al. BK viremia and nephropathy in pediatric renal transplant recipients. Pediatr Transplant. 2019;23:e13460. [DOI] [PubMed] [Google Scholar]
- 314.Hymes LC, Warshaw BL. Polyomavirus (BK) in pediatric renal transplants: evaluation of viremic patients with and without BK associated nephritis. Pediatr Transplant. 2006;10:920–922. [DOI] [PubMed] [Google Scholar]
- 315.Hymes LC, Warshaw BL. Five-year experience using sirolimus-based, calcineurin inhibitor-free immunosuppression in pediatric renal transplantation. Pediatr Transplant. 2011;15:437–441. [DOI] [PubMed] [Google Scholar]
- 316.Montini G, Murer L, Ghio L, et al. One-year results of basiliximab induction and tacrolimus associated with sequential steroid and MMF treatment in pediatric kidney transplant recipient. Transpl Int. 2005;18:36–42. [DOI] [PubMed] [Google Scholar]
- 317.Anyaegbu EI, Almond PS, Milligan T, et al. Intravenous immunoglobulin therapy in the treatment of BK viremia and nephropathy in pediatric renal transplant recipients. Pediatr Transplant. 2012;16:E19–E24. [DOI] [PubMed] [Google Scholar]
- 318.Araya CE, Lew JF, Fennell RS, 3rd, et al. Intermediate-dose cidofovir without probenecid in the treatment of BK virus allograft nephropathy. Pediatr Transplant. 2006;10:32–37. [DOI] [PubMed] [Google Scholar]
- 319.Al Khasawneh E, Araya CE, Dharnidharka VR. Missed viral surveillance testing visits associate with full blown viral diseases in children receiving kidney transplants. Pediatr Transplant. 2013;17:129–132. [DOI] [PubMed] [Google Scholar]
- 320.Herman J, Van Ranst M, Snoeck R, et al. Polyomavirus infection in pediatric renal transplant recipients: evaluation using a quantitative real-time PCR technique. Pediatr Transplant. 2004;8:485–492. [DOI] [PubMed] [Google Scholar]
- 321.Kizilbash SJ, Rheault MN, Bangdiwala A, et al. Infection rates in tacrolimus versus cyclosporine-treated pediatric kidney transplant recipients on a rapid discontinuation of prednisone protocol: 1-year analysis. Pediatr Transplant. 2017;21:e12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Puliyanda DP, Toyoda M, Traum AZ, et al. Outcome of management strategies for BK virus replication in pediatric renal transplant recipients. Pediatr Transplant. 2008;12:180–186. [DOI] [PubMed] [Google Scholar]
- 323.Celik B, Shapiro R, Vats A, et al. Polyomavirus allograft nephropathy: sequential assessment of histologic viral load, tubulitis, and graft function following changes in immunosuppression. Am J Transplant. 2003;3:1378–1382. [DOI] [PubMed] [Google Scholar]
- 324.Zarauza Santoveña A, García Meseguer C, Martínez Mejía S, et al. BK virus infection in pediatric renal transplantation. Transplant Proc. 2015;47:62–66. [DOI] [PubMed] [Google Scholar]
- 325.Araya CE, Garin EH, Neiberger RE, et al. Leflunomide therapy for BK virus allograft nephropathy in pediatric and young adult kidney transplant recipients. Pediatr Transplant. 2010;14:145–150. [DOI] [PubMed] [Google Scholar]
- 326.Cho YH, Hyun HS, Park E, et al. Higher incidence of BK virus nephropathy in pediatric kidney allograft recipients with Alport syndrome. J Clin Med. 2019;8:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Jung YH, Moon KC, Ha JW, et al. Leflunomide therapy for BK virus allograft nephropathy after pediatric kidney transplantation. Pediatr Transplant. 2013;17:E50–E54. [DOI] [PubMed] [Google Scholar]
- 328.Launay M, Baudouin V, Guillemain R, et al. Leflunomide for BK virus: report of seven kidney-transplanted children. Int J Organ Transplant Med. 2018;9:178–183. [PMC free article] [PubMed] [Google Scholar]
- 329.Araya CE, Lew JF, Fennell RS, et al. Intermediate dose cidofovir does not cause additive nephrotoxicity in BK virus allograft nephropathy. Pediatr Transplant. 2008;12:790–795. [DOI] [PubMed] [Google Scholar]
- 330.Vats A, Shapiro R, Singh Randhawa P, et al. Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults. Transplantation. 2003;75:105–112. [DOI] [PubMed] [Google Scholar]
- 331.Sharma AP, Moussa M, Casier S, et al. Intravenous immunoglobulin as rescue therapy for BK virus nephropathy. Pediatr Transplant. 2009;13:123–129. [DOI] [PubMed] [Google Scholar]
- 332.Funk GA, Steiger J, Hirsch HH. Rapid dynamics of polyomavirus type BK in renal transplant recipients. J Infect Dis. 2006;193:80–87. [DOI] [PubMed] [Google Scholar]
- 333.Acott PD, O’Regan PA, Lee SH, et al. In vitro effect of cyclosporin A on primary and chronic BK polyoma virus infection in Vero E6 cells. Transpl Infect Dis. 2008;10:385–390. [DOI] [PubMed] [Google Scholar]
- 334.Li YJ, Weng CH, Lai WC, et al. A suppressive effect of cyclosporine A on replication and noncoding control region activation of polyomavirus BK virus. Transplantation. 2010;89:299–306. [DOI] [PubMed] [Google Scholar]
- 335.Hirsch HH, Yakhontova K, Lu M, et al. BK polyomavirus replication in renal tubular epithelial cells is inhibited by sirolimus, but activated by tacrolimus through a pathway involving FKBP-12. Am J Transplant. 2016;16:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Barraclough KA, Isbel NM, Staatz CE, et al. BK virus in kidney transplant recipients: the influence of immunosuppression. J Transplant. 2011;2011:750836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Moens U, Subramaniam N, Johansen B, et al. A steroid hormone response unit in the late leader of the noncoding control region of the human polyomavirus BK confers enhanced host cell permissivity. J Virol. 1994;68:2398–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Johnson HJ, Swan SK, Heim-Duthoy KL, et al. The pharmacokinetics of a single oral dose of mycophenolate mofetil in patients with varying degrees of renal function. Clin Pharmacol Ther. 1998;63:512–518. [DOI] [PubMed] [Google Scholar]
- 339.Meier-Kriesche HU, Shaw LM, Korecka M, et al. Pharmacokinetics of mycophenolic acid in renal insufficiency. Ther Drug Monit. 2000;22:27–30. [DOI] [PubMed] [Google Scholar]
- 340.Fernandez E, Perez R, Hernandez A, et al. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics. 2011;3:53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19:262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Trompeter R, Filler G, Webb NJ, et al. Randomized trial of tacrolimus versus cyclosporin microemulsion in renal transplantation. Pediatr Nephrol. 2002;17:141–149. [DOI] [PubMed] [Google Scholar]
- 343.Grenda R, Watson A, Trompeter R, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transplant. 2010;10:828–836. [DOI] [PubMed] [Google Scholar]
- 344.Offner G, Toenshoff B, Hocker B, et al. Efficacy and safety of basiliximab in pediatric renal transplant patients receiving cyclosporine, mycophenolate mofetil, and steroids. Transplantation. 2008;86:1241–1248. [DOI] [PubMed] [Google Scholar]
- 345.Staskewitz A, Kirste G, Tönshoff B, et al. Mycophenolate mofetil in pediatric renal transplantation without induction therapy: results after 12 months of treatment. Transplantation. 2001;71:638–644. [DOI] [PubMed] [Google Scholar]
- 346.Henne T, Latta K, Strehlau J, et al. Mycophenolate mofetil-induced reversal of glomerular filtration loss in children with chronic allograft nephropathy. Transplantation. 2003;76:1326–1330. [DOI] [PubMed] [Google Scholar]
- 347.Brunkhorst LC, Fichtner A, Hocker B, et al. Efficacy and safety of an everolimus- vs. a mycophenolate mofetil-based regimen in pediatric renal transplant recipients. PLoS One. 2015;10:e0135439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Hocker B, Zencke S, Krupka K, et al. Cytomegalovirus Infection in pediatric renal transplantation and the impact of chemoprophylaxis with (val-)ganciclovir. Transplantation. 2016;100:862–870. [DOI] [PubMed] [Google Scholar]
- 349.Tönshoff B, Ettenger R, Dello Strologo L, et al. Early conversion of pediatric kidney transplant patients to everolimus with reduced tacrolimus and steroid elimination: results of a randomized trial. Am J Transplant. 2019;19:811–822. [DOI] [PubMed] [Google Scholar]
- 350.Tönshoff B, Tedesco-Silva H, Ettenger R, et al. Three-year outcomes from the CRADLE study in de novo pediatric kidney transplant recipients receiving everolimus with reduced tacrolimus and early steroid withdrawal. Am J Transplant. 2021;21:123–137. [DOI] [PubMed] [Google Scholar]
- 351.Smith F, Panek R, Kiberd BA. Screening to prevent polyoma virus nephropathy in kidney transplantation: a cost analysis. Am J Transplant. 2009;9:2177–2179. [DOI] [PubMed] [Google Scholar]
- 352.Kiberd BA. Screening to prevent polyoma virus nephropathy: a medical decision analysis. Am J Transplant. 2005;5:2410–2416. [DOI] [PubMed] [Google Scholar]
- 353.Wong G, Myint TM, Lee YJ, et al. Economic evaluation of screening for polyomavirus infection in kidney transplant recipients: a cost-utility analysis. Transplant Direct. 2022;8:e1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.de Weerd AE, Betjes MGH. ABO-incompatible kidney transplant outcomes: a meta-analysis. Clin J Am Soc Nephrol. 2018;13:1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Nguyen K, Diamond A, Carlo AD, et al. Characterization of kidney retransplantation following graft failure due to BK virus nephropathy. J Surg Res. 2022;269:110–118. [DOI] [PubMed] [Google Scholar]
- 356.Ramos E, Vincenti F, Lu WX, et al. Retransplantation in patients with graft loss caused by polyoma virus nephropathy. Transplantation. 2004;77:131–133. [DOI] [PubMed] [Google Scholar]
- 357.Huang J, Danovitch G, Pham PT, et al. Kidney retransplantation for BK virus nephropathy with active viremia without allograft nephrectomy. J Nephrol. 2015;28:773–777. [DOI] [PubMed] [Google Scholar]
- 358.Cooper JE, Huskey J, Chan L, et al. Preemptive retransplant for BK virus nephropathy without concurrent transplant nephrectomy. Transplantation. 2010;90:331–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.