Abstract
Background:
The impact of esophageal dysmotility among patients with post-fundoplication esophageal symptoms is not fully understood. This study aimed to investigate secondary peristalsis and esophagogastric junction (EGJ) opening biomechanics using functional lumen imaging probe (FLIP) panometry in symptomatic post-fundoplication patients.
Methods:
Eighty-seven adult patients post-fundoplication who completed FLIP for symptomatic esophageal evaluation were included. Secondary peristaltic contractile response (CR) patterns and EGJ opening metrics (EGJ distensibility index (EGJ-DI) and maximum EGJ diameter) were evaluated on FLIP panometry and analyzed against high-resolution manometry (HRM), patient-reported outcomes, and fundoplication condition seen on esophagram and/or endoscopy.
Key Results:
FLIP CR patterns included 14 (16%) normal CR, 30 (34%) borderline CR, 28 (32%) impaired/disordered CR, 13 (15%) absent CR, and 2 (2%) spastic reactive CR. Compared with normal and borderline CRs (i.e., CR patterns with distinct, antegrade peristalsis), patients with impaired/disordered and absent CRs demonstrated significantly greater time since fundoplication (2.4 (0.6–6.8) vs. 8.9 (2.6–14.5) years; p = 0.002), greater esophageal body width on esophagram (n = 50; 2.3 (2.0–2.8) vs. 2.9 (2.4–3.6) cm; p = 0.013), and lower EGJ-DI (4.3 (2.7–5.4) vs. 2.6 (1.7–3.7) mm2/mmHg; p = 0.001). Intact fundoplications had significantly higher rates of normal CRs compared to anatomically abnormal (i.e., tight, disrupted, slipped, herniated) fundoplications (9 (28%) vs. 5 (9%); p = 0.032), but there were no differences in EGJ-DI or EGJ maximum diameter.
Conclusions & Inferences:
Symptomatic post-fundoplication patients were characterized by frequent abnormal secondary peristalsis after fundoplication, potentially worsening with time after fundoplication or related to EGJ outflow resistance.
Keywords: dysphagia, fundoplication, impedance, peristalsis, reflux
1 |. INTRODUCTION
Laparoscopic fundoplication aims to anatomically restore the anti-reflux barrier and reduce gastroesophageal reflux for treatment of gastroesophageal reflux disease (GERD).1 Nissen fundoplication (360°) and partial fundoplication techniques (Toupet (270°) and Dor (180°)) involve varying degrees of circumferential plication of the gastric fundus around the esophagus to reinforce the defective esophagogastric junction (EGJ).2 However, despite reflux alleviation in most patients, chronic post-fundoplication symptoms are prevalent, including dysphagia (7.5%−42%), recurrent reflux (17.7%), and gas-bloat syndrome (10%−32%).3–12
Esophageal symptoms after fundoplication, specifically dysphagia, have been attributed to a variety of structural mechanisms including the fundoplication being too tight, disrupted, herniated, and/or slipped.9 Additionally, impaired peristalsis on manometry has been shown to be associated with both dysphagia and reflux pathology before and after anti-reflux surgery.13,14 These findings warrant further investigation into the role of impaired esophageal motility and EGJ mechanics in the development of post-fundoplication symptoms.
Functional lumen imaging probe (FLIP) panometry provides a well-tolerated, unique modality to evaluate both esophageal secondary peristalsis and EGJ opening biomechanics using high-resolution impedance planimetry during stepwise volumetric distension.15 Through FLIP panometry, the secondary peristaltic contractile response (CR) to distension can be classified into CR patterns ranging from normal to borderline to abnormal (impaired-disordered, spastic reactive, or absent) esophageal motility.16 Both FLIP panometry CR patterns and FLIP evaluation of EGJ distensibility and opening parallel and complement high-resolution manometry (HRM) diagnoses based on the Chicago classification v4.0.17, 18 However, the full spectrum of FLIP CR patterns has not yet been described in an adult post-fundoplication cohort, nor have they been analyzed against clinical and patient-reported outcomes in this context. Hence, this study aimed to investigate FLIP panometry CR patterns and EGJ opening biomechanics in relationship to clinical outcomes in symptomatic post-fundoplication patients.
2 |. MATERIALS AND METHODS
2.1 |. Subjects
Adult patients (ages 18–85) presenting to the Esophageal Center of Northwestern between January 2015 and August 2021 who completed FLIP with upper endoscopy were retrospectively accessed from a prospectively maintained esophageal motility data registry. Consecutive symptomatic patients with a history of Nissen, Toupet, Dor, or Belsey fundoplication were subsequently included and evaluated using a cross-sectional study design. Patients with technically limited FLIP studies were excluded as were patients with a history of transoral incisionless fundoplication, magnetic sphincter augmentation, Heller myotomy, tracheoesophageal fistula repair, or takedown of prior fundoplication without redo.
If completed within 3months of upper endoscopy with FLIP (and without interval surgery or endoscopic treatment, i.e., dilation), HRM and/or barium esophagram were also reviewed. Fundoplications were assessed via upper endoscopy by experienced endoscopists and (when available) barium esophagrams evaluated by radiologists. Endoscopy and radiology reports were reviewed for fundoplication status; in the setting of discrepancies, abnormal findings were favored. Fundoplication wrap anatomy was classified as “abnormal” if described as tight, disrupted, herniated (sliding vs. paraesophageal), slipped, and/or twisted; otherwise, fundoplications were classified as anatomically “intact.” HRM performed prior to fundoplication were also reviewed when available. The study protocol was approved by the Northwestern University Institutional Review Board.
2.2 |. Functional lumen imaging probe study protocol
The FLIP study, using 16-cm FLIP (EndoFLIP® EF-322N; Medtronic, Inc, Shoreview, MN), was performed during sedated endoscopy as previously described.15,17,19 Endoscopy and FLIP were conducted in the left-lateral decubitus position using conscious sedation with midazolam and fentanyl. Other medications, e.g., propofol, were also used with monitored anesthesia care in some cases at the discretion of the performing endoscopist. Although these medications can alter esophageal motility, the patterns of motility during the FLIP protocol are reproducible and have been shown to correspond with motility patterns during standard manometry performed without these medications.15,19–21
After completion of the endoscopy and calibration to atmospheric pressure, the FLIP was placed transorally and positioned for the remainder of the study with 1–3 impedance sensors situated beyond the EGJ. Stepwise 10-mL FLIP distensions were then performed, beginning with 40-mL and increasing to a target volume of 70-mL; each stepwise distension volume was maintained for 30–60s.
2.3 |. Functional lumen imaging probe panometry analysis
FLIP panometry analysis utilized a customized program (available open source at http://www.wklytics.com/nmgi) and was conducted blinded to clinical characteristics, (e.g., HRM and esophagram results). The CR pattern was defined using the total duration of the 50–70mL FLIP fill volumes. Studies were reviewed for specific features and patterns of contractility that were then applied to assign a CR pattern (Figure SI).16,22 Normal CR or borderline CR involved distinct, antegrade contractions, consistent with normal secondary peristalsis. Impaired/disordered CR, absent CR, and spastic reactive CR were considered abnormal CR patterns.16,22
EGJ distensibility index (Dl) was measured at the 60-mL FLIP fill volume while the maximum EGJ diameter was determined during the 60-mL or 70-mL fill volume as previously described.22 Areas at the EGJ that were affected by dry catheter artifact (i.e., artifact that distorts diameter measurement when occlusion of the FLIP bag disrupts the electrical current utilized for the impedance planimetry technology) were omitted from EGJ analysis.22 Normal EGJ opening was classified as EGJ-DI >2.0mm2/mmHg and maximum EGJ diameter >16mm.18 Reduced EGJ opening was defined as EGJ-DI <2.0mm2/mmHg and maximum EGJ diameter <12mm. Borderline EGJ opening was classified as EGJ-DI <2.0mm2/mmHg or maximum EGJ diameter <16mm, but not meeting reduced EGJ opening criteria.
2.4 |. High-resolution manometry protocol and analysis
After a minimum 6-h fast, HRM studies were completed using a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals (Medtronic Inc, Shoreview, MN). The HRM assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with approximately three intragastric pressure sensors. After a 2-min baseline recording (during which the basal EGJ pressure was measured during end expiration), the HRM protocol was performed with 10, 5-mL liquid swallows in a supine position and five 5-mL liquid swallows in an upright, seated position.23
HRM studies were analyzed independent of other clinical details including FLIP results and were interpreted according to the Chicago classification v4.0.23 Although the Chicago classification is intended for patients without previous foregut surgery, the metric thresholds and motility classifications were applied to this cohort to define the motility patterns in a standardized manner. Peristalsis classifications included normal primary peristalsis, ineffective esophageal motility, absent, hypercontractile, and premature contractility. The integrated relaxation pressure (IRP), was measured for the 10 supine swallows and 5 upright swallows; median values for each position were then applied. A median IRP of >15mmHg was considered abnormal for supine swallows; a median IRP of >12 mmHg was considered abnormal for upright swallows.23
2.5 |. Clinical evaluation and assessment of fundoplication
Some patients completed the baseline brief esophageal dysphagia questionnaire (BEDQ), GERD questionnaire (GerdQ), and esophageal hypervigilance and anxiety scale (EHAS) assessing for dysphagia, GERD, and esophageal hypervigilance and anxiety symptom burden, respectively; greater scores correlated with greater symptom severity.24–26 Additional clinical features were collected at the time of motility testing and maintained in the data registry included demographic information, primary indication for motility evaluation, proton pump inhibitor (PPI) use, time since fundoplication, type of fundoplication, and details of hiatal hernia repair at the time of fundoplication(s).
Barium esophagram was performed around time of FLIP in some patients. In some of these patients, a timed barium esophagram (TBE) protocol was further performed, which was conducted in upright position and involved drinking 200-mL of low-density barium sulfate with images obtained at 1 and 5min. 12.5-mm barium tablet was also ingested if liquid barium cleared. TBE was assessed for esophageal retention, with abnormal (tablet impaction, 1-min column height ≥5cm, or 5-min column height ≥2cm) and normal (i.e., not meeting preceding criteria considered “abnormal”).27 The esophagram was further assessed for esophageal body width and fundoplication condition.
2.6 |. Statistical analysis
Results were reported as mean (standard deviation; SD), or median (interquartile range; IQR) depending on data distribution. Groups were compared using the chi-square or Fisher exact tests for categorical variables and ANOVA/t-tests or Kruskal-Wallis/Mann-Whitney U for continuous variables, depending on data distribution. Comparative analysis of CR subgroups primarily included normal and borderline CR combined versus impaired/disordered and absent CR combined, i.e., omitted patients with spastic reactive CR (n = 2) given the small sample size. Comparative analysis between primary indications for evaluation included dysphagia versus heartburn/reflux with omission of patients with ‘other’ indications given the small sample size (n = 8). Statistical significance was considered at a two-tailed p-value <0.05. Post-hoc comparison testing, as appropriate, was completed using a Bonferroni correction.
3 |. RESULTS
3.1 |. Subjects
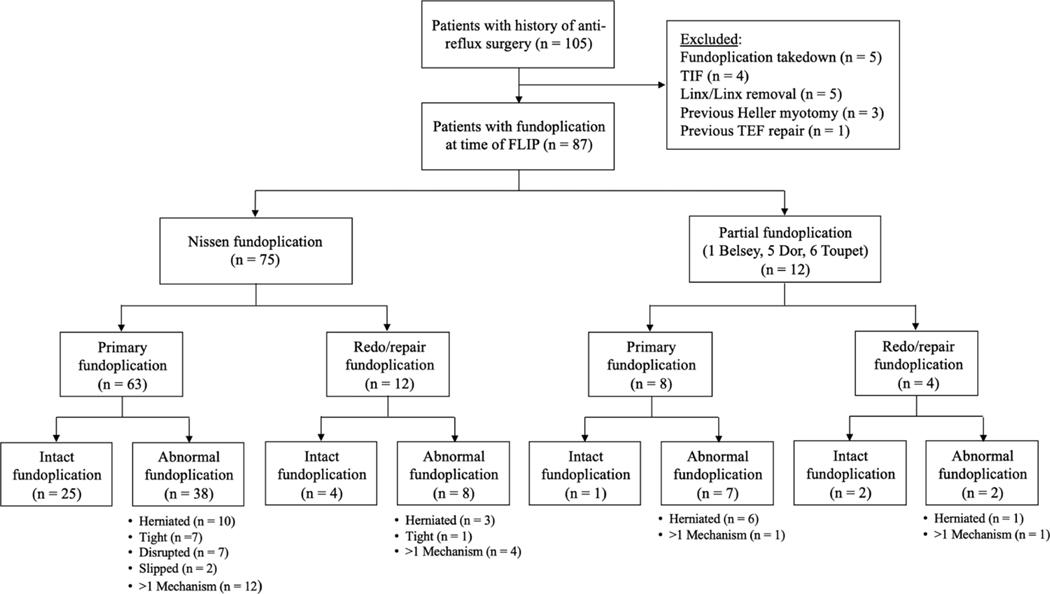
A total of 87 patients (median (IQR) age, 59 (47–70) years; 72% female) were included; Figure 1, Table 1. The cohort consisted of 75 (86%) Nissen fundoplications and 12 (14%) partial fundoplications. 16 (18%) patients had a least one redo fundoplication prior to FLIP evaluation. 26 (30%) fundoplications were performed at our institution, while 61 (70%) fundoplications were completed externally. The most common indication for post-fundoplication evaluation was dysphagia (72%), followed by heartburn/reflux symptoms (17%) and chest pain (6%). Fundoplication wrap anatomy was assessed as 37% intact and 63% abnormal. Anatomically abnormal fundoplications included 9% tight, 23% herniated (35% sliding hiatal hernia, 65% paraesophageal hernia), 8% disrupted, 2% slipped, and 21% with a combination of problems (namely 61% disrupted and herniated). 66% of patients were on PPI therapy at time of FLIP evaluation.
FIGURE 1.
Post-fundoplication patient cohort composition and wrap failure mechanisms. FLIP–functional lumen imaging probe. TlF–transoral incisionless fundoplication. TEF–tracheoesophageal fistula.
TABLE 1.
Cohort characteristics.
| Full cohort | Intact fundoplication | Abnormal fundoplication anatomy | |
|---|---|---|---|
| n (%) | 87 | 32 (37) | 55 (63) |
| Age, y | 59 (47–70) | 55 (48–67) | 61 (47–70) |
| Biological sex, n (%) female | 63 (72) | 23 (72) | 40 (73) |
| Body mass index (BMI) | 26.3 (24.0–32.0) | 27.3 (23.2–34.4) | 26.3 (24.7–31.1) |
| Primary indication for evaluation, n (%) | |||
| Dysphagia | 63 (72) | 23 (72) | 40 (73) |
| Heartburn/reflux | 15 (17) | 5 (16) | 10 (18) |
| Chest pain | 5 (6) | 3 (9) | 2 (4) |
| Other | 4 (5) | 1 (3) | 3 (5) |
| Symptom scores | |||
| GerdQ score (IQR), [n (%) completed] | 9 (7–11) [47 (54)] | 9 (7–11) [16 (50)] | 9 (8–11) [31 (56)] |
| BEDQ score (IQR), [n (%) completed] | 15 (8–22) [51 (59)] | 16 (6–28) [18 (56)] | 14 (10–19) [33 (60)] |
| EHAS score (IQR), [n (%) completed] | 33 (21–39) [44 (51)] | 37 (29–38) [13 (41)] | 34 (25–41) [31 (56)] |
| Time since fundoplication, y | 4.9 (1.0–11.2) | 6.7 (0.7–13.8) | 4.3 (1.3–9.5) |
| Fundoplication type, n (%) | |||
| Nissen fundoplication | 75 (86) | 29 (91) | 46 (84) |
| Partial fundoplication, n (%) | 12 (14) | 3 (9) | 9 (16) |
| Hiatal hernia repair, n (%) | |||
| With hiatal hernia repair | 64 (74) | 23 (72) | 41 (75) |
| Without hiatal hernia repair | 4 (5) | 2 (6) | 2 (4) |
| Hiatal hernia repair unknown | 19 (22) | 7 (22) | 12 (22) |
| Redo/repaired fundoplication, n (%) | 16 (18) | 6 (19) | 10 (18) |
| Esophagram completed, n (%) | 50 (27) | 17 (53) | 33 (60) |
| Esophageal body width, cm | 2.6 (2.2–3.1) | 2.4 (2.2–3.0) | 2.7 (2.2–3.3) |
| Tablet impaction/Used barium tablet, n/n (%) | 7/33 (21) | 2/12 (17) | 5/21 (24) |
| TBE completed, n (%) | 28 (56) | 10 (59) | 18 (55) |
| 1-min column height, cm | 0.0 (0.0–5.0) | 0.0 (0.0–0.0) | 0.0 (0.0–11.2) |
| 5-min column height, cm | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–5.5) |
Note: All values were reported as median (IQR–interquartile range) unless indicated otherwise.
p < 0.05 in comparison between intact and abnormal fundoplications.
Abbreviations: BEDQ–brief esophageal dysphagia Questionnaire. GerdQ–GERD questionnaire. EHAS–esophageal hypervigilance and anxiety scale. TBE–timed barium esophagram.
Patients evaluated primarily for dysphagia demonstrated a significantly higher rate of Nissen fundoplications (p = 0.031), ≥1 redo fundoplications (p = 0.032), and BEDQ scores (p = 0.013) compared to those evaluated primarily for heartburn/reflux; Table S1. Meanwhile, patients evaluated for heartburn/reflux versus dysphagia showed a higher rate of partial fundoplications (p = 0.031) and Toupet fundoplications (p = 0.011). There were no significant differences in symptom severity scores among fundoplication conditions, EGJ opening classification, or between full versus partial fundoplication (results not displayed).
3.2 |. Secondary peristalsis in symptomatic post-fundoplication patients
Antegrade secondary peristalsis was seen in 44 (50%) patients: 16% with normal CR and 34% with borderline CR. Abnormal FLIP panometry CRs included 28 (32%) impaired/disordered CR. 13 (15%) absent CR, and 2 (2%) spastic reactive CR.
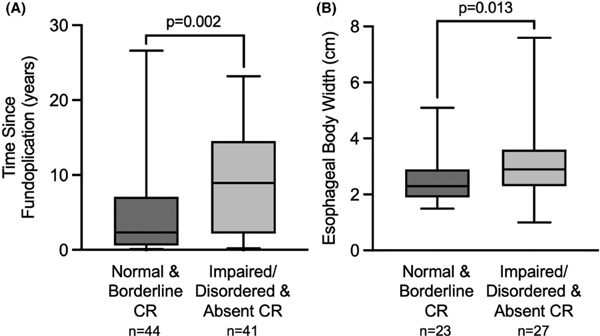
EGJ-DI and maximum EGJ diameters were lower in patients with impaired/disordered or absent CRs compared to patients with normal/borderline CRs (p = 0.001, p = 0.008, respectively); Table 2. On post-fundoplication HRM, supine IRP was greater in impaired/disordered or absent CRs than normal/borderline CRs (p = 0.045; Table 2), while upright IRP and basal EGJ pressure did not differ (results not displayed). Patients with impaired/disordered or absent CRs had a greater interval between surgery and evaluation than patients with normal/borderline CRs; Figure 2. Additionally, intact fundoplications exhibited higher rates of normal CRs in comparison to anatomically abnormal fundoplications (9 (28%) vs. 5 (9%); p = 0.032); Table 3.
TABLE 2.
Comparisons by secondary peristaltic contractile responses among symptomatic post-fundoplication patients.
| Normal and Borderline CRs | Impaired/disordered and absent CRs | |
|---|---|---|
| n (%) | 44 (51) | 41 (47) |
| FLIP metrics | ||
| Maximum EGJ diameter, mm | 17.4 (15.6–19.6) | 15.4 (12.0–18.1)* |
| EGJ distensibility index (EGJ-DI), mm2/mmHg | 4.3 (2.7–5.4) | 2.6 (1.7–3.7)* |
| Pressure, 60mL fill volume, mmHg | 44.1 (35.0–53.3) | 35.3 (26.1–43.8)* |
| FLIP EGJ opening classification, n (%) | ||
| Normal EGJ opening | 29 (66) | 18 (44) |
| Borderline EGJ opening | 13 (30) | 16 (39) |
| Reduced EGJ opening | 2 (5) | 7 (17) |
| Esophagram completed, n (%) | 23 (52) | 27 (66) |
| Used barium tablet, n (%) | 16 (70) | 16 (59) |
| Tablet impaction, n (%) | 2 (13) | 5 (31) |
| TBE completed, n (%) | 13 (57) | 15 (56) |
| TBE: 1-min column height, cm | 0.0 (0.0–0.0) | 0.0 (0.0–14.1)* |
| TBE: 5-min column height, cm | 0.0 (0.0–0.0) | 0.0 (0.0–7.45)* |
| HRM completed, n (%) | 33 (75) | 29 (71) |
| HRM metrics | ||
| Supine IRP, mmHg | 12.3 (8.5–17.4) | 17.0 (14.1–21.0)* |
| Upright IRP, mmHg | 13.0 (8.5–20.7) | 15.0 (12.3–19.0) |
| Basal EGJ Pressure, mmHg | 16.0 (10.0–23.0) | 13.0 (9.0–22.0) |
| HRM peristalsis/contractility–CCv4.0, n (%) | ||
| Normal peristalsis | 24 (73) | 13 (45)* |
| Ineffective esophageal motility | 5 (15) | 9 (31) |
| Absent contractility | 1 (3) | 6 (21)* |
| Hypercontractile | 2 (6) | 0 (0) |
| Premature | 1 (3) | 1 (3) |
| symptom scores | ||
| GerdQ score, [n (%) completed GerdQ] | 9 (7–12) [25 (57)] | 9 (7–10) [22 (54)] |
| BEDQ score, [n (%) completed BEDQ] | 17 (13–24) [27 (61)] | 11 (7–17) [23 (56)] |
| EHAS score, [n (%) completed EHAS] | 37 (30–41) [21 (48)] | 31 (22–39) [23 (56)] |
Note:
p <0.05 in comparison with normal/borderline CRs.
Abbreviations: CCv4.0, Chicago classification version 4.0; EGJ, esophagogastric junction; EHAS, esophageal hypervigilance and anxiety scale; FLIP, functional lumen imaging probe; GerdQ, GERD questionnaire; HRM, high-resolution manometry; IRP, integrated relaxation pressure; TBE, timed barium esophagram.
FIGURE 2.
Longer time since fundoplication and wider esophageal body width associated with abnormal secondary peristalsis. (A) Time since fundoplication (years) and (B) Esophageal body width (CM) in relation to normal & borderline contractile response (CR) versus impaired/disordered & absent CR.
TABLE 3.
Stratified subgroup analysis of motility parameters relative to fundoplication details.
| Intact fundoplication | Abnormal fundoplication anatomy | Nissen fundoplication | Partial fundoplication | No redo fundoplication | ≥1 redo fundoplication | |
|---|---|---|---|---|---|---|
| FLIP, n (96) | 32 (37) | 55 (63) | 75 (86) | 12 (14) | 71 (82) | 16 (18) |
| FLIP contractile response (CR), n (%) | ||||||
| Normal CR | 9 (28) | 5 (9)* | 13 (17) | 1 (8) | 13 (18) | 1 (6) |
| Borderline CR | 10 (31) | 20 (36) | 25 (33) | 5 (42) | 23 (32) | 7 (44) |
| Impaired/disordered CR | 9 (28) | 19 (35) | 26 (35) | 2 (17) | 21 (30) | 7 (44) |
| Absent CR | 3 (9) | 10 (18) | 9 (12) | 4 (33) | 12 (17) | 1 (6) |
| Spastic reactive CR | 1 (3) | 1 (2) | 2 (3) | 0 (0) | 2 (3) | 0 (0) |
| EGJ-DI, mm2/mmHg, median (IQR) | 3.0 (2.3–5.0) | 3.5 (1.9–5.3) | 3.2 (2.2–5.2) | 2.5 (1.1–5.3) | 3.1 (1.9–5.2) | 3.1 (2.6–5.2) |
| Maximum EGJ diameter, mm, median (IQR) | 17.2 (14.9–18.3) | 16.5 (13.0–18.6) | 16.5 (14.2–18.5) | 16.0 (10.8–18.8) | 16.5 (13.8–18.6) | 16.6 (13.7–18.7) |
| FLIP pressure, 60mL, mmHg, median (IQR) | 45.3 (36.2–60.1) | 36.8 (29.3–46.4)* | 39.2 (30.9–51.2) | 34.9 (25.0–50.7) | 39.4 (30.3–51.2) | 34.9 (29.6–50.5) |
| HRM (n = 63), n (%) | 25 (78) | 38 (69) | 55 (73) | 8 (67) | 51 (72) | 12 (75) |
| Primary peristalsis, n (%) | ||||||
| Normal primary peristalsis | 18 (72) | 19 (50) | 33 (60) | 4 (50) | 29 (57) | 8 (66) |
| Ineffective esophageal motility | 2 (8) | 12 (32)* | 10 (18) | 4 (50) | 10 (20) | 4 (33) |
| Absent contractility | 1 (4) | 6 (16) | 7 (13) | 0 (0) | 7 (14) | 0 (0) |
| Hypercontractile/premature | 4 (16) | 1 (3) | 5 (9) | 0 (0) | 5 (10) | 0 (0) |
| Supine IRP, mmHg median (IQR) | 15.1 (10.3–20.0) | 15.3 (10.2–20.7) | 15.2 (10.9–20.7) | 13.5 (8.0–19.9) | 15.2 (10.9–21.4) | 12.8 (7.9–16.9) |
Note:
p <0.05 in comparison to intact fundoplications.
Abbreviations: EGJ-DI, esophagogastric junction distensibility index; FLIP, functional lumen imaging probe; HRM, high-resolution manometry; IQR, interquartile range; IRP, integrated relaxation pressure.
On esophagram, esophageal body width was greater in patients with impaired/disordered or absent CRs than in normal/borderline CRs; Figure 2. Among patients that completed TBE that also had normal EGJ opening on FLIP (n = 18), normal and borderline CRs (n = 9) demonstrated 1-min and 5-min column heights of median (IQR) 0.0 (0.0–0.0) cm and 0.0 (0.0–0.0) cm, respectively, both significantly lower than in patients with impaired/disordered and absent CRs (n = 9), who had 1-min and 5-min column heights of 3.7 (0.0–14.5) cm (p = 0.050) and 4.7 (0.0–9.1) cm (p = 0.029), respectively, (results not displayed). Among patients that completed TBE with a borderline or reduced EGJ opening (n = 10), normal and borderline CRs demonstrated 1-min and 5-min column heights of 0.0 (0.0–0.0) cm and 0.0 (0.0–0.0) cm, respectively, with no significant difference in comparison to patients with impaired/disordered and absent CRs (n = 9), 0.0 (0.0–8.9) cm and 0.0 (0.0–0.0) cm, respectively, (results not displayed).
Of the patients with spastic reactive CR (n = 2), one had a Nissen 1.2 years prior, which was intact at time of FLIP with sustained occluding contractions (SOCs) on FLIP and hypercontractile esophagus on post-fundoplication HRM; pre-fundoplication HRM showed normal primary peristalsis. The other patient with spastic reactive CR had a Nissen 11.3 years prior, which was herniated at time of FLIP with sustained lower esophageal sphincter contractions (sLESC); no HRM studies were available. Repetitive retrograde contractions (RRCs), using current criteria, were not observed in the present cohort.
3.3 |. Associations with primary peristalsis in symptomatic post-fundoplication patients
63 (72%) patients completed HRM after fundoplication. 10/11 (91%) patients with normal CR and 14/22 (64%) of patients with borderline CR on FLIP had normal peristalsis on HRM; Table 4.
TABLE 4.
Relationship of secondary peristalsis (FLIP contractile response) and primary peristalsis (HRM) on follow-up testing after fundoplication.
| HRM CCv4.0 peristalsis patterns | |||||||
|---|---|---|---|---|---|---|---|
| Normal | Ineffective esophageal motility | Absent contractility | Hypercontractile | Premature | Total | ||
| FLIP contractile Response | Normal | 10 (16) | 0 (0)* | 0 (0) | 0 (0) | 1 (2) | 11 (17) |
| Borderline | 14 (22) | 5 (8) | 1 (2) | 2 (3) | 0 (0) | 22 (35) | |
| Impaired/disordered | 12 (19) | 6 (10) | 1 (2) | 0 (0) | 1 (2) | 20 (32) | |
| Absent | 1 (2) | 3 (5) | 5 (8)* | 0 (0) | 0 (0) | 9 (14) | |
| Spastic reactive | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 1 (2) | |
| Total | 37 (59) | 14 (22) | 7 (11) | 3 (5) | 2 (3) | 63 (100) | |
Note: Values in each cell are n (% of the total cohort that completed both high-resolution manometry (HRM) and functional lumen imaging probe (FLIP)).
p <0.05 in comparison to normal primary peristalsis.
Abbreviation: CCv4.0 – Chicago Classification version 4.0.
Ineffective esophageal motility was significantly less common among patients with intact versus abnormal fundoplication anatomy (p = 0.028); Table 3. There were no differences in time since fundoplication between primary peristalsis patterns on HRM; Figure S2. On esophagram, esophageal body width was greater among patients with absent contractility (2.4 (2.2–2.9) cm) versus those with normal primary peristalsis (3.1 (2.7–3.5) cm; p=0.043); Figure S2. Symptom severity scores did not differ among HRM peristalsis patterns (results not displayed). Maximum EGJ diameter was lower in patients with absent primary peristalsis compared to normal primary peristalsis (p=0.031); Figure S3.
There were 21 patients with pre-fundoplication HRM data available, 16 of whom also had post-fundoplication HRM data for comparison. Among the patients with baseline normal HRM, post-fundoplication FLIP CRs were normal in 3 (19%), borderline in 5 (31%), impaired/disordered in 5 (31%), absent in 2 (13%), and spastic reactive in 1 (6%). 15/16 of these patients with baseline normal primary peristalsis also had post-fundoplication HRM, which were normal in 11 (73%), ineffective esophageal motility in 2 (18%), hypercontractile in 1 (7%), and premature contractility in 1 (7%) (results not displayed).
3.4 |. Post-fundoplication egj opening characteristics
There were no significant differences in EGJ-DI or maximum EGJ diameter between the intact and anatomically abnormal fundoplication groups; Table 2. However, median FLIP pressure (at 60-mL fill volume) was significantly greater in intact compared to anatomically abnormal fundoplications, p = 0.009; Table 2. Additionally, tight Nissen fundoplications identified on endoscopy/esophagram were found to have a significantly lower EGJ-DI in comparison to herniated Nissen fundoplications; Figure 3. There were no significant differences in the proportions of normal EGJ opening, borderline EGJ opening, or reduced EGJ opening between intact and anatomically abnormal fundoplications (results not displayed).
FIGURE 3.
Association of FLIP EGJ opening parameters with fundoplication status. (A) EGJ Distensibility Index (Dl) (mm2/mmHg) from the 60mL FLIP fill volume and (B) Maximum EGJ Diameter (mm) in Nissen and partial fundoplications (Toupet, Dor, and Belsey) across intact versus abnormal (tight, herniated, disrupted, slipped, >1 mechanism) fundoplication wraps.
There were 50 (57%) patients who completed an esophagram, of which 66% used barium tablet and 56% were TBEs; Table 1. Among patients who completed esophagram with tablet, only 1/20 (5%) with normal EGJ opening, specifically a long fundoplication, had tablet impaction in comparison to 5/11 (45%) (p = 0.013) with borderline EGJ opening and 1/2 (50%) with reduced EGJ opening. EGJ-DI (4.1 (2.7–5.3) versus 2.4 (1.3–3.1) mm2/mmHg; p = 0.003) and maximum EGJ diameter (18.2 (16.1–19.4) versus 13.0 (12.9–15.2) mm; p = 0.002) were also greater in patients with tablet passage compared to those with tablet impaction (results not displayed). 2 (29%) tablet impactions were associated with intact fundoplication anatomy, and 5 (71%) were with abnormal fundoplication anatomy; Table 1.
4 |. DISCUSSION
The main findings of this study were that abnormal secondary peristalsis on FLIP Panometry was commonly observed in this symptomatic post-fundoplication cohort and was associated with abnormal primary peristalsis on HRM, increased time after fundoplication, and increased esophageal body diameter (dilatation). Abnormal CRs were also associated with markers of EGJ obstruction including lower EGJ distensibility, lower maximum EGJ diameter, and higher IRP. Therefore, this study shows that abnormal secondary peristalsis is common after fundoplication and suggests that impairment may potentially worsen over time or be related to increased EGJ outflow resistance; Figure 4.
FIGURE 4.
Association of esophageal dilatation with peristaltic function. (A) This patient demonstrates normal motility (both on FLIP (top) and HRM (bottom)), normal caliber esophagus (width: 2.3cm) and clearance, and intact fundoplication (FP) anatomy on endoscopy/esophagram. (B) This patient demonstrates absent motility (both on FLIP and HRM), with esophageal body dilatation (width: 7.6cm) and impaired esophageal clearance, and tight FP anatomy on endoscopy/esophagram. CR–contractile response. EGJ-DI–esophagogastric junction distensibility index.
With respect to fundoplication surgery, FLIP has largely been used as a means of calibrating EGJ opening dynamics intraoperatively.28–31 However, this is the first study to explore the full spectrum of CR patterns on FLIP panometry in a symptomatic post-fundoplication adult cohort. In a pediatric study of 42 patients (including 16 fundoplications), presence of repetitive antegrade contractions on FLIP was significantly associated with greater bolus flow on HRM with impedance.32 Secondary peristalsis has also previously been investigated after fundoplication using manometry with focal esophageal distension. One such study noted impaired triggering of secondary peristalsis in 13 GERD patients before and 3 months after fundoplication with no changes to primary or secondary peristaltic amplitude or propagation rate.33 Another such study (n = 44) also reported impaired secondary peristalsis in both chronic GERD patients and patients >3years after fundoplication with the post-fundoplication cohort importantly having higher thresholds for triggering secondary peristalsis.13 Similarly, our study demonstrated frequent abnormalities with triggering secondary peristalsis on FLIP panometry in symptomatic patients post-fundoplication, potentially also in relationship to chronicity after fundoplication; Figure 4.
In congruence with what has been previously described among patients evaluated for primary esophageal motility disorders,16,17 secondary peristaltic CRs on FLIP in this post-fundoplication cohort generally paralleled primary peristaltic patterns on HRM. This study was able to highlight the reliability of normal CR on FLIP panometry in identifying normal peristalsis on HRM and ruling out significant, relevant motility abnormalities. However, primary peristaltic function on HRM did not appear to carry the same association with duration since fundoplication, suggesting that secondary peristalsis may be more vulnerable to chronic impairment. While a limitation of the study was incomplete availability of pre-fundoplication HRM (or FLIP), even patients with normal primary peristalsis prior to surgery commonly (50% of patients) had abnormal FLIP CRs after fundoplication. For comparison, a study of 164 patients without prior foregut surgery and normal motility on HRM demonstrated abnormal FLIP CRs in 23%.22
This study also demonstrated that abnormal peristalsis was significantly associated with esophageal dilatation and markers of EGJ obstruction in post-fundoplication patients. This suggested a relationship between esophageal anatomic change and physiologic function potentially related to remodeling in response to increased EGJ outflow resistance; Figure 4. Additionally, even in patients with a normal EGJ opening, there was significantly worsened esophageal clearance seen on TBE in patients with impaired/disordered or absent CRs, emphasizing the role of secondary peristalsis in effective esophageal clearance post-fundoplication and supporting a NeuroMyogenic model that incorporates both primary and secondary peristalsis to characterize esophageal motility.34 Meanwhile, normal CR on FLIP panometry was significantly associated with intact fundoplication anatomy.
EGJ parameters on FLIP remained heterogenous in relation to anatomical fundoplication status (intact vs. abnormal). However, consistent with previous studies,35,36 we observed reduced EGJ distensibility with abnormal CRs on FLIP panometry. Of note, reduced EGJ opening (REO), previously shown to identify a large proportion of achalasia and conclusive EGJ outflow obstruction,17,18,37 was only found in 10% of this symptomatic post-fundoplication cohort (and without apparent relationship to the anatomic assessment of the fundoplication). This suggests that even mild reduction in EGJ distensibility may lead to esophageal remodeling given sufficient time. While these results appear to differ from previous descriptions of EGJ distensibility in post-fundoplication dysphagia, our study differs by including a wide range of fundoplication conditions, including disrupted and herniated fundoplications. However, similar to previous studies, “tight” fundoplications identified on endoscopy were found to have significantly lower EGJ-DI in comparison to herniated fundoplications, supporting the growing role of EGJ-DI-directed care in the setting of fundoplication surgery and post-operative dilation.31,38 Furthermore, patients evaluated primarily for dysphagia demonstrated decreased maximum EGJ diameter in comparison to those with heartburn/reflux, emphasizing the clinical relevance of the EGJ maximum diameter in symptoms post-fundoplication; Table S1.
Although this study reports on a comprehensive evaluation of symptomatic post-fundoplication patients, it has limitations. There was poor correspondence between the endoscopic assessment of the fundoplication and the FLIP measures of EGJ distensibility (with the exception of “tight” fundoplications). This may be attributable to the inherent subjectivity in the endoscopic evaluation39–41 or that the relevant features of the surgery are other than what one can see with an endoscope such as the tightness of the hiatal repair or axial immobilization of the distal esophagus. While we complemented the endoscopic assessment of fundoplication with esophagram when available, esophagram also carries inherent limitations, thus future investigation involving other methods such as intraoperative assessment is anticipated. Further, studying an asymptomatic post-fundoplication cohort might shed light on this but to date, FLIP has not yet been incorporated into our workflow for these patients (and thus asymptomatic patients are not included in this study). Another limitation was that data prior to fundoplication was incomplete as were post-fundoplication HRM, esophagram, and TBE datasets, thus, we are unable to determine whether these differences are not related to the baseline GERD physiology likely present in all of these patients. Pre- and post-studies are being performed. Nonetheless, this study still offers valuable insights and, hopefully, future investigation will further our understanding of chronic esophageal remodeling, its clinical ramifications in the context of fundoplication, and re-operation outcomes.
In conclusion, impaired secondary peristalsis was frequently observed after fundoplication, which may potentially worsen with time and occur related to EGJ outflow resistance. Additionally, heterogeneous EGJ opening mechanics on FLIP panometry were seen regardless endoscopic/radiographic assessment of the fundoplication (with the exception of “tight” fundoplications). This study also demonstrated the utility of FLIP panometry as a complementary diagnostic tool during clinical evaluation post-fundoplication and highlighted its reliability in identifying intact peristalsis. While additional studies will further establish the clinical significance of impaired secondary peristalsis in this setting, impaired secondary peristalsis and/or reduced EGJ distensibility may represent early stages of post-fundoplication esophageal dysfunction with potential for timely intervention.
Supplementary Material
Key points.
Functional lumen imaging probe (FLIP) Panometry evaluates esophageal secondary peristalsis and esophageal wall biomechanics, both of which may impact clinical outcomes after anti-reflux surgery with fundoplication.
In this observational study of 87 symptomatic patients with previous fundoplication, abnormal secondary peristalsis on FLIP was associated with abnormal primary peristalsis on manometry, increased time since fundoplication, and reduced esophagastric junction distensibility.
FLIP Panometry appeared to serve as a useful complementary diagnostic tool during clinical evaluation of patients post-fundoplication.
ACKNOWLEDGMENTS
We thank Wenjun (Walter) Kou for his contributions implementing the FLIP panometry analysis software.
FUNDING INFORMATION
This work was supported by P01 DK117824 from the Public Health Service (JEP).
Footnotes
CONFLICT OF INTEREST STATEMENT
JEP, PJK, and Northwestern University hold shared intellectual property rights and ownership surrounding FLIP panometry systems, methods, and apparatus with Medtronic Inc. DAC: Medtronic (Speaking, Consulting, License); Phathom Pharmaceuticals (Consulting); Braintree (Consulting); Medpace (Consulting). PJK: Reckitt (Consulting). JEP: Sandhill Scientific (Consulting, Speaking, Grant), Takeda (Speaking), Astra Zeneca (Speaking), Medtronic (Speaking, Consulting, Patent, License), Torax (Speaking, Consulting), Ironwood (Consulting). ENT: Boston Scientific (Education, Consulting). MML: Nothing to disclose.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author on reasonable request and completion of necessary privacy and ethical approvals.
REFERENCES
- 1.Richter JE, Rubenstein JH. Presentation and epidemiology of gas-troesophageal reflux disease. Gastroenterology. 2018;154(2):267–276. doi: 10.1053/j.gastro.2017.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dallemagne B, Weerts JM, Jehaes C, Markiewicz S, Lombard R. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc. 1991;1(3):138–143. [PubMed] [Google Scholar]
- 3.Ackroyd R, Watson Dl, Majeed AW, Troy G, Treacy PJ, Stoddard CJ. Randomized clinical trial of laparoscopic versus open fundoplication for gastro-oesophageal reflux disease. Br J Surg. 2004;91(8):975–982. doi: 10.1002/bjs.4574 [DOI] [PubMed] [Google Scholar]
- 4.de Beaux AC,Watson Dl,O’Boyle C,Jamieson GG. Roleoffundoplication in patient symptomatology after laparoscopic antireflux surgery. Brit J Surg. 2001;88(8):1117–1121. doi: 10.1046/j.0007-1323.2001.01839.x [DOI] [PubMed] [Google Scholar]
- 5.Hasak S, Brunt LM, Wang D, Gyawali CP. Clinical characteristics and outcomes of patients with postfundoplication dysphagia. Clin Gastroenterol Hepatol. 2019;17(10):1982–1990. doi: 10.1016/j.cgh.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 6.Pessaux P, Arnaud JP, Delattre JF, et al. Laparoscopic antireflux surgery–five-year results and beyond in 1340 patients. Arch Surg-Chicago. 2005;140(10):946–951. doi: 10.1001/archsurg,140.10.946 [DOI] [PubMed] [Google Scholar]
- 7.Triponez F, Dumonceau JM, Azagury D, et al. Reflux, dysphagia, and gas bloat after laparoscopic fundoplication in patients with incidentally discovered hiatal hernia and in a control group. Surgery. 2005;137(2):235–242. doi: 10.1016/j.surg.2004.07.016 [DOI] [PubMed] [Google Scholar]
- 8.Walle KV, Funk LM, Xu YW, et al. Persistent dysphagia rate after Antireflux surgery is similar for Nissen fundoplication and partial fundoplication. J Surg Res. 2019;235:52–57. doi: 10.1016/j.jss.2018.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadlapati R, Hungness ES, Pandolfino JE. Complications of antireflux surgery. Am J Gastroenterol. 2018;113(8):1137–1147. doi: 10.1038/s41395-018-0115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azagury D, Morton J. Surgical anti-reflux options beyond fundoplication. Curr Gastroenterol Rep. 2017;19(7):35. doi: 10.1007/s11894-017-0582-9 [DOI] [PubMed] [Google Scholar]
- 11.Sobrino-Cossio S, Soto-Perez JC, Coss-Adame E, et al. Post-fundoplication symptoms and complications: diagnostic approach and treatment. Rev Gastroenterol Me. 2017;82(3):234–247. doi: 10.1016/j.rgmx.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 12.Maret-Ouda J, Wahlin K, El-Serag HB, Lagergren J. Association between laparoscopic Antireflux surgery and recurrence of gastroesophageal reflux. J Am Med Assoc. 2017;318(10):939–946. doi: 10.1001/jama.2017.10981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rydberg L, Ruth M, Lundell L. Characteristics of secondary oesophageal peristalsis in operated and non-operated patients with chronic gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2000;12(7):739–743. doi: 10.1097/00042737-200012070-00004 [DOI] [PubMed] [Google Scholar]
- 14.Schoeman MN, Holloway RH. Secondary oesophageal peristalsis in patients with non-obstructive dysphagia. Gut. 1994;35(11):1523–1528. doi: 10.1136/gut.35.11.1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol. 2016;111(12):1726–1735. doi: 10.1038/ajg.2016.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson DA. Baumann AJ, Prescott JE, et al. Validation of secondary peristalsis classification using FLIP panometry in 741 subjects undergoing manometry. Neurogastroenterol Motil. 2022;34(l):el4192. doi: 10.1111/nmo.14192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson DA, Gyawali CP, Khan A, et al. Classifying esophageal motility by FLIP panometry: a study of 722 subjects with manometry. Am J Gastroenterol. 2021;116(12):2357–2366. doi: 10.14309/ajg.0000000000001532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson DA, Prescott JE, Baumann AJ, et al. Validation of clinically relevant thresholds of esophagogastric junction obstruction using FLIP panometry. Clin Gastroenterol Hepatol. 2022;20(6):el250–el262. doi: 10.1016/j.cgh.2021.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson DA, Kou W, Lin Z, et al. Normal values of esophageal distensibility and distension-induced contractility measured by functional luminal imaging probe panometry. Clin Gastroenterol Hepatol. 2019;17(4):674–681 el. doi: 10.1016/j.cgh.2018.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther. 2010;31(5):601–606. doi: 10.1111/j.1365-2036.2009.04212.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittal RK, Frank EB, Lange RC, McCallum RW. Effects of morphine and naloxone on esophageal motility and gastric emptying in man. Dis Dis Sci. 1986;31(9):936–942. doi: 10.1007/BF01303214 [DOI] [PubMed] [Google Scholar]
- 22.Carlson DA, Baumann AJ, Donnan EN, Krause A, Kou W, Pandolfino JE. Evaluating esophageal motility beyond primary peristalsis: assessing esophagogastric junction opening mechanics and secondary peristalsis in patients with normal manometry. Neurogastroenterol Motil. 2021;33(10):el4116. doi: 10.1111/nmo.l4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0((c)). Neurogastroenterol Motil. 2021;33(l):el4058. doi: 10.1111/nmo.14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonasson C, Wernersson B, Hoff DA, Hatlebakk JG. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37(5):564–572. doi: 10.1111/apt.12204 [DOI] [PubMed] [Google Scholar]
- 25.Taft TH, Riehl M, Sodikoff JB, et al. Development and validation of the brief esophageal dysphagia questionnaire. Neurogastroenterol Motil. 2016;28(12):1854–1860. doi: 10.1111/nmo,12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taft TH, Triggs JR, Carlson DA, et al. Validation of the oesophageal hypervigilance and anxiety scale for chronic oesophageal disease. Aliment Pharmacol Ther. 2018;47(9):1270–1277. doi: 10.1111/apt.14605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blonski W, Kumar A, Feldman J, Richter JE. Timed barium swallow: diagnostic role and predictive value in untreated achalasia, esophagogastric junction outflow obstruction, and non-achalasia dysphagia. Am J Gastroenterol. 2018;113(2):196–203. doi: 10.1038/ajg.2017.370 [DOI] [PubMed] [Google Scholar]
- 28.Ahuja NK, Agnihotri A, Lynch KL, et al. Esophageal distensibility measurement: impact on clinical management and procedure length. D/s Esophagus. 2017;30(8):1–8. doi: 10.1093/dote/dox038 [DOI] [PubMed] [Google Scholar]
- 29.Su B, Callahan ZM, Kuchta K, et al. Use of impedance planimetry (Endoflip) in foregut surgery practice: experience of more than 400 cases. J Am Coll Surg. 2020;231(1):160–171. doi: 10.1016/j.jamcollsurg.2020.02.017 [DOI] [PubMed] [Google Scholar]
- 30.Su B, Wong HJ, Attaar M, et al. Comparing short-term patient outcomes after fundoplication performed over a traditional bougie versus a functional lumen imaging probe. Surgery. 2021:169(3):533–538. doi: 10.1016/j.surg.2020.07.027 [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Attaar M, Wong HJ, et al. Impedance planimetry (EndoFLIP) reveals changes in gastroesophageal junction compliance during fundoplication. Surg Endosc. 2022:36(9):6801–6808. doi: 10.1007/S00464-021-08966-1 [DOI] [PubMed] [Google Scholar]
- 32.Rosen R, Stayn Z, Garza JM, et al. The utility of functional luminal imaging probes measurements to diagnose dysmotility and their relationship to impaired bolus clearance. J Pediatr Gastroenterol Nutr. 2022:74(4):523–528. doi: 10.1097/MPG.0000000000003394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tew S, Jamieson GG, Holloway RH, Ferguson S, Tew P. A prospective study of the effect of fundoplication on primary and secondary peristalsis in the esophagus. Dis Esophagus. 1997;10(4):247–252. doi: 10.1093/dote/10.4.247 [DOI] [PubMed] [Google Scholar]
- 34.Koop AH, Kahrilas PJ, Schauer J, Pandolfino JE. Carlson DA. The impact of primary peristalsis, contractile reserve, and secondary peristalsis on esophageal clearance measured by timed barium esophagogram. Neurogastroenterol Motil. 2023;35:el4638. doi: 10.1111/nmo.14638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwiatek MA, Kahrilas K, Soper NJ, et al. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg. 2010;14(2):268–276. doi: 10.1007/sll605-009-1086-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen R, Garza JM, Nurko S. Functional luminal imaging probe assessment in postfundoplication patients changes management beyond manometry. J Pediatr Gastroenterol Nutr. 2020;70(6):e119–e123. doi: 10.1097/MPG.0000000000002658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rooney KP, Baumann AJ, Donnan E, et al. Esophagogastric junction opening parameters are consistently abnormal in untreated achalasia. Clin Gastroenterol Hepatol. 2021;19(5):1058–1060. doi: 10.1016/j.cgh.2020.03.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samo S, Mulki R, Godiers ML, et al. Utilizing functional lumen imaging probe in directing treatment for post-fundoplication dysphagia. Surg Endosc. 2021;35(8):4418–4426. doi: 10.1007/s00464-020-07941-6 [DOI] [PubMed] [Google Scholar]
- 39.Abdelmoaty WF, Swanstrom LL . Endoscopic evaluation of post-fundoplication anatomy. Curr Gastroenterol Rep. 2017;19(10):51. doi: 10.1007/s11894-017-0592-7 [DOI] [PubMed] [Google Scholar]
- 40.Juhasz A, Sundaram A, Hoshino M, Lee TH, Filipi CJ, Mittal SK. Endoscopic assessment of failed fundoplication: a case for standardization. Surg Endosc. 2011;25(12):3761–3766. doi: 10.1007/s00464-011-1785-z [DOI] [PubMed] [Google Scholar]
- 41.Song EJ, Yadlapati R, Chen JW, et al. Variability in endoscopic assessment of Nissen fundoplication wrap integrity and hiatus herniation. Dis Esophagus. 2022;35:doab078. doi: 10.1093/dote/doab078 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request and completion of necessary privacy and ethical approvals.