Abstract
In αβ T cells, immunosurveillance is enabled by the αβ T cell receptor (TCR), which co-recognises peptide, lipid or small molecule antigens presented by MHC and MHC-I like antigen-presenting molecules, respectively. Although αβ TCRs vary in their antigen recognition modes, in general they co-recognise the presented antigen and the antigen presenting molecule and do so in an invariable ‘end-to-end’ manner. Quite distinctly, γδ T cells – by way of their γδ TCR - can recognise ligands that extend beyond the confines of MHC and MHC-I like restrictions. From structural studies, it is now becoming apparent that γδ TCR recognition modes can break the co-recognition paradigm and deviate markedly from the ‘end-to-end’ docking mechanisms of αβ TCR counterparts. This minireview highlights the emerging portrait of how γδ TCRs can recognise diverse epitopes of their antigens in a manner reminiscent to how antibodies recognise antigens.
Keywords: γδ T cells, γδ T cell receptor, MR1, CD1, MHC-I like
Introduction
The adaptive immune system is often simplified to a bipartite system comprising of B cells and αβ T cells. As a part of the humoral arm of adaptive immunity, B cells mediate the recognition of intact pathogen or damage-derived antigens via the production of soluble antibodies. In contrast, as drivers of the cell-mediated arm of adaptive immunity, αβ T cells typically recognise short peptides presented by MHC class I and II molecules through an αβ T cell receptor (TCR) complex. In addition, unconventional αβ T cell populations can recognise lipid or other small molecule antigens presented on MHC I-like molecules, namely CD1 and MR1, respectively (1, 2). Thus, γδ T cells can recognise a diverse repertoire of antigens when presented by a suitable antigen-presenting MHC or MHC I-like molecule and do so with high specificity to elicit protective immune responses. Many of these B cell and αβ T cell interactions with antigens have been comprehensively characterised, including structural biology studies that revealed the molecular, and even atomic, details of antigen recognition and thus, cellular activation.
The adaptive immune system in fact includes a third lymphocyte lineage, yielding a tri-cellular arsenal of B cells, αβ, and γδ T cells that has persisted over 450 million years of jawed vertebrate evolution (3). It has become increasingly evident that γδ T cells have a central role in immunology with expanding evidence for homeostatic pathogen, cancer, and tissue repair (3). Indeed, an expanding literature implicates peripheral blood γδ T cell populations in pathogen-driven clonal expansion and potent protection in tuberculosis, malaria and cancers (4–10). Furthermore, γδ T cells are implicated in cancer-induced stress surveillance (11) although it is currently contested whether the tumour infiltration capacity of γδ T cells correlate with anti or protumour outcomes in various malignancies and solid tumours (10, 12–14). Such studies have contributed to a burgeoning field of study investigating γδ T cells as novel cellular immunotherapies. This immunotherapeutic development proceeds despite a lack of clarity around γδ T cell activation mechanisms, and the role of the γδ TCR in directing γδ T cell biology.
While the γδ TCR can recognise antigens presented on MHC and MHC I-like molecules, the γδ TCR is not restricted to MHC presentation like its αβ TCR counterpart. Indeed, the repertoire of known γδ TCR ligands is incredibly diverse and ever expanding as the literature characterising this enigmatic complex broadens. Several isolated γδ T cell populations have been characterised from human peripheral blood mononuclear cells (PBMCs), with reactivity to unconventional T cell antigens beyond the confines of the MHC architecture. These antigens exhibit diverse protein architectures such as phycoerythrin (15), Ephrin type-A receptor 2 (16), Annexin A2 (17), histidyl tRNA synthetase (18), ATP synthase, and apolipoprotein A-1 (apoA-I) (19). Other peripheral γδ T cell populations exhibit reactivity to stress-induced phosphoantigens presented by butyrophilin (BTN) proteins (4, 20–23). γδ TCRs have also demonstrated the ability to recognise self-ligands in barrier tissues such as the mucosal lining of the gut as well as the peripheral blood as means of stress surveillance and maintenance of homeostasis. In the blood, stress inducible markers such as endothelial protein C receptor (EPCR) (24) or lipids presented by CD1 molecules were found to be antigens for select γδ T cell populations (25–28).
Tissue-homing γδ T cells locate to epithelial tissues (skin, lungs, and gut) where they mediate homoeostasis, immune surveillance and protection (29–33). Intriguingly, tissue-homing γδ T cells express similar TCRs suggesting tissue-specific functions. Enteric γδ T cells are an intra-epithelial lymphocyte subset known to bind BTN-like (BTNL) proteins, with roles in homeostasis and enteropathies including celiac disease, Ulcerative colitis, Crohn’s disease and colorectal cancer (34–43).
Thus, to better inform therapeutic approaches, a comprehensive understanding of structural paradigms underpinning γδ TCR interactions with cognate ligands is needed. Presently there have only been seven examples of γδ TCR-ligand complex structures to date, six of which are human (25, 27, 28, 44–47). Here we review recent progress in this nascent field and make comparisons to αβ T cell receptors.
Structures of αβ TCR complexed to conventional and unconventional ligands
Thanks to a concerted global effort over recent decades, a multitude of αβ TCR structures have been determined in complex with peptides bound to MHC allomorphs, reviewed in (48). This catalogue of αβ TCR-pMHC structures has invariably shown a conserved end-to-end docking mode whereupon αβ TCRs sit atop the MHC antigen presenting platform. This end-to-end docking mode allows for the hypervariable complementarity determining region (CDR) loops of the αβ TCR to co-contact the presented peptide and the MHC molecule, termed co-recognition (49). Within this conserved ‘end-to-end’ TCR-pMHC binding, the αβ TCR has, with notable exceptions (50–52), demonstrated a canonical docking topology that typically transcends differences in affinity, MHC molecules, and presented antigens. Namely, αβTCR recognition of MHC I adopts a well-established ‘diagonal’ docking modality in which Vα contacts the α2 helix of MHC I and the Vβ is positioned over the α1 helix (48, 53). Here, the CDR1 and CDR2 loops contact the α helices, leaving the hypervariable CDR3 loop to predominantly recognise the presented peptide. αβ TCR recognition of pMHC II is similar to that of MHC I, where overall docking topology remains largely consistent across both MHC classes whereby the Vα contacts the β chain ɑ helix and the Vβ contacts the ɑ chain ɑ helix (48).
The αβ TCR can also recognise unconventional MHC I-like ligands such as MR1 that present small molecule metabolites, and those of the CD1 family that present lipids (1). These αβ TCR-ligand-MHC-I like ternary complexes can markedly differ from TCR-pMHC complexes. For example, in the case of MR1, small metabolites such as those generated from vitamin B metabolism are encased in its antigen binding cleft (2). Due to the size of these metabolites, they are often less accessible the TCR to during recognition. Further, characterisation of Natural Killer T cell TCRs revealed a parallel docking mode over the distal end of the antigen presentation cleft of CD1d (54). Autoreactive TCRs from CD1-reactive T cells can bind CD1a and CD1c without co-contacting the lipid, thereby breaking the co-recognition paradigm (55, 56). Despite variability in orientation and docking angle, the αβ TCR invariably sits atop these MHC I-like molecules in a highly conserved ‘end-to-end’ manner.
First insights into the γδ TCR
The first insights into how the γδ TCR endows this cell lineage with unique antigen recognition principles was garnered from early work characterising unbound receptor structures. The first such study determined the structure of a single variable (V) δ domain of the human ES204 (Vγ1Vδ3) TCR, an HLA-A2 reactive clone isolated from peripheral blood (57, 58). This was followed by a structure of the human G115 (Vγ9Vδ2) TCR isolated from peripheral blood with reactivity to phosphoantigen stimulation (20, 59). These structures revealed that the γδ TCR variable domains formed a typical IgV fold comprised of two β-sheets of antiparallel β-strands that expose the CDR loops for antigen engagement, analogous to αβ TCRs and immunoglobulins (Ig). The ES204 TCR structure provided the first structural evidence for the antibody-like capacity of the γδ TCR, as analysis of the Vδ3 structure revealed close structural similarity to the VH domain of human IgM, despite sequence similarity to the Vα domain (57). Here, the close structural alignments between antibodies and the ES204 Vδ domain were theorised to stem from fundamental similarities in the framework region (57). Namely, the ES204 Vδ3 structure revealed that the C” β-strand that follows the CDR2 loop is three-residues longer in Vδ domains than in the Vα domain, and more comparable to those observed in the VH or VL domains of antibodies. The Vδ2 domain of the G115 TCR exhibited a shorter C” β-strand due a shortened CDR2δ loop, yet the carbon backbone of this framework region remained similar to an antibody.(59). This shortening of the CDR2δ loop in compensation for shorter β-strands is observed in the VL domain of antibodies (57).
In both the ES204 TCR and G115 TCR, the CDR loops display a unique surface with vestiges of both αβ TCRs and antibodies. Namely, the CDR1δ loop positioning is nearly identically to the Vα domain in both the ES204 TCR and the G115 TCR. Conversely, the CDR2δ loop most closely resembles antibody CDR2 loops as a consequence of the shared pairing of the C’ β-strand prior to the CDR2δ loop and the C” β-strand subsequent to the CDR2 loop, however similarity to antibodies manifests differently between the ES204 TCR and the G115 TCR (57, 59). Both CDR2δ loops are positioned at an angle congruent with antibodies and in the ES204 TCR, the CDR2δ loop is more aligned with the VH domain than that of the Vɑ domain (57). Meanwhile, in the G115 TCR, the CDR2δ is positioned more similarly to the VL domain as they share a shortened CDR2 loop (59). The two γδ TCR structures differ markedly when comparing their CDR3δ loop structure. In the ES204 TCR, the 10 amino acid (aa) long CDR3δ loop is positioned more in line with the Vα domain whist the 15 aa long CDR3δ loop in the G115 TCR is similar to the VH domain, suggesting that CDR3δ loop may exist on a spectrum in degree of similarity to either the Vα or VH domains depending on loop length (57, 59). Broad analysis of an array of γδ TCRs shows that the range of CDR3δ loop length (8–21 aa) is more similar to long CDR3 loop lengths of the VH domains (3–25 aa) of antibodies rather than αβ TCRs (6–12 aa) (60). This large range leads to greater protrusions when the CDR3δ loop is longer, and this is more reminiscent of antibodies instead of the relatively flatter surface of the αβ TCR. Together these structures showed for the first time that the γδ TCR displays elements of both αβ TCR and antibody structures, leading to many questions and speculation about how these receptors would bind antigens, a question that remained hotly contested until further structural studies emerged.
Peculiarities in γδ TCR interactions
Despite a diverse breadth of potential cognate antigens, current γδ TCR-ligand complexes have focused upon recognition of MHC I-like molecules via unprecedented mechanisms. Superposition of the γδ TCR-ligand complex structures solved to date immediately reveals the extreme diversity of docking interactions with MHC I-like molecules (Figure 1A). Below, we will summarise the key aspects of each individual complex and identify unifying principles underpinning these interactions.
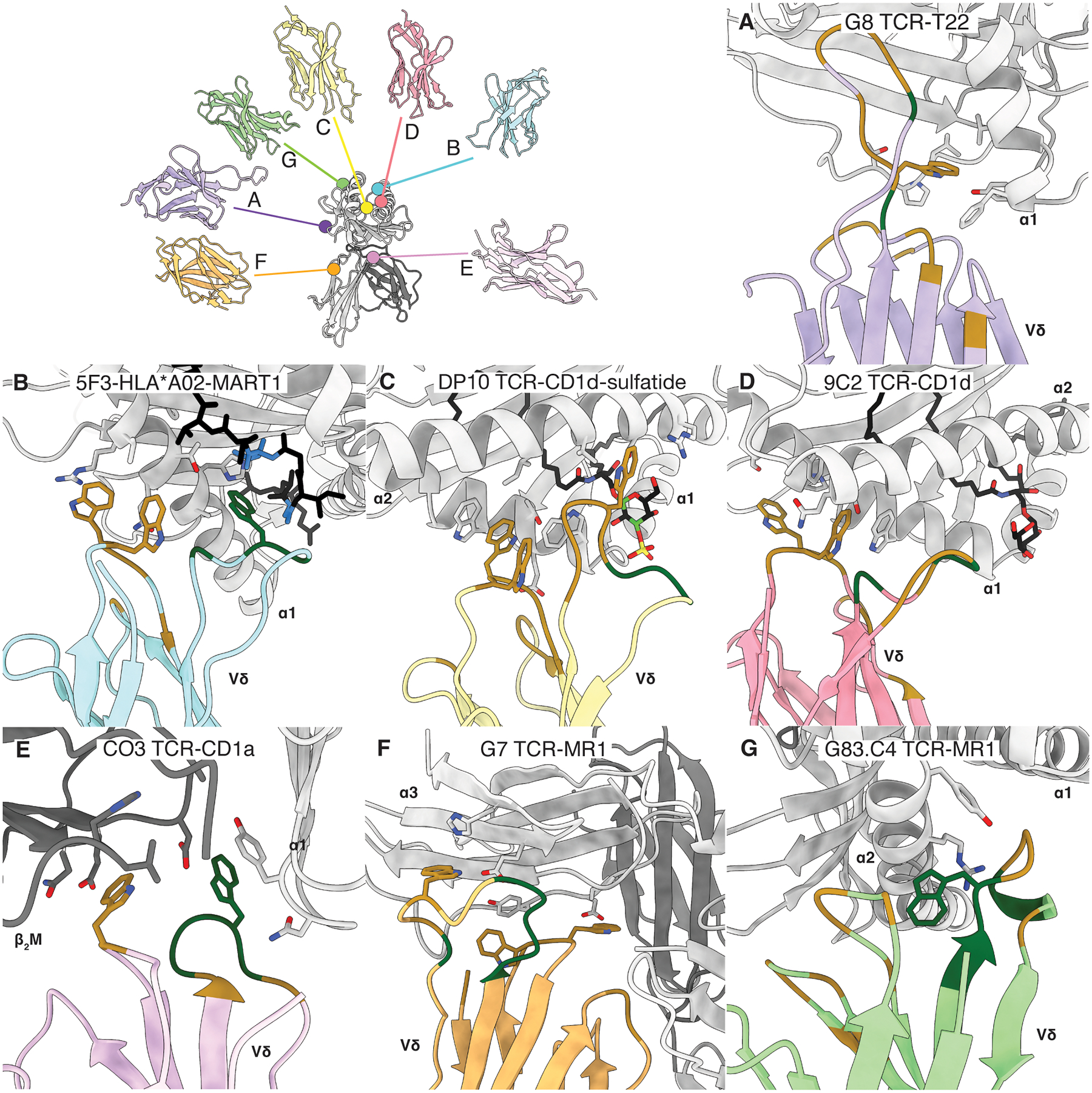
Figure 1. Docking topology of γδ TCRs upon MHC-like molecules.

A. Expanded alignment of γδ TCR complex structures upon an MHC I-like molecule. All molecules are represented as a surface with the γ-chain of each TCR in a lighter shade and the δ-chain in a darker shade of the orange (G7 TCR), purple (G8 TCR), green (G83.C4 TCR), yellow (DP10 TCR), red (9C2 TCR), blue (5F3 TCR), and pink (CO3 TCR). Dots and projections symbolise the approximate centre of the interface. B-H) Contribution of Vγ and Vδ towards BSA at the interface of the TCR and MHC-like molecule. Complexes are initially displayed in cartoon form within a silhouette of the surface of the complex. The BSA of the MHC-like molecule corresponding to each chain of the γδ TCR is highlighted in their respective colours with the Vγ chain being depicted in the lighter shade. B. Murine G8 TCR in complex with T22. PDB: 1YPZ. C. 5F3 TCR in complex with HLA-A*02 presenting MART-1 peptide. PDB: 6D7G. D. DP10 TCR in complex with CD1d presenting sulfatide. PDB: 4MNG. E. 9C2 TCR in complex with CD1d presenting αGalCer. PDB: 4LHU. F. CO3 TCR in complex with CD1a presenting sulfatide. PDB: 7RYN. G. G7 TCR in complex with MR1 presenting 5-OP-RU. PDB: 6MWR. H. G83.C4 in complex with MR1 presenting 5-OP-RU. PDB: 7LLI. Molecular graphics and analyses were performed with UCSF ChimeraX, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, USA.
Murine γδ TCR – T22
The first γδ TCR complex structure solved was that of murine G8 (Vγ4Vδ10) TCR recognising murine non-classical MHC-I like molecule, T22 (Figure 1B) (44). Approximately 0.4% of murine splenic γδ T cells react to T22 and its close relative T10 (61). As T22 is unable to present antigens due to a partially collapsed antigen presenting platform, the G8 TCR bound the T22 molecule itself at ~ 67 nM, a remarkably strong affinity for a TCR that is more typical for antibodies (62). The G8 TCR docked onto T22 in a manner that deviated from the canonical docking mode observed in αβ TCR-pMHC complexes. Namely, the γδ TCR binds at an almost a sideways angle, distal from what would be the antigen binding cleft of T22. The interface is dominated by the δ-chain which contributes 89% of buried surface area (BSA) with a minor contribution of 11% from the CDR3γ loop (Table 1). This contrasts with αβ TCR-pMHC complexes which typically display a more balanced contribution from the two chains and the six CDR loops encoded within, reviewed in (48). For the G8 TCR, the 12 aa long CDR3δ loop contributes ~70% of the BSA as it protrudes into the cavity created by T22’s ɑ1 helix and the underlying β-sheet (Figure 2A) (44). Interacting residues or contacts were spread across germline-encoded (conserved across generations) and non-germline residues that resulted from recombination. Specifically, the germline-encoded hydrophobic tryptophan (Trp) residue Trp93 was shown to be a driver of reactivity, with alanine mutagenesis of this residue implied that absence of this residue and other hydrophobic residues had significant impact on TCR binding (62). Intriguingly, the hydrophobic residues driving this reactivity were shared across all T22 reactive γδ TCRs (63), including the murine KN6 (Vγ4Vδ5) TCR which had a non-germline encoded Trp within the CDR3δ loop that was implicated via alanine mutagenesis as a driver of T22 binding (62). This mode of recognition was shown to mimic a single-chain shark antibody which utilised a homologous CDR3 loop to bind a similar cavity within lysozyme (62). The G8 TCR-T22 complex provided a prefatory glimpse into the unique rules of γδ TCR-ligand binding, suggesting a prominent role for apical CDR3δ Tryptophan’s that were common across the T22-reactive γδ T cells isolated.
Table 1:
Buried surface areas of all known γδ TCR-ligand complex structures
| Complex | KD | BSA (Å2) | BSA (%) | BSA (%) | sc |
|---|---|---|---|---|---|
| G8 TCR-T22 (Vγ4Vδ10) |
67 nM | T22: 930 | |||
| TCR: 940 | γ: 11 | CDR1γ: 0 | 0.66 | ||
| CDR2γ: 0 | |||||
| CDR3γ: 11 | |||||
| δ: 89 | CDR1δ: 12 | ||||
| CDR2δ: 2 | |||||
| CDR3δ: 71 | |||||
| DP10 TCR-CD1d-sulfatide (Vγ4Vδ1) |
5.6 μM | CD1d: 590 | |||
| TCR: 720 | γ: 0 | 0.82 | |||
| δ: 100 | CDR1δ: 32 | ||||
| CDR2δ: 1 | |||||
| CDR3δ: 57 | |||||
| 9C2 TCR-CD1d-αGalCer (Vγ5Vδ1) |
16 μM | CD1d: 930 | |||
| TCR: 900 | γ: 25 | CDR1γ: 4 | 0.75 | ||
| CDR2γ: 0 | |||||
| CDR3γ: 18 | |||||
| δ: 75 | CDR1δ: 40 | ||||
| CDR2δ: 0 | |||||
| CDR3δ: 28 | |||||
| G7 TCR-MR1–5-OP-RU (Vγ9Vδ1) |
4.7 μM | MR1: 1030 | |||
| TCR: 1090 | γ: 16 | CDR1γ: 0 | 0.56 | ||
| CDR2γ: 0 | |||||
| CDR3γ: 0.1 | |||||
| δ: 84 | CDR1δ: 22 | ||||
| CDR2δ: 0 | |||||
| CDR3δ: 42 | |||||
| 5F3 TCR-HLA-A*02-MART1 (Vγ8Vδ1) |
2.9 μM | MHC: 990 | |||
| TCR: 1000 | γ: 41 | CDR1γ: 7 | 0.71 | ||
| CDR2γ: 22 | |||||
| CDR3γ: 10 | |||||
| δ: 59 | CDR1δ: 22 | ||||
| CDR2δ: 0 | |||||
| CDR3δ: 32 | |||||
| G83.C4 TCR-MR1–5-OP-RU (Vγ5Vδ3) |
0.57 μM | MR1: 1000 | |||
| TCR: 970 | γ: 29 | CDR1γ: 3 | 0.62 | ||
| CDR2γ: 11 | |||||
| CDR3γ: 11 | |||||
| δ: 71 | CDR1δ: 17 | ||||
| CDR2δ: 19 | |||||
| CDR3δ: 37 | |||||
| CO3 TCR-CD1a-sulfatide (Vγ4Vδ1) |
22 μM | CD1a: 550 | |||
| β2M: 210 | |||||
| TCR: 730 | γ: 35 | CDR1γ: 1 | 0.65 | ||
| CDR2γ: 6 | |||||
| CDR3γ: 5 | |||||
| δ: 65 | CDR1δ: 20 | ||||
| CDR2δ: 0 | |||||
| CDR3δ: 44 |
Figure 2: Key tryptophan residues in the germline and non-germline encoded regions of γδ TCR paratopes.
Cartoon representation of the interfacial tryptophan residues from the TCR δ-chain, with germline and non-germline residues coloured brown and dark green, respectively for the following: A. G8 TCR in complex with murine T22. PDB: 1YPZ. B. 5F3 TCR in complex with HLA-A*02 presenting MART-1 peptide. PDB: 6D7G. C. DP10 TCR in complex with CD1d presenting sulfatide. PDB: 4MNG. D. 9C2 TCR in complex with CD1d presenting αGalCer. PDB: 4LHU. E. CO3 TCR in complex with CD1a presenting sulfatide. PDB: 7RYN. F. G7 TCR in complex with MR1 presenting 5-OP-RU. PDB: 6MWR. G. G83.C4 in complex with MR1 presenting 5-OP-RU. PDB: 7LLI. Contact residues on the MHC I-like molecule are also displayed. Molecular graphics and analyses were performed with UCSF ChimeraX, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, USA.
γδ TCR-pMHC-I
Despite sometimes being overlooked as bona fide ligands, multiple examples of MHC I and MHC II reactive γδ TCRs have been identified, including several human γδ TCR clones reactive to various MHC molecules such as HLA-B*07 (64), HLA-A*24 (65) and HLA-A*02 (antigen for ES204 TCR) (58). Currently, there is one structure of a γδ TCR interacting with a classical MHC I molecule, namely the 5F3 (Vγ8Vδ1) TCR binding HLA-A*02 presenting melanoma associated antigen, MART-1 peptide (Figure 1C) (45). 5F3 TCR was isolated from human hematopoietic stem and progenitor cells and bound HLA-A*02-MART1 at relatively strong affinity for physiological TCRs at ~2.9 μM (45, 66). The overall docking topology of this interaction was highly reminiscent of canonical αβ TCR-pMHC-I interactions as the Vγ and Vδ co-recognises HLA-A*02 and MART-1 peptide in an ‘end-to-end’ manner. However, there are some distinctions. As the δ and α chain are homologs, and the Vδ1 domain sits atop the α1 helix and the Vγ8 domain atop the ɑ2 helix, this is considered a 180° flip of what is typical with αβ TCRs (45, 48). Here, there is also a δ-chain bias as it is responsible for ~59% of the interface (Table 1). All loops, with the exception of CDR2δ loop are involved in binding HLA-A*02-MART1 to varying degrees. Germline CDR1δ loop residues Trp29 and Trp30, in particular, contribute predominantly by forming extensive van der Waals interactions with the α1 helix on the antigen binding platform of HLA-A*02 (Figure 2B). However, only the CDR3δ and CDR1γ loops are directly in contact with the MART-1 peptide with non-germline encoded Trp98 of the CDR3δ loop being the major contributor (Figure 2A), forming more van der Waals interactions cross the peptide with Ala3, Gly4, and Ile7 (45). Conversely, the CDR2γ loop is responsible for most of the contacts to HLA-A*02 with positively charged Arg56 underpinning a network of hydrogen bonds, van der Waals interactions, and a salt bridge with Glu154 on the α1 helix of the MHC I molecule. The 5F3 TCR-HLA-A*02-MART1 structure revealed a recurrence of both a δ-chain bias and Trp residues acting as major contacts, the latter may have implications for γδ TCR recognition broadly, as the Trptophans were germline and thus conserved across all Vδ1+ TCRs.
γδ TCR-CD1-lipid
Several populations of human γδ T cell populations isolated from peripheral blood and the intestinal epithelium have exhibited reactivity towards several members of the CD1 family (25–28, 67–70). To date there are three γδ TCR-CD1 structures, two with CD1d presenting different lipid antigens (25, 27) and one with CD1a (28).
The two CD1d structures include the DP10 (Vγ4Vδ1) and 9C2 (Vγ5Vδ1) TCRs, in complex with CD1d presenting sulfatide or ɑ-Galactosylceramide (ɑ-GalCer) (Figure 1D & 1E) (25, 27). Both DP10 and 9C2 TCRs were isolated from peripheral blood whereby, 0.004–0.01% of CD3+ PBMCs and 0.05–3.5% of γδ TCR+ PBMCs were reactive to CD1d-sulfatide and CD1d-ɑ-GalCer respectively (25, 27, 71). The DP10 and 9C2 TCRs bound CD1d-sulfatide and CD1d-ɑ-GalCer with comparable affinities (~5.6 μM & ~16.0 μM, respectively), which demonstrated moderate affinity interactions that fell within the range expected of physiological TCRs (66). Of the two, only the DP10 TCR displayed lipid-specific reactivity (sulfatide), whilst the 9C2 TCR was broadly reactive to endogenous lipids. The DP10 and 9C2 γδ TCRs bound atop the antigen presentation cleft of the CD1d molecule although DP10 was positioned centrally above the presented sulfatide lipid, whereas 9C2 was positioned slightly closer to the distal end of the antigen presentation platform (25, 27). Interestingly, both γδ TCR-CD1d-lipid complexes were dominated by the δ-chain, the DP10 δ-chain exclusively mediated CD1d-sulfatide recognition whereas the 9C2 δ-chain comprised 75% of the CD1d-ɑ-GalCer interface. More specifically, the DP10-CD1d-sulfatide interface involved germline encoded CDR1δ and CDR3δ loop residues as the main mediators of the interaction with the CD1d molecule, contributing 32% and 57% to BSA, respectively (Table 1). Here, the germline encoded Trp29, Trp30 of the CDR1δ loop, and Trp99 of the CDR3δ loop (Figure 2C) made key contributions to the recognition of CD1d. These three aromatic residues formed extensive covalent bonds and van der Waals interactions with the α1 and α2 helices of the CD1d antigen presentation platform. However, only the CDR3δ loop contacted the presented sulfatide as junctionally encoded aromatic Phe101 formed a hydrogen bond with the sulfate moiety of the sulfatide. With the 9C2 TCR, the CDR1δ loop contributed a majority 40% to CD1d binding, whereby Trp29 and Trp30 formed mainly van der Waals interactions with the α2 helix of CD1d (Figure 2D). The 9C2 CDR3δ loop also contributed 28% to BSA as almost the entirety of the loop (Asp98 to Lys106) formed extensive van der Waals interactions with both α helices of CD1d. In contrast to the DP10 TCR, the γ-chain of 9C2 TCR is also involved in recognition of the CD1d-lipid complex. Although the 9C2γ only minorly contributed to BSA (25%) (Table 1), Arg103 and Tyr111 of the CDR3γ loop were the sole mediators of α-GalCer recognition, forming extensive hydrogen bonds and van der Waals interactions with the galactose moiety.
The most recently published γδ TCR-CD1-lipid structure is that of CO3 (Vγ4Vδ1) TCR in complex with CD1a presenting sulfatide (Figure 1F) (28). Here, ~0.070% of CD3+ γδ TCR+ PBMCs isolated from the peripheral blood were broadly CD1a reactive (28). The CO3 TCR binds the CD1a-sulfatide complex at ~22 μM, consistent with other γδ TCR-antigen interactions and αβ TCR-CD1a interactions (55). The CO3 TCR was also found to be lipid permissive as the loading of different lipopeptides and endogenously loaded lipids had little effect on binding (28). This CO3 γδ TCR-CD1a-lipid structure was a highly novel interaction whereby the γδ TCR co-contacted CD1a and β2 microglobulin (β2m) at the extreme end of the antigen binding cleft in a fashion described as ‘sideways’ as opposed to the canonical ‘end-to-end’. Notably, there is no co-recognition of the presented sulfatide as the CO3 TCR only contacted CD1a but nevertheless a CD1a-bound lipopeptide can impact CO3 TCR recognition via indirect means, namely by altering the conformation of the CD1a cleft. Here, the δ-chain once again dominated the majority of the interaction, contributing 65% to BSA (Table 1). The CDR1δ and CDR3δ loops were primarily responsible for the interactions with both the CD1a molecule and the β2M, contributing 20% and 40% to BSA respectively. Once more, the germline encoded Trp31 formed extensive van der Waals interactions with the β2M while the non-germline encoded Trp99 formed a mixture of hydrogen bonds, van der Waals, and pi-stacking interactions with the CD1a heavy chain (Figure 2E). As with previously examined gdTCR complexes, there is a persistence of tryptophan residues on the δ-chain in mediating interactions with the MHC-I like molecule. The γ-chain only contributed ~35% of the BSA, where framework residues (Arg48, Tyr51, Leu61, Glu62) proximal to the CDR2γ loop primarily formed van der Waals interactions with His21 of the CD1a heavy chain. Together these CD1-γδ TCR structures demonstrate a continued involvement of germline Tryptophan’s in the recognition of MHC-I like molecules, despite unprecedented variances in antigens or docking mechanisms.
γδ TCR-MR1
Following on from the interest in γδ T cell-CD1 interactions, novel interactions with human MR1 were observed (46). Here, <0.1–5% of γδ T cells in the peripheral blood were broadly specific for MR1 and from these γδ T cell clones, G7 (Vγ9Vδ1) and G83.C4 (Vγ5Vδ3) were isolated (46). The co-complex structure of G7 γδ TCR-MR1 presenting 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU), a pyrimidine formed as a metabolite of the riboflavin pathway (72), was solved first (Figure 1G) (46). G7 TCR bound MR1–5-OP-RU at moderate affinity of ~ 4.7 μM akin to αβ MAIT TCR recognition of MR1–5-OP-RU (~1.65 μM) (2). Like the CO3 TCR, G7 TCR overturned any preconceived notions of a canonical binding modes adopted by γδ TCRs as it binds to the underside of the MR1 molecule within the α3 domain without co-contacting the presented 5-OP-RU (46). Again, there is a prevailing dominance of the δ chain in ligand interaction as the Vδ1 contributed a majority 84% to BSA (Table 1). Here, CDR1δ and CDR3δ loops were the primary mediators of contacting the α3 domain, contributing 22% and 42% to BSA respectively. This is due to the predominance of aromatic residues in the CDR1δ and CDR3δ loops at the interface, a recurring motif in MHC I-like molecule recognition. Again, key Tryptophan’s (Trp29, Trp30, and Trp106) formed key van der Waals interactions with the α3 domain of MR1 (Figure 2F). Similarly to the CO3 TCR, the framework regions of the γ-chain made crucial contacts to the MR1 molecule and were responsible for almost the entire γ-chain contributions to the BSA. Here, Arg59, Lys60, Glu61, and Ser62 on the γ chain formed a mixture of van der Waals and hydrogen interactions with Glu219 of the MR1 α3 domain.
Next, the Vδ3+ G83.C4 TCR was determined to bind MR1–5-OP-RU at strong affinity of ~0.57 μM. This affinity is relatively stronger that of αβ TCR-MR1 interactions and is also considered a strong affinity for αβ TCRs overall (2, 66). The co-complex structure revealed that it too, had a docking topology that deviated from the norm of αβ TCR binding (47). The G83.C4 TCR binds MR1 on the upper side away from the antigen presentation cleft without co-recognition of 5-OP-RU (Figure 1H). Being the first human non-Vδ1+ TCR in complex with an antigen solved, underlying patterns observed in Vδ1+ complexes were also observed here with key differences. The δ-chain continues to dominate the interaction with MR1, contributing 71% towards BSA as the CDR3δ loop (37%) is a driver for the unconventional docking topology (Table 1). In contrast to Vδ1+ structures, Trp29 and Trp30 were absent from the Vδ3+ domain. Instead, key contacts to MR1 were primarily made via germline-encoded negatively charged Asp103 and Asp107 on the CDR3δ loop. Here salt bridges were formed with positively charged Arg61 on the α1 helix and Arg167 on the α2 helix respectively. Additional hydrogen bonds and van der Waals interactions were formed between Asp103 and both the α1 and α2 helices. Here, non-germline encoded Trp100 on the CDR3δ loop contributed indirectly to the interaction, forming van de Waals interactions with the polar end of the α1 helix (Figure 2G) (47). Despite the lesser role aromatics may play in Vδ3+ TCR bind to MHC I-like structure, its presence in the interaction is consistent with human Vδ1+ and murine γδ TCR recognition of MHC I-like molecules. Minor contribution from the γ domain is mainly evenly distributed between the CDR2γ and the CDR3γ loops (11%) where each loop forms a complex of hydrogen bonds, salt bridges and van der Waals interactions with the α1 and α2 helices respectively.
Vδ-domain centric binding
A striking observation of the four Vδ1+ TCR structures in complex with MHC I-like molecules (DP10, 9C2, G7, and CO3) is their markedly different angles and paratopes of recognition, highlighting an unpredictability of γδ TCR ligand binding. Despite such variances, there are commonalties across these γδ TCR-ligand interfaces including the conserved involvement of germline-encoded CDR1 and CDR3 loop residues yet, this does not lead to common docking mechanisms as observed in some αβ TCR-peptide-MHC interactions (73). Specifically, CDR1δ loop encoded Tryptophan’s are major mediators in all of the Vδ1+ TCR complex structures yet do so uniquely within each complex (Figure 2). From the singular Vδ3+ TCR-antigen complex solved; hydrophobic residues still played a key role in supporting the interaction with MHC-like molecule. However, whether this underlying pattern can be observed in other Vδ3+ interactions remain to be seen. Furthermore, whether these observations hold true for Vδ2+ and Vδ5+ populations is unknown.
An emergent trend across all these γδ TCR complexes is the Vδ domain dominance at the binding interface. So far, all published complex structures are of γδ TCRs bound to an MHC or MHC I-like molecules, leaving open an unexplored realm of co-complex structures with other ligands (Table 2). Further support for a prevailing Vδ reliance has been demonstrated in γδ TCR activation assays involving CD1b (70) and CD1c (67) which showed reactive Vδ1+ populations with varying Vγ domains. Reactivity towards other ligands, including MICA (74) and non-MHC I-like ligands like phycoerythrin (15), and Ephrin type-a receptor 2 (75) has also been observed in γδ T cell populations isolated from human peripheral blood. For many of these highly unconventional ligands, reactive populations expressed a common δ-chain, implying a high degree of δ-chain reliance in binding. Phycoerythrin and Ephrin type-a receptor reactive γδ T cells all expressed a Vδ1 chain (15, 75). It is possible that the capabilities of the CDR3δ for generating long and hyper-diverse loops may play a role in driving the dominance of the δ-chain in these interactions.
Table 2:
All presently known human γδ TCR ligands.
| Known Ligands | γδ TCR | Known Presentation | Ref. | Structure |
|---|---|---|---|---|
| MHC/MHC-like | ||||
| HLA:peptide | Vδ1+ | Peripheral blood | (45, 87) | 5F3 TCR-HLA-A*02-MART1 |
| MICA | Vδ1+ | Peripheral blood | (74) | N/A |
| CD1a | Vδ1+ | Peripheral blood | (28) | CO3 TCR-CD1a-sulfatide |
| CD1b | Vδ1+ | Peripheral blood | (70) | N/A |
| CD1d | Vδ1+/Vδ3+ | Peripheral blood | (25, 27) | 9C2 TCR-CD1d-αGalCer DP10 TCR-CD1d-sulfatide |
| CD1c | Vδ1+ | Peripheral blood | (67) | N/A |
| MR1 | Vδ1+/Vδ3+ | Peripheral blood | (46, 47) | G7 TCR-MR1–5-OP-RU G83.C4 TCR-MR1–5-OP-RU |
| Butyrophilins | ||||
| BTN2A1/BTN3A1 | Vγ9Vδ2+ | Peripheral blood | (4, 20–23). | G115 TCR-BTN2A1 |
| BTNL3/BTNL8 | Vγ4+ | Enteric organs | (42) | N/A |
| Other | ||||
| Phycoerythrin (PE) | Vδ1+ | Unknown | (15) | N/A |
| EphA2 | Vγ9Vδ1+ | Peripheral blood | (75) | N/A |
| hMSH2 | Vδ2+ | Peripheral blood | (88) | N/A |
| histidyl tRNA synthetase | Vγ3Vδ2+ | Peripheral blood | (18) | N/A |
| Annexin A2 | Vδ3+ | Unknown | (11) | N/A |
| EPCR | Vγ4Vδ5+ | Peripheral blood | (24) | N/A |
γδ TCR-B7-like
Potential exceptions to the Vδ centric binding paradigms are a population which respond to the B7-like molecules butyrophilin or butyrophilin-like (BTNL) molecules. BTNs are a family of immunoglobulin proteins related to B7 proteins such as PD1, a checkpoint inhibitor for αβ T cell activation (20). The former utilises a Vγ9 domain and to recognise BTN2A1 (21–23), whereas the latter relies upon a Vγ4 domain to bind BTNL3 in a BTNL3-BTNL8 heterodimer (42, 43). These γδ T cell populations are some of the most numerous with Vγ9Vδ2+ T cells accounting for up to ~20% of circulating peripheral blood γδ T cells (20, 22).
A number of studies have been made into understanding the underpinnings of γδ TCR mediated recognition of phosphoantigens (pAgs) via butyrophilins (BTN), BTN2A1 and BTN3A1, drivers of the recognition of intracellular pAgs (22, 23, 76, 77). Phosphoantigens are accumulated in cells either due to infection with pAg-producing microbes and parasites or due to dysfunctional metabolic pathways often present in cancers or cells under stress (4, 20, 78, 79). Phosphoantigens bind to the intracellular domain of BTN3A1 (76), and are thought to induce a conformational change that promotes BTN2A1 association (80, 81). It is hypothesised that this conformational change propagates to the extracellular region, leading the exposure of a γδ TCR binding surface on BTN2A1 (82). Whilst there is an established restriction of BTN2A1 binding to the Vγ9 domain, direct recognition of the extracellular V domain of BTN3A1 by the positively charged CDR2δ residues of the Vδ2 domain has only recently been demonstrated via mutational analyses (83). BTN3A1 was also recently demonstrated to form a dimer with non-pAg sensing isoforms BTN3A2 or BTN3A3, which enable the cell surface trafficking of BTN3A1 and the subsequent recognition of pAgs and BTN3A1 by Vγ9Vδ2 TCRs (23, 84).
Recently, the first glimpses into how a Vγ9Vδ2 TCR interacts with BTN2A1 was afforded by an elegant structural study and resultant complex crystal structure (PDB: 8DFW), described in detail in (83). This novel structure revealed that the BTN2A1 IgV domain was bound by the side of the Vγ9 domain. The CDRγ loops had little involvement in BTN2A1 binding, with all major contacts stemming from germline-encoded framework regions. This interaction centred upon a positively charged Arginine (Arg20) on the β strand preceding the CDR1γ loop. This residue is absent from other Vγ domains and could explain the specificity of Vγ9+ TCRs for BTN2A1. Thus, the Vδ domain dominance that underpins γδ TCR interactions MHC-like molecules is starkly contrasted in the first example of a BTN reactive γδ TCR structure where the γ chain drove binding.
Shifting shape complementarity and buried surface area contributions
Although the BSA contribution of each chain varies across γδ TCR complexes, we aimed to probe how BSA and shape complementarity (sc) (85) of the entire interface compares to that of αβ TCRs. Previous analyses of a large repertoire of αβ TCR-MHC complexes revealed that average total BSA was ~1910 or 2030 Å2 between MHC I and MHC II complexes, respectively (48). This value, amongst the γδ TCR complexes is slightly lower on average, at ~1780 Å. The smallest value seen in DP10 TCR-CD1d presenting sulfatide with a total BSA of ~1310 Å, this is within the range of αβ MHC I complexes but is smaller than known αβ TCR-MHC II complexes.
In regard to sc, with 0 being considered not at all fitting and, a value of 1 being considered to be a perfect fit, most of the γδ TCR-complexes were similarly well fitting. The lowest sc values amongst existing αβ TCR complexes were 0.41 and 0.48 for MHC I and MHC II respectively. Whereas the lowest sc value for a γδ TCR-ligand complexes was 0.56 for the G7 gdTCR-MR1 complex. (Table 1). Shape complementarity has also been observed to be a poor correlate for affinity in αβ-TCR-pMHC complexes (48). Shape complementarity in antibody-antigen complexes (0.64 – 0.68) is generally higher than those of αβ TCR-pMHC complexes (85). Considering the structural attributes that γδ TCRs share with both the αβ TCR and antibodies, sc is yet another aspect worth examination as further γδ TCR-antigen complexes are solved.
Conclusions
Inroads have been made in recent decades in the effort to unravel the complexity of the γδ TCR interactions. As the new structural evidence suggests, γδ TCR complexes can be entirely unpredictable. When viewing all existing γδ TCR-MHC I complexes superposed to together, it becomes apparent that each complex is distinct in docking topology, akin to antibody-antigen complexes rather than the canonical ‘end-to-end’ binding of conventional αβ TCR-pMHC complexes. Nevertheless, some recurring patterns are emerging in the γδ TCR recognition of MHC-like molecules. For example, there is a strong dominance of the δ-chain and the omnipresence of a tryptophan’s in mediating interactions, this is a concept that grows as the portrait of γδ TCR recognition continues to evolve. These recurring patterns are only observed in γδ TCR-MHC I-like structures, perhaps suggesting that these motifs are unique to MHC I-like recognition. Notably, the increased presence of hydrophobic CDR loop residues in αβ TCRs have been linked to reactivity to self-antigens presented via MHC molecules(86).
Furthermore, the antigen co-recognition paradigm is absent in multiple existing γδ TCR- MHC I-like structures. Instead, direct recognition of the MHC-like molecule proceeds and is reminiscent of antibody-like recognition. These observations corroborate noted structural similarities of γδ TCR variable domains and antibodies which suggested that the conformational flexibility of γδ TCR CDR3 loops were reminiscent of immunoglobulins (57, 60).
The veracity of these emerging trends as solid rules for γδ TCR engagement is yet to be established and exemplifies the importance of further structural studies to deconvolute the nature of how γδ TCRs interact with ligands. In this vein, study of the γδ TCR with the context of CD3 signalling apparatus could be highly informative as would further insight into the signalling capacity of γδ TCRs with conflicting observations of hypo or hyper responsive γδ T cells following antigen stimulation (28, 46, 47). Such structural studies will be revealing for γδ T cell biology and highly informative for the burgeoning field of γδ T cell-based immunotherapies.
Acknowledgments
This work is supported by the Australian Research Council (ARC) Grant DP220102401 & DP230102073, the National Institutes of Health (NIH) Grant RO1 AI148407-01A1, and the NHMRC Investigator Grant 2008981.
Footnotes
Disclosures
The authors declare no competing interests.
References
- 1.Brigl M, and Brenner MB. 2004. CD1: antigen presentation and T cell function. Annu Rev Immunol 22: 817–890. [DOI] [PubMed] [Google Scholar]
- 2.Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B, Reantragoon R, Sandoval-Romero ML, Sullivan LC, Brooks AG, Chen Z, Fairlie DP, McCluskey J, and Rossjohn J. 2013. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun 4: 2142–2151. [DOI] [PubMed] [Google Scholar]
- 3.Hayday AC 2000. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 18: 975–1026. [DOI] [PubMed] [Google Scholar]
- 4.Morita CT, Jin C, Sarikonda G, and Wang H. 2007. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 215: 59–76. [DOI] [PubMed] [Google Scholar]
- 5.Costa G, Loizon S Fau - Guenot M, Guenot M Fau - Mocan I, Mocan I Fau - Halary F, Halary F Fau - de Saint-Basile G, de Saint-Basile G Fau - Pitard V, Pitard V Fau - Déchanet-Merville J, Déchanet-Merville J Fau - Moreau J-F, Moreau Jf Fau - Troye-Blomberg M, Troye-Blomberg M Fau - Mercereau-Puijalon O, Mercereau-Puijalon O Fau - Behr C, and Behr C. 2011. Control of Plasmodium falciparum erythrocytic cycle: γδ T cells target the red blood cell-invasive merozoites. Blood 118: 6952–6962. [DOI] [PubMed] [Google Scholar]
- 6.Davey MS, Willcox CR, Joyce SP, Ladell K, Kasatskaya SA, McLaren JE, Hunter S, Salim M, Mohammed F, Price DA, Chudakov DM, and Willcox BE. 2017. Clonal selection in the human Vdelta1 T cell repertoire indicates gammadelta TCR-dependent adaptive immune surveillance. Nat Commun 8: 14760–14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujishima N, Hirokawa M, Fujishima M, Yamashita J, Saitoh H, Ichikawa Y, Horiuchi T, Kawabata Y, and Sawada KI. 2007. Skewed T cell receptor repertoire of Vdelta1(+) gammadelta T lymphocytes after human allogeneic haematopoietic stem cell transplantation and the potential role for Epstein-Barr virus-infected B cells in clonal restriction. Clin Exp Immunol 149: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taupin JL, Halary F Fau - Déchanet J, Déchanet J Fau - Peyrat MA, Peyrat Ma Fau - Ragnaud JM, Ragnaud Jm Fau - Bonneville M, Bonneville M Fau - Moreau JF, and Moreau JF. 1999. An enlarged subpopulation of T lymphocytes bearing two distinct gammadelta TCR in an HIV-positive patient. International Immunology 11: 545–552. [DOI] [PubMed] [Google Scholar]
- 9.von Borstel A, Chevour P, Arsovski D, Krol Jelte MM, Howson Lauren J, Berry Andrea A, Day Cheryl L, Ogongo P, Ernst Joel D, Nomicos Effie YH, Boddey Justin A, Giles Edward M, Rossjohn J, Traore B, Lyke Kirsten E, Williamson Kim C, Crompton Peter D, and Davey Martin S. Repeated Plasmodium falciparum infection in humans drives the clonal expansion of an adaptive γδ T cell repertoire. Sci Transl Med 13: eabe7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, Hu Q, Li Y, Lu L, Xiang Z, Yin Z, Kabelitz D, and Wu Y. 2023. gammadelta T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct Target Ther 8: 434–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marlin R, Pappalardo A, Kaminski H, Willcox CR, Pitard V, Netzer S, Khairallah C, Lomenech AM, Harly C, Bonneville M, Moreau JF, Scotet E, Willcox BE, Faustin B, and Dechanet-Merville J. 2017. Sensing of cell stress by human gammadelta TCR-dependent recognition of annexin A2. Proc Natl Acad Sci U S A 114: 3163–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, and Alizadeh AA. 2015. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med 21: 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chabab G, Barjon C, Bonnefoy N, and Lafont V. 2020. Pro-tumor gammadelta T Cells in Human Cancer: Polarization, Mechanisms of Action, and Implications for Therapy. Front Immunol 11: 2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, and Wang RF. 2007. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 27: 334–348. [DOI] [PubMed] [Google Scholar]
- 15.Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA, Kuhns MS, Waters RW, Davis MM, Weaver CT, and Chien YH. 2012. gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity 37: 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harly C, Joyce SP, Domblides C, Bachelet T, Pitard V, Mannat C, Pappalardo A, Couzi L, Netzer S, Massara L, Obre E, Hawchar O, Lartigue L, Claverol S, Cano C, Moreau J-F, Mahouche I, Soubeyran I, Rossignol R, Viollet B, Willcox CR, Mohammed F, Willcox BE, Faustin B, and Déchanet-Merville J. 2021. Human γδ T cell sensing of AMPK-dependent metabolic tumor reprogramming through TCR recognition of EphA2. Science Immunology 6: eaba9010. [DOI] [PubMed] [Google Scholar]
- 17.Marlin R, Pappalardo A, Kaminski H, Willcox CR, Pitard V, Netzer S, Khairallah C, Lomenech A-M, Harly C, Bonneville M, Moreau J-F, Scotet E, Willcox BE, Faustin B, and Déchanet-Merville J. 2017. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proceedings of the National Academy of Sciences 114: 3163–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruder J, Siewert K, Obermeier B, Malotka J, Scheinert P, Kellermann J, Ueda T, Hohlfeld R, and Dornmair K. 2012. Target specificity of an autoreactive pathogenic human gammadelta-T cell receptor in myositis. J Biol Chem 287: 20986–20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, Monsarrat B, Saulquin X, Maillet S, Esteve JP, Lopez F, Perret B, Collet X, Bonneville M, and Champagne E. 2005. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 22: 71–80. [DOI] [PubMed] [Google Scholar]
- 20.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, and Fournie JJ. 1994. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science 264: 267–270. [DOI] [PubMed] [Google Scholar]
- 21.Harly C, Guillaume Y, Nedellec S, Peigne CM, Monkkonen H, Monkkonen J, Li J, Kuball J, Adams EJ, Netzer S, Dechanet-Merville J, Leger A, Herrmann T, Breathnach R, Olive D, Bonneville M, and Scotet E. 2012. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood 120: 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z, McWilliam HEG, Hudson C, Tutuka C, Wheatley AK, Kent SJ, Villadangos JA, Pal B, Kurts C, Simmonds J, Pelzing M, Nash AD, Hammet A, Verhagen AM, Vairo G, Maraskovsky E, Panousis C, Gherardin NA, Cebon J, Godfrey DI, Behren A, and Uldrich AP. 2020. Butyrophilin 2A1 is essential for phosphoantigen reactivity by gammadelta T cells. Science 367: eaay5516. [DOI] [PubMed] [Google Scholar]
- 23.Karunakaran MM, Subramanian H, Jin Y, Mohammed F, Kimmel B, Juraske C, Starick L, Nohren A, Lander N, Willcox CR, Singh R, Schamel WW, Nikolaev VO, Kunzmann V, Wiemer AJ, Willcox BE, and Herrmann T. 2023. A distinct topology of BTN3A IgV and B30.2 domains controlled by juxtamembrane regions favors optimal human gammadelta T cell phosphoantigen sensing. Nat Commun 14: 7617–7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, Moreau JF, Hayday AC, Willcox BE, and Dechanet-Merville J. 2012. Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol 13: 872–879. [DOI] [PubMed] [Google Scholar]
- 25.Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, Anderson B, Scharf L, Kung JE, Sibener LV, Savage PB, Jabri B, Bendelac A, and Adams EJ. 2013. Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity 39: 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, and Brenner MB. 2000. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med 191: 937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, Patel O, Beddoe T, Gras S, Rossjohn J, and Godfrey DI. 2013. CD1d-lipid antigen recognition by the gammadelta TCR. Nat Immunol 14: 1137–1145. [DOI] [PubMed] [Google Scholar]
- 28.Wegrecki M, Ocampo TA, Gunasinghe SD, von Borstel A, Tin SY, Reijneveld JF, Cao TP, Gully BS, Le Nours J, Moody DB, Van Rhijn I, and Rossjohn J. 2022. Atypical sideways recognition of CD1a by autoreactive gammadelta T cell receptors. Nat Commun 13: 3872–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita CT, Parker CM, Brenner MB, and Band H. 1994. TCR usage and functional capabilities of human gamma delta T cells at birth. J Immunol 153: 3979–3988. [PubMed] [Google Scholar]
- 30.Deusch K, Lüling F, Reich K, Classen M, Wagner H, and Pfeffer K. 1991. A major fraction of human intraepithelial lymphocytes simultaneously expresses the γ/δ T cell receptor, the CD8 accessory molecule and preferentially uses the Vδ1 gene segment. European Journal of Immunology 21: 1053–1059. [DOI] [PubMed] [Google Scholar]
- 31.Brandtzaeg P, Bosnes V, Halstensen TS, Scott H, Sollid LM, and Valnes KN. 1989. T Lymphocytes in Human Gut Epithelium Preferentially Express the α/β Antigen Receptor and are often CD45/UCHL1-Positive. Scandinavian Journal of Immunology 30: 123–128. [DOI] [PubMed] [Google Scholar]
- 32.Bos JD, Teunissen Mb Fau - Cairo I, Cairo I Fau - Krieg SR, Krieg Sr Fau - Kapsenberg ML, Kapsenberg Ml Fau - Das PK, Das Pk Fau - Borst J, and Borst J. 1990. T-cell receptor gamma delta bearing cells in normal human skin. Journal of Investigative dermatology 94: 37–42. [DOI] [PubMed] [Google Scholar]
- 33.Gully BS, Rossjohn J, and Davey MS. 2021. Our evolving understanding of the role of the gammadelta T cell receptor in gammadelta T cell mediated immunity. Biochem Soc Trans 49: 1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen MM, Witherden DA, and Havran WL. 2017. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nature Reviews Immunology 17: 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, Deban L, Cipolat S, Hart R, Iannitto ML, Laing A, Spencer-Dene B, East P, Gibbons D, Irving PM, Pereira P, Steinhoff U, and Hayday A. 2016. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific γδ T Cell Compartments. Cell 167: 203–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eggesbø LM, Risnes LF, Neumann RS, Lundin KEA, Christophersen A, and Sollid LM. 2020. Single-cell TCR sequencing of gut intraepithelial γδ T cells reveals a vast and diverse repertoire in celiac disease. Mucosal Immunology 13: 313–321. [DOI] [PubMed] [Google Scholar]
- 37.Vantourout P, Laing A, Woodward MJ, Zlatareva I, Apolonia L, Jones AW, Snijders AP, Malim MH, and Hayday AC. 2018. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing gammadelta T cell biology. Proc Natl Acad Sci U S A 115: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catalan-Serra I, Sandvik AK, Bruland T, and Andreu-Ballester JC. 2017. Gammadelta T Cells in Crohn’s Disease: A New Player in the Disease Pathogenesis? Journal of Crohn’s and Colitis 11: 1135–1145. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Hayman L, Kilbey A, Edwards J, and Coffelt SB. 2020. Gut γδ T cells as guardians, disruptors, and instigators of cancer. Immunological Reviews 298: 198–217. [DOI] [PubMed] [Google Scholar]
- 40.Mayassi T, Ladell K, Gudjonson H, McLaren JE, Shaw DG, Tran MT, Rokicka JJ, Lawrence I, Grenier J-C, van Unen V, Ciszewski C, Dimaano M, Sayegh HE, Kumar V, Wijmenga C, Green PHR, Gokhale R, Jericho H, Semrad CE, Guandalini S, Dinner AR, Kupfer SS, Reid HH, Barreiro LB, Rossjohn J, Price DA, and Jabri B. 2019. Chronic Inflammation Permanently Reshapes Tissue-Resident Immunity in Celiac Disease. Cell 176: 967–981.e919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dart RJ, Zlatareva I, Vantourout P, Theodoridis E, Amar A, Kannambath S, East P, Recaldin T, Mansfield JC, Lamb CA, Parkes M, Irving PM, Prescott NJ, and Hayday AC. 2023. Conserved gammadelta T cell selection by BTNL proteins limits progression of human inflammatory bowel disease. Science 381: eadh0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melandri D, Zlatareva I, Chaleil RAG, Dart RJ, Chancellor A, Nussbaumer O, Polyakova O, Roberts NA, Wesch D, Kabelitz D, Irving PM, John S, Mansour S, Bates PA, Vantourout P, and Hayday AC. 2018. The gammadeltaTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol 19: 1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willcox CR, Vantourout P, Salim M, Zlatareva I, Melandri D, Zanardo L, George R, Kjaer S, Jeeves M, Mohammed F, Hayday AC, and Willcox BE. 2019. Butyrophilin-like 3 Directly Binds a Human Vgamma4(+) T Cell Receptor Using a Modality Distinct from Clonally-Restricted Antigen. Immunity 51: 813–825 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adams EJ, Chien YH, and Garcia KC. 2005. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science 308: 227–231. [DOI] [PubMed] [Google Scholar]
- 45.Benveniste PM, Roy S, Nakatsugawa M, Chen ELY, Nguyen L, Millar DG, Ohashi PS, Hirano N, Adams EJ, and Zuniga-Pflucker JC. 2018. Generation and molecular recognition of melanoma-associated antigen-specific human gammadelta T cells. Sci Immunol 3: eaav4036. [DOI] [PubMed] [Google Scholar]
- 46.Le Nours J, Gherardin NA, Ramarathinam SH, Awad W, Wiede F, Gully BS, Khandokar Y, Praveena T, Wubben JM, Sandow JJ, Webb AI, von Borstel A, Rice MT, Redmond SJ, Seneviratna R, Sandoval-Romero ML, Li S, Souter MNT, Eckle SBG, Corbett AJ, Reid HH, Liu L, Fairlie DP, Giles EM, Westall GP, Tothill RW, Davey MS, Berry R, Tiganis T, McCluskey J, Pellicci DG, Purcell AW, Uldrich AP, Godfrey DI, and Rossjohn J. 2019. A class of gammadelta T cell receptors recognize the underside of the antigen-presenting molecule MR1. Science 366: 1522–1527. [DOI] [PubMed] [Google Scholar]
- 47.Rice MT, von Borstel A, Chevour P, Awad W, Howson LJ, Littler DR, Gherardin NA, Le Nours J, Giles EM, Berry R, Godfrey DI, Davey MS, Rossjohn J, and Gully BS. 2021. Recognition of the antigen-presenting molecule MR1 by a Vdelta3(+) gammadelta T cell receptor. Proc Natl Acad Sci U S A 118: e2110288118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossjohn J, Gras S, Miles JJ, Turner SJ, Godfrey DI, and McCluskey J. 2015. T cell antigen receptor recognition of antigen-presenting molecules. Annu Rev Immunol 33: 169–200. [DOI] [PubMed] [Google Scholar]
- 49.La Gruta NL, Gras S, Daley SR, Thomas PG, and Rossjohn J. 2018. Understanding the drivers of MHC restriction of T cell receptors. Nat Rev Immunol 18: 467–478. [DOI] [PubMed] [Google Scholar]
- 50.Beringer DX, Kleijwegt FS, Wiede F, van der Slik AR, Loh KL, Petersen J, Dudek NL, Duinkerken G, Laban S, Joosten A, Vivian JP, Chen Z, Uldrich AP, Godfrey DI, McCluskey J, Price DA, Radford KJ, Purcell AW, Nikolic T, Reid HH, Tiganis T, Roep BO, and Rossjohn J. 2015. T cell receptor reversed polarity recognition of a self-antigen major histocompatibility complex. Nat Immunol 16: 1153–1161. [DOI] [PubMed] [Google Scholar]
- 51.Gras S, Chadderton J, Del Campo CM, Farenc C, Wiede F, Josephs TM, Sng XYX, Mirams M, Watson KA, Tiganis T, Quinn KM, Rossjohn J, and La Gruta NL. 2016. Reversed T Cell Receptor Docking on a Major Histocompatibility Class I Complex Limits Involvement in the Immune Response. Immunity 45: 749–760. [DOI] [PubMed] [Google Scholar]
- 52.Zareie P, Szeto C, Farenc C, Gunasinghe SD, Kolawole EM, Nguyen A, Blyth C, Sng XYX, Li J, Jones CM, Fulcher AJ, Jacobs JR, Wei Q, Wojciech L, Petersen J, Gascoigne NRJ, Evavold BD, Gaus K, Gras S, Rossjohn J, and La Gruta NL. 2021. Canonical T cell receptor docking on peptide-MHC is essential for T cell signaling. Science 372: eabe9124. [DOI] [PubMed] [Google Scholar]
- 53.Wang JH, and Reinherz EL. 2012. The structural basis of alphabeta T-lineage immune recognition: TCR docking topologies, mechanotransduction, and co-receptor function. Immunol Rev 250: 102–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, and Rossjohn J. 2007. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448: 44–49. [DOI] [PubMed] [Google Scholar]
- 55.Birkinshaw RW, Pellicci DG, Cheng TY, Keller AN, Sandoval-Romero M, Gras S, de Jong A, Uldrich AP, Moody DB, Godfrey DI, and Rossjohn J. 2015. alphabeta T cell antigen receptor recognition of CD1a presenting self lipid ligands. Nat Immunol 16: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wun KS, Reijneveld JF, Cheng TY, Ladell K, Uldrich AP, Le Nours J, Miners KL, McLaren JE, Grant EJ, Haigh OL, Watkins TS, Suliman S, Iwany S, Jimenez J, Calderon R, Tamara KL, Leon SR, Murray MB, Mayfield JA, Altman JD, Purcell AW, Miles JJ, Godfrey DI, Gras S, Price DA, Van Rhijn I, Moody DB, and Rossjohn J. 2018. T cell autoreactivity directed toward CD1c itself rather than toward carried self lipids. Nat Immunol 19: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Lebedeva MI, Llera AS, Fields BA, Brenner MB, and Mariuzza RA. 1998. Structure of the Vdelta domain of a human gammadelta T-cell antigen receptor. Nature 391: 502–506. [DOI] [PubMed] [Google Scholar]
- 58.Spits H, Paliard X, Engelhard VH, and de Vries JE. 1990. Cytotoxic activity and lymphokine production of T cell receptor (TCR)-alpha beta+ and TCR-gamma delta+ cytotoxic T lymphocyte (CTL) clones recognizing HLA-A2 and HLA-A2 mutants. Recognition of TCR-gamma delta+ CTL clones is affected by mutations at positions 152 and 156. J Immunol 144: 4156–4162. [PubMed] [Google Scholar]
- 59.Allison TJ, Winter CC, Fournie JJ, Bonneville M, and Garboczi DN. 2001. Structure of a human gammadelta T-cell antigen receptor. Nature 411: 820–824. [DOI] [PubMed] [Google Scholar]
- 60.Rock EP, Sibbald PR, Davis MM, and Chien YH. 1994. CDR3 length in antigen-specific immune receptors. J Exp Med 179: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, and Chien Y. 2000. A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science 287: 314–316. [DOI] [PubMed] [Google Scholar]
- 62.Adams EJ, Strop P, Shin S, Chien YH, and Garcia KC. 2008. An autonomous CDR3delta is sufficient for recognition of the nonclassical MHC class I molecules T10 and T22 by gammadelta T cells. Nat Immunol 9: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, and Chien YH. 2005. Antigen recognition determinants of gammadelta T cell receptors. Science 308: 252–255. [DOI] [PubMed] [Google Scholar]
- 64.Del Porto P, D’Amato M, Fiorillo MT, Tuosto L, Piccolella E, and Sorrentino R. 1994. Identification of a novel HLA-B27 subtype by restriction analysis of a cytotoxic gamma delta T cell clone. J Immunol 153: 3093–3100. [PubMed] [Google Scholar]
- 65.Ciccone E, Viale O, Pende D, Malnati M, Battista Ferrara G, Barocci S, Moretta A, and Moretta L. 1989. Specificity of human T lymphocytes expressing a gamma/delta T cell antigen receptor. Recognition of a polymorphic determinant of HLA class I molecules by a gamma/delta clone. Eur J Immunol 19: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 66.Stone JD, Chervin AS, and Kranz DM. 2009. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology 126: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roy S, Ly D, Castro CD, Li NS, Hawk AJ, Altman JD, Meredith SC, Piccirilli JA, Moody DB, and Adams EJ. 2016. Molecular Analysis of Lipid-Reactive Vdelta1 gammadelta T Cells Identified by CD1c Tetramers. J Immunol 196: 1933–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russano AM, Agea E, Corazzi L, Postle AD, De Libero G, Porcelli S, de Benedictis FM, and Spinozzi F. 2006. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted gamma delta T cells. J Allergy Clin Immunol 117: 1178–1184. [DOI] [PubMed] [Google Scholar]
- 69.Russano AM, Bassotti G, Agea E, Bistoni O, Mazzocchi A, Morelli A, Porcelli SA, and Spinozzi F. 2007. CD1-restricted recognition of exogenous and self-lipid antigens by duodenal gammadelta+ T lymphocytes. J Immunol 178: 3620–3626. [DOI] [PubMed] [Google Scholar]
- 70.Reijneveld JF, Ocampo TA, Shahine A, Gully BS, Vantourout P, Hayday AC, Rossjohn J, Moody DB, and Van Rhijn I. 2020. Human gammadelta T cells recognize CD1b by two distinct mechanisms. Proc Natl Acad Sci U S A 117: 22944–22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B, Adams EJ, Savage PB, and Bendelac A. 2012. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur J Immunol 42: 2505–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, Williamson NA, Strugnell RA, Van Sinderen D, Mak JY, Fairlie DP, Kjer-Nielsen L, Rossjohn J, and McCluskey J. 2014. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509: 361–365. [DOI] [PubMed] [Google Scholar]
- 73.Feng D, Bond CJ, Ely LK, Maynard J, and Garcia KC. 2007. Structural evidence for a germline-encoded T cell receptor-major histocompatibility complex interaction ‘codon’. Nat Immunol 8: 975–983. [DOI] [PubMed] [Google Scholar]
- 74.Xu B, Pizarro JC, Holmes MA, McBeth C, Groh V, Spies T, and Strong RK. 2011. Crystal structure of a gammadelta T-cell receptor specific for the human MHC class I homolog MICA. Proc Natl Acad Sci U S A 108: 2414–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harly C, Joyce SP, Domblides C, Bachelet T, Pitard V, Mannat C, Pappalardo A, Couzi L, Netzer S, Massara L, Obre E, Hawchar O, Lartigue L, Claverol S, Cano C, Moreau JF, Mahouche I, Soubeyran I, Rossignol R, Viollet B, Willcox CR, Mohammed F, Willcox BE, Faustin B, and Dechanet-Merville J. 2021. Human gammadelta T cell sensing of AMPK-dependent metabolic tumor reprogramming through TCR recognition of EphA2. Sci Immunol 6: eaba9010. [DOI] [PubMed] [Google Scholar]
- 76.Sandstrom A, Peigne CM, Leger A, Crooks JE, Konczak F, Gesnel MC, Breathnach R, Bonneville M, Scotet E, and Adams EJ. 2014. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity 40: 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rhodes DA, Chen HC, Price AJ, Keeble AH, Davey MS, James LC, Eberl M, and Trowsdale J. 2015. Activation of human gammadelta T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J Immunol 194: 2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, and Jomaa H. 2001. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett 509: 317–322. [DOI] [PubMed] [Google Scholar]
- 79.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, and De Libero G. 2003. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 197: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsiao CH, Lin X, Barney RJ, Shippy RR, Li J, Vinogradova O, Wiemer DF, and Wiemer AJ. 2014. Synthesis of a phosphoantigen prodrug that potently activates Vgamma9Vdelta2 T-lymphocytes. Chem Biol 21: 945–954. [DOI] [PubMed] [Google Scholar]
- 81.Gu S, Sachleben JR, Boughter CT, Nawrocka WI, Borowska MT, Tarrasch JT, Skiniotis G, Roux B, and Adams EJ. 2017. Phosphoantigen-induced conformational change of butyrophilin 3A1 (BTN3A1) and its implication on Vgamma9Vdelta2 T cell activation. Proc Natl Acad Sci U S A 114: E7311–E7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan L, Ma X, Yang Y, Qu Y, Li X, Zhu X, Ma W, Duan J, Xue J, Yang H, Huang JW, Yi S, Zhang M, Cai N, Zhang L, Ding Q, Lai K, Liu C, Zhang L, Liu X, Yao Y, Zhou S, Li X, Shen P, Chang Q, Malwal SR, He Y, Li W, Chen C, Chen CC, Oldfield E, Guo RT, and Zhang Y. 2023. Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vgamma9Vdelta2 T cells. Nature 621: 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fulford TS, Soliman C, Castle RG, Rigau M, Ruan Z, Dolezal O, Seneviratna R, Brown HG, Hanssen E, Hammet A, Li S, Redmond SJ, Chung A, Gorman MA, Parker MW, Patel O, Peat TS, Newman J, Behren A, Gherardin NA, Godfrey DI, and Uldrich AP. 2023. Vγ9Vδ2 T cells recognize butyrophilin 2A1 and 3A1 heteromers (preprint). bioRxiv: 2023.2008.2030.555639. [DOI] [PubMed] [Google Scholar]
- 84.Willcox CR, Salim M, Begley CR, Karunakaran MM, Easton EJ, von Klopotek C, Berwick KA, Herrmann T, Mohammed F, Jeeves M, and Willcox BE. 2023. Phosphoantigen sensing combines TCR-dependent recognition of the BTN3A IgV domain and germline interaction with BTN2A1. Cell Rep 42: 112321. [DOI] [PubMed] [Google Scholar]
- 85.Lawrence MC, and Colman PM. 1993. Shape complementarity at protein/protein interfaces. J Mol Biol 234: 946–950. [DOI] [PubMed] [Google Scholar]
- 86.Stadinski BD, Shekhar K, Gomez-Tourino I, Jung J, Sasaki K, Sewell AK, Peakman M, Chakraborty AK, and Huseby ES. 2016. Hydrophobic CDR3 residues promote the development of self-reactive T cells. Nat Immunol 17: 946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deseke M, Rampoldi F, Sandrock I, Borst E, Boning H, Ssebyatika GL, Jurgens C, Pluckebaum N, Beck M, Hassan A, Tan L, Demera A, Janssen A, Steinberger P, Koenecke C, Viejo-Borbolla A, Messerle M, Krey T, and Prinz I. 2022. A CMV-induced adaptive human Vdelta1+ gammadelta T cell clone recognizes HLA-DR. J Exp Med 219: e20212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dai Y, Chen H, Mo C, Cui L, and He W. 2012. Ectopically expressed human tumor biomarker MutS homologue 2 is a novel endogenous ligand that is recognized by human gammadelta T cells to induce innate anti-tumor/virus immunity. J Biol Chem 287: 16812–16819. [DOI] [PMC free article] [PubMed] [Google Scholar]


