Abstract
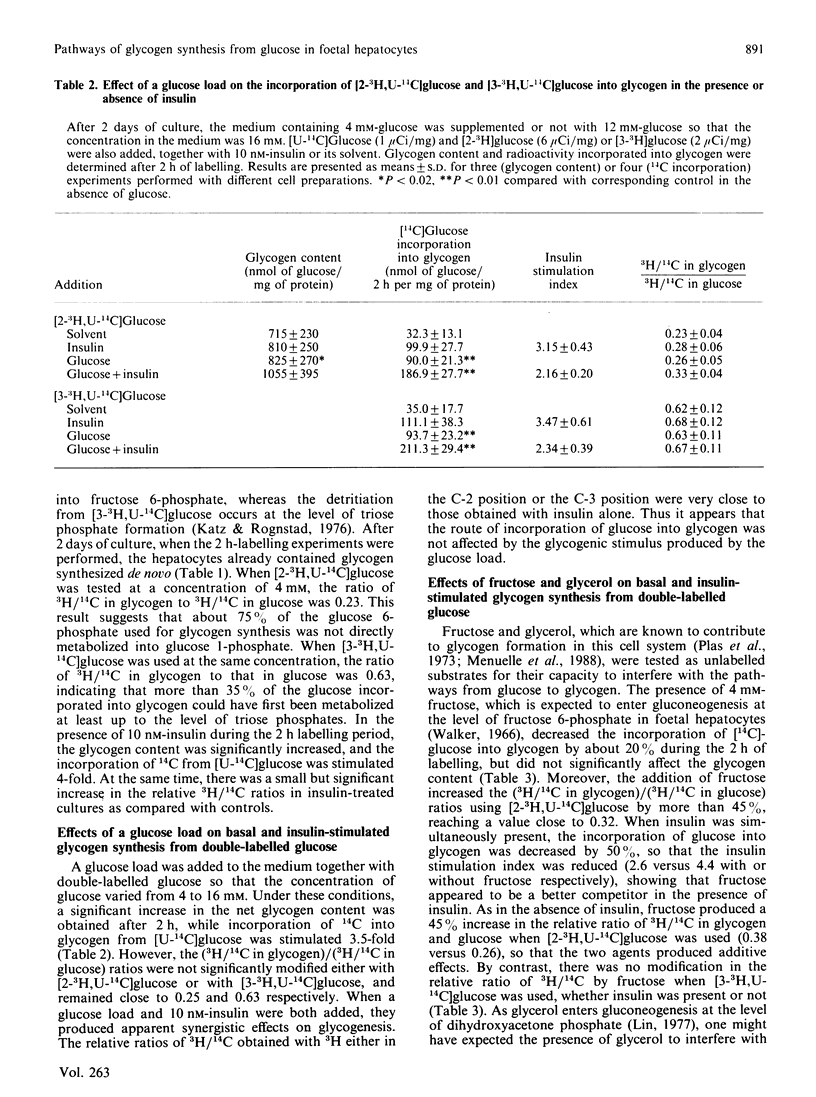
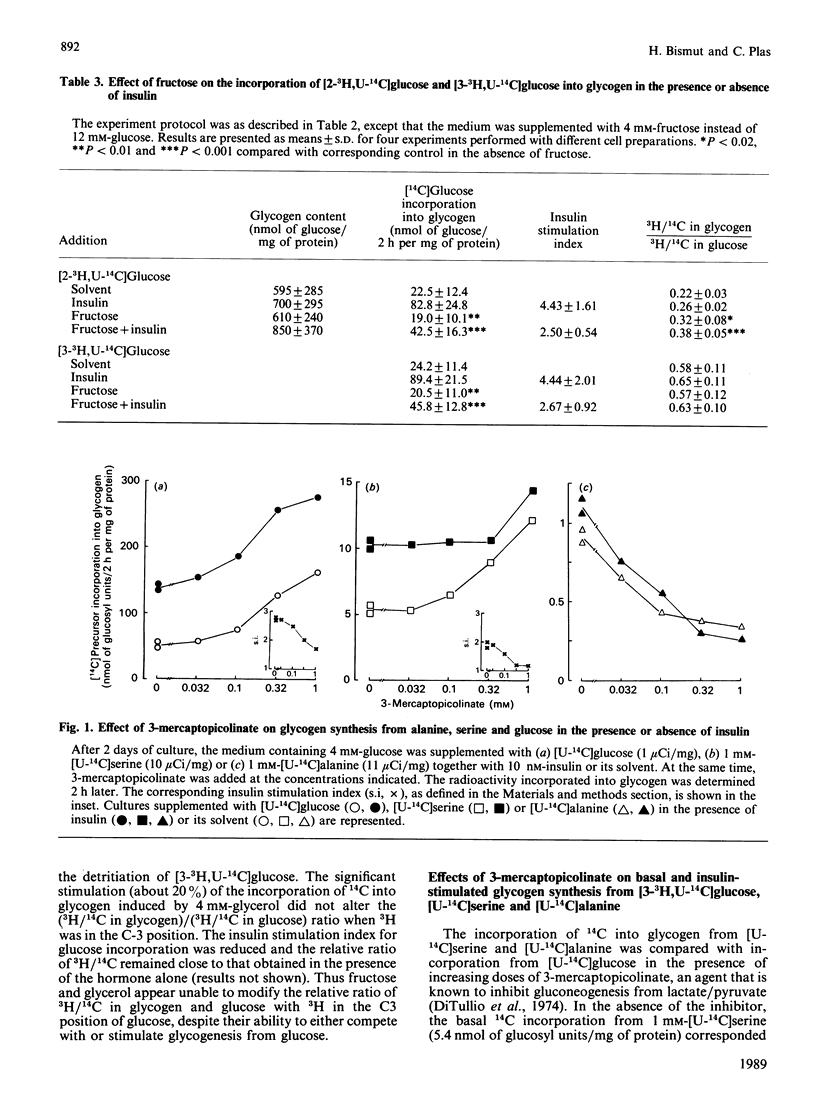
The pathways of glycogen synthesis from glucose were studied using double-isotope procedures in 18-day cultured foetal-rat hepatocytes in which glycogenesis is strongly stimulated by insulin. When the medium containing 4 mM-glucose was supplemented with [2-3H,U-14C]glucose or [3-3H,U-14C]glucose, the ratios of 3H/14C in glycogen relative to that in glucose were 0.23 +/- 0.04 (n = 6) and 0.63 +/- 0.09 (n = 8) respectively after 2 h. This indicates that more than 75% of glucose was first metabolized to fructose 6-phosphate, whereas 40% reached the step of the triose phosphates prior to incorporation into glycogen. The stimulatory effect of 10 nM-insulin on glycogenesis (4-fold) was accompanied by a significant increase in the (3H/14C in glycogen)/(3H/14C in glucose) ratio with 3H in the C-2 position (0.29 +/- 0.05, n = 6, P less than 0.001) or in the C-3 position (0.68 +/- 0.09, n = 8, P less than 0.01) of glucose, whereas the effect of a 12 mM-glucose load (3.5-fold) did not alter these ratios. Fructose (4 mM) displaced [U-14C]glucose during labelling of glycogen in the presence and absence of insulin by 50 and 20% respectively, and produced under both conditions a similar increase (45%) in the (3H/14C in glycogen)/(3H/14C in glucose) ratio when 3H was in the C-2 position. 3-Mercaptopicolinate (1 mM), an inhibitor of gluconeogenesis from lactate/pyruvate, further decreased the already poor labelling of glycogen from [U-14C]alanine, whereas it increased both glycogen content and incorporation of label from [U-14C]serine and [U-14C]glucose with no effect on the relative 3H/14C ratios in glycogen and glucose with 3H in the C-3 position of glucose. These results indicate that an alternative pathway in addition to direct glucose incorporation is involved in glycogen synthesis in cultured foetal hepatocytes, but that insulin preferentially favours the classical direct route. The alternative foetal pathway does not require gluconeogenesis from pyruvate-derived metabolites, contrary to the situation in the adult liver.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd M. E., Albright E. B., Foster D. W., McGarry J. D. In vitro reversal of the fasting state of liver metabolism in the rat. Reevaluation of the roles of insulin and glucose. J Clin Invest. 1981 Jul;68(1):142–152. doi: 10.1172/JCI110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSante D. C., Little L., Peavy D. E., Vinicor F. Insulin-responsive cultured foetal-rat hepatocytes. Their preparation and characterization. Biochem J. 1984 Oct 1;223(1):39–46. doi: 10.1042/bj2230039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiTullio N. W., Berkoff C. E., Blank B., Kostos V., Stack E. J., Saunders H. L. 3-mercaptopicolinic acid, an inhibitor of gluconeogenesis. Biochem J. 1974 Mar;138(3):387–394. doi: 10.1042/bj1380387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS V. J., BRYANT J. C., KERR H. A., SCHILLING E. L. CHEMICALLY DEFINED MEDIA FOR CULTIVATION OF LONG-TERM CELL STRAINS FROM FOUR MAMMALIAN SPECIES. Exp Cell Res. 1964 Dec;36:439–474. doi: 10.1016/0014-4827(64)90302-7. [DOI] [PubMed] [Google Scholar]
- Girard J. Gluconeogenesis in late fetal and early neonatal life. Biol Neonate. 1986;50(5):237–258. doi: 10.1159/000242605. [DOI] [PubMed] [Google Scholar]
- Goodman M. N. Effect of 3-mercaptopicolinic acid on gluconeogenesis and gluconeogenic metabolite concentrations in the isolated perfused rat liver. Biochem J. 1975 Jul;150(1):137–139. doi: 10.1042/bj1500137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. W., Ballard J. Hormonal regulation of hepatic P-enolpyruvate carboxykinase (GTP) during development. Fed Proc. 1975 Feb;34(2):166–171. [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Taylor E. A. Glycogen synthesis in the perfused liver of the starved rat. Biochem J. 1972 Sep;129(3):529–538. doi: 10.1042/bj1290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G., Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Jungermann K. Metabolic zonation of liver parenchyma: significance for the regulation of glycogen metabolism, gluconeogenesis, and glycolysis. Diabetes Metab Rev. 1987 Jan;3(1):269–293. doi: 10.1002/dmr.5610030112. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Lin E. C. Glycerol utilization and its regulation in mammals. Annu Rev Biochem. 1977;46:765–795. doi: 10.1146/annurev.bi.46.070177.004001. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Kuwajima M., Newgard C. B., Foster D. W., Katz J. From dietary glucose to liver glycogen: the full circle round. Annu Rev Nutr. 1987;7:51–73. doi: 10.1146/annurev.nu.07.070187.000411. [DOI] [PubMed] [Google Scholar]
- Menuelle P., Buc H. A., Plas C. Differences between glucose and insulin stimulation of glycogenesis in cultured fetal hepatocytes. Biochim Biophys Acta. 1987 May 18;928(3):332–340. doi: 10.1016/0167-4889(87)90193-5. [DOI] [PubMed] [Google Scholar]
- Menuelle P., M'zali H., Forest N., Plas C. Compared roles of glucose, galactose and fructose as glycogen precursors during the acute response to insulin in cultured rat foetal hepatocytes. Int J Biochem. 1988;20(8):777–782. doi: 10.1016/0020-711x(88)90063-8. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Newgard C. B., Moore S. V., Foster D. W., McGarry J. D. Efficient hepatic glycogen synthesis in refeeding rats requires continued carbon flow through the gluconeogenic pathway. J Biol Chem. 1984 Jun 10;259(11):6958–6963. [PubMed] [Google Scholar]
- Okajima F., Katz J. Effect of mercaptopicolinic acid and of transaminase inhibitors on glycogen synthesis by rat hepatocytes. Biochem Biophys Res Commun. 1979 Mar 15;87(1):155–162. doi: 10.1016/0006-291x(79)91660-7. [DOI] [PubMed] [Google Scholar]
- Parniak M. A., Kalant N. Enhancement of glycogen concentrations in primary cultures of rat hepatocytes exposed to glucose and fructose. Biochem J. 1988 May 1;251(3):795–802. doi: 10.1042/bj2510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniak M., Kalant N. Incorporation of glucose into glycogen in primary cultures of rat hepatocytes. Can J Biochem Cell Biol. 1985 May;63(5):333–340. doi: 10.1139/o85-049. [DOI] [PubMed] [Google Scholar]
- Plas C., Chapeville F., Jacquot R. Development of glycogen storage ability under cortisol control in primary cultures of rat fetal hepatocytes. Dev Biol. 1973 May;32(1):82–91. doi: 10.1016/0012-1606(73)90221-2. [DOI] [PubMed] [Google Scholar]
- Plas C., Forest N., Pringault E., Menuelle P. Contribution of glucose and gluconeogenic substrates to insulin-stimulated glycogen synthesis in cultured fetal hepatocytes. J Cell Physiol. 1982 Dec;113(3):475–480. doi: 10.1002/jcp.1041130317. [DOI] [PubMed] [Google Scholar]
- Plas C., Menuelle P., Moncany M. L., Fulchignoni-Lataud M. C. Time dependence of the glycogenic effect of insulin in cultured fetal hepatocytes. Diabetes. 1979 Aug;28(8):705–712. doi: 10.2337/diab.28.8.705. [DOI] [PubMed] [Google Scholar]
- Plas C., Nunez J. Role of cortisol on the glycogenolytic effect of glucagon and on the glycogenic response to insulin in fetal hepatocyte culture. J Biol Chem. 1976 Mar 10;251(5):1431–1437. [PubMed] [Google Scholar]
- Scofield R. F., Kosugi K., Schumann W. C., Kumaran K., Landau B. R. Quantitative estimation of the pathways followed in the conversion to glycogen of glucose administered to the fasted rat. J Biol Chem. 1985 Jul 25;260(15):8777–8782. [PubMed] [Google Scholar]
- Shikama H., Ui M. Glucose load diverts hepatic gluconeogenic product from glucose to glycogen in vivo. Am J Physiol. 1978 Oct;235(4):E354–E360. doi: 10.1152/ajpendo.1978.235.4.E354. [DOI] [PubMed] [Google Scholar]
- Snell K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv Enzyme Regul. 1984;22:325–400. doi: 10.1016/0065-2571(84)90021-9. [DOI] [PubMed] [Google Scholar]
- Snell K. Liver enzymes of serine metabolism during neonatal development of the rat. Biochem J. 1980 Aug 15;190(2):451–455. doi: 10.1042/bj1900451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Walker D. G. Regulation of hepatic L-serine dehydratase and L-serine-pyruvate aminotransferase in the developing neonatal rat. Biochem J. 1974 Dec;144(3):519–531. doi: 10.1042/bj1440519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. T., Koudelka A. P. Pathway of glycogen synthesis from glucose in hepatocytes maintained in primary culture. J Biol Chem. 1985 Feb 10;260(3):1521–1526. [PubMed] [Google Scholar]
- Stalmans W. The role of the liver in the homeostasis of blood glucose. Curr Top Cell Regul. 1976;11:51–97. doi: 10.1016/b978-0-12-152811-9.50009-2. [DOI] [PubMed] [Google Scholar]
- WALKER D. G. The post-natal development of hepatic fructokinase. Biochem J. 1963 Jun;87:576–581. doi: 10.1042/bj0870576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. F., Arora K. K., Longenecker J. P. The pentose pathway: a random harvest. Impediments which oppose acceptance of the classical (F-type) pentose cycle for liver, some neoplasms and photosynthetic tissue. The case for the L-type pentose pathway. Int J Biochem. 1987;19(9):749–817. doi: 10.1016/0020-711x(87)90239-4. [DOI] [PubMed] [Google Scholar]


