Abstract
Several known biomarkers have been used to understand the physiological responses of humans to various short and long-term interventions such as exercise or dietary interventions. However, little exploratory work has been conducted to identify novel biomarkers in human saliva that could enable non-invasive physiological research to understand acute responses to interventions such as reducing sedentary time. The purpose of this study was to identify novel biomarkers in the saliva (cytokines, growth factors and vascular factors) that respond to prolonged (4 hours) and interrupted sitting (4 hours of sitting interrupted by 3 minutes of walking at 60% of maximal heart rate every 27 minutes) in young, healthy males and females. We also sought to determine whether responsive biomarkers would differ by sex. Participants (n = 24, 21.2 ± 2.2 years, 50% female) completed a prolonged sitting (PS) session and an interrupted sitting (IS) session in random order. Individual saliva samples were pooled into a male sample and a female sample to identify responsive biomarkers using a human cytokine antibody membrane array (42 targets). Several novel biomarkers were responsive in both sexes (e.g., IL-8, Angiogenin, VEGF, and EGF), in females only (e.g., TNF-α and IL-13), and in males only (e.g., IL-3, RANTES, and IL-12p40/p70). Importantly, several biomarkers appear to be responsive to the 4-hour prolonged and interrupted sitting sessions (e.g., TNF-α, IL-8, IL-3, RANTES, EGF, Angiogenin, and VEGF). This work highlights new directions for researchers aiming to investigate the effect of short-term or acute interventions on different physiological pathways using non-invasive methods. Our work clearly indicates that human saliva samples can provide a wealth of insight into physiological responses, and that a number of biomarkers can be used to understand changes induced by acute interventions such as interrupting prolonged sitting.
Introduction
Biomarkers can be measured using different bodily fluids (e.g., blood, saliva, and urine), and muscle biopsies. Previously, blood and muscle biopsies were considered the “gold standard” in the analysis of biomarkers in exercise physiology [1]. However, these techniques are invasive, creating challenges for data collection and participant recruitment. Furthermore, while serum and plasma have been used widely, and therefore have established efficacy and accuracy [2,3], a consequence of drawing blood is that acute inflammation can occur due to the stress induced within the participant during blood collection. As such, invasive methods may be problematic for studies aiming to understand the physiological changes observed from an acute stressor or intervention. Alternatively, saliva is a safe and non-invasive method, that does not pose the same risk of inducing inflammation during collection [2,3].
Saliva sampling has been shown to be feasible and insightful for the measurement of cytokine responses to stressors. It is also more stable than blood, and therefore requires less preparation for analysis while providing a large number of analytes [4]. A review by Szabo et al. found that 17 cytokines found in the saliva were reported as detectable and responsive to acute stressors across multiple studies [3]. Saliva has also been shown to be an accurate method of sampling and to be applicable across multiple clinical settings [5,6]. However, few experimental studies assessing acute responses have used saliva. This is also the case in the area of sedentary physiology.
Sedentary behavior is defined as any waking behavior that has an energy expenditure of ≤ 1.5 metabolic equivalents, while in a sitting, reclining, or lying posture [7]. Data indicates that Canadians spend over 10 hours per day engaging in sedentary activities [8]. Accumulating high volumes of sedentary time is associated with increased risk of mortality [9–12], cardiovascular disease, and metabolic diseases such as diabetes [13–17]. It has also been shown to negatively affect cardiometabolic biomarkers [12], vascular function [18–21] and pro-inflammatory biomarkers [22,23]. Importantly, a growing body of research indicates that acute bouts of sitting can lead to negative physiological outcomes [24–26]. Two studies from our laboratory have investigated the acute response of Interleukin (IL)-8, a pro-inflammatory cytokine, in the saliva in this context. Both studies used a prolonged sitting session (4 hours of sitting) and compared this to an interrupted sitting session where 4 hours of sitting was interrupted by physical activity every 30–60 minutes [27,28]. Analysis revealed that salivary IL-8 levels increased during the prolonged sitting session, and the response was either attenuated or abolished during the interrupted session depending on the intensity of the physical activity. While IL-8 appears to be a promising marker, it is not clear if other markers, particularly those related to cardiovascular or pro-inflammatory pathways, would respond to such protocols.
Another gap in this area relates to sex differences. In our previous work, sex differences were noted when comparing IL-8 responses to prolonged and interrupted sitting [28]. Sex-differences have been noted in observational studies and in intervention studies in response to a variety of short and longer-term interventions related to movement and diet. These responses have been observed for vascular function markers (flow mediated dilation), and biomarkers such as IL-6, IL-8, Fibrinogen, CRP, glucose, cortisol, and RANTES [24,28–33]. It has been hypothesized that these differences are due to hormones, physical activity levels, inflammatory responses, body mass index, chronic disease states, diet composition, or cardiovascular fitness [28,29,31–34]. Whether these sex differences are also detected using salivary measures is unclear.
Saliva sampling is a promising method of assessing changes in biomarkers in response to acute interventions. However, no studies to our knowledge have investigated the array of biomarkers that are either detectable or responsive in the saliva in acute experimental studies such as our prolonged and interrupted sitting protocols. As such, we conducted an exploratory study aimed at identifying 1) detectable biomarkers present in the saliva and 2) biomarkers that respond to prolonged and interrupted sitting.
Methods
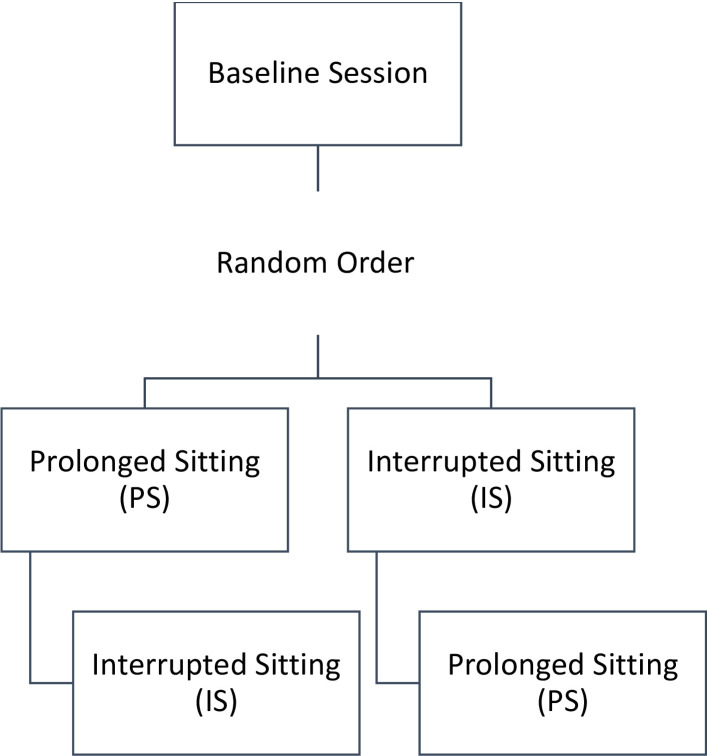
Study Design: A randomized crossover design was used (Fig 1). Participants were randomly allocated to complete either the Prolonged Sitting (PS) session or the Interrupted Sitting (IS) session first using a number generator, with balanced randomization. Sessions took place at least one week apart.
Fig 1. Experimental design.
Visual representation of the experimental design. Participants completed a baseline session and then completed the Prolonged Sitting (PS) or Interrupted Sitting (IS) sessions in random order.
Participants
Eligible participants were males and females between the ages of 18–30 years, with a body mass index (BMI) of <30 kg/m2, who were non-smokers. Individuals were excluded if they had an existing respiratory, cardiovascular, or metabolic condition, an acute infection, were pregnant, were taking any medication that would influence their inflammatory or exercise response, had an acute injury, or had recent dental surgery and/or any known oral disease. All participants provided written informed consent prior to participation in the study. This study was approved by the Research Ethics Board at Ontario Tech University (REB #16473). Data collection began on September 22, 2021 and ended on April 27, 2022.
Due to the exploratory and novel nature of this research, as well as the sex-based pooled analysis approach, a sample size calculation was not possible as there was no one effect size. Literature on microarray-based analyses to examine effects of exercise [35–37] and of microarray-based analyses of saliva [27,38,39] have used a sample size of n = 10–24; based on this we chose a sample size of 24 participants. Additionally, a previous study from our laboratory using the same interruption protocol, investigating salivary and plasma IL-8, completed a sample size calculation that indicated an n = 21 was sufficient [28].
Protocols
Participants completed three laboratory sessions: 1) baseline session, 2) PS, and 3) IS.
Session 1: Baseline session
Resting HR, resting blood pressure (A&D Medical Digital Blood Pressure Monitor, Model UA-767FAM, A&D Engineering, Inc. San Jose, CA, USA), height (cm), and body mass (kg) (Detecto Weight Beam Eye-Level, Webb City Missouri) were measured. An incremental to maximal exercise test using a stepwise protocol was performed on a treadmill (Trackmaster, FullVision, Newton, KS). Participants were fitted with a portable HR monitor (Polar Electro Oy, Professorintie 5, FI-90440 Kempele, Finland) for continuous measurement of HR. A metabolic cart was used for breath-by-breath gas analysis (Parvo Medics 2400, USA). Concentrations of expired O2 and CO2 were analyzed, and ventilation was measured. Test termination criteria included a Respiratory Exchange Ratio (RER) >1.15, HR ± 10 beats per minute of age-predicated maximal HR (220-age), a plateau in oxygen uptake (VO2), or volitional fatigue. The highest HR recorded during the test was used for HRmax. VO2max was calculated as the highest VO2 that was attained during the test. After finding the peak VO2, VO2max was calculated as a mean of ±5 breaths, including this value.
Session 2 and 3: Prolonged and interrupted sitting sessions
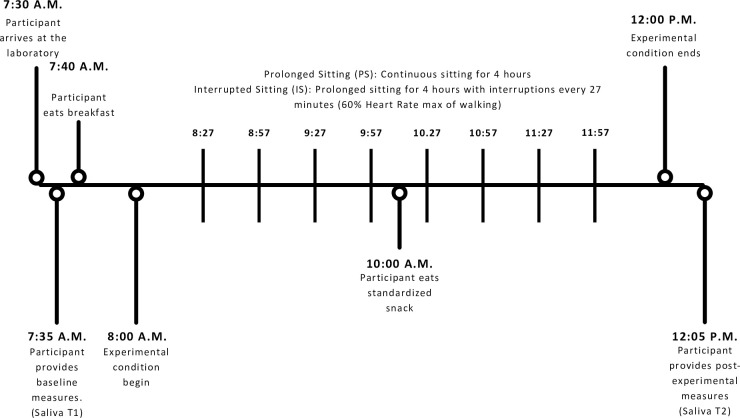
These sessions were conducted in random order. Data collection was conducted from October 2021 to February 2022. For the PS, participants were seated continuously for four hours (Fig 2). For the IS, the four-hour prolonged session was interrupted with three-minute activity interruptions (60% Heart Rate (HR)max) every 27 minutes (at times: 27, 57, 87, 117, 147, 177, 207, and 237 minutes), for a total of eight interruptions. The four-hour duration was chosen as it best mimics a typical sitting pattern. Participants were instructed to perform as little lower limb movement as possible, however, they were permitted to use their upper limbs for the study duration. Participants were instructed to arrive fasted and drink 1L of water prior to arrival. They were provided a standardized breakfast and snack during the sessions (590 Calories; Fat: 6.5g, Carbohydrates: 127g, Protein: 11.1g).
Fig 2. Prolonged and interrupted sitting sessions timeline.
Visual representation of the Prolonged Sitting (PS) and Interrupted Sitting (IS) protocols. This timeline provides an outline of the sample collection timing and interruption timings.
Saliva collection and analysis
Whole saliva samples were taken upon arrival (T1) and at the end (T2) of each of the PS and IS sessions. Participants were instructed to refrain from alcohol, smoking, strenuous exercise, and the use of anti-inflammatory medications in the 24-hours prior to the session. They were also instructed to refrain from caffeine/stimulants, supplements, or mouthwash on the day of the session.
Saliva samples were collected using oral swabs (Salimetrics SalivaBio, Salimetrics LLC, State College, PA, USA). The participant was instructed to rinse their mouth with water to remove any debris or particulates and then the swab was placed under the tongue or against the cheek for five minutes and then immediately centrifuged (VWR Clinical 2000, Germany) at 4000 rpm for five minutes allowing collection of 1.5–3.0 mL of saliva, which was subsequently stored at -80°C. Saliva sample collection timing was matched between the two conditions, samples at T1 were collected between 7:30–8:00 a.m., while saliva samples at T2 were collected between 12:00–12:30 p.m. for both the PS and IS protocols. Samples were thawed on the day of analysis and were centrifuged for 15 minutes at 1500 x g at 4°C to remove mucins and particulate matter that could potentially interfere with antibody binding. Samples of individual participants were then pooled into 8 samples by sex (female and male), session (PS and IS), and time (T1 (before sitting) and T2 (end of 4-hour session)).
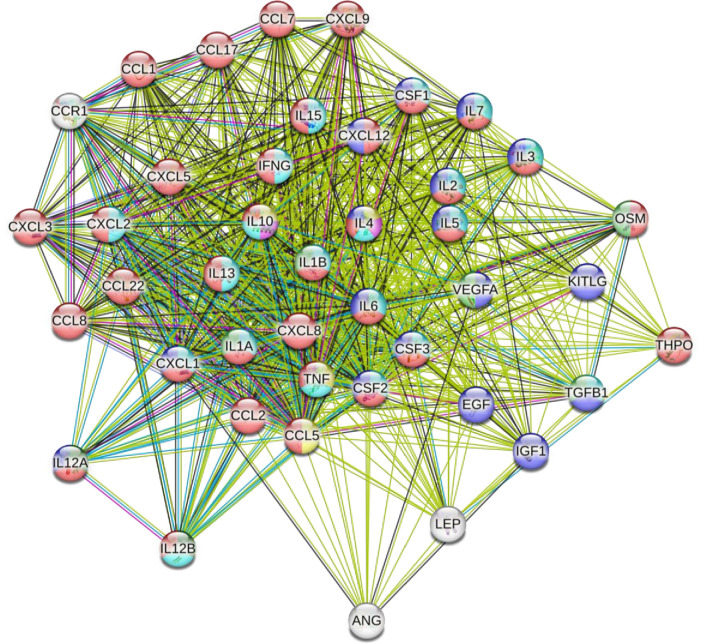
Total salivary protein concentrations of the 8 pooled samples were determined using the Coomassie PLUS 138 Protein Assay Reagent (Thermo Fisher Scientific, MA, USA) prior to sample analysis. Cytokines, including several chemokines, growth factors and vascular/endothelial factors within the saliva samples were quantified using Human Cytokine Antibody Array Membranes following manufacturer’s protocols (Abcam, Catalog # ab133997). The full list of biomarkers analyzed by the microarray kit and their alternate names are presented in S1 Table. This particular array was chosen based on the pathways indicated in Fig 3, and their importance for sedentary physiology.
Fig 3. Physiological pathways and connections between the 42-Targets of the antibody array.
Connections between 42 cytokines and growth factors with potential interactions are displayed using STRING v11.5 analysis. The 42 targets are connected using green (text mining), grey (co-expression), pale blue (protein homology), royal blue (gene co-occurrence), red (gene fusions), lime green (gene neighbourhood), blue (from curated databases), and pink (experimentally determined). Nodes are coloured to represent different functional pathways and to identify the biomarker as either a cytokine (red), growth factor (blue), or mitogen (light green). IL-3, IL-4, Il-5, CSF1 (MCSF), OSM (Oncostatin M), CSF2 (GM-CSF), THPO (Thrombopoietin), IL-10, IL-12A/IL-12B (IL-12), KITLG (SCF), IL-1A (IL-1⍺), IL-1B (IL-1β), IFNG (IFN-γ), LEP (Leptin), CCL2 (MCP-1), IL-2, CCL8 (MCP-2), CCL7 (MCP-3), CXCL5 (ENA-78), CCL22 (MDC), CXCL9 (MIG), CXCL8 (IL-8), CXCL1/CXCL2/CXCL3 (GRO), CCL5 (RANTES), CXCL1 (GRO-⍺), CCL1 (I-309), CCL12 (SDF-1), TNF (TNF-⍺/TNF-β), IL-6, IL-7, IL-13, Il-15, EGF, ANG (Angiogenin), CSF3 (GCSF), VEGFA (VEGF), CCR1 (MIP-1δ), IGF-1, CCL17 (Thymus and Activation Regulated Chemokine (TARC)), TGF-Β1 (TGF-β1).
For analysis of samples, 250 μg of total protein (allowing for standardization across samples) from the pooled samples were loaded onto the membranes and subsequently incubated overnight at 4°C under gentle rotation. Following the overnight incubation, an extra wash was performed prior to loading the biotin-conjugated anti-cytokine antibody onto the membranes. Next, a wash was performed and the HRP (Horseradish Peroxidase)-conjugated Streptavidin was added to the membrane and incubated for 2-hours at room temperature. Lastly, a detection buffer containing HRP substrate was added and incubated for 2-minutes at room temperature and then chemiluminescence was measured. Chemiluminescence detection and semi-quantitative determination of cytokine expression within the different samples was performed using a LiCor C-DiGit® Blot Scanner and Image Studio™ imaging software. To calculate relative cytokine and growth factor expression levels, the summed signal density of each spot was background corrected and normalized to the positive controls across all membranes. Therefore, the mean spot pixel density is normalized to the positive controls across all membranes and indicates relative expression levels, despite not having a standard concentration-based unit of measurement. The concentration of targets detected by the Abcam ab133997 membrane array is typically in the pg/mL range, based on information provided by the manufacturer.
Data analysis
Descriptive statistics (Means ± SD) were performed on sample characteristics. Human cytokine antibody array results from the pooled samples were compiled. As this study took on an exploratory approach, similar to previous exploratory work [40], samples were pooled into male and female subgroups. As such, samples were not used to detect individual level marker concentrations, and therefore no statistical analysis can be performed to compare changes, instead the detection of biomarkers and their responsiveness to the different conditions can be visually inspected using average pixel density.
The Search Tool for the Retrieval of Interacting Genes/Proteins database (STRING v11.5, https://string-db.org/) was used to examine relationships between responsive and detectable cytokines and growth factors. Pathways were generated through the use of Ingenuity Pathways Analysis (IPA QIAGEN Inc.) to examine the different pathways the biomarkers are involved in (https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis) [41,42].
Results
Participant characteristics (n = 24, mean 21.1 years ± 2.2, 50% female) can be found in Table 1. Of the 42 targets, 26 were detectable. Several cytokines and growth factors were below detectable levels in the saliva (IL-2, MCP-2, TNF-β, MCP-3, IL-5, IL-6, IL-7, MIP-1δ, IL-10, I-309, IL-1α, TARC, IFN-γ, G-CSF, GM-CSF, and Leptin). Some cytokines and growth factors were only detectable in females (TNF-α, Thrombopoietin (only IS-T2), and IL-13 (only PS-T2)), while some cytokines and growth factors were only detectable in males (IL-3, IL-4 (only IS-T2), GRO, RANTES, GRO-α/CXCL-1, IL-12, SCF, PDGF-BB, and SDF-1/CXCL-12).
Table 1. Participant characteristics.
| Female (n = 12) | Male (n = 12) | |
|---|---|---|
| Age (years) | 20.9 ± 1.8 | 21.3 ± 2.5 |
| Body Mass Index (kg / m2) | 24.4 ± 2.1 | 25 ± 3.6 |
| Physical Activity (minutes · week -1) | 154.2 ± 74.9 | 158.8 ± 189.6 |
| Maximal Aerobic Capacity–VO2max (mL · kg-1 · min-2) | 37.2 ± 4.9 | 45.6 ± 8.5 |
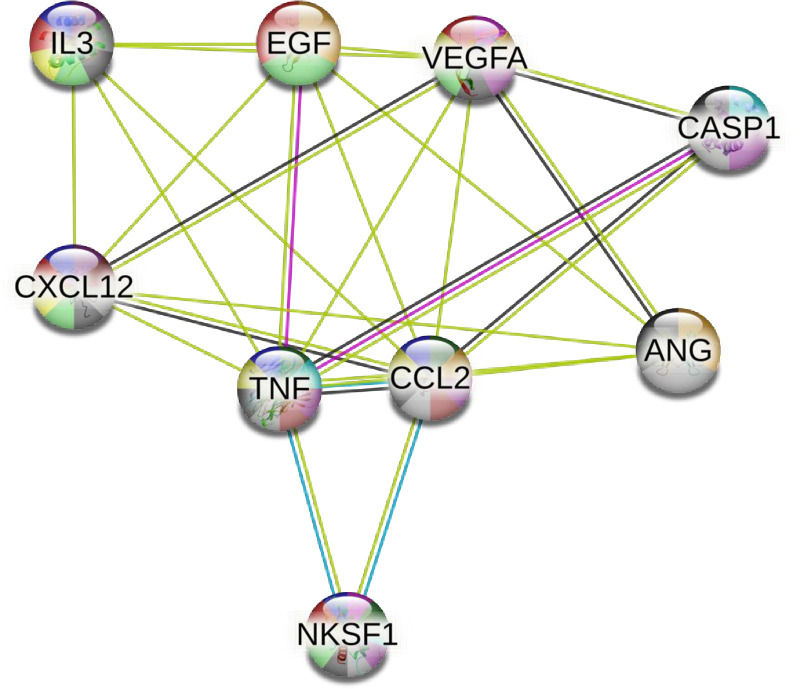
Relationships between responsive and detectable cytokines and growth factors were assessed using STRING v11.5 [41]. All 42 targets from the antibody array were inputted into STRING v11.5 (Fig 3), to detect potential relationships between all targets. Functional pathways between biomarkers were identified using the STRING v11.5 database and correspond to the color coding of the nodes. The nine biomarkers that appeared to differ between males and females were inputted into STRING v11.5 (Fig 4). Associations, such as protein interactions, co-expression, and experimentally determined interactions, were determined between eight of the nine cytokines and growth factors. This can be used to provide insight into which pathways may be meaningful to investigate further.
Fig 4. Physiological pathways and connections between cytokines and growth factors that demonstrated sex differences.
Connections between nine cytokines and growth factors that demonstrated some sex differences, displayed using STRING v11.5 analysis. Nodes are coloured to represent different functional pathways and to identify the biomarker as either a growth factor (green), or cytokine (blue). CASP1 (IL-1β), IL-3, TNF (TNF-α), VEGFA (VEGF), CCL2 (MCP-1), EGF, ANG (Angiogenin), CXCL12 (SDF-1), and NKSF1 (IL-12) are connected using green (text mining), pink (experimentally determined), blue (from curated databases), and grey (co-expression) lines.
Edges- Text mining: these biomarkers are identified as having a significant protein interaction group in the abstracts of scientific literature within the String database; Experimentally determined: these biomarkers are identified as having a protein-protein interaction, determined experimentally, within the String data sets; From curated databases: these biomarkers are identified as having a link via the String curated database; Co-expression: these biomarkers are simultaneously expressed, within homo sapiens, in response to a stimulus.
Colour Coding of Nodes Based on Functional Pathways- Growth factor activity: Green; Cytokine activity: Yellow; Regulation of blood vessels endothelial cell proliferation involved in sprouting angiogenesis: Pink; Negative regulation of endothelial cell proliferation: Dark green; Cytokine production: Light blue; Angiogenesis: Orange; Cytokine-mediated signalling pathway: Purple; Negative regulation of immune system process: Brown; Immune response: Grey; Immune system process: Black.
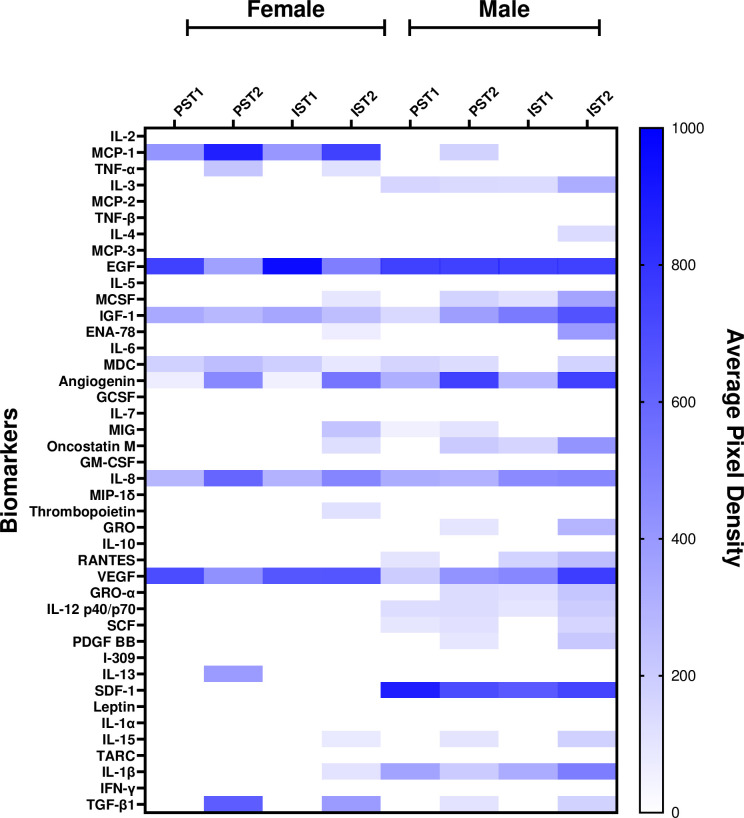
Mean spot pixel densities, indicating relative levels of detectable cytokines and growth factors in saliva, from PS and IS at T1 and T2 are presented in Fig 5 using heat maps. The heat maps indicate the relative levels of biomarkers in males and females within each condition and the potential variation in the presence of biomarkers. The heat maps clearly indicate differences in relative levels of biomarker presence in the saliva across different conditions and between sexes.
Fig 5. Heat map of the pixel density for the 42-Targets of the antibody array in males and females.
Heat map presentation of the average pixel density for the pooled samples for males and females in each condition. Interrupted Sitting Time 1 (IS-T1), Interrupted Sitting Time 2 (IS-T2), Prolonged Sitting Time 1 (PS-T1), and Prolonged Sitting Time 2 (PS-T2). White space indicates unresponsive biomarkers for the specified condition.
The biomarkers identified in the pooled male and females sample were then inputted into Ingenuity Pathway Analysis (IPA) [42]. Examples of two relevant pathways to sedentary physiology that indicate a clear linkage to vascular and immune responses are presented in Table 2. This table displays the number of cytokines present in each condition between males and females, and the associated pathways.
Table 2. Examples of pathways identified from Ingenuity Pathway Analysis (IPA) in females and males.
| Pathway | Molecules | |||||||
|---|---|---|---|---|---|---|---|---|
| Females | ||||||||
| PS-T1 | PS-T2 | IS-T1 | IS-T2 | |||||
| Biomarkers | Total Number of Biomarkers | Biomarkers | Total Number of Biomarkers | Biomarkers | Total Number of Biomarkers | Biomarkers | Total Number of Biomarkers | |
| Atherosclerosis Signaling | CXCL8 | 1 | CXCL8, TGF-β1, TNF | 3 | CXCL8 | 1 | CSF1, CXCL8, IL-1β, TGF-β1, TNF | 5 |
| Chemokine Signaling | 0 | 0 | 0 | 0 | ||||
| Males | ||||||||
| Atherosclerosis Signaling | CXCL12, CXCL8, IL-1 β | 3 | CSF1, CXCL12, CXCL8, IL-1β, PDGFΒ, TGF-β1 | 6 | CSF1, CXCL12, CXCL8, IL-1β | 4 | CSF1, CXCL12, CXCL8, IL-1β, PDGFΒ, TGF- β1 | 6 |
| Chemokine Signaling | CCL5, CXCL12 | 2 | CXCL12 | 1 | CCL5, CXCL12 | 2 | CCL5, CXCL12 | 2 |
Discussion
We sought to identify novel biomarkers in the saliva that are responsive to acute interventions such as our sedentary protocol. Our primary finding is that saliva provides a rich source of biomarker data that can be used to understand physiological changes in response to acute stressors or interventions. Furthermore, based on pooled male and female samples, it appears that several biomarkers may be responsive to acute interventions, and these response may vary by sex. These findings provide interesting insights into the potential use of salivary biomarkers in understanding acute response to interventions.
Our exploratory study provided us with more robust findings than we anticipated. Specifically, of the 42 targets on the microarray, 26 were detected in the pooled male and female saliva samples, including 18 cytokines and 8 growth factors. As such, we were unable to follow-up with individual sample analysis for each biomarker; however, such an analysis was conducted for IL-8 and is available elsewhere [28]. Furthermore, of the markers detected in the pooled saliva samples, many have also been detected in response to sedentary interventions in blood, such as IL-8, IL-6, IL-10, TNF-α, and RANTES [27,28,43].
Our analysis using STRING v11.5 revealed several functional pathways between the 42 targets, such as regulation of the chronic inflammatory response and negative regulation of the vascular endothelial growth factor signaling pathway. The STRING v11.5 analysis and Figs 1 and 2 allow us to conceptualize how the biomarkers connect and work together to produce measurable immune, endothelial, and vascular responses actions. For example, STRING v11.5 identified a functional pathway between RANTES, TNF-α, IL-10, and IL-4 which builds on previous research that has shown that pro-inflammatory biomarkers TNF-α and RANTES/CCL-5 have higher concentrations with prolonged sedentary time [43–45], and have been linked to chronic diseases, including rheumatoid arthritis, inflammatory bowel disease, psoriasis, and cardiovascular disease [46–49]. Thus, the work conducted in this study lays the foundation for several lines of future inquiry using salivary biomarkers for research aimed at understanding sedentary physiology and/or sex-differences, as well as other fields assessing acute physiological responses to stressors.
Our analysis using IPA also reveals the potential to see sex differences in acute and short duration interventions. For example, IPA analysis identified that the atherosclerosis signaling pathway might be responsive to prolonged and interrupted sitting. Specifically, biomarker detection changed across the 4 conditions in females—PS-T1 (IL-8), PS-T2 (IL-8, TGF-β1, TNF), IS-T1 (IL-8), and IS-T2 (CSF-1, IL-8, IL-1β, TGF-β1, TNF)- and in males—PS-T1 (IL-8, IL-1β, CXCL12), PS-T2 (CSF-1, CXCL12, IL-8, IL-1β, PDGFB, TGF-β1), IS-T1(CSF-1, CXCL12, IL-8, IL-1β), and IS-T2 (CSF-1, CXCL12, IL-8, IL-1β, PDGFB, TGF-β1). This highlights that even in an acute study, saliva may allow us to observe sex-differences in the activation of different pathways in different conditions. The IPA software provides an interesting insight into the potential health impacts of prolonged sitting, as several pathways were noted, however, due to the exploratory nature of the work the direction of change for these pathways cannot be determined. Future work should look to confirm the change and direction of the pathways found in this study.
The heat map (Fig 5) shows the variation between average pixel density of the biomarkers detected in the pooled saliva samples of males and females. Overall, it appears that cytokines and growth factors were responsive to prolonged and interrupted sitting protocols, however sedentary time and sex may not be the only factors influencing salivary biomarkers. In fact, a growing body of physiological research emphasizes the need to look at individual differences when assessing the response to interventions such as exercise [50–53]. Factors such as age, sex, cardiorespiratory fitness, oral health, and more could confound changes in salivary biomarkers. As such, future work analyzing individual saliva samples should address these confounders in their analysis.
To our knowledge, this is the first study to use an antibody array-based approach to investigate a range of salivary biomarkers in the context of acute responses, particularly in the context of sedentary physiology. Previous research in the field has focused on a few well-known cytokines [54], with limited research into growth and endothelial factors. We identified eight growth and endothelial factors as well as eighteen cytokines in the saliva of healthy males and females. Thus, our work provides further support for analysis of saliva, a non-invasive and simple sampling method that can be applied across different areas of study, including sedentary and exercise physiology [55,56]. The use of the bioinformatic tools, STRING v11.5 and IPA [41,42], allowed for the determination of functional relationships between the detected biomarkers and to better understand how these biomarkers may work together to elicit measurable responses. Another strength of the current study is the homogenous sample of young, healthy individuals and the randomized cross-over design.
The major limitation of this study was the lack of individual level analysis of saliva samples. Our intention was to follow up with ELISA on markers detected using the microarray kit; however, far more markers were detected than originally anticipated making it impossible for individual level analysis on all of the markers. Nevertheless, individual level analysis was conducted using IL-8 and shows promise for use of saliva to detect changes in acute response interventions [28]. Other limitations pertaining to saliva analysis and confounders are also important to consider in interpreting the results of the present study. Previous studies have varied in the timing of their biomarker sample collection, typically occurring immediately post-intervention, 2-hours post-intervention, or 24-hours post-intervention [28,57–60]. A salivary sample collection 2-hours or 24-hours post-protocol may have allowed for the detection of other biomarkers or biomarkers to be collected at their peak of activity. Future research should consider having multiple interruption sessions with different exercise intensities for comparison. Future studies should also explore the correlation between serum and saliva samples for individual biomarkers. Regardless of validity however, it is important to note that saliva remains a valid measure [61–63] and has been shown to be an accurate method of sampling [5,6]. Finally, several confounders may influence the acute responses of salivary markers. Although we asked individuals regarding their oral health, prevalent conditions such as gingivitis or blood in the mouth could have tainted our samples. Similarly, salivary flow may have influenced salivary content. The time of collection of the samples may also influence biomarker detection as previous work has shown that cytokine profiles may be influenced by circadian rhythm and that salivary biomarkers may follow a circadian pattern [64–66]. Future work is also needed to investigate whether these factors are influenced by individual differences in addition to sex, age, and cardiovascular fitness.
In conclusion, saliva provides a promising new avenue for future research aimed at understanding acute responses to interventions in humans without the added stress of invasive procedures. Of 42 targets, 26 biomarkers were detected in the pooled saliva samples of young, healthy males and females. Future research is needed to determine how these biomarkers respond at an individual level, and what individual characteristics are associated with responsiveness of these markers.
Supporting information
Table listing the biomarkers and their alternate name(s) investigated using the Abcam Human Cytokine Antibody Array Membranes. Biomarkers are organized by cytokine, chemokine, myokine, and growth, vascular, or endothelial factor.
(TIF)
Average Spot Pixel Density of Cytokines (a) and Growth and Vascular/Endothelial Factors (b) to Prolonged and Interrupted Sitting Sessions at T1 and T2 in females (n = 12) and males (n = 12). Table of average spot pixel density of cytokines (a) and growth and vascular/endothelial factors (b) from both conditions, prolonged sitting (PS) and interrupted sitting (IS), pre-intervention (T1) and post-intervention (T2) for males and females.
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Dr. Dogra received funding for this work by the Natural Sciences and Engineering Research Council in Canada (DDG-2021-00007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lindsay A, Costello JT. Realising the potential of urine and saliva as diagnostic tools in sport and exercise medicine. Sports medicine. 2017;47(1):11–31. doi: 10.1007/s40279-016-0558-1 [DOI] [PubMed] [Google Scholar]
- 2.Slavish DC, Graham-Engeland JE, Smyth JM, Engeland CG. Salivary markers of inflammation in response to acute stress. Brain, Behavior, and Immunity. 2015;44:253–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabo YZ, Slavish DC, Graham-Engeland JE. The effect of acute stress on salivary markers of inflammation: A systematic review and meta-analysis. Brain, behavior, and immunity. 2020;88:887–900. doi: 10.1016/j.bbi.2020.04.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutherfurd-Markwick K, Starck C, Dulson DK, Ali A. Salivary diagnostic markers in males and females during rest and exercise. Journal of the International Society of Sports Nutrition. 2017;14(1):1–8. doi: 10.1186/s12970-017-0185-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufman E, Lamster IB. The diagnostic applications of saliva—a review. Critical Reviews in Oral Biology & Medicine. 2002;13(2):197–212. [DOI] [PubMed] [Google Scholar]
- 6.Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, et al. Current developments in salivary diagnostics. Biomarkers in Medicine. 2010;4(1):171–89. doi: 10.2217/bmm.09.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, et al. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. International Journal of Behavioral Nutrition and Physical Activity. 2017;14(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian adults: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Reports. 2011;22(1):7. [PubMed] [Google Scholar]
- 9.Koster A, Caserotti P, Patel KV, Matthews CE, Berrigan D, Van Domelen DR, et al. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PloS one. 2012;7(6):e37696. doi: 10.1371/journal.pone.0037696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel AV, Maliniak ML, Rees-Punia E, Matthews CE, Gapstur SM. Prolonged leisure time spent sitting in relation to cause-specific mortality in a large US cohort. American Journal of Epidemiology. 2018;187(10):2151–8. doi: 10.1093/aje/kwy125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Archives of Internal Medicine. 2012;172(6):494–500. doi: 10.1001/archinternmed.2011.2174 [DOI] [PubMed] [Google Scholar]
- 12.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults: a systematic review of longitudinal studies, 1996–2011. American Journal of Preventive Medicine. 2011;41(2):207–15. doi: 10.1016/j.amepre.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 13.Proper KI, Singh AS, Van Mechelen W, Chinapaw MJ. Sedentary behaviors and health outcomes among adults: a systematic review of prospective studies. American Journal of Preventive Medicine. 2011;40(2):174–82. doi: 10.1016/j.amepre.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 14.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–67. doi: 10.2337/db07-0882 [DOI] [PubMed] [Google Scholar]
- 15.Chinapaw M, Proper K, Brug J, Van Mechelen W, Singh A. Relationship between young peoples’ sedentary behaviour and biomedical health indicators: a systematic review of prospective studies. Obesity Reviews. 2011;12(7):e621–e32. doi: 10.1111/j.1467-789X.2011.00865.x [DOI] [PubMed] [Google Scholar]
- 16.Foster JA, Gore SA, West DS. Altering TV viewing habits: an unexplored strategy for adult obesity intervention? American Journal of Health Behavior. 2006;30(1):3–14. doi: 10.5555/ajhb.2006.30.1.3 [DOI] [PubMed] [Google Scholar]
- 17.Marshall SJ, Biddle SJ, Gorely T, Cameron N, Murdey I. Relationships between media use, body fatness and physical activity in children and youth: a meta-analysis. International Journal of Obesity. 2004;28(10):1238–46. doi: 10.1038/sj.ijo.0802706 [DOI] [PubMed] [Google Scholar]
- 18.Padilla J, Fadel PJ. Prolonged sitting leg vasculopathy: contributing factors and clinical implications. American Journal of Physiology-Heart and Circulatory Physiology. 2017;313(4):H722–H8. doi: 10.1152/ajpheart.00326.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, et al. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. American Journal of Physiology-Heart and Circulatory Physiology. 2016;310(5):H648–H53. doi: 10.1152/ajpheart.00943.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vranish JR, Young BE, Kaur J, Patik JC, Padilla J, Fadel PJ. Influence of sex on microvascular and macrovascular responses to prolonged sitting. American Journal of Physiology-Heart and Circulatory Physiology. 2017;312(4):H800–H5. doi: 10.1152/ajpheart.00823.2016 [DOI] [PubMed] [Google Scholar]
- 21.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Padilla J. Prior exercise and standing as strategies to circumvent sitting-induced leg endothelial dysfunction. Clinical Science. 2017;131(11):1045–53. doi: 10.1042/CS20170031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falconer C, Cooper A, Walhin J, Thompson D, Page A, Peters T, et al. Sedentary time and markers of inflammation in people with newly diagnosed type 2 diabetes. Nutrition, Metabolism and Cardiovascular Diseases. 2014;24(9):956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yates T, Khunti K, Wilmot EG, Brady E, Webb D, Srinivasan B, et al. Self-reported sitting time and markers of inflammation, insulin resistance, and adiposity. American journal of preventive medicine. 2012;42(1):1–7. doi: 10.1016/j.amepre.2011.09.022 [DOI] [PubMed] [Google Scholar]
- 24.Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro‐and macrovascular dilator function. Experimental Physiology. 2015;100(7):829–38. doi: 10.1113/EP085238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc. 2015;47(4):843–9. doi: 10.1249/MSS.0000000000000479 [DOI] [PubMed] [Google Scholar]
- 26.Widlansky ME, Gokce N, Keaney JF, Vita JA. The clinical implications of endothelial dysfunction. Journal of the American College of Cardiology. 2003;42(7):1149–60. doi: 10.1016/s0735-1097(03)00994-x [DOI] [PubMed] [Google Scholar]
- 27.Dogra S, Wolf M, Jeffrey MP, Foley RC, Logan-Sprenger H, Jones-Taggart H, et al. Disrupting prolonged sitting reduces IL-8 and lower leg swell in active young adults. BMC Sports Science, Medicine and Rehabilitation. 2019;11(1):1–7. doi: 10.1186/s13102-019-0138-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Rourke N, Meens-Miller E, Jeffrey M, Saleem L, Green-Johnson J, Dogra S. Short bouts of walking attenuates the response of IL-8 to prolonged sitting in healthy adults. European Journal of Applied Physiology. 2023:1–11. doi: 10.1007/s00421-023-05153-z [DOI] [PubMed] [Google Scholar]
- 29.Bergens O, Nilsson A, Papaioannou K-G, Kadi F. Sedentary patterns and systemic inflammation: sex-specific links in older adults. Frontiers in Physiology. 2021;12:69. doi: 10.3389/fphys.2021.625950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García JJ, Bote E, Hinchado MD, Ortega E. A single session of intense exercise improves the inflammatory response in healthy sedentary women. Journal of physiology and biochemistry. 2011;67(1):87–94. doi: 10.1007/s13105-010-0052-4 [DOI] [PubMed] [Google Scholar]
- 31.Loh R, Stamatakis E, Folkerts D, Allgrove JE, Moir HJ. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: a systematic review and meta-analysis. Sports Medicine. 2020;50(2):295–330. doi: 10.1007/s40279-019-01183-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tvarijonaviciute A, Martinez-Lozano N, Rios R, de Teruel MCM, Garaulet M, Cerón JJ. Saliva as a non-invasive tool for assessment of metabolic and inflammatory biomarkers in children. Clinical nutrition. 2020;39(8):2471–8. doi: 10.1016/j.clnu.2019.10.034 [DOI] [PubMed] [Google Scholar]
- 33.Pearlmutter P, DeRose G, Samson C, Linehan N, Cen Y, Begdache L, et al. Sweat and saliva cortisol response to stress and nutrition factors. Scientific reports. 2020;10(1):19050. doi: 10.1038/s41598-020-75871-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Experimental biology and medicine. 2010;235(1):111–8. doi: 10.1258/ebm.2009.009186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lammers G, Poelkens F, van Duijnhoven NT, Pardoel EM, Hoenderop JG, Thijssen DH, et al. Expression of genes involved in fatty acid transport and insulin signaling is altered by physical inactivity and exercise training in human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 2012;303(10):E1245–E51. doi: 10.1152/ajpendo.00356.2012 [DOI] [PubMed] [Google Scholar]
- 36.Liu D, Wang R, Grant AR, Zhang J, Gordon PM, Wei Y, et al. Immune adaptation to chronic intense exercise training: new microarray evidence. BMC Genomics. 2017;18(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundberg TR, Fernandez-Gonzalo R, Tesch PA, Rullman E, Gustafsson T. Aerobic exercise augments muscle transcriptome profile of resistance exercise. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2016;310(11):R1279–R87. doi: 10.1152/ajpregu.00035.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hidayat M, Milne T, Cullinan M, Seymour G. Feasibility of the salivary transcriptome as a novel biomarker in determining disease susceptibility. Journal of Periodontal Research. 2018;53(3):369–77. doi: 10.1111/jre.12522 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhou X, St. John M, Wong D. RNA profiling of cell-free saliva using microarray technology. Journal of Dental Research. 2004;83(3):199–203. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Xiao H, Karlan S, Zhou H, Gross J, Elashoff D, et al. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PloS one. 2010;5(12):e15573. doi: 10.1371/journal.pone.0015573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–d13. doi: 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krämer A, Green J, Pollard J, Jr., Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arouca AB, Santaliestra-Pasías AM, Moreno LA, Marcos A, Widhalm K, Molnár D, et al. Diet as a moderator in the association of sedentary behaviors with inflammatory biomarkers among adolescents in the HELENA study. European Journal of Nutrition. 2019;58(5):2051–65. doi: 10.1007/s00394-018-1764-4 [DOI] [PubMed] [Google Scholar]
- 44.Nayak M, Peinhaupt M, Heinemann A, Eekhoff ME, van Mechelen W, Desoye G, et al. Sedentary behavior in obese pregnant women is associated with inflammatory markers and lipid profile but not with glucose metabolism. Cytokine. 2016;88:91–8. doi: 10.1016/j.cyto.2016.08.031 [DOI] [PubMed] [Google Scholar]
- 45.Borel J-C, Roux-Lombard P, Tamisier R, Arnaud C, Monneret D, Arnol N, et al. Endothelial dysfunction and specific inflammation in obesity hypoventilation syndrome. PloS one. 2009;4(8):e6733. doi: 10.1371/journal.pone.0006733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milan-Mattos JC, Anibal FdF, Perseguini NM, Minatel V, Rehder-Santos P, Castro C, et al. Effects of natural aging and gender on pro-inflammatory markers. Brazilian Journal of Medical and Biological Research. 2019;52. doi: 10.1590/1414-431X20198392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flynn MG, Markofski MM, Carrillo AE. Elevated inflammatory status and increased risk of chronic disease in chronological aging: inflamm-aging or inflamm-inactivity? Aging & Disease. 2019;10(1). doi: 10.14336/AD.2018.0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Croft M, Duan W, Choi H, Eun S-Y, Madireddi S, Mehta A. TNF superfamily in inflammatory disease: translating basic insights. Trends in immunology. 2012;33(3):144–52. doi: 10.1016/j.it.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeng Z, Lan T, Wei Y, Wei X. CCL5/CCR5 axis in human diseases and related treatments. Genes & diseases. 2022;9(1):12–27. doi: 10.1016/j.gendis.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouchard C. Genomic predictors of trainability. Experimental physiology. 2012;97(3):347–52. doi: 10.1113/expphysiol.2011.058735 [DOI] [PubMed] [Google Scholar]
- 51.Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Medicine & Science in Sports & Exercise. 2001;33(6):S446–S51. doi: 10.1097/00005768-200106001-00013 [DOI] [PubMed] [Google Scholar]
- 52.Hecksteden A, Kraushaar J, Scharhag-Rosenberger F, Theisen D, Senn S, Meyer T. Individual response to exercise training-a statistical perspective. Journal of applied physiology. 2015;118(12):1450–9. doi: 10.1152/japplphysiol.00714.2014 [DOI] [PubMed] [Google Scholar]
- 53.Sparks LM. Exercise training response heterogeneity: physiological and molecular insights. Diabetologia. 2017;60:2329–36. doi: 10.1007/s00125-017-4461-6 [DOI] [PubMed] [Google Scholar]
- 54.Sahabudeen A, Rao CR, Chandrasekaran B, Pedersen SJ. Dose-response effects of periodic physical activity breaks on the chronic inflammatory risk associated with sedentary behavior in high-and upper-middle income countries: A systematic review and meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2023:102730. [DOI] [PubMed] [Google Scholar]
- 55.Papacosta E, Nassis GP. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. Journal of Science and Medicine in Sport. 2011;14(5):424–34. doi: 10.1016/j.jsams.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 56.Szabo YZ, Slavish DC. Measuring salivary markers of inflammation in health research: A review of methodological considerations and best practices. Psychoneuroendocrinology. 2021;124:105069. doi: 10.1016/j.psyneuen.2020.105069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moldoveanu AI, Shephard RJ, Shek PN. Exercise elevates plasma levels but not gene expression of IL-1β, IL-6, and TNF-α in blood mononuclear cells. Journal of Applied Physiology. 2000;89(4):1499–504. [DOI] [PubMed] [Google Scholar]
- 58.O’Neill C O ’Rourke N, Jeffrey M, Green-Johnson J, Dogra S. Salivary concentrations of IL-8 and IL-1ra after HIIT and MICT in young, healthy adults: A randomized exercise study. Cytokine. 2022;157:155965. [DOI] [PubMed] [Google Scholar]
- 59.Smith L, Anwar A, Fragen M, Rananto C, Johnson R, Holbert D. Cytokines and cell adhesion molecules associated with high-intensity eccentric exercise. European Journal of Applied Physiology. 2000;82:61–7. doi: 10.1007/s004210050652 [DOI] [PubMed] [Google Scholar]
- 60.Suzuki K, Yamada M, Kurakake S, Okamura N, Yamaya K, Liu Q, et al. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. European Journal of Applied Physiology. 2000;81:281–7. doi: 10.1007/s004210050044 [DOI] [PubMed] [Google Scholar]
- 61.Cullen T, Thomas A, Webb R, Hughes MG. The relationship between interleukin-6 in saliva, venous and capillary plasma, at rest and in response to exercise. Cytokine. 2015;71(2):397–400. doi: 10.1016/j.cyto.2014.10.011 [DOI] [PubMed] [Google Scholar]
- 62.Eriksson M, Sartono E, Martins C, Balé C, Garly ML, Whittle H, et al. A comparison of ex vivo cytokine production in venous and capillary blood. Clinical & Experimental Immunology. 2007;150(3):469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williamson S, Munro C, Pickler R, Grap MJ, Elswick R. Comparison of biomarkers in blood and saliva in healthy adults. Nursing Research and Practice. 2012;2012. doi: 10.1155/2012/246178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izawa S, Miki K, Liu X, Ogawa N. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain, behavior, and immunity. 2013;27:38–41. doi: 10.1016/j.bbi.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 65.Reinhardt ÉL, Fernandes PACM, Markus RP, Fischer FM. Night work effects on salivary cytokines TNF, IL-1β and IL-6. Chronobiology international. 2019;36(1):11–26. doi: 10.1080/07420528.2018.1515771 [DOI] [PubMed] [Google Scholar]
- 66.Sarkar A, Kuehl MN, Alman AC, Burkhardt BR. Linking the oral microbiome and salivary cytokine abundance to circadian oscillations. Scientific reports. 2021;11(1):2658. doi: 10.1038/s41598-021-81420-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table listing the biomarkers and their alternate name(s) investigated using the Abcam Human Cytokine Antibody Array Membranes. Biomarkers are organized by cytokine, chemokine, myokine, and growth, vascular, or endothelial factor.
(TIF)
Average Spot Pixel Density of Cytokines (a) and Growth and Vascular/Endothelial Factors (b) to Prolonged and Interrupted Sitting Sessions at T1 and T2 in females (n = 12) and males (n = 12). Table of average spot pixel density of cytokines (a) and growth and vascular/endothelial factors (b) from both conditions, prolonged sitting (PS) and interrupted sitting (IS), pre-intervention (T1) and post-intervention (T2) for males and females.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.