Abstract
Introduction
Retinal focal nodular gliosis (FNG), also known as vasoproliferative tumors (VPTs), are rare, benign vascular tumors associated with exudation with no current consensus on management. Herein, we describe the varied clinical course and management of 3 patients with retinal FNG, one of whom is associated with retinitis pigmentosa.
Case Presentations
Case 1 is a 76-year-old female who presented with reduced vision and distortion secondary to a vitreous hemorrhage and epiretinal membrane (ERM) as complications of a known small peripheral retinal FNG. She underwent vitrectomy for the hemorrhage to relieve vascular traction and the ERM peel, and the tumor was kept under observation. Case 2 is a 24-year-old female with genetically uncharacterized retinitis pigmentosa-like phenotype who presented with gradual loss of central vision in one eye due to cystoid macular oedema (CMO). She was found to have two peripheral retinal areas of FNG located inferonasally. Tumors were treated with cryotherapy and adjuvant intraocular steroid implant to control the CMO. Case 3 is a 28-year-old female with retinitis pigmentosa secondary to genetically confirmed variant in CRB1 gene who presented with intractable right eye CMO and localized inferior serous retinal detachment secondary to a large inferotemporal FNG. Her left eye has no light perception vision due to previous extensive serous retinal detachment and anterior segment ischemia. The right eye tumor was managed with multiple rounds of cryotherapy and laser therapy to control the serous detachment. Despite this, the condition progressed and was ultimately treated with plaque brachytherapy. Unfortunately, this resulted in extensive retinal inflammation causing annular tractional retinal detachment which was treated with combined pars plana vitrectomy and scleral buckle.
Conclusion
We characterized the retinal phenotype of 3 patients with retinal FNG (VPTs) and found them to have varied clinical courses requiring tailored surgical management. The case associated with retinitis pigmentosa had a known pathogenic variant in Crumbs homolog-1 (CRB1) gene affecting retinal structure and exhibited a more severe clinical course. It is therefore important for patients with retinal dystrophies to undergo thorough peripheral examinations and detect FNG early as they may require prompt, aggressive treatment.
Keywords: Retinal vasoproliferative tumors, Retinal focal nodular gliosis, Retinitis pigmentosa, CRB1
Introduction
Retinal focal nodular gliosis (FNG) or vasoproliferative tumors (VPTs) are rare, benign disease entities that may occur as an idiopathic primary lesion (80%) or secondary tumors (in association with other ocular pathologies such as pars planitis, Coats disease, retinitis pigmentosa (RP), familial exudative vitreoretinopathy, and toxoplasmosis) and are thought to manifest as a result of gliosis and vascular proliferation [1, 2]. They are usually characterized as pink to yellow vascular lesions with a predilection for the inferior retina and can produce an exudative and hemorrhagic retinopathy [3]. Secondary FNG tend to manifest at an earlier age of onset, are often bilateral and multifocal, and confer a poorer visual prognosis [1]. Complications associated with retinal FNG are extensive and can result in severe vision loss. These include lipid exudation, epiretinal membrane (ERM), cystoid macular oedema, vitreous hemorrhage, and tractional retinal detachment [1, 3]. No standardized treatment for FNG is currently established, but management options include photocoagulation, photodynamic therapy (PDT), cryotherapy, plaque radiotherapy, transscleral local resection [2]. Pars plana vitrectomy may be performed for complications associated with VPTs [4]. Herein, we describe the clinical features and management of 3 cases of retinal FNG, one without and two with RP phenotype. Each case needed tailored medical and surgical management, specific to their retinal phenotype. In particular, we found that the case with genetically confirmed CRB1-related retinopathy had the most complex clinical course requiring aggressive management and multiple surgical interventions.
Case 1
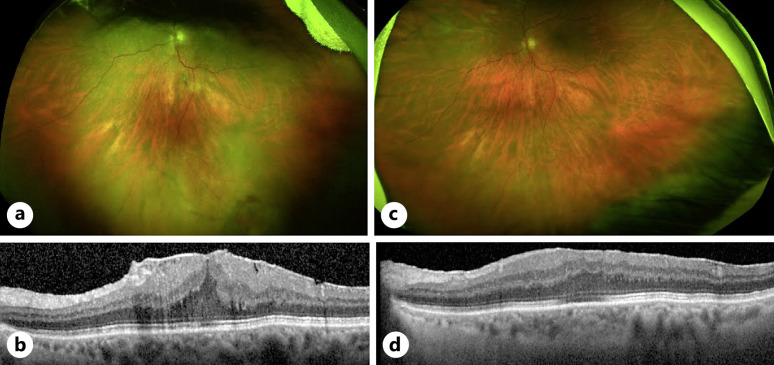
A 76-year-old female was referred to the vitreoretinal service for a left ERM and vitreous hemorrhage with visual acuity of OD 20/25 and OS 20/160. She had a background of a stable inferior FNG (Fig. 1a) in the left eye and previously declined treatment with plaque radiotherapy. She was offered pars plana vitrectomy with ERM peeling due to worsening metamorphopsia from ERM formation (Fig. 1b) without associated CMO and was noted to have a quiescent FNG in the inferior peripheral retina. She underwent routine 25-gauge pars plana vitrectomy with ERM and inner limiting membrane peel and was noted to have a regressed FNG lesion intraoperatively (Fig. 1c). She remained stable 6 months postoperatively with best corrected visual acuity improving to 20/60 and no recurrence of ERM (Fig. 1d).
Fig. 1.
Phenotype and clinical management of case 1. a Inferotemporal solitary retinal FNG/VPT (denoted by black arrow) in the left fundus on presentation with unilateral ERM secondary to the VPT shown in (b). c Quiescent inferior FNG after routine ERM peel (d) with resolution of symptoms.
Case 2
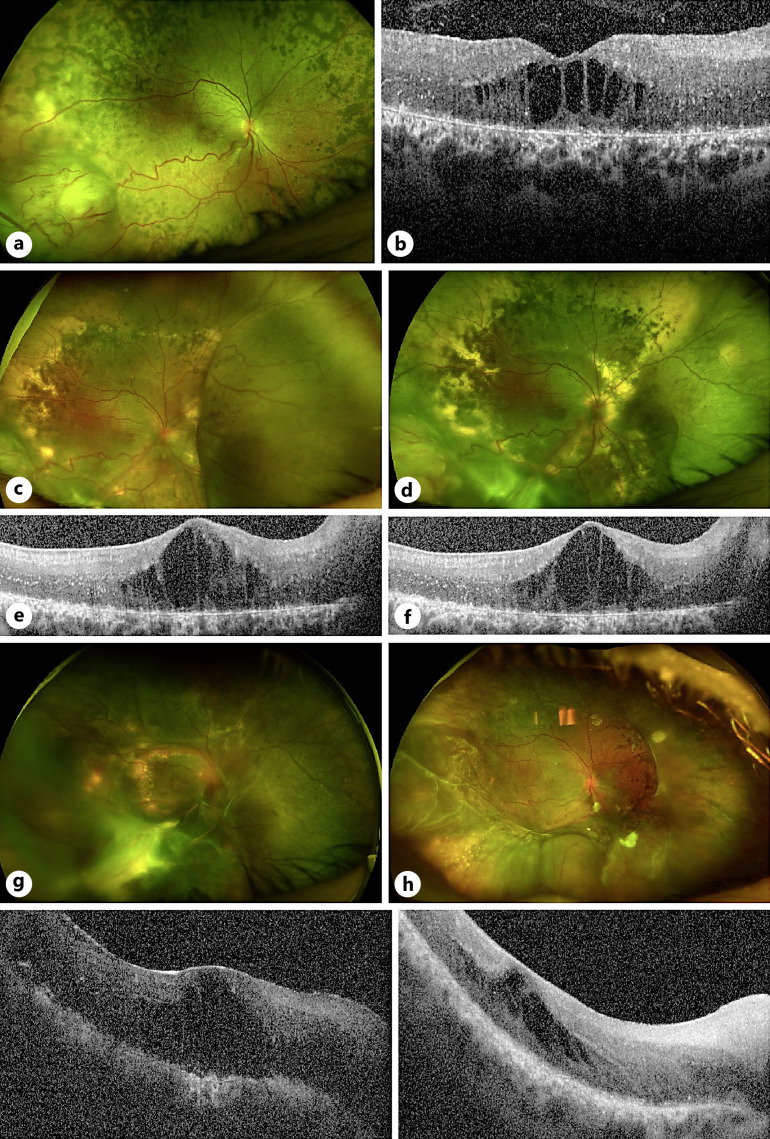
A 24-year-old female was referred to our genetics service for management of unilateral chronic CMO and an inferonasal retinal FNG. She had a known phenotypic resemblance of RP with peripheral bony spicule changes, but no causative mutation found on whole genome testing. Visual acuity was OD 20/40 and OS 20/125. Examination demonstrated unilateral CMO in the left eye associated with two inferonasal peripheral retinal yellow-red lesions and localized exudation (Fig. 2a–d).
Fig. 2.
Background of RP-like phenotype and clinical management of case 2. a–d Presentation of unilateral CMO associated with inferior FNG OS (denoted by red arrows) on background of bilateral RP-like phenotype. e, f Attenuation of CMO 1 month of post-cryotherapy and dexamethasone intravitreal implant (Ozurdex). g Recurrence of CMO during pregnancy – no further treatment given during this period. h, i Resolution of CMO 3 months of post-cryotherapy and intravitreal Ozurdex after the patient had given birth, confirmed on ultrasonography (j).
She underwent initial intravitreal dexamethasone implant (Ozurdex) for CMO and cryotherapy to the inferior VPT. One month postoperatively revealed good response to treatment with reduction of CMO and flat FNG lesions without exudate, with vision improving to 20/60 (Fig. 2e, f). Six months postoperatively, lesions remained stable, though there was evidence of mild recurrence of CMO with stable vision (Fig. 2g). The decision was made for conservative management with observation due to concurrent pregnancy. Review at 9 months of post-cryotherapy showed further visual deterioration due to progressing CMO as well as peripheral exudation from the FNG. Management options including verteporfin PDT, cryotherapy, and intravitreal steroids were offered. The patient proceeded with cryotherapy and intravitreal Ozurdex implant once she had given birth. A good response to treatment was shown with complete resolution of CMO and regression of the FNG (Fig. 2h, i), which was also confirmed on b-scan ultrasonography (Fig. 2j). Best corrected visual acuity remained at 20/60 due to the development of a small posterior subcapsular cataract.
Case 3
A 28-year-old female is a case of recurrent chronic right eye CMO on a background of severe childhood onset retinal dystrophy secondary to confirmed pathogenic homozygous variants in CRB1 and inferotemporal FNG lesion with Coats’ type phenotype.
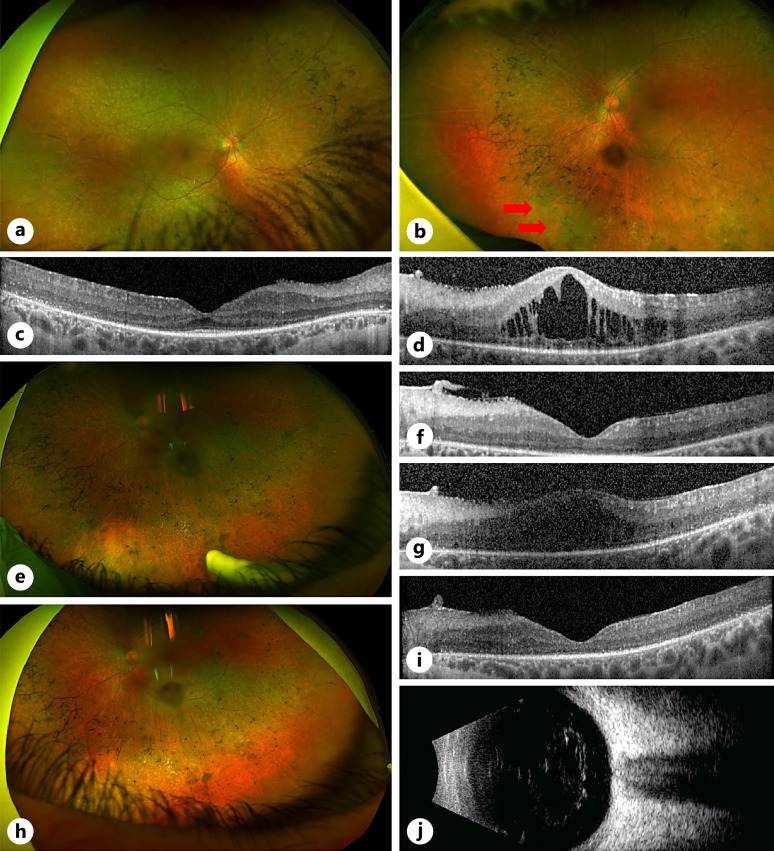
VA on presentation in 2014 was 20/80 OD with longstanding no light perception vision OS from previous extensive serous detachment and subsequent anterior segment ischemia/pupillary block glaucoma (Fig. 3). Examination revealed a right localized inferior serous retinal detachment associated with an inferior vascular complex, vitreous cells, and CMO (Fig. 4a, b). The left eye showed a phthisical eye due to chronic retinal detachment.
Fig. 3.
Timeline of clinical presentation and management of case 3.
Fig. 4.
Phenotype and clinical management of case 3. a, b Presenting phenotype with right inferotemporal FNG associated with CMO. c, e Plaque radiotherapy was offered due to increasing peripheral engorgement and exudation from the FNG, associated with worsening CMO, 7 years of post-initial presentation. d, f Attenuated appearance of FNG lesion 2 months after plaque brachytherapy with persistent CMO due to concomitant postradiation inflammation. g Development of progressive annular tractional retinal detachment with increasing macular edema 14 months after brachytherapy. h Stable retinal appearance with improved appearance of chronic CMO 12 months after undergoing vitrectomy and scleral buckle with removal of silicone oil and 24 months of post-brachytherapy.
She exhibited initial good control of CMO with acetazolamide alone (250 mg daily), with a waxing and waning disease course of a 7-year period (from age 20 years old). In addition, she required periodic cryotherapy to the lesion and barrier laser retinopexy to control her inferior serous retinal detachment.
However, 7 years after initial presentation, due to worsening CMO associated with increasing peripheral engorgement and exudation and deteriorating vision to 20/100 in her right eye (Fig. 4c and e), medical therapy was escalated, and she received increasing doses of acetazolamide to 250 mg twice daily for 4 months. In addition, she received two intravitreal anti-VEGF (bevacizumab 1.25 mg/0.05 mL) injections, 1 month apart. Despite this, the exudative detachment progressed with subsequent visual decline to hand movements, and her tumor thickness was measured to be 3.7 mm on b-scan ultrasonography. She was referred to our ocular oncology colleagues at this point, and management options were discussed. Due to concerns that further cryotherapy may instigate a massive exudative response and PDT laser could potentially cause a tractional retinal detachment with tumor regression, she ultimately underwent plaque radiotherapy with further intravitreal bevacizumab to the lesion. This stabilized her fundal appearance (Fig. 4d), but concomitant postradiation retinopathy and pan-ocular inflammation ensued with worsening CMO (Fig. 4f). The inflammation was treated with a course of systemic oral prednisolone (60 mg daily) for 1 week, tapering by 10 mg per week over 6 weeks, and topical dexamethasone 0.1% four times daily. The CMO continued to have a protracted course but was eventually mitigated with the addition of sub-tenon triamcinolone 4 months after plaque brachytherapy, with improvement in both subjective visual acuity and OCT appearance after 4 weeks post sub-tenon triamcinolone.
At 14 months post-brachytherapy, she developed progressive annular tractional retinal detachment involving the macula (Fig. 4g) in her right eye and was therefore offered further surgical intervention. A 25-gauge pars plana vitrectomy was performed with segmentation of her tractional membranes, cryotherapy to the tumor, and endotamponade with sulfur hexafluoride (SF6). At routine 1-month postoperative review, she was found to have retinal redetachment due to reopening of an entry site break and underwent further 25-gauge pars plana vitrectomy with 276 circumferential scleral buckle and 240 encirclement, endolaser retinopexy, and 5,000 centistoke silicone oil. Four-months post-vitrectomy (18 months post-brachytherapy), review showed successful retinal reattachment but significant periocular and intraocular inflammation which were treated with high doses of oral prednisolone. While the retina remained attached, she developed early and significant cataract and subsequently underwent phacoemulsification and intraocular lens insertion combined with silicone oil removal. Six months post-vitrectomy, the steroids were tapered off, there were no signs of clinical inflammation, and the retina was reattached with stable level of chronic CMO and hand movement vision. The patient’s visual acuity and retinal appearance continued to be stable 1 year of post-retinal detachment surgery and 2 years of post-brachytherapy at her most recent clinical review (Fig. 4h).
Discussion
Our cases demonstrate the varied clinical course of retinal FNG (VPTs) and the need for tailored management based on visually threatening characteristics. In particular, the case of FNG secondary to CRB1-RP underwent a more complicated clinical course and required more aggressive treatment. Although there is no genetically confirmed variant of RP in case 2, the retina also exhibits early features of CRB1-related retinopathy, including retinal thickening and loss of lamination [5], so it is possible that this patient could also carry unknown pathogenic variants in CRB1 gene.
Solitary primary retinal FNG as in case 1, tend to confer a better visual prognosis compared to secondary FNG (cases 2 and 3) with poor visual acuity of worse than 20/200 occurring in 15% compared to 28% in secondary tumors [1]. ERMs remain a significant cause of visual loss in patients affected by FNG, though the relationship between these tumors and the vitreomacular interface remains unclear [1, 4]. Moreover, secondary ERMs are associated with worse visual outcomes and increased central macular thickness compared to idiopathic ERMs and should be observed more closely [6]. Although spontaneous avulsion of retinal FNG with resolution of ERM has been described [7], case 1 had persistent and progressive ERM despite involution of their FNG. This case demonstrated the role of pars plana vitrectomy in managing complications of a FNG that is otherwise quiescent. The role of inner limiting membrane removal in addition to ERM removal remains uncertain in VPT-related ERMs, but we found that peeling of both layers in this patient to be effective, without evidence of recurrence at 6 months of postoperative review.
RP accounts for up to 22% of eyes affected by secondary FNG and may even occur as the initial presentation of RP [1, 8]. While rare, exudative vasculopathies have been reported in association with RP and can manifest on a spectrum from retinal telangiectasias to FNG (VPTs) – the latter of which were demonstrated in cases 2 and 3 [9, 10]. Both cases demonstrated a prolonged disease course with partial clinical regression of vasculopathy and exudation following laser photocoagulation and cryotherapy. Case 3 with CRB1 variant exhibited a particularly aggressive retinal disease phenotype, resulting in early loss of vision in the left eye due to neovascular glaucoma from chronic retinal detachment. Similarly, a combination of CRB1 retinopathy and VPTs led to a protracted disease course in the right eye, ultimately requiring radiotherapy treatment to VPTs. For this patient, this unfortunately evoked a persistent uveitic response and more aggressive recurrence of tractional and serous peripheral retinal detachments which soon became macula-threatening and required complex repair by surgical procedures via pars plana vitrectomy and tamponading with oil and encircling band. This aggressive inflammatory response is likely to be linked to the underlying CRB1 variants which can be associated with chronic inflammation [11, 12].
CRB1 gene encodes for a transmembrane protein that functions as cell polarity regulators and has a role in long-term retinal integrity, with mutations disrupting the spatiotemporal aspects of retinogenesis and resulting in retinal degeneration. In addition, there are typical structural retinal changes including increased retinal thickness, loss of lamination, and photoreceptor degeneration [5, 11]. Pathogenic variants in CRB1 account for up to 6.5% of patients with autosomal recessive RP and are implicated in the cause of a Coats-like manifestation of RP, as in case 3 [10]. Moreover, CRB1 variants themselves have a predilection for CMO [11]. The protracted nature of the CMO in cases 2 and 3 implies that the cause of the macular edema was due to the concurrent VPT, but the underlying CRB1 variation in case 3 is likely to have a predisposition for ocular inflammation and macular edema and more likely to be refractory to simple management. A thorough peripheral search for these lesions should thus be performed in such presentations of unilateral CMO.
Despite notable advances in managing FNG (VPTs), secondary VPTs are known to confer a poorer prognosis [1] and remain a clinical challenge as there is still no consensus on management. Vision loss occurs owing to hemorrhagic and exudative sequelae as well as macular complications (ERM formation, exudation, and CMO) with end-stage disease culminating in neovascular glaucoma [1, 13]. Peripheral small tumors with minimal exudation, as in case 1 and 2, can be effectively treated with laser therapy or cautious application of cryotherapy [13] with adjunctive pars plana vitrectomy for macular complications of ERM. Larger FNG (>2 mm) may be amenable to plaque radiotherapy (ruthenium106 or iodine125) with several large case series showing regression of tumor size and exudation [2, 14]. However, it is important to note that secondary FNG have been associated with poorer visual outcomes than primary FNG irrespective of tumor size when treated with I-125 plaque brachytherapy and therefore may explain the poor response for case 3, whose underlying CRB1 mutation may have provided an impetus for ongoing exudation and retinal thickening refractory to treatment. PDT laser could be considered for these patients, although extensive exudation is more likely to be treatment resistant [13, 14]. Therefore, early aggressive treatment for patients with FNG secondary to retinal dystrophies, particularly CRB1 mutation, is recommended.
Conclusion
There is currently no consensus on the management of FNG (VPTs), which may be seen in patients with RP and can result in significant vision loss. The cases presented reiterate the need for a tailored management paradigm according to patient factors. More severe presentations may occur with FNG associated with inherited retinal dystrophies. In our series, FNG in the presence of CRB1-associated retinopathy led to a complicated clinical course with poor visual outcome.
Statement of Ethics
Ethical approval is not required for this study in accordance with local or national guidelines.
Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
None of the above authors have any conflicts of interests.
Funding Sources
The following authors receive funding from the following sources: MRC and NIHR Oxford BRC to J.C.-K., NIHR Oxford BRC to R.E.M.
Author Contributions
A.I., M.S.S., R.E.M., and J.C.-K. attest they meet the current ICMJE criteria for authorship and all were involved in the clinical care and management of these patients. A.I. wrote the manuscript with the consultation of M.S.S., R.E.M., and J.C.-K., all of whom provided critical feedback with regards to the review and editing of the manuscript.
Funding Statement
The following authors receive funding from the following sources: MRC and NIHR Oxford BRC to J.C.-K., NIHR Oxford BRC to R.E.M.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding authors.
References
- 1. Shields CL, Kaliki S, Al-Dahmash S, Rojanaporn D, Shukla SY, Reilly B, et al. Retinal vasoproliferative tumors: comparative clinical features of primary vs secondary tumors in 334 cases. JAMA Ophthalmol. 2013;131(3):328–34. [DOI] [PubMed] [Google Scholar]
- 2. Heimann H, Bornfeld N, Vij O, Coupland SE, Bechrakis NE, Kellner U, et al. Vasoproliferative tumours of the retina. Br J Ophthalmol. 2000;84(10):1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jain K, Berger AR, Yucil YH, McGowan HD. Vasoproliferative tumours of the retina. Eye. 2003;17(3):364–8. Preprint at. [DOI] [PubMed] [Google Scholar]
- 4. Castro-Navarro V, Saktanasate J, Say EAT, Chiang A, Shields CL. Role of pars plana vitrectomy and membrane peel in vitreomacular traction associated with retinal vasoproliferative tumors. Oman J Ophthalmol. 2016;9(3):167–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daich Varela M, Georgiou M, Alswaiti Y, Kabbani J, Fujinami K, Fujinami-Yokokawa Y, et al. CRB1-Associated retinal dystrophies: genetics, clinical characteristics, and natural history. Am J Ophthalmol. 2023;246:107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim DI, Kim BH, Bae KW, Park UC. Comparison of surgical outcomes after removal of epiretinal membrane associated with retinal break and idiopathic epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2022;260(7):2121–8. [DOI] [PubMed] [Google Scholar]
- 7. McGrath LA, Rundle P. Spontaneous avulsion of a retinal vasoproliferative tumour with resolution of epiretinal membrane. Can J Ophthalmol. 2019;54(4):e173–4. [DOI] [PubMed] [Google Scholar]
- 8. Li HK, Shields JA, Shields CL, Maguire JI, Garg SJ. Retinal vasoproliferative tumour as the initial manifestation of retinitis pigmentosa. Clin Exp Ophthalmol. 2008;36(9):895–7. [DOI] [PubMed] [Google Scholar]
- 9. Bromeo AJ, Lerit SJ, Veloso A, Mercado GJ. Retinal vasoproliferative tumour secondary to retinitis pigmentosa sine pigmento. BMJ Case Rep. 2021;14(5):e240878. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magliyah M, Alshamrani AA, Schatz P, Taskintuna I, Alzahrani Y, Nowilaty SR. Clinical spectrum, genetic associations and management outcomes of Coats-like exudative retinal vasculopathy in autosomal recessive retinitis pigmentosa. Ophthalmic Genet. 2021;42(2):178–85. [DOI] [PubMed] [Google Scholar]
- 11. Slavotinek AM. The family of Crumbs genes and human disease. Mol Syndromol. 2016;7(5):274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li AS, Pasricha MV, Mishra K, Nguyen QD, Beres SJ, Wood EH. CRB1-associated retinal dystrophy presenting as self-resolving opsoclonus and posterior uveitis. Am J Ophthalmol Case Rep. 2022;26:101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hussain RN, Jmor F, Damato B, Heimann H. Verteporfin photodynamic therapy for the treatment of retinal vasoproliferative tumors. Ophthalmology. 2015;122(11):2361–3. [DOI] [PubMed] [Google Scholar]
- 14. Cohen VML, Shields CL, Demirci H, Shields JA. Iodine I 125 plaque radiotherapy for vasoproliferative tumors of the retina in 30 eyes. Arch Ophthalmol. 2008;126(9):1245–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding authors.