Abstract
Objective:
Early life experiences, including attachment-related experiences, inform internal working models that guide adult relationship behaviors. Few studies have examined the association between adolescent attachment and adult relationship behavior on a neural level. The current study examined attachment in adolescence and its associations with neural correlates of relationship behaviors in adulthood.
Method:
85 participants completed the Adult Attachment Interview (AAI) at age 14. Ten years later, at age 24, participants underwent functional brain image when participants were under the threat of electric shock alone, holding the hand of a stranger, or their partner.
Results:
We found that adolescents who were securely attached at age 14 showed increased activation in regions commonly associated with cognitive, affective, and reward processing when they held the hand of their partner and stranger compared to being alone. Adolescents with higher preoccupied attachment scores showed decreased activation in similar regions only during the stranger handholding condition compared to being alone.
Conclusions:
These findings suggest that adolescent attachment predicts adult social relationship behaviors on a neural level, in regions largely consistent with previous literature. Broadly, this study has implications for understanding long-term links between attachment and adult relationship behaviors and has potential for informing intervention.
Keywords: Adolescence, adult attachment interview, adult relationshi, attachment, functional magnetic resonance imaging, handholding, posterior cingulate cortex, reward
Introduction
Interpersonal theories such as the Social Baseline Theory (SBT; Beckes & Coan, 2011) assert that human brains assume proximity to other humans—their primary ecological niche or habitat. When this assumption fails, humans perceive increasing physiological demand for threat detection and emotion regulation. Metabolic and vascular resources are optimized for rapid responses to potential threats, and when maintained for long periods, cause detrimental impacts on individual health and longevity (Sterling, 2020). In accordance with this account, individuals demonstrated less activation of the threat-responsive neural circuits while holding the hand of a relational partner compared to when they were alone (Coan et al., 2017). These effects were observed most consistently in the dorsal anterior cingulate cortex (dACC) and dorsolateral prefrontal cortex (dlPFC), In other words, the presence of a relational partner conserves bioenergetic resources, as reflected by reduced activity in regions commonly associated with emotion, cognitive-control, and self-regulation (Coan et al., 2006, 2017; Gonzalez et al., 2015; MacDonald et al., 2000).
However, little work has considered developmental sources of individual differences, and in particular, attachment in adolescence (Long et al., 2020). Gross and Medina-DeVilliers (2020) argued that early life experiences and individual traits, including attachment, can alter expectations regarding the availability of social resources and contribute to internal working models that in turn influence physiological behaviors, epigenetics, and neural responses to threat. Additionally, fewer studies assessed attachment in adolescence—a sensitive period for social brain development, peer relationship development, and increasing independence from parents (Gee et al., 2014; Loeb et al., 2021). Thus, the current study aims to understand links of attachment in adolescence to adult brain reactivity during interpersonal processes. Examining the developmental roots of inter-individual differences particularly in attachment domains can move us towards a better understanding of affective and cognitive processes in social situations and adult relationship development.
Attachment in adolescence
Attachment theory proposes that children evolved to seek proximity to others for protection and regulation of physiological resources in times of threat. Those early experiences shape adaptive strategies to maintain closeness in individual social environment–that is, patterns of attachment (Bowlby, 1969; Main, 1990). Based on caregivers’ availability, sensitivity, and responsivity, children form a secure or insecure attachment. Secure attachment is characterized by autonomous exploration of the environment and trusting that close others will provide a secure base when needed. Such experiences are abstracted and represented as a secure internal working model of relationships, including expectations that close others will be available and helpful in times of need. On the other hand, inconsistent, rejecting, and painful attachment-related experiences shape an insecure model of relationships, characterized by a sense of doubt in the availability of a secure base when needed.
Internal working models of attachment tend to show moderate stability across development (Ammaniti et al., 2000; Waters et al., 2000), linking early experiences to later development by informing predictions about future relationships (Main et al., 1985). In adolescence, research has linked internal working models of attachment to both self- and socially-mediated emotion regulation (Allen & Miga, 2010; Mikulincer & Shaver, 2008). Secure attachment representations in adolescence are associated with healthy emotion regulation (balancing autonomy and relatedness), greater support-seeking behaviors, lower risk for psychopathology, higher-quality peer relationships, and less hostile conflict in adult romantic relationships (e.g., Loeb et al., 2021; Tan et al., 2016).
By adolescence, attachment representations can be measured via the Adult Attachment Interview (AAI; George et al., 1985), a semi-structured interview that assesses recollection of childhood attachment related experiences, in terms of participants’ ability to discuss emotionally intense experiences coherently, with emphasis on perspective taking, balance, clarity of communication, and lack of anger or avoidance of the topics. The AAI examines the “current state of mind regarding attachment” rather than childhood attachment to parents per se (Main et al., 1985). The AAI is among the most widely used measures of attachment in adolescence and shows strong links to early caregiving experiences. Adolescents with high scores on the security dimension of the AAI show autonomous emotion regulation, narrative coherence, emotional balance, and a sense of valuing relationships. In contrast, dismissing adolescents tend to avoid attachment-related topics and downplay the importance of close relationships, while preoccupied adolescents may show anger and preoccupation with negative attachment experiences.
Teens’ attachment assessed by the AAI is associated with individual differences in social functioning with parents, peers, and romantic partners (Allen, 2008; Allen & Manning, 2007; Allen & Miga, 2010). For example, Loeb and colleagues (2021) found that insecure attachment measured via the AAI at age 14 predicted decreasing abilities to seek and receive help from friends at ages 14–18 and more negative interactions and hostile attitudes with romantic partners from ages 14–27. Thus, state of mind regarding attachment in adolescence should form the foundation through which adults approach interpersonal relationships (Bowlby, 1969; Mikulincer & Shaver, 2012), and the current study makes a further contribution by examining attachment during this critical development period.
Attachment, social relationships, and neural correlates
Empirical neuroimaging studies related to social relationships have identified functionally specialized brain regions commonly associated with emotion processing, reward circuits, and cognition implicated in both romantic attachment (Bartels & Zeki, 2000; Kircher et al., 2001) and mother-child caregiving relationships (Lorberbaum et al., 1999; Nitschke et al., 2004; Strathearn et al., 2008). Both types of maternal and romantic relationships showed deactivation in a set of regions associated with negative emotions, social judgment, and mentalizing and activation of regions associated with reward circuitry (Bartels & Zeki, 2004), forming a push-pull mechanism that overcomes social distance via deactivated negative emotions and social judgment and enables bonding via activated reward circuits.
To integrate this body of work, Long and colleagues (2020) proposed a comprehensive functional neuroanatomical model of human attachment (NAMA; see also Vrtička, 2017; Vrtička & Vuilleumier, 2012). According to NAMA, the human attachment system is comprised of an affective and a cognitive system. Within the affective system, an approach-oriented component (e.g., the ventral tegmental area, substantia nigra, Aron et al., 2005; Kim et al., 2017) encodes social interactions as innately rewarding, while an aversion-oriented component (e.g., insula, Seeley et al., 2007; Vrtička, 2017) encodes negative affect and pain. On the other hand, the cognitive system reflects top-down volitional control of the emotion through an emotion-regulation component (e.g., the lateral orbitofrontal cortex, Callaghan & Tottenham, 2016; Lieberman, 2007) and perspective taking through a mental state representation component (e.g., posterior cingulate cortex and precuneus, Kanske, 2018; Spreng & Grady, 2010). The different components act as independent but complementary neurobiological systems encoding positive and negative emotional states relevant for the attachment pathway, as well as conscious mental regulations of the cognitive processes.
Study design and hypotheses
Expectations of the availability of attachment figures during threat are critical to influencing how one allocates their bioenergetic resources. According to Bowlby (1969), seeking proximity to a secure base is the primary attachment strategy when an individual faces external threat from infancy (Main, 1990) to adulthood (Fraley & Shaver, 1998). This strategy encompasses a variety of nonverbal behaviors, among which handholding is a common nonverbal mode of proximity-seeking that could be easily implemented in the fMRI environment (Coan et al., 2006). Handholding is an important form of interaction for humans as well as other species (Gliga et al., 2019), and is critical to intrapersonal and interpersonal emotion regulation. From a developmental perspective, handholding and other affectionate nonverbal behaviors are encoded in attachment relationships during which one’s security depends on another’s supportive and responsive caregiving (Schachner et al., 2005). For example, decreased exposure to social touch as an infant has been associated with subsequent brain, social, and cognitive development (Gliga et al., 2019). Understanding the neurobehavioral pathway between attachment and social functioning can facilitate a better characterization of human affective and cognitive processes in social contexts.
Thus, in this study, we examined associations between adolescent attachment, measured via Adult Attachment Interview at age 14, and adult neural responses to threat while holding the hand of a partner, a stranger, or while alone measured via fMRI ten years later, at age 24. We hypothesized that secure attachment assessed in adolescence would be associated with (a) reduced activation in regions commonly implicated with cognitive control and (b) greater activation in regions associated with positive affect and reward, when holding the hand of a relational partner (and to a lesser extent, holding the hand of a stranger), compared to alone. Conversely, we predicted that insecure attachment assessed in adolescence would be associated with greater activation in regions implicated with cognitive control and reduced activation in regions associated with positive affect and reward when accompanied by a close partner or stranger, compared to being alone. Comparing the partner (and stranger) to the alone condition zones in on capturing effects that are unique to the partner handholding and stranger handholding condition.
Methods
Participants
Participants were recruited via email and phone call from a larger longitudinal investigation of adolescent social development in familial and peer context (Hare et al., 2011) and adolescents were initially recruited from the seventh and eighth grades of a public middle school drawing from suburban and urban populations in the Southeastern United States. At around age 14 (Meanage = 14.18, Medianage = 14.14, SDage = 0.77, Rangeage = 12.69 – 15.85), participants completed the Adult Attachment Interview (AAI). Then, at around age 24 (Meanage = 23.66, Medianage = 23.68, SDage = 0.97, Rangeage = 21.87 −25.64), participants brought a partner to the scanner and completed a series of fMRI tasks. Following safety standards for fMRI practice, possible participants were excluded if pregnant, claustrophobic, or if they had ferromagnetic items in their body. Participants were also excluded if they could not bring a well-known partner, either a friend, dating partner, dating cohabitating partner, or married partner to the scanning session. All participants provided written consent and were compensated for their participation at both time points.
A total of 85 participants (22 Black/African American, 2 Hispanic/Latinx, 51 White/European, 7 Mixed Race, 3 Other identities) completed both the AAI and the fMRI task. When the study began in 1998, participants were provided with binary options to indicate their gender identity; in the present sample, 39 participants identified as male, and 46 participants identified as female. Adolescents reported an annual baseline median family income in the $40,000–59,999 range. At around age 24, 71 participants identified as heterosexual, 4 participants identified as bisexual, 2 participants reported unsure, and 8 participants did not report information regarding their sexual orientation. Of scanned dyads, 25 identified as friends, 29 were dating, 27 were cohabiting, and 4 were married. Thus, for the current study, “partner” refers to any of these types of relationships, as contrasted with “stranger”. Both partner and stranger are members of different gender. Previous lab work demonstrated that relationship status did not moderate the neuroimaging findings (Coan et al., 2017).
All experiments in the study were approved by the Institutional Review Board. Participants’ data are protected by a Confidentiality Certificate issued by the U.S. Department of Health and Human Services, which protected information from subpoena by federal, state, and local courts. The current dataset was derived from a larger study, and the whole-brain exploratory analysis plan was pre-registered. We made no deviation from the pre-registered analysis plan. Pre-registration is available online: https://osf.io/38ck2/. Adult participants and participating dyads provided informed consent and were paid for participation.
Measures
Adult Attachment Interview (AAI; George et al., 1985): Age 14.
The Adult Attachment Interview (AAI; George et al., 1985) and Q-set (Kobak et al., 1993) were designed to assess adults’ current states of mind with regard to attachment. The AAI is a semi-structured, hour-long interview, consisting of 18 questions regarding the interviewees’ childhood experiences with their attachment figures. Participants begin by providing a general description of their experience with caregivers during childhood. Then, participants answered to what extent they were rejected by their caregivers when they were upset, hurt, or sick as a child; threatened or abused by their caregivers; and experiences of separation, loss, or other trauma. Lastly, participants explain how their current state of mind is informed by the attachment experiences described, recent changes in their relationship with caregivers, and their hopes and expectations for their own childrearing. The current study employed slight modifications to the adult version, so it was easier to understand by adolescent populations. Interviews were audiotaped and transcribed verbatim for coding.
The AAI Q-set (Kobak et al., 1993) was designed to parallel the AAI classification system (Main & Goldwyn, 1998) and yield continuous measures of attachment organization, consistent with current recommendations (Roisman et al., 2007a). Continuous data reduced via an algorithm to classifications were largely consistent with the three-category ratings from the AAI Classification system in the broad research and a sub-sample of this population, using the coding system developed by this laboratory (Allen et al., 1998). To code the transcripts, at least two coders with extensive training in the Q-sort and AAI classification systems independently rated the interviews. Each coder read the transcript and provided a Q-set description by sorting 100 items into 9 categories ranging from the most characteristic of the interview to the least characteristic of the interview. Validity was established by comparing the Q-sets with dimensional prototypes for secure strategies, preoccupied strategies, and dismissing strategies (see Kobak et al., 1993). Scale score was then calculated as the correlation of the 100 items of the individual’s Q-sort with each dimension (ranging on an absolute scale from −1.00 to 1.00). The current sample showed good Spearman-Brown reliability of the security scale score (r = 0.82) and good inter-rater reliability (ICC = 0.82), which is considered in the excellent range for this statistic (Cicchetti & Sparrow, 1981).
Handholding paradigm: Age 24.
The following paradigm is based on a well-validated and replicable handholding design (Coan et al., 2017). Two Ag–AgCl shock electrodes were applied to the participant’s ankle (left or right, counterbalanced across participants). The participant was then taken into the scanning chamber where he or she first received a high-resolution anatomical scan.
Participants brought a romantic partner or friend who were willing to visit the lab and provided handholding while participants were in the scanner. Participants viewed stimuli projected onto a screen behind the magnet’s bore through a mirror and responded to stimuli by button box. Each participant underwent three blocks of “threat of shock paradigm” in counterbalanced order, where they held the hand of their partner, an unseen confederate (stranger), or were alone. Each block was composed of 24 trials, including an equal number of threat and safety trials. A threat trial is consisted of 1-s threat cue (a red “X” on a black background), followed by 4–10 seconds of anticipation period (a fixation cross), and 17% chance of receiving electric shock, prior to the end cue (a small dot). A safety trial is consisted of 1-s safety cue (a blue “O” on a black background), followed by 4–10 seconds of anticipation period, with no chance of shock, prior to the end cue. Participants’ right hands were used for handholding, whereas their left hands held the button box. Shocks were generated by an isolated physiological stimulator (Coulbourn Instruments, Allentown, PA, USA) and lasted for 20 ms at 4 mA.
Image acquisition
Functional images were acquired using a Siemens 3.0 Tesla MAGNETOM Trio high-speed magnetic imaging device, with a circularly polarized transmit/receive head coil with integrated mirror. A total of 216 functional T2*-weighted echo planar images (EPIs) sensitive to blood-oxygen-level-dependent contrasts were collected per block, in volumes of 28 3.5-mm transversal echo-planar slices (1-mm slice gap) covering the whole brain (1-mm slice gap, repetition time (TR) 2000 ms, echo time (TE) 40 ms, flip angle90, field of view (FOV) 192 mm, matrix 64 × 64, voxel size 3 × 3 × 3.5 mm). Before collection of functional images, 176 high-resolution T1-magnetization-prepared rapid-acquisition gradient echo images were acquired to determine the localization of function (1-mm slices, TR 1900 ms, TE 2.53 ms, flip angle 90, FOV 50 mm, voxel size 1 × 1 × 1 mm).
Data were preprocessed and analyzed using FMRIB’s Software Library (FSL) software (Version 5.98; https://www.fmrib.ox.ac.uk/fsl, Worsley, 2001). Motion correction involved FMRIB’s Linear Image Registration Tool, an intra-modal correction algorithm tool (MCFLIRT; Jenkinson et al., 2002), with slice scan time correction and a high-pass filtering cutoff point of 100 s, removing irrelevant signals. We used BET (Smith, 2002) brain extraction, eliminating non-brain material voxels in the fMRI data, and a 5-mm full width at half minimum Gaussian kernel for smoothing. Images were registered to the Montreal Neurological Institute (MNI) space by FLIRT (Jenkinson et al., 2002). Trials in which participants received shocks were excluded due to movement artifacts and our primary interest in anticipatory threat.
Analytical plan
First and second-level analyses were conducted using FMRI Expert Analysis Tool (FEAT; Version 6.00). The threat response was first defined as a contrast of brain activation during the threat cue and brain activation during the safe cue. First level analyses began with a threat minus safety trial contrast applied separately to each handholding condition (alone, partner, stranger) for each subject. Within this model, the main effects of threat yielded robust neural activity when experiencing distress alone compared to with a partner or stranger. During second-level analyses, the alone condition was then contrasted with the partner and stranger conditions using fixed effect model.
Main effects for the handholding contrast (alone (threat – safe) > partner (threat – safe)) have been reported elsewhere (Coan et al., 2017). Briefly, main effects of handholding showed greater activation in dlPFC and posterior cingulate cortex when participants were alone compared to when they were holding the hands of their partner. Using centered attachment secure, dismissing, and preoccupation scores as explanatory variables (EV), we conducted a whole-brain corrected (z = 3.1, p = .05) covariate analysis of attachment scores and neural threat reaction, using contrast codes [1 –1] representing positive and negative associations between individual EV (e.g., centered secure, dismissing, and preoccupation scores) and main effects of handholding in alone > partner and alone > stranger conditions from second-level analyses.
Preliminary analyses
The mean and standard deviations of attachment scores were as shown (Msecure = 0.26, SDsecure = 0.44; Mdismissing = 0.02, SDdismissing = −0.44; Mpreoccupation = 0.04, SDpreoccupation = 0.23). Descriptive statistics of the attachment scores showed significant correlations (secure and dismissing scores: r = −0.95, p < .001; secure and preoccupation scores: r = −0.55, p < .001; dismissing and preoccupation scores: r = 0.48, p < .001). In line with previous reports (Nivison et al., 2021), we regard zero-order correlations lower than .30 as relatively distinct, zero-order correlations between .30 and .70 as partially distinct, and zero-order correlations higher than .70 as indicating something commonly latent. Drawing on these guidelines, our security and preoccupation scores are partially distinct, our dismissing and preoccupation scores are also partially distinct, but our secure and dismissing scores likely represent a common underlying latent factor associated with secure attachment. Given the high correlation between dismissing and secure attachment scores, and the greater primacy of security as a focus in the research literature, dismissing scores were dropped from further analyses.
Results
Attachment and alone condition relative to partner handholding condition
We conducted a whole-brain corrected (z = 3.1, p = .05) covariate analysis looking at the association between attachment scores and the main effects of experiencing threat in the alone condition compared to the partner condition. Clusters were defined both by structural probability maps in FSL and by the continuity of functional activation. Locations were specified using three-dimensional coordinates where each image has an origin, usually the anterior commissure, which specifies the coordinates of x = 0, y = 0, and z = 0. The x-axis goes towards the right side of the brain, the y-axis goes towards the front of the brain, and the z-axis goes towards the top of the brain. The orientation indicates whether direction relative to the origin is positive or negative. Results suggested reduced posterior cingulate cortex (x = −4, y = 62, z = 26; p < .001; Figure 1) activity during the alone condition compared to the partner condition as security scores increased. There was no association between preoccupation scores and the main effects of neural threats in alone relative to partner condition.
Figure 1.
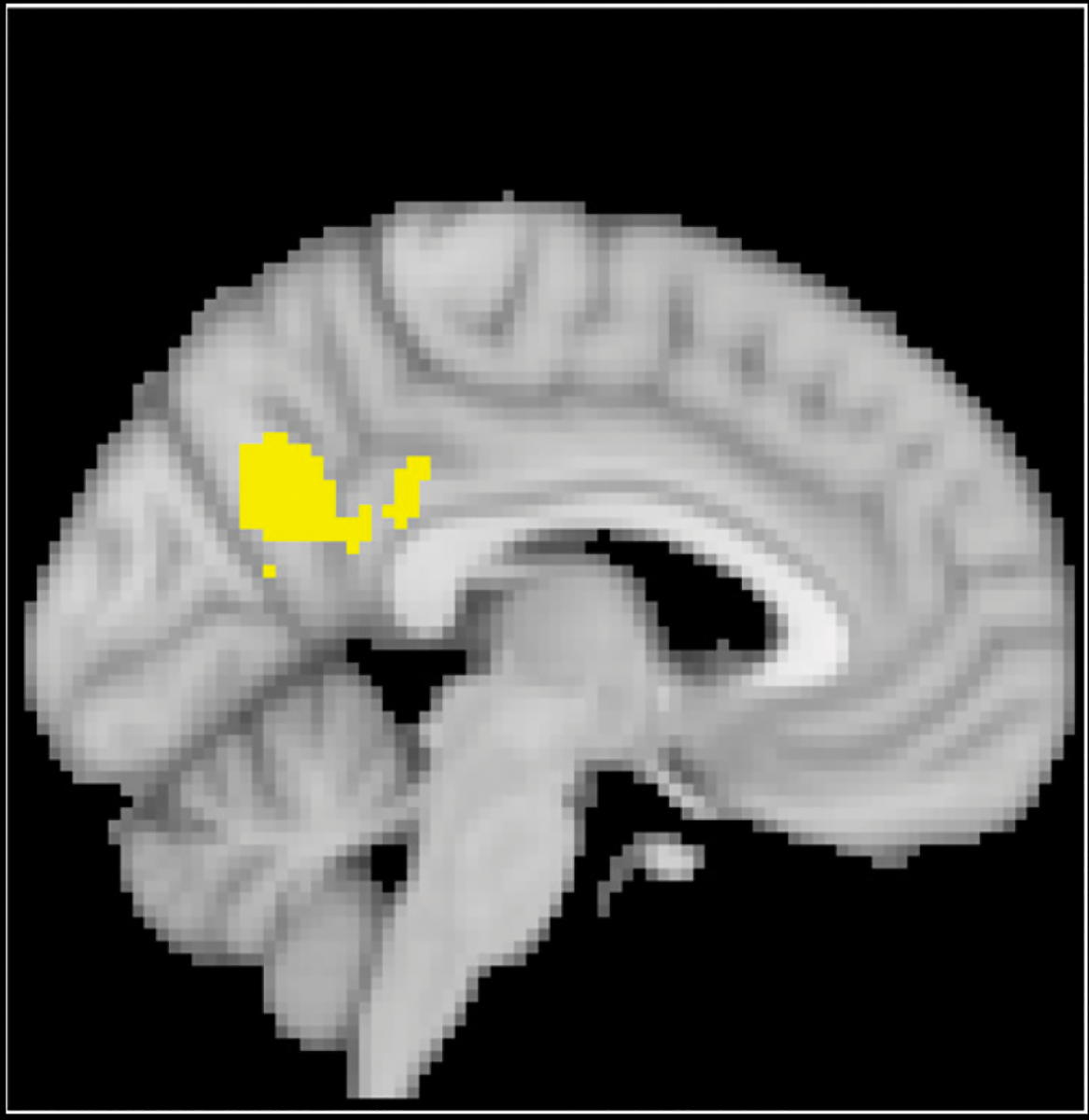
The posterior cingulate cortex (x = −4, y = 62, z = 26). We observed that while alone, participants with higher secure attachment scores showed reduced posterior cingulate activity compared to when they are holding the hand of their partner.
Attachment and alone condition relative to stranger handholding condition
We used the same whole-brain corrected (z = 3.1, p = .05) covariate analysis, this time looking at the association of attachment scores and the main effects of experiencing threat in the alone condition compared to the stranger condition (Figure 2). Brain areas of activation at this contrast as a function of security scores are summarized in Table 1. Clusters were defined both by structural probability maps in FSL and by the continuity of functional activation. Results suggested reduced activity in the posterior cingulate cortex (x = −2, y = −28, z = 26; p < .001), lateral occipital cortex clusters (x = 34, y = −70, z = 50; x = −32, y = −68, z = 50; p < .001), right pallidum/putamen (x = 18, y = 4, z = 6; p < .001), middle frontal gyrus (x = 40, y = 20, z = 44; p = .008), inferior frontal gyrus (x = 52, y = 20, z = 12; p = .020), and insular cortex (x = −42, y = 4, z = −12; p = .041) as secure attachment scores increased.
Figure 2.
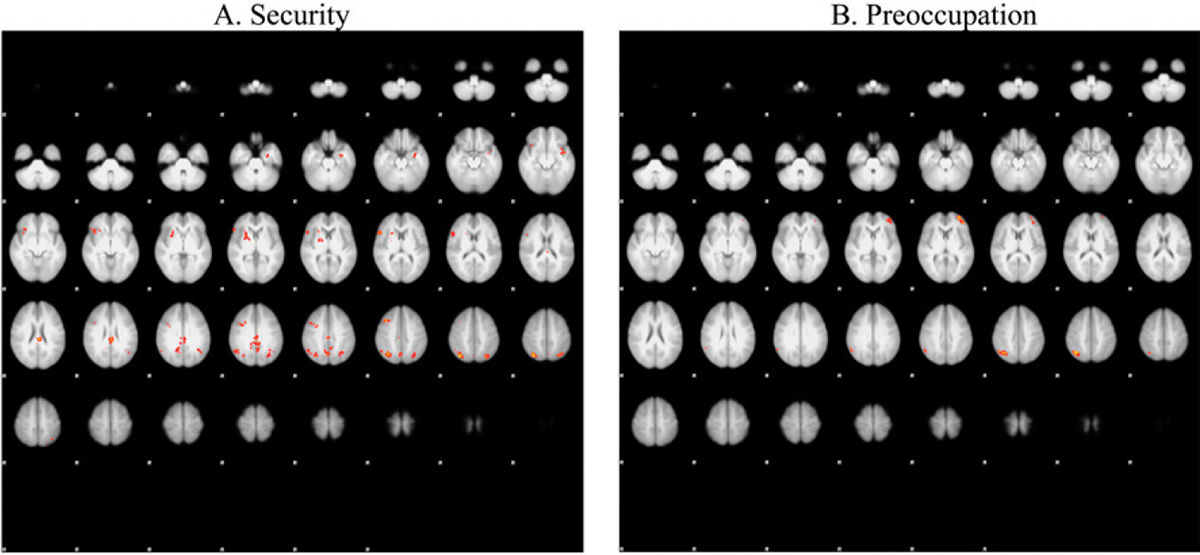
Depicts lightbox images showing significant activations in A: alone > stranger condition as the AAI continuous security scores decreased; B: alone > stranger condition as the AAI continuous preoccupation scores increased.
Table 1.
Significant clusters of activity for the alone < stranger condition with decreasing security and increasing preoccupation scores.
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Structural location | Cluster size in voxels | Z-max | X | Y | Z |
| Alone-stranger with security scores | |||||
| Posterior cingulate gyrus | 576 | 4.37 | −2 | −28 | 26 |
| Lateral occipital cortex | 382 | 4.47 | 34 | −70 | 50 |
| Lateral occipital cortex | 324 | 4.05 | −32 | −68 | 50 |
| Right pallidum/Putamen | 264 | 4.03 | 18 | 4 | 6 |
| Middle frontal gyrus | 163 | 4.2 | 40 | 20 | 44 |
| Inferior frontal gyrus | 134 | 4.07 | 52 | 20 | 12 |
| Insular cortex | 113 | 3.88 | −42 | 4 | −12 |
| Alone-stranger with preoccupation scores | |||||
| Lateral occipital cortex | 251 | 4.15 | 38 | −64 | 46 |
| Frontal pole | 192 | 3.98 | −32 | 56 | 10 |
Note. X, Y, and Z represent coordinates in relation to an origin, usually the anterior commissure, which specifies the coordinates of x = 0, y = 0, and z = 0. The x-axis goes towards the right side of the brain, the y-axis goes towards the front of the brain, and the z-axis goes towards the top of the brain. The orientation indicates whether direction relative to the origin is positive or negative.
Using the same whole-brain corrected (z = 3.1, p = .05) covariate analysis above, we also observed greater activity in the lateral occipital cortex (x = 38, y = −64, z = 46; p < .001) and frontal pole (x = −32, y = 56, z = 10; p = .004) cluster activity as preoccupation scores increased.
Discussion
This study provides evidence of a brain-behavior link that can advance understanding of adolescent attachment as a predictor of downstream social consequences in adulthood, such as seeking support when facing external threats. Specifically, individuals higher in secure attachment in adolescence showed, 10 years later as adults, higher activations in the posterior cingulate cortex—a region linked to empathy and perspective taking—as they anticipated an external threat when holding the hand of their partner compared to when alone. Additionally, securely attached adolescents also showed, as adults, higher activations in regions involved in perspective taking (e.g., posterior cingulate cortex), volitional control (e.g., lateral occipital cortex, middle and inferior frontal gyrus), reward-related circuitry (e.g., right pallidum/putamen), and affective systems (e.g., insular cortex) as they anticipated a threat while holding the hand of a stranger compared to when alone.
By contrast, higher preoccupied attachment in adolescence predicted reduced adult activations in the lateral occipital cortex and frontal pole—regions involved in cognitive control and perception when participants were holding the hand of a stranger compared to alone. No significant differences were found for preoccupation in the alone relative to partner condition. This study was among the first to examine the relation between attachment in adolescence and social-regulatory responses to threat in adulthood using fMRI. The findings advance our understanding of attachment and social relationships from a neurodevelopmental perspective.
In the current study, brain activation in regions such as posterior cingulate cortex, precuneus, striatum (right pallidum and putamen), and insula, map largely to the functional neuroanatomical model of human attachment (NAMA; Long et al., 2020) and broad existing neuroimaging literature on attachment and social relationships (Bartels & Zeki, 2004). Particularly, existing activations found in the current study point to a reward circuitry that receives integrated inputs from core regions, including the insula (associated with affective processes and pleasant feelings of touch; Olausson et al., 2002) and the striatum (putamen and pallidum), which plays a key role in rewarding responses (Elliott et al., 2003; Knutson et al., 2001). On the other hand, the posterior cingulate cortex is implicated in empathy (Völlm et al., 2006) and plays a prominent role in both positive and negative emotions (Beauregard et al., 1998; Cabeza & Nyberg, 2000). Other regions including the inferior and middle frontal gyrus and frontal pole are involved in cognitive control (e.g., inferior frontal gyrus, Tops & Boksem, 2011; middle frontal gyrus, Banich et al., 2009; frontal pole, Bramson et al., 2020), by modulating affective responses. Bartels and Zeki (2004) argued that the involvement of these regions in both positive and negative emotions suggests that activity in these regions may be modulated through limbic/paralimbic regions, which either facilitate or inhibit the effects of mood on cognitive processing (Liotti et al., 2000; Mayberg et al., 1999).
Together, the current findings suggest that secure attachment in adolescence, assessed by the AAI, was associated with increased activation in affective processes and reward-related circuitry; in other words, securely attached adolescents experienced handholding as innately rewarding, drawing closeness and connection with a partner and even a stranger. On the other hand, insecure attachment assessed in adolescence was associated with reduced activation in positive affective system and reward circuitry, leading to less cognitive processes involved in mental inference and empathy, and suggesting that insecurely atptached adolescents found handholding with strangers and partners to be less rewarding.
Based on attachment theory, experiences of social connection in times of threat are innately rewarding, because of the reduction of the fear response and the rewarding qualities of the provision of comfort and social connection (Atzil et al., 2018). Several studies have shown that social interactions with parents, partners, or peers are associated with the experience of positive emotions and increased activity in the reward circuitry (Vrtička & Vuilleumier, 2012). The current neuroimaging findings corroborate the existing literature and suggests the critical involvement of reward circuitry in attachment-based interactions. In other words, individuals with a history of a secure state of mind regarding attachment in adolescence may find handholding with partners and strangers more rewarding compared to individuals with insecure attachment. In turn, effects related to the reward facilitate and promote the positive effects of mood on how one thinks about others and regulates cognitive processes—a key role of the posterior cingulate cortex found in the current study.
Results regarding preoccupied attachment, on the other hand, was slightly discrepant from this pattern, such that increased preoccupation was associated with reduced activity in the lateral occipital cortex and frontal pole–regions involved in cognition and perception, only during the stranger (vs. alone) handholding condition, not during the partner (vs. alone) handholding condition. Theoretically, individuals with preoccupied attachment desire closeness and intimacy, fear the consequences of abandonment and rejection (Bartholomew, 1990), and may use negative relationship maintenance behaviors to seek information in order to reduce relational anxiety (Goodboy & Bolkan, 2011). Although research examining preoccupied individuals interacting with confederates is limited, previous research suggested that preoccupied attachment did not predict negative reaction to close peers’ provision of support; instead, preoccupied attachment was associated with minimal increases in support provisional behaviors in response to their peers’ needs. Another study found that preoccupied individuals only reciprocated disclosure to a high-disclosing confederate (Feeney et al., 2008; Mikulincer & Nachshon, 1991). As such, uncertainty and perceived availability of others might be key deciding factors for support provision of individuals with preoccupied attachment. Our existing findings, together with previous literature, suggested that when information about the availability of others is uncertain (i.e., in the stranger condition), individuals with preoccupied attachment may be overwhelmed by negative affect; in contrast, when such information is available (i.e., in the partner condition), they behave similarly to the securely attached individuals. Briefly, we note that in the current study, our preoccupation and security scores were only partially distinct (r = −0.55, p < .001) so interpretations should be made with caution.
The current findings also diverged in some ways from findings from prior research. For example, a previous study that followed the same neuroimaging handholding design found that higher maternal support in adolescence corresponded with less threat-related activation in adulthood—particularly in the bilateral orbitofrontal cortex, inferior frontal gyrus, and left insula— when participants were holding the hands of their friends, compared to when alone (Coan et al., 2013). The effects (compared to the current study) were in similar regions but opposite directions. However, key differences exist between the two studies. For instance, the prior study comprised a much smaller sample (N = 22), where inter-individual variability was limited. Second, maternal support was measured via an observationally coded dyadic interaction (the Supportive Behavior Task), where participants and their mothers engaged in a conversation focusing on a problem where they were having and mothers’ emotional engagement during the task was coded as maternal support (vs. assessing attachment representations broadly). Third, maternal support was assessed when participants were 16–17, 2 to 3 years later than when the adolescents’ attachment were assessed in the current investigation. Lastly and perhaps most importantly, attachment during adolescence is a much more ambivalent process (Allen, 2021). During infancy and childhood, primary caregivers provide sensitive responsiveness to the needs of their children, providing the basis for secure attachment in early development. As childhood transitions to adolescence, the primary responsibility of caregivers also changes from providing 24/7 nurture to fostering emotional independence and autonomy. Additionally, as adolescents grow, changes in family relationships, peer friendships, and larger societal contextual factors such as aggression, bullying, poverty, and racism, further overwhelm the attachment system (Allen, 2021). Thus, more research focusing on attachment during adolescence is needed to understand this complex developmental period interplayed by a multitude of familial and societal influences.
The current study employed the Adult Attachment Interview, which measures one’s “state of mind regarding attachment” and defines security as a state of “autonomous, yet valuing of attachment” (Main et al., 2002). The interview itself assesses participants in terms of their ability to discuss emotionally intense experiences coherently, with emphasis on perspective taking, balance, clarity of communication, and lack of anger or avoidance of the topics—all abilities closely tied to emotion regulation. Accordingly, Hesse (2008) described the goal of AAI as, “to bring into relief individual differences in what are presumed to be deeply internalized strategies for regulating emotion and attention when speakers are discussing attachment-related experiences.” Allen and Miga (2010) further argued that adolescents’ AAI status is indeed a reflection of emotion regulation, measured in the context of discussing attachment-related experiences. In particular, they found a strong relation between peer functioning and AAI status. Peer factors (e.g., teen popularity, teen calls for emotional support from peers, and fewer experiences of peer pressure) were more strongly linked overall to adolescent AAI status than were parental factors, accounting for a full 24% of the variance. If the AAI in adolescence measures teens’ autonomy and emotion regulation, it makes sense for adolescents who were more autonomous and self-regulated to show reduced neural reactivity in regions implicated in the cognitive control system (e.g., inferior frontal gyrus) when they were alone compared to when they were with a relational partner or stranger.
Additionally, different attachment instruments likely correspond to different neurological processes, especially when comparing the AAI to self-report measures (Roisman et al., 2007b). Yaseen and colleagues (2016) argued that the AAI emphasizes an overall implicit representation of the positive valence of primary self-other relation through an adequately attuned and responsive caregiving bond. The authors compared neural activity to maternal presence and their correlations with two different attachment measures – the AAI and a self-report measure, the Relationship Scales Questionnaire (RSQ). Comparing the differential brain regions associated with AAI and RSQ-assessed attachment, the authors conclude that AAI-assessed security was associated with enhanced activity in midline regions involved in empathy (e.g., the posterior cingulate cortex), suggesting more implicit processing of self-other relation. These regions are similarly implicated in the current study within the expected direction, further corroborating that the interpretation of the current finding relies heavily on the attachment measure utilized. The AAI approach might have assessed non-conscious processes as a product of the “core self,” while other self-report scales of attachment such as the RSQ relate to the higher-order cognitive systems at the level of conscious awareness.
Strengths, limitations, and future directions
The current study contributes to a line of research establishing longitudinal links between early life experiences such as attachment and brain activation in the same individual at a later point when they seek support in the presence of social others. Furthermore, it sheds light on attachment during adolescence–a unique period for maternal independence and peer relationship development.
This study also has several limitations. First, most participants who completed the Adult Attachment Interview had a high inverse correlation between secure and dismissing attachment scores (r = −0.95 p < .001). On the other hand, there was also a moderate correlation between secure and preoccupied attachment scores. There was also limited variability in the preoccupation scores (common in U.S. community samples); thus, future studies should examine potential unique effects of preoccupied attachment, drawing on samples with greater variability. Future studies could also benefit from understanding the developmental changes of the social brain in association with attachment through repeated neuroimaging measure. Moreover, although the neuroimaging design of the study restricted sample size, we note relatively modest power as a limitation of the current study. Previous literature suggested that a sample size of 80 can reliably recover regions with medium effect sizes (0.5 < Cohen’s d < 0.8; Geuter et al., 2018). It is also worth noting that the “intensity” of handholding was not specified as part of the script during the experiment, which may convey different relational messages. Lastly, the explanations provided here are post-hoc, and as such results from the current study warrant replication.
Conclusions
The current results suggest that attachment assessed via the AAI at age 14 predicts the degree to which one’s brain responds to threat in the presence of social resources 10 years later. Specifically, secure adolescent attachment score prospectively predicts adults’ increased activation in regions commonly associated with empathy, affective and reward processing during partner and stranger handholding compared to being alone. On the other hand, preoccupation attachment score is associated with decreased activation in similar regions, but only during stranger handholding in comparison to being alone. These findings add to a broader understanding of attachment and social relationships from a neurodevelopmental perspective.
Open research statement
As part of IARR’s encouragement of open research practices, the authors have provided the following information: This research was pre-registered. The aspects of the research that were pre-registered were study hypotheses and analyses plan. The registration was submitted to OSF. The data used in the research cannot be publicly shared with any person. Participants’ data are protected by a Confidentiality Certificate issued by the U.S. Department of Health and Human The materials used in the research can be publicly posted. The pre-registration can be obtained at: https://osf.io/38ck2/.
Acknowledgments
We acknowledge that this work would not be possible without the hard work of research coordinators and assistants of the KLIFF/VIDA project at UVA.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from the National Institute of Child Health and Human Development and the National Institute of Mental Health (R01 HD058305-16A1; R01-MH58066; and R01MH080725).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Allen JP (2008). The attachment system in adolescence. In Cassidy J, & Shaver PR (Eds.), Handbook of attachment: Theory, research, and clinical applications (2nd ed., pp. 419–435). Guilford. [Google Scholar]
- Allen JP (2021). Beyond stability. Attachment (p. 161). The Fundamental Questions. [Google Scholar]
- Allen JP, & Manning N (2007). From safety to affect regulation: Attachment from the vantage point of adolescence. New Directions for Child and Adolescent Development, 2007(117), 23–39. 10.1002/cd.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, & Miga EM (2010). Attachment in adolescence: A move to the level of emotion regulation. Journal of Social and Personal Relationships, 27(2), 181–190. 10.1177/0265407509360898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Moore C, Kuperminc G, & Bell K (1998). Attachment and adolescent psychosocial functioning. Child Development, 69(5), 1406–1419. 10.2307/1132274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammaniti M, Van Ijzendoorn MH, Speranza AM, & Tambelli R (2000). Internal working models of attachment during late childhood and early adolescence: An exploration of stability and change. Attachment and Human Development, 2(3), 328–346. 10.1080/14616730010001587 [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li HF, & Brown LL (2005). Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology, 94(1), 327–337. 10.1152/jn.00838.2004 [DOI] [PubMed] [Google Scholar]
- Atzil S, Gao W, Fradkin I, & Barrett LF (2018). Growing a social brain. Nature Human Behaviour, 2(9), 624–636. 10.1038/s41562-018-0384-6 [DOI] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, & Heller W (2009). Cognitive control mechanisms, emotion and memory: A neural perspective with implications for psychopathology. Neuroscience and Biobehavioral Reviews, 33(5), 613–630. 10.1016/j.neubiorev.2008.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, & Zeki S (2000). The neural basis of romantic love. NeuroReport, 11(17), 3829–3834. 10.1097/00001756-200011270-00046 [DOI] [PubMed] [Google Scholar]
- Bartels A, & Zeki S (2004). The neural correlates of maternal and romantic love. NeuroImage, 21(3), 1155–1166. 10.1016/j.neuroimage.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Bartholomew K (1990). Avoidance of intimacy: An attachment perspective. Journal of Social and Personal Relationships, 7(2), 147–178. 10.1177/0265407590072001 [DOI] [Google Scholar]
- Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, & Stip E (1998). The functional neuroanatomy of major depression: An fMRI study using an emotional activation paradigm. NeuroReport, 9(14), 3253–3258. 10.1097/00001756-199810050-00022 [DOI] [PubMed] [Google Scholar]
- Beckes L, & Coan JA (2011). Social baseline theory: The role of social proximity in emotion and economy of action. Social and Personality Psychology Compass, 5(12), 976–988. 10.1111/j.1751-9004.2011.00400.x [DOI] [Google Scholar]
- Bowlby J (1969). Attachment and loss: Vol. 1. Attachment (original ed. 1969; 2nd ed. 1982). Basic Books. [Google Scholar]
- Bramson B, Folloni D, Verhagen L, Hartogsveld B, Mars RB, Toni I, & Roelofs K (2020). Human lateral frontal Pole contributes to control over emotional approach-avoidance actions. Journal of Neuroscience, 40(14), 2925–2934. 10.1523/JNEUROSCI.2048-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, & Nyberg L (2000). Neural bases of learning and memory: Functional neuroimaging evidence. Current Opinion in Neurology, 13(4), 415–421. 10.1097/00019052-200008000-00008 [DOI] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016). The neuroenvironmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology, 41(1), 163–176. 10.1038/npp.2015.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti DV, & Sparrow SA (1981). Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. American Journal of Mental Deficiency, 86(2), 127–137. Retrieved from.https://psycnet.apa.org/psycinfo/1982-00095-001 [PubMed] [Google Scholar]
- Coan JA, Beckes L, & Allen JP (2013). Childhood maternal support and social capital moderate the regulatory impact of social relationships in adulthood. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 88(3), 224–231. 10.1016/j.ijpsycho.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Beckes L, Gonzalez MZ, Maresh EL, Brown CL, & Hasselmo K (2017). Relationship status and perceived support in the social regulation of neural responses to threat. Social Cognitive and Affective Neuroscience, 12(10), 1574–1583. 10.1093/scan/nsx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, & Davidson RJ (2006). Lending a hand: Social regulation of the neural response to threat. Psychological Science, 17(12), 1032–1039. 10.1111/j.1467-9280.2006.01832.x [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, & Deakin JF (2003). Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. Journal of Neuroscience, 23(1), 303–307. 10.1523/JNEUROSCI.23-01-00303.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney BC, Cassidy J, & Ramos-Marcuse F (2008). The generalization of attachment representations to new social situations: Predicting behavior during initial interactions with strangers. Journal of Personality and Social Psychology, 95(6), 1481–1498. 10.1037/a0012635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley RC, & Shaver PR (1998). Airport separations: A naturalistic study of adult attachment dynamics in separating couples. Journal of Personality and Social Psychology, 75(5), 1198–1212. 10.1037/0022-3514.75.5.1198 [DOI] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, & Tottenham N (2014). Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychological Science, 25(11), 2067–2078. 10.1177/0956797614550878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George C, Main M, & Kaplan N (1985). Adult attachment interview (AAI) [database record]. APA PsycTests. 10.1037/t02879-000 [DOI] [Google Scholar]
- Geuter S, Qi G, Welsh RC, Wager TD, & Lindquist MA (2018). Effect size and power in fMRI group analysis. Biorxiv, 295048. 10.1101/295048 [DOI] [Google Scholar]
- Gliga T, Farroni T, & Cascio CJ (2019). Social touch: A new vista for developmental cognitive neuroscience? Developmental Cognitive Neuroscience, 35, 1–4. 10.1016/j.dcn.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MZ, Beckes L, Chango J, Allen JP, & Coan JA (2015). Adolescent neighborhood quality predicts adult dACC response to social exclusion. Social Cognitive and Affective Neuroscience, 10(7), 921–928. 10.1093/scan/nsu137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodboy AK, & Bolkan S (2011). Attachment and the use of negative relational maintenance behaviors in romantic relationships. Communication Research Reports, 28(4), 327–336. 10.1080/08824096.2011.616244 [DOI] [Google Scholar]
- Gross EB, & Medina-DeVilliers SE (2020). Cognitive processes unfold in a social context: A review and extension of social baseline theory. Frontiers in Psychology, 11, 378. 10.3389/fpsyg.2020.00378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare AL, Marston EG, & Allen JP (2011). Maternal acceptance and adolescents’ emotional communication: A longitudinal study. Journal of Youth and Adolescence, 40(6), 744–751. 10.1007/s10964-010-9586-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse E (2008). The Adult Attachment Interview: Protocol, method of analysis, and empirical studies. In Cassidy J, & Shaver PR (Eds.), Handbook of attachment: Theory, research, and clinical applications (2nd ed., pp. 552–598). Guilford. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- Kanske P (2018). The social mind: Disentangling affective and cognitive routes to understanding others. Interdisciplinary Science Reviews, 43(2), 115–124. 10.1080/03080188.2018.1453243 [DOI] [Google Scholar]
- Kim S, Iyengar U, Mayes LC, Potenza MN, Rutherford HJV, & Strathearn L (2017). Mothers with substance addictions show reduced reward responses when viewing their own infant’s face. Human Brain Mapping, 38(11), 5421–5439. 10.1002/hbm.23731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher TT, Senior C, Phillips ML, Rabe-Hesketh S, Benson PJ, Bullmore ET, Brammer M, Simmons A, Bartels M, & David AS (2001). Recognizing one’s own face. Cognition, 78(1), B1–B15. 10.1016/s0010-0277(00)00104-9 [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, & Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159. 10.1523/JNEUROSCI.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobak RR, Cole H, Ferenz-Gillies R, Fleming W, & Gamble W (1993). Attachment and emotion regulation during mother-teen problem-solving: A control theory analysis. Child Development, 64(1), 231–245. 10.2307/1131448 [DOI] [PubMed] [Google Scholar]
- Lieberman MD (2007). Social cognitive neuroscience: A review of core processes. Annual Review of Psychology, 58, 259–289. 10.1146/annurev.psych.58.110405.085654 [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, & Fox PT (2000). Differential limbic--cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biological Psychiatry, 48(1), 30–42. 10.1016/s0006-3223(00)00874-x [DOI] [PubMed] [Google Scholar]
- Loeb EL, Stern JA, Costello MA, & Allen JP (2021). With(out) a little help from my friends: Insecure attachment in adolescence, support-seeking, and adult negativity and hostility. Attachment and Human Development, 23(5), 624–642. 10.1080/14616734.2020.1821722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Verbeke W, Ein-Dor T, & Vrtička P (2020). A functional neuro-anatomical model of human attachment (NAMA): Insights from first- and second-person social neuroscience. Cortex, 126, 281–321. 10.1016/j.cortex.2020.01.010 [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, Bloomer CW, Bohning DE, Vincent D, Johnson MR, Emmanuel N, Brawman-Mintzer O, Book SW, Lydiard RB, Ballenger JC, & George MS (1999). Feasibility of using fMRI to study mothers responding to infant cries. Depression and Anxiety, 10(3), 99–104. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, & Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science, 288(5472), 1835–1838. 10.1126/science.288.5472.1835 [DOI] [PubMed] [Google Scholar]
- Main M (1990). Cross-cultural studies of attachment organization: Recent studies, changing methodologies, and the concept of conditional strategies. Human Development, 33(1), 48–61. 10.1159/000276502 [DOI] [Google Scholar]
- Main M, & Goldwyn R (1998). Adult attachment scoring and classification system. University of California. Unpublished manuscript. [Google Scholar]
- Main M, Goldwyn R, & Hesse E (2002). Adult attachment scoring and classification systems. Version 7.1. University of California. Unpublished Manuscript. [Google Scholar]
- Main M, Kaplan N, & Cassidy J (1985). Security in infancy, childhood, and adulthood: A move to the level of representation (pp. 66–104). Monographs of the Society for Research in Child Development. 10.2307/3333827 [DOI] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, & Fox PT (1999). Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry, 156(5), 675–682. 10.1176/ajp.156.5.675 [DOI] [PubMed] [Google Scholar]
- Mikulincer M, & Nachshon O (1991). Attachment styles and patterns of self-disclosure. Journal of Personality and Social Psychology, 61(2), 321–331. 10.1037/0022-3514.61.2.321 [DOI] [Google Scholar]
- Mikulincer M, & Shaver PR (2008). Adult attachment and affect regulation. In Cassidy J, & Shaver PR (Eds.), Handbook of attachment: Theory, research, and clinical applications (2nd ed., pp. 503–531). Guilford. [Google Scholar]
- Mikulincer M, & Shaver PR (2012). An attachment perspective on psychopathology. World Psychiatry, 11(1), 11–15. 10.1016/j.wpsyc.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, & Davidson RJ (2004). Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. NeuroImage, 21(2), 583–592. 10.1016/j.neuroimage.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Nivison MD, Dagan O, Booth-LaForce C, Roisman GI, & Waters TEA (2021). Caregiving antecedents of secure base script knowledge inferred from the adult attachment interview: A comparative, pre-registered analysis. Infant and Child Development, e2410. 10.1002/icd.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, & Bushnell MC (2002). Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience, 5(9), 900–904. 10.1038/nn896 [DOI] [PubMed] [Google Scholar]
- Roisman GI, Fraley RC, & Belsky J (2007). A taxometric study of the adult attachment interview. Developmental Psychology, 43(3), 675–686. 10.1037/0012-1649.43.3.675 [DOI] [PubMed] [Google Scholar]
- Roisman GI, Holland A, Fortuna K, Fraley RC, Clausell E, & Clarke A (2007). The adult attachment interview and self-reports of attachment style: An empirical rapprochement. Journal of Personality and Social Psychology, 92(4), 678–697. 10.1037/0022-3514.92.4.678 [DOI] [PubMed] [Google Scholar]
- Schachner DA, Shaver PR, & Mikulincer M (2005). Patterns of nonverbal behavior and sensivity in the context of attachment relations. Journal of Nonverbal Behavior, 29(3), 141–169. 10.1007/s10919-005-4847-x [DOI] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, & Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. 10.1523/jneurosci.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, & Grady CL (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience, 22(6), 1112–1123. 10.1162/jocn.2009.21282 [DOI] [PubMed] [Google Scholar]
- Sterling P (2020). What is health? Allostasis and the evolution of human design. MIT Press. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, & Montague PR (2008). What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics, 122(1), 40–51. 10.1542/peds.2007-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JS, Hessel ET, Loeb EL, Schad MM, Allen JP, & Chango JM (2016). Long-term predictions from early adolescent attachment state of mind to romantic relationship behaviors. Journal of Research on Adolescence, 26(4), 1022–1035. 10.1111/jora.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, & Boksem MA (2011). A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Frontiers in Psychology, 2, 330. 10.3389/fpsyg.2011.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völlm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, Deakin JF, & Elliott R (2006). Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. NeuroImage, 29(1), 90–98. 10.1016/j.neuroimage.2005.07.022 [DOI] [PubMed] [Google Scholar]
- Vrtička P (2017). The social neuroscience of attachment. In Ibáñez A, Sedeño L, & García A (Eds.), Neuroscience and social science. Springer. 10.1007/978-3-319-68421-5_5 [DOI] [Google Scholar]
- Vrtička P, & Vuilleumier P (2012). Neuroscience of human social interactions and adult attachment style. Frontiers in Human Neuroscience, 6, 212. 10.3389/fnhum.2012.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E, Hamilton CE, & Weinfield NS (2000). The stability of attachment security from infancy to adolescence and early adulthood: General introduction. Child Development, 71(3), 678–683. 10.1111/1467-8624.00175 [DOI] [PubMed] [Google Scholar]
- Worsley KJ (2001). Statistical analysis of activation images. In Functional MRI: An introduction to methods. Oxford University Press. 10.1093/acprof:oso/9780192630711.003.0014 [DOI] [Google Scholar]
- Yaseen ZS, Zhang X, Muran JC, Winston A, & Galynker II (2016). Comparison of brain activity correlating with self-report versus narrative attachment measures during conscious appraisal of an attachment figure. Frontiers in Human Neuroscience, 10, 90. 10.3389/fnhum.2016.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]


