Abstract
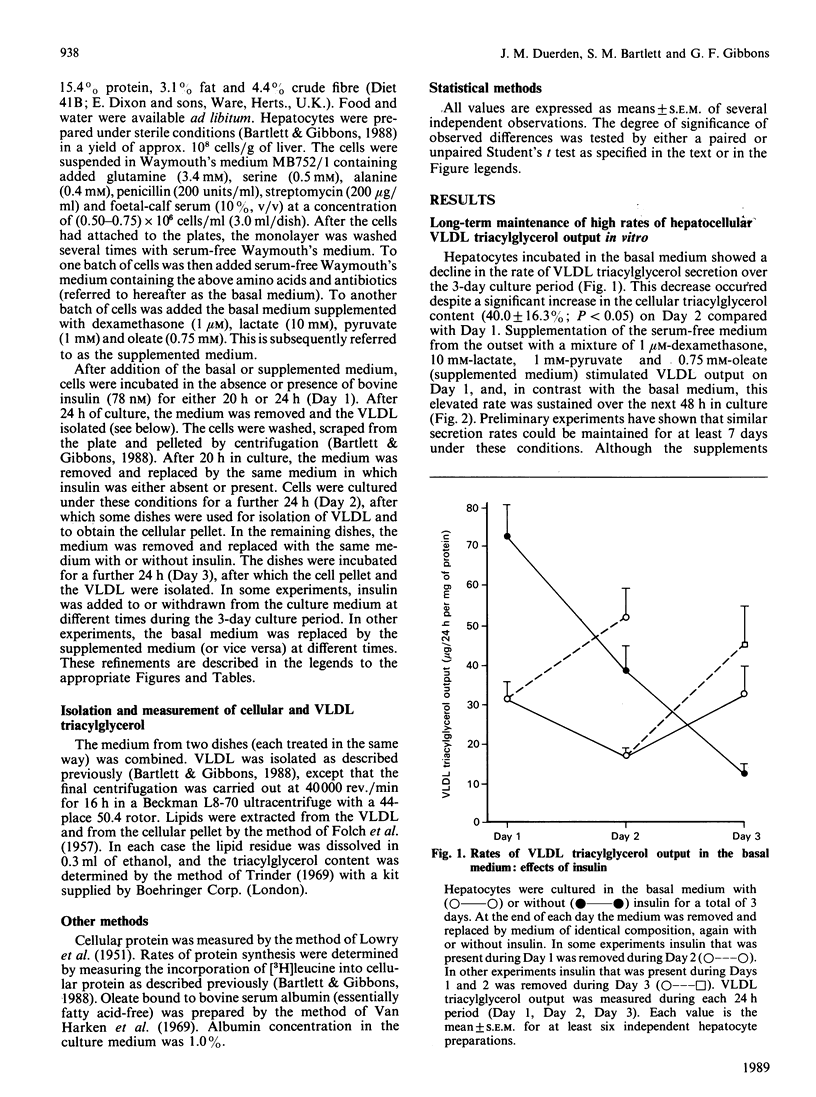
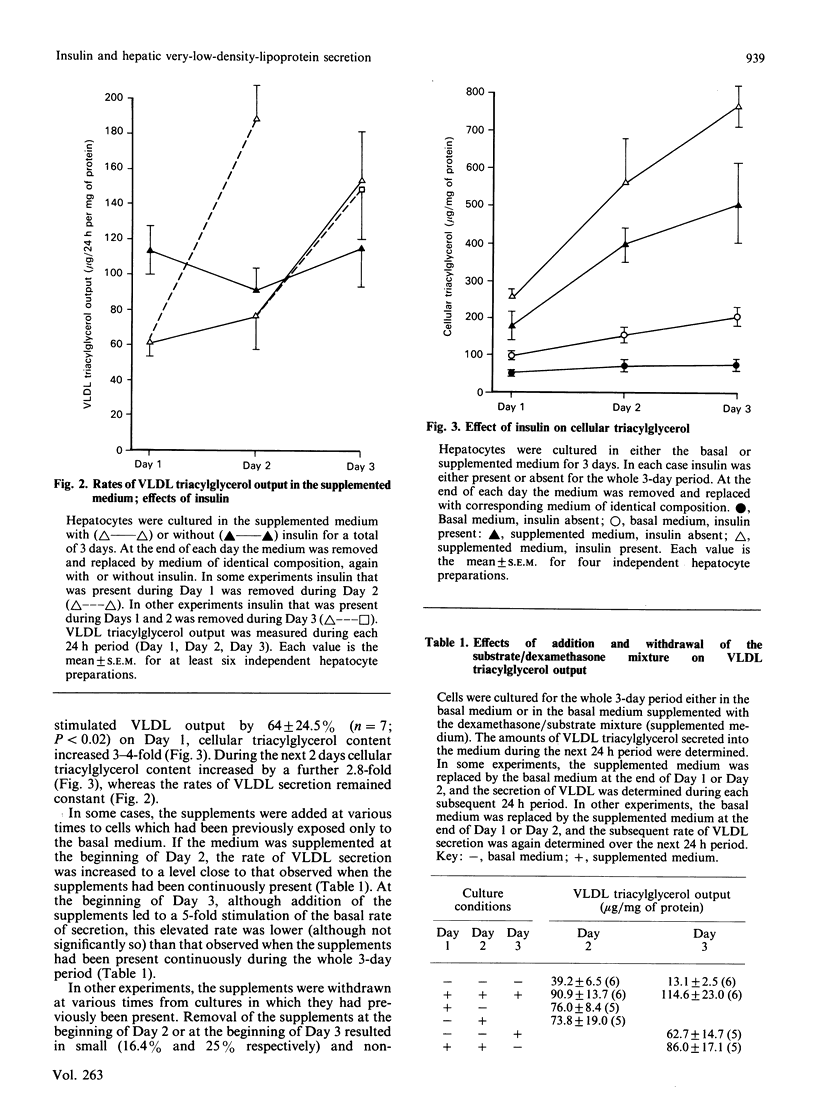
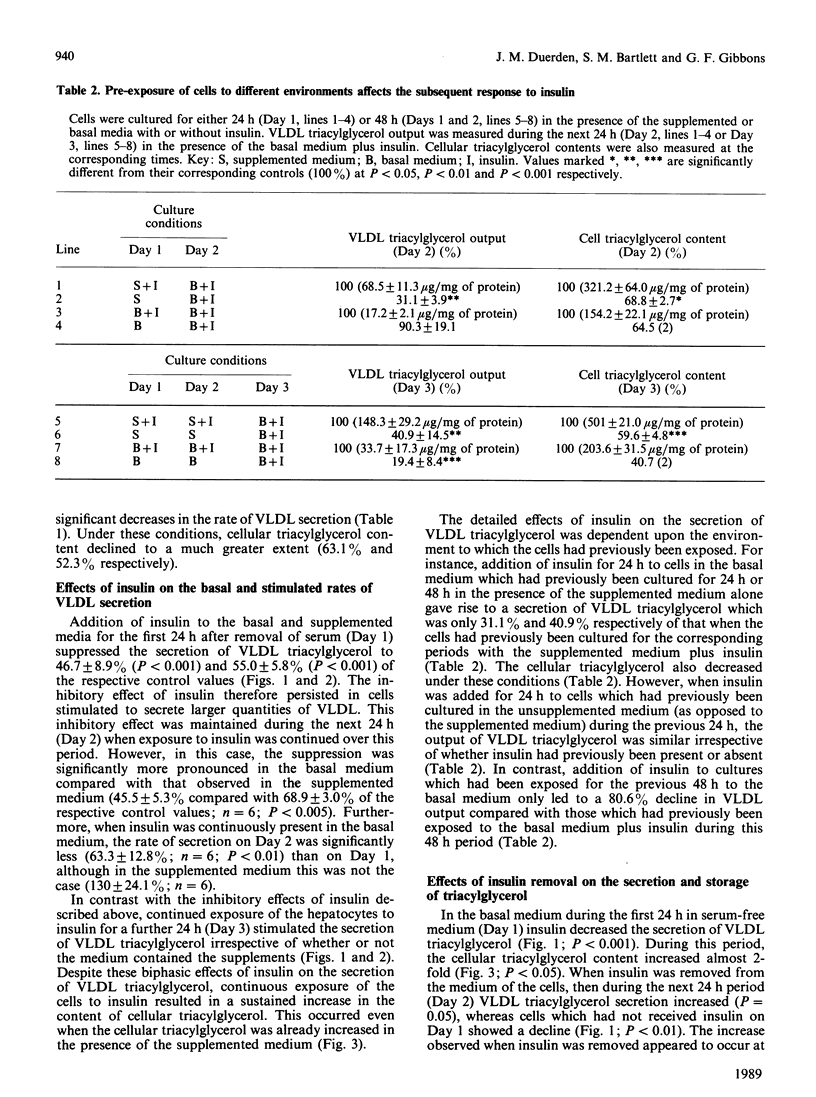
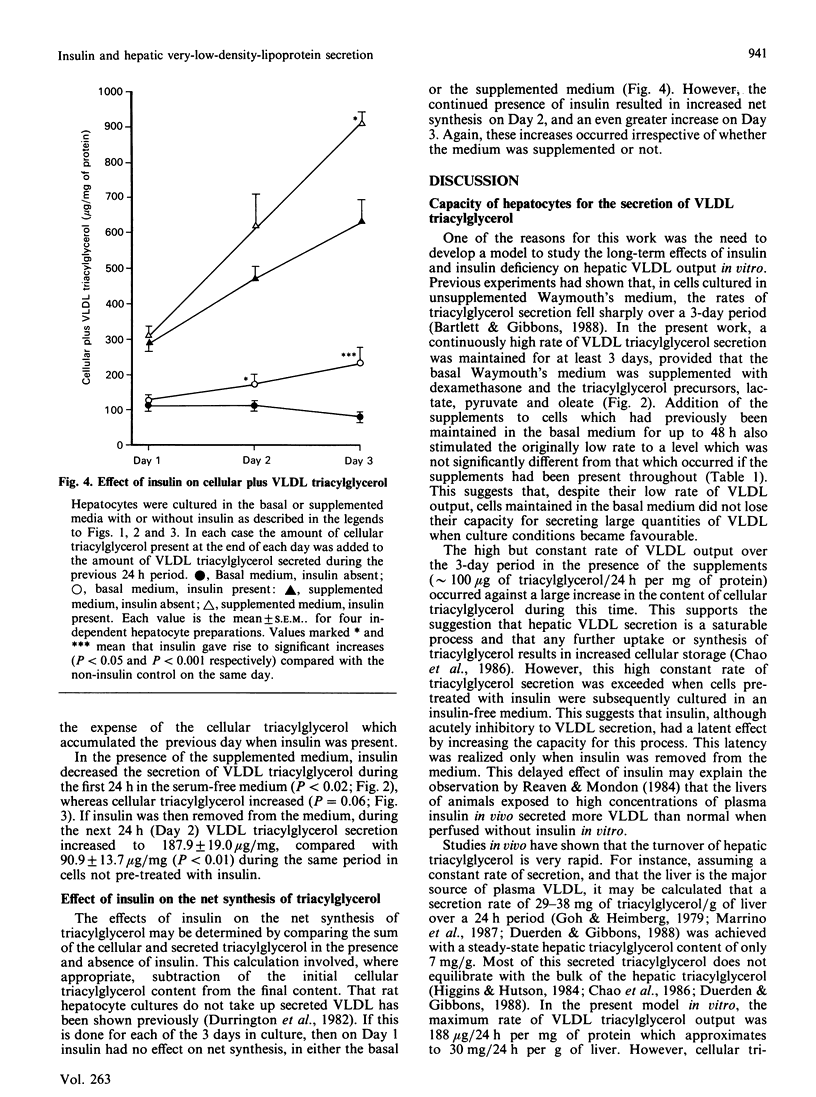
High rates of hepatic cellular triacylglycerol synthesis and very-low-density-lipoprotein (VLDL) triacylglycerol output were maintained in vitro for at least 3 days when hepatocytes were cultured in a medium lacking insulin but supplemented with 1 microM-dexamethasone, 10 mM-lactate, 1 mM-pyruvate and 0.75 mM-oleate (supplemented medium). Under these conditions VLDL output remained constant, whereas cell triacyglycerol content increased 10-fold over 3 days, suggesting that the secretory process was saturated. Insulin, present during the first 24 h period, enhanced the storage of cellular triacylglycerol by inhibiting the secretion of VLDL. This stored triacyglycerol was subsequently released into the medium as VLDL if insulin was removed. With the supplemented medium the increased rate of VLDL secretion after insulin removal exceeded that observed under 'saturating' conditions, suggesting that pre-treatment with insulin enhanced the capacity for VLDL secretion. In contrast with the short-term (24 h) effects of insulin, longer-term exposure (greater than 48 h) to insulin enhanced the secretion of VLDL compared with insulin-untreated cultures. Under these conditions, insulin increased the net rates of triacylglycerol synthesis. The results suggest that insulin affects the secretion of VLDL triacylglycerol by two distinct and opposing mechanisms: first, by direct inhibition of secretion; second by increasing triacylglycerol synthesis, which stimulates secretion. The net effect at any time depends upon the relative importance of each of these processes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcindor L. G., Infante R., Soler-Argilaga C., Polonovski J. Effect of a single insulin administration on the hepatic release of triglycerides into the plasma. Biochim Biophys Acta. 1973 Jun 21;306(3):347–352. doi: 10.1016/0005-2760(73)90173-2. [DOI] [PubMed] [Google Scholar]
- Amatruda J. M., Chang C. L. The regulation of lipid synthesis in primary cultures of hepatocytes from nonketotic streptozotocin diabetic rats. Metabolism. 1983 Mar;32(3):224–229. doi: 10.1016/0026-0495(83)90186-5. [DOI] [PubMed] [Google Scholar]
- Amatruda J. M., Danahy S. A., Chang C. L. The effects of glucocorticoids on insulin-stimulated lipogenesis in primary cultures of rat hepatocytes. Biochem J. 1983 Apr 15;212(1):135–141. doi: 10.1042/bj2120135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasse E. O., Bier D. M., Havel R. J. Early effects of anti-insulin serum on hepatic metabolism of plasma free fatty acids in dogs. Diabetes. 1972 May;21(5):280–288. doi: 10.2337/diab.21.5.280. [DOI] [PubMed] [Google Scholar]
- Bartlett S. M., Gibbons G. F. Short- and longer-term regulation of very-low-density lipoprotein secretion by insulin, dexamethasone and lipogenic substrates in cultured hepatocytes. A biphasic effect of insulin. Biochem J. 1988 Jan 1;249(1):37–43. doi: 10.1042/bj2490037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry E. M., Ziv E., Bar-On H. Lipoprotein secretion by isolated perfused livers from streptozotocin-diabetic rats. Diabetologia. 1981 Oct;21(4):402–408. doi: 10.1007/BF00252689. [DOI] [PubMed] [Google Scholar]
- Chao F. F., Stiers D. L., Ontko J. A. Hepatocellular triglyceride synthesis and transfer to lipid droplets and nascent very low density lipoproteins. J Lipid Res. 1986 Nov;27(11):1174–1181. [PubMed] [Google Scholar]
- Duerden J. M., Gibbons G. F. Secretion and storage of newly synthesized hepatic triacylglycerol fatty acids in vivo in different nutritional states and in diabetes. Biochem J. 1988 Nov 1;255(3):929–935. doi: 10.1042/bj2550929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gibbons G. F. Insulin, diabetes and hepatic very-low-density lipoprotein metabolism. Biochem Soc Trans. 1989 Feb;17(1):49–51. doi: 10.1042/bst0170049. [DOI] [PubMed] [Google Scholar]
- Goh E. H., Heimberg M. Relationship between activity of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase and secretion of very-low-density-lipoprotein cholesterol by the isolated perfused liver and in the intact rat. Biochem J. 1979 Oct 15;184(1):1–6. doi: 10.1042/bj1840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Hutson J. L. The roles of Golgi and endoplasmic reticulum in the synthesis and assembly of lipoprotein lipids in rat hepatocytes. J Lipid Res. 1984 Dec 1;25(12):1295–1305. [PubMed] [Google Scholar]
- Howard B. V. Lipoprotein metabolism in diabetes mellitus. J Lipid Res. 1987 Jun;28(6):613–628. [PubMed] [Google Scholar]
- Kostner G. M., Karádi I. Lipoprotein alterations in diabetes mellitus. Diabetologia. 1988 Oct;31(10):717–722. doi: 10.1007/BF00274772. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laker M. E., Mayes P. A. Investigations into the direct effects of insulin on hepatic ketogenesis, lipoprotein secretion and pyruvate dehydrogenase activity. Biochim Biophys Acta. 1984 Sep 12;795(2):427–430. doi: 10.1016/0005-2760(84)90094-8. [DOI] [PubMed] [Google Scholar]
- Mangiapane E. H., Brindley D. N. Effects of dexamethasone and insulin on the synthesis of triacylglycerols and phosphatidylcholine and the secretion of very-low-density lipoproteins and lysophosphatidylcholine by monolayer cultures of rat hepatocytes. Biochem J. 1986 Jan 1;233(1):151–160. doi: 10.1042/bj2330151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrino P., Gavish D., Shafrir E., Eisenberg S. Diurnal variations of plasma lipids, tissue and plasma lipoprotein lipase, and VLDL secretion rates in the rat. A model for studies of VLDL metabolism. Biochim Biophys Acta. 1987 Aug 15;920(3):277–284. doi: 10.1016/0005-2760(87)90105-6. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Farquhar J. W., Reaven G. M. Reappraisal of the role of insulin in hypertriglyceridemia. Am J Med. 1974 Oct;57(4):551–560. doi: 10.1016/0002-9343(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Gotto A. M., Jr, Patsch J. R. Effects of insulin on lipoprotein secretion in rat hepatocyte cultures. The role of the insulin receptor. J Biol Chem. 1986 Jul 25;261(21):9603–9606. [PubMed] [Google Scholar]
- Pietri A. O., Dunn F. L., Grundy S. M., Raskin P. The effect of continuous subcutaneous insulin infusion on very-low-density lipoprotein triglyceride metabolism in type I diabetes mellitus. Diabetes. 1983 Jan;32(1):75–81. doi: 10.2337/diab.32.1.75. [DOI] [PubMed] [Google Scholar]
- Pullinger C. R., Gibbons G. F. Effects of hormones and pyruvate on the rates of secretion of very-low-density lipoprotein triacylglycerol and cholesterol by rat hepatocytes. Biochim Biophys Acta. 1985 Jan 9;833(1):44–51. doi: 10.1016/0005-2760(85)90251-6. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Greenfield M. S. Diabetic hypertriglyceridemia: evidence for three clinical syndromes. Diabetes. 1981;30(Suppl 2):66–75. doi: 10.2337/diab.30.2.s66. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Mondon C. E. Effect of in vivo plasma insulin levels on the relationship between perfusate free fatty acid concentration and triglyceride secretion by perfused rat livers. Horm Metab Res. 1984 May;16(5):230–232. doi: 10.1055/s-2007-1014753. [DOI] [PubMed] [Google Scholar]
- Sparks J. D., Sparks C. E., Bolognino M., Roncone A. M., Jackson T. K., Amatruda J. M. Effects of nonketotic streptozotocin diabetes on apolipoprotein B synthesis and secretion by primary cultures of rat hepatocytes. J Clin Invest. 1988 Jul;82(1):37–43. doi: 10.1172/JCI113597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. Insulin and non-esterified fatty acids. Acute regulators of lipogenesis in perfused rat liver. Biochem J. 1982 May 15;204(2):433–439. doi: 10.1042/bj2040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L., Storer G. B., Trimble R. P. Effects of flow rate and insulin on triacylglycerol secretion by perfused rat liver. Am J Physiol. 1988 Sep;255(3 Pt 1):E306–E313. doi: 10.1152/ajpendo.1988.255.3.E306. [DOI] [PubMed] [Google Scholar]
- Van Harken D. R., Dixon C. W., Heimberg M. Hepatic lipid metabolism in experimental diabetes. V. The effect of concentration of oleate on metabolism of triglycerides and on ketogenesis. J Biol Chem. 1969 May 10;244(9):2278–2285. [PubMed] [Google Scholar]
- Vogelberg K. H., Gries F. A., Moschinski D. Hepatic production of VLDL-triglycerides. Dependence of portal substrate and insulin concentration. Horm Metab Res. 1980 Dec;12(12):688–694. doi: 10.1055/s-2007-999233. [DOI] [PubMed] [Google Scholar]
- Weiland D., Mondon C. E., Reaven G. M. Evidence for multiple causality in the development of diabetic hypertriglyceridaemia. Diabetologia. 1980 Apr;18(4):335–340. doi: 10.1007/BF00251016. [DOI] [PubMed] [Google Scholar]
- Woodside W. F., Heimberg M. Effects of anti-insulin serum, insulin, and glucose on output of triglycerides and on ketogenesis by the perfused rat liver. J Biol Chem. 1976 Jan 10;251(1):13–23. [PubMed] [Google Scholar]


