Key Points
Endometriotic lesions secrete exosomes expressing MICA/B, ULBP1–3, FasL, and TRAIL.
These exosomes downregulate the NKG2D cytotoxic pathway and upregulate apoptosis.
This promotes immune escape by suppressing cytotoxicity and killing lymphocytes.
Visual Abstract
Abstract
Endometriosis, affecting 10% of women, is defined as implantation, survival, and growth of endometrium-like/endometriotic tissue outside the uterine cavity, causing inflammation, infertility, pain, and susceptibility to ovarian cancer. Despite extensive studies, its etiology and pathogenesis are poorly understood and largely unknown. The prevailing view is that the immune system of endometriosis patients fails to clear ectopically disseminated endometrium from retrograde menstruation. Exosomes are small extracellular vesicles that exhibit immunomodulatory properties. We studied the role of endometriotic tissue–secreted exosomes in the pathophysiology of endometriosis. Two exosome-mediated mechanisms known to impair the immune response were investigated: 1) downregulation of NKG2D-mediated cytotoxicity and 2) FasL- and TRAIL-induced apoptosis of activated immune cells. We showed that secreted endometriotic exosomes isolated from supernatants of short-term explant cultures carry the NKG2D ligands MICA/B and ULBP1-3 and the proapoptotic molecules FasL and TRAIL on their surface, i.e., signature molecules of exosome-mediated immune suppression. Acting as decoys, these exosomes downregulate the NKG2D receptor, impair NKG2D-mediated cytotoxicity, and induce apoptosis of activated PBMCs and Jurkat cells through the FasL- and TRAIL pathway. The secreted endometriotic exosomes create an immunosuppressive gradient at the ectopic site, forming a “protective shield” around the endometriotic lesions. This gradient guards the endometriotic lesions against clearance by a cytotoxic attack and creates immunologic privilege by induction of apoptosis in activated immune cells. Taken together, our results provide a plausible, exosome-based mechanistic explanation for the immune dysfunction and the compromised immune surveillance in endometriosis and contribute novel insights into the pathogenesis of this enigmatic disease.
Introduction
Endometriosis is a disease characterized by endometrial-like tissue growing outside the uterine cavity. This causes inflammation, severe pelvic pain, dysmenorrhea, infertility, and an increased risk of ovarian cancer. Endometriosis is a common gynecological condition, affecting ∼10% of women of reproductive age worldwide (1–3). Ectopic dissemination of endometrial tissue by retrograde menstruation is the most widely accepted hypothesis for the development of the disease. The displaced endometrial glands implant, proliferate, and invade the underlying tissue, forming endometriotic lesions that grow under the hormonal influence of the menstrual cycle (4, 5). Notably, as many as 90% of fertile women show evidence of retrograde menstruation, but only ∼10% develop endometriosis (6). First described in 1860 (7), the etiology and pathogenesis of endometriosis are still largely unknown. Heritability has been proposed to confer susceptibility to the disease (8). It is suggested that aberrant immune reactions allow the ectopically disseminated endometrium to escape immune surveillance (9, 10). Dysfunctional immune cells and suppressive immune mediators result in poor detection and insufficient clearance of the endometriotic tissue. Compromised NK cell function (11–13) and apoptosis (14) are factors proposed to influence the pathogenesis in endometriosis patients. Higher levels of soluble FasL were found in the serum and peritoneal fluid of endometriosis patients with later-stage disease compared with early-stage and healthy controls (15). We have reported that the inflammation caused by the implantation of endometriotic tissue evokes compensatory expression of immunoregulatory cytokines that deviate the immune response toward local immune suppression and T-regulatory cell priming (16).
It has been reported that eutopic endometrium in healthy subjects produces exosomes that function in the menstrual cycle and regulate trophoblast cell adhesion, migration, invasion, and extracellular matrix remodeling (17–19). Recently, attention has turned to studies of extracellular vesicles (EVs), including exosomes, in endometriosis. However, few reports deal with the role of EVs in the pathophysiology of endometriosis, as previously reviewed (20). Most studies focus on the EV’s nucleic acid cargo, showing that they carry lncRNA and miRNAs with immunoregulatory effects (21–23). Huang et al. (24) found that endometriotic tissue exosomes decreased the phagocytotic capacity of macrophages and promoted their polarization to M2 type; similar results were demonstrated in murine models (25).
EVs, defined by their size as large (200 nm) or small (200 nm) (26), are involved in intercellular communication and serve as important players in physiological and pathological processes in health and disease (27). Small EVs (30–200 nm in size) originating from the endosomal cellular compartment are called exosomes (28) (reviewed in Refs. 29 and 30). Exosomes carry, on their surface and inside, proteins, DNA, mRNA, and noncoding RNAs and can be either immunostimulatory or -suppressive (31–34). According to MISEV2018 (26), the term exosomes should be used when appropriate specific isolation methods and morphological analyses at the ultrastructural level are applied. We have long-term experience in exosome research (35–41), use methods and morphological analyses approved by the International Society for Extracellular Vesicles for exosome studies, and accordingly will use the term exosomes for small EV isolated from endometriotic tissue explant cultures.
Thus, in this study, we investigated the role of exosomes secreted by endometriotic tissue explants in two processes: 1) suppression of the NKG2D receptor-ligand–mediated cytotoxic response of NK cells critical for effective immune surveillance and 2) apoptosis of activated immune cells as possible mechanisms promoting immune privilege of ectopically implanted endometriotic tissue.
Materials and Methods
Study population, inclusion criteria, and collection of samples
Endometriotic tissue and peripheral blood were collected at surgery from ten patients at the Women’s Clinics at Norrland’s University Hospital, Umeå, and Örnsköldsvik’s Hospitals after ethical permission by the Regional Ethical Review Board in Umeå, Sweden (d.nr 09-108M) and written informed consent. Patients were enrolled via GYNOS (Gynecologic Cohort Northern Sweden), comprising consecutively collected ovarian tissue and blood samples from women removing one or both ovaries for any reason since 2005. The criteria for enrollment of patients in this study were as follows: 1) severe endometriosis stage III to IV according to the revised American Society for Reproductive Medicine classification (42); 2) hormonally untreated patients were desired; 3) no anti-inflammatory treatment such as COX inhibitors or glucocorticoids; 4) fertile age, i.e., still having recurrent menstrual bleeding, including those patients with hormonal treatment; and 5) histopathologically verified endometriosis with both endometrial glands and stroma, according to international pathology standards (43). Because our healthcare region is sparsely inhabited, and today, most patients with suspected endometriosis are put on hormonal treatment before surgery, it was difficult to collect enough samples from hormonally untreated patients. To increase the number of study patients, one patient with a low dose of systemically administrated gestagen (5 mg medroxyprogesterone acetate/day) and two patients with hormonal intrauterine devices were enrolled; for the latter, only serum exosomes were analyzed. Histopathological diagnoses and selection of tissue for explant cultures were performed by an experienced pathologist at the accredited clinical pathology laboratory at Norrland’s university hospital. The tissue samples used in explant cultures were taken from the cyst of endometriomas with viable stromal and glandular cells and were put in culture within 2–6 h after surgery. There was no information about the phase of the menstrual cycle when the sampling took place. For controls, serum samples were collected from age-matched healthy donors (n = 6) not subjected to hormonal, anti-inflammatory, or any other treatment. Serum samples from peripheral blood were kept at −80°C until use. Information about the patients and the healthy donors is summarized in Table I.
Table I. Characteristics of endometriosis patients and controls.
| Characteristics | Patients | Controls |
|---|---|---|
| Number | 10 | 6 |
| Explants | 5 | NA |
| Serum | 7 | 6 |
| Age | ||
| Mean | 39.5 | 37.8 |
| Maximum | 52 | 47 |
| Minimum | 24 | 19 |
| Parity | ||
| 0 | 5 | 1 |
| 1 | 1 | 0 |
| 2 | 4 | 3 |
| 3 or more | 0 | 2 |
| Hormonal treatment | ||
| None | 7 | 6 |
| IUD | 2 | 0 |
| IUD + MPA | 1 | 0 |
| Anti-inflammatory drugs* | ||
| Yes | 0 | 0 |
| No | 10 | 6 |
| Dysmenorrhea | ||
| None | 1 | 3 |
| Mild | 0 | 2 |
| Moderate | 3 | 1 |
| Severe | 6 | 0 |
| Histopathology-verified endometriosis | ||
| Yes | 10 | NA |
| No | 0 | NA |
| Stage according to rASRM | ||
| III | 5 | NA |
| IV | 5 | NA |
COX inhibitors or glucocorticoids.
IUD, intrauterine device (levonorgestrel 20 µg/24 h); NA, not applicable; MPA, medroxyprogesterone; rASRM, revised American Society for Reproductive Medicine.
Antibodies
The following Abs were used: anti-CA125 (OV185:1, Imgenex), anti-MICA (159227, R&D Systems), anti-MICB (236511, R&D; bs-6933R, Bioss), anti-ULBP1 (H-46), anti-ULBP2 (H-48; N-16), anti-ULBP3 (H-45), anti-CD63 (MX-49.129.5), and anti-CD81 (5A6) all from Santa Cruz; anti-NKG2D (BD Biosciences); FITC-conjugated anti-CD3 (SK7, BioLegend); mouse anti-human FasL (G247-4; BD Pharmingen); goat anti-human TRAIL (K-18; Santa Cruz Biotechnology); and subclass control Abs anti-mouse IgG1 and IgG2b (DAK-GO1 and DAK-GO9), and rabbit IgG, FITC-conjugated goat anti-mouse IgG all from DAKO.
Short-term endometriotic explant cultures
Endometriotic tissue samples were processed as previously described (38, 39, 44), i.e., the tissue was extensively washed in HBSS, cut into pieces of 5–10-mg wet weight, and cultured in RPMI 1640 with 10% ultracentrifuged, exosome-free FCS, ascorbic acid and antibiotics at 37°C with 5% CO2 for 24 h. The supernatants were collected and kept at −20°C until use.
Isolation and characterization of exosomes
The exosomes were isolated using sucrose gradient ultracentrifugation as described (38, 39, 44). After precentrifugation at 4,000g for 30 min and at 17, 000g for 30 min to remove cell debris and large particles and filtration through a 0.2-µm filter, a 30% sucrose gradient ultracentrifugation at 110,000g for 2 h was applied. After collection of the band with exosomes and washing, the exosomes were stored at −20°C in sterile PBS supplemented with protease inhibitors (Roche Diagnostics). Yield and size distribution were evaluated by nanoparticle tracking analysis (NTA) with ZetaView (Particle Metrix, Germany). The average of five measurements of size and concentration (number of particles/ml) was calculated.
Electron microscopy of endometriotic tissue–derived exosomes
For negative contrast staining, isolated exosomes were absorbed on Formvar/carbon-coated nickel grids, washed with PBS, fixed with 2% paraformaldehyde, and stained with 1.9% methylcellulose containing 0.3% uranyl acetate. Excess fluid was removed, and the samples were dried before examination with transmission electron microscopy (TEM). Immunoelectron microscopy (IEM) was used to assess exosomal markers (tetraspanins CD63 and CD81), CA-125, MICA/Bm, ULBP 1–3, FasL, and TRAIL. After blocking in 0.1 M glycine and 0.3% BSA, grids with exosomes were incubated with appropriate mAbs or isotype-matched control mAbs. After washing, the grids were incubated with secondary Abs conjugated with 10-nm gold particles for 1 h. After washing, silver enhancement, and fixation with 2.5% glutaraldehyde, the grids were negatively stained as described above and analyzed in a JEOL1600 electron microscope.
Protein expression analysis by immunoflow cytometry of isolated endometriotic tissue–derived exosomes coupled to latex beads
Aldehyde/sulfate latex beads, 4% (w/v), 4 µm (Invitrogen Molecular Probes) were used as previously described (38, 44). In brief, for Ab coating, the beads were incubated at 4°C overnight with end-to-end rotation with appropriate concentrations of anti-MICA/B, anti-ULBP1-3, anti-FasL, anti-TRAIL, or isotype-matched control Abs. After washing and blocking of uncoupled sites, exosomes were added and incubated at 4°C overnight with end-to-end rotation (45). After washing, FITC-conjugated CD63 mAbs were added and incubated for 30 min. The beads were then washed and analyzed with an Accuri 6C flow cytometer (BD Biosciences).
Isolation of PBMCs and culture of Jurkat cells
PBMCs from healthy donors were isolated by Lymphoprep (Nycomed) gradient centrifugation (46). The PBMCs were used as readout cells in the receptor studies and cytotoxic and apoptotic experiments. Jurkat cells were cultured in RPMI 1640 and supplemented with 0.5% BSA (Sigma-Aldrich) and antibiotics at 37°C and 5% CO2 with humidity.
NKG2D receptor-ligand studies
Receptor downregulation of NKG2D expression with exosomes was done as described (38, 44). PBMCs from healthy donors were incubated in 5% CO2, at 37°C for 4 h in the absence/presence of native exosomes or in the presence of the same type of exosomes treated with a mixture of specific mAbs against NKG2D ligands or CD63. After incubation, the cells were stained by immunofluorescence with mAbs against the NKG2D receptor. Ten thousand events/sample were collected in Accuri 6C flow cytometer (BD Biosciences) and analyzed in CFlow Plus program (BD Biosciences).
Cytotoxicity experiments
NKG2D-mediated cytotoxicity was assessed using PBMCs from healthy donors as effector cells and the erythroid cell line K562 as targets in an effector:target ratio of 40:1 (38). Cell death was assessed by CytoTox96 nonradioactive cytotoxicity assay (Promega), a calorimetric alternative to the 51Cr release cytotoxicity assay that measures quantitatively lactate dehydrogenase (LDH), a stable cytosolic enzyme that is released upon cell lyses in a similar way as 51Cr is released in radioactive assays. In brief, K562 targets and PBMC effectors were incubated at 37°C for 4 h to assess baseline cytotoxicity. Effector and target cells were incubated with native exosomes or Ab-blocked exosomes at a concentration of 40 μg/ml. Simultaneously, anti-NKG2D blocking Abs (10 μg/ml, clone 1D11, BD Biosciences) were used for receptor blocking, anti-CD63 mAbs were used for exosome blocking, and a mixture of ULBP1-3 and MICA/B Abs (5 μg/ml of each Ab) were used for ligand blocking. The LDH release due to cell death is revealed by development of a color reaction after addition of CytoTox96 reagent, a LDH substrate, provided by the manufacturer. Specific lysis was calculated with the manufacturer-provided standard formula:
| (1) |
Assessment of apoptosis
To detect exosome-induced apoptosis in Jurkat cells and activated PBMCs, the PE annexin V apoptosis detection kit I (BD Pharmingen) and immunoflow cytometry was used, according to the manufacturer’s instructions. Jurkat cells, recommended by the manufacturer for this assay and PMA-ionomycin–activated PBMCs were used as readout cells. PMA and ionomycin at final concentrations of 5 ng/ml and 0.5 µg/ml, respectively, were used for stimulation of PBMCs from healthy donors. In short, Jurkat cells (1 × 106/ml) or activated PBMCs (1 × 106/ml) were cultured on a 96-well plate, treated with native- or mAb-blocked endometriotic tissue–derived exosomes or endometriosis serum exosomes for 24 h, then harvested, washed with PBS, stained with annexin V/7-aminoactinomycin D, and analyzed on an Accuri 6C flow cytometer (BD Biosciences).
Statistical analysis
Expression of ligands/receptors on the surface of exosomes or cells of endometriosis patients compared with healthy controls were analyzed using Student t tests. Pairwise Student t tests were used to compare receptor expression before and after addition/treatment with exosomes, the cytotoxicity experiment before and after addition/treatment with exosomes, and the apoptosis experiment before and after the addition of exosomes. The statistical analyses were performed in MATLAB R2022a.
Results
Short-term endometriotic-tissue cultures secret exosomes
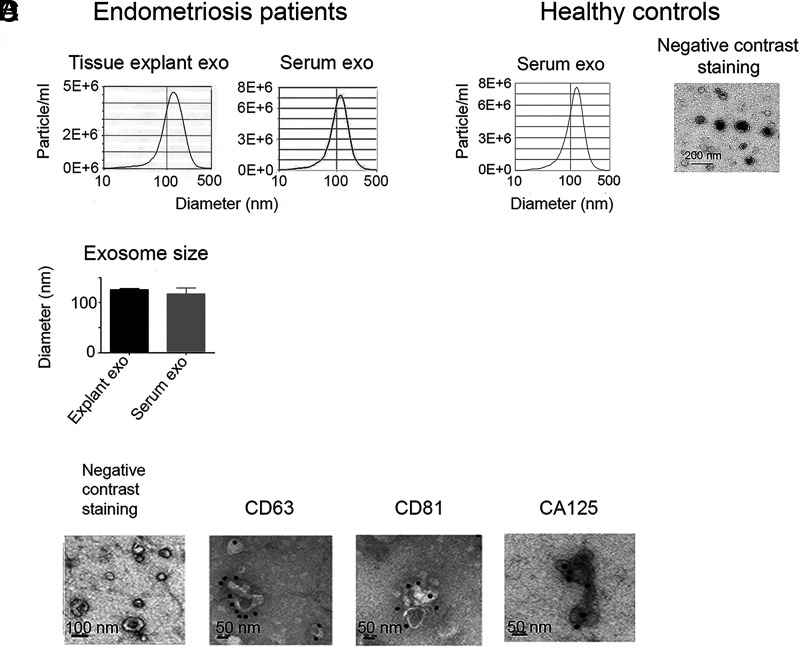
Exosomes were isolated from the short-term culture supernatants and serum from endometriosis patients and healthy controls and characterized by NTA, IEM, and TEM as shown in Fig. 1. The purity, size, and concentration of the isolated tissue-derived exosomes were compared with exosomes isolated from serum of endometriosis patients (Fig. 1A, 1B). In addition to exosomes from endometriotic lesions, various other exosomes from organs/tissues/cells are circulating in the peripheral blood. Negative contrast staining and TEM showed the typical exosomal cup shape (Fig. 1C, 1D). IEM revealed expression of the tetraspanins CD63 and CD81, regularly used as exosomal markers (Fig. 1C). Interestingly, we also found an expression of CA125 on the endometriotic-tissue exosomes, a marker traditionally used in the diagnosis of ovarian cancer.
FIGURE 1.
Nanoparticle tracking analysis (NTA) and electron micrographs of exosomes (exo) isolated from short-term endometriotic tisesue cultures and serum from endometriosis patients and healthy controls. (A and B) Size distribution of representative endometriotic tissue exosomes and serum samples (A) and mean endometriotic tissue exosome size (n = 5) (B) compared with the size of endometriosis serum exosomes (n = 7). (C) Representative micrographs of negatively stained endometriotic tissue exosomes showing size, purity, and the typical cup shape, and immunoelectron microscopy with mAbs and silver-enhanced immunogold staining of the tetraspanins CD63 and CD81; and CA125, often used as an ovarian cancer marker. (D) Representative experiment of NTA analysis and negative contrast staining of exosomes from serum of healthy controls.
Endometriotic tissue–derived exosomes carry NKG2D ligands that downregulate the cognate receptor
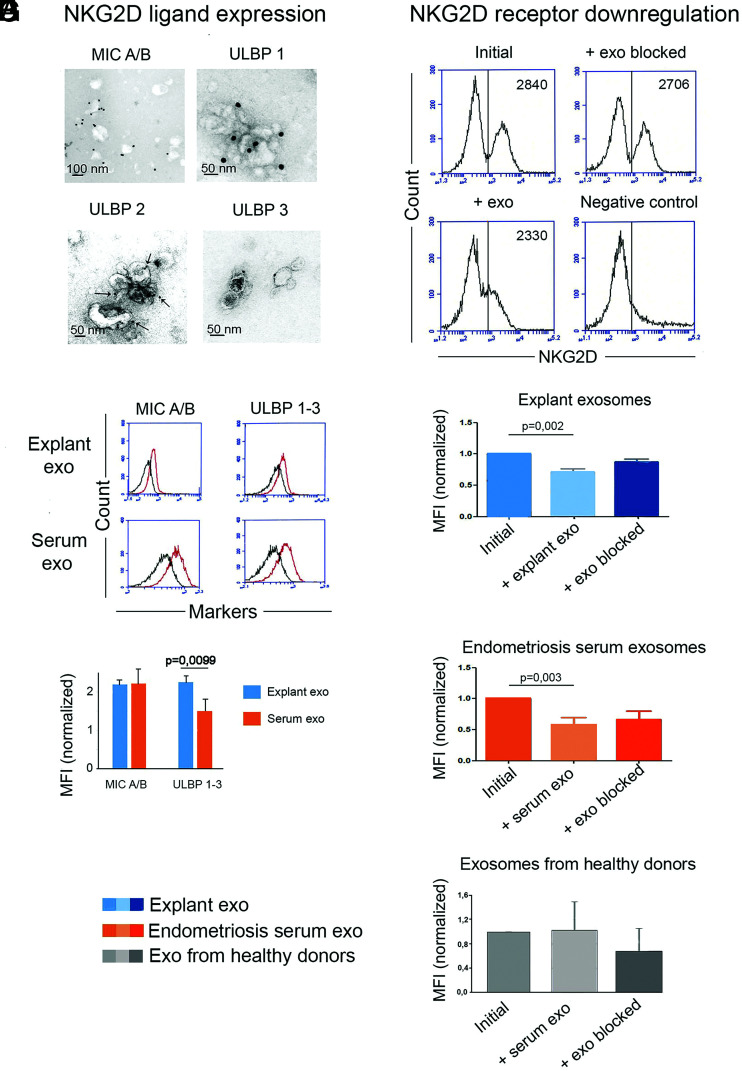
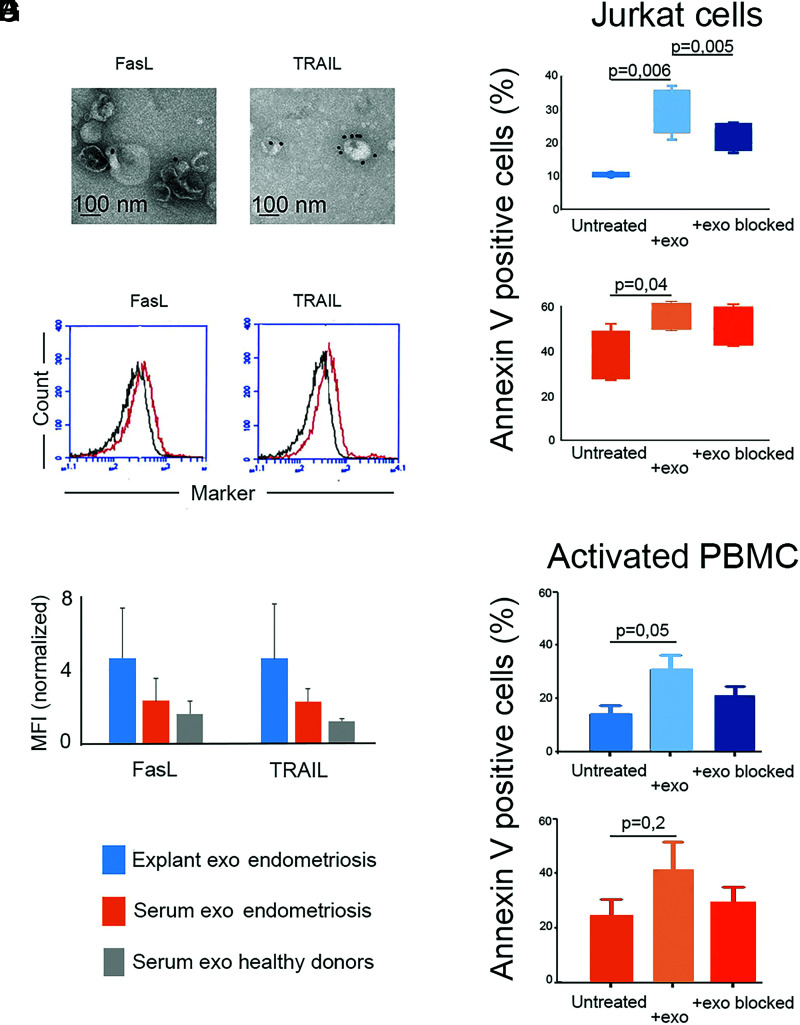
Because NK cells have impaired function in endometriosis (11–13), we specifically studied whether endometriotic-tissue exosomes had an impact on NK-cells’ function. Endometriotic-tissue exosomes and serum exosomes were examined for surface expression of the NKG2D ligands, MICA/B, and ULBP1-3 by IEM and immunoflow cytometry with latex beads. Fig. 2A illustrates representative IEM micrographs of exosomes stained with mAb against MICA/B and ULBP1-3, and in Fig. 2B and 2C, immunoflow cytometry with exosomes captured on latex microbeads coated with mAbs against NKG2D ligands are presented. These experiments confirmed that endometriotic tissue–derived exosomes carry NKG2D ligands on their surface.
FIGURE 2.
Endometriotic tissue–derived exosomes (exo) carry NKG2D ligands that downregulate the NKG2D receptor on PBMCs from healthy donors. (A) Immunoelectron micrographs of endometriotic tissue exosomes expressing the NKG2D ligands MICA/B and ULBP1-3. (B) Immunoflow cytometry of endometriotic tissue exosomes captured on mAb-coated latex beads showing surface expression of members of the MICA/B and ULBP families. The black curve represents negative controls with isotype-matched mAbs. (C) MICA/B and ULBP1-3 expression, measured by MFI normalized to isotype-matched mAb control (= 1), on endometriotic tissue- and endometriosis serum exosomes; note the higher ULBP ligand expression on endometriotic tissue exosomes (n = 4). (D) A representative experiment showing downregulation of the NKG2D receptor expression on PBMCs from healthy donors, assessed by MFI before and after 24-h incubation with native endometriotic-tissue exosomes or with the same exosomes blocked by anti-CD63 Abs. Isotype-matched control mAbs (DAKO) were used in the negative control. (E) Average results of seven experiments like the one shown in (D), measuring NKG2D receptor downregulation in the absence and presence of native or blocked exosomes from endometriotic tissue, p < 0.001. (F and G) Average results of six experiments with endometriosis serum exosomes (F) and four experiments with healthy donors’ serum exosomes (G) like the ones shown in (D) and (E).
We then tested the ability of the NKG2D ligand-bearing exosomes to downregulate the cognate receptor expression, assessed by normalized mean fluorescence intensity (MFI). The results are summarized in Fig. 2D–G. Histograms of one representative experiment show a decrease in MFI after the addition of endometriotic-tissue exosomes and recovery of the MFI after blocking the exosomes by anti-CD63 mAbs (Fig. 2D). A summary of incubation experiments with endometriotic tissue–derived exosomes (Fig. 2E, n = 7) and with endometriotic serum-derived exosomes (Fig. 2F, n = 6) showed a significantly decreased MFI for the NKG2D receptor expression on PBMCs from healthy donors, whereas in the summary of experiments with serum exosomes isolated from healthy donors (Fig. 2G, n = 4), no significance was reached. When the exosomes were pretreated with mAbs against CD63, the surface expression of the NKG2D receptor was restored close to initial values. Thus, we conclude that endometriotic tissue–secreted exosomes, concordant with other NKG2D ligand-bearing exosomes, downregulate the NKG2D receptor. The endometriosis serum-derived exosomes contain endometriotic exosomes and thus had a similar downregulating effect on the NKG2D receptor.
The exosome-mediated NKG2D receptor downregulation impairs the killing ability of NK cells in PBMCs from healthy donors
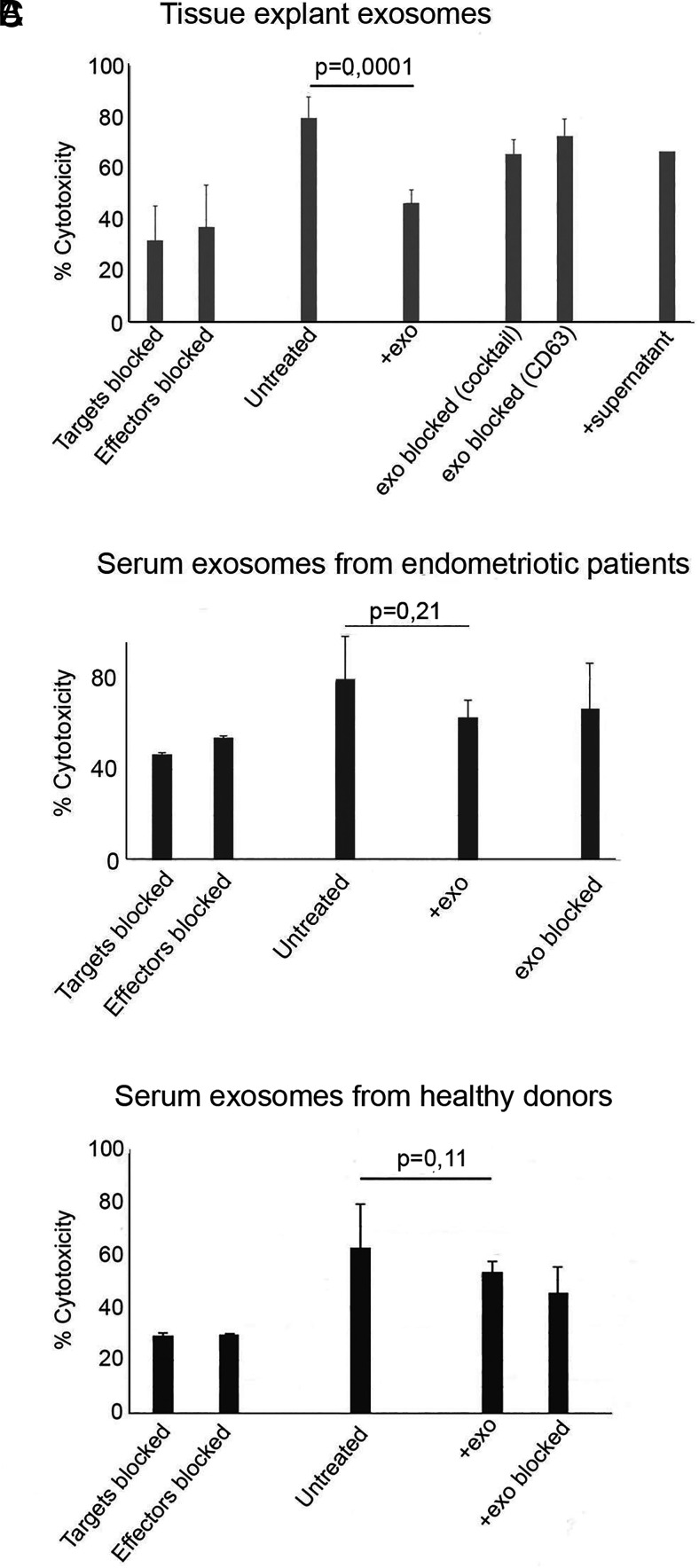
We assessed whether the exosome-mediated downregulation of the NKG2D receptor impaired the killing ability of NK cells (Fig. 3). PBMCs from healthy donors were used as effector cells, and the cells of the human NKG2D ligand-expressing erythroleukemia cell line K562, purchased from American Type Culture Collection, were used as targets. As stated by American Type Culture Collection and the manufacturer and as found in previous reports (47), the K562 cell line’s MHC class I expression is extremely low/negligible. Because MHC expression is mandatory for CTL activation, this assay is vastly assessing NK-cell cytotoxicity and is particulary recommended for evaluation of the NKG2D receptor-mediated NK-cell cytotoxicity. If some scarce cytotoxic signals from other cells might be present, their contribution is negligible. The NK-cell cytotoxicity was assessed with: 1) untreated effector and target cells to assess baseline cytotoxicity; 2) effector and target cells in the presence of native exosomes or exosomes blocked by mAb against NKG2D ligands or anti-CD63 mAbs; 3) anti-NKG2D receptor-blocked effector cells; and 4) anti-NKG2D ligand-blocked target cells. In addition, the effect of supernatant depleted of exosomes on the cytotoxicity was tested. As shown in Fig. 3A, in the presence of native endometriotic-tissue exosomes, the cytotoxic response was downregulated to levels comparable to blocking effector or target cells. The cytotoxicity was restored when the native exosomes were blocked with a mAb mixture of NKG2D ligands or anti-CD63 mAb. Used supernatant after exosome isolation had no effect on cytotoxicity. In contrast, serum exosomes isolated from the sera of endometriosis patients (Fig. 3B) and from healthy donors (Fig. 3C) showed a tendency to downregulate cytotoxicity but did not reach statistical significance. As an explanation, it is plausible to assume that even if endometriotic NKG2D ligand-expressing exosomes are/could be present in the blood of the endometriosis patients together with exosomes from other organs and cells, their concentration and suppressive effect is diluted. From these experiments, we concluded that NKG2D ligand-carrying endometriotic tissue–derived exosomes significantly impair the cytotoxic function of NK cells.
FIGURE 3.
Endometriotic tissue–derived NKG2D ligand-carrying exosomes (exo) impair the killing ability of PBMCs from healthy donors. (A) NKG2D-mediated cytotoxicity was assessed with PBMCs as effector cells and the erythroleukemia cell line K562 as target cells (E:T ratio 40:1) in the presence/absence of native or mAb-blocked endometriotic tissue exosomes (n = 4). Note that the presence of native exosomes reduced the cytotoxicity to a degree comparable to blocking the target or effector cells (p = 0.0001). Treatment of the exosomes with a mAb mixture of NKG2D ligands or anti-CD63 mAb reversed the suppression to nonsignificant levels, compared with untreated target and effector cells. Used supernatant, left after isolation of exosomes, did not affect cytotoxicity. (B) Experiments similar to those in (A) with exosomes isolated from serum from endometriosis patients (n = 3). (C) Experiments similar to those in (A) with exosomes from healthy donors (n = 6).
Endometriotic tissue–derived exosomes carry on their surface FasL and TRAIL that trigger in-vitro apoptosis of Jurkat cells
The expression of the proapoptotic molecules FasL and TRAIL on endometriotic tissue–derived exosomes was confirmed by IEM and immunoflow cytometry of exosomes captured on latex microbeads coated with mAbs against FasL and TRAIL (Fig. 4A, 4B). It is notable that the FasL and TRAIL expression on exosomes from endometriotic tissue, measured by normalized MFI, was much higher than that in endometriosis serum exosomes (Fig. 4C, blue- and orange-colored staples, respectively). The healthy donors’ serum exosomes had low expression (Fig. 4C; and gray-colored staples, respectively). These differences represent the fact that FasL/TRAIL-carrying exosomes are diluted in the peripheral blood and represent only a part of the total moiety of isolated serum exosomes. It is logical to assume that in the endometriosis serum, an inflow of exosomes from the endometriotic lesions takes place. The FasL/TRAIL-carrying exosomes in the serum of healthy donors are much fewer and come from other sources. Apoptosis was assessed using Jurkat cells, as recommended by the manufacturer, and activated PBMCs from healthy donors as readout cells. As shown in Fig. 4D and 4F, the endometriotic tissue–derived exosomes induced a significant apoptotic effect on Jurkat cells and activated PBMCs, respectively, that was reduced after blocking with anti-CD63 mAb. Endometriosis serum exosomes had a significant apoptotic effect on Jurkat cells (Fig. 4E) and a visible apoptotic effect on activated PBMCs (Fig. 4G) without reaching statistical significance.
FIGURE 4.
Endometriotic tissue–derived exosomes (exo) carry functional FasL and TRAIL that trigger apoptosis of Jurkat cells in vitro. (A) Immunoelectron microscopy of endometriotic tissue exosomes stained with mAbs against the proapoptotic ligands FasL and TRAIL. (B) Immunoflow cytometry of endometriotic tissue exosomes captured on latex beads coated with mAbs against FasL and TRAIL. The black curves represent negative isotype-matched mAb controls. (C) Average expression of FasL and TRAIL, measured by MFI normalized to isotype-matched mAb control (= 1) on endometriotic tissue exosomes compared with endometriosis serum exosomes and exosomes from healthy donors (n = 4). (D and E) Apoptosis of Jurkat cells induced by endometriotic tissue–derived exosomes (D) and by endometriosis serum exosomes (E) (n = 4). (F and G) Apoptosis of activated PBMCs induced by endometriotic tissue–derived exosomes (F) and by endometriosis serum exosomes (G) (n = 3).
Discussion
Despite extensive research, the etiology of endometriosis remains unknown, and its pathogenesis is not fully understood. Previous reports suggest that the immune surveillance might be compromised in patients, suffering from endometriosis. Their immune system fails to clear the retrograde dissemination of endometrium to ectopic sites that normally occurs in all women during menstruation. In this article, we present evidence that endometriotic tissue–derived exosomes emerge as common denominators for the impairment of the immune surveillance in endometriosis via downregulation of the NKG2D-mediated NK-cell cytotoxicity and enhancement of apoptotic killing of activated immune cells. In support for this suggestion, our previous cytokine studies in endometriosis showed vigorous ongoing local inflammation (16), a process known to promote and enhance exosome secretion (37).
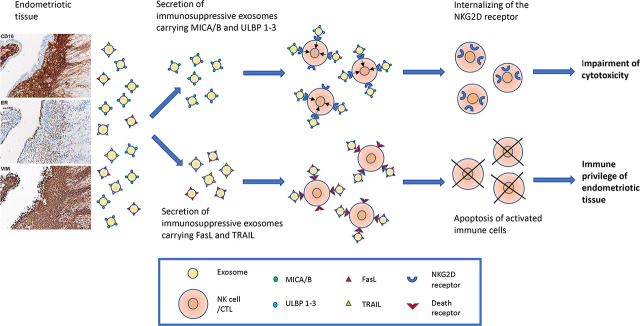
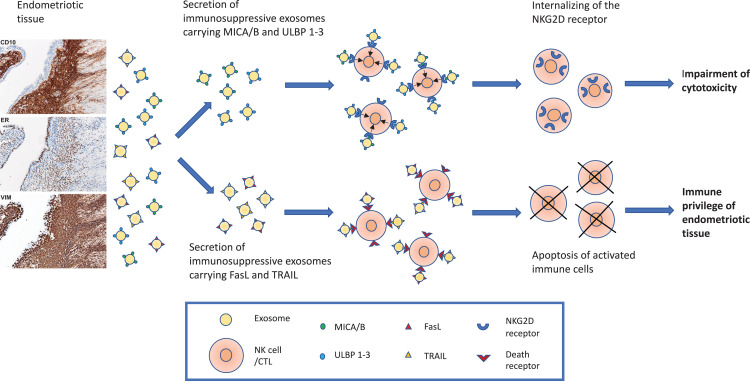
Taken together, our results can be summarized as follows: 1) endometriotic tissue explants ubiquitously secrete small EV with the size and morphology of exosomes; 2) the endometriotic tissue–derived exosomes carry MICA/B and ULBP1‐3 ligands on their surface that downregulate the cognate NK‐cell receptor NKG2D and impair the cytotoxic response of NK cells; and 3) in addition, the endometriotic‐tissue exosomes carry FasL and TRAIL molecules, which induce apoptosis in activated immune cells and thus protect the ectopic endometriotic implants from immune attack. Our results, schematically summarized in Fig. 5, suggest that endometriotic tissue–derived exosomes express immunosuppressive signatures (41) that help the endometriotic tissue to escape immune surveillance and create a local site of immunologic privilege where endometriotic lesions can persist.
FIGURE 5.
Schematic presentation of two immunosuppressive mechanisms driven by endometriotic tissue–derived exosomes. The endometriotic tissue, here presented by diagnostic micrographs stained for CD 10, estrogen receptor (ER), and vimentin (VIM), secretes exosomes carrying the NKG2D receptor ligands MICA/B and ULBP 1–3 and the proapoptotic ligands FasL and TRAIL. MICA/B and ULBP 1–3 carrying exosomes internalize the NKG2D receptor on NK cells and impair their cytotoxic ability. Exosomes carrying FasL and TRAIL induce apoptosis of activated immune cells. These mechanisms protect the endometriotic tissue from immune attack and allow its persistence at ectopic sites.
EVs, including exosomes, in endometriosis have been studied before. Most/all of the EV isolations in the published reports were done from cell lines, enzyme-treated tissue, or serum and peritoneal fluid, in which exosome contamination of other sources could be expected (24, 48, 49). We used another approach: short-term explant cultures of endometriotic tissue obtained by surgery. The tissue immediately emerged in culture medium after extraction and was put in culture within 2–6 h, and the supernatant for exosome isolation was collected after 24 h. In addition to mimicking as closely as possible the in vivo exosome secretion from endometriotic tissue, the choice of 24-h short-term culture aimed to eliminate tissue deterioration, a condition that would increase contamination with other microvesicles, such as apoptotic bodies, or with exosomes not secreted but released from multivesicular bodies of destroyed cells. To our knowledge, this approach for studying exosomes has not been performed before in endometriosis. Huang et al. (24) isolated exosomes from trypsinized endometriotic tissue. Tissue trypsinization is not recommended for exosome harvest because it causes cell damage, which could release immature exosomes from multivesicular bodies of dying cells. This might explain why our exosomes, derived from spontaneous secretion from endometriotic tissue, differ in size (average 120 nm) compared with the exosomes studied by Huang et al. (average 80 nm) (24). Similarly, Zhang et al. (48) used collagenase- and trypsin-treated endometriotic tissue for exosome isolation. Nazri et al. (49) performed isolation of exosomes from peritoneal fluid, which naturally contains a mixture of exosomes from a variety of organs and cells, including immune cells, making it difficult to assess which exosomes are produced by the endometriotic tissue. Because our focus was on endometriotic tissue–secreted exosomes, this approach was not suitable for our study.
It could be of interest to compare exosomes from short-term endometriotic cultures with exosomes from short-term eutopic endometrium cultures. We have not found studies of epithelial cell–derived exosomes from eutopic endometrium in endometriosis. Hsu et al. (50) studied extracellular vesicles produced by stromal cells of endometrium from three endometriosis patients and found that they carry annexin A2 and could promote proliferation, motility, and angiogenesis. A recently published meta-analysis (51) revealed that most of the studies of eutopic endometrium in endometriosis compared with endometrium of healthy women gave conflicting results, probably due to differences in patient cohorts, hormonal influence from the menstrual cycle, and differences in the experimental set-up and methodology. Future studies are needed to address this question.
We showed that endometriotic tissue–derived exosomes carry the NKG2D ligands MICA/B and ULBP 1–3 on their surface and that these exosomes downregulate the NKG2D receptor on PBMCs from healthy controls. We and others have previously reported that NKG2D ligand–bearing exosomes act as a decoy and downregulate cytotoxicity in a dose-dependent manner by internalizing the cognate receptor from the surface of the NK, CTL, and γδ T cells without destroying it (38, 52). Thus, it can be recirculated back to the cell surface. One can assume that the downregulation of the NKG2D receptor is strongest locally at the ectopic implantation site where the constant exosome secretion produces the highest concentration of endometriotic tissue–derived exosomes. It could be expected that the downregulating effect fades when the immune cells move away following the bloodstream and start to recycle the NKG2D receptor expression. We found that exosomes isolated from endometriosis serum and expressing NKG2D ligands on their surface downregulated the NKG2D receptor, whereas serum exosomes from healthy donors did not. The functional cytotoxic experiments confirmed that the exosome-mediated NKG2D receptor downregulation impaired cytotoxicity as shown in Fig. 3. Significant impairment of the cytotoxic response, measured by functional experiments, was only obtained when exosomes isolated from endometriotic cultures were used, whereas experiments with serum exosomes from endometriosis and healthy donors did not reach statistical significance. This is probably due to dilution in the blood. Endometriotic exosomes are present in the serum exosomes from endometriosis but comprise only a part of the total moiety of serum exosomes, of which the majority is produced and secreted in the blood by several organs. In general, all types of serum exosomes per se represent a moiety from different organs and have a very short halftime in the blood, and their composition in serum in real time is rapidly changing. Thus, they exert their function locally on their target cells in various organs and at different sites. The systemic immunosurveillance may not be downregulated to the same extent (38) as at the local level, and the cytotoxic immune function in endometriosis patients might be only partially immunocompromised. Our results are in line with previous reports on increased levels of soluble MICA/B and ULBP in peritoneal fluid from endometriosis patients compared with controls that correlated with disease severity (53, 54). In this study, we show that the increased levels of “soluble” MICA/B and ULBP comprise endometriotic tissue exosomes carrying MICA/B and ULBP. Exosome-carried NKG2D ligands are significantly more potent inhibitors of cytotoxicity than the cleaved and thus truncated soluble ligands (55). Our results are also confirmed by Xu et al. (56), who found decreased expression of NKG2D receptors on NK cells in the peritoneal fluid of endometriosis patients compared with controls. To our knowledge, we have shown for the first time that the NK cell dysfunction in endometriosis is due to secretion of endometriotic tissue–derived exosomes that carry NKG2D ligands, which act as a decoy and interfere with the NKG2D receptor–mediated cytotoxicity. This mechanism is also found in pregnancy, for protection of the semiallogeneic fetus from maternal immune attack (33, 38, 44), and in many cancers, in which NKG2D ligands carried by the cancer exosomes downregulate the NKG2D receptor and impair the cytotoxic anti-tumor response in a similar way (52).
In addition to NKG2D-mediated cytotoxicity, we studied exosomal FasL- and TRAIL-induced apoptosis. Apoptosis, a pathway for clearing infected and damaged cells without mounting an inflammatory response, is also a mechanism of tolerance at immunologically privileged sites such as the eye, testis, and pregnant uterus (57–59). Cancers take advantage of apoptotic mechanisms to escape immune surveillance. Exosomes can induce apoptosis in a dose-dependent manner as shown in our earlier studies (39).
The exosomal membrane and its composition resemble the cellular membrane’s lipid rafts, known to facilitate attachment and aggregation of signaling proteins including the proapoptotic FasL and TRAIL (60). Expression of FasL and TRAIL on exosomes enhance their concentration on the exosomal membrane and protect these molecules from cleavage by matrix metalloproteases (39, 61, 62). We found that endometriotic tissue–derived exosomes express FasL and TRAIL and thus trigger apoptosis in activated PBMCs and Jurkat cells (39, 63). As could be expected, the endometriotic tissue–derived exosomes had the strongest apoptotic effect. An influx of these exosomes to the peripheral blood takes place, and they can induce apoptosis albeit, due to their dilution in the blood, significant but not as strong as when a pure moiety of endometriotic tissue exosomes is used. Our results are in line with a study by Sturlese et al. (64) that showed high levels of mRNA for Fas in mononuclear cells and high soluble FasL in peritoneal fluid of endometriosis patients, which increased with the increasing stage of the disease. They suggested that Fas-bearing mononuclear cells became targets for killing by FasL-expressing endometriotic cells. Our results provide evidence that the killing is mediated “by proxy” by the FasL- and TRAIL-bearing exosomes, produced and secreted by endometriotic cells. Sbracia et al. (65) found reduced expression of Fas and increased levels of FasL in ectopic endometrium compared with controls, suggesting an immune privilege for the endometriotic tissue. Confirming this suggestion, we show that the immune privilege is effectuated by exosomes that induce apoptosis of activated mononuclear cells through the FasL/Fas pathway. This mechanism is most prominent locally in the endometriotic tissue because expression of FasL and TRAIL on serum exosomes was less pronounced.
Exosomes have a potential as diagnostic markers for several diseases (66, 67). We found that the exosomes secreted by endometriotic tissue carry CA 125 on their surface. CA 125 is known to be elevated in serum of endometriosis patients (68). CA 125 is also elevated in most patients suffering from ovarian cancer and thus is not a specific marker in either of these diseases. However, in combination with clinical symptoms, it could still be a useful marker for diagnostic purposes and an address marker for endometriotic exosomes and for separating these exosomes from the peripheral blood.
Our study includes a limited number of patients partly due to the sparsely populated health district of Northern Sweden and partly because we attempted to obtain exosomes from hormonally untreated cases of severe endometriosis, which are very rare to find. An advantage of our study is our experimental set-up of isolating exosomes from spontaneous secretion in short-term endometriotic-tissue explant cultures. This approach has not been previously used in endometriosis. It reflects in the best possible way the in vivo secretion of endometriotic tissue–derived exosomes in the peritoneal cavity of endometriosis patients. Furthermore, all experiments of marker expression and the functional studies are done with exosomes isolated from individual donors; no mixtures of exosomes from several donors were used. Additional studies with much larger and well defined patient cohorts are needed to prove/disprove the results of this investigation. In our further studies, we would like to investigate the NKG2D receptor–ligand system in the eutopic endometrium in endometriosis and compare it to endometrium in healthy subjects and endometriotic lesions. Another interesting question is whether the endometriotic exosomes carry other NK-cell receptor ligands that can affect their cognate receptors.
In summary, the results presented here show, for the first time, that endometriotic lesions secrete exosomes carrying the NKG2D ligands MICA/B and ULBP1-3 and the proapoptotic molecules FasL and TRAIL, i.e., molecules defined as molecular signatures of immunosuppressive/inhibitory exosomes (41). These exosomes downregulate NKG2D-mediated cytotoxicity and induce apoptosis in activated immune cells. The exosomes form a protective “shield” around the endometriotic lesions that prevents a cytotoxic attack and renders the tissue an immune privilege. We provide a plausible exosome-based mechanistic explanation for the immune dysfunction in endometriosis patients that causes insufficient endometrial tissue clearance in the peritoneal cavity and leads to the development of the disease. Our results explain to a great extent the reported NK-cell dysfunction in endometriosis. We do not exclude the possibility that there also could be other unknown or intrinsic factors that could contribute to the NK-cell dysfunction.
Acknowledgments
We are grateful to all donors and the staff at the Women’s Clinic at the University Hospital in Umeå and at the Örnsköldsvik’s Hospital for collecting samples. This study was performed at the Department of Clinical Microbiology/Infection and Immunology of Umeå University.
Footnotes
This work was supported by Grant 18-20-345240311 from the Swedish Research Council/Vetenskapsrådet (to L.M.-N.); Grant CAN 2018/350 (no. 18 07 17) from the Swedish National Cancer Research Foundation/Cancerfonden (to L.M.-N.); Grant from Central ALF Funding (to L.M.-N. and E.L.); Grant LVNFOU992727 from Forskning Utveckling Innovation Region Västernorrland (to E.B.); and grants from the Lion’s Cancer Research Foundation (to E.L., U.O., and P.I.), Avtal om Läkarutbildning och Forskning Funding (to U.O., E.L., and L.M.-N.), the Faculty of Medicine of Umeå University, and Västerbotten County Council.
- EV
- extracellular vesicle
- IEM
- immunoelectron microscopy
- LDH
- lactate dehydrogenase
- MFI
- mean fluorescence intensity
- NTA
- nanoparticle tracking analysis
- TEM
- transmission electron microscopy.
Disclosures
The authors have no financial conflicts of interest.
References
- 1. Zondervan, K. T., Becker C. M., Missmer S. A.. 2020. Endometriosis. N. Engl. J. Med. 382: 1244–1256. [DOI] [PubMed] [Google Scholar]
- 2. Harada, T., Iwabe T., Terakawa N.. 2001. Role of cytokines in endometriosis. Fertil. Steril. 76: 1–10. [DOI] [PubMed] [Google Scholar]
- 3. Cramer, D. W., Missmer S. A.. 2002. The epidemiology of endometriosis. Ann. N. Y. Acad. Sci. 955: 11–22. [DOI] [PubMed] [Google Scholar]
- 4. Sampson, J. A. 1927. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am. J. Pathol. 3: 93–110.43. [PMC free article] [PubMed] [Google Scholar]
- 5. Giudice, L. C., Kao L. C.. 2004. Endometriosis. Lancet 364: 1789–1799. [DOI] [PubMed] [Google Scholar]
- 6. Halme, J., Hammond M. G., Hulka J. F., Raj S. G., Talbert L. M.. 1984. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 64: 151–154. [PubMed] [Google Scholar]
- 7. Knapp, V. J. 1999. How old is endometriosis? Late 17th- and 18th-century European descriptions of the disease. Fertil. Steril. 72: 10–14. [DOI] [PubMed] [Google Scholar]
- 8. Saha, R., Pettersson H. J., Svedberg P., Olovsson M., Bergqvist A., Marions L., Tornvall P., Kuja-Halkola R.. 2015. Heritability of endometriosis. Fertil. Steril. 104: 947–952. [DOI] [PubMed] [Google Scholar]
- 9. Izumi, G., Koga K., Takamura M., Makabe T., Satake E., Takeuchi A., Taguchi A., Urata Y., Fujii T., Osuga Y., et al. 2018. Involvement of immune cells in the pathogenesis of endometriosis. J. Obstet. Gynaecol. Res. 44: 191–198. [DOI] [PubMed] [Google Scholar]
- 10. Ahn, S. H., Monsanto S. P., Miller C., Singh S. S., Thomas R., Tayade C.. 2015. Pathophysiology and immune dysfunction in endometriosis. Biomed. Res. Int. 2015: 795976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang, Y.-J., Jeung I. C., Park A., Park Y.-J., Jung H., Kim T.-D., Lee H. G., Choi I., Yoon S. R.. 2014. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 29: 2176–2189. [DOI] [PubMed] [Google Scholar]
- 12. Gilmore, S. M., Aksel S., Hoff C., Peterson R. D.. 1992. In vitro lymphocyte activity in women with endometriosis–an altered immune response? Fertil. Steril. 58: 1148–1152. [PubMed] [Google Scholar]
- 13. Oosterlynck, D. J., Cornillie F. J., Waer M., Vandeputte M., Koninckx P. R.. 1991. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil. Steril. 56: 45–51. [DOI] [PubMed] [Google Scholar]
- 14. Cho, Y. J., Lee S. H., Park J. W., Han M., Park M. J., Han S. J.. 2018. Dysfunctional signaling underlying endometriosis: current state of knowledge. J. Mol. Endocrinol. 60: R97–R113. [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Velasco, J. A., Mulayim N., Kayisli U. A., Arici A.. 2002. Elevated soluble Fas ligand levels may suggest a role for apoptosis in women with endometriosis. Fertil. Steril. 78: 855–859. [DOI] [PubMed] [Google Scholar]
- 16. Björk, E., Vinnars M.-T., Nagaev I., Nagaeva O., Lundin E., Ottander U., Mincheva-Nilsson L.. 2020. Enhanced local and systemic inflammatory cytokine mRNA expression in women with endometriosis evokes compensatory adaptive regulatory mRNA response that mediates immune suppression and impairs cytotoxicity. Am. J. Reprod. Immunol. 84: e13298. [DOI] [PubMed] [Google Scholar]
- 17. Greening, D. W., Nguyen H. P. T., Elgass K., Simpson R. J., Salamonsen L. A.. 2016. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: insights into endometrial–embryo interactions. Biol. Reprod. 94: 38. [DOI] [PubMed] [Google Scholar]
- 18. Sun, H., Li D., Yuan M., Li Q., Li N., Wang G.. 2019. Eutopic stromal cells of endometriosis promote neuroangiogenesis via exosome pathway. Biol. Reprod. 100: 649–659. [DOI] [PubMed] [Google Scholar]
- 19. Ng, Y. H., Rome S., Jalabert A., Forterre A., Singh H., Hincks C. L., Salamonsen L. A.. 2013. Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo-endometrial cross talk at implantation. PLoS One 8: e58502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freger, S., Leonardi M., Foster W. G.. 2021. Exosomes and their cargo are important regulators of cell function in endometriosis. Reprod. Biomed. Online 43: 370–378. [DOI] [PubMed] [Google Scholar]
- 21. Chen, Y., Wang K., Xu Y., Guo P., Hong B., Cao Y., Wei Z., Xue R., Wang C., Jiang H., et al. 2019. Alteration of myeloid-derived suppressor cells, chronic inflammatory cytokines, and exosomal miRNA contribute to the peritoneal immune disorder of patients with endometriosis. Reprod. Sci. 26: 1130–1138. [DOI] [PubMed] [Google Scholar]
- 22. Khalaj, K., Miller J. E., Lingegowda H., Fazleabas A. T., Young S. L., Lessey B. A., Koti M., Tayade C.. 2019. Extracellular vesicles from endometriosis patients are characterized by a unique miRNA-lncRNA signature. JCI Insight 4: e128846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang, L., Li H.-H., Yuan M., Li D., Wang G.-Y.. 2020. Exosomal miR-22-3p derived from peritoneal macrophages enhances proliferation, migration, and invasion of ectopic endometrial stromal cells through regulation of the SIRT1/NF-κB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 24: 571–580. [DOI] [PubMed] [Google Scholar]
- 24. Huang, Y., Zhu L., Li H., Ye J., Lin N., Chen M., Pan D., Chen Z.. 2022. Endometriosis derived exosomal miR-301a-3p mediates macrophage polarization via regulating PTEN-PI3K axis. Biomed. Pharmacother. 147: 112680. [DOI] [PubMed] [Google Scholar]
- 25. Sun, H., Li D., Yuan M., Li Q., Zhen Q., Li N., Wang G.. 2019. Macrophages alternatively activated by endometriosis-exosomes contribute to the development of lesions in mice. Mol. Hum. Reprod. 25: 5–16. [DOI] [PubMed] [Google Scholar]
- 26. Théry, C., Witwer K. W., Aikawa E., Alcaraz M. J., Anderson J. D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G. K., et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7: 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Niel, G., D’Angelo G., Raposo G.. 2018. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 19: 213–228. [DOI] [PubMed] [Google Scholar]
- 28. Huang, C., Quinn D., Sadovsky Y., Suresh S., Hsia K. J.. 2017. Formation and size distribution of self-assembled vesicles. Proc. Natl. Acad. Sci. U. S. A. 114: 2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sadovsky, Y., Ouyang Y., Powell J. S., Li H., Mouillet J.-F., Morelli A. E., Sorkin A., Margolis L.. 2020. Placental small extracellular vesicles: Current questions and investigative opportunities. Placenta 102: 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taylor, D. D., Gercel-Taylor C.. 2011. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin. Immunopathol. 33: 441–454. [DOI] [PubMed] [Google Scholar]
- 31. Chaput, N., Théry C.. 2011. Exosomes: immune properties and potential clinical implementations. Semin. Immunopathol. 33: 419–440. [DOI] [PubMed] [Google Scholar]
- 32. Clayton, A., Mason M. D.. 2009. Exosomes in tumour immunity. Curr. Oncol. 16: 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mincheva-Nilsson, L., Baranov V.. 2010. The role of placental exosomes in reproduction. Am. J. Reprod. Immunol. 63: 520–533. [DOI] [PubMed] [Google Scholar]
- 34. Kalluri, R., LeBleu V. S.. 2020. The biology, function, and biomedical applications of exosomes. Science 367: eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mincheva-Nilsson, L., Baranov V., Nagaeva O., Dehlin E.. 2016. Isolation and Characterization of Exosomes from Cultures of Tissue Explants and Cell Lines. Curr. Protoc. Immunol. 115: 14.42.1–14.42.21. [DOI] [PubMed] [Google Scholar]
- 36. Labani-Motlagh, A., Israelsson P., Ottander U., Lundin E., Nagaev I., Nagaeva O., Dehlin E., Baranov V., Mincheva-Nilsson L.. 2015. Differential expression of ligands for NKG2D and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes and its influence on NK cell cytotoxicity. Tumour Biol. 37: 5455–5466. [DOI] [PubMed] [Google Scholar]
- 37. Hedlund, M., Nagaeva O., Kargl D., Baranov V., Mincheva-Nilsson L.. 2011. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One 6: e16899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hedlund, M., Stenqvist A.-C., Nagaeva O., Kjellberg L., Wulff M., Baranov V., Mincheva-Nilsson L.. 2009. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J. Immunol. 183: 340–351. [DOI] [PubMed] [Google Scholar]
- 39. Stenqvist, A.-C., Nagaeva O., Baranov V., Mincheva-Nilsson L.. 2013. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 191: 5515–5523. [DOI] [PubMed] [Google Scholar]
- 40. Israelsson, P., Björk E., Nagaev I., Nagaeva O., Lundin E., Mincheva-Nilsson L., Ottander U.. 2022. NKG2D-mediated cytotoxicity improves after primary surgery for high-grade serous ovarian cancer. Am. J. Reprod. Immunol. 89: e13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mincheva-Nilsson, L. 2021. Immunosuppressive protein signatures carried by syncytiotrophoblast-derived exosomes and their role in human pregnancy. Front. Immunol. 12: 717884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. 1997. Fertil. Steril. 67: 817–821. [DOI] [PubMed] [Google Scholar]
- 43. Han, L., García R., Busca A., Parra-Herran C.. 2021. Endometriosis . Pathology Outlines. Available at: https://www.pathologyoutlines.com/topic/ovarynontumorendometriosis.html.
- 44. Mincheva-Nilsson, L., Nagaeva O., Chen T., Stendahl U., Antsiferova J., Mogren I., Hernestål J., Baranov V.. 2006. Placenta-derived soluble MHC class I chain-related molecules down-regulate NKG2D receptor on peripheral blood mononuclear cells during human pregnancy: a possible novel immune escape mechanism for fetal survival. J. Immunol. 176: 3585–3592. [DOI] [PubMed] [Google Scholar]
- 45. Lamparski, H. G., Metha-Damani A., Yao J.-Y., Patel S., Hsu D.-H., Ruegg C., Le Pecq J.-B.. 2002. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods 270: 211–226. [DOI] [PubMed] [Google Scholar]
- 46. Nagaeva, O., Jonsson L., Mincheva-Nilsson L.. 2002. Dominant IL-10 and TGF-beta mRNA expression in gammadeltaT cells of human early pregnancy decidua suggests immunoregulatory potential. Am. J. Reprod. Immunol. 48: 9–17. [DOI] [PubMed] [Google Scholar]
- 47. Michaëlsson, J., Teixeira de Matos C., Achour A., Lanier L. L., Kärre K., Söderström K.. 2002. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J. Exp. Med. 196: 1403–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang, Y., Chang X., Wu D., Deng M., Miao J., Jin Z.. 2021. Down-regulation of exosomal miR-214-3p targeting CCN2 contributes to endometriosis fibrosis and the role of exosomes in the horizontal transfer of miR-214-3p. Reprod. Sci. 28: 715–727. [DOI] [PubMed] [Google Scholar]
- 49. Nazri, H. M., Imran M., Fischer R., Heilig R., Manek S., Dragovic R. A., Kessler B. M., Zondervan K. T., Tapmeier T. T., Becker C. M., et al. 2020. Characterization of exosomes in peritoneal fluid of endometriosis patients. Fertil. Steril. 113: 364–373.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hsu, C.-Y., Hsieh T.-H., Lin H.-Y., Lu C.-Y., Lo H.-W., Tsai C.-C., Tsai E.-M.. 2021. Characterization and proteomic analysis of endometrial stromal cell-derived small extracellular vesicles. J. Clin. Endocrinol. Metab. 106: 1516–1529. [DOI] [PubMed] [Google Scholar]
- 51. Prašnikar, E., Knez J., Kovačič B., Kunej T.. 2020. Molecular signature of eutopic endometrium in endometriosis based on the multi-omics integrative synthesis. J. Assist. Reprod. Genet. 37: 1593–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clayton, A., Mitchell J. P., Court J., Linnane S., Mason M. D., Tabi Z.. 2008. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 180: 7249–7258. [DOI] [PubMed] [Google Scholar]
- 53. González-Foruria, I., Santulli P., Chouzenoux S., Carmona F., Batteux F., Chapron C.. 2015. Soluble ligands for the NKG2D receptor are released during endometriosis and correlate with disease severity. PLoS One 10: e0119961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Groh, V., Wu J., Yee C., Spies T.. 2002. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419: 734–738. [DOI] [PubMed] [Google Scholar]
- 55. Ashiru, O., Boutet P., Fernández-Messina L., Agüera-González S., Skepper J. N., Valés-Gómez M., Reyburn H. T.. 2010. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer. Res. 70: 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu, H. 2019. Expressions of natural cytotoxicity receptor, NKG2D and NKG2D ligands in endometriosis. J. Reprod. Immunol. 136: 102615. [DOI] [PubMed] [Google Scholar]
- 57. Green, D. R., Ferguson T. A.. 2001. The role of Fas ligand in immune privilege. Nat. Rev. Mol. Cell. Biol. 2: 917–924. [DOI] [PubMed] [Google Scholar]
- 58. Benhar, I., London A., Schwartz M.. 2012. The privileged immunity of immune privileged organs: the case of the eye. Front. Immunol. 3: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lee, H.-O., Herndon J. M., Barreiro R., Griffith T. S., Ferguson T. A.. 2002. TRAIL: a mechanism of tumor surveillance in an immune privileged site. J. Immunol. 169: 4739–4744. [DOI] [PubMed] [Google Scholar]
- 60. Lingwood, D., Simons K.. 2010. Lipid rafts as a membrane-organizing principle. Science 327: 46–50. [DOI] [PubMed] [Google Scholar]
- 61. Yamada, A., Arakaki R., Saito M., Kudo Y., Ishimaru N.. 2017. Dual role of Fas/FasL-mediated signal in peripheral immune tolerance. Front. Immunol. 8: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Klinker, M. W., Lizzio V., Reed T. J., Fox D. A., Lundy S. K.. 2014. Human B cell–derived lymphoblastoid cell lines constitutively produce Fas ligand and secrete MHCII+FasL+ killer exosomes. Front. Immunol. 5: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shen, T., Huang Z., Shi C., Pu X., Xu X., Wu Z., Ding G., Cao L.. 2020. Pancreatic cancer-derived exosomes induce apoptosis of T lymphocytes through the p38 MAPK-mediated endoplasmic reticulum stress. FASEB J. 34: 8442–8458. [DOI] [PubMed] [Google Scholar]
- 64. Sturlese, E., Salmeri F. M., Retto G., Pizzo A., De Dominici R., Ardita F. V., Borrielli I., Licata N., Laganà A. S., Sofo V., et al. 2011. Dysregulation of the Fas/FasL system in mononuclear cells recovered from peritoneal fluid of women with endometriosis. J. Reprod. Immunol. 92: 74–81. [DOI] [PubMed] [Google Scholar]
- 65. Sbracia, M., Valeri C., Antonini G., Biagiotti G., Pacchiarotti A., Pacchiarotti A.. 2016. Fas and Fas-ligand in eutopic and ectopic endometrium of women with endometriosis: the possible immune privilege of ectopic endometrium. Reprod. Sci. 23: 81–86. [DOI] [PubMed] [Google Scholar]
- 66. Gurunathan, S., Kang M. H., Kim J. H.. 2021. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int. J. Nanomed. 16: 1281–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Agarwal, S. K., Chapron C., Giudice L. C., Laufer M. R., Leyland N., Missmer S. A., Singh S. S., Taylor H. S.. 2019. Clinical diagnosis of endometriosis: a call to action. Am. J. Obstet. Gynecol. 220: 354.e1–354.12. [DOI] [PubMed] [Google Scholar]
- 68. Hirsch, M., Duffy J., Davis C. J., Nieves Plana M., Khan K. S., International Collaboration to Harmonise Outcomes and Measures for Endometriosis . 2016. Diagnostic accuracy of cancer antigen 125 for endometriosis: a systematic review and meta-analysis. BJOG 123: 1761–1768. [DOI] [PubMed] [Google Scholar]