Abstract
Background.
Heart transplantation is always an emergency because the transplant needs to occur within 6 h after procurement to prevent primary graft dysfunction. Static cold storage (SCS) is the gold-standard preservation method. This study describes the outcomes of hearts preserved after prolonged SCS (12 and 24 h); those are then resuscitated with a novel normothermic ex situ heart perfusion (NEHP) system.
Methods.
Anesthetized piglets (n = 10) were used as heart donors. Hearts were procured and stored at 5 °C CoStorSol following standard SCS protocols. Two groups were studied: SCS-12 h and SCS-24 h. After SCS, 8 h of NEHP (37 °C blood-based perfusate) was performed at 0.7–1.0 mL/min/g of cardiac tissue. NEHP parameters were monitored continuously. Results were corroborated with 3 additional hearts transplanted orthotopically in healthy recipients (n = 3) after SCS (24 h) + NEHP (5 h). Recipients were observed for 90 min after weaning off cardiopulmonary bypass support.
Results.
All hearts (after 12 and 24 h of SCS) regained normal function and metabolism within 10 min and retained it throughout 8 h of NEHP. No differences were observed in NEHP parameters and histopathology between groups. Three hearts were successfully transplanted after a total ~30 h of preservation (24 h of SCS + 5 h of NEHP + 1 h of second cold ischemia time). The 3 recipients were weaned off cardiopulmonary bypass with mild vasopressor support.
Conclusions.
NEHP has the potential to routinely resuscitate porcine hearts that have undergone SCS for up to 24 h, restoring them to viable function. By objectively assessing heart function before transplant, NEHP may enhance the success rate of transplants. If these resuscitated hearts can be successfully transplanted, it would support the effectiveness of NEHP in ensuring heart viability.
Cardiac transplantation is the gold standard for treating end-stage heart failure unresponsive to optimal medical treatment.1 A key factor affecting posttransplant outcomes is the organ preservation technique.2 Traditionally, static cold storage (SCS) has been used, but it is limited to 6 h.3 Prolonged SCS can lead to delayed graft function (DGF), oxidative stress, and cell death, and it prevents objective assessment of heart function before transplantation.4 Most centers exclude hearts preserved with SCS for >6 h. Mechanical support is an alternative to transplantation, but long-term outcomes are better with heart transplants.1 To address organ scarcity, hearts from marginal donors5 and donations after circulatory death (DCD)6 are used, but they carry higher risks of early complications. Despite increased donations and transplant procedures, waiting times for heart transplants remain unchanged.1,7
Improving preservation methods to ensure successful heart transplants is crucial. Normothermic ex situ perfusion (NEHP) is a promising technique, but the only Food and Drug Administration–approved system (Transmedics, Organ Care System) is currently limited to 6–8 h of preservation,8,9 entails complicated logistics, and presents a significant economic burden.10 In the clinical setting, longer preservation periods with NEHP have led to DGF and required mechanical support with extracorporeal membrane oxygenation for up to 72 h.11,12
Our group successfully used 3-d NEHP with a blood-derived perfusate and plasma cross-circulation with a live animal, which added critical components to the perfusate.13 We have also preserved pediatric and adult hearts in porcine models for 24 h using plasma exchange and hemofiltration without a live animal.14-16 Continuous hemofiltration removes toxic molecules, enabling perfusion of 24 h without plasma exchange.15 This study uses NEHP to resuscitate and evaluate porcine hearts after 12 and 24 h of SCS, corroborated by orthotopic heart transplants in healthy piglets.
MATERIALS AND METHODS
All animals received humane care in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Protocol No. 011170 was approved by the University of Michigan Institutional Animal Care and Use Committee.
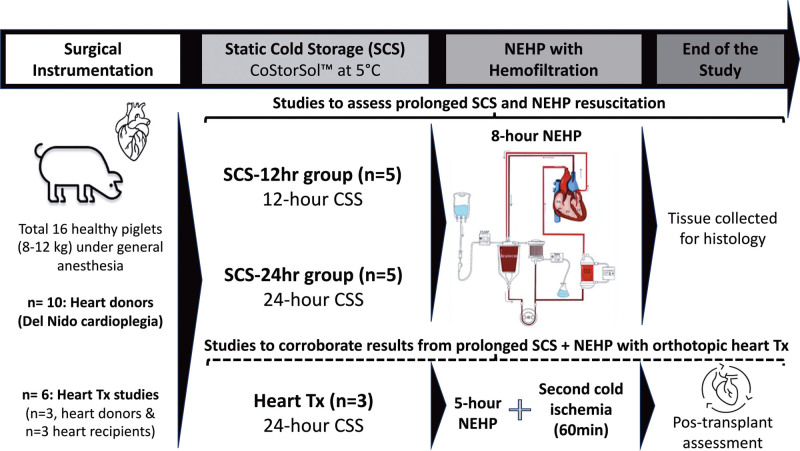
A total of 16 healthy Yorkshire piglets (8–12 kg, 6–8 wk of age) were used in this study as heart donors (n = 13) or heart transplant recipients (n = 3). Hearts from donor pigs were assigned to one of the following groups: SCS-12 h (n = 5, hearts placed in SCS for 12 h followed by 8 h of NEHP) or SCS-24 h (n = 5, hearts placed in SCS for 24 h followed by 8 h of NEHP). The experimental hearts were instrumented (<50 min) for NEHP (Langendorff perfusion) with 37 °C blood-based perfusate at 0.7–1.0 mL/min/g of cardiac tissue for 8 h. Six pigs were used for transplant studies to corroborate the viability of the hearts after prolonged SCS (24 h) + NEHP (5 h). Recipient pigs (n = 3) were observed for 90 min after weaning from cardiopulmonary bypass (CPB) support. The experimental timeline and experimental groups are described in Figure 1.
FIGURE 1.
Experimental timeline and experimental groups description. CSS, CoStorSol; NEHP, normothermic ex situ perfusion; SCS, static cold storage; Tx, transplant.
Surgical Instrumentation and Heart Procurement
Protocols for animal preparation, induction of anesthesia and general anesthesia, and surgical interventions for hemodynamic monitoring were the same as described previously by our laboratory.14-16 An intravenous bolus of lidocaine (1 mg/kg) was administered before median sternotomy. The aorta and venae cavae were isolated and loosely encircled with ligatures, and the donor pig was anticoagulated with unfractionated heparin 400 IU/kg. The pericardium was preserved to prevent heart desiccation during NEHP. Then, the branch vessels from the aortic arch and the venae cavae were ligated, the mid-descending thoracic aorta was cross-clamped, and the left heart was decompressed by transecting the right inferior pulmonary veins. Cold del Nido cardioplegia (CAPS Inc, Detroit, MI) 50 mL/kg was infused antegrade via the proximal innominate artery. Concurrently, topical cooling with sterile iced saline was applied. After cardioplegia administration, 25 mL/kg CoStorSol solution (Preservation Solutions, Inc, Elkhorn, WI) was infused in an antegrade manner. The hearts were excised, weighed, and placed in a saline ice bath for cannula placement (see back table preparation) and then placed in bags with CoStorSol at 5 °C for 12 or 24 h of SCS.
Normothermic Ex Situ Heart Perfusion
Back Table Preparation
A 10-Fr venous cannula (Medtronic, Minneapolis, MN) and a high-compliance balloon connected to a pressure-transducing apparatus for left ventricular (LV) pressure monitoring were inserted via a left atriotomy. A 5-0 polypropylene suture was placed into the posterior leaflet of the mitral valve and secured to the LV balloon to prevent its migration. An 18-Fr DLP cannula (Medtronic Inc, Dublin, Ireland) was placed into the right ventricle (RV) via the pulmonary artery (PA). A ¼ × ¼ inch Luer Lock connector (Medtronic, Minneapolis, MN) was secured in the aortic root for antegrade coronary perfusion. All remaining branches were ligated.
NEHP Circuit
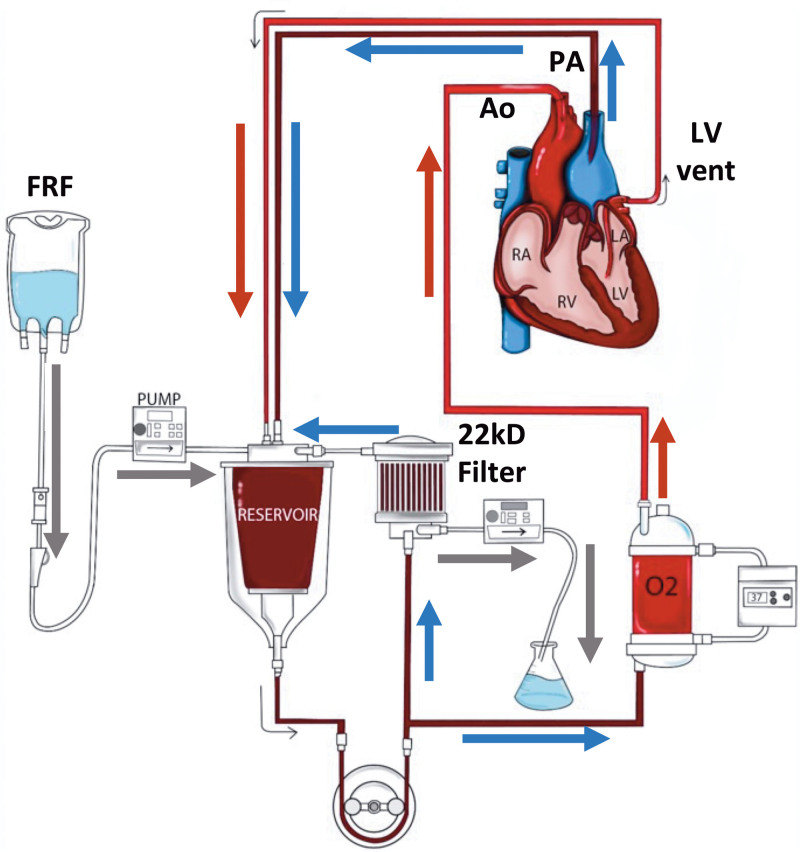
The perfusion circuit, as illustrated in Figure 2, consisted of commercially available components: a reservoir (Terumo, Ann Arbor, MI), an FX05 Baby Capiox Oxygenator (Terumo CVS, Ann Arbor, MI), a roller pump (Stockert, Munich, Germany), and a hemofilter Plasmaflex HF 1000 (Baxter Inc, Deerfield, IL).
FIGURE 2.
NEHP circuit with hemofiltration (Prismaflex HF1000 filter [22 Kd] Baxter Inc, Deerfield, IL). Ao, aortic flow; FRF, filtrate replacement fluid; LA, left atrium; LV, left ventricle; NEHP, normothermic ex situ perfusion; PA, pulmonary artery flow; RA, right atrium.
NEHP Perfusate
The perfusate was platelet- and leukocyte-reduced pig blood with a hemoglobin (Hb) concentration goal of >8 g/dL for both experimental and control hearts. Perfusate electrolytes and metabolic abnormalities were corrected to normal physiological ranges at 37 °C with a priming volume of approximately 300 mL before connecting the heart to the system. Anesthetized healthy pigs (100–120 kg) were exsanguinated for blood donation. Blood (5–6 L) was collected using the 3-bag Teruflex (Terumo Corp, Tokyo, Japan) blood bag system with citrate phosphate dextrose adenine solution anticoagulant. The volume of blood per kit was 450 mL. The collected blood was then stored in a refrigerator at 5 °C for up to 10 d on a rocking system until use. Whole blood was then separated using the collecting blood bags via centrifugation (Sorvall Legend XFR Centrifuge, Thermo Fisher Scientific, Waltham, MA) for 20 min at 25 °C with 3600 RPM. Plasma and packed red blood cells (pRBCs) were then collected using the plasma extractor and Fenwal transfer set (Fresenius Kabi AG, Bad Homburg, Germany) and the buffy coat (platelet and white blood cells) was discarded.
NEHP System Prime and Perfusate Exchange
The priming of the circuit had several steps: (1) 300 mL of Plasma-lyte A (balanced crystalloid solution) to remove any air in the system, (2) Plasma-lyte A is removed from the system (as much as possible), and (3) addition of blood-derived perfusate from the intravenous transfer bag (see below) into the NEHP reservoir. The priming volume was approximately 250–300 mL of platelet- and leukocyte-reduced blood with a Hb concentration goal of >8 g/dL and hematocrit of ≥24% for all studies. The blood-derived perfusate was then oxygenated and conditioned to normothermic conditions (37 °C) before connecting the heart to NEHP. Washed pRBCs from our animal blood bank were used to maintain a Hb concentration of >8 g/dL. Normal saline was used to wash pRBCs if the K values were >9 mmol/L. In addition, calcium is monitored immediately after pRBC and hourly during NEHP and replenished if ionized Ca <1.1 mmol/L with 250 mg calcium gluconate (1 mL bolus). Glucose was replaced if perfusate levels were <60 mg/dL, but this rarely occurs during NEHP with the addition of hemofiltration.
The perfusate was exchanged at 60-min NEHP to eliminate residual cardioplegia and toxins that may have accumulated from reperfusion. The perfusate exchange followed CPB practices. The volume in the reservoir was depleted to a safe level (~30 mL), and the new perfusate was added while the outflow from the heart was collected and discarded. This maneuver mitigated air embolism, whereas most of the perfusate was exchanged without stopping perfusion of the heart.
NEHP With Hemofiltration
Perfusion was performed in parallel with hemofiltration. The hemofilter was Prismaflex HF 1000 (Baxter Inc, Deerfield, IL), which filters molecules up to 22 kD. Perfusate hemofiltration was maintained at 1 mL/h/g cardiac tissue using an intravenous pump. Isotonic filtrate replacement fluid (FRF) was added to the perfusate at a 1:1 ratio. One liter of FRF consisted of 750 mL 0.9% saline solution and 250 mL with 3.3 g glucose, 400 mg calcium gluconate, 30 mEq bicarbonate, 160 mg magnesium, 4 mEq potassium, 250 mg nafcillin, and 40 mg gentamicin.
NEHP Parameters
After completion of the SCS time, the hearts and cannulas were de-aired and connected to the NEHP system. Aortic blood flows were slowly adjusted to maintain a physiologically normal coronary flow rate of 1.0 mL/min/g of cardiac tissue because the heart was rewarmed. Hemofiltration was maintained at 1.0 mL/h/g of cardiac tissue. FRF was infused at an equal rate to maintain circuit euvolemia. Temperature was maintained at 37 °C using a water heater (CSZ Cincinnati Sub-Zero ECMO Heather, Cincinnati, OH). The sweep gas (50% O2, 45% N2, 5% CO2) was adjusted to maintain pCO2 at 45 ± 5 mm Hg. If fibrillation occurred, the heart was defibrillated with 3–5 J using internal defibrillation paddles (Philips, Andover, MA). After 60 min of NEHP, the perfusate was exchanged using 300 mL of blood-derived perfusate to eliminate residual storage solution circulation during reperfusion. The perfusate exchange followed CPB practices. The volume in the reservoir was depleted to a safe level (~30 mL), and the new perfusate was added while the outflow from the heart was collected and discarded. This maneuver mitigated air embolism, whereas most of the perfusate was exchanged without stopping perfusion of the heart.
Range of Normal NEHP Parameters
Normal ranges of all NEHP parameters are as follow: sinus rhythm; LV systolic (LVS) pressure: 35–90 mm Hg; calculated coronary vascular resistance: 0.25–1.0 mm Hg/mL/min; venous oxygen saturation: 70%–90%; buffer base deviation: 40–50 mmol/L; PA lactate levels: <2 mmol/L; PA potassium levels: 3–5 mmol/L.
Data collected during NEHP included perfusion flows and pressures, calculated coronary resistance (CCR), oxygen kinetics (calculated oxygen delivery [DO2], consumption, and oxygen extraction ratio), perfusate biomarkers (potassium and lactate values), and arterial and venous perfusate gases. Heart rate (HR), LVS pressure, and cardiac rhythm were used to assess cardiac function. Formulas used for calculated variables are described in Table 1.
TABLE 1.
Formulas used for calculated variables during NEHP
| Variable | Formula |
|---|---|
| CaO2, mL/dL | |
| CvO2, mL/dL | |
| A – VO2, mL/dL | |
| CCR, mm Hg/mL/min | |
| DO2,a mL/min/100 g cardiac tissue | |
| VO2,a mL/min/100 g cardiac tissue | |
| EOR | |
| WDr | |
| Normalization to 100 g cardiac tissue |
aValues normalized to 100 g of cardiac tissue.
Ao, aortic; A – VO2, oxygen content difference; CaO, arterial oxygen content; CBF, coronary flood flow (same as pulmonary artery blood flow) in mL/min; CCR, calculated coronary resistance; CvO2, venous oxygen content; DO2, oxygen delivery; EOR, oxygen extraction ratio; Hb, hemoglobin; NEHP, normothermic ex situ heart perfusion; paO2, partial pressure of arterial oxygen (mm Hg); pvO2, partial pressure of venous oxygen (mm Hg); SaO2%, arterial oxygen saturation; SvO2%, venous oxygen saturation; VO2, oxygen consumption; WDr, wet-dry weight ratio.
Termination of NEHP
Ex situ perfusion was maintained for 8 h before elective termination in all 10 SCS studies. The hearts not selected for transplantation (n = 10) were decannulated, weighed, and sent to pathology in 10% buffered formalin. The 3 hearts used for transplantation into healthy recipient piglets after 24 h of SCS and 5 h of NEHP were rearrested via antegrade administration of 250 mL of del Nido cardioplegia using the NEHP system at a pressure not exceeding 50 mm Hg. After cardioplegia administration, hearts were removed from the NEHP system, decannulated, and prepared for implantation. The second cold ischemia time did not exceed 60 min.
Orthotopic Heart Transplantation Studies
Heart Explantation
Healthy piglets were selected (n = 3) as heart donors and managed as described earlier. After 24 h of SCS and 5 h of NEHP, hearts were cooled down and put in SCS for 1 h then transplanted into healthy pigs. Recipients were induced as stated earlier and maintained under general anesthesia via inhaled isoflurane. Central venous access and arterial access were obtained via bilateral femoral vessels for anesthesia monitoring and intravenous administration of medications. A midline sternotomy was performed to gain access to the thoracic cavity. The great vessels were isolated. Bicaval cannulation was achieved via 16-Fr right angle DLP cannulas for drainage. Aortic arterial cannulation was achieved using a 12-Fr DLP cannula via the ascending aorta for reinfusion. Animals were fully supported on normothermic CPB at a blood flow of 50–80 mL/min/kg, maintaining a mean arterial pressure (MAP) of >45 mm Hg. After the initiation of CPB, the ascending aorta was cross-clamped and the recipient’s heart was explanted using standard clinical methods.
Heart Implantation
After donor preparation and steroid administration, implantation began with anastomosis of the left atrium, the inferior and superior caval anastomosis, and the aortic anastomosis. Careful attention was given to maintain the patency of the recipient pulmonary veins. The cross-clamp was subsequently removed, and the PA anastomosis was completed.
Posttransplantation Management (90 min)
After successful transplantation, animals were weaned from CPB (based on MAP >45 mm Hg) and decannulated. All animals were maintained under general anesthesia and hemodynamically supported to achieve physiologic perfusing pressures. Hemodynamic and metabolic data were monitored throughout the study. At study termination, animals were euthanized.
Vasopressor Support
Vasoactive-inotropic score (VIS) was used to assess the total inotrope exposure and support during the recipient procedure (CPB support and posttransplant period). The agents included in VIS were 1 × dopamine dose (µg/kg/min) + 1 × dobutamine dose (µg/kg/min) + 100 × epinephrine dose (µg/kg/min) + 10 × milrinone dose (µg/kg/min) + 10 000 vasopressin dose (U/kg/min) + 100 × norepinephrine dose (µg/kg/min) were the agents used to support the heart transplant recipients. In addition, the VIS was used to calculate the vasopressor dependency index (VDI) = VIS/MAP. VDI was the main tool to assess hemodynamic response based on the relationship between the vasopressor doses and MAP. The higher the VDI, the higher the vasopressor doses. A score of <10 µg/kg/min is associated with better postoperative outcomes compared with a score of >10 µg/kg/min.
Tissue Analysis
Macroscopic Tissue Analysis
After NEHP, tissue samples (1 cm × 1 cm) from each cardiac chamber were weighed (wet weight), stored in a desiccator for 7 d, weighed again (dry weight), and the ratio of wet weight to dry weight was calculated (wet-dry ratio; see Table 1).
Microscopic Analysis
Hearts were sent to pathology for routine hematoxylin and eosin staining. The samples were examined and scored from 0 to 3. Lower scores suggested proper tissue preservation (0: absent injury; +1: mild injury; +2: moderate injury; and +3: severe irreversible injury) as assessed by a blinded veterinary pathologist using a previously described cardiac histopathological injury score17 based on myofiber degeneration, myocardial hemorrhage, interstitial edema, and endothelial changes. Average scores using the ordinal data for each injury type were reported for each cardiac chamber and the total cumulative score for each heart in both groups.
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism version 10.0.0 for Windows (GraphPad Software, Boston, MA, www.graphpad.com) and Microsoft Excel 365 Version 16.79.1 for Mac. Differences were analyzed and compared within each test group and between the test groups. A mixed model was performed to examine the effect of SCS time or NEHP time between groups. The heart/experiment number is the repeated measure variable, and the independent variables were the experimental group (length of SCS time) and the NEHP time. Finally, post hoc analysis using a Bonferroni-corrected confidence interval was used to determine differences between experimental groups for all dependent variables (NEHP characteristics, cardiac function, perfusate values, and oxygen kinetics). Wilcoxon rank-sum tests were used to test the difference in wet-dry ratios between treatment groups. Histopathologic data were compared using the Mann-Whitney U test, and box-and-whisker plots were constructed for illustration. All P values of <0.05 were considered statistically significant. Results are expressed as mean values with error bars representing standard error.
RESULTS
All hearts were successfully resuscitated after 12 h (n = 5) and 24 h (n = 5) of SCS during the full duration of NEHP (8 h). The average heart weight at the beginning of the study was 54.4 ± 8.5 and 51.6 ± 2.3 g in the SCS-12 h and SCS-24 h groups, respectively, or 53.8 ± 2.5 g across groups. At the end of the study, the wet weight gain was <10% in both groups, specifically 8.4% ± 5.7% and 8.5% ± 2.2% in the SCS-12 h and SCS-24 h groups, respectively, without statistical significance.
Throughout the NEHP period, all hearts maintained adequate function, with perfusion and metabolic parameters remaining within the normal range, as detailed in the Materials and Methods section, along with the biomarkers and oxygen kinetics. The 3 transplanted hearts had the same NEHP characteristics, were successfully transplanted into healthy recipients, and were weaned off CPB with mild vasopressor support.
Cardiac Function During NEHP
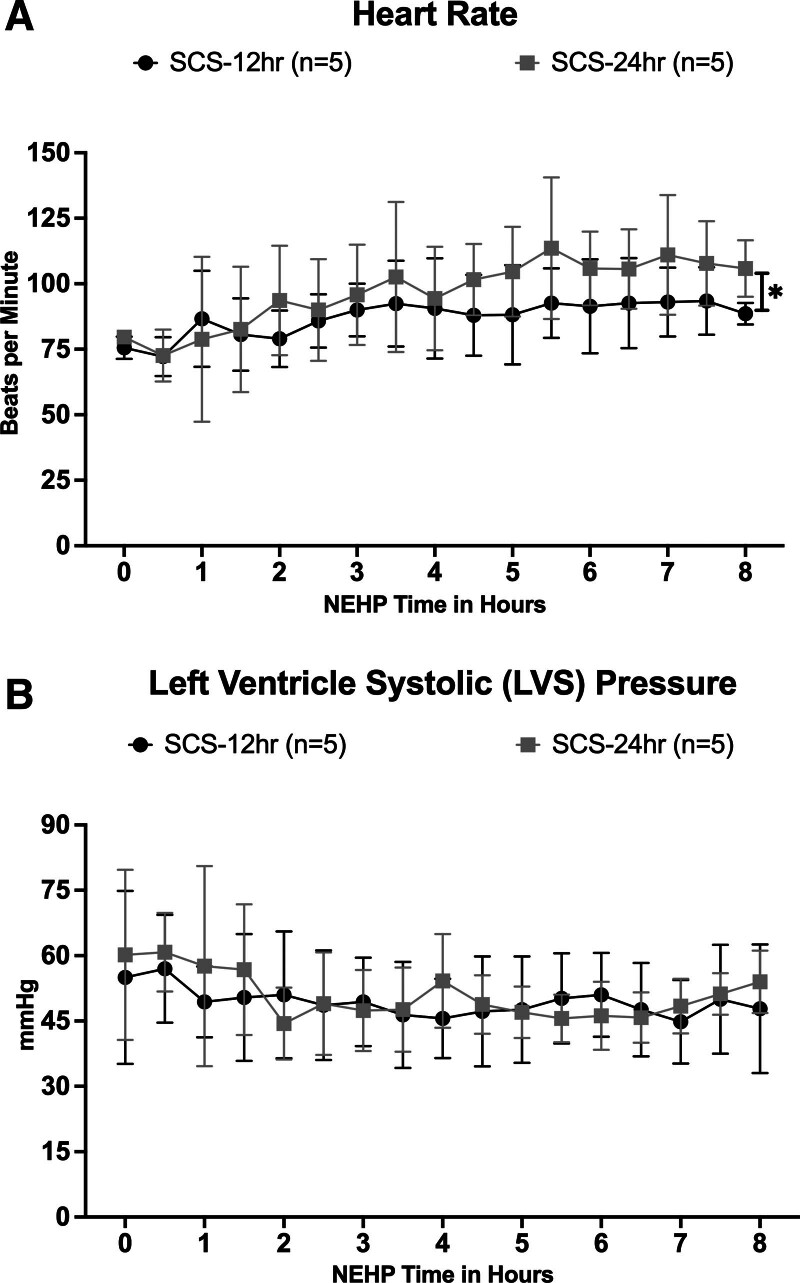
All hearts achieved the restoration of normal sinus contraction after the perfusion temperature reached >34 °C during NEHP without pacing (Figure 3). No arrhythmias were observed during ex situ perfusion, and the sinus rhythm was maintained during the whole NEHP time. The average HR during NEHP was 87 ± 6 and 97 ± 12 bpm in the SCS-12 h and SCS-24 h groups, respectively, without significant differences between groups (Figure 3A). However, HR was significantly higher (P = 0.02) in the SCS-24 h group than in the SCS-12 h group at the end of NEHP (hour 8). Average LVS pressure was 49.4 ± 3.1 and 50.8 ± 5.3 mm Hg in the SCS-12 h and the SCS-24 h groups, respectively, without significant difference between groups. Similarly, LVS pressure at baseline versus the end of NEHP did not change over the 8 h of ex situ perfusion in both groups, demonstrating normal preservation of LV contractility (Figure 3B).
FIGURE 3.
Cardiac function during NEHP: HR (A) and LVS pressure (B). HR, heart rate; LVS, left ventricle systolic; NEHP, normothermic ex situ perfusion; SCS, static cold storage. *Statistically significant with P = 0.02, SCS-12 h group, higher HR than SCS-24 h group at the end of NEHP (8 h).
NEHP Parameters and Characteristics After Prolonged SCS
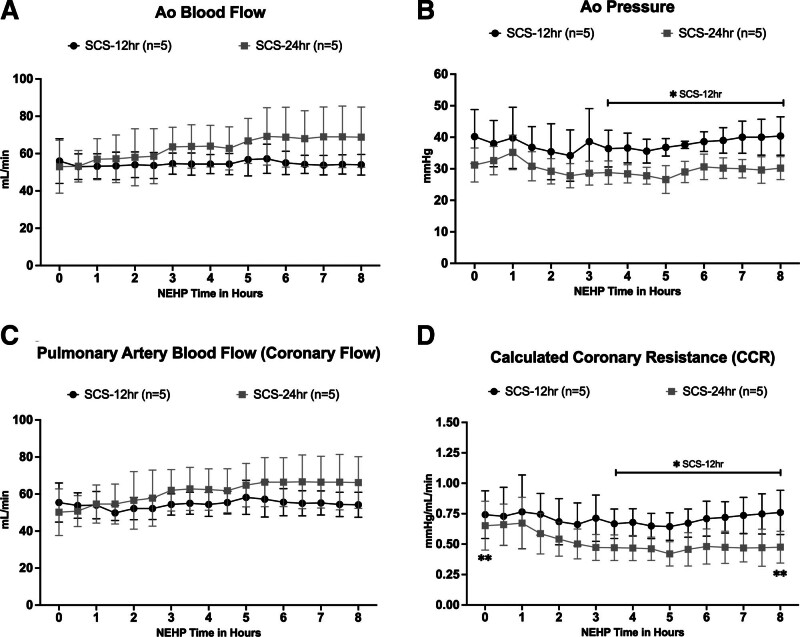
Per protocol, aortic blood flow (Ao flow) was adjusted to maintain coronary artery blood flow (PA flow) at a target rate of 1 mL/min/g cardiac tissue (Figure 4). The average Ao flow was 54.5 ± 1.2 and 63.9 ± 5.8 mL/min for the SCS-12 h and SCS-24 h groups, respectively. No difference was observed between and within groups; however, in the SCS-24 h group, there was a trend to higher flows during 5–8 h of NEHP (Figure 4A). The average Ao pressure was 37.9 ± 1.9 and 29.8 ± 2.0 mm Hg. Higher pressures were observed with significant difference (P < 0.05) in the SCS-12 h group after hour 3 of NEHP until the end of ex situ perfusion when compared with the SCS-24 h group (Figure 4B). Average coronary blood flow (measured via PA) was 54.5 ± 2.0 and 61.0 ± 5.7 mL/min in the SCS-12 h and SCS-24 h group, respectively, without significant difference between and within groups (Figure 4C). No difference was observed between Ao flow and coronary flow, suggesting competency of the aortic valve during NEHP. As expected, there was a significant difference in CCR after hour 3 of NEHP between groups (0.71 ± 0.04 and 0.52 ± 0.08 mm Hg/mL/min, in SCS-12 h and SCS-24 h groups, respectively). In addition, CCR was statistically higher (P = 0.02) at the beginning of perfusion versus the end of perfusion in the SCS-24 h group only; however, it decreased during NEHP (Figure 4D).
FIGURE 4.
Cardiac function during NEHP: Ao blood flow (A); Ao pressure (B); PA blood flow, as surrogate of coronary blood flow (C); and calculated coronary resistance (D). Ao, aortic; CCR, calculated coronary resistance; NEHP, normothermic ex situ perfusion; PA, pulmonary artery; SCS, static cold storage. *Statistically significant with P < 0.05, for the SCS-12 h group after hour 3 of NEHP until termination. **Statistically significant with P = 0.02 at the beginning of perfusion vs end of perfusion in the SCS-24 h group.
NEHP, Perfusate Characteristic
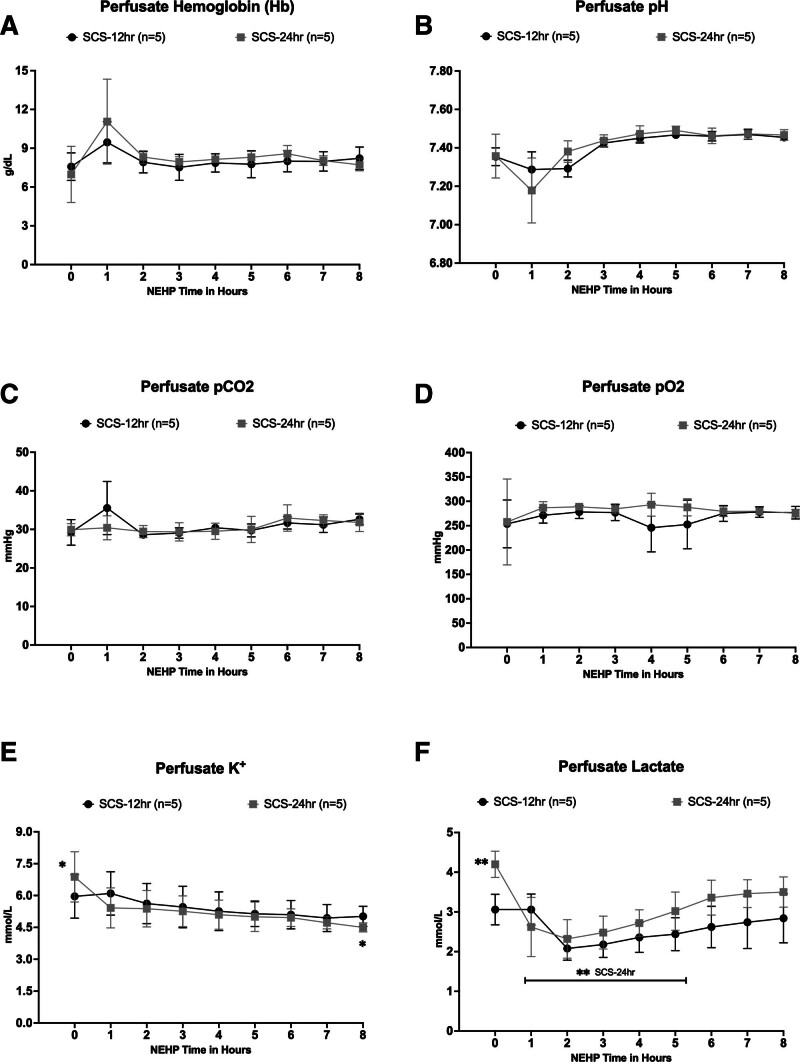
Perfusate Hb was maintained at the target value of >8.0 g/dL throughout NEHP (Figure 5). There were no significant differences between groups (SCS-12 h group = 8.0 ± 0.6 g/dL and SCS-24 h group = 8.3 ± 1.1 g/dL; Figure 5A). Perfusate arterial samples for pH, pCO2, and pO2 were maintained within the normal range during NEHP without significant differences between groups. Average values for these variables were pH= 7.41 ± 0.08 and 7.41 ± 0.09; pCO2 = 30.9 ± 2.2 and 30.6 ± 1.4 mm Hg; and pO2 = 267.6 ± 13.0 and 281.4 ± 10.5 mm Hg in the SCS-12 h and SCS-24 h groups, respectively (Figure 5B–D). Perfusate potassium (K+) levels were similar in both groups without significant differences between them (average K+ values were 5.4 ± 0.4 and 5.2 ± 0.7 mmol/L in the SCS-12 h and SCS-24 h groups, respectively). However, initial perfusate K+ values were higher in both groups compared with the termination of NEHP but only significant (P < 0.05) in the SCS-24 h group (Figure 5E). Finally, perfusate lactate values were 2.6 ± 0.4 and 3.1 ± 0.6 mmol/L without significant differences between them during NEHP. However, as observed with K+ values, lactate values at the beginning of NEHP were significantly higher (P < 0.04) in the SCS-24 h group, but this value decreased immediately and remained low during NEHP (Figure 5F).
FIGURE 5.
Perfusate characteristics during NEHP: Hb (A), perfusate pH (B), Perfusate pCO2 (C), perfusate pO2 (D), perfusate K+ levels (E), and perfusate lactate levels (F). Hb, hemoglobin; NEHP, normothermic ex situ perfusion; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; SCS, static cold storage. *Statistically significant (P < 0.05) in the SCS-24 h group. **Statistically significant higher lactate values (P < 0.04) in the SCS-24 h group.
Oxygen Kinetics During NEHP
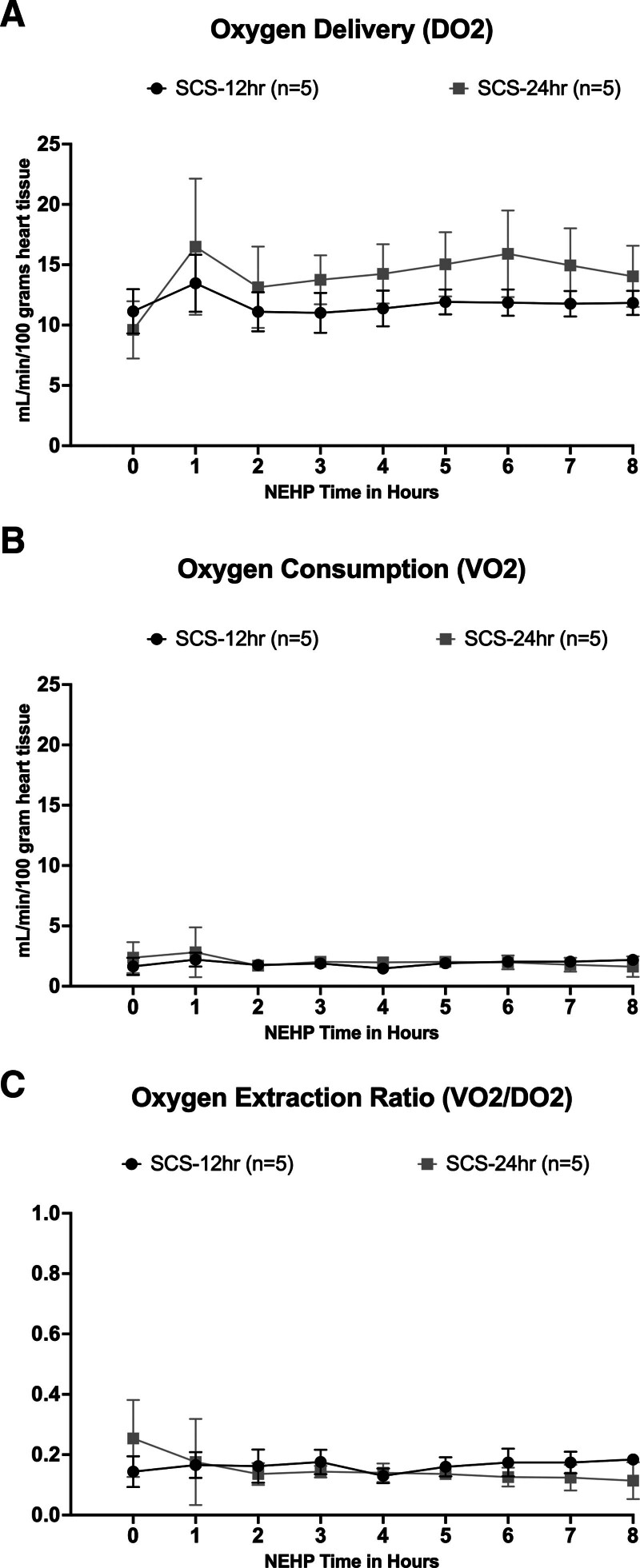
DO2 per 100 g of cardiac tissue was 11.7 ± 0.74 and 14.1 ± 1.9 mL/min in the SCS-12 h and SCS-24 h groups, respectively (Figure 6). However, DO2 was significantly lower (P < 0.05) at the beginning of perfusion compared with the other NEHP points in both groups (Figure 6A). Oxygen consumption (VO2) was similar in both groups throughout NEHP without significant differences between and within groups (1.9 ± 0.2 and 2.0 ± 0.4 for the SCS-12 h and SCS-24 h groups, respectively; Figure 6B). Similarly, the oxygen extraction ratio averaged 0.16 ± 0.02 and 0.15 ± 0.04 in the SCS-12 h and SCS-24 h groups, respectively (Figure 6C), and no differences were observed between and within groups.
FIGURE 6.
Oxygen kinetics during NEHP: DO2 (A), VO2 (B), and OER (C). DO2, oxygen delivery; NEHP, normothermic ex situ perfusion; OER, oxygen extraction ratio; SCS, static cold storage; VO2, oxygen consumption.
Wet-Dry Ratio
The wet-dry ratio of the LV was 3.1 ± 1.8 in the SCS-12 h group and 2.3 ± 1.4 in the SCS-24 h group without significant difference between and within groups. A similar observation was made in the wet-dry ratio of the RV 4.7 ± 3.4 and 2.2 ± 1.2 in the SCS-12 h and SCS-24 h groups, respectively.
Histology Score
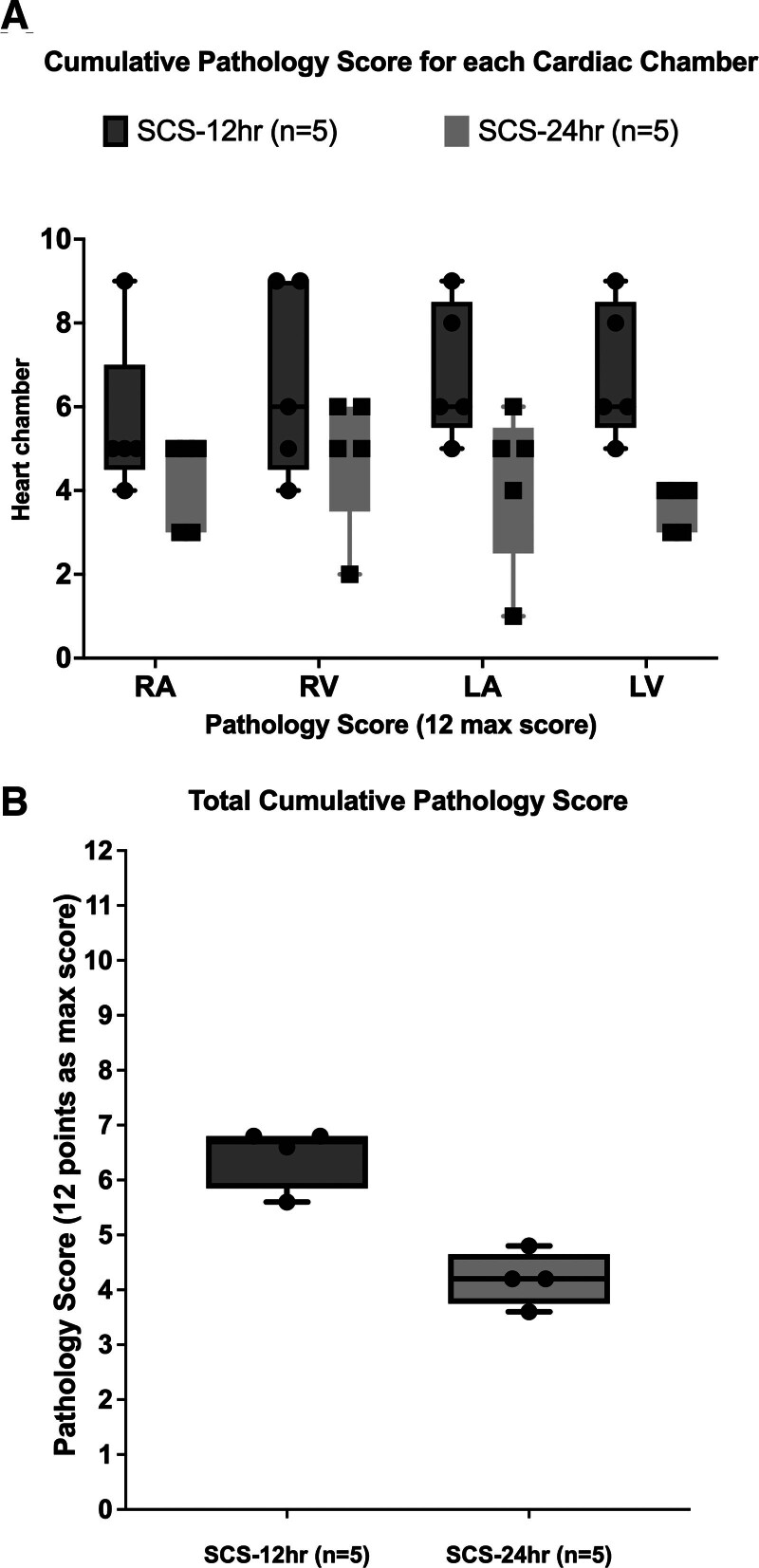
The average cumulative pathological injury score was 6.5 ± 1.2 and 4.2 ± 1.1 in the SCS-12 h and SCS-24 h groups, respectively, without significant difference despite a trend to higher total cumulative score in the SCS-12 h group (Figure 7A). Regarding the cumulative score for each cardiac chamber, there were no significant differences between right atrium, right ventricle, and left atrium between groups. However, the LV in the SCS-12 h group had a significantly higher (P = 0.007) score than the SCS-24 h group (average LV scores were 1.7 ± 0.3 and 0.9 ± 0.1 for each group, respectively; Figure 7B).
FIGURE 7.
Histopathology score. A, Cumulative pathology score for each cardiac chamber. B, Total cumulative pathology score.
Transplanted Hearts After 24 h of SCS + 5 h of NEHP
The 3 hearts used for orthotopic transplantation had trends during the 5 h of NEHP similar to the SCS-24 h group (Table 2). CPB time was between 68 and 240 min and aortic cross-clamp was between 87 and 120 min. All recipients were successfully weaned off CPB and heart function was observed for 90 min. Posttransplant mean HR after weaning off CPB was 129 ± 10 bpm and MAP was 41.4 ± 3.5 mm Hg, with normal arterial blood gases (electrolytes and acid-base balance). Lactate at the end of the study in all recipients was mildly elevated at 4–5 mmol/L compared with baseline (<1.5 mmol/L). Pressors were limited unless required for animal viability following MAP and HR. Therefore, the VDI was used to assess pharmacological support during CPB and post–heart transplant. All animals required vasopressor support during CPB with an average VDI of 0.2 ± 0.05. VDI ranges after weaning off CPB were the following: transplant 1 VDI = 0.3–0.4 µg/min/kg/mm Hg, transplant 2 VDI = 0.2–2.1 µg/min/kg/mm Hg, and transplant 3 VDI = 0.5–1.3 µg/kg/min/mm Hg. Before the termination of the study, an intraoperative ultrasound was performed to assess ventricular contraction and quantify cardiac output. All transplanted hearts had a cardiac output between 0.8 and 1.1 L/min, ejection fraction of >50%, and normal LV wall motion.
TABLE 2.
NEHP (5 h) parameters of transplanted hearts
| Variable | Average | SE | Range |
|---|---|---|---|
| Heart rate, bpm | 113 | 28 | 59–157 |
| LVS pressure, mm Hg | 45.6 | 8.4 | 31–76 |
| Ao perfusion pressure, mL/min | 31.7 | 2.7 | 25–42 |
| CBF, mL/min | 84.6 | 12.0 | 60–104 |
| Calculated coronary resistance, mm Hg/mL/min | 0.38 | 0.05 | 0.28–0.44 |
| Hb, g/dL | 9.0 | 0.5 | 8.1–10.8 |
| Perfusate K+, mmol/L | 6.3 | 0.2 | 5.5–9.5 |
| Perfusate lactate, mmol/L | 4.1 | 0.4 | 2.6–6.8 |
Ao, aortic; bpm, beats per minute; CBF, coronary flood flow (same as pulmonary artery blood flow); Hb, hemoglobin; LVS, left ventricle systolic; NEHP, normothermic ex situ heart perfusion.
DISCUSSION
Despite increased heart donor offers and record-high transplants in the past decade, the transplant-to-donor ratio has decreased because of logistical issues such as limited preservation time, surgical team availability, recipient location, and strict donor criteria.1,7 Heart donation from brain-dead donors involves anticoagulation, cold cardiac arrest, vascular washout with preservation solution, cooling with ice, and maintaining hypothermia until transplantation. The process for DCD includes premortem anticoagulation, rapid washout, or normothermic regional perfusion before following standard methods or using normothermic perfusion systems.18-20
Hypothermic preservation slows organ metabolism and enzyme functions, with a 50% reduction in metabolic activity for every 10 °C decrease, resulting in 10%–12% activity at 4–5 °C.21,22 However, it can cause tissue injury because of cellular edema, acidosis, and reactive oxygen species upon reperfusion, with these effects increasing with longer SCS times.
In the clinical setting, when using cardiac grafts that have been preserved for prolonged periods of time (>4 h), the incidence of DGF increases.2 In addition, when normothermic perfusion is also extended after 8 h as reported by Stamp et al11 and Kaliyev et al,12 DGF has been observed, and in these 2 reports, extracorporeal support was required for up to 72 h.
This work continues our series of studies on prolonged (24 h) heart preservation using normothermic ex situ perfusion.13-16 The main purpose of this study was to assess the outcomes of hearts that sustained prolonged (12 and 24 h) cold ischemia followed by 8 h of NEHP for organ resuscitation and objective assessment of cardiac function. To corroborate the findings, 3 hearts were transplanted into 3 healthy recipients. All hearts were successfully resuscitated after 12 and 24 h of SCS, followed by 8 h of NEHP. During perfusion, objective parameters of cardiac function (HR, LVS pressure, and cardiac rhythm) were within normal range at low perfusion flows (coronary blood flow). All NEHP parameters and perfusate values were within normal ranges (sinus rhythm; LVS pressure: 35–90 mm Hg; calculated coronary vascular resistance: 0.25–1.0 mm Hg/mL/min; venous oxygen saturation: 70%–90%; buffer base deviation: 40–50 mmol/L; PA lactate levels: <2 mmol/L; PA potassium levels: 3–5 mmol/L). In the SCS-24 h group, we observed lower coronary resistance; this is likely explained by a potential vasoplegic syndrome (VS) observed and documented in the clinical practice when patients have prolonged pump times or hearts are preserved for longer periods.23 VS is secondary to multifactorial conditions, including heart preservation time, recipient management before transplant, intraoperative management, and postoperative management. Pathophysiology is not well understood, but NEHP may provide a tool for studying the cause and management of VS.
After the first 4 h of NEHP, K+ levels normalized. Lactate values remained in the mild range (<4 mmol/L) in both groups without modifying the filtration rate. Being able to modify the K+ and lactate values in our system is a unique feature with the addition of hemofiltration. This simple intervention may be the first step to individual organ management during preservation. Lactate values are not used to adjust for perfusion and are primarily an outcome of perfusion. However, as stated, using hemofilters or plasma filters, lactate, and other biomarkers cannot be used, and objective parameters (organ function) are required to assess viability. An important issue currently performed in our laboratory is the evaluation of lactate generation and lactate clearance rates, but we have seen that lactate values in the blood-derived perfusate and filtrate increase with cardiac activity and metabolic rate. Another potential explanation for the increase in lactate is hemolysis because blood products from our animal blood bank are used. Most importantly, organ edema was <10% in all hearts. The pathology scores during the preservation-only stage demonstrated reversible myocardial injuries (mild to moderate, total score <9 points). Based on these results, the decision to transplant 3 hearts was made to corroborate the findings in a piglet model of orthotopic heart transplants. All recipients were successfully weaned off CPB with normal HR for this size pigs under general anesthesia (95–140 bpm), a VDI (<10 µg/kg/min/ mm Hg), cardiac output between 08 and 1.1 L/min, and adequate LV contraction. These parameters were observed for 90 min until planned termination. All recipient pigs required vasopressors (see methods) during CPB; 2 of them had long CPB times of >180 min under general anesthesia with inhaled isoflurane, a major cardiovascular depressor. However, despite observing higher requirements of vasopressor support after weaning off CPB, the VDI score was low, and trends of significance were not seen with this low number. VDI is commonly used in the clinical setting to manage septic patients; however, several reports show that VDI and its adaptations are an excellent tool to measure illness severity and facilitate select interventions in pediatric and adult cardiac surgery.24,25
If successful, the translation of this research into clinical practice could be used in the “resuscitation” and “objective assessment of organ function” of further discarded organs (lungs, livers, kidneys, and pancreas). It could also be used in other applications, such as gene therapy,26 mitochondrial infusions,27 immunomodulation,28 repair of damaged organs29-31 while on NEHP, and the study of pharmacology,32 physiology,33 and pathology and treatment of diseases,34 such as cancer. Ex situ therapy for reasons other than transplantation is not currently practiced, but there is significant innovative potential in these areas.
Limitations
This is a translational model of heart preservation and transplantation using piglets. The animal model does not simulate the scenario of human heart donation because the animals are young and healthy. The posttransplant evaluation time was short, and the only purpose was to assess the option to withdraw acutely CPB support. The positive effects of NEHP will be confirmed with specific (SCS) controls where reversal of histological injury can be proven. It is possible that SCS alone was sufficient to preserve the hearts of these porcine donors, but this was not studied as a control group. Access to vasopressors and extracorporeal support is available, but this cannot be replicated in large animal studies in clinical practice. Only 3 hearts were transplanted, which is admittedly a small number of animals; however, the data provide enough information to corroborate the findings and prove the concept of using NEHP for organ resuscitation after 12 and 24 h of SCS and objective assessment of cardiac function. The selection of medications posttransplant was limited because many of the medications used in the clinical setting were not approved in the animal protocol; therefore, only vasopressors approved and available were used.
CONCLUSIONS
Porcine hearts that underwent 12 and 24 h of SCS can be routinely resuscitated to viable function using NEHP. In addition, NEHP allows for objective assessment of heart function (working and nonworking beating hearts) before transplantation. Successful transplantation of these hearts corroborates adequate resuscitation, and it confirms viability after resuscitation using NEHP, demonstrating that it could play an important role after prolonged SCS and mitigate early reperfusion injury as this occurs ex vivo and not in the recipient. This work suggests that novel preservation techniques such as normothermic heart perfusion could change the practice of transplantation by eliminating common problems observed with organ allocation because of the availability of surgical teams (logistics), geographical location of the recipient/donor, and prolonged cold ischemia times. This work also opens the possibility to consider the use of organs from marginal brain-dead donors and DCD. The most important critical factor of the NEHP technique described by our group is the option to objectively evaluate heart function (ventricular pressures, echocardiography, etc) before transplantation without relying on biomarkers that filtration methods can easily modify. Finally, prolonged NEHP opens the possibility to develop protocols to create organ-specific therapies such as gene therapy, immunoregulation, and surgical procedures.
It is reasonable to state that the hearts can be used but not conclude that the perfusion technique is essential; this was not demonstrated. Some speculation in the discussion can be carefully argued that the perfusion method is a likely factor. To be clear, for this article to reach priority for publication, the conclusions need to be concise based on the experiments performed and not extrapolated.
Footnotes
Support for this study was provided by the Maxine and Stuart Frankel Foundation, the Frankel Fast Forward initiative at the University of Michigan and the National Institutes of Health (grant R01HL161139-02).
R.H.B. and A.R.-P. indicate receipt of supplies from Xvivo and Terumo for the ECLS Lab. A.R.-P. is on the Advisory Board of the New Leadership Academy, University of Utah and is a paid consultant with Terumo Cardiovascular. D.H.D. receives royalties from Thompson Surgical and has unpaid board and committee positions with the American Society of Echocardiography and the American Society for Thoracic Surgery. The other authors declare no conflicts of interest.
A.R.-P., J.B.N., G.E.B., J.W.H., R.H.B., and D.H.D. participated in study design. A.R.-P., M.D.J., K.A.U., B.L.D., J.S., J.B.N., and R.H.B. participated in data analysis. A.R.-P., M.D.J., K.A.U., G.E.B., J.W.H., and R.H.B. participated in article writing.
Contributor Information
Matthew D. Johnson, Email: matthew.johnson2018@gmail.com.
Kristopher A. Urrea, Email: kurrea@umich.edu.
Brianna L. Spencer, Email: blspence@med.umich.edu.
Jasnoor Singh, Email: jasnoors@umich.edu.
Joseph B. Niman, Email: joebniman@gmail.com.
Gabe E. Owens, Email: gabeo@med.umich.edu.
Jonathan W. Haft, Email: haft@med.umich.edu.
Robert H. Bartlett, Email: robbar@med.umich.edu.
Daniel H. Drake, Email: daniel.h.drake@gmail.com.
REFERENCES
- 1.Schladt DP, Israni AK. OPTN/SRTR 2021 annual data report: introduction. Am J Transplant. 2023;23(2 Suppl 1):S12–S20. [DOI] [PubMed] [Google Scholar]
- 2.Valero-Masa MJ, González-Vílchez F, Almenar-Bonet L, et al. Cold ischemia >4 hours increases heart transplantation mortality. An analysis of the Spanish heart transplantation registry. Int J Cardiol. 2020;319:14–19. [DOI] [PubMed] [Google Scholar]
- 3.Minasian SM, Galagudza MM, Dmitriev YV, et al. Preservation of the donor heart: from basic science to clinical studies. Interact Cardiovasc Thorac Surg. 2015;20:510–519. [DOI] [PubMed] [Google Scholar]
- 4.Lauzier B, Sicard P, Bouchot O, et al. After four hours of cold ischemia and cardioplegic protocol, the heart can still be rescued with postconditioning. Transplantation. 2007;84:1474–1482. [DOI] [PubMed] [Google Scholar]
- 5.Bifulco O, Bottio T, Caraffa R, et al. Marginal versus standard donors in heart transplantation: proper selection means heart transplant benefit. J Clin Med. 2022;11:2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi Y, Scheuer S, Chew H, et al. Heart transplantation from DCD donors in Australia: lessons learned from the first 74 cases. Transplantation. 2023;107:361–371. [DOI] [PubMed] [Google Scholar]
- 7.Dharmavaram N, Hess T, Jaeger H, et al. National trends in heart donor usage rates: are we efficiently transplanting more hearts? J Am Heart Assoc. 2021;10:e019655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu J, Buchwald JE, Martins PN. Review of current machine perfusion therapeutics for organ preservation. Transplantation. 2020;104:1792–1803. [DOI] [PubMed] [Google Scholar]
- 9.Pahuja M, Case BC, Molina EJ, et al. Overview of the FDA’s Circulatory System Devices Panel virtual meeting on the TransMedics Organ Care System (OCS) Heart—portable extracorporeal heart perfusion and monitoring system. Am Heart J. 2022;247:90–99. [DOI] [PubMed] [Google Scholar]
- 10.Alomari M, Garg P, Yazji JH, et al. Is the Organ Care System (OCS) still the first choice with emerging new strategies for donation after circulatory death (DCD) in heart transplant? Cureus. 2022;14:e26281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stamp NL, Shah A, Vincent V, et al. Successful heart transplant after ten hours out-of-body time using the transmedics organ care system. Heart Lung Circ. 2015;24:611–613. [DOI] [PubMed] [Google Scholar]
- 12.Kaliyev R, Bekbossynov S, Nurmykhametova Z. Sixteen-hour ex vivo donor heart perfusion during long-distance transportation for heart transplantation. Artif Organs. 2019;43:319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLeod JS, Poling C, Church JT, et al. Ex vivo heart perfusion for 72 hours using plasma cross circulation. ASAIO J. 2020;66:753–759. [DOI] [PubMed] [Google Scholar]
- 14.Tchouta L, Drake D, Hoenerhoff M, et al. ; University of Michigan Department of Surgery Extracorporeal Life Support Laboratory. Twenty-four-hour normothermic perfusion of isolated ex vivo hearts using plasma exchange. J Thorac Cardiovasc Surg. 2022;164:128–138. [DOI] [PubMed] [Google Scholar]
- 15.Johnson MD, Fallon BP, Langley M, et al. Prolonged (24-hour) normothermic ex vivo heart perfusion facilitated by perfusate hemofiltration. ASAIO J. 2022;68:1282–1289. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MD, Zimmerman KG, Nakashima T, et al. Artificial intelligence-assisted strain echocardiography in an ex vivo heart. ASAIO J. 2023;69:e523–e525. [DOI] [PubMed] [Google Scholar]
- 17.Trahanas JM, Witer LJ, Alghanem F, et al. Achieving 12 hour normothermic ex situ heart perfusion: an experience of 40 porcine hearts. ASAIO J. 2016;62:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copeland H, Hayanga JWA, Neyrinck A, et al. Donor heart and lung procurement: a consensus statement. J Heart Lung Transplant. 2020;39:501–517. [DOI] [PubMed] [Google Scholar]
- 19.Camp PC. Heart transplantation: donor operation for heart and lung transplantation. Oper Tech Thorac Cardiovasc Surg. 2010;15:125–137. [Google Scholar]
- 20.Buckberg GD. Myocardial temperature management during aortic clamping for cardiac surgery. Protection, preoccupation, and perspective. J Thorac Cardiovasc Surg. 1991;102:895–903. [PubMed] [Google Scholar]
- 21.Jahania MS, Sanchez JA, Narayan P, et al. Heart preservation for transplantation: principles and strategies. Ann Thorac Surg. 1999;68:1983–1987. [DOI] [PubMed] [Google Scholar]
- 22.Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–247. [DOI] [PubMed] [Google Scholar]
- 23.Kumar N, Fitzsimons MG, Iyer MH, et al. Vasoplegic syndrome during heart transplantation: a systematic review and meta-analysis. J Heart Lung Transplant. 2024;43:931–943. [DOI] [PubMed] [Google Scholar]
- 24.Kumar M, Sharma R, Sethi SK, et al. Vasoactive inotrope score as a tool for clinical care in children post cardiac surgery. Indian J Crit Care Med. 2014;18:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koponen T, Karttunen J, Musialowicz T, et al. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth. 2019;122:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishawi M, Roan JN, Milano CA, et al. A normothermic ex vivo organ perfusion delivery method for cardiac transplantation gene therapy. Sci Rep. 2019;9:8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowan DB, Yao R, Akurathi V, et al. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS One. 2016;11:e0160889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brasile L, Glowacki P, Castracane J, et al. Pretransplant kidney-specific treatment to eliminate the need for systemic immunosuppression. Transplantation. 2010;90:1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maheswaran S, Haber DA. Ex vivo culture of CTCs: an emerging resource to guide cancer therapy. Cancer Res. 2015;75:2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed MSA. Combined ex vivo organ perfusion and mesenchymal stem cells transplantation. J Stem Cells. 2016;11:213–217. [PubMed] [Google Scholar]
- 31.de Hart J, de Weger A, van Tuijl S, et al. An ex vivo platform to simulate cardiac physiology: a new dimension for therapy development and assessment. Int J Artif Organs. 2011;34:495–505. [DOI] [PubMed] [Google Scholar]
- 32.Brasile L, Stubenitsky BM, Haisch CE, et al. Repair of damaged organs in vitro. Am J Transplant. 2005;5:300–306. [DOI] [PubMed] [Google Scholar]
- 33.Vidavalur R, Swarnakar S, Thirunavukkarasu M, et al. Ex vivo and in vivo approaches to study mechanisms of cardioprotection targeting ischemia/reperfusion (I/R) injury: useful techniques for cardiovascular drug discovery. Curr Drug Discov Technol. 2008;5:269–278. [DOI] [PubMed] [Google Scholar]
- 34.Qin G, Wohlfart B, Zuo L, et al. Intact coronary and myocardial functions after 24 hours of non-ischemic heart preservation. Scand Cardiovasc J. 2020;54:59–65. [DOI] [PubMed] [Google Scholar]