Abstract
The development of monoclonal antibodies (mAbs) presents an emerging, highly specific therapeutic strategy for the treatment of multiple sclerosis (MS). mAbs target selective molecules and have shown early promise, along with notable risks, in the treatment of MS and other immune-mediated diseases. The mechanism of action of the 4 mAbs under active investigation for MS (natalizumab, rituximab, alemtuzumab, and daclizumab) are reviewed, with a discussion of how mAb interaction with each target antigen may produce direct and indirect effects (proven and hypothesized) on immune cell activity, CNS-related inflammatory processes, and clinical outcomes.
INTRODUCTION
Monoclonal antibodies (mAbs) offer clinicians a highly selective group of therapeutic agents that target specific molecules expressed on the cell surface. mAbs in development for multiple sclerosis (MS) currently target only molecules of the immune system. These emerging therapies have potent immunomodulatory effects, both direct and indirect. This review summarizes the known or hypothesized mechanisms of action of four mAbs in use or under study (with demonstrated efficacy in Phase II trials) for the treatment of MS.
OVERVIEW
Monoclonal antibodies, which have been under investigation for human therapeutic use since the mid-1980s, are inherently limited by their immunogenicity if they are of nonhuman origin. Researchers have sought to overcome these limits with the development of humanized, chimeric, and fully human mAbs. Humanized mAbs consist of more than 90% human components, with the balance from the original (usually murine) structure; these include natalizumab, alemtuzumab, and daclizumab. (The nomenclature of mAbs is coded to indicate their origin, as seen in the italicized syllables.) Chimeric mAbs are ~66% human structure and ~34% murine (from the variable region of the original mouse Ab) and include rituximab and basiliximab. Fully human mAbs, such as ofatumumab and adalimumab, have a 100% human structure and may be derived from either human cells or genetically engineered mice.1, 2
Monoclonal antibodies consist of fragment antigen-binding (Fab) and fragment-crystallizable (Fc) domains arranged in a rough Y shape. The Fc, or complement-binding, domains, located on the stem, modulate immune cell activity, determining whether the mAb binds to different Fc receptors expressed on immune cells or whether it activates complement. The Fab, or antigen-binding, regions, found at the terminal ends of the arms, determine how the antibody will bind to the epitope of its target molecule and with what degree of affinity.
DETERMINANTS OF mAb ACTION
Four main factors determine how a mAb acts upon its target: the function and distribution of the targeted molecule; the antibody’s mode of interaction with its target; its effector functions; and its efficacy in reaching the target tissues (in this setting, the central nervous system [CNS]).
Characteristics of target molecule.
Each mAb binds only to its target molecule, which is expressed on particular cells for specific functions, such as cell activation, survival, or migration. The monoclonal antibodies currently most relevant to MS therapy and their targeted molecules are shown in Table 1. Alemtuzumab (Campath®, Genzyme, Cambridge, MA) binds to CD52, an antigen widely expressed in the immune system, including on the surface of T and B lymphocytes, natural killer (NK) cells, and a majority of monocytes and macrophages. Daclizumab (Facet Biotech, Redwood City, CA, and Biogen Idec, Cambridge, MA) targets CD25, expressed on activated T cells and some regulatory T cells, among others. The target of natalizumab (Tysabri®, Biogen Idec, Cambridge, MA, and Elan Pharmaceuticals, Dublin, Ireland) is CD49d (a subunit of adhesion molecule VLA-4), another molecule with wide expression in the immune system. CD20, expressed only on B cells, is the target of rituximab (Rituxan®, Genentech, South San Francisco, CA, and Biogen Idec, Cambridge, MA).
Table 1.
| Monoclonal Ab | Targeted molecule | Cell expression |
|---|---|---|
| Alemtuzumab (Campath-1H) |
CD 52 | All T and B cells, NK cells, majority of MФ, DCs, most granulocytes (except neutrophils), tissue of the male reproductive system |
| Daclizumab (Zenapax) |
CD 25 (IL-2Rα) | Activated T cells, some regulatory T cells (FoxP3+), CD56bright NK cells, MФ, some DCs, oligodendrocytes |
| Natalizumab (Tysabri) |
CD49d (VLA-4) | All T and B cells, NK cells, majority of monocytes and MФ, most granulocytes (except neutrophils) |
| Rituximab (Rituxan) |
CD20 | B cells (but not plasma cells) |
NK, natural killer; MФ, macrophages; DCs, dendritic cells, VLA-4, very late activating antigen-4
How the mAb interacts with the target molecule.
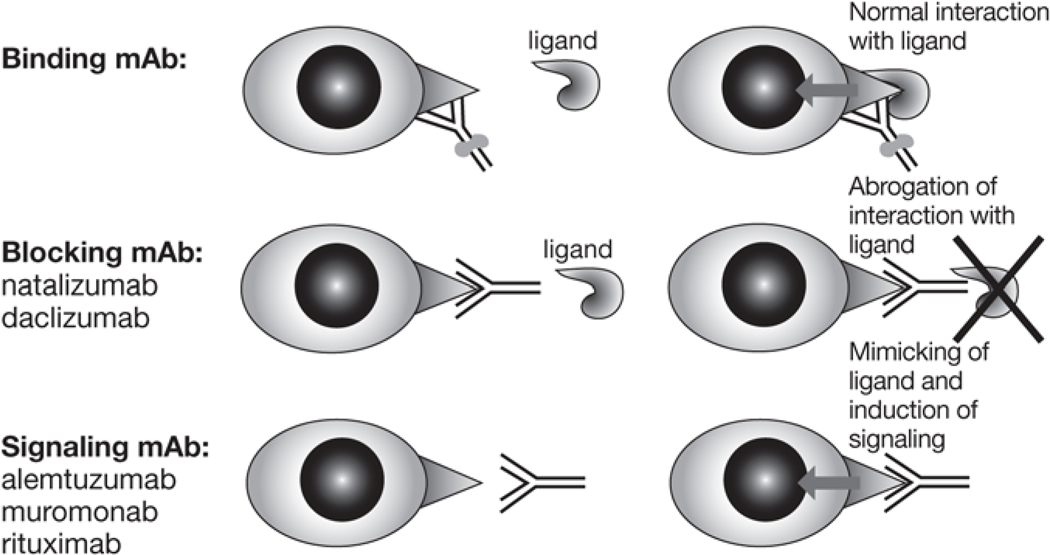
A particular mAb may interact with its target by binding, blocking, or signaling, depending on its Fab activity (figure 1). Binding mAbs bind to the specific molecule but do not interfere with its function. Therefore, the physiologic ligand is still able to bind to the molecule targeted by a mAb and to signal. A binding mAb can mark a target cell for destruction through its effector functions (see below) or through conjugation of mAb to toxin (currently developed only for cancer applications). Blocking mAbs block the epitope needed for ligand interaction, preventing the target molecule from signaling but (usually) sparing the cell itself. Both natalizumab and daclizumab are blocking mAbs. Signaling mAbs mimic the ligand and induce signaling between molecules. Depending on the level of cellular activation the signaling mAb induces, treatment with it may be associated with in vivo activation of targeted cells and their release of cytokines. This cytokine release syndrome, first observed in association with muromonab (Orthoclone OKT®3, Ortho-Biotech Products, Bridgewater, NJ), an anti-rejection agent, is also to a lesser degree associated with the use of alemtuzumab and rituximab,3 2 signaling mAbs in development for the treatment of MS.4,5
Figure 1.
The properties of the mAb in regard to the target molecule (Fab-dependent)18,31,49,65,79
mAb effector functions.
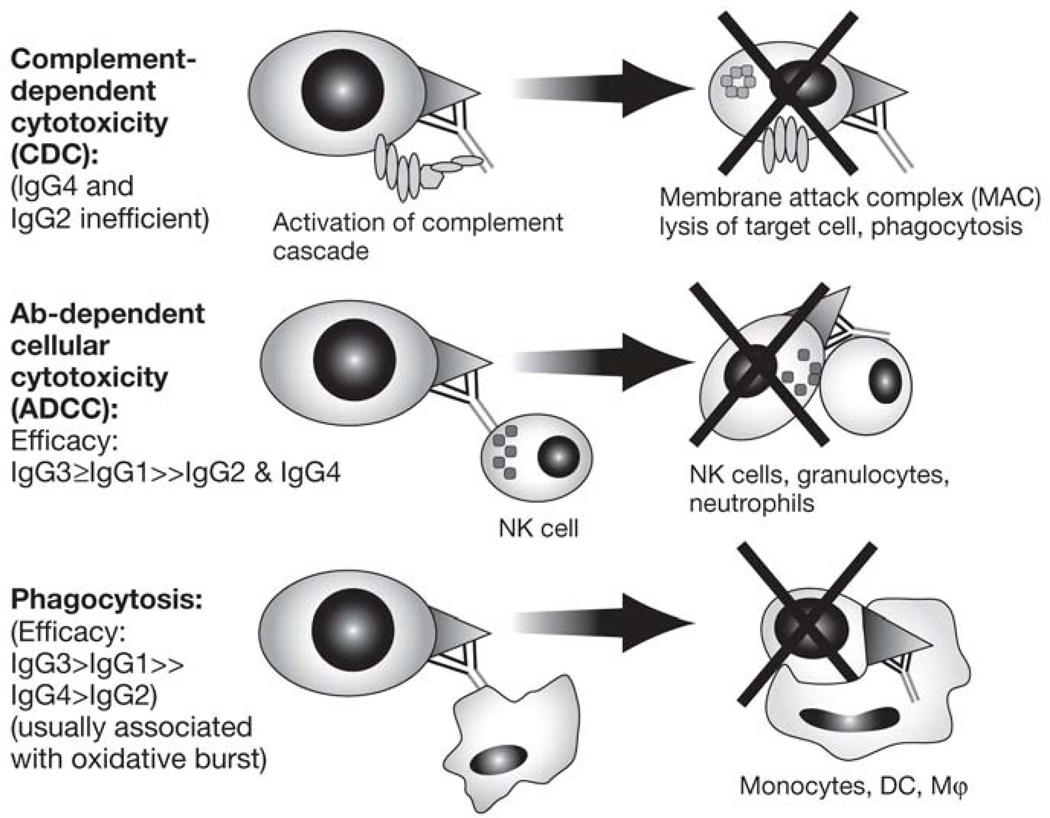
Monoclonal antibodies may mediate cytotoxic immune responses and destroy targeted cells. These effector functions depend on the Fc domain of the Ig molecule, and their efficacy varies by IgG subgroup (figure 2).6–8
Figure 2.
The effector function of mAb (Fc-dependent)7,8
NK, natural killer; DCs, dendritic cells; MФ, macrophages
Complement-dependent cytotoxicity (CDC).
In this scenario, the Fc receptor activates the complement system, forming a membrane attack complex (MAC) that may destroy the target cells. In theory, IgG1 and IgG3 should both activate CDC; human IgG2, on the other hand, binds complement inefficiently, and IgG4 does not activate complement at all, making these IgG subtypes unlikely to provoke CDC.6
Ab-dependent cellular cytotoxicity (ADCC).
In this process, the mAb binds to Fcγ receptors on the surface of potentially cytotoxic immune cells such as NK cells, monocytes, and macrophages, triggering their degranulation and destruction of the target cell.8,9
Phagocytosis.
Targeted cells may be engulfed and destroyed and their proteins used for antigen presentation if the mAb binds to immune cells capable of phagocytosis, such as macrophages, monocytes, dendritic cells (DCs), and neutrophils.8,9 For both ADCC and phagocytosis, the IgG subtypes vary in Fc receptor binding affinity, with IgG3 generally demonstrating the highest affinity, followed by IgG1 with intermediate affinity, IgG4 with low affinity, and IgG2 with the lowest affinity.6–8
Although mAbs exert direct effects only on cells that express their target molecule, mAb therapy may cause more widespread immune perturbation than would be expected solely from their direct effects on targeted cells. Examples of these secondary effects, which usually take longer to occur, include the functional inhibition of T cells upon B-cell depletion by rituximab10,11; increased levels of FoxP3+ T-reg cells upon immune reconstitution after alemtuzumab12 and rituximab13therapy; and upregulation of CD56bright NK cells by daclizumab14 and rituximab.15 Based on this foundation, what follows is a synopsis of the known or theorized mechanisms of action of the mAbs under study or in current use for therapy of MS.
Access of mAb to CNS tissue
mAbs are large molecules and therefore need to be transported across the intact blood-brain barrier (BBB) via limited active transport mechanisms. It has been documented that only approximately 0.1% of systemically administered mAbs reach the intrathecal compartment.16,17 This proportion is probably significantly higher in patients with gross disruption of the BBB, such as MS patients with contrast-enhancing lesions (CELs) on brain magnetic resonance imagining (MRI). The positive therapeutic results in patients with relapsing-remitting MS (RRMS) with CELs indicate either that a sufficient amount of mAb reaches the CNS compartment in these patients or that the therapeutic benefit required only systemic effects.
NATALIZUMAB
Adhesion molecules mediate the entry of immune cells into the CNS. Natalizumab, a humanized antibody with an IgG4 framework, is a selective adhesion molecule (SAM) inhibitor whose target is CD49, the α4 subunit of the very late activating antigen-4 (VLA-4) receptor (Table 2). VLA-4 interacts with vascular cell adhesion molecule-1 (VCAM-1) in order for immune cells to migrate across the blood-brain barrier (BBB). Natalizumab binds to α4-integrin expressed on the surface of activated T-cells and other mononuclear leukocytes, where it prevents adhesion between the endothelial cell and the immune cell. This action inhibits the migration of leukocytes into CNS tissue.9, 18,19
Table 2.
| Direct effects | Indirect effects | Consequences (therapeutic and side effects) |
|---|---|---|
| Inhibition of migration of immune cells into CNS | Stabilization of BBB | Probably most important early therapeutic MoA Decreased CNS immunosurveillance |
| Inhibition of APC turnover in the CNS | Decreased antigen presentation in the CNS | Possibly MoA that underlies prolonged efficacy, but also further limits CNS immunosurveillance |
| Mobilization of lymphoid (especially pre-B) and lymphoid/myeloid (CD34+) precursors from the BM | Likely contributes to risk of PML |
CNS, central nervous system; BBB, blood-brain barrier; MoA, mechanism of action, APC, antigen-presenting cell; PML, progressive multifocal leukoencephalopathy; BM, bone marrow
Direct effects.
Two phase III clinical trials have shown natalizumab to effectively inhibit brain inflammatory activity as measured on MRI.20,21 High efficacy of natalizumab in blocking the migration of immune cells into the CNS is likely the most important early therapeutic mechanism of action (MoA) for this mAb in MS.22 However, the same MoA also underlies, or at least contributes to, the one problematic effect of natalizumab treatment: decreased CNS immunosurveillance,23 especially as it relates to the risk of progressive multifocal leukoencephalopathy (PML). The risk of PML with natalizumab therapy has been estimated at ~1/1,000 patients treated for a mean of 17.9 months.24 Natalizumab treatment has been shown to result in a prolonged decrease of lymphocytes in cerebrospinal fluid (CSF), up to 6 months after therapy has been stopped.25 The explanation for this prolonged effect may reside in poor antigen presentation in the CNS, as natalizumab treatment is associated with significantly decreased frequency of DCs in the CNS perivascular spaces.19 Nevertheless, it remains controversial whether lack of CNS immune surveillance is solely responsible for the increased incidence of PML in patients treated with natalizumab. Other supporting mechanisms may involve the increased release of B-cell precursors from the bone marrow into the blood observed in natalizumab-treated individuals, because bone marrow B cells are known to harbor JC virus.26 Accelerating the clearance of mAbs from the body through plasma exchange has been proposed as one way to restore immune function in the CNS and curb the risk of rare but serious complications such as PML.27
Clinical trial evidence.
The detailed analysis of the outcomes of two major phase III clinical trials of natalizumab for MS suggest that this mAb may be most effective for treatment of highly inflammatory early MS and less effective in later stages of the disease, characterized more by neurodegenerative than by inflammatory processes.
In the SENTINEL trial, 1,196 patients with RRMS who experienced relapse despite at least a year of interferon beta-1a (IFN β−1a) were randomized to combined treatment with natalizumab and IFN β−1a (Avonex®, Biogen Idec) or to continued therapy with IFN β−1a alone.21 Combination therapy resulted in a 24% reduction in the relative risk of sustained disability progression compared to IFN β−1a alone (p = 0.02).
In the AFFIRM trial, monotherapy with natalizumab was compared to placebo for RRMS in a total of 942 patients, 627 of whom received the mAb. After 2 years, natalizumab reduced the risk of sustained disability progression by 42% (almost twice the effect on this end point as in the mAb-treated group in SENTINEL) and reduced the rate of clinical relapse at 1 year by 68% (both p = 0.001).20
There is evidence that the pathophysiologic mechanisms of MS evolve over the course of the disease, with inflammation characterizing the early relapsing-remitting stage and neurodegeneration more important in later stages.28 The disparate results of these 2 trials of natalizumab may reflect this progression; the population in SENTINEL was somewhat older than that in AFFIRM (38.9 years vs 36 years), and had a longer duration of disease (7 years vs 5 years). Detailed post hoc analysis of patient subgroups in both trials fully supports the notion that natalizumab (and likely all agents targeting the inflammatory component of MS) may be most effective in younger patients with early MS and in highly active disease, when inflammation is the dominant process.29
RITUXIMAB
Although investigations into MS pathophysiology have focused mainly on T cells, growing evidence suggests a key role for B lymphocytes as a potential therapeutic target. Rituximab is a chimeric murine/human IgG1K mAb that targets the CD20 antigen, which is expressed only by pre-B and mature B lymphocytes, not on Ab-producing plasma cells. The primary MoA of rituximab, at least early in therapy, is a complete but transient depletion of B cells. Although rituximab has some intrinsic cytotoxic activity toward B cells in vitro, it is believed that its main in vivo B-cell–depleting activity involves ADCC30 and, to a lesser extent, CDC (Table 3).31, 32 To date, rituximab has been approved by the Food and Drug Administration (FDA) for use in rheumatoid arthritis (RA) and B-cell lymphomas. However, several published studies of rituximab in MS10, 32–35 indicate that this B-cell–targeting therapy may be highly effective in inhibiting MS-related inflammation, perhaps leading us to critically reconsider our belief that MS is a predominantly T-cell–mediated autoimmune process.
Table 3.
| Direct effects | Indirect effects | Consequences (therapeutic and side effects) |
|---|---|---|
| Depletion of B cells (via ADCC and CDC) | Inhibition of function of FcγR-bearing cells such as macrophages (“immune-decoy hypothesis”) | Probably most important early therapeutic MoA |
| Lack of B-cell help/loss of APC function for T-cell-mediated immune responses | Possibly a contributing MoA | |
| Prolonged depletion of memory B cells may lead to lack of turnover of long-lived plasma cells | Possible effect on Ab-mediated immunity | |
| Early repopulation of B cells by increased egress of pre-B and naïve B cells from the BM | May be beneficial for MS (naïve B cells tend to produce anti-inflammatory cytokines like IL-10) but may also contribute to risk of PML |
ADCC, Ab-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity; MoA, mechanism of action; Ab, antibody; BM, bone marrow; MS, multiple sclerosis; PML, progressive multifocal leukoencephalopathy
Direct effects.
The depletion of B cells by rituximab is rapid (within 2 weeks); it lasts for approximately 6 months, and even longer for CD27+ memory B cells.36 B cells are also depleted in secondary lymphoid tissues, but with slower kinetics.37, 38 Due to lack of expression of CD20 on plasma cells, rituximab depletes only those autoantibodies that are produced by memory B cells, not those that are produced by plasma cells. Consequently, most of the protective Abs against infectious agents and vaccines are not affected by rituximab therapy.39 Similarly, rituximab therapy does not cause disappearance of CSF oligoclonal bands (OCBs) or normalization of the IgG index.10
Possible indirect effects.
The action of rituximab extends beyond B-cell depletion and suppression of autoantibody synthesis. Many studies have documented inhibition of innate immune responses and adaptive T-cell responses associated with the use of rituximab in treatment of human autoimmune diseases;38,40 however, the mechanisms for these observations are insufficiently understood. According to a proposed “immune complex decoy hypothesis,” the rapid formation of a large number of immune complexes of rituximab-bound B cells may exhaust the functional activity of macrophages and other cells expressing FcγR, redirecting them from foci of chronic inflammation and leading to their functional inhibition.41 Indeed, rituximab therapy has also been associated with reductions in various global biomarkers of inflammation, such as IL-6 and C-reactive protein.9
Rituximab therapy has been shown to reduce T cells in the CSF of MS patients10 and in the inflamed synovia of RA patients,42 possibly due to lack of B-cell help for T-cell–mediated immune responses, such as antigen presentation and production of proinflammatory cytokines. Longitudinal data on the effect of long-term (ie, repeated) B-cell depletion on IgG levels of specific antibodies are not available. However, the current model of long-term human B-cell memory (ie, humoral immunity) indicates that “long-lived” plasma cells have a lifespan of only several months and therefore need to be constantly replenished by antigen-independent homeostatic activation of memory B cells.43 This suggests that long-term depletion of memory B cells (eg, by repeated doses of rituximab) should lead to an eventual decrease in humoral immunity, reflected by a decline in IgG levels.
Early repopulation of peripheral B cells by increased egress of pre-B and naïve B cells from bone marrow might contribute to the therapeutic benefit of rituximab, because naïve B cells produce anti-inflammatory cytokines,44 but, as mentioned earlier in discussion of natalizumab treatment, it may also contribute to the risk of PML.
Clinical trial evidence.
Rituximab demonstrated a rapid and sustained improvement in disease activity (fewer relapses, Gd-enhancing lesions, new T2 lesions, and T2 lesion volumes) in a 72-week open-label Phase 1 trial of patients with RRMS.34 A comparable early and robust effect was reported in a 48-week, double-blind, phase II trial of 104 patients with RRMS.32 In this trial, rituximab treatment (1,000 mg IV on Days 1 and 15; N = 69) was associated with a highly significant reduction in CELs on brain MRI at weeks 12, 16, 20, and 24 post infusion (80.3% vs 51.4% of patients were lesion free, and the mean number of lesions was 91% lower; p <0.001) compared to placebo (N = 35). While the level of efficacy on CELs may be overestimated in this trial due to uneven distribution of CELs in the placebo and active-treatment groups at baseline, there is little doubt that rituximab is highly effective in suppressing BBB disruption in MS.
Given its apparent mediation of the inflammatory cascade, rituximab, like natalizumab, might be expected to show its greatest efficacy in early-stage, inflammatory MS. A large, multicenter, phase II trial of rituximab in primary-progressive MS (PPMS)45 failed to reach the primary efficacy end point, but it confirmed that in patients with active CNS inflammation, rituximab significantly decreases CELs.46 While more than 11 cases of PML have been reported after rituximab therapy in PML-prone systemic lupus erythematosus (SLE) patients, only a single case of PML has so far been identified in an RA patient, and no case has been associated with MS therapy.47 Only future exposure of a large number of patients who are not prone to development of PML to rituximab therapy will define the exact risk of PML associated with this mAb.
ALEMTUZUMAB
Alemtuzumab is a humanized IgG1K mAb, currently approved by the FDA for the treatment of B-cell chronic lymphocytic leukemia. Its target is CD52, a glycoprotein expressed widely throughout the immune system on T and B lymphocytes, NK cells, DCs, most monocytes and macrophages, and some granulocytes (but not neutrophils).48 It is also expressed in tissues of the male reproductive system. Investigation of alemtuzumab for use in MS by a team at the University of Cambridge, UK, suggests potentially impressive efficacy, offset by significant risks.
Direct and indirect effects.
Alemtuzumab produces swift and almost complete depletion of CD52-bearing cells (Table 4). This is probably the mAb’s most important early therapeutic MoA. While alemtuzumab was shown to induce caspase-independent apoptosis in B-lymphoid cell lines in vitro,59 it is unclear to what extent this is direct cytotoxicity relevant for its in vivo effect.50 Because alemtuzumab effectively depletes cells that mediate ADCC (such as NK cells),51 it is likely that CDC represents the main effector mechanism of alemtuzumab-mediated depletion of immune cells in vivo.52
Table 4.
| Direct effects | Indirect effects | Consequences (therapeutic and side effects) |
|---|---|---|
| Depletion of CD52-bearing cells (T and B, NK cells, monocytes and MФ, DCs, some granulocytes) | Stabilization of BBB | Probably most important early therapeutic MoA Decreased CNS immunosurveillance Theoretically immunosuppressive, but only modest rate of infectious complications observed so far |
| Repopulation with skewed repertoire: early B-cell repopulation, late T-cell repopulation; CD25high T cells, Caspase3+ T cells | Theoretical benefits for long-term efficacy but may also underlie Ab-mediated autoimmune complications |
NK, natural killer cells; MФ, macrophages; DCs, dendritic cells; BBB, blood-brain barrier; MoA, mechanism of action; CNS, central nervous system
The depletion of immune cells is associated with dramatic decrease in CELs, indicating stabilization of BBB.53,54 Profound lymphocyte depletion in theory should cause immunosuppression and decreased CNS immunosurveilance, although rates of infectious complications in clinical trials of MS have not been very high.54,55 After alemtuzumab therapy, immune-cell repopulation takes place, although with a skewed repertoire: early B-cell repopulation is followed by enrichment of CD25high T cells and possibly caspase 3+ T cells during later T-cell repopulation.9,54 In theory, these repertoire changes induced by alemtuzumab may improve long-term efficacy (repopulation by naïve B cells and FoxP3+ T-regs may provide a tolerance-enhancing environment for T-cell immunity), but it could also underlie development of Ab-mediated autoimmune complications, such as Ab-mediated thyroid disorders, immune thrombocytopenic purpura (ITP) and Goodpasture’s syndrome, reported in association with alemtuzumab treatment.53,54,56 The development of these autoimmune complications have been now linked by very elegant work to high levels of IL-21.57
Clinical trial evidence.
Based on its apparent MOA, alemtuzumab would be expected to confer clinical benefit in early, inflammatory MS and clinical trial data clearly support this. Early clinical experience with alemtuzumab demonstrated that despite its profound effect on inhibition of formation of focal inflammatory lesions measured by CEL on MRI, alemtuzumab did not halt the progression of disability and development of brain atrophy in the secondary-progressive stage of the disease.55 In striking comparison, when applied to very early MS patients with little accumulated disability but high level of clinical activity, alemtuzumab therapy stabilized disease progression.53 This prompted initiation of a large, 36-month, phase II trial comparing 2 IV doses of alemtuzumab (12 mg/day or 24 mg/day in annual cycles) to subcutaneously (SC) administered IFN β−1a (44 μg 3 times/week).54 This study was single blind (ie, patients were aware of their treatment allocation, but the evaluating physician was blinded). Alemtuzumab significantly reduced the rate of sustained accumulation of disability in comparison to SC IFN β−1a (9.0% vs 29.2%, hazard ratio 0.29, p <0.001). In fact, disability scores improved in alemtuzumab-treated patients by 0.39 points on the EDSS scale, whereas scores worsened by 0.38 points for patients treated with SC IFN β−1a. These robust clinical efficacy data were fully supported by MRI data (CELs, T2 lesion load, and development of brain atrophy). Even though the higher alemtuzumab dose seemingly performed better on virtually all measured parameters, the difference between the 2 mAb doses did not reach statistical significance. Alemtuzumab dosing in this trial was suspended after 3 patients developed ITP, 1 of whom eventually developed fatal intracerebral hemorrhage. Documented adverse reactions to alemtuzumab include an infusion reaction believed to be mediated by cytokine release from lysed lymphocytes. Other adverse effects relate to Ab-mediated autoimmunity, particularly Grave’s disease,58 ITP,54 and Goodpasture’s syndrome.56 Finally, patients on alemtuzumab experienced a significantly higher rate of infections than SC IFN β−1a–treated patients (66% vs 47%). Development of melanoma has been described following alemtuzumab therapy,59 and only future studies can determine the exact risk/benefit ratio of alemtuzumab treatment for chronic autoimmune disease such as MS. Two such large phase III trials of alemtuzumab are ongoing: in treatment-naïve MS patients (CARE-MSSM I) and in patients with inadequate response to current immunomodulatory therapies (CARE-MSSM II).
DACLIZUMAB
Daclizumab, widely used to prevent rejection after allogeneic tissue transplantation, is a promising new therapeutic possibility for MS. Its complex MOA, which has yet to be fully elucidated, has so far demonstrated several intriguing and unexpected features. This humanized IgG1 mAb blocks CD25, the IL-2 binding epitope (Tac epitope) of the α chain of the IL-2 receptor (IL-2R).
The rationale for the development of daclizumab was to curb proliferation of effector T cells by blocking the formation of high-affinity IL-2 receptor.60,61 CD25 is found only at low levels in resting human T cells (except for FoxP3+ T-regulatory cells, which express high levels of CD25) but is significantly upregulated on activated T cells, permitting them to receive a high-affinity IL-2 signal. IL-2 is mostly produced by activated T cells (and to a lesser degree by activated DCs),62 and it was widely believed to drive expansion of activated T cells and their development of effector functions. However, this assumption, which was based on the use of IL-2 for expansion of T cells in vitro, has been questioned after IL-2–deficient or CD25-deficient transgenic animals developed generalized lymphoproliferation rather than immunodeficiency.63 The current view is that IL-2 plays an important role both in regulating expansion of lymphocytes and in mediating their contraction, through the process of reactivation-induced cell death.64
Direct and indirect effects.
In contrast to rituximab and alemtuzumab treatment, daclizumab therapy does not lead to immediate deletion of CD25-bearing cells, despite its shared IgG1 structure.14 Why this mAb is nondepleting in vivo remains unclear; its nondepletion may be caused by low expression levels of CD25 and by the ability of cells both to downregulate and to shed this molecule in soluble form. Nevertheless, it is clear that the downmodulation/shedding of CD25 during daclizumab therapy is incomplete (~40%), and many T cells survive in the circulation with daclizumab bound to their cell surfaces.14 Although daclizumab clearly inhibits IL-2 signaling on T cells in vitro,65 it has relatively little effect on polyclonally stimulated T cells ex vivo.14 Instead, daclizumab therapy in MS leads to profound expansion of the regulatory CD56bright NK cells.14,66 These regulatory CD56bright NK cells are present in lymph nodes,67 where they are in a position to influence T-cell priming through early production of cytokines (Table 5) and can kill immature DCs.68 However, these cells also migrate to inflammatory lesions and participate in termination of the immune response by killing autologous activated T cells.14 It remains unclear whether expansion and activation of CD56bright NK cells represent the only MoA of daclizumab in MS, but a strong correlation of expansion of CD56bright NK cells with clinical and MRI response to daclizumab14,66 indicates that this unexpected MoA is indeed important in vivo. Daclizumab also inhibits survival of CD25 FoxP3+ T-regulatory cells,69,70 an effect that may underlie some of its observed side effects, such as development of skin rashes.71
Table 5.
| Direct effects | Indirect effects | Consequences (therapeutic and side effects) |
|---|---|---|
| Inhibition of IL-2 signaling via high-affinity IL-2R Apparently nondepleting, despite IgG1 structure |
Expansion of CD56bright NK cells → killing of activated T cells | Probably most important therapeutic MoA Prolonged duration of NK cell expansion may lead to lymphadenopathy but may provide some protection against viral infections |
| Mild inhibition of T-cell proliferation | Possibly some degree of immunosuppression | |
| Inhibition of survival of CD25-expressing FoxP3+ T-regs | Likely risk factor for development of skin lesions/autoimmune complications? |
IL, interleukin; IL-2R, interleukin-2 receptor; NK, natural killer; MoA, mechanism of action; Ig = immunoglobulin, T reg = T regulatory
Clinical trial evidence.
Three small, open-label, baseline-to-treatment phase II trials established daclizumab as a potential therapy for MS.66,72–74 In 2 of these, RRMS patients with incomplete clinical and MRI responses to IFN-β received add-on therapy with daclizumab. Daclizumab was well tolerated and was associated with a reduction (>70%) in new CELs and also with stabilization or improvement in disability scores.72–74 The third trial addressed the question of synergy between IFN-β and daclizumab and demonstrated that while daclizumab monotherapy is sufficient for inhibition of MS disease activity in the majority of MS patients, IFN-β + daclizumab combination therapy or, alternatively, higher-dose daclizumab monotherapy may be required for inhibition of disease activity in very active patients.66 This study demonstrated stabilization and also long-term improvement of neurologic disability in treated patients, similar to the stabilization observed in the alemtuzumab trial.54 Nevertheless, due to the open nature of the trial, these data need to be interpreted cautiously and require confirmation in a blinded trial. The data from these small open-label trials were reproduced in a double-blind, placebo-controlled trial of daclizumab vs placebo in RRMS patients failing IFN-β therapy (CHOICE study). (Results were presented at a recent meeting,75 but remain unpublished so far.) This study evaluated 2 doses of daclizumab: 1 mg/kg or 2 mg/kg administered SC every 2 weeks for a total of 24 weeks vs placebo. The patients receiving the higher dose (which is close in bioavailability to 1 mg/kg IV every 4 weeks, the dosage used in previous open-label trials) experienced 72% reduction in CELs compared to patients on placebo. The drug is generally well tolerated, with the most common side effects identified in open-label studies being skin rashes and lymphadenopathy.71–73 One case of CNS vasculitis was observed outside of the NIH trial, which developed upon interruption of daclizumab therapy and was assessed as likely to be related to treatment (unpublished data). Therefore, the full side-effect profile of this therapy will have to be determined from large multicenter phase III trials.
CONCLUSIONS
Ongoing research continues to expand our understanding of the complex and varied MOAs of various mAbs targeting the immune system for treatment of MS. While mAbs target highly specific molecules, their in vivo MoA is remarkably pleiotropic, thanks to (often unpredicted) indirect effects. It is rather surprising that mAbs with such divergent targets as B cells (rituximab) or CD25-expressing cells (daclizumab) have demonstrated similar robust therapeutic efficacy in a single disease. This should make us reconsider our views of the complex interactions between different immune cells that underlie the development of the immune responses in humans in general and in MS in particular. Because of their large size, immunogenicity, and requirement for parenteral administration, binding mAbs may not be ideal long-term therapeutics in a chronic disease such as MS, as oral therapies are coming to the market. However, mAbs may be used as specific “probes” that help to identify therapeutic targets, and their development may be followed by searches for small molecules that explore identical MoAs. The prime example of this is the current development of small molecular antagonists of VLA-4 as a second generation of therapeutics following successful natalizumab therapy.
All mAbs reviewed in this article seem most likely to provide benefit in the earlier, inflammatory stages of MS, before the disease’s natural course evolves into a neurodegenerative phase of irreversible axonal loss. To what degree such early aggressive treatment prevents the development of clinical disability and of the progressive stages of MS remains to be determined. Promising results in clinically and MRI-documented disease progression must be balanced against the still-unfolding picture of risk for these powerful and highly specific immunomodulating agents. Further study of the basic molecular activity of mAbs and their effects in clinical trials should provide more data to guide the safest and most effective use of mAb therapy in MS.
Acknowledgments
This supplement was supported by an educational grant from Teva Neuroscience. Expert Medical Education contributed to the editorial refinement of this article and to the production of this supplement.
Footnotes
Disclosures:
Dr. Bielekova is co-inventor on 2 NIH patents related to daclizumab therapy and as such has received patent royalty payments.
Contributor Information
Bibiana Bielekova, Neuroimmunological Diseases Unit, Neuroimmunology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland.
Brenda L. Becker, Expert Medical Education, Washington, DC.
References
- 1.Pavlou AK, Belsey MJ. The therapeutic antibodies market to 2008. Eur J Pharm Biopharm 2005;59:389–396. [DOI] [PubMed] [Google Scholar]
- 2.Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv Drug Deliv Rev 2006;58:640–656. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal A, Vieira CA, Book BK, et al. Rituximab, anti-CD20, induces in vivo cytokine release but does not impair ex vivo T-cell responses. Am J Transplant. 2004;4:1357–1360. [DOI] [PubMed] [Google Scholar]
- 4.Hederer RA, Guntermann C, Miller N, et al. The CD45 tyrosine phosphatase regulates Campath-1H (CD52)-induced TCR-dependent signal transduction in human T cells. Int Immunol 2000;12:505–516. [DOI] [PubMed] [Google Scholar]
- 5.Walshe CA, Beers SA, French RR, et al. Induction of cytosolic calcium flux by CD20 is dependent upon B cell antigen receptor signaling. J Biol Chem 2008;283:16971–16984. [DOI] [PubMed] [Google Scholar]
- 6.Tao MH, Smith RI, Morrison SL. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med 1993;178:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijstelbloem HM, van de Winkel JG, Kallenberg CG. Inflammation in autoimmunity: receptors for IgG revisited. Trends Immunol 2001;22:510–516. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Solal JF, Cassard L, Fridman WH, Sautès-Fridman C. Fc gamma receptors. Immunol Lett 2004;92:199–205. [DOI] [PubMed] [Google Scholar]
- 9.Muraro PA, Bielekova B. Emerging therapies for multiple sclerosis. Neurotherapeutics. 2007;4:676–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons JA. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 2006;180:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurlings RM, Vos K, Wijbrandts CA, et al. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis 2008;67:917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloom DD, Chang Z, Fechner JH, et al. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant 2008;8:793–802. [DOI] [PubMed] [Google Scholar]
- 13.Sfikakis PP, Souliotis VL, Fragiadaki KG, et al. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clin Immunol 2007;123:66–73. [DOI] [PubMed] [Google Scholar]
- 14.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2R{alpha}-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A 2006;103:5941–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reis EA, Athanazio DA, Lima I, et al. NK and NKT cell dynamics after rituximab therapy for systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int 2009;29:469–475. [DOI] [PubMed] [Google Scholar]
- 16.Rubenstein JL, Combs D, Rosenberg J,et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood 2003;101:466–468. [DOI] [PubMed] [Google Scholar]
- 17.Petereit H, Rubbert-Roth A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult Scler. 2009;15:189–192. [DOI] [PubMed] [Google Scholar]
- 18.Rudick RA, Sandrock A. Natalizumab: α4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother 2004;4:571–580. [DOI] [PubMed] [Google Scholar]
- 19.del Pilar Martin M, Cravens PD, Winger R et al. Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Arch Neurol 2008; 65:1596–1603. [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 21.Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006;354:911–923. [DOI] [PubMed] [Google Scholar]
- 22.Niino M, Bodner C, Simard M-L, et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol 2006;59:748–754. [DOI] [PubMed] [Google Scholar]
- 23.Yao K, Gagnon S, Akhyani N, et al. Reactivation of human herpesvirus-6 in natalizumab treated multiple sclerosis patients. PLoS One 2008;3:e2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006;354:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stüve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006;59:743–747. [DOI] [PubMed] [Google Scholar]
- 26.Ransohoff RM. “Thinking without thinking” about natalizumab and PML. J Neurol Sci 2007;259:50–52. [DOI] [PubMed] [Google Scholar]
- 27.Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 2009;72:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson M, Kappos L, Calabresi PA, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol Epub 2009. Mar;256:405–415. [DOI] [PubMed] [Google Scholar]
- 29.Dhib-Jalbut S, Arnold DL, Cleveland DW,et al. Neurodegeneration and neuroprotection in multiple sclerosis and other neurodegenerative diseases. J Neuroimmunol 2006;176:198–215. [DOI] [PubMed] [Google Scholar]
- 30.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002;99:754–758. [DOI] [PubMed] [Google Scholar]
- 31.Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood 2000;95:3900–3908. [PubMed] [Google Scholar]
- 32.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. [DOI] [PubMed] [Google Scholar]
- 33.Monson NL, Cravens PD, Frohman EM, Hawker K, Racke MK. Effect of rituximab on the peripheral blood and cerebrospinal fluid B cells in patients with primary progressive multiple sclerosis. Arch Neurol 2005;62:258–264. [DOI] [PubMed] [Google Scholar]
- 34.Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 2008;63:395–400. [DOI] [PubMed] [Google Scholar]
- 35.Stuve O, Cepok S, Elias B, et al. Clinical stabilization and effective B-lymphocyte depletion in the cerebrospinal fluid and peripheral blood of a patient with fulminant relapsing-remitting multiple sclerosis. Arch Neurol 2005;62:1620–1623. [DOI] [PubMed] [Google Scholar]
- 36.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony H-P. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum 2006;54:2377–2386. [DOI] [PubMed] [Google Scholar]
- 37.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol 2006;6:394–403. [DOI] [PubMed] [Google Scholar]
- 38.Kessel A, Rosner I, Toubi E. Rituximab: beyond simple B cell depletion. Clin Rev Allergy Immunol 2008;34:74–79. [DOI] [PubMed] [Google Scholar]
- 39.Gurcan HM, Keskin DB, Stern JN, Nitzberg MA, Shekhani H, Ahmed AR. A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol. 2009;9:10–25. [DOI] [PubMed] [Google Scholar]
- 40.Zambruno G, Borradori L. Rituximab immunotherapy in pemphigus: therapeutic effects beyond B-cell depletion. J Invest Dermatol 2008;128:2745–2747. [DOI] [PubMed] [Google Scholar]
- 41.Taylor RP, Lindorfer MA. Drug insight: the mechanism of action of rituximab in autoimmune disease—the immune complex decoy hypothesis. Nat Clin Pract Rheumatol 2007;3:86–95. [DOI] [PubMed] [Google Scholar]
- 42.Kavanaugh A, Rosengren S, Lee SJ, et al. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann Rheum Dis 2008;67:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanzavecchia A, Bernasconi N, Traggiai E, Ruprecht CR, Corti D, Sallusto F. Understanding and making use of human memory B cells. Immunol Rev 2006;211:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanz I, Anolik JH, Looney RJ. B cell depletion therapy in autoimmune diseases. Front Biosci 2007;12:2546–2567. [DOI] [PubMed] [Google Scholar]
- 45.Hawker K. B-cell-targeted treatment for multiple sclerosis: mechanism of action and clinical data. Curr Opin Neurol 2008;21(suppl 1):S19–S25. [DOI] [PubMed] [Google Scholar]
- 46.Genentech and Biogen Idec Announce Top-Line Results From a Phase II/III Clinical Trial of Rituxan In Primary-Progressive Multiple Sclerosis [press release]. South San Francisco and Cambridge, Mass.: Genentech and Biogen Idec; April 14, 2008. [Google Scholar]
- 47.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy following rituximab therapy in HIV negative patients: a report of 57 cases from the Research on Adverse Drug Event and Reports (RADAR) project. Blood 2009;113:4834–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ratzinger G, Reagan JL, Heller G, Busam KJ, Young JW. Differential CD52 expression by distinct myeloid dendritic cell subsets: implications for alemtuzumab activity at the level of antigen presentation in allogeneic graft-host interactions in transplantation. Blood 2003;101:1422–1429. [DOI] [PubMed] [Google Scholar]
- 49.Stanglmaier M, Reis S, Hallek M. Rituximab and alemtuzumab induce a nonclassic, caspase-independent apoptotic pathway in B-lymphoid cell lines and in chronic lymphocytic leukemia cells. Ann Hematol 2004;83:634–645. [DOI] [PubMed] [Google Scholar]
- 50.Zent CS, Chen JB, Kurten RC, Kaushal GP, Lacy HM, Schichman SA. Alemtuzumab (CAMPATH 1H) does not kill chronic lymphocytic leukemia cells in serum free medium. 2004;Leuk Res 28:495–507. [DOI] [PubMed] [Google Scholar]
- 51.Stauch D, Dernier A, Sarmiento Marchese E, et al. Targeting of natural killer cells by rabbit antithymocyte globulin and campath-1H: similar effects independent of specificity. PLoS ONE 2009;4:e4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zent CS, Secreto CR, LaPlant BR, et al. Direct and complement dependent cytotoxicity in CLL cells from patients with high-risk early-intermediate stage chronic lymphocytic leukemia (CLL) treated with alemtuzumab and rituximab. Leuk Res 2008;32:1849–185654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol 2006;253:98–108. [DOI] [PubMed] [Google Scholar]
- 54.CAMMS223 Trial Investigators, Coles AJ, Compston DA, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008;359:1786–1801. [DOI] [PubMed] [Google Scholar]
- 55.Coles AJ, Wing MG, Molyneux P, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol 1999;46:296–304. [DOI] [PubMed] [Google Scholar]
- 56.Clatworthy MR, Wallin EF, Jayne DR. Anti-glomerular basement membrane disease after alemtuzumab. N Engl J Med 2008;359:768–769. [DOI] [PubMed] [Google Scholar]
- 57.Jones JL, Phuah CL, Cox AL, et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest 2009;119:2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coles AJ, Wing M, Smith S, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1995;354:1691–1695. [DOI] [PubMed] [Google Scholar]
- 59.Pace AA, Zajicek JP. Melanoma following treatment with alemtuzumab for multiple sclerosis. Eur J Neurol. 2009;66:471–479. [DOI] [PubMed] [Google Scholar]
- 60.Waldmann T. The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res 2002;4(suppl 3):S161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vlad G, Ho EK, Vasilescu ER et al. Anti-CD25 treatment and FOXP3-positive regulatory T cells in heart transplantation. Transpl Immunol 2007;18:13–21. [DOI] [PubMed] [Google Scholar]
- 62.Granucci F, Feau S, Angeli V, Trottein F, Ricciardi-Castagnoli P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. 2003;J Immunol 170:5075–5081. [DOI] [PubMed] [Google Scholar]
- 63.Waldmann TA. The IL-2/IL-15 receptor systems: targets for immunotherapy. J Clin Immunol 2002;22:51–56. [DOI] [PubMed] [Google Scholar]
- 64.Lenardo MJ. Interleukin-2 programs mouse T lymphocytes for apoptosis. Nature 1991;353:858–861. [DOI] [PubMed] [Google Scholar]
- 65.Goebel J, Stevens E, Forrest K, Roszman TL. Daclizumab (Zenapax) inhibits early interleukin-2 receptor signal transduction events. Transpl Immunol 2000;8:153–159. [DOI] [PubMed] [Google Scholar]
- 66.Bielekova B, Howard T, Packer AN, et al. Effect of anti-CD25 antibody daclizumab in the inhibition of inflammation and stabilization of disease progression in multiple sclerosis. Arch Neurol 2009;66:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 2003;101:3052–3057. [DOI] [PubMed] [Google Scholar]
- 68.Della Chiesa M, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol 2003;33:1657–1666. [DOI] [PubMed] [Google Scholar]
- 69.Binder M, Vögtle F-N, Michelfelder S, et al. Identification of their epitope reveals the structural basis for the mechanism of action of the immunosuppressive antibodies basiliximab and daclizumab. Cancer Res 2007;67:3518–3523. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Xiao L, Shi B-Y, et al. Short-term anti-CD25 monoclonal antibody treatment and neogenetic CD4+CD25high regulatory T cells in kidney transplantation. Transpl Immunol 2008;19:69–73. [DOI] [PubMed] [Google Scholar]
- 71.Oh U, Blevins G, Griffith C,et al. Regulatory T cells are reduced during anti-CD25 antibody treatment of multiple sclerosis. Arch Neurol 2009;66:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bielekova B, Richert N, Howard T, et al. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon {beta}. Proc Natl Acad Sci U S A 2004;101:8705–8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rose JW, Watt HE, White AT, Carlson NG. Treatment of multiple sclerosis with an anti-interleukin-2 receptor monoclonal antibody. Ann Neurol 2004;56:864–867. [DOI] [PubMed] [Google Scholar]
- 74.Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology 2007;69:785–789. [DOI] [PubMed] [Google Scholar]
- 75.Montalban X, Wynn D, Kaufman M, Wang M, Fong A. Preliminary CHOICE results: a phase 2, randomised, double-blind, placebo-controlled multicentre study of subcutaneous daclizumab in patients with active, relapsing forms of multiple sclerosis on interferon beta. Presented at the 23rd Congress of the European Committee for Treatment and Research of Multiple Sclerosis (ECTRIMS) in Prague, Czech Republic, October 11–14, 2007. [Google Scholar]
- 76.Tkaczuk J, Milford E, Yu C, et al. Intracellular signaling consequences of anti-IL-2Ralpha blockade by daclizumab. Transplant Proc. 200;33:212–213. [DOI] [PubMed] [Google Scholar]
- 77.Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–1342. [DOI] [PubMed] [Google Scholar]
- 78.Rastetter W, Molina A, White CA. Rituximab: expanding role in therapy for lymphomas and autoimmune diseases. Annu Rev Med. 2004;55:477–503. [DOI] [PubMed] [Google Scholar]
- 79.Carpenter PA, Tso JY, Press OW, Yu X, Anasetti C.Non-FcR-binding, humanized anti-CD3 antibody Hu291 induces apoptosis of human T cells more effectively than OKT3 and is immunosuppressive in vivo. Transplant Proc. 2000;32:1545–1546. [DOI] [PubMed] [Google Scholar]
- 80.Krumbholz M, Meinl I, Kümpfel T, Hohlfeld R, Meinl E.et al. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology 2008;71:1350–1354. [DOI] [PubMed] [Google Scholar]
- 81.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172:3422–3427. [DOI] [PubMed] [Google Scholar]
- 82.Cree B. Emerging monoclonal antibody therapies for multiple sclerosis. Neurologist 2006;12:171–178. [DOI] [PubMed] [Google Scholar]