Abstract
The TAM receptor family of TYRO3, AXL and MERTK regulates tissue and immune homeostasis. Aberrant TAM receptor signalling has been linked to a range of diseases, including cancer, fibrosis and viral infections. Specifically, the dysregulation of TAM receptors can enhance tumour growth and metastasis due to their involvement in multiple oncogenic pathways. For example, TAM receptors have been implicated in the epithelial–mesenchymal transition, maintaining the stem cell phenotype, immune modulation, proliferation, angiogenesis and resistance to conventional and targeted therapies. Therapeutically, multiple TAM receptor inhibitors are in preclinical and clinical development for cancers and other indications, with those targeting AXL being the most clinically advanced. Although there has been notable clinical advancement in recent years, challenges persist. This Review aims to provide both biological and clinical insights into the current therapeutic landscape of TAM receptor inhibitors, and evaluates their potential for the treatment of cancer and non-malignant diseases.
Introduction
TYRO3, AXL (also known as UFO) and MERTK comprise the TAM receptor tyrosine kinase (RTK) family, which was identified in 1991 (refs. 1–4). Genetic knockout studies demonstrated that the inactivation of individual TAM receptors has little effect on embryonic development, suggesting they are functionally redundant, and developmental defects in mice only occur after the loss of two or more TAM family members2.
Experimental evidence indicates that the TAM receptors have critical roles in promoting phagocytosis of apoptotic cells, functioning as gatekeepers to prevent hyperreactive immune responses, and regulating vascular integrity and blood vessel permeability3–6. In addition, TAM receptors are also referred to as phosphatidylserine virus entry enhancing receptors (PVEERs), due to their ability to interact with phosphatidylserine (PtdSer) present on the viral envelope that facilitates viral entry7.
In cancer, TAM receptor activation promotes cell proliferation and metastasis8–11. Historically, AXL cDNA was isolated from primary human myeloid leukaemia cells and, when overexpressed in NIH3T3 cells, induced neoplastic transformation12,13. This was followed by the identification of MERTK (c-MER) from a human B lymphoblastoid cDNA expression library in 1994 (ref. 14). Although Tyro3 cDNA was isolated from the mouse central nervous system using a similar screening approach, it later showed the ability to promote anchorage-independent growth when transfected into untransformed fibroblasts, a hallmark of oncogenicity15–17.
Unlike many RTKs, TAM receptors are rarely mutated, and dysregulation of receptor activity is mostly due to gene amplification or activation by their microenvironment, making them ideal therapeutic targets with minimal concerns of developing mutation-driven drug resistance18. The majority of TAM receptor inhibitors, including small molecules and biological agents (biologics), have been developed to target or disrupt the AXL signalling pathway. Clinically, AXL inhibitors are in multiple clinical trials for various oncology indications, and MERTK inhibitors are following very similar developmental plans19. This Review provides biological and clinical insights into the functional consequences of TAM receptor activation and surveys the current therapeutic landscape of inhibitors that target the TAM receptor family for both cancer and non-cancer indications.
Protein structure of the TAM receptor family
Similarly to many other trans-membrane RTKs, the TAM receptors have an extracellular domain, which consists of two immunoglobulin domains that are essential for ligand binding followed by two fibronectin type III repeats20. The intracellular kinase domain is highly conserved between the three receptors, which all feature a KWIAIES sequence that is unique to this family of RTKs21 (Fig. 1). TAM receptors primarily interact with two ligands, growth arrest-specific protein 6 (GAS6) and vitamin K-dependent protein S1 (PROS1)22–26 (Box 1). Although structural data for receptor–ligand complexes are not available for TYRO3 and MERTK, the structure of AXL in complex with GAS6 provides insight into the binding configuration between TAM receptors and their ligands20,27. An initial 1:1 interaction between the AXL receptor and GAS6 leads to a 2:2 formation of AXL and GAS6, resulting in receptor homodimerization and activation of the intracellular catalytic domain28. Autophosphorylation of the TAM receptor catalytic domains promotes binding to the p85 subunit of phosphatidylinositol 3-kinase (PI3K), SRC family members and growth factor receptor-bound protein 2 (GRB2), leading to activation of MEK–ERK signalling27,29–31.
Fig. 1 |. TAM receptors and their interacting ligands.
TAM receptors are activated by growth arrest-specific protein 6 (GAS6) and vitamin K-dependent protein S1 (PROS1). The biological consequences of GAS6 and PROS1 signalling are listed. The three TAM receptors AXL, MERTK and TYRO3 interact with their ligands in the form of a dimer. Ligands also form dimers and bind to the receptor amino-terminal immunoglobulin-like domains, which are connected to two fibronectin type III domains. The intracellular kinase domains of all three TAM family members share close sequence homology and have autophosphorylation sites throughout the domain. Enzyme-induced cleavage can occur in proximity to the cell membrane to release soluble forms of the TAM receptors.
Box 1. GAS6 and PROS1 expression and their roles in regulating receptor activities.
Growth arrest-specific protein 6 (GAS6) is expressed in various tissues and cell types, including vascular endothelial cells in the heart, kidney and lungs; platelets; immune cells, such as myeloid cells184–186; and in the brain, particularly in astrocytes187. GAS6 expression is induced in response to various stimuli, including growth factors, cytokines and oxidative stress. GAS6 has been implicated in the regulation of physiological processes such as coagulation and vascular remodelling, and immune functions such as phagocytosis188.
Vitamin K-dependent protein S1 (PROS1) expression has been observed in a wide range of tissues and cell types. High PROS1 expression was reported in the neural tissue, liver, kidney, lung and gonads6. Cell types that are positive for PROS1 expression include neuronal cells, vascular endothelial cells, platelets, Sertoli cells, retinal pigment epithelium cells and osteoblasts3,116,189,190. PROS1 has many overlapping functions with GAS6, including promoting blood coagulation, maintaining vascular function and facilitating phagocytosis62,72,191. Dysregulation of PROS1 signalling is associated with the pathogenesis of diseases including thrombosis, inflammation and cancer188. Importantly, PROS1 activates MERTK and TYRO3 but not AXL, leaving GAS6 the predominant, if not only, ligand capable of activating AXL signalling45.
Ligand-mediated interactions
The GAS6 and PROS1 ligands have sex hormone-binding globulin (SHBG) domains, composed of two LG domains on their carboxy termini, which interact with the extracellular immunoglobulin domains of the TAM receptors and initiate receptor activation. A vitamin K-dependent carboxylation domain (Gla domain) is located in the amino terminus of both GAS6 and PROS1, which is important for binding phospholipids in the cell membrane27,32. Another unique feature of the Gla domain is its ability to bind to PtdSer through interaction with γ-carboxyglutamic acid, a common gateway hijacked by viruses to gain entry into mammalian cells33–35 (Fig. 2a).
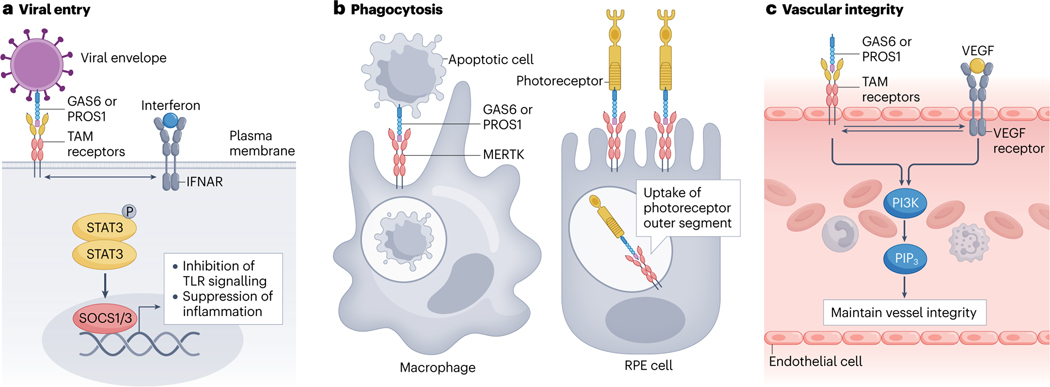
Fig. 2 |. TAM receptor signalling maintains physiological homeostasis.
a, Viral entry into host cells can occur through the binding of growth arrest-specific protein 6 (GAS6) or vitamin K-dependent protein S1 (PROS1), which are expressed on phosphatidylserine-presenting membranes as part of the viral envelope. Upon receptor–ligand binding and activation, TAM receptors suppress the immune response by engaging in crosstalk with the interferon-α/β receptor (IFNAR)–STAT pathway. This pathway leads to SOCS1- and SOCS3-mediated inhibition of Toll-like receptor (TLR) signalling and suppression of inflammatory responses, helping the virus evade immune surveillance. b, TAM receptors, particularly MERTK, are required for the phagocytic clearance of apoptotic cells and cellular debris. MERTK is expressed by phagocytes such as macrophages and retinal pigment epithelium (RPE) cells, which recognize and engulf GAS6-expressing and PROS1-expressing apoptotic cells and cellular debris such as photoreceptor outer segments. Phagocytosis restores tissue homeostasis and avoids inflammatory responses and autoimmune disease. c, GAS6-mediated and PROS1-mediated TAM receptor activation is essential for blood vessel growth and vascular integrity. Crosstalk between TAM receptors and VEGF receptor signalling facilitates downstream phosphatidylinositol 3-kinase (PI3K)–PIP3 pathways. Crosstalk between TAM receptors and receptor tyrosine kinase signalling can also occur.
GAS6 is a 75-kDa protein that binds to all three TAM receptors, with strongest binding affinity towards AXL, followed by TYRO3 and weakest affinity for MERTK36. Although binding to MERTK is weak, this does not undermine the critical role of GAS6-induced MERTK activation in some tissues24. Structural studies revealed two contact points between GAS6 immunoglobulin domains and AXL immunoglobulin domains20. The major contact groove or so-called ‘high-affinity’ interaction occurs between Ig2GAS6 and Ig1AXL, and the minor contact is between Ig1GAS6 and Ig2AXL. This distinctive binding conformation facilitates the binding of two GAS6 molecules to homodimeric AXL receptors as seen in the co-complex structure20. Although the exact binding conformation between GAS6 and MERTK or TYRO3 requires further clarification, the structural similarities among TAM receptors and their ligands strongly suggest that a similar co-complex structure is likely.
PROS1 is a 73-kDa, vitamin K-dependent protein that bears close structural resemblance to GAS6. Moreover, similar to GAS6, PROS1 forms dimers when binding to TAM receptors32,37. The blood PROS1 concentration is significantly higher than that of GAS6 under normal physiological conditions (300 nM versus 0.2 nM)38–41. These differences in blood concentration are consistent with PROS1 playing a pivotal role in regulating blood coagulation and having additional functions beyond TAM receptor activation. Biologically, PROS1 acts as a cofactor for protein C to prevent blood coagulation through the inhibition of factor Vα and factor VIIIα. Thus, a deficiency in PROS1 significantly increases the risk of venous thrombosis42–44. PROS1 is capable of activating MERTK and TYRO3 but not AXL, leaving GAS6 the predominant, if not only, ligand capable of activating AXL signalling45.
In addition to GAS6 and PROS1, the proteins TUBBY, TULP1 and GALECTIN 3 have been identified as potential ligands of MERTK that are capable of activating macrophage phagocytosis in retinal epithelium and hepatocytes46,47. However, further studies are required to provide more clarity on their roles as activating ligands of TAM receptors.
All three TAM receptors rely on ligand-mediated activation for receptor signalling. Efficient activation of TAM receptors by GAS6 and PROS1 is dependent on vitamin K-dependent γ-carboxylation of the Gla domain48,49. Interestingly, inhibition of GAS6 carboxylation or loss of the Gla domain does not prevent GAS6 from binding TAM receptors but inhibits receptor activation50. Importantly, PtdSer is found on the surfaces of stressed or apoptotic cells and vesicles such as exosomes. Therefore, all of these membrane surfaces have the potential to interact with GAS6 and PROS1, representing a vital step in TAM receptor activation50 (Fig. 2a).
Some studies have reported that AXL formed heterodimers with other RTKs in a tissue-dependent manner51. In some cases, ligand-independent receptor dimerization and activation have been reported for AXL and, to some extent, TYRO3 (refs. 52,53). Because there is little definitive structural or biochemical evidence to support these observations, additional studies are necessary54.
Physiological roles of TAM receptors
TAM receptors have important physiological roles in various cellular processes, such as fetal development and adult tissue homeostasis. They function as negative regulators of the immune response and facilitate phagocytic removal of apoptotic cells4,55. TAM receptor signalling is equally critical for the preservation of vascular integrity56. In addition, aberrant activation of TAM receptors results in abnormalities in the immune, vasculature and male reproductive systems3,6,57,58.
Immune response functions
TAM receptor expression in the immune system is localized to activated dendritic cells, macrophages, myeloid-derived suppressor cells and natural killer cells, all of which are essential regulators of the innate immune response56,59. Mice with genetic ablation of Tyro3−/−, Axl−/− and Mertk−/− (TAM TKO) have provided insights into the functional consequences of TAM receptor activation within the immune landscape56. For example, receptor crosstalk can occur between TAM receptors and the interferon-α/β receptor (IFNAR)–STAT signalling pathway, which downregulates the inflammatory response through SOCS1 and SOCS3, inhibiting inflammation mediated by Toll-like receptors (TLRs)60,61. The role of TAM receptors in natural killer cell maturation and T cell activation has also been reported and is likely to be driven by PROS1 (ref. 54). Activated T cells secrete PROS1 as part of their negative feedback response and animals lacking PROS1 exhibit an exacerbated T cell response upon immunization62. Clearly, TAM receptors and their ligands are important players in modulating the magnitude and intensity of the immune response and in maintaining the intricate homeostasis of the innate and adaptive immune pathways.
Phagocytic clearance of apoptotic cells
TAM receptors contribute to phagocytosis and the clearance of cell debris in various cell types (Fig. 2b). Complete loss of all three TAM receptors in mice leads to diminished phagocytic clearance of apoptotic cells and cellular debris by macrophages and dendritic cells, and this failure to maintain tissue homeostasis results in hyperactive and pathogenic inflammatory responses56. For example, the elimination of the photoreceptor outer segment in the retina is typically performed by MERTK-expressing phagocytic retinal pigment epithelium. Retinal pigment epithelium cells lacking MERTK fail to remove toxic debris and the resulting hyperactive inflammatory response leads to the death of photoreceptors, retinal degeneration and, ultimately, blindness, as demonstrated in Mertk−/− rodent models63–65. However, a recent study suggests that TYRO3 function might be concomitantly lost in this model, resulting in exacerbation of the retinal degeneration phenotype66. Loss of all three TAM receptors has also been proposed to be responsible for male infertility due to the failure of phagocytic Sertoli cells to clear apoptotic germ cells3. Clearly, TAM receptors have a critical role in maintaining appropriate immune homeostasis, and the dysregulation of TAM receptors will result in a plethora of pathogenic inflammatory responses.
Vascular functions
Because GAS6 and PROS1 are essential to the growth and maintenance of endothelial cells and platelets, TAM receptor signalling has been implicated in maintaining blood vessel integrity and permeability, as exemplified by the susceptibility of TAM TKO mice to haemorrhage56. In particular, reports have proposed that AXL promotes vessel growth through interaction with VEGFA and signalling through PI3K and AKT (Fig. 2c). For example, loss of AXL leads to the impairment of blood vessel formation both in vitro and in vivo67,68, whereas inhibition of MERTK or TYRO3 only results in mild effects69. However, the role of AXL in blood vessel development needs additional investigation as Axl−/− mice appear phenotypically normal without obvious blood vessel dysfunction.
In addition, TAM receptor ligands are known to promote angiogenesis and platelet aggregation6,70. Pros1−/− mice are embryonically lethal due to substantial vessel leakage and haemorrhage71,72. Interestingly, unlike Pros1−/− mice, Gas6−/− mice appear to be phenotypically normal with adequate platelet counts and clotting factor levels with no reports of spontaneous bleeding or thrombosis73. However, when challenged with agonists such as thrombin, Gas6−/− mice produced significantly smaller thrombi compared with wild-type controls74. These data suggest that pharmacological inhibition of GAS6 could be effective at reducing thrombosis with reduced risk of haemorrhagic complications.
TAM receptor signalling in cancer
Aberrant TAM receptor signalling in cancer growth and progression is well documented75. Although not considered conventional oncoproteins, TAM receptors that become aberrantly activated can drive metastasis, facilitate therapeutic resistance and promote cancer cell survival during hypoxia and nutrient deprivation11,76–78 (Table 1). Activating mutations within the TAM receptor kinase domain are rare, which supports the requirement for ligand-mediated activation.
Table 1 |.
Expression of TAM receptors and ligands and their functional relevance in cancer
| Cancer type | AXL | MERTK | TYRO3 | GAS6 | PROS1 |
|---|---|---|---|---|---|
| Haematological cancer | |||||
| AML | Yes (E, B, F) | Yes (E, F) | Yes (E) | Yes (E, B) | Yes (E) |
| Chronic myeloid leukaemia | Yes (E, F) | Yes (E) | |||
| B-CLL | Yes (E) | ||||
| Acute lymphoblastic leukaemia | Yes (E, F) | Yes (E, F) | |||
| Mantle cell | Yes (E) | ||||
| Multiple myeloma | Yes (E) | Yes (E) | Yes (E) | ||
| Solid tumour | |||||
| Breast | Yes (E, B, F) | Yes (F) | Yes (E, F) | ||
| Head and neck | Yes (B) | ||||
| Colorectal | Yes (E, B, F) | Yes (F) | Yes (E) | ||
| Pancreatic | Yes (E, B, F) | Yes (F) | Yes (F) | Yes (E) | |
| Oesophageal | Yes (E, B, F) | ||||
| Gastric | Yes (E, B, F) | Yes (B) | Yes (E, F) | ||
| Melanoma | Yes (E, F) | Yes (E, F) | Yes (F) | Yes (F) | |
| Hepatocellular | Yes (F) | ||||
| Squamous cell | Yes (E, F) | ||||
| NSCLC | Yes (E, B, F) | Yes (E, F) | Yes (E) | Yes (B) | Yes (E) |
| Ovarian | Yes (E, B, F) | Yes (E) | |||
| Cervical | Yes (F) | ||||
| Endometrial | Yes (E) | Yes (E) | Yes (E) | ||
| Prostate | Yes (E, F) | Yes (E) | Yes (E) | Yes (F) | Yes (E, B, F) |
| Thyroid | Yes (E, F) | Yes (E, F) | Yes (E, F) | Yes (E) | |
| Bladder | Yes (E, B, F) | ||||
| Renal | Yes (E, F) | Yes (E, B, F) | |||
| Mesothelioma | Yes (E, F) | ||||
| Oral squamous | Yes (B) | ||||
| Glioblastoma | Yes (E, B, F) | Yes (E, F) | Yes (E, B) | Yes (E) | |
| Neuroblastoma | Yes (F) | ||||
| Schwannoma | Yes (E, F) | Yes (E) | Yes (E) | Yes (E, F) | |
| Kaposi’s sarcoma | Yes (E, F) | ||||
| Osteosarcoma | Yes (E, B) | Yes (F) | |||
| Rhadomyosarcoma | Yes (E) | ||||
AML, acute myeloid leukaemia; B, validated as a prognostic biomarker; B-CLL, B cell chronic lymphocytic leukaemia; E, elevated expression in tumours; F, positive correlation between expression and functional relevance; GAS6, growth arrest-specific protein 6; NSCLC, non-small-cell lung cancer; PROS1, vitamin K-dependent protein S1.
AXL expression and function
Dysregulated expression of AXL has been reported in cancers of diverse histological origins (Table 1), and GAS6–AXL signalling is associated with various pro-tumorigenic functions79. AXL expression is regulated at the transcriptional level in response to external stimuli. For example, under hypoxic conditions, cancer cells stabilize the hypoxia inducible transcription factors 1 and 2 (HIF1 and HIF2), to activate AXL and promote low oxygen adaptation78. In contrast, STAT1 regulates AXL mRNA downstream of TLR signalling54,80. Thus, different stresses can regulate AXL through numerous different transcriptional regulatory elements.
Given that gain-of-function AXL mutations are found in less than 1.5% of AXL-positive cancers, it might be concluded that GAS6 is the primary regulator of AXL activation79. However, although elevated GAS6 levels are associated with many AXL-positive cancers, the stoichiometric relationship between GAS6 and AXL is not an exact 1:1 ratio. Such discrepancy is, in part, the result of protease-mediated receptor ectodomain shedding. The extracellular domains of AXL and MERTK can be cleaved by proteases such as ADAM10 and ADAM17, leading to proteolytic shedding of the receptors, which act as soluble receptor traps to regulate free GAS6 and PROS1 levels in blood81.
AXL facilitates epithelial–mesenchymal transition (EMT), a critical cancer metastasis initiation event that promotes the migratory and invasive capability of tumour cells79. Intrinsic AXL signalling positively regulates transcription factors required for EMT, including TWIST, ZEB1, ZEB2 and SLUG, and also promotes the expression of N-cadherin and downregulation of E-cadherin to facilitate EMT79. In addition, AXL can facilitate the activation of other key oncogenic pathways, such as those stimulated by MET, SRC and Ras, to promote EMT78 (Fig. 3).
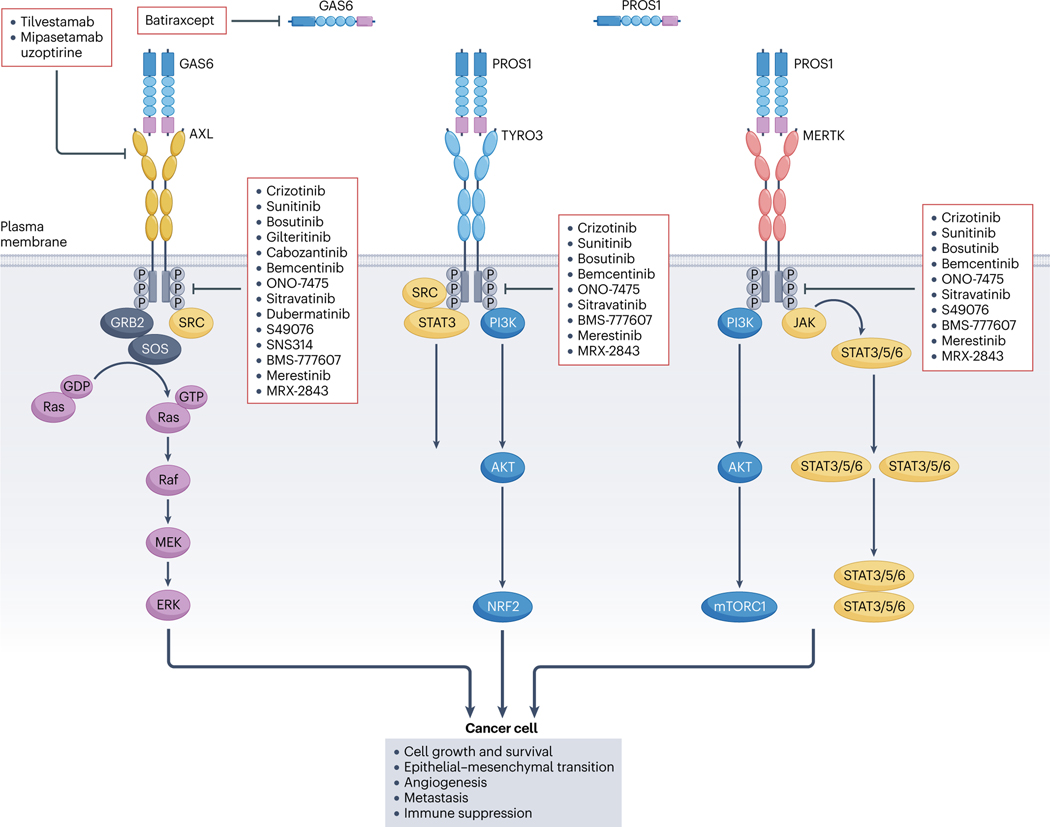
Fig. 3 |. The role of TAM receptor signalling in cancer and the action of specific inhibitors.
A subset of the key oncogenic signalling pathways and biological outcomes driven by the TAM receptors AXL, TYRO3 and MERTK in facilitating cancer progression. When activated by growth arrest-specific protein 6 (GAS6) and vitamin K-dependent protein S1 (PROS1), AXL engages the SRC protein, which recruits GRB2 and SOS to facilitate the Ras–Raf–MEK–ERK signalling pathway. TYRO3 signals through the SRC and phosphatidylinositol 3-kinase (PI3K)–AKT pathways, promoting the activation of downstream molecules such as mTORC1 and NRF2. MERTK activation channels into various signalling pathways, notably PI3K–AKT, JAK–STAT3/5/6 and SRC. TAM receptors can recruit and activate overlapping downstream signalling pathways. However, distinct temporal and spatial regulation can produce varied biological outcomes. Upon activation, TAM receptors enhance cancer cell proliferation, tumour cell survival and metastasis. They also stimulate angiogenesis and contribute to immune suppression. Two categories of TAM inhibitors are illustrated. The first includes molecules such as crizotinib, sunitinib and bemcentinib, which directly target the kinase domain of the TAM receptors, inhibiting kinase activity. The second category, represented by agents such as tilvestamab, mipasetamab uzopritine and batiraxcept, disrupts receptor–ligand binding.
In response to stress, cancer cells upregulate AXL signalling as a protective measure to ensure survival. In addition, elevated AXL expression is often associated with resistance to therapy in both haematological and solid tumours82,83. Specifically, AXL signalling is thought to mediate resistance to the EGFR inhibitor cetuximab and radiation through the activation of AKT and ERK signalling pathways, and decreases tumour cell apoptosis by modulating the activity of c-ABL84. Pharmacologic inhibition of AXL restores sensitivity of tumour cells to cytotoxic therapy and significantly reduces the tumour burden in xenograft models of metastatic ovarian cancer85. Furthermore, upregulation of AXL signalling is frequently observed in cancers resistant to various tyrosine kinase inhibitors. For example, aberrant AXL expression strongly correlates with erlotinib resistance in non-small-cell lung cancer (NSCLC) and sunitinib resistance in renal cell carcinoma86. In both cases, inhibition of AXL genetically or pharmacologically results in re-sensitization to these targeted molecules. Similarly, single-cell profiling identified high AXL levels as a hallmark associated with treatment relapse in BRAF V600E mutant melanoma, indicating that targeting AXL in melanoma might enhance the response to BRAF V600E inhibitors87. The success of immune checkpoint therapies has increased interest in targeting the immune system to control tumour growth and metastasis. AXL expression is elevated in patients unresponsive to PD1 immunotherapy, and inhibition of AXL in tumours that are resistant to both immunotherapy and radiation can facilitate the release of tumour antigens, promoting T cell-mediated tumour killing82. Taken together, these findings provide a scientific rationale for the addition of AXL inhibitors to immunotherapy or radiation treatment to enhance efficacy.
A subset of cancer cells retain the ability to self-renew and are referred to as cancer stem cells. These cells promote aggressive tumour growth, facilitate metastasis and foster therapeutic resistance88. Interestingly, a correlation between AXL expression and cancer stem cell markers, including CD44, ALDH1, ISL1 and CDC2A, has been reported in many cancer types such as cutaneous squamous cell carcinoma, breast cancer and glioblastoma89–91. Loss of AXL signalling genetically or through pharmacologic inhibition greatly reduces the ability of cancer cells to form organoids and restores sensitivity to chemotherapy.
A mechanistic link between GAS6–AXL signalling and angiogenesis has recently been established. AXL signalling promotes plasmin production, endothelial cell invasion and angiogenesis in clear cell renal cell carcinoma by positively regulating the plasminogen receptor S100A10. Inhibition of AXL with the small-molecule inhibitor cabozantinib or a high-affinity soluble AXL (sAXL) Fc fusion decoy receptor reduced blood vessel density and tumour growth in a pazopanib-resistant patient-derived xenograft model of clear cell renal cell carcinoma92. This study highlights the role of AXL in regulating angiogenesis through S100A10 signalling in a cancer resistant to a tyrosine kinase inhibitor.
Published data consistently support a role for AXL signalling in positively regulating tumour survival, metastasis and drug resistance, but how AXL governs these multiple functions remains elusive. A likely scenario is that AXL signalling acts to promote cancer cell survival when encountering both intrinsic and external stress stimuli. These stress stimuli could include oxygen or nutrient deprivation, immune cell-mediated attack, cytotoxic chemotherapy or ionizing radiation. Tumour cells elevate AXL expression as part of their survival strategy when encountering an unfavourable growth environment. Therefore, eliminating AXL in cancer cells facilitates enhanced tumour killing and prevents drug resistance85.
MERTK expression and function
Unlike AXL, which has ubiquitous expression in cancer, elevated MERTK expression is particularly associated with haematological cancers93–96 (Table 1). MERTK expression in haematological cancers is important for the evasion of innate immune surveillance, thus promoting the growth and proliferation of malignancies such as acute lymphoblastic leukaemia and acute myeloid leukaemia (AML)97,98. Aberrant expression of MERTK occurs in 30% of paediatric B cell acute lymphoblastic leukaemias97. MERTK expression is upregulated in macrophages during efferocytosis, a process that involves the removal of apoptotic cells through phagocytosis99. In glioblastoma, inhibition of MERTK led to a reduction in CD206+ anti-inflammatory M2-like macrophages. Although this effect alone was insufficient to prolong survival, the combination of MERTK inhibition with radiation resulted in a significant reduction in tumour growth, a shift in M2-like to M1-like macrophage polarization and a heightened pro-inflammatory immune response in animal models of glioblastoma95 (Fig. 3).
Although there are only a few studies reporting high levels of MERTK in solid tumours such as melanoma and prostate cancer, this restricted expression pattern supports its highly controlled regulation in tissues54. A role for MERTK in promoting tumour growth has been established in multiple melanoma tumour models. Furthermore, pharmacological inhibition of MERTK can effectively suppress oncogenic signalling in melanoma and reduce tumour growth independently of BRAF status96. Although the functional significance of MERTK activity in solid tumours is yet to be fully understood, the inhibition of MERTK has the potential to augment immune responses by amplifying neoantigen production and attracting pro-inflammatory immune cells. Consequently, this implies that MERTK inhibitors could enhance the effectiveness of immune checkpoint therapies and might represent a promising strategy that should be tested in the clinic.
TYRO3 expression and function
Elevated TYRO3 expression has been reported in multiple malignancies such as squamous cell cancer, lung cancer, prostate cancer, thyroid cancer, schwannoma and multiple myeloma (Table 1), but its functional roles and clinical relevance remain unclear100–103. The shared expression pattern of all three TAM receptors in certain cancers implies a degree of functional redundancy. This redundancy is notable because critical oncogenic pathways, such as PI3K–AKT and STAT signalling, can be activated either directly or indirectly through TAM receptor activation. Furthermore, similar biological outcomes, such as the promotion of cancer progression through EMT signalling pathways and the development of therapeutic resistance, have been documented104,105 (Fig. 3).
TYRO3 demonstrates its distinctive biological activity in the regulation of bone homeostasis. TYRO3 regulates the differentiation and function of osteoclasts, which are responsible for bone resorption in normal bone remodelling. Nevertheless, aberrant TYRO3 signalling can worsen pathological conditions, including osteoporosis and the development of bone metastases106. These findings suggest that TYRO3 could be a promising therapeutic target for restoring osteodynamics and to prevent cancer metastasis to bone. Further research is needed to fully elucidate the role of TYRO3 in cancer, as well as to evaluate the potential clinical benefits of TYRO3-targeted therapies.
TAM receptors in non-malignant diseases
Whereas TAM receptors are essential in maintaining the homeostatic balance of the immune, haematopoietic and vascular systems56, aberrant TAM receptor signalling is often the underlying cause of diseases such as fibrosis, autoimmune disorders and viral infections56,107,108. In this section, we will explore the biological consequences of TAM receptor signalling in various non-malignant diseases.
Fibrosis
Tissue fibrosis is characterized by extensive accumulation of extracellular matrix (ECM) and stromal elements in response to chronic inflammation and tissue injury109. The TAM receptor family has critical roles during tissue remodelling in fibrotic diseases (Fig. 4). The same GAS6–TAM receptor pathway that transforms epithelial-like cancer cells into mesenchymal cells seems to be adopted by tissues under chronic inflammation to form fibrotic scars110. In liver cirrhosis, AXL signalling is activated to induce the transcription of SLUG, a critical regulator of EMT111. Furthermore, AXL interacts with TGFβ, promoting the deposition of α-smooth muscle actin (α-SMA), collagen and matrix metalloproteinases (MMPs) in a SMAD3-dependent manner112 (Fig. 4). In a Gas6−/− non-alcoholic steatohepatitis model, animals deficient in GAS6 had reduced hepatic inflammation, fibrosis and decreased expression of TGFβ and collagen I113. Idiopathic pulmonary fibrosis (IPF) is another potentially deadly fibrotic disease leading to progressive loss of lung function114. IPF tissues express elevated levels of TAM receptors and GAS6, and small-molecule inhibitors that target multiple TAM receptor kinases exhibit enhanced anti-fibrotic activity when compared with biologics that specifically target the GAS6 and AXL pathway, suggesting that IPF has co-opted the entire TAM receptor family115.
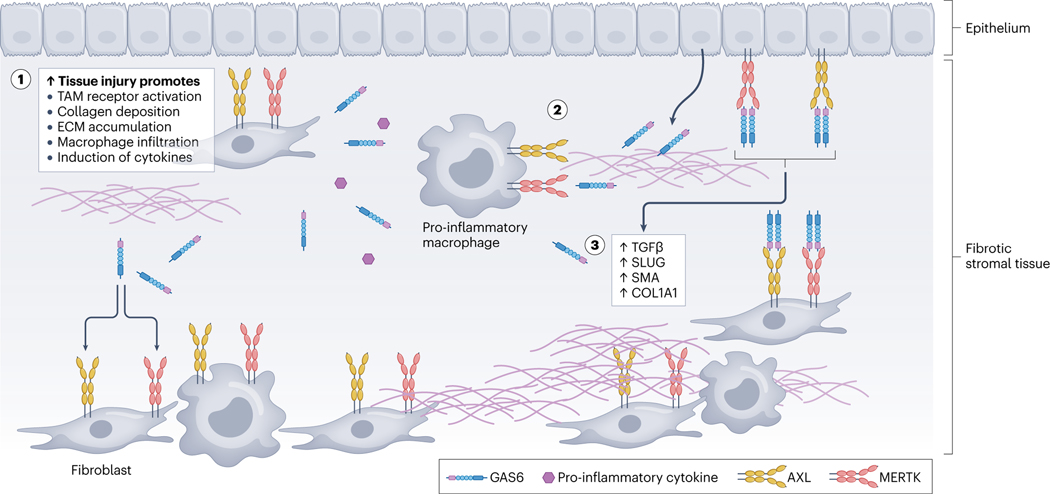
Fig. 4 |. GAS6-mediated TAM receptor signalling promotes fibrosis in response to tissue injury.
Chronic inflammation in stromal tissues promotes TAM receptor activity within the tissue microenvironment in different cell types such as epithelial cells, fibroblasts and pro-inflammatory macrophages. This triggers a sequence of biological events leading to tissue fibrosis. Elevated TAM receptor activation boosts the secretion of pro-inflammatory cytokines and promotes macrophage infiltration, leading to epithelial–mesenchymal transition and the deposition of fibrotic matrix (1). Stromal cell-derived growth arrest-specific protein 6 (GAS6) is upregulated in response to tissue injury, resulting in TAM receptor activation on cells within the extracellular matrix (ECM). TAM receptor activation facilitates the generation and transformation of myofibroblasts, which further contribute to the deposition of ECM and fibrosis. Additionally, TAM receptor signalling modulates the activity of immune cells, and promotes the release of pro-fibrotic cytokines (2). TAM receptors promote TGFβ signalling, a critical mediator of fibrosis. The molecular interplay between TAM receptors and TGFβ signalling results in the upregulation of pro-fibrotic factors, such as SLUG, α-smooth muscle actin (α-SMA) and type I collagen COL1A1(3).
Autoimmune disease
The pathology of autoimmune disease is diverse and extremely complex. However, most clinical presentations are associated with abnormal immune complexes and the production of autoantibodies. Autoimmune diseases promote chronic inflammatory responses, leading to destruction of normal tissue56. An important role of TAM receptors in immune regulation is in clearing apoptotic cells, which is primarily driven by MERTK signalling116. Loss of MERTK signalling has been described in numerous autoimmune diseases, including systemic lupus erythematosus and multiple sclerosis. For example, Ballantine et al. reported a reduction of intact MERTK receptors on monocytes and macrophages from juvenile patients with systemic lupus erythematosus, consistent with decreased phagocytosis, supporting the essential role of MERTK in apoptotic cell clearance in systemic lupus erythematosus117. Myelin is a critical component of the central nervous system, and its disruption is a hallmark of multiple sclerosis118. MERTK has an important role in regulating myelin phagocytosis by microglia and macrophages, which are key immune cells involved in multiple sclerosis pathology. MERTK is required for microglial activation and subsequent remyelination119. Considering these findings, activation of MERTK emerges as a promising therapeutic avenue in multiple sclerosis, with the potential to alleviate myelin phagocytosis, enhance debris clearance and decrease neuroinflammation120.
Viral entry
Throughout evolution, viruses have acquired the capability to evade innate immune responses for successful host entry. Among their evasion mechanisms is the ability of virions to encapsulate in a lipid bilayer comprising the host cell’s plasma membrane, leading to viral engulfment and release121. Viruses that utilize this mode of host cell entry include the Zika, Ebola, Marburg, dengue and West Nile viruses122–124. In particular, these enveloped viruses use PVEERs, including AXL, MER and TYRO3, to enter human host cells125. It is probable that enveloped viruses exposing PtdSer can form complexes with GAS6 or PROS1 on the Gla domain, thereby enabling them to exploit all three TAM receptors for cellular entry72. Because AXL is the most abundant and ubiquitously expressed TAM receptor, it has a higher probability of being used for viral entry. In addition to this mechanism, apoptotic mimicry is another strategy employed by enveloped viruses to gain entry into host cells in an ‘immunologically silent’ fashion. Viruses expressing PtdSer dock onto host cells expressing TAM receptors, facilitating endocytic engulfment. Upon docking, the virus can also suppress the inflammatory response by facilitating TAM receptor-mediated inhibition of TLR signalling, allowing it to evade immune surveillance122 (Fig. 2a). Recent Zika and Ebola virus outbreaks have generated significant interest in targeting TAM receptors as a method to inhibit virus uptake by host cells126,127.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters the host cell via binding to angiotensin converting enzyme 2 (ACE2). Bohan et al. reported that the presence of the GAS6–AXL receptor complex can enhance SARS-CoV-2 infection when ACE2 levels are low, even though SARS-CoV-2 does not use AXL receptors for cell entry. Therefore, disruption of the GAS6–AXL–PtdSer complex might be a potential therapeutic approach to lessen the severity of SARS-CoV-2 infection128.
Translational landscape of TAM receptor inhibition
TAM receptor inhibitors have shown promising results in preclinical and clinical studies for both malignant and non-malignant diseases. Strong evidence has shown that TAM receptor inhibitors can promote antitumour immune responses, reduce inflammation and improve responses to cancer therapy when combined with standard-of-care treatments. With the growing understanding of TAM receptor signalling and the development of new therapeutic agents, TAM receptor inhibition represents a promising approach for the treatment of a wide range of diseases.
The two major strategies that have been investigated for the inhibition of TAM signalling are inhibiting receptor–ligand interactions and inhibiting kinase activity. The majority of TAM receptor inhibitors are small molecules that block kinase activity, but given the high degree of homology between the kinase domains of TAM receptors, most of these small molecules promiscuously inhibit all three TAM family members as well as other kinase receptors.
Anti-AXL antibodies and sAXL decoy receptors that specifically block the binding between AXL and its ligand GAS6 have been developed and are currently in late-phase clinical trials36,129. More recently, an AXL aptamer that inhibits AXL-mediated signalling has also been developed130. In addition, two AXL antibody–drug conjugates are currently being tested in the clinic for the treatment of various oncology indications131,132. In the section below, we provide a description of TAM inhibitors and their biological properties (Table 2 and Fig. 3).
Table 2 |.
Inhibitors of TAM receptors under clinical development
| Compound (sponsor) | IC50 in vitro (nM) | Clinical stage | Indications | Adverse effects |
|---|---|---|---|---|
| Crizotinib (Pfizer) | 294 (AXL) | Approved | ALK+ advanced NSCLC, advanced solid or haematological cancers | Abdominal pain, headache, pyrexia |
| Sunitinib (Pfizer) | 5 (AXL) | Approved | Renal cell carcinoma, GIST, pancreatic neuroendocrine tumours | Diarrhoea, fatigue, hypertension |
| Bosutinib (Pfizer) | 560 (AXL) | Approved | Ph+ chronic myeloid leukaemia, solid tumours, Lewy body dementia | Diarrhoea, rash, liver toxicity |
| Gilteritinib (Astellas/Kotobuk) | 0.73 (AXL) | Approved | Relapsed or refractory FLT3-AML | Neutropenia, anaemia, thrombocytopenia |
| Cabozantinib (Exelixis/Ipsen) | 7 (AXL) | Approved | Advanced renal cancer, hepatocellular cancer, thyroid cancer | Fistulas, intra-abdominal, pelvic abscess |
| Batiraxcept (Aravive) | NA | Phase III | Platinum-resistant ovarian cancer, renal and pancreatic cancers | NA |
| Bemcentinib (Bergen Bio) | 14 (AXL) | Phase II | TNBC, NSCLC, pancreatic cancer, brain tumour, mesothelioma, COVID-19 | NA |
| Amuvatinib (Astex) | 10 (AXL) | Phase II (discontinued) | Small cell lung cancer, metastatic solid tumours | Fatigue, alopecia, diarrhoea |
| Foretinib (GSK) | 11 (AXL) | Phase II (discontinued) | TNBC, HER2+ breast cancer, NSCLC | Fatigue, nausea, hypertension |
| ONO-7475 (Ono) | 2.2 (AXL) 0.4 (MERTK) 1.9 (TYRO3) |
Phase I/II | Advanced cancer | NA |
| Sitravatinib (Mirati) | <1 (AXL) <1 (MERTK) <1 (TYRO3) |
Phase I/II | Sarcoma, breast cancer; combination with PD1 inhibitors for solid tumours | NA |
| Dubermatinib (Sumitomo/Tolero) | 27 (AXL) | Phase I | Advanced solid tumours, FLT3-AML | NA |
| S49076 (Servier) | 7 (AXL) 2 (MERTK) |
Phase I | Advanced solid tumours | Peripheral oedema, albumin anaemia |
| SNS314 (Sunesis) | 84 (AXL) | Phase I | Advanced solid tumours | NA |
| BMS-777607 (BMS/Aslan) | 1.1 (AXL) 14 (MERTK) 4.3 (TYRO3) |
Phase I | Advanced or metastatic solid cancer | Anaemia, nausea, constipation |
| RXDX-106 (Ignyta) | 7 (AXL) 29 (MERTK) 19 (TYRO3) |
Phase I (discontinued) | Locally advanced solid tumour | NA |
| Tilvestamab (Bergen Bio) | NA | Phase I | Ovarian cancer | NA |
| Mipasetamab uzoptirine (Bergen Bio/ADC) | NA | Phase I | Advanced solid tumour in combination with gemcitabine | NA |
| Merestinib (Eli Lilly/Dana-Farber) | 2 (AXL) 10 (MERTK) |
Phase I | Biliary tract carcinoma, NSCLC, refractory AML, mantle cell lymphoma, colorectal cancer | Anaemia, thrombocytopenia, leukopenia, neutropenia, nausea, constipation |
| MRX-2843 (Meryx) | 1.3 (AXL) 15 (MERTK) 17 (TYRO3) |
Phase I | Advanced cancer | NA |
ALK+, anaplastic lymphoma kinase-positive; AML, acute myeloid leukaemia; GIST, gastrointestinal stromal tumour; NA, not available; NSCLC, non-small-cell lung cancer; Ph+, Philadelphia chromosome-positive; TNBC, triple-negative breast cancer.
Small-molecule inhibitors
Most TAM receptor inhibitors are chemically synthesized small molecules designed to interfere with the ATP binding moiety located on the intracellular kinase domain. Multiple small molecules with low nanomolar IC50 values against TAM receptors are in preclinical and clinical development.
Clinical development programmes.
Approximately 20 small-molecule inhibitors targeting TAM receptors have been tested in clinical trials, with 15 of them currently undergoing active commercial development (Table 2).
Bemcentinib (BGB324) is a clinical stage AXL inhibitor with an IC50 of 14 nM that has 50-fold to 100-fold selectivity for AXL compared with MERTK and TYRO3. The efficacy of bemcentinib was validated in preclinical models of ovarian cancer, glioblastoma, mesothelioma, pancreatic cancer, breast cancer and lung cancer91,133,134. In all of these models, bemcentinib was proposed to exhibit its therapeutic efficacy in an AXL-dependent manner134. Bemcentinib is now one of the most advanced AXL inhibitors currently in clinical development, with five phase I/II programmes in NSCLC, triple-negative breast cancer (TNBC) and melanoma (NCT03184571, NCT02872259, NCT03184558). In addition to clinical studies in patients with cancer, clinical studies investigating bemcentinib as a treatment for COVID-19 are also ongoing (NCT04890509).
RXDX-106 was developed as a pan-TAM inhibitor and has a slow dissociation rate to improve its potency. RXDX-106 inhibits AXL (IC50 = 7 nM), TYRO3 (IC50 = 19 nM), MERTK (IC50 = 29 nM) and c-MET (IC50 = 12 nM). Yokoyama et al. reported that tumour growth inhibition in mice by RXDX-106 was associated with increased tumour-infiltrating lymphocytes, polarization of M1-like intratumoural macrophages and activation of natural killer cells135. Combining RXDX-106 with an anti-PD1 antibody resulted in an enhanced antitumour effect. However, a phase 1 study of RXDX-106 that commenced in March 2018 (NCT03454243) was terminated in 2019 by the sponsor.
MRX-2843 is a MERTK inhibitor (IC50 = 1.3 nM) that also inhibits FLT3 with high potency (IC50 = 0.64 nM). In comparison with previous generations of the compound, UNC1062 and UNC20205, MRX-2843 showed improved oral bioavailability, enhanced solubility, drug metabolism and pharmacokinetic properties. The in vivo efficacy of MRX-2843 was reported in quizartinib-resistant FLT3-ITD mutant preclinical AML models demonstrating that MRX-2843-treated animals had significantly prolonged overall survival136. MRX-2843 was also tested in EGFR inhibitor-resistant NSCLC models and acute lymphoblastic leukaemia, where it exhibited strong anticancer activity and decreased PD1 expression on T cells, leading to immune-mediated therapeutic activity97,137. Multiple clinical trials are ongoing to investigate the efficacy of MRX-2843 in relapsed/refractory metastatic solid tumours (NCT03510104), in multiple subtypes of acute leukaemia (NCT04872478, NCT04946890) and in combination with the EGFR inhibitor osimertinib for the treatment of NSCLC (NCT04762199).
Sitravatinib (MGCD516) inhibits all members of the TAM receptor family. Cell-free assays revealed that the IC50 for all TAM receptors is below 2 nM. This molecule shows potent antitumour efficacy in preclinical models of renal cell carcinoma, NSCLC, sarcoma and breast cancer138,139. However, it is likely that the strong antitumour effect observed is attributable to multi-kinase inhibition beyond the TAM family. Sitravatinib is in late-stage clinical development in more than 30 clinical trials targeting various solid tumour types, including NSCLC and melanoma. It is also being evaluated as part of a combination treatment with anti-PD1 antibodies and chemotherapy (NCT04921358, NCT05461794).
ONO-7475 is a potent inhibitor of AXL (IC50 = 0.7 nM) and MERTK (IC50 = 1.0 nM). In preclinical models of AXL-expressing, EGFR mutant NSCLC, ONO-7475 treatment sensitized cells to EGFR tyrosine kinase inhibitors, leading to tumour regression and delayed tumour growth. AML cells bearing FLT3 mutations exhibited sensitivity to ONO-7475 treatment, leading to cell death. Furthermore, enhanced efficacy was observed when ONO-7475 was utilized in combination with the BCL-2 inhibitor venetoclax, resulting in a further reduction in the tumour burden140,141. However, recruitment was terminated in clinical trials of ONO-7475 for patients with acute leukaemias (NCT03176277).
Multi-kinase Inhibitors that inhibit TAM receptors include crizotinib, sunitinib, bosutinib, gilteritinib, cabozantinib, S49076, SNS314, BMS-777607 and merestinib. These are included in Table 2 and Fig. 3 but not discussed further here. In most cases they lack selectivity for the TAM kinase family and have not demonstrated TAM inhibition as a mechanism of antitumour activity in patients.
Preclinical development programmes.
New small molecules aimed at targeting TAM receptors are in active development and undergoing testing in preclinical settings. Some of these molecules are showcasing novel mechanisms of action.
NPS-1034 is an inhibitor with an IC50 of 10.3 nM for AXL and 48 nM for c-MET. This molecule also possesses inhibitory activity at a lower potency against MERTK and several other kinases such as SCF1R, DDR and FLT3. NPS-1034 has anti-proliferative and pro-apoptotic activity in vitro as a single agent. When used in combination with the EGFR inhibitor gefitinib in tumour cell lines, it can overcome drug resistance caused by MET amplification142.
LDC1267 was developed as a pan-TAM receptor inhibitor and has nanomolar IC50 values against all three TAM receptors, with highest inhibitory activity for MERTK at IC50 < 5 nM. LDC1267 treatment resulted in the enhancement of natural killer cell tumour killing activity in vivo, leading to a significant reduction in murine melanoma metastasis143.
SGI-7079 is a type II ATP-competitive inhibitor that binds to the hydrophobic pocket of the ‘DFG-out’ conformation of kinases (often associated with an inactive kinase state). SGI-7079 inhibits AXL kinase with an IC50 of 58 nM, and partially restored treatment sensitivity to mesenchymal NSCLC cells that were resistant to the EGFR inhibitor erlotinib in an EGFR mutation-independent manner. This molecule also demonstrated potent inhibition of MERTK, TYRO3, FLT1, SYK and PDGFRβ in vitro144.
The field of TAM receptor-targeted therapies is evolving rapidly, with promising molecules in clinical development and novel mechanisms of action on the horizon. These advancements offer new hope for more effective cancer treatment.
Biologics and novel therapeutic strategies
Biologics have been designed to specifically target and bind TAM receptors, thereby reducing off-target effects and improving therapeutic selectivity compared with small molecules. Biologics have a long half-life, which can lead to sustained and durable inhibition, making them an attractive option for TAM receptor inhibition.
Clinical development programmes.
The landscape of TAM receptor-targeted therapies, including biologics and other novel therapeutic strategies, has witnessed significant advancements in recent years, offering promising avenues in the treatment of various cancer types. Here, we highlight several therapies currently in clinical development.
Batiraxcept (AVB-S6–500) is an engineered ultra-high-affinity sAXL decoy receptor that is distinct from other AXL inhibitors. It was designed to bind and neutralize GAS6 at femtomolar binding affinity. By adopting a directed evolution-based strategy through an unbiased mutation screening approach, sAXL decoy receptor mutants were generated with a 320-fold improvement in binding affinity towards GAS6 compared with the wild-type AXL receptor36. Preclinically, batiraxcept displayed significant antitumour activities in preclinical models of ovarian cancer, AML, breast cancer, renal cancer and pancreatic cancer85. It also significantly enhances the therapeutic efficacy of cytotoxic chemotherapy, radiation therapy, immune checkpoint blockade (ICB) and anti-angiogenic therapy92. In a phase Ib trial, patients dosed with batiraxcept in combination with paclitaxel showed an overall response rate of 34.8% with two complete responses (NCT03639246)145. Based on data presented in the phase Ib trial, the US Food and Drug Administration (FDA) granted ‘Fast Track Designation’ to batiraxcept in platinum-resistant recurrent ovarian cancer. However, results from a phase III study, combining batiraxcept with paclitaxel, did not demonstrate a significant improvement in therapeutic outcomes compared with the control treatment. (NCT04729608). Currently, this molecule is being explored in other cancers, including clear cell renal cell carcinoma (NCT04300140), and pancreatic cancer (NCT04983407).
Tilvestamab is a fully humanized AXL blocking antibody that has an affinity of 5–500 pM for the AXL receptor and exhibits little binding to MERTK or TYRO3. Functionally, Tilvestamab blocks GAS6 binding to AXL and exhibits antitumour activity in AML, NSCLC, pancreatic cancer and renal cancer models. Tilvestamab is being tested in a phase I clinical trial in relapsed, platinum-resistant high-grade serous ovarian cancer (NCT04893551).
Enapotamab vedotin (HuMax-AXL-ADC) is an antibody–drug conjugate comprising an anti-AXL antibody conjugated to cytotoxic auristatin E for targeted elimination of AXL-expressing cancer cells. HuMax-AXL-ADC selectively targets cells expressing high levels of AXL to improve the potency of BRAF and MEK inhibitors in melanoma treatment146. A phase I/II open-label, dose-escalation trial with an expansion cohort to evaluate the safety and antitumour activity of HuMax-AXL-ADC in patients with solid tumours was completed in 2020 (NCT02988817). Unfortunately, a lack of sufficient clinical efficacy led to the discontinuation of this programme.
Mecbotamab vedotin (CAB-AXL-ADC) is another antibody–drug conjugate targeting AXL-expressing tumour cells. Although there are no research data available in the public domain, a company press release highlighted that this molecule demonstrates conditional activation within the tumour microenvironment, thereby reducing the risk of normal tissue toxicity. Three phase II clinical studies evaluating the safety and efficacy of CAB-AXL-ADC as a monotherapy or in combination with PD1 inhibitors for the treatment of NSCLC, ovarian cancer and other solid tumours are ongoing (NCT04681131, NCT03425279, NCT04918186).
Biologic therapeutics targeting TAM receptors have witnessed significant advancements in recent years. These biologics represent an exciting frontier in oncology research and offer new avenues for improving patient outcomes.
Preclinical development programmes.
YW327.6S2 is an affinity-matured anti-AXL antibody developed using a phage-display platform. This molecule recognizes both human and mouse AXL with a KD of 1 nM and 545 pM, respectively. YW327.6S2 blocks the ligand–receptor interaction between GAS6 and AXL in a dose-dependent manner and does not cross-react with MERTK or TYRO3. Unfortunately, YW327.6S2 monotherapy did not demonstrate satisfactory efficacy in preclinical in vivo studies and was not pursued as a clinical candidate. It is worth highlighting that the binding of native GAS6 to AXL is very strong (KD = 32 pM), and the lack of in vivo efficacy observed with YW327.6S2 was likely due to its weaker affinity compared with GAS6 (ref. 147).
AXL aptamer GL21 is a 34-mer, RNA-based aptamer that was generated from a library of single-stranded RNAs using the SELEX platform. In human U87MG glioma cells, GL21 binds to the extracellular binding domain of AXL with a KD of 12 nM and can disrupt GAS6-mediated AXL activation in vitro. Treatment with GL21 also inhibited tumour growth in a NSCLC lung xenograft model148. Due to their small size and high specificity, nucleotide-based aptamers represent a highly versatile class of molecules for clinical use through forming conjugates with drugs such as cytotoxic chemotherapy to deliver target-mediated killing of tumour cells. However, development of GL21 could run into the same problem as YW327.6S2 given its weaker binding affinity towards AXL compared with wild-type GAS6.
Combination therapies
Inhibition of TAM receptor activity can lead to therapeutic resistance, but TAM receptor inhibitors can be combined with cytotoxic therapeutics such as chemotherapy and radiotherapy to overcome treatment resistance36,149,150. Importantly, inhibition of individual TAM receptors seems to have minimal toxicity towards normal tissue, with the exception of MERTK inhibition in photoreceptors56. For example, a phase I clinical study of the engineered AXL decoy receptor batiraxcept demonstrated a remarkable safety profile in normal healthy volunteers, where biomarker analysis showed complete neutralization of GAS6 (ref. 145). Molecules of this class presented the opportunity to combine TAM receptor inhibitors with standard-of-care treatments without adding further toxicity. Clinically, enhanced antitumour efficacies have been reported with TAM receptor inhibitors used in combination with cytotoxic chemotherapies, immune checkpoint inhibitors or other kinase inhibitors151–153. In this section, we will discuss the biological rationale of combining TAM receptor inhibitors with other therapies.
Combinations with RTK inhibitors.
Dysregulated kinase signalling enhances the growth and dissemination of cancer cells, and inhibitors against kinases such as EGFR, BRAF and VEGF have been validated in the clinic to prolong survival of patients with cancer154,155. Unfortunately, intrinsic and acquired drug resistance is common for all kinase inhibitors and poses a significant clinical challenge156. Both acquired resistance due to crosstalk between kinase receptors and intrinsic, mutation-driven resistance can result in the loss of therapeutic efficacy in patients157.
TAM receptors share similar downstream effectors with many oncogenic RTKs, and therefore TAM receptor activation can promote drug resistance by signalling to the same downstream effectors when oncogenic RTKs are inhibited75. In NSCLC cell lines, elevated AXL expression alone is sufficient to mediate resistance to EGFR inhibition by maintaining the downstream activities of the PI3K–AKT and MEK–ERK signalling pathways86. In NSCLC clinical samples derived from patients resistant to EGFR inhibitors, AXL signalling is increased and, potentially, a resistance mechanism.
In addition to compensating for the loss of RTK signalling to critical downstream effectors, AXL can also crosstalk with other RTKs by forming receptor dimers157. Dimerization of AXL and EGFR has been reported in head and neck cancer and could be a contributing factor towards persistent EGFR signalling in the presence of cetuximab84. Resistance to PI3K inhibitors can also occur due to AXL–EGFR dimerization, leading to compensatory activation of the PLCγ–PKC–mTOR pathway instead of the PI3K–AKT signalling cascade51. In preclinical studies of breast cancer, AXL induces acquired resistance to the HER2 inhibitor lapatinib by promoting dimerization with HER3, bypassing HER2-mediated signalling158.
Although AXL activity is frequently reported when drug resistance induced by RTK inhibitors occurs, the role of MERTK and TYRO3 in this context remains poorly investigated. Given the overlapping expression of TAM receptors in some cancers, MERTK and TYRO3 are likely to have a role in promoting therapeutic resistance105,159. In some tumours, increased MERTK expression compensates for AXL inhibition, and inhibition of MERTK activity can re-sensitize head and neck squamous cell carcinoma, TNBC and NSCLC to AXL inhibition137,159. Collectively, these data support the use of TAM inhibitors with other kinase inhibitors to enhance antitumour efficacy and block acquired drug resistance.
Combinations with immune checkpoint blockade.
ICB has achieved impressive clinical success in recent years. However, the patient response varies significantly with a high proportion of non-responders lacking a cytotoxic T cell response160. Given that innate immunity has a crucial role in the priming and activation of T cells, research has been focused on the regulation of dendritic cells, antigen-presenting cells, macrophages, myeloid-derived suppressor cells and natural killer cells, which all govern the innate immune response and, interestingly, express high levels of TAM receptors upon ICB treatment54. Under physiological conditions, TAM receptor activity is required for preventing hyper-stimulated, uncontrolled inflammatory responses. However, during cancer progression, TAM activation promotes immunosuppression and protects tumour cells from immune surveillance, suggesting that therapeutic blockade of TAM receptor activity in tumours might facilitate immune-mediated tumour killing. Given that AXL and MERTK have highly differentiated but important roles in regulating the immune response, selective inhibition of each receptor can be used to target different components of the innate immune system.
The potential therapeutic benefit of inhibiting AXL signalling in combination with ICB has been reported in preclinical and clinical studies. Aguilera et al. showed that an ICB-resistant subclone of MMTV-PyMT breast cancer possessed higher expression levels of AXL compared with radiation-sensitive and ICB-sensitive cells. Mechanistically, this resistance to ICB therapy can be explained by AXL-expressing tumour cells mediating the downregulation of MHC class I molecules and increasing the numbers of immune-suppressive tumour macrophages. Genetic deletion of AXL in this breast cancer model resulted in significantly enhanced CD8+ T cell infiltration and tumours sensitized to ICB and radiation treatment82. Additionally, Tsukita et al. reported a correlation between AXL, PDL1 and CXCR6 mRNA transcripts in a model of EGFR mutant lung adenocarcinoma. In this model, pharmacologic inhibition of AXL was sufficient to decrease the expression of PDL1 and CXCR6 at the transcriptional level161. Clinically, the AXL inhibitor bemcentinib is being tested in combination with the immune checkpoint inhibitor pembrolizumab for the treatment of chemotherapy and ICB refractory NSCLC (NCT03184571).
Under physiological conditions, MERTK is essential for maintaining efferocytosis and macrophage polarization. In the tumour microenvironment, elevated MERTK facilitates immune evasion by elevating expression of the tumour-promoting cytokines IL-4, IL-10 and TGFβ162. Increased MERTK expression is found on tumour-associated myeloid-derived suppressor cells, suggesting that MERTK signalling favours the recruitment of tumour-promoting immune suppressor cells163. However, inhibition of MERTK in patients can potentially have opposing effects due to its non-redundant role in phagocytosis. A soluble MERTK receptor that traps both GAS6 and PROS1 resulted in defective engulfment of apoptotic cells by macrophages and deficient platelet aggregation164. Therefore, it will be challenging to identify a MERTK inhibitor that can safely and effectively promote an immune response in tumours.
Combinations with DNA damaging agents.
Cytotoxic agents are often used as front-line therapies for cancer treatment165,166. These agents cause cell death in a non-selective manner by inducing lethal amounts of DNA damage that force cancer cells to undergo apoptosis or reproductive cell death. The most common DNA damaging agents that are used in cancer therapy include platinum-based chemotherapies167, paclitaxel (Taxol)168, doxorubicin and poly(ADP-ribose) polymerase (PARP) inhibitors151,169. Kariolis et al. demonstrated that a sAXL decoy receptor inhibited GAS6-mediated AXL signalling in ovarian cancer cells, leading to increased DNA damage. A combination of this sAXL decoy receptor with doxorubicin resulted in a greater than additive effect in decreasing the tumour burden in vivo compared with either agent alone85. Furthermore, Quinn et al. reported that high expression of AXL predicts chemoresistance in patients with ovarian cancer, and treatment of chemoresistant patient-derived xenografts with an AXL inhibitor improved the tumour response to paclitaxel and carboplatin133. These studies support the rationale of using AXL inhibitors in tumours that have developed resistance to chemotherapy.
Ionizing radiation is highly effective at treating cancers including head and neck squamous cell carcinoma, breast cancer, lung cancer, prostate cancer and some forms of gliomas170. Further enhancement of the therapeutic efficacy of radiotherapy can be achieved when combined with ICB and anti-angiogenic agents. However, despite its clinical success, the failure of radiation therapy to eradicate a tumour can be attributed to both intrinsic and exogenous factors171. Elevated expression of AXL is well described in radiation-resistant tumours and inhibitors of AXL are being investigated as radiosensitizers. AXL-induced resistance of tumours to radiotherapy can be achieved through different mechanisms82. Specifically, activation of tyrosine 821 on the AXL kinase domain is linked to radiation resistance through c-ABL signalling84. AXL signalling has also been implicated as a mechanism of resistance to the combination of EGFR antibody therapy and radiation treatment in head and neck squamous cell carcinoma.
A synergism in tumour cell killing when AXL inhibitors and PARP inhibitors are combined has been reported. PARP inhibitors selectively prevent the addition of branched PAR chains to histones and chromatin-associated proteins to aid in the recruitment of repair proteins for DNA single-strand break repair. Inhibition of PARP in homologous recombination-deficient cancer cells results in synthetic lethality. Interestingly, AXL inhibition has been proposed to induce a ‘transient’ state of homologous recombination deficiency in cancer cells, sensitizing them to PARP inhibition and providing a scientific rationale for combining AXL and PARP inhibitors172.
Unfortunately, the importance of MERTK and TYRO3 in the DNA damage and repair response of cells is less well studied. However, as MERTK signalling promotes phagocytic clearance and is required for apoptotic induced immune cell tolerance, MERTK inhibition might increase the therapeutic efficacy of DNA damaging agents by promoting an inflammatory response173. As TYRO3 activity is important for the physiological homeostasis of neuronal cells, inhibition of TYRO3 could be beneficial for the treatment of gliomas.
TAM receptor inhibitors for non-malignant indications
Emerging biological evidence supports the expansion of TAM receptor inhibitors for non-oncology indications. GAS6, AXL and MERTK are associated with the pathological development of many fibrotic diseases including non-alcoholic steatohepatitis, alcoholic liver disease, hepatitis C (HCV), IPF and IgA nephropathy174–177. The AXL inhibitor bemcentinib has been evaluated in a carbon tetrachloride (CCl4)-induced mouse liver fibrosis model. In this model, bemcentinib inhibited the activation of hepatic stellate cells, diminished collagen deposition in association with reduced MM9 and reduced α-SMA expression174. Similarly, Kariolis et al. showed that inhibition of GAS6–AXL signalling by treatment with a non-clinical version of batiraxcept led to reduced collagen deposition in highly desmoplastic, chemoresistant pancreatic tumours, allowing better tissue penetration and cytotoxic activity of gemcitabine85. Therefore, targeting TAM receptor signalling is a potential clinical strategy for treating various types of fibrosis. In addition, the MERTK inhibitor UNC569 (a derivative of MRX-2843) has demonstrated the ability to inhibit the activation of hepatic stellate cells178.
As discussed earlier, TAM receptor signalling is used by enveloped viruses as a route of entry into mammalian cells. In light of the recent Zika and Ebola virus outbreaks, the clinical use of AXL inhibitors to prevent viral entry through PtdSer-mediated cell internalization has been proposed. Pharmacological inhibition of AXL leads to a reduction in viral titre and viral-induced inflammatory responses in Zika virus-infected human cells179. The question remains whether AXL inhibitors possess sufficiently potent antiviral activity to be considered for use in the clinic.
There is some evidence suggesting that TAM receptors serve as a point of entry for SAR-CoV-2 into host cells when ACE2 expression is limited128, and the AXL inhibitor bemcentinib has been tested in a phase II clinical trial for COVID-19 (NCT04890509). Although the trial reported mixed outcomes, a subset of patients treated with bemcentinib showed symptom improvement with measurable clinical responses. Although this study is intriguing, the use of TAM receptor inhibitors in treatment of COVID-19 will require additional investigation.
Most studies have focused on understanding the pathological outcomes of TAM receptor signalling, and the therapeutic effect of TAM receptor inhibition. However, TAM receptor activation is also necessary as a negative regulator of the inflammatory response, and its activity is often suppressed in autoimmune diseases. Therefore, development of TAM receptor agonists for autoimmune diseases should be considered180. Experimentally, recombinant GAS6 and PROS1 reduce collagen-induced arthritis when administered both systemically and locally181. In addition, an AXL activating antibody administered in vivo demonstrated anti-inflammatory effects through the suppression of IFNβ1 and IFNα4 at the transcriptional level 2 h post administration182. These studies indicate that activating TAM receptors and their ligands is a potential therapeutic strategy for autoimmune diseases.
Summary and outlook
Significant progress has been made in recent years towards understanding the roles of TAM receptors in normal tissue homeostasis and disease states. In particular, the mechanistic consequences of TAM receptor activation in cancer have been the subject of intense study. A large body of literature now supports a critical role for AXL and MERTK in tumour progression and therapy resistance. Compelling preclinical studies have led to the development of therapeutic agents targeting TAM receptors as a family, as well as inhibitors specific to AXL and MERTK. Many of these molecules are demonstrating promising results, with efficacy as a single agent or in combination with other targeted and cytotoxic therapies in preclinical settings, but exactly which tumours will benefit from targeting TAM receptors alone or in combination in the clinic is still under investigation.
More than 20 TAM receptor inhibitors are being clinically developed for treating various malignancies and other diseases (Table 2). Although the majority of these inhibitors are small molecules, biologics in the form of soluble receptor ligand traps, antibodies and antibody–drug conjugates are also being evaluated. Small molecules are cost-effective to synthesize, yet a significant challenge lies in their lack of specificity, which is primarily attributed to structural homology within the catalytic domain of TAM family members. It is important to note that most small molecules are developed to inhibit TAM receptor activities as part of a multi-kinase inhibition approach, and therefore they exhibit potent inhibitory activity against many kinases. Because cancer growth and metastasis are likely to occur due to the dysregulation of many kinases, these types of inhibitors could provide more efficacious antitumour responses than selective agents towards the TAM family alone. However, inhibiting multiple kinases often leads to dose-limiting toxicities. For example, foretinib is a highly potent multi-kinase inhibitor of MET, VEGFR2 and AXL183, but unfortunately exhibited dose-limiting toxicities during phase II clinical trials that prevented its further clinical development (NCT00920192)18. Biologics in the form of antibodies and ligand traps provide a path forward for selectively disrupting TAM receptor–ligand interactions. However, these approaches have their own challenges. In particular, molecules with strong binding affinities are required to successfully disrupt the GAS6–AXL signalling axis due its high native binding affinity in the picomolar range. Additional challenges associated with TAM receptor inhibition are further discussed in Box 2.
Box 2. Challenges of TAM receptor inhibition.
Despite the clinical advances in TAM receptor therapies, several challenges still persist. Although genetic and pharmacological inhibition of individual TAM receptors can suppress tumour growth and improve the antitumour response in the setting of combination therapy, redundancy can occur amongst TAM receptors and generate compensatory signalling. For example, inhibiting AXL leads to MERTK upregulation in some cell types, promoting tumour growth and therapy resistance159. Given that TAM family members are functionally redundant, targeting multiple TAM receptors or their ligands, such as growth arrest-specific protein 6 (GAS6), might be more beneficial for certain tumours.
Identifying the precise mechanisms associated with the antitumour effects of TAM inhibitors in the clinic is difficult, given their essential role in fostering the tumour microenvironment. Finding biomarkers that predict the response to TAM inhibition might help overcome these difficulties, enabling patient selection and the monitoring of treatment response and resistance development. Potential biomarkers under investigation include TAM receptor expression in tumour biopsies and circulating soluble AXL and GAS6 levels. Pending positive clinical trial outcomes, biomarkers could become essential for patient selection and improving the clinical utility of TAM inhibitors.
The inhibition of TAM receptors and their ligands GAS6 and vitamin K-dependent protein S1 (PROS1) could have potential undesirable effects in the clinic. For example, Maimon et al. reported that myeloid cell-derived PROS1 has an unexpected function in inhibiting metastasis in lung and breast tumour models192. Also, inhibiting TAM receptors and ligands can impair the phagocytosis of apoptotic cells by macrophages and dendritic cells, leading to the accumulation of apoptotic cells. Subsequent autoimmune responses occur, resulting in ocular and central nervous system toxicity and, in some cases, promoting cancer growth193,194.
A study demonstrated that MERTK activation by PROS1 serves as a co-stimulatory signal for human CD8+ T cells, indicating that MERTK has a pleiotropic function in enhancing both immune activation and immune suppression and presenting challenges in targeting MERTK in cancer195. In addition, the inhibition of TAM receptors might have potential undesirable effects on platelet function and coagulation, given that the receptors regulate thrombosis and haemostasis. Inhibiting TAM receptors can also increase PDL1 expression, potentially limiting the therapeutic effects of TAM inhibitors in immune blockade therapy196. However, combining TAM inhibitors with PD1 or PDL1 inhibitors could potentially have synergistic antitumour effects and be a promising therapeutic strategy for cancer.
Collectively, our understanding of the biology of TAM receptor signalling during physiological and pathological states has led to the development and advancement of TAM receptor inhibitors and, in some cases, activators. In the next 5 years, the clinical success of these molecules will be determined, in the hope that they benefit patients.
Acknowledgements
This work was supported by P01CA257907 (to A.J.G. and E.B.R.) and R01CA272432-01 (to E.B.R.) from the National Cancer Institute (NCI).
Footnotes
Competing interests
A.J.G. and Y.R.M. received stock from Aravive Biologics Inc. E.B.R. declares no competing interests.
References
- 1.Lai C. & Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron 6, 691–704 (1991). [DOI] [PubMed] [Google Scholar]
- 2.Burstyn-Cohen T. TAM receptor signaling in development. Int. J. Dev. Biol 61, 215–224 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Lu Q. et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398, 723–728 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Scott RS et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207–211 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Angelillo-Scherrer A. et al. Role of Gas6 in erythropoiesis and anemia in mice. J. Clin. Invest 118, 583–596 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelillo-Scherrer A. et al. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J. Clin. Invest 115, 237–246 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morizono K. et al. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe 9, 286–298 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Challier C, Uphoff CC, Janssen JW & Drexler HG Differential expression of the ufo/axl oncogene in human leukemia-lymphoma cell lines. Leukemia 10, 781–787 (1996). [PubMed] [Google Scholar]
- 9.Graham DK et al. Ectopic expression of the proto-oncogene Mer in pediatric T-cell acute lymphoblastic leukemia. Clin. Cancer Res 12, 2662–2669 (2006). [DOI] [PubMed] [Google Scholar]
- 10.De Vos J. et al. Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays. Blood 98, 771–780 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Rankin EB et al. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 70, 7570–7579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen JW et al. A novel putative tyrosine kinase receptor with oncogenic potential. Oncogene 6, 2113–2120 (1991). [PubMed] [Google Scholar]
- 13.O’Bryan JP et al. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol. Cell Biol 11, 5016–5031 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham DK, Dawson TL, Mullaney DL, Snodgrass HR & Earp HS Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 5, 647–657 (1994). [PubMed] [Google Scholar]
- 15.Fujimoto J. & Yamamoto T. brt, a mouse gene encoding a novel receptor-type protein-tyrosine kinase, is preferentially expressed in the brain. Oncogene 9, 693–698 (1994). [PubMed] [Google Scholar]
- 16.Biesecker LG, Gottschalk LR & Emerson SG Identification of four murine cDNAs encoding putative protein kinases from primitive embryonic stem cells differentiated in vitro. Proc. Natl Acad. Sci. USA 90, 7044–7048 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biscardi JS et al. Rek, a gene expressed in retina and brain, encodes a receptor tyrosine kinase of the Axl/Tyro3 family. J. Biol. Chem 271, 29049–29059 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Ling L, Templeton D. & Kung HJ Identification of the major autophosphorylation sites of Nyk/Mer, an NCAM-related receptor tyrosine kinase. J. Biol. Chem 271, 18355–18362 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Lee-Sherick AB et al. Aberrant Mer receptor tyrosine kinase expression contributes to leukemogenesis in acute myeloid leukemia. Oncogene 32, 5359–5368 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki T. et al. Structural basis for Gas6–Axl signalling. EMBO J. 25, 80–87 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagata M, Sanes JR & Weiner JA Synaptic adhesion molecules. Curr. Opin. Cell Biol 15, 621–632 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Stitt TN et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell 80, 661–670 (1995). [DOI] [PubMed] [Google Scholar]
- 23.Varnum BC et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth-arrest-specific gene 6. Nature 373, 623–626 (1995). [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Carey K. & Godowski PJ Identification of Gas6 as a ligand for Mer, a neural cell adhesion molecule related receptor tyrosine kinase implicated in cellular transformation. Oncogene 14, 2033–2039 (1997). [DOI] [PubMed] [Google Scholar]
- 25.Godowski PJ et al. Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell 82, 355–358 (1995). [DOI] [PubMed] [Google Scholar]
- 26.Nagata K. et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem 271, 30022–30027 (1996). [DOI] [PubMed] [Google Scholar]
- 27.Sasaki T. et al. Crystal structure of a C-terminal fragment of growth arrest-specific protein Gas6. Receptor tyrosine kinase activation by laminin G-like domains. J. Biol. Chem 277, 44164–44170 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Fisher PW et al. A novel site contributing to growth-arrest-specific gene 6 binding to its receptors as revealed by a human monoclonal antibody. Biochem. J 387, 727–735 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mark MR, Chen J, Hammonds RG, Sadick M. & Godowsk PJ Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J. Biol. Chem 271, 9785–9789 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Heiring C, Dahlback B. & Muller YA Ligand recognition and homophilic interactions in Tyro3: structural insights into the Axl/Tyro3 receptor tyrosine kinase family. J. Biol. Chem 279, 6952–6958 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Huang X. et al. Structural insights into the inhibited states of the Mer receptor tyrosine kinase. J. Struct. Biol 165, 88–96 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahlback B. & Villoutreix BO Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure–function relationships and molecular recognition. Arterioscler. Thromb. Vasc. Biol 25, 1311–1320 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Calianese DC & Birge RB Biology of phosphatidylserine (PS): basic physiology and implications in immunology, infectious disease, and cancer. Cell Commun. Signal 18, 41 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharyya S. et al. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe 14, 136–147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dayoub AS & Brekken RA TIMs, TAMs, and PS-antibody targeting: implications for cancer immunotherapy. Cell Commun. Signal 18, 29 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kariolis MS et al. An engineered Axl ‘decoy receptor’ effectively silences the Gas6–Axl signaling axis. Nat. Chem. Biol 10, 977–983 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perera L, Li L, Darden T, Monroe DM & Pedersen LG Prediction of solution structures of the Ca2+-bound γ-carboxyglutamic acid domains of protein S and homolog growth arrest specific protein 6: use of the particle mesh Ewald method. Biophys. J 73, 1847–1856 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pintao MC et al. Protein S levels and the risk of venous thrombosis: results from the MEGA case–control study. Blood 122, 3210–3219 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Nonagase Y. et al. Tumor tissue and plasma levels of AXL and GAS6 before and after tyrosine kinase inhibitor treatment in EGFR-mutated non-small cell lung cancer. Thorac. Cancer 10, 1928–1935 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balogh I, Hafizi S, Stenhoff J, Hansson K. & Dahlback B. Analysis of Gas6 in human platelets and plasma. Arterioscler. Thromb. Vasc. Biol 25, 1280–1286 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Griffin JH, Gruber A. & Fernandez JA Reevaluation of total, free, and bound protein S and C4b-binding protein levels in plasma anticoagulated with citrate or hirudin. Blood 79, 3203–3211 (1992). [PubMed] [Google Scholar]
- 42.Hackeng TM, Sere KM, Tans G. & Rosing J. Protein S stimulates inhibition of the tissue factor pathway by tissue factor pathway inhibitor. Proc. Natl Acad. Sci. USA 103, 3106–3111 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heeb MJ, Mesters RM, Tans G, Rosing J. & Griffin JH Binding of protein S to factor Va associated with inhibition of prothrombinase that is independent of activated protein C. J. Biol. Chem 268, 2872–2877 (1993). [PubMed] [Google Scholar]
- 44.Heeb MJ et al. Protein S binds to and inhibits factor Xa. Proc. Natl Acad. Sci. USA 91, 2728–2732 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rezende SM, Simmonds RE & Lane DA Coagulation, inflammation, and apoptosis: different roles for protein S and the protein S–C4b binding protein complex. Blood 103, 1192–1201 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Caberoy NB Synergistic interaction of tubby and tubby-like protein 1 (Tulp1). Adv. Exp. Med. Biol 801, 503–509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caberoy NB, Alvarado G, Bigcas JL & Li W. Galectin-3 is a new MerTK-specific eat-me signal. J. Cell Physiol 227, 401–407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsou WI et al. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J. Biol. Chem 289, 25750–25763 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lew ED et al. Differential TAM receptor–ligand–phospholipid interactions delimit differential TAM bioactivities. eLife 3, e03385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geng K. et al. Requirement of γ-carboxyglutamic acid modification and phosphatidylserine binding for the activation of Tyro3, Axl, and mertk receptors by growth arrest-specific 6. Front. Immunol 8, 1521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elkabets M. et al. AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell 27, 533–546 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellosta P, Costa M, Lin DA & Basilico C. The receptor tyrosine kinase ARK mediates cell aggregation by homophilic binding. Mol. Cell Biol 15, 614–625 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor IC, Roy S. & Varmus HE Overexpression of the Sky receptor tyrosine kinase at the cell surface or in the cytoplasm results in ligand-independent activation. Oncogene 11, 2619–2626 (1995). [PubMed] [Google Scholar]
- 54.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB & Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131, 1124–1136 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Anderson HA et al. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat. Immunol 4, 87–91 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Lu Q. & Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 293, 306–311 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Cohen PL et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med 196, 135–140 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allen MP et al. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol. Endocrinol 13, 191–201 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Caraux A. et al. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat. Immunol 7, 747–754 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Kinjyo I. et al. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17, 583–591 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Zong C, Yan R, August A, Darnell JE Jr. & Hanafusa H. Unique signal transduction of Eyk: constitutive stimulation of the JAK–STAT pathway by an oncogenic receptor-type tyrosine kinase. EMBO J. 15, 4515–4525 (1996). [PMC free article] [PubMed] [Google Scholar]
- 62.Carrera Silva EA et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity 39, 160–170 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gal A. et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet 26, 270–271 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Nandrot E. et al. Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol. Dis 7, 586–599 (2000). [DOI] [PubMed] [Google Scholar]
- 65.D’Cruz PM et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet 9, 645–651 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Akalu YT et al. Tissue-specific modifier alleles determine Mertk loss-of-function traits. eLife 11, e80530 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melaragno MG et al. Increased expression of Axl tyrosine kinase after vascular injury and regulation by G protein-coupled receptor agonists in rats. Circ. Res 83, 697–704 (1998). [DOI] [PubMed] [Google Scholar]
- 68.Melaragno MG et al. Gas6 inhibits apoptosis in vascular smooth muscle: role of Axl kinase and Akt. J. Mol. Cell Cardiol 37, 881–887 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Murao K. et al. A product of growth arrest-specific gene 6 modulates scavenger receptor expression in human vascular smooth muscle cells. FEBS Lett. 459, 363–366 (1999). [DOI] [PubMed] [Google Scholar]
- 70.Calzavarini S. et al. Platelet protein S limits venous but not arterial thrombosis propensity by controlling coagulation in the thrombus. Blood 135, 1969–1982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saller F. et al. Generation and phenotypic analysis of protein S-deficient mice. Blood 114, 2307–2314 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burstyn-Cohen T, Heeb MJ & Lemke G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J. Clin. Invest 119, 2942–2953 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salian-Mehta S. et al. Loss of growth arrest specific gene 6 (Gas6) results in altered GnRH neuron migration, delayed vaginal opening and sexual maturation in mice. Mol. Cell Endocrinol 393, 164–170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cosemans JM et al. Potentiating role of Gas6 and Tyro3, Axl and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization. J. Thromb. Haemost 8, 1797–1808 (2010). [DOI] [PubMed] [Google Scholar]
- 75.Graham DK, DeRyckere D, Davies KD & Earp HS The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat. Rev. Cancer 14, 769–785 (2014). [DOI] [PubMed] [Google Scholar]
- 76.Burchert A, Attar EC, McCloskey P, Fridell YW & Liu ET Determinants for transformation induced by the Axl receptor tyrosine kinase. Oncogene 16, 3177–3187 (1998). [DOI] [PubMed] [Google Scholar]
- 77.McCloskey P. et al. GAS6 mediates adhesion of cells expressing the receptor tyrosine kinase Axl. J. Biol. Chem 272, 23285–23291 (1997). [DOI] [PubMed] [Google Scholar]
- 78.Rankin EB et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc. Natl Acad. Sci. USA 111, 13373–13378 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rankin EB & Giaccia AJ The receptor tyrosine kinase AXL in cancer progression. Cancers 8, 103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Badarni M. et al. Repression of AXL expression by AP-1/JNK blockage overcomes resistance to PI3Ka therapy. JCI Insight 5, e125341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller MA, Sullivan RJ & Lauffenburger DA Molecular pathways: receptor ectodomain shedding in treatment, resistance, and monitoring of cancer. Clin. Cancer Res 23, 623–629 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aguilera TA et al. Reprogramming the immunological microenvironment through radiation and targeting Axl. Nat. Commun 7, 13898 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park IK et al. Receptor tyrosine kinase Axl is required for resistance of leukemic cells to FLT3-targeted therapy in acute myeloid leukemia. Leukemia 29, 2382–2389 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDaniel NK et al. AXL mediates cetuximab and radiation resistance through tyrosine 821 and the c-ABL kinase pathway in head and neck cancer. Clin. Cancer Res 26, 4349–4359 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kariolis MS et al. Inhibition of the GAS6/AXL pathway augments the efficacy of chemotherapies. J. Clin. Invest 127, 183–198 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Z. et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet 44, 852–860 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zuo Q. et al. AXL/AKT axis mediated-resistance to BRAF inhibitor depends on PTEN status in melanoma. Oncogene 37, 3275–3289 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clara JA, Monge C, Yang Y. & Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells — wa clinical update. Nat. Rev. Clin. Oncol 17, 204–232 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Cichon MA et al. The receptor tyrosine kinase Axl regulates cell–cell adhesion and stemness in cutaneous squamous cell carcinoma. Oncogene 33, 4185–4192 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Asiedu MK et al. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene 33, 1316–1324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sadahiro H. et al. Activation of the receptor tyrosine kinase AXL regulates the immune microenvironment in glioblastoma. Cancer Res. 78, 3002–3013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao Y. et al. S100A10 is a critical mediator of GAS6/AXL-induced angiogenesis in renal cell carcinoma. Cancer Res. 79, 5758–5768 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Linger RM et al. Mer receptor tyrosine kinase is a therapeutic target in pre-B-cell acute lymphoblastic leukemia. Blood 122, 1599–1609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cummings CT et al. Mer590, a novel monoclonal antibody targeting MER receptor tyrosine kinase, decreases colony formation and increases chemosensitivity in non-small cell lung cancer. Oncotarget 5, 10434–10445 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su YT et al. MerTK inhibition decreases immune suppressive glioblastoma-associated macrophages and neoangiogenesis in glioblastoma microenvironment. Neurooncol. Adv 2, vdaa065 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sinik L. et al. Inhibition of MERTK promotes suppression of tumor growth in BRAF mutant and BRAF wild-type melanoma. Mol. Cancer Ther 18, 278–288 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee-Sherick AB et al. MERTK inhibition alters the PD-1 axis and promotes anti-leukemia immunity. JCI Insight 3, e97941 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koda Y, Itoh M. & Tohda S. Effects of MERTK inhibitors UNC569 and UNC1062 on the growth of acute myeloid leukaemia cells. Anticancer. Res 38, 199–204 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Nishi C, Yanagihashi Y, Segawa K. & Nagata S. MERTK tyrosine kinase receptor together with TIM4 phosphatidylserine receptor mediates distinct signal transduction pathways for efferocytosis and cell proliferation. J. Biol. Chem 294, 7221–7230 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ekyalongo RC et al. TYRO3 as a potential therapeutic target in breast cancer. Anticancer. Res 34, 3337–3345 (2014). [PubMed] [Google Scholar]
- 101.Ammoun S. et al. Axl/Gas6/NFκB signalling in schwannoma pathological proliferation, adhesion and survival. Oncogene 33, 336–346 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Dantas-Barbosa C. et al. Expression and role of TYRO3 and AXL as potential therapeutical targets in leiomyosarcoma. Br. J. Cancer 117, 1787–1797 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsu PL, Jou J. & Tsai SJ TYRO3: a potential therapeutic target in cancer. Exp. Biol. Med 244, 83–99 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chien CW et al. Targeting TYRO3 inhibits epithelial–mesenchymal transition and increases drug sensitivity in colon cancer. Oncogene 35, 5872–5881 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Jiang Z. et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J. Clin. Invest 131, e139434 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruiz-Heiland G. et al. Deletion of the receptor tyrosine kinase Tyro3 inhibits synovial hyperplasia and bone damage in arthritis. Ann. Rheum. Dis 73, 771–779 (2014). [DOI] [PubMed] [Google Scholar]
- 107.Korshunov VA, Mohan AM, Georger MA & Berk BC Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ. Res 98, 1446–1452 (2006). [DOI] [PubMed] [Google Scholar]
- 108.Shimojima M. et al. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J. Virol 80, 10109–10116 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zeisberg M. & Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol 304, C216–C225 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bellan M. et al. Gas6/TAM system: a key modulator of the interplay between inflammation and fibrosis. Int. J. Mol. Sci 20, 5070 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee HJ, Jeng YM, Chen YL, Chung L. & Yuan RH Gas6/Axl pathway promotes tumor invasion through the transcriptional activation of Slug in hepatocellular carcinoma. Carcinogenesis 35, 769–775 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Reichl P. et al. Axl activates autocrine transforming growth factor-β signaling in hepatocellular carcinoma. Hepatology 61, 930–941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fourcot A. et al. Gas6 deficiency prevents liver inflammation, steatohepatitis, and fibrosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol 300, G1043–G1053 (2011). [DOI] [PubMed] [Google Scholar]
- 114.Nathan SD et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest 140, 221–229 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Espindola MS et al. Targeting of TAM receptors ameliorates fibrotic mechanisms in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med 197, 1443–1456 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burstyn-Cohen T. et al. Genetic dissection of TAM receptor–ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron 76, 1123–1132 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ballantine L. et al. Increased soluble phagocytic receptors sMer, sTyro3 and sAxl and reduced phagocytosis in juvenile-onset systemic lupus erythematosus. Pediatr. Rheumatol. Online J 13, 10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shen K. et al. Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Rep. 34, 108835 (2021). [DOI] [PubMed] [Google Scholar]
- 119.Healy LM et al. MerTK-mediated regulation of myelin phagocytosis by macrophages generated from patients with MS. Neurol. Neuroimmunol. Neuroinflamm 4, e402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bellan M, Pirisi M. & Sainaghi PP The Gas6/TAM system and multiple sclerosis. Int. J. Mol. Sci 17, 1807 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Helenius A. Virus entry: looking back and moving forward. J. Mol. Biol 430, 1853–1862 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meertens L. et al. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 12, 544–557 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jemielity S. et al. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 9, e1003232 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zwernik SD et al. AXL receptor is required for Zika virus strain MR-766 infection in human glioblastoma cell lines. Mol. Ther. Oncolytics 23, 447–457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moller-Tank S. & Maury W. Phosphatidylserine receptors: enhancers of enveloped virus entry and infection. Virology 468–470, 565–580 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen J. et al. AXL promotes Zika virus infection in astrocytes by antagonizing type I interferon signalling. Nat. Microbiol 3, 302–309 (2018). [DOI] [PubMed] [Google Scholar]
- 127.Hunt CL, Kolokoltsov AA, Davey RA & Maury W. The Tyro3 receptor kinase Axl enhances macropinocytosis of Zaire ebolavirus. J. Virol 85, 334–347 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bohan D. et al. Phosphatidylserine receptors enhance SARS-CoV-2 infection. PLoS Pathog. 17, e1009743 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zammarchi F. et al. Preclinical development of ADCT-601, a novel pyrrolobenzodiazepine dimer-based antibody–drug conjugate targeting AXL-expressing cancers. Mol. Cancer Ther 21, 582–593 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quirico L. et al. Axl-148b chimeric aptamers inhibit breast cancer and melanoma progression. Int. J. Biol. Sci 16, 1238–1251 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boshuizen J. et al. Cooperative targeting of immunotherapy-resistant melanoma and lung cancer by an AXL-targeting antibody–drug conjugate and immune checkpoint blockade. Cancer Res. 81, 1775–1787 (2021). [DOI] [PubMed] [Google Scholar]
- 132.Boshuizen J. et al. Cooperative targeting of melanoma heterogeneity with an AXL antibody–drug conjugate and BRAF/MEK inhibitors. Nat. Med 24, 203–212 (2018). [DOI] [PubMed] [Google Scholar]
- 133.Quinn JM et al. Therapeutic inhibition of the receptor tyrosine kinase AXL improves sensitivity to platinum and taxane in ovarian cancer. Mol. Cancer Ther 18, 389–398 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ludwig KF et al. Small-molecule inhibition of Axl targets tumor immune suppression and enhances chemotherapy in pancreatic cancer. Cancer Res. 78, 246–255 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yokoyama Y. et al. Immuno-oncological efficacy of RXDX-106, a novel TAM (TYRO3, AXL, MER) family small-molecule kinase inhibitor. Cancer Res. 79, 1996–2008 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Minson KA et al. The MERTK/FLT3 inhibitor MRX-2843 overcomes resistance-conferring FLT3 mutations in acute myeloid leukemia. JCI Insight 1, e85630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yan D, Earp HS, DeRyckere D. & Graham DK Targeting MERTK and AXL in EGFR mutant non-small cell lung cancer. Cancers 13, 5639 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang Y. et al. Modulating the function of ABCB1: in vitro and in vivo characterization of sitravatinib, a tyrosine kinase inhibitor. Cancer Commun. 40, 285–300 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu CP et al. Sitravatinib sensitizes ABCB1- and ABCG2-overexpressing multidrug-resistant cancer cells to chemotherapeutic drugs. Cancers 12, 195 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Okura N. et al. ONO-7475, a novel AXL inhibitor, suppresses the adaptive resistance to initial EGFR-TKI treatment in EGFR-mutated non-small cell lung cancer. Clin. Cancer Res 26, 2244–2256 (2020). [DOI] [PubMed] [Google Scholar]
- 141.Post SM et al. AXL/MERTK inhibitor ONO-7475 potently synergizes with venetoclax and overcomes venetoclax resistance to kill FLT3-ITD acute myeloid leukemia. Haematologica 107, 1311–1322 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shin JS et al. NPS-1034, a novel MET inhibitor, inhibits the activated MET receptor and its constitutively active mutants. Invest. N. Drugs 32, 389–399 (2014). [DOI] [PubMed] [Google Scholar]
- 143.Zdzalik-Bielecka D. et al. Bemcentinib and gilteritinib inhibit cell growth and impair the endo-lysosomal and autophagy systems in an AXL-independent manner. Mol. Cancer Res 20, 446–455 (2021). [DOI] [PubMed] [Google Scholar]
- 144.Byers LA et al. An epithelial–mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res 19, 279–290 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fuh KC et al. Phase 1b study of AVB-500 in combination with paclitaxel or pegylated liposomal doxorubicin platinum-resistant recurrent ovarian cancer. Gynecol. Oncol 163, 254–261 (2021). [DOI] [PubMed] [Google Scholar]
- 146.Koopman LA et al. Enapotamab vedotin, an AXL-specific antibody–drug conjugate, shows preclinical antitumor activity in non-small cell lung cancer. JCI Insight 4, e128199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ye X. et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 29, 5254–5264 (2010). [DOI] [PubMed] [Google Scholar]
- 148.Cerchia L. et al. Targeting Axl with an high-affinity inhibitory aptamer. Mol. Ther 20, 2291–2303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu G. et al. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Mol. Cancer 17, 20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Linger RM, Keating AK, Earp HS & Graham DK TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res 100, 35–83 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mullen MM et al. GAS6/AXL inhibition enhances ovarian cancer sensitivity to chemotherapy and PARP inhibition through increased DNA damage and enhanced replication stress. Mol. Cancer Res 20, 265–279 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toboni MD et al. Inhibition of AXL and VEGF-A has improved therapeutic efficacy in uterine serous cancer. Cancers 13, 5877 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ben-Batalla I. et al. Axl blockade by BGB324 inhibits BCR-ABL tyrosine kinase inhibitor-sensitive and -resistant chronic myeloid leukemia. Clin. Cancer Res 23, 2289–2300 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Yang Z. et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int. J. Cancer 140, 2805–2819 (2017). [DOI] [PubMed] [Google Scholar]
- 155.Puzanov I, Burnett P. & Flaherty KT Biological challenges of BRAF inhibitor therapy. Mol. Oncol 5, 116–123 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alexander PB & Wang XF Resistance to receptor tyrosine kinase inhibition in cancer: molecular mechanisms and therapeutic strategies. Front. Med 9, 134–138 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Du Z. & Lovly CM Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 17, 58 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu L. et al. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 69, 6871–6878 (2009). [DOI] [PubMed] [Google Scholar]
- 159.McDaniel NK et al. MERTK mediates intrinsic and adaptive resistance to AXL-targeting agents. Mol. Cancer Ther 17, 2297–2308 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Morad G, Helmink BA, Sharma P. & Wargo JA Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184, 5309–5337 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tsukita Y. et al. Axl kinase drives immune checkpoint and chemokine signalling pathways in lung adenocarcinomas. Mol. Cancer 18, 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mohd Idrus FN et al. Differential polarization and the expression of efferocytosis receptor MerTK on M1 and M2 macrophages isolated from coronary artery disease patients. BMC Immunol. 22, 21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zizzo G. & Cohen PL The PPAR-γ antagonist GW9662 elicits differentiation of M2c-like cells and upregulation of the MerTK/Gas6 axis: a key role for PPAR-γ in human macrophage polarization. J. Inflamm 12, 36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sather S. et al. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood 109, 1026–1033 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Slade D. PARP and PARG inhibitors in cancer treatment. Genes. Dev 34, 360–394 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goldstein M. & Kastan MB The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu. Rev. Med 66, 129–143 (2015). [DOI] [PubMed] [Google Scholar]
- 167.Wang D. & Lippard SJ Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov 4, 307–320 (2005). [DOI] [PubMed] [Google Scholar]
- 168.Weaver BA How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 25, 2677–2681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Carvalho C. et al. Doxorubicin: the good, the bad and the ugly effect. Curr. Med. Chem 16, 3267–3285 (2009). [DOI] [PubMed] [Google Scholar]
- 170.Baskar R, Lee KA, Yeo R. & Yeoh KW Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci 9, 193–199 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim BM et al. Therapeutic implications for overcoming radiation resistance in cancer therapy. Int. J. Mol. Sci 16, 26880–26913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Balaji K. et al. AXL inhibition suppresses the DNA damage response and sensitizes cells to PARP inhibition in multiple cancers. Mol. Cancer Res 15, 45–58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lindsay RS et al. MERTK on mononuclear phagocytes regulates T cell antigen recognition at autoimmune and tumor sites. J. Exp. Med 218, e20200464 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barcena C. et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J. Hepatol 63, 670–678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang J, Qiao L, Hou Z. & Luo G. TIM-1 promotes hepatitis C virus cell attachment and infection. J. Virol 91, e01583–e01616 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tutusaus A. et al. A functional role of GAS6/TAM in nonalcoholic steatohepatitis progression implicates AXL as therapeutic target. Cell Mol. Gastroenterol. Hepatol 9, 349–368 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nagai K. et al. Dual involvement of growth arrest-specific gene 6 in the early phase of human IgA nephropathy. PLoS ONE 8, e66759 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bernsmeier C. et al. Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology 148, 603–615 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Meertens L. et al. Axl mediates Zika virus entry in human glial cells and modulates innate immune responses. Cell Rep. 18, 324–333 (2017). [DOI] [PubMed] [Google Scholar]
- 180.Lemke G. & Rothlin CV Immunobiology of the TAM receptors. Nat. Rev. Immunol 8, 327–336 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.van den Brand BT et al. Therapeutic efficacy of Tyro3, Axl, and Mer tyrosine kinase agonists in collagen-induced arthritis. Arthritis Rheum. 65, 671–680 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zagorska A, Traves PG, Lew ED, Dransfield I. & Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat. Immunol 15, 920–928 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Logan TF Foretinib (XL880): c-MET inhibitor with activity in papillary renal cell cancer. Curr. Oncol. Rep 15, 83–90 (2013). [DOI] [PubMed] [Google Scholar]
- 184.Avanzi GC et al. GAS6, the ligand of Axl and Rse receptors, is expressed in hematopoietic tissue but lacks mitogenic activity. Exp. Hematol 25, 1219–1226 (1997). [PubMed] [Google Scholar]
- 185.Gallicchio M. et al. Inhibition of vascular endothelial growth factor receptor 2-mediated endothelial cell activation by Axl tyrosine kinase receptor. Blood 105, 1970–1976 (2005). [DOI] [PubMed] [Google Scholar]
- 186.Giroud P. et al. Expression of TAM-R in human immune cells and unique regulatory function of MerTK in IL-10 production by tolerogenic DC. Front. Immunol 11, 564133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gilchrist SE, Pennelli GM & Hafizi S. Gas6/TAM signalling negatively regulates inflammatory induction of GM-CSF in mouse brain microglia. Cells 10, 3281 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lemke G. Biology of the TAM receptors. Cold Spring Harb. Perspect. Biol 5, a009076 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gely-Pernot A. et al. An endogenous vitamin K-dependent mechanism regulates cell proliferation in the brain subventricular stem cell niche. Stem Cell 30, 719–731 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Burstyn-Cohen T. & Hochberg A. TAM signaling in the nervous system. Brain Plast. 7, 33–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.ten Kate MK & van der Meer J. Protein S deficiency: a clinical perspective. Haemophilia 14, 1222–1228 (2008). [DOI] [PubMed] [Google Scholar]
- 192.Maimon A. et al. Myeloid cell-derived PROS1 inhibits tumor metastasis by regulating inflammatory and immune responses via IL-10. J. Clin. Invest 131, e126089 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ubil E. et al. Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J. Clin. Invest 128, 2356–2369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bosurgi L. et al. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proc. Natl Acad. Sci. USA 110, 13091–13096 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Peeters MJW et al. MERTK acts as a costimulatory receptor on human CD8+ T cells. Cancer Immunol. Res 7, 1472–1484 (2019). [DOI] [PubMed] [Google Scholar]
- 196.Kasikara C. et al. Phosphatidylserine sensing by TAM receptors regulates AKT-dependent chemoresistance and PD-L1 expression. Mol. Cancer Res 15, 753–764 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]