Abstract
Introduction:
Biportal endoscopic spine surgery (BESS) has gained traction for lumbar laminectomy and diskectomy. To justify the transition to BESS, outcomes and the surgical learning curve should be known. This study evaluates rates of complications with BESS and how these rates change with increased surgeon experience.
Methods:
A single surgeon's consecutive patients who underwent BESS were evaluated. Patients older than 18 years who underwent BESS for lumbar laminectomy and diskectomy were included. Patients with previous spine surgery, multiple levels, or BESS for fusion were excluded. Demographics, length of surgery, intraoperative complications, postoperative complications, and revision surgery were recorded. The learning phase group and mastery phase group were based on a cumulative summation analysis based on surgical time.
Results:
A total of 63 patients, with 31 and 32 patients in the learning and mastery group, respectively, were included. Surgical time decreased from 87 to 52 minutes in the mastery phase. Conversion to open decreased from 3 to 0 cases (P = 0.1803), intraoperative complications decreased from 3 to 0 (P = 0.1803), postoperative complications decreased from 7 to 2 (P = 0.017), and rates of revision surgery decreased from 4 to 1 (P = 0.4233).
Conclusion:
This study suggests a learning curve of 31 cases for adequate performance of BESS for lumbar laminectomy and diskectomy.
Laminectomy and diskectomy are commonplace surgeries in modern-day orthopaedics, and their efficacy in treating offending pathology, spinal stenosis, and herniated nucleus pulposus is proven.1 Within these treatment options, different techniques on how to perform them have been developed and practiced over the years. In the United States, standard open surgery and “minimally invasive” tubular surgeries have predominated and subsequently been taught in most spine surgery training programs. Endoscopic spine surgery (ESS) is a relatively newer technique that has had greater adoption into practice in other countries but has not become a large portion of spine practices in the United States.2
As interest in minimally invasive spine surgery continues to grow, interest in ESS techniques and technology continue to be developed and improved on. Endoscopic spine surgery techniques involve procedures using small incisions to insert an endoscope and accompanying tools to the affected spine segment.3 Different procedures and pathologies can be addressed including laminectomy, diskectomy, and even fusions.
There are a multitude of proposed and recognized benefits of minimally invasive spine surgery and, furthermore, ESS. These include decreased postoperative pain and potentially faster recovery secondary to decreased local tissue and muscle trauma; safer and more effective surgery due to improved aspects of surgical visualization provided by an endoscope; and decreased neck and back pain to the surgeon due to ergonomic advantages from using an endoscope similar to orthopaedic arthroscopic procedures.3
Standard ESS has been performed from a single portal that allowed viewing and instrument access.4 A relatively new technique, biportal ESS (BESS) or unilateral biportal endoscopy is a technique that has been developed, which uses two ipsilateral portals. One is primarily the viewing portal, and the second is the working portal. This technique more closely resembles the hand-eye coordination used by several orthopaedic surgeons during training in large joint arthroscopy.
Endoscopic spine surgery has known benefits but continues to have a slow widespread adoption. This is due to the lack of exposure to the technique in most training programs. Without training in the technique, the adoption of ESS into an established practice is met with a perceived steep learning curve. There are courses available to learn and practice the technique, but it is unclear to the established surgeon when the cost of learning a new technique will be outweighed by the benefits of endoscopy. Furthermore, in the United States, with relatively few surgeons adopting this technique, courses and support groups within the United States is limited, which can affect the learning curve and adoption. Finally, BESS can be performed without a dedicated spine company that hinders courses and support systems typically provided by industry.
The purpose of this study was to evaluate the learning curve, as defined by the number of surgeries to stabilization, surgical time, and rate of complications, of a US fellowship-trained surgeon after the addition of BESS to his practice. The secondary aim was to evaluate patient-reported outcomes after BESS.
Methods
This was a single-center retrospective review of a single US fellowship-trained orthopaedic spine surgeon. The surgeon's clinical practice database was evaluated for patients who underwent BESS. This included all cases performed in the first 18 months after the surgeon's initial adoption of this technique.
The study included any patient older than 18 years. Procedures included single-level lumbar laminectomy or diskectomy by a BESS technique. Patients with previous lumbar spine surgery or who underwent BESS for fusion or multiple levels were excluded. Demographics, length of surgery, intraoperative blood loss, intraoperative complications, postoperative complications, revision surgery, visual analog scale (VAS) pain scores, Oswestry Disability Index (ODI), EuroQol-5 Dimension (EQ-5D), and Patient Reported Outcome Measure Information System (PROMIS)-Physical and PROMIS-Mental patient-reported outcome measures were obtained preoperatively and at 1 and 3 months postoperatively. Any patient who did not follow up to 3 months was excluded. It is the typical practice of the senior surgeon to follow up patients after a single-level laminectomy and/or diskectomy up to 3 months. After that period, people were seen on an as-needed basis. Finally, we only included patients with at least 12 months of follow-up. Patients were evenly categorized into early and late groups based on the date of surgery. The amount of pain medication postoperatively was calculated based on prescriptions sent and filled. Morphine milligram equivalents (MMEs) were calculated.
Techniques of BESS have been published elsewhere, but a brief description is as follows: A 0° or 30° arthroscope is typically used. The patient is placed on a spinal Jackson table with the patient's legs in a sling to open up the interlaminar space. General anesthesia is typically used. A C-arm fluoroscope is used to localize the level of interest. The use of systemic tranexamic acid can be considered to help minimize bony bleeding during surgery.
Two incisions are made approximately just lateral to the spinous process. For right-handed surgeons, a left-sided approach makes surgery easier because the dominant hand coincides with the caudal working portal. Landmarks for cephalad/caudal orientation are as follows: The working portal is typically made at the superior edge of the lower laminar margin of the target level. The camera portal is then made typically 2 cm above that target. A lateral radiograph is then used to confirm the portal placements.
The first step of BESS is to create a working space. A radiofrequency ablator or shaver is used to create the working space at the interlaminar space of the level of interest. One should see the base of the spinous process, inferior edge of the superior lamina, medial edge of the facet joint, and superior edge of the inferior lamina. Using a burr, the ipsilateral lamina is then thinned and removed with a Kerrison ronguer. If a central and contralateral laminectomy needs to be done, the spinous process is undercut and an over-the-top contralateral bony decompression is accomplished similar to tubular surgery. Once all bony work is finished, the ligament flavum is removed en bloc to minimize risk of durotomy. Once the traversing nerve roots ipsilaterally and contralaterally are freed from compression, the laminectomy is finished. Finally, if a diskectomy needs to be performed, the offending disk herniation can be removed. If only an ipsilateral diskectomy needs to be performed, the contralateral bony decompression is not performed and only an ipsilateral bony decompression and ligamentum flavum removal is accomplished.
For statistical analysis, the data were evaluated by multiple ways. The overall averages of each variable were reported. The incidence of complications was plotted against the surgery case number to find the trend of the complication rate as surgeon experience increased.
The learning curve based on surgical time was calculated using a cumulative summation technique similar to that demonstrated by Xu et al.5 For this analysis, it was assumed that mastery is achieved after n → ∞. The average length of surgery (LOS) for all cases will equal the ideal amount of time it takes to perform the surgery after it is learned. Consequently, each trial's operation time can be compared with the average LOS time as a metric to determine the learning curve, LOS. A positive value means that it took longer to perform the surgery than the ideal average time, and a negative will be shorter than the ideal average time. Adding the differences produced a curve that will become more positive as each surgery performed takes longer than the average, or more negative as each surgery performed takes less time than the average. The equation is defined by CUSUM = ∑(LOSi – LOS), where LOSi is the length of the i-th consecutive surgery. A scatter plot of the cumulative summation (CUSUM) values was generated in MATLAB R2022a, and the line of best fit was generated. The R2 value was used to judge the fit of the model, and the model with the highest R2 value was then used for subsequent analysis. The derivative of this line was then taken to determine the local maxima because this represents improving surgical time and defined the threshold for achieving mastery. Measured complications included intraoperative conversion to open procedure, dural tear, epidural hematoma, postoperative reherniation, radiculopathy, or revision surgery. Outcome measures at 1 and 3 months postoperatively were compared with baseline using a paired Student t test.
Results
In total, 63 patients were included in the study. These patients underwent lumbar laminectomy and diskectomy by the biportal endoscopic technique.
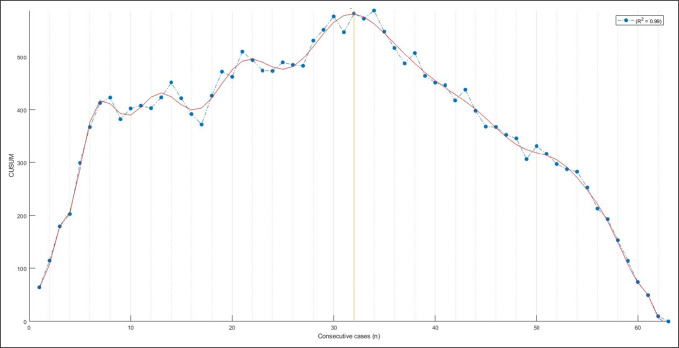
After learning curve analysis, it was determined that there were 31 patients in the learning phase group and 32 patients in the mastery group. No difference was observed in age or sex between groups (P > 0.05); a marginally lower body mass index was found in the mastery phase than in the learning phase group (27.16 ± 0.78 and 29.48 ± 0.80, respectively; P = 0.0425). Figure 1 demonstrates the plot used for determining the learning phase versus mastery phase.
Figure 1.
Line graph demonstrating the development of the learning and mastery phase based on cumulative summation analysis versus case number. The yellow line is at 32 cases. The initial portion of the curve to the left of the yellow line represents the learning phase; the portion of the curve to the right of the yellow line represents the approach of individual surgical time to the average surgical time and can be interpreted as the mastery phase.
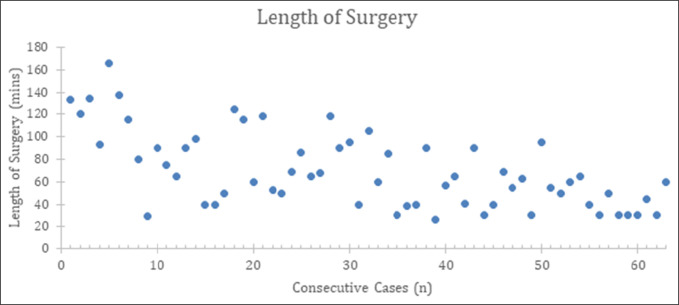
The total average surgical time was 70 minutes, with an average surgical time of 87 and 52 minutes for the learning phase and mastery phase groups, respectively. The overall trend as the number of cases increases is a decrease in surgical time overall, as shown in Figure 2.
Figure 2.
Scatter plot showing the length of surgery plotted against total number of cases. This demonstrates the overall trend of decreased surgical time as the number of cases increased.
Conversion to open decreased from 3 to 0 cases (P = 0.1803). In the cases that were converted to open, one was due to excessive bleeding obscuring the procedure and two were for durotomy.
Intraoperative complications decreased from 3 to 0 (P = 0.1803). The intraoperative complications were a durotomy in two cases and an episode of malignant hyperthermia in one case.
Postoperative complications decreased from 7 to 2 (P = 0.0577) between phases. The learning phase complications were urinary retention resolved medically, persistent radicular-like pain unexplained by repeat MRI, recurrent herniation at the same level but the opposite side, recurrent herniation at the same level but the same side, a return to emergency room for radicular pain within 30 days that returned after a motor vehicle accident and subsided without intervention, and recurrent symptoms of stenosis in two patients who went on to require open decompression and fusion. Complications in the mastery phase included a vertebral compression fracture that occurred after a fall on postoperative day 9 and recurrent herniation at the same level and same side.
The rates of revision surgery decreased from 4 to 1 (P = 0.4233). Of the five patients that required revision surgery, one was an early reherniation, but at the same level on the opposite side. Another was a reherniation after 6 months that occurred at the same level on the same side. The patient reported an acute increase in pain directly after heavy lifting activity. Two patients developed recurrent stenosis over a year after surgery. The patient in the mastery phase who required a revision surgery was a patient with recurrent herniation.
The number of intraoperative complications, postoperative complications, conversion to open, revision surgeries, and recurrent herniations are listed in Table 1.
Table 1.
The Actual Complications That Occurred Broken Down Into Conversion to Open, Intraoperative Complications, Postoperative Complications, Number of Reherniations, and Number of Revision Surgeries
| Conversion to Open | Intraoperative Complications | Postoperative Complications | No. of Revision Surgeriess | No. of Recurrent Herniations | |
| Learning phase (n < 32) | 3 | 3 | 7 | 4 | 2 |
| Mastery phase (n > 32) | 0 | 0 | 2 | 1 | 1 |
These were divided into the learning and mastery phase and demonstrated that all complications occurred within the learning phase.
No patient received a lumbar drain, and there were no cases of postoperative epidural hematoma. Length of hospital stay was not statistically significant between the two groups. All but two patients left the same day of surgery. One patient stayed overnight, and another stayed for a prolonged time related to exacerbation of medical comorbidities and was discharged 8 days after surgery.
Patient-reported outcome scores were collected at 1 and 3 months and compared with preoperative baseline scores. The average VAS score for the left leg at baseline was 4.34 and improved to 1.29 (P = 1E-7) at 1 month and 1.12 (P = 0.0003) at 3 months postoperatively. The average VAS score for the right leg at baseline was 4.31 and improved to 1.19 (P = 1E-6) at 1 month and 0.74 (P = 0.0001) at 3 months postoperatively. The average VAS score for the back at baseline was 6.54 and improved to 2.38 (P = 0.0000) at 1 month and 2.06 (P = 0.0000) at 3 months postoperatively. The average ODI score at baseline was 46.06 and improved to 32.94 (P = 0.00013) at 1 month and 25.94 (P = 0.000013) at 3 months postoperatively. The average EQ5D score at baseline was 0.75 and improved to 0.79 (P = 0.03119) at 1 month and 0.8275 (P = 0.00968) at 3 months postoperatively. The average PROMIS scores did not show a statistically significant difference between 1 and 3 months postoperatively.
Regarding narcotic pain medications, patients received tramadol, hydrocodone, oxycodone, or oxycodone-acetaminophen. Preoperatively, 12 patients (19%) were on narcotics for pain control. Postoperatively, no patient received an initial quantity of more than 30 days of narcotic pain medication. Patients on average received 32 MME (range, 20 to 50 MME) after surgery. No patient received more than 50 MME during the postoperative period. All patients not on preoperative narcotics and 9 of 12 patients (75%) on preoperative narcotics did not require narcotics past 30 days postoperatively. Three patients (<5%) remained on narcotics past 30 days managed by pain management. Those three patients were on preoperative narcotics and, postoperatively, remained at or below their preoperative level of medication.
The patient comorbidities were also collected. All patients had obtained preoperative clearance from their primary care physician. The three most common comorbidities were diabetes (25.4%; 16/63), peripheral vascular disease (17.5%; 11/63), and chronic obstructive pulmonary disease (12.7%; 8/63). Results are presented in Table 2.
Table 2.
Significant Comorbidities Found Among the Patient Population Included in the Study
| Learning Phase | Mastery Phase | Total | |
| Myocardial infarction | 1 | 3 | 4 |
| Congestive heart failure | 3 | 2 | 5 |
| Peripheral vascular disease | 4 | 7 | 11 |
| Cerebrovascular accident | 0 | 2 | 2 |
| Dementia | 1 | 0 | 1 |
| Chronic obstructive pulmonary disease | 7 | 1 | 8 |
| Connective tissue disorder | 0 | 0 | 0 |
| Liver disease | 2 | 2 | 4 |
| Diabetes | 8 | 8 | 16 |
| Hemiplegia | 0 | 0 | 0 |
| Chronic kidney disease | 3 | 3 | 6 |
| History of cancer | 1 | 3 | 4 |
| Leukemia | 0 | 0 | 0 |
| Lymphoma | 0 | 0 | 0 |
| AIDS | 1 | 0 | 1 |
Discussion
The primary purpose of this study was to evaluate the learning curve for a surgeon adding ESS to an established practice especially in the United States. As stated previously, in the United States, there is a lack of industry-sponsored training. Furthermore, the support system from peers is lacking compared with Asia and Europe, which could prolong the learning curve. A previous study evaluating biportal decompressive laminectomy reported that a surgeon with no experience reached adequate performance at the 58th operation. In that study of 60 patients, the average surgical time in the first 30 patients was 10.3 minutes versus 62.4 minutes in the second 30 patients.6 This study found that a “mastery phase” was achieved after 31 separate BESS cases. This translates to not only a decreased average surgical time of 87 to 56 minutes for these cases but also decreased variance in surgical time after the 31 cases. The statistical analysis in this study showed that the mastery phase was achieved after 31 cases. There are several things to consider when interpreting these data. The mastery phase was defined by surgical time compared with an aggregate sum of surgical time during the period. This was done in an attempt to provide a more accurate value for consistent surgical time. When plainly evaluating the length of surgery, the line of best fit in Figure 2 shows a clear overall decrease in length of surgery from the onset of the study.
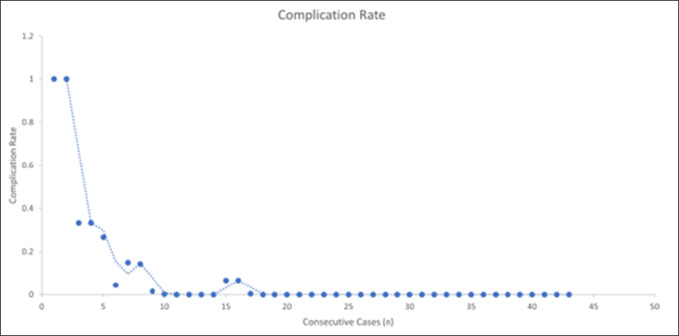
Although ESS can minimize intraoperative complications, it is important to realize that it comes with its own complications that require management in a unique way compared with standard open surgery. The overall complication rate with full ESS has been reported between 2.5% and 9.8%.7 Complications have also been subdivided by severity, with one study reporting a rate of only 2.7% for complications that require intervention, which most often was a reherniation in patients who underwent a diskectomy. Park et al recently published a meta-analysis of 3673 cases reporting 2.23% of cases with a dural tear, 1.29% of cases with incomplete decompression, and 3.79% of cases with an epidural hematoma. Less common complications were transient nerve root injury (0.24%) and infection (0.08%).8 In our study, the complication rate was overall low, with their incidence decreased as the number of cases increased. We found that our incomplete decompression rate was 3.17% (2/63), the durotomy rate was 4.76% (3/63), and the recurrent herniation rate was 4.76% (3/63). Furthermore, our complication rate was much higher in the learning phase compared with the mastery phase. Figure 3 demonstrates graphically the trend of complications as it pertains to the number of cases performed.
Figure 3.
Forest plot showing the cumulative complication rate—calculated as the number of complications over the total number of cases performed—plotted against consecutive case numbers. This shows an overall low complication rate, with all complications occurring within the learning phase and asymptotically approaching zero as the case number increases.
When compared with open procedures, Guerin et al found a durotomy rate of 3.84%.9 Stromquist found in the Swedish registry that the overall durotomy rate was 5.0%, but for lumbar disk herniation, it was lower at 2.8% compared with 6.5% in stenosis.10 Specific to postoperative epidural hematoma, Djiurasovic found a range of 0.01% to 0.69%11 while Aona et al found a rate of 0% for lumbar diskectomy and a rate of 0.5% for decompression.12 However, since in ESS, surgery is done in a restrained space, irrigation can mask bleeding during the surgery. A study by Kim et al13 using postoperative MRI showed a symptomatic hematoma rate of 1.9% and a radiographic rate of 23.6%. These findings are in line with the results discussed in an American Academy of Orthopaedic Surgeons instructional course lecture that compared open and endoscopic surgery. Rates for durotomy were found to be 0% to 9.1% in endoscopy and 0% to 7.6% in open surgery.14 Durotomy is treated with either conversion to open surgery and primary suture closure or with application of fibrin sealant. This decision is dependent on the size of the tear and the surgeon's experience. Rates for recurrent herniation were 1.8% to 11.4% for endoscopic procedures versus 0% to 7.1% for open groups.
It is important to understand the justification for pursuing endoscopic technique as a skill in even an established spine surgeon's practice. The potential benefits of ESS include minimal soft-tissue damage, rapid recovery, and less scar tissue. Faster recovery and improved immediate postoperative pain scores have been shown in previous studies as well as discussed in detail in the aforementioned AAOS instructional course lecture by Zakko et al.14,15 This study again demonstrated low postoperative VAS and ODI scores. Minimum clinically important difference values have previously been published for VAS, ODI, and EQ5D scores, corresponding to 1.37, 12.8, and 0.019, respectively. In our population, scores improved by 4.48 for VAS, 20.12 for ODI, and 0.078 for EQ5D, which all surpass the minimum clinically important difference threshold for respective scores. This is summarized further in Table 3.
Table 3.
Baseline, 1-Month, and 3-Month PROs for the Statistically Significant Findings That Include VAS Right Leg, VAS Left Leg, VAS Back, ODI, and EQ5D Scores
| Baseline | 1 mo | 3 mo | |
| VAS left leg | 4.34 | 1.29 | 1.12 |
| VAS right leg | 4.31 | 1.19 | 0.74 |
| VAS back | 6.54 | 2.38 | 2.06 |
| ODI | 46.06 | 32.94 | 25.94 |
| EQ5D | 0.75 | 0.79 | 0.828 |
ODI = Oswestry Disability Index, MCID = minimum clinically important difference, PRO = patient-reported outcome measure, VAS = visual analog scale.
These are shown with their respective minimum clinically important difference, or MCID.
Surgical invasiveness indices have been established, which have shown that postoperative complications correlate with blood loss. Endoscopic spine surgery has repeatedly decreased blood loss when compared with open procedures of similar magnitude (number of levels, etc).16 In addition, narcotic use after BESS in this study was not inferior to the benchmark for a standard lumbar diskectomy. In 2015, Harris et al17 found that patients who underwent primary lumbar diskectomy had a median duration of postoperative opioid use of 44 days and 17% of patients remained on chronic opioids after surgery. In addition, Schoenfeld et al18 found, in a healthy military population in the United States, that only 8% of patients continued to use narcotics after 3 months with simple procedures like laminectomy and discectomy. Other US data found that the prolonged narcotic use after spinal surgery in the United States after 3 months was as high as 17.3%. Risks were higher if they were using narcotics preoperatively.19 While our study was conducted a few years later, no patient required initial postoperative narcotics of more than 30 days, with the majority requiring narcotics for less than 10 days. Furthermore, only three patients (<5%) remained on chronic opiate use, and all three were on preoperative narcotics.
The design of this study intentionally only followed one surgeon's experience with BESS. A limitation of this is there may not be generalizability to all surgeons. Although it does provide valuable information, the learning curve established should be compared/combined with other published findings to increase generalizability. Second, this study was a retrospective review which is not as strong as a prospective study. Third, this patient population is heterogenous because it includes single-level laminectomy and/or diskectomy. However, we included this group because this patient clinical scenario would be the initial cohort a beginner endoscopy physician would encounter. Fourth, in biportal endoscopy, both surgeries involve the interlaminar approach and are identical compared with uniportal techniques, which can vary markedly based on whether a translaminar diskectomy or interlaminar decompression is chosen. Fifth, some patients required both a laminectomy and a diskectomy. Finally, a long-term study could provide more information, but as in the United States, laminectomy and diskectomy patients are only followed for short periods of time.
It should be emphasized that the technique used in this study was a biportal technique. There are multiple options for ESS at present; however, the biportal technique is relatively new and continues to be examined. To our knowledge, no studies have been published directly comparing the learning curve between different types of ESS. From expert opinion, it is argued that a biportal technique allows for faster adoption, given its similarity to joint arthroscopy regularly practiced in orthopaedic residency. The two-handed technique with a viewing portal and a separate working portal mirrors the technique used in knee, shoulder, etc.
Conclusion
This study suggests that mastery of the biportal endoscopic technique could be achieved after 31 cases of lumbar laminectomy and diskectomy. This mastery is shown not only through continued decrease in length of surgery but also with more consistency in the length of surgery as the number of cases increases. The complications associated with this technique are overall low and comparable with standard open surgery, and the benefits of the endoscopic technique are demonstrated with low postoperative patient-reported pain scores.
Footnotes
None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Easthardt, Dr. Zakko, Dr. Jawad, Mr. Lee, and Dr. Park.
References
- 1.Binder DK, Schmidt MH, Weinstein PR: Lumbar spinal stenosis. Semin Neurol 2002;22:157-166. [DOI] [PubMed] [Google Scholar]
- 2.Lewandrowski KU, Soriano-Sánchez JA, Zhang X, et al. : Regional variations in acceptance, and utilization of minimally invasive spinal surgery techniques among spine surgeons: Results of a global survey. J Spine Surg 2020;6(suppl 1):S260-S274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang JW, Lee DG, Park CK: Rationale and advantages of endoscopic spine surgery. Int J Spine Surg 2021;15:S11-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi CM: Biportal endoscopic spine surgery (BESS): Considering merits and pitfalls. J Spine Surg 2020;6:457-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Wang D, Liu J, et al. : Learning curve and complications of unilateral biportal endoscopy: Cumulative sum and risk-adjusted cumulative sum analysis. Neurospine 2022;19:792-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SM, Kim HJ, Kim GU, et al. : Learning curve for lumbar decompressive laminectomy in biportal endoscopic spinal surgery using the cumulative summation test for learning curve. World Neurosurg 2019;122:e1007-e1013. [DOI] [PubMed] [Google Scholar]
- 7.Ju CI, Lee SM: Complications and management of endoscopic spinal surgery. Neurospine 2023;20:56-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park DY, Upfill-Brown A, Curtin N, et al. : Clinical outcomes and complications after biportal endoscopic spine surgery: A comprehensive systematic review and meta-analysis of 3673 cases. Eur Spine J 2023;32:2637-2646. [DOI] [PubMed] [Google Scholar]
- 9.Guerin P, El Fegoun AB, Obeid I, et al. : Incidental durotomy during spine surgery: Incidence, management and complications. A retrospective review. Injury 2012;43:397-401. [DOI] [PubMed] [Google Scholar]
- 10.Strömqvist F, Sigmundsson FG, Strömqvist B, Jönsson B, Karlsson MK: Incidental durotomy in degenerative lumbar spine surgery - a register study of 64,431 operations. Spine J 2019;19:624-630. [DOI] [PubMed] [Google Scholar]
- 11.Djurasovic M, Campion C, Dimar JR, II, Glassman SD, Gum JL: Postoperative epidural hematoma. Orthop Clin North Am 2022;53:113-121. [DOI] [PubMed] [Google Scholar]
- 12.Aono H, Ohwada T, Hosono N, et al. : Incidence of postoperative symptomatic epidural hematoma in spinal decompression surgery. J Neurosurg Spine 2011;15:202-205. [DOI] [PubMed] [Google Scholar]
- 13.Kim JE, Choi DJ, Kim MC, Park EJ: Risk factors of postoperative spinal epidural hematoma after biportal endoscopic spinal surgery. World Neurosurg 2019;129:e324-e329. [DOI] [PubMed] [Google Scholar]
- 14.Zakko P, Lipphardt M, Park DK: Endoscopic spine surgery: Advertisement or game changer? Instr Course Lect 2023;72:675-687. [PubMed] [Google Scholar]
- 15.Hasan S, Härtl R, Hofstetter CP: The benefit zone of full-endoscopic spine surgery. J Spine Surg 2019;5(suppl 1):S41-S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MJ, Konodi MA, Cizik AM, Bransford RJ, Bellabarba C, Chapman JR: Risk factors for medical complication after spine surgery: A multivariate analysis of 1,591 patients. Spine J 2012;12:197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris AB, Zhang B, Marrache M, et al. : Chronic opioid use following lumbar discectomy: Prevalence, risk factors, and current trends in the United States. Neurospine 2020;17:879-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoenfeld AJ, Nwosu K, Jiang W, et al. : Risk factors for prolonged opioid use following spine surgery, and the association with surgical intensity, among opioid-naive patients. J Bone Joint Surg Am 2017;99:1247-1252. [DOI] [PubMed] [Google Scholar]
- 19.Kowalski C, Ridenour R, McNutt S, et al. : Risk factors for prolonged opioid use after spine surgery. Glob Spine J 2023;13:683-688. [DOI] [PMC free article] [PubMed] [Google Scholar]