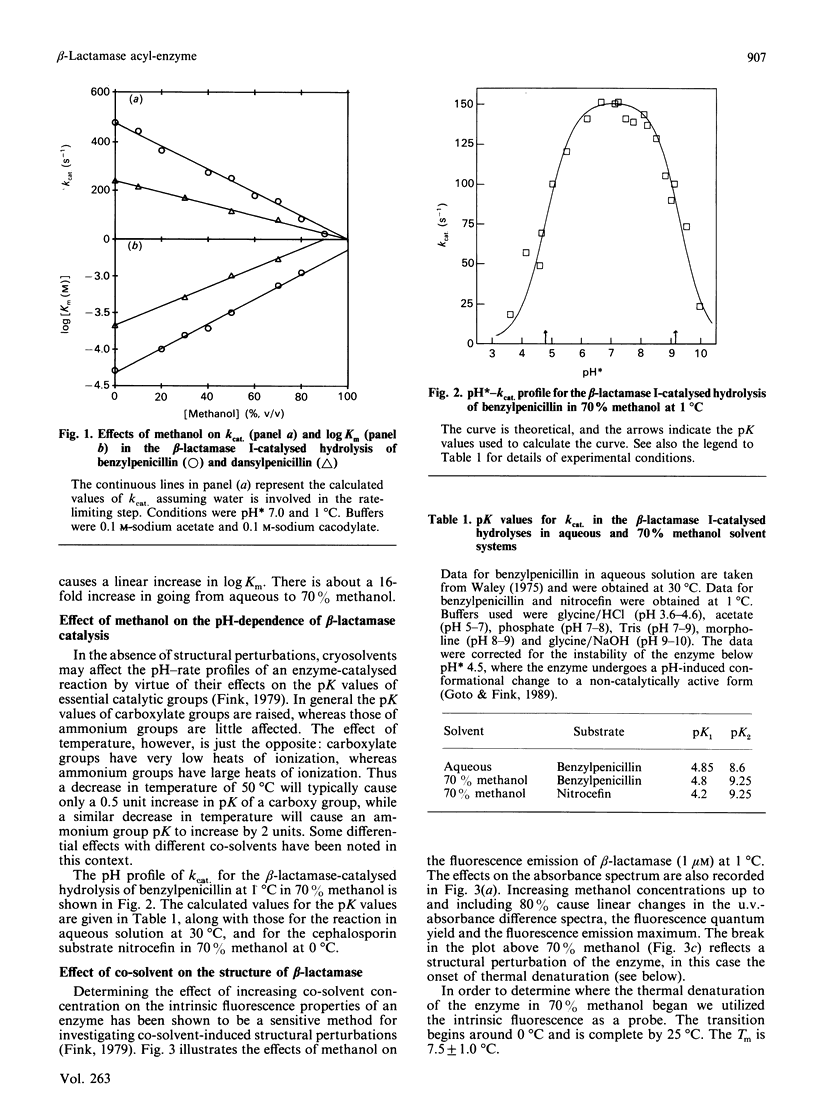
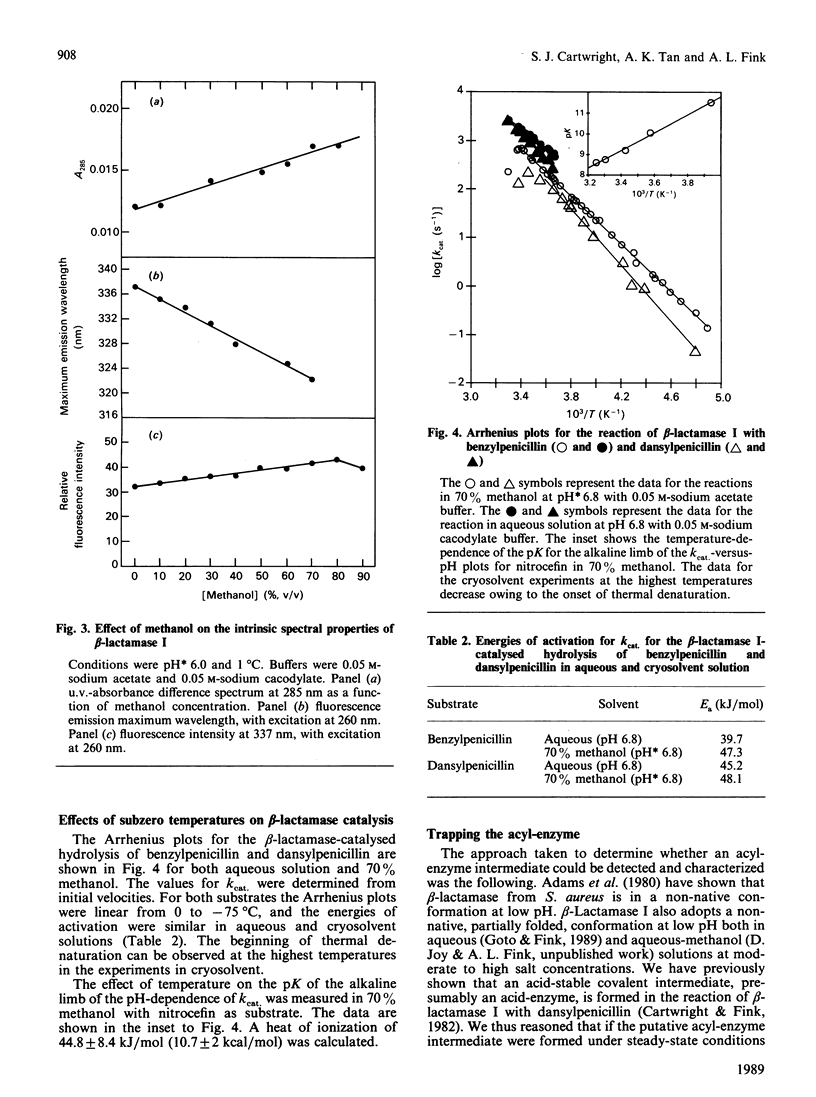
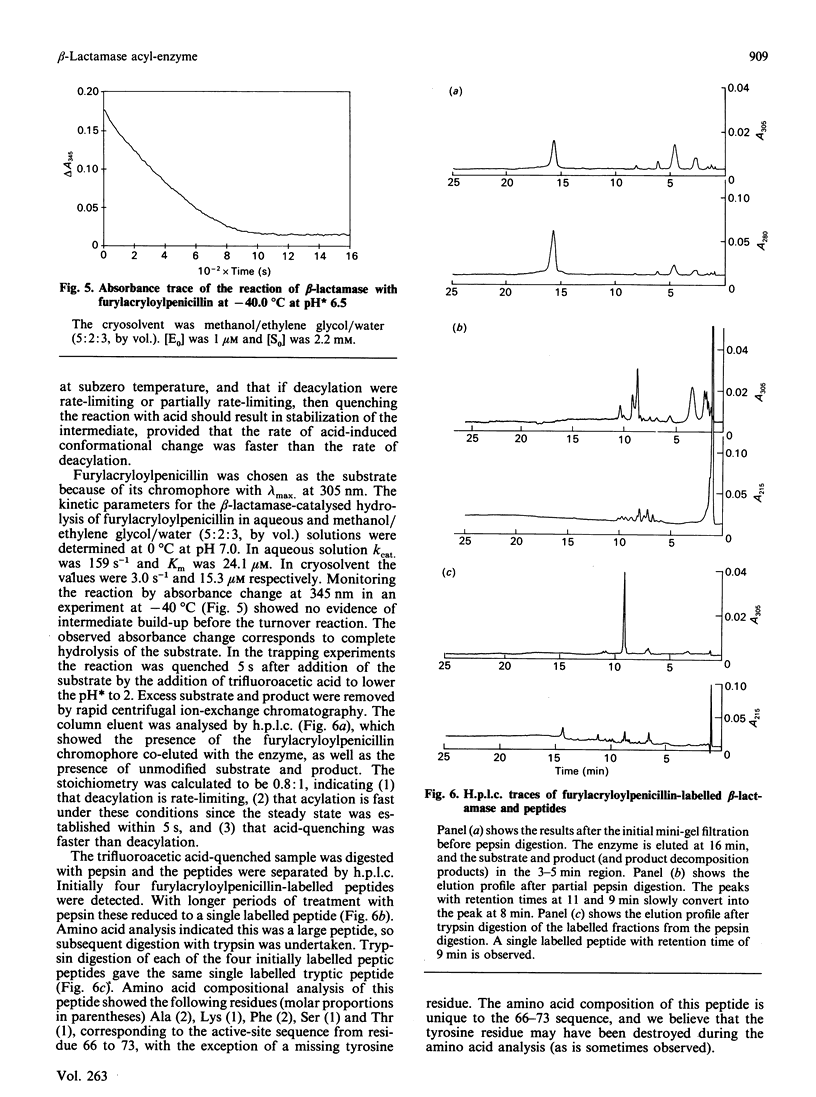
Abstract
Cryoenzymology techniques were used to facilitate trapping an acyl-enzyme intermediate in beta-lactamase I catalysis. The enzyme (from Bacillus cereus) was investigated in aqueous methanol cryosolvents over the 25 to -75 degrees C range, and was stable and functional in 70% (v/v) methanol at and below 0 degree C. The value of kcat. decreased linearly with increasing methanol concentration, suggesting that water is a reactant in the rate-determining step. In view of this, the lack of incorporation of methanol into the product means that the water molecule involved in the deacylation is shielded from bulk solvent in the enzyme-substrate complex. From the lack of adverse effects of methanol on the catalytic and structural properties of the enzyme we conclude that 70% methanol is a satisfactory cryosolvent system for beta-lactamase I. The acyl-enzyme intermediate from the reaction with 6-beta-(furylacryloyl)amidopenicillanic acid was accumulated in steady-state experiments at -40 degrees C and the reaction was quenched by lowering the pH to 2. H.p.l.c. experiments showed covalent attachment of the penicillin to the enzyme. Digestion by pepsin and trypsin yielded a single labelled peptide fragment; analysis of this peptide was consistent with Ser-70 as the site of attachment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. G., Pratt R. F. Pre-steady state beta-lactamase kinetics. Observation of a covalent intermediate during turnover of a fluorescent cephalosporin by the beta-lactamase of STaphylococcus aureus PC1. J Biol Chem. 1981 Nov 25;256(22):11401–11404. [PubMed] [Google Scholar]
- Anderson E. G., Pratt R. F. Pre-steady state beta-lactamase kinetics. The trapping of a covalent intermediate and the interpretation of pH rate profiles. J Biol Chem. 1983 Nov 10;258(21):13120–13126. [PubMed] [Google Scholar]
- Cartwright S. J., Coulson A. F. Active site of staphylococcal beta-lactamase. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):370–372. [PubMed] [Google Scholar]
- Cartwright S. J., Fink A. L. Isolation of a covalent intermediate in beta -lactamase I catalysis. FEBS Lett. 1982 Jan 25;137(2):186–188. doi: 10.1016/0014-5793(82)80345-1. [DOI] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. Cryoenzymology of beta-lactamases. Biochemistry. 1987 Aug 25;26(17):5329–5337. doi: 10.1021/bi00391a017. [DOI] [PubMed] [Google Scholar]
- Clarke A. J., Mezes P. S., Vice S. F., Dmitrienko G. I., Viswanatha T. Inactivation of Bacillus cereus 569/H beta-lactamase I by 6-beta-(trifluoromethane sulfonyl)amidopenicillanic acid sulfone and its N-methyl derivative. Biochim Biophys Acta. 1983 Nov 14;748(3):389–397. doi: 10.1016/0167-4838(83)90184-x. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Pratt R. F. Inactivation of Bacillus cereus beta-lactamase I by 6 beta-bromopencillanic acid: mechanism. Biochemistry. 1980 Aug 19;19(17):3996–4003. doi: 10.1021/bi00558a017. [DOI] [PubMed] [Google Scholar]
- Dalbadie-McFarland G., Cohen L. W., Riggs A. D., Morin C., Itakura K., Richards J. H. Oligonucleotide-directed mutagenesis as a general and powerful method for studies of protein function. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6409–6413. doi: 10.1073/pnas.79.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A. L., Cartwright S. J. Cryoenzymology. CRC Crit Rev Biochem. 1981;11(2):145–207. [PubMed] [Google Scholar]
- Fink A. L., Cartwright S. J. Cryoenzymology. CRC Crit Rev Biochem. 1981;11(2):145–207. [PubMed] [Google Scholar]
- Fink A. L., Geeves M. A. Cryoenzymology: the study of enzyme catalysis at subzero temperatures. Methods Enzymol. 1979;63:336–370. doi: 10.1016/0076-6879(79)63015-x. [DOI] [PubMed] [Google Scholar]
- Fisher J., Belasco J. G., Khosla S., Knowles J. R. beta-Lactamase proceeds via an acyl-enzyme intermediate. Interaction of the Escherichia coli RTEM enzyme with cefoxitin. Biochemistry. 1980 Jun 24;19(13):2895–2901. doi: 10.1021/bi00554a012. [DOI] [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Bradley S. M., Knowles J. R. Inactivation of the RTEM beta-lactamase from Escherichia coli. Interaction of penam sulfones with enzyme. Biochemistry. 1981 May 12;20(10):2726–2731. doi: 10.1021/bi00513a004. [DOI] [PubMed] [Google Scholar]
- Goto Y., Fink A. L. Conformational states of beta-lactamase: molten-globule states at acidic and alkaline pH with high salt. Biochemistry. 1989 Feb 7;28(3):945–952. doi: 10.1021/bi00429a004. [DOI] [PubMed] [Google Scholar]
- Hardy L. W., Kirsch J. F. Diffusion-limited component of reactions catalyzed by Bacillus cereus beta-lactamase I. Biochemistry. 1984 Mar;23(6):1275–1282. doi: 10.1021/bi00301a040. [DOI] [PubMed] [Google Scholar]
- Herzberg O., Moult J. Bacterial resistance to beta-lactam antibiotics: crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.5 A resolution. Science. 1987 May 8;236(4802):694–701. doi: 10.1126/science.3107125. [DOI] [PubMed] [Google Scholar]
- Knott-Hunziker V., Waley S. G., Orlek B. S., Sammes P. G. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett. 1979 Mar 1;99(1):59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- Martin M. T., Waley S. G. Kinetic characterization of the acyl-enzyme mechanism for beta-lactamase I. Biochem J. 1988 Sep 15;254(3):923–925. doi: 10.1042/bj2540923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel P., Hoa G. H., Douzou P. The pH dependence of the hydrolysis of benzoyl-L-arginine ethyl ester in cooled mixed solvents. J Biol Chem. 1975 Feb 25;250(4):1376–1382. [PubMed] [Google Scholar]
- Maurel P. Relevance of dielectric constant and solvent hydrophobicity to the organic solvent effect in enzymology. J Biol Chem. 1978 Mar 10;253(5):1677–1683. [PubMed] [Google Scholar]
- Pratt R. F., McConnell T. S., Murphy S. J. Accumulation of acyl-enzyme intermediates during turnover of penicillins by the class A beta-lactamase of Staphylococcus aureus PC1. Biochem J. 1988 Sep 15;254(3):919–922. doi: 10.1042/bj2540919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Sigal I. S., DeGrado W. F., Thomas B. J., Petteway S. R., Jr Purification and properties of thiol beta-lactamase. A mutant of pBR322 beta-lactamase in which the active site serine has been replaced with cysteine. J Biol Chem. 1984 Apr 25;259(8):5327–5332. [PubMed] [Google Scholar]
- Sigal I. S., Harwood B. G., Arentzen R. Thiol-beta-lactamase: replacement of the active-site serine of RTEM beta-lactamase by a cysteine residue. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7157–7160. doi: 10.1073/pnas.79.23.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. The pH-dependence and group modification of beta-lactamase I. Biochem J. 1975 Sep;149(3):547–551. doi: 10.1042/bj1490547. [DOI] [PMC free article] [PubMed] [Google Scholar]


