Abstract
This review discusses available evidence on the mechanisms of action of bacterial lysates, and the clinical effects of their sublingual administration. Bacterial lysates act through many immunological effects, including dendritic cell activation, modification of circulating lymphocyte subsets and antibody production. The production of salivary IgA was repeatedly shown to be induced by the sublingual administration of a prototype bacterial lysate containing soluble and corpuscular antigens. Bacterial lysates are a useful tool for the prevention of recurrent respiratory tract infections. Sublingual administration should be the preferred option.
Keywords: bacterial lysate, immunological mechanisms, sublingual administration
Introduction
Fear of increased immunity debt after the universal use of personal protective equipment during the recent COVID-19 pandemic, and the consequent increased risk of respiratory infections, prompted further attention on the relevance of prophylaxis.1 Bacterial lysates were first produced in the 1960s and introduced for the treatment and prophylaxis of human respiratory tract infections (RTI). They are obtained by either physical or chemical lysis; the first method produces fragments of bacterial bodies, characterized by slow diffusion, whereas the second method yields either low-molecular-weight proteins or a mixture of bacterial bodies and soluble proteins with greater diffusion capability. According to these different physical characteristics, lysates can be administered orally, sublingually or nasally.2 Bacterial antigens are generally obtained from the most frequently isolated strains during RTI, such as Streptococcus pneumoniae, Streptococcus pyogenes, Branhamella catarrhalis, Staphylococcus aureus, Haemophilus influenzae and Klebsiella pneumoniae.3 Despite the more recent introduction of many drugs to treat RTI, prophylaxis with bacterial lysates still attracts the interest of clinicians as a promising tool to reduce acute infection, and limit the use of antibiotics, local corticosteroids and anti-inflammatory drugs. Along this line, the European Medicines Agency recently revised the evidence for the safety and efficacy of bacterial lysates, and recommended their use for the prophylaxis of recurrent respiratory diseases.2 More importantly, in recent years, the mechanism of action of bacterial lysates administered sublingually has been investigated, showing relevant immunological effects. Their clinical efficacy was also confirmed by well-designed clinical studies over the same period, both when used alone or in addition to recommended therapies.3 A very recent revision of the literature has also demonstrated their positive effect on the reduction of the number of respiratory infections and their related symptoms.4
This article describes some findings on the mechanisms of action of bacterial lysates, supporting their clinical use. In addition, recent evidence on the clinical effects of sublingually administered bacterial lysates is reported.
Review
Mechanisms of action
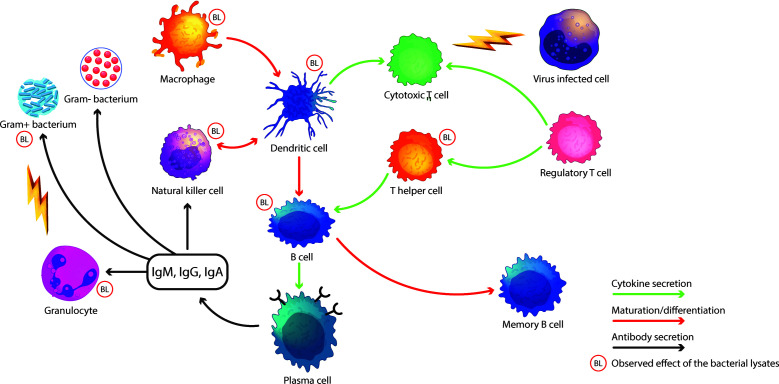
The rationale for the production of bacterial lysates was originally based on the hypothesis that they could elicit a specific immune response against bacterial antigens, which were considered the main aetiology of RTI in the 1960s. Later, it was shown that viruses are often responsible for RTI and that bacterial lysates help in activating both adaptive and innate immune defense.3 Meanwhile, several immunological effects were discovered that could be linked to the effects of lysates, including dendritic cell (DC) activation,5–7 modification of circulating lymphocyte subsets8,9 and antibody production10–12 (Figure 1). Mature DCs are necessary to drive an efficient immune response. Several bacterial lysates induced significant DC activation and maturation in vitro.5,6 More recently, it has been reported that in vitro treatment of immature DCs with fragments of bacterial strains obtained by mechanical lysis can induce maturation of DCs and the secretion of a wide array of cytokines and chemokines.7 Lanzilli et al.,8 in an ex vivo study, found that treatment with a polyvalent mechanical bacterial lysate (PMBL) modified the subsets of circulating lymphocytes in older patients with chronic obstructive pulmonary disease (COPD). T and natural killer (NK) cells increased significantly in number but not in their percentage of circulating cells, whilst B cells remained unmodified. Amongst T cells, CD3+CD4+ and activated cells were increased. Amongst B cells, transitional B cells increased in their late maturation step, whilst only early naive B cells increased; other naive subpopulations were not modified. Memory B cells were reduced globally, but the most immature form of memory B cells was significantly increased.8 These results were observed ex vivo in blood samples from elderly patients with COPD. In the same group, NK cells were positively affected, regardless of the age of patients. Another study recently confirmed the capacity of a bacterial lysate to increase NK cell activity.9 Despite the broad availability of data on the mechanism of action of bacterial lysates, there is still the potential for further studies on recently discovered new immunology targets such as innate lymphoid cells. Moreover, bacterial lysates, through the activation of DCs, induce a predominant T helper 1 (TH1) response, with increased IL-12 and interferon-γ (IFNγ),14,15 weakening the TH2 pattern of the immune system. Parallel to the enhancement of the TH1 response, the oral administration of bacterial lysates aids the production of IL-10, favouring the conversion of FoxP3− T cells into FoxP3+ regulatory T cells.16,17 Nevertheless, taken together, the effects of TH1/TH2 balance and the stimulated differentiation of DC and T regulatory cells lay the basis for possible use of bacterial lysates in the prevention of allergic diseases.
Figure 1.
Immune mechanisms affected by bacterial lysates.
BL, bacterial lysate
The production of salivary IgA was repeatedly shown to be induced by the sublingual administration of a prototype of polyvalent chemical bacterial lysate (PCBL) containing soluble and corpuscular antigens.10,11 Subsequently, locoregional antibody production was demonstrated for the sublingual administration of a PMBL. A prospective study in 40 patients with recurrent upper RTI found that treatment for 6 months reduced the number of episodes and enhanced the locoregional immune response, with increased IgA and IgG levels and an induced ability to opsonize living bacteria.12 Interestingly, opsonizing capacity was also observed for antibiotic-resistant bacteria. Finally, IgA and innate responses can act toward all types of microbes, including viruses.12
An improvement in locoregional antibody-mediated immune response was also found in older patients with COPD treated with PMBL in a randomized clinical trial.13 Concomitantly with the secretion of specific antibodies, the treated group experienced fewer seroconversions, infectious episodes and COPD exacerbations than the group receiving a placebo.13 Overall, the results obtained with sublingually administered bacterial lysates suggest that this way of administration can locally activate the innate and adaptive immune response at the route of entry of pathogens responsible for respiratory infections. Additionally, these results indicate that the generation of a ‘specific’ immune response – as demonstrated by the presence of IgA directed towards surface antigens of bacteria – only represents a fraction of the whole immune response generated by the treatment. Further different mechanisms should also be involved and, for this reason, researchers have lately focused on different characteristics of PMBL.18–20
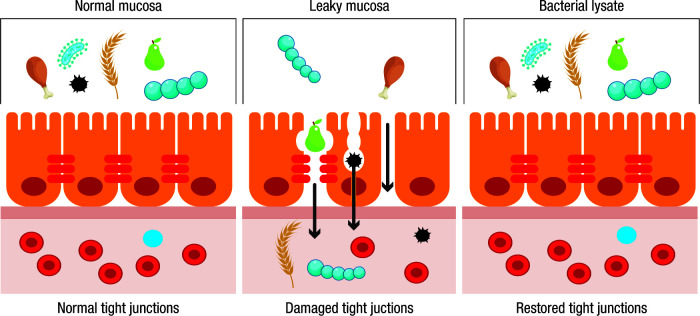
Recent reports have shown that PMBL is able to enhance antimicrobial barrier mechanisms in human airway epithelial cells.18 It is well established that microbes entering the bodies of multicellular eukaryotes must first cross an epithelial cell layer. Besides functioning as physical barriers to prevent infection, mammalian epithelial cells can detect the presence of microbes and respond by increasing their barrier function, signalling to leukocytes and directly killing pathogens. Whilst signalling to leukocytes has received considerable attention, their augmented barrier function and pathogen-killing features have so far received less.18
It has been demonstrated that human airway epithelial cells can recognize PMBL and, in response, undergo a potent activation resulting in a significant increase in the expression of cellular adhesion molecules, such as intercellular adhesion molecule 1 and E-cadherin, as well as the expression of growth factors critical for supporting human airway epithelial cell proliferation such as amphiregulin and IL-22.19,20 Cell proliferation in damaged epithelial tissues and the expression of molecules involved in tight junction formation can locally contribute to provide a more efficient physical barrier to pathogens, including viruses, but also to relieve inflammation and regenerate damaged airway tissues (Figure 2). These findings are relevant, considering that inflamed tissues display increased permeability favouring the access of pathogens to the sub-mucosal compartment, resulting in an increased risk of locoregional infections.
Figure 2.
Activity of bacterial lysates on tight junctions of the mucosa.
Remarkably, polyvalent mechanical bacterial lysates also promoted the de novo expression of human β-defensin 2, a major antimicrobial peptide, in human bronchial epithelial cells, conferring them a direct antimicrobial activity.21 Moreover, PMBL-stimulated human bronchial epithelial cells provided signals for increased IL-22 production by innate lymphoid cells via IL-23, which could further contribute to the release of antimicrobial peptides by epithelial cells. In agreement with these in vitro data, the concentration of both IL-23 and antimicrobial peptides (human β-defensin-2 and LL-37) increased in the saliva of healthy volunteers after sublingual administration of PMBL.20
Another new and interesting field of research on sublingual bacterial lysates was highlighted during the COVID-19 pandemic. In vitro treatment of epithelial cells from the upper respiratory airways with PCBL has been observed to negatively regulate the expression of angiotensin-converting enzyme 2 (ACE2), a main SARS-CoV-2 receptor.22 At the same time, PCBL does not affect the expression of other surface molecular structures such as CD54 (the rhinovirus receptor). Further tests using epithelial cells infected in vitro with a wild strain of SARS-CoV-2 (Epsilon variant) showed that cells pretreated with the bacterial lysate had a significantly reduced amount of virus copies, thus supporting the idea that the decreased expression of ACE2 in vitro could ‘shut the door’ to the virus. This capacity is shared with other drugs such as OM-85.18 However, OM-85 is administered orally and the doses required to downregulate ACE2 would be far higher than those achievable in human therapy.18,23 For example, reducing ACE2 expression with OM-85 in vitro required the stimulation of primary normal human bronchial epithelial cells with 1.92 mg/mL OM-85 for 48 hours, a concentration that cannot easily be obtained using capsules containing 7 mg lysate per oral administration; the number of capsules to be administered would be impossible to manage.18,23 On the contrary, PCBL appears to downregulate ACE2 by doses easily obtained by sublingual administration.22
Clinical studies
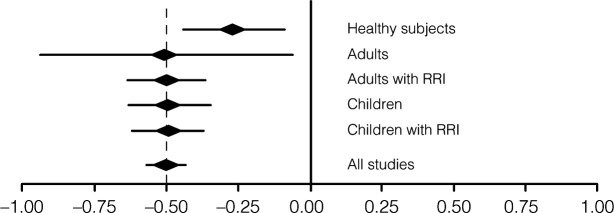
Several clinical studies have been conducted over the past 40 years concerning sublingually administered bacterial lysates. The most recent publication is an extensive review of available PCBL clinical data from 22 randomized controlled trials involving 4,571 patients, clearly indicating that all studies confirmed the clinical efficacy of this therapeutic approach (Figure 3).4
Figure 3.
Efficacy of Lantigen B in clinical studies. Reduction of respiratory tract infections measured according to study end points. RRI, recurrent respiratory infections.
Data from Braido et al.4
Studies in adults with recurrent RTI
Most of all available studies on the efficacy and tolerability of PCBL in recurrent RTI (RRTI) have been performed in adult patients, showing an overall reduction of −47% in different outcomes, including the number of infectious episodes, number of days with fever or number of days of absence from work/school.4
In 2014, a multicentre, randomized, placebo-controlled trial on the use of PCBL in adults with RRTI3 showed that not only the overall sample of patients with RRTI but also of patients with allergies and RRTI experienced a significant reduction in the number of recurrences. Notably, the efficacy of PCBL was detected despite a relevant placebo effect as long as recurrences were reduced in control patients, but the difference between groups was significant.
Studies in adults with COPD
COPD is a major cause of death worldwide. It is characterized by persistent respiratory symptoms and airflow limitation; it is also associated with parenchymal destruction (emphysema), the relative contribution of which varies from person to person.24 Pharmacological management of COPD has progressively improved during the past decade.24 Decades ago, treatment with PMBL was considered useful in comparison with other available options, as confirmed by a systematic review with a meta-analysis25 showing significant efficacy in preventing RRTI in adults, including patients with COPD and children.
Following the radical change in standard treatments, new regimens were explored. A phase IV multicentre, double-blind, randomized, controlled study investigated the efficacy of sublingual administration of PMBL in addition to the recommended treatment in patients with moderate to very severe COPD.26 The primary end point of a reduction in the number of exacerbations by 25% compared to placebo could not be reached as the efficacy of the recommended therapy in the control group was high and the number of exacerbations lower than expected in both groups. However, in the PMBL group, the number of days with fever (21 versus 40.15 days/year; p<0.001) and the days of hospitalization (65 versus 162 days; p<0.001) were significantly reduced. Similarly, the interval between the first and second exacerbations (123.89 versus 70.36 days; p=0.03) was prolonged, and the number of days in poor health (109 versus 171 days/year; p<0.001) was also significantly decreased in the PMBL group. These results show that adding bacterial lysates to recommended treatments may improve patient quality of life and reduce healthcare costs.
Studies in paediatric patients
The recently published review of the literature on PCBL suggests its efficacy in reducing the number, and severity of RRTI in children as all available studies report a positive effect.4 Moreover, in a randomized, double-blind, placebo-controlled, parallel-group study conducted on 152 children with allergic asthma, those treated with sublingually administered PMBL had a lower mean number of asthma exacerbations at week 12 (0.3±0.6 versus 0.8±1.1; p=0.009), and over the total study period (1.1±1.3 versus 1.9±2.0; p=0.01) than the placebo group.27 Despite the primary end point of asthma control, based on the Asthma Control Test/Childhood Asthma Control Test (ACT/C-ACT) score, not being reached, treatment with PMBL was associated with a lower mean number of days with exacerbation per patient (13.3±11.2 versus 19.8±15.7 days throughout the study; p=0.009); additionally, the time to the second and third exacerbation was prolonged by 55% and 74%, respectively, in the PMBL group. No serious adverse event related to PMBL administration was recorded.27 Subsequently, it was shown that the sublingual administration of PMBL was acceptable to preschoolers, demonstrating that the formulation is appropriate for young children.28
Another randomized, placebo-controlled study in paediatric patients with seasonal allergic rhinitis found that the sublingual administration of PMBL during the grass pollen season relieves seasonal allergic rhinitis symptoms. The authors concluded that PMBL could affect mucosal immunity, weakening the response of TH2 cells.29 The same group also ruled out the possibility that PMBL could eradicate S. aureus nasal carriage in children with allergies.30
In general, recent, well-designed, placebo-controlled clinical studies have proved that the sublingual administration of bacterial lysates in children is safe, well accepted, and results in a reduction in the number of respiratory infections and specific allergy-related symptoms.3,4,27,30,31
Discussion
The sublingual administration of bacterial lysates (both corpuscular and as a mixture of soluble and corpuscular antigens) reduces the number of RRTIs not only in patients without allergies but also offers great advantages in those with allergies, which represents a significant fraction of patients with RRTI. Indeed, it is well known that allergies are associated with a higher incidence of respiratory infections. On the other hand, viral respiratory infections are often the trigger of allergic asthma attacks, and some of the cellular and molecular bases of these events have been identified.32 As reported, many studies described associations between bacterial lysate administration and immunological mechanisms, including DC activation, recruitment of lymphoid subsets, and production of specific antibodies directed towards bacterial lysate proteins.5–13
More recently, novel and interesting properties of PMBL were described, such as the impact on activation of airway epithelial cell response.20 Proliferation of damaged epithelial cells and expression of molecules involved in tight junction formation, both induced by PMBL, could play a relevant role not only in improving the physical barrier to pathogens but also in controlling inflammation and regenerating the epithelial layer upon injury in the airway mucosa, which may also be caused by non-infective factors, such as smoke, pollution and chronic airway diseases (allergy, COPD). On the other hand, conditional pathogen killing by epithelial cells is an important aspect of innate resistance to infection that has now been shown to be induced by PMBL via the production of antimicrobial peptides.20 However, these results only appear relevant if bacterial lysates are administered sublingually because this route of administration allows bacterial fragments to come into contact with mucosal epithelial cells of oropharynx – the gate of entry of virtually every airway pathogen. New evidence indicates that sublingual administration of PMBL might support mucosal barrier integrity, the impairment of which can facilitate microbial invasion, and promote mechanisms of antimicrobial activity in the upper respiratory tract.
Several clinical studies, designed based on preclinical evidence during the last two decades, have demonstrated the efficacy and safety of bacterial lysates in the prophylaxis of RRTI, observing also relevant benefits in allergic diseases and COPD.3,25–31,33,34
Since the current maintenance treatment of asthma and COPD is based on inhaled corticosteroids and bronchodilators, and the former has been related to a possible enhanced susceptibility to pneumonia due to their impact on immune responses, there is a strong rationale to foster the innate and adaptive immune response with bacterial lysates in these patients.35 Moreover, despite the availability of effective triple combination therapies with inhaled corticosteroids and dual bronchodilator, COPD exacerbations still represent a burden for patients and for the healthcare system. As long as COPD exacerbations are mainly triggered by respiratory viral infections (although bacterial infections and environmental factors may also trigger and/or amplify these events),24 the rationale for RRTI prophylaxis in these patients is strong. Treatments aimed at the activation of innate and adaptive immunity are a valuable tool in the management of RRTI on top of maintenance treatments, such as long-acting bronchodilators and inhaled corticosteroids for COPD. In fact, the growing risk of bacterial resistance suggests that prophylaxis should be favoured over treatment in the management of RRTI. Furthermore, activation of the immune system by bacterial lysate administration seems a reasonable strategy to face the risk of immunity debt, which may be associated with many subjective and environmental conditions, including extensive use of protective devices during the SARS-CoV-2 pandemic, allergies, air pollution, concomitant diseases and polypharmacy. Specifically, sublingual administration appears to be able to boost immune defences over the route of respiratory infections.
Finally, bacterial lysates should be deeply investigated for the prevention of allergic sensitization based on their immune activity, which mimics the protective effects of natural exposure to microbe-rich environments, resulting in a probable induction of tolerogenic DCs and FOXP3+ T cells, as suggested in certain experimental models.36,37
Conclusion
In conclusion, bacterial lysates are currently emerging as a useful tool for the prevention of RRTI due to their efficient activation of innate and adaptive immunity associated with a favourable tolerability profile. Sublingual administration of bacterial lysates is supported by a strong immunological rationale and clinical evidence and should be the preferred option in the prophylaxis of RRTI.
Acknowledgements
Editorial assistance was provided by Laura Brogelli, PhD, Aashni Shah, Massimiliano Pianta and Valentina Attanasio (Polistudium Srl, Milan, Italy).
Footnotes
Contributions: Study conception and design: FB, GM, GN, GWC; collection and interpretation of data: FB, GM; manuscript drafting: GM; manuscript editing: FB, GM, GN, MF, SDG, MDG, GWC; approval to submit: FB, GM, GN, MF, SDG, MDG, GWC. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: GN is the Medical Director of Bruschettini srl, Genova, Italy; GM and FB act as consultant advisors of Bruschettini srl, Genova, Italy; SDG, MDG, MF and GWC have no competing interests. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2024/06/dic.2024-1-5-COI.pdf
Funding declaration: Editorial assistance was supported by Bruschettini Srl, Genoa, Italy.
Correct attribution: Copyright © 2024 Braido F, Melioli G, Nicolini G, Ferraris M, Di Girolamo S, Di Gioacchino M, Canonica GW. https://doi.org/10.7573/dic.2024-1-5. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights, and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Cohen R, Ashman M, Taha MK, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51:418–423. doi: 10.1016/j.idnow.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Medicines Agency. Bacterial lysates-containing medicinal products for respiratory conditions. [Accessed July 22, 2024];Active substances Assessment report. EMA/457345/2019 https://www.ema.europa.eu/en/documents/referral/bacterial-lysate-medicines-article-31-referral-chmp-assessment-report_en.pdf. [Google Scholar]
- 3.Braido F, Melioli G, Candoli P, et al. The bacterial lysate Lantigen B reduces the number of acute episodes in patients with recurrent infections of the respiratory tract: the results of a double blind, placebo controlled, multicenter clinical trial. Immunol Lett. 2014;162:185–193. doi: 10.1016/j.imlet.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braido F, Melioli G, Nicolini G, et al. Prevention of recurrent respiratory tract infections: a literature review of the activity of the bacterial lysate Lantigen B. Eur Rev Med Pharmacol Sci. 2023;27:7756–7767. doi: 10.26355/eurrev_202308_33430. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Zelle-Rieser C, Ramoner R, Bartsch G, et al. A clinically approved oral vaccine against pneumotropic bacteria induces the terminal maturation of CD83+ immunostimulatory dendritic cells. Immunol Lett. 2001;76:63–67. doi: 10.1016/S0165-2478(00)00326-6. [DOI] [PubMed] [Google Scholar]
- 7.Morandi B, Agazzi A, D’Agostino A, et al. A mixture of bacterial mechanical lysates is more efficient than single strain lysate and of bacterial-derived soluble products for the induction of an activating phenotype in human dendritic cells. Immunol Lett. 2011;138:86–91. doi: 10.1016/j.imlet.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Lanzilli G, Traggiai E, Braido F, et al. Administration of a polyvalent mechanical bacterial lysate to elderly patients with COPD: Effects on circulating T, B and NK cells. Immunol Lett. 2013;149:62–67. doi: 10.1016/j.imlet.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Lee YK, Haam JH, Suh E, et al. A case-control study on the changes in natural killer cell activity following administration of polyvalent mechanical bacterial lysate in Korean adults with recurrent respiratory tract infection. J Clin Med. 2022;11:3014. doi: 10.3390/jcm11113014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake CH, Smith JE. Letter: salivary antibody response to oral vaccine. Lancet. 1975;2:614–615. doi: 10.1016/S0140-6736(75)90215-9. [DOI] [PubMed] [Google Scholar]
- 11.Dirienzo W, Merli A, Ciprandi G, et al. Risposta anticorpale salivare e sierica ad una vaccinoterapia orale. Arch Med Intern. 1984;36:2–8. [Google Scholar]
- 12.Braido F, Schenone G, Pallestrini E, et al. The relationship between mucosal immunoresponse and clinical outcome in patients with recurrent upper respiratory tract infections treated with a mechanical bacterial lysate. J Biol Regul Homeost Agents. 2011;25:477–485. [PubMed] [Google Scholar]
- 13.Ricci R, Palmero C, Bazurro G, et al. The administration of a polyvalent mechanical bacterial lysate in elderly patients with COPD results in serological signs of an efficient immune response associated with a reduced number of acute episodes. Pulm Pharmacol Ther. 2014;27:109–113. doi: 10.1016/j.pupt.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Butcher MJ, Zhu J. Recent advances in understanding the Th1/Th2 effector choice. Fac Rev. 2021;10:30. doi: 10.12703/r/10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanzilli G, Falchetti R, Tricarico M, et al. In vitro effects of an immunostimulating bacterial lysate on human lymphocyte function. Int J Immunopathol Pharmacol. 2005;182:245–254. doi: 10.1177/039463200501800207. [DOI] [PubMed] [Google Scholar]
- 16.Navarro S, Cossalter G, Chiavaroli C, et al. The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal Immunol. 2011;4(1):53–65. doi: 10.1038/mi.2010.51. [DOI] [PubMed] [Google Scholar]
- 17.Pivniouk V, Gimenes-Junior JA, Ezeh P, et al. Airway administration of OM-85, a bacterial lysate, blocks experimental asthma by targeting dendritic cells and the epithelium/IL-33/ILC2 axis. J Allergy Clin Immunol. 2022;149(3):943–956. doi: 10.1016/j.jaci.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pivniouk V, Pivniouk O, DeVries A, et al. The OM-85 bacterial lysate inhibits SARS-CoV-2 infection of epithelial cells by downregulating SARS-CoV-2 receptor expression. J Allergy Clin Immunol. 2022;149:923–933e6. doi: 10.1016/j.jaci.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidoti Migliore G, De Pasquale C, Carrega P, et al. Human airway epithelial cells directly recognize mechanical bacterial lysates eliciting tight junction sealing, antimicrobial peptides production and epithelial cell proliferation. XII World Congress on COPD, Asthma & Respiratory Allergy; Dubai, UAE. February 2–5, 2018. [Google Scholar]
- 20.Sidoti Migliore G, Campana S, Barberi C, et al. Mechanical bacterial lysate enhances antimicrobial barrier mechanisms in human airway epithelial cells. J Leukoc Biol. 2023;113:535–540. doi: 10.1093/jleuko/qiad003. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Ding J, Liao C, et al. Defensins: the natural peptide antibiotic. Adv Drug Deliv Rev. 2021;179:114008. doi: 10.1016/j.addr.2021.114008. [DOI] [PubMed] [Google Scholar]
- 22.Pizzimenti C, D’Agostino A, Pirrello P, et al. The SARS-CoV-2 cellular receptor ACE2 is expressed in oropharyngeal cells and is modulated in vitro by the bacterial lysate lantigen B. Arch Clin Biomed Res. 2023;7:13–18. doi: 10.26502/acbr.50170315. [DOI] [Google Scholar]
- 23.Broncho vaxom RCP. [Accessed July 22, 2024]. https://medicinali.aifa.gov.it/it/#/it/dettaglio/0000017573 .
- 24. [Accessed September 19, 2023];Global initiative for chronic obstructive lung disease report. 2023 https://goldcopd.org/2023-gold-report-2/ [Google Scholar]
- 25.Cazzola M, Anapurapu S, Page CP. Polyvalent mechanical bacterial lysate for the prevention of recurrent respiratory infections: a meta-analysis. Pulm Pharmacol Ther. 2012;25:62–68. doi: 10.1016/j.pupt.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Braido F, Melioli G, Cazzola M, et al. Sub-lingual administration of a polyvalent mechanical bacterial lysate (PMBL) in patients with moderate, severe, or very severe chronic obstructive pulmonary disease (COPD) according to the GOLD spirometric classification: a multicentre, double-blind, randomised, controlled, phase IV study (AIACE study: Advanced Immunological Approach in COPD Exacerbation) Pulm Pharmacol Ther. 2015;33:75–80. doi: 10.1016/j.pupt.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Emeryk A, Bartkowiak-Emeryk M, Raus Z, et al. Mechanical bacterial lysate administration prevents exacerbation in allergic asthmatic children-the EOLIA study. Pediatr Allergy Immunol. 2018;29:394–401. doi: 10.1111/pai.12894. [DOI] [PubMed] [Google Scholar]
- 28.Emeryk A, Vallet T, Wawryk-Gawda E, et al. Acceptability of a sublingual drug formulation for respiratory tract infections in children aged 3 to 5 years. Pharmaceutics. 2021;13:294. doi: 10.3390/pharmaceutics13020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janeczek K, Emeryk A, Rachel M, et al. Polyvalent mechanical bacterial lysate administration improves the clinical course of grass pollen-induced allergic rhinitis in children:a randomized controlled trial. J Allergy Clin Immunol Pract. 2021;9:453–462. doi: 10.1016/j.jaip.2020.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Janeczek K, Emeryk A, Zimmer Ł, et al. Nasal carriage of Staphylococcus aureus in children with grass pollen-induced allergic rhinitis and the effect of polyvalent mechanical bacterial lysate immunostimulation on carriage status: a randomized controlled trial. Immun Inflamm Dis. 2022;10:e584. doi: 10.1002/iid3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pozzi E, Serra C. Efficacy of Lantigen B in the prevention of bacterial respiratory infections. Monaldi Arch Chest Dis. 2004;61:19–27. [PubMed] [Google Scholar]
- 32.Melioli G, Passalacqua G, Baena-Cagnani CE, et al. Allergens and bacteria interaction in the induction of basophil activation: is this the lost ring between allergy and infections in pediatric patients? Curr Opin Allergy Clin Immunol. 2012;12:164–170. doi: 10.1097/ACI.0b013e328350fd91. [DOI] [PubMed] [Google Scholar]
- 33.Price HC, Henley G. Trial of an oral antigen in upper respiratory tract infections in two Bristol schools. Practitioner. 1974;213:720–726. [PubMed] [Google Scholar]
- 34.Price HC, Henley G. Trial of an oral antigen against upper respiratory-tract infection. Results in the second year (1973–74) Practitioner. 1976;216:341–346. [PubMed] [Google Scholar]
- 35.Suissa S, Patenaude V, Lapi F, et al. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68:1029–1036. doi: 10.1136/thoraxjnl-2012-202872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein MM, Hrusch CL, Gozdz J, et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med. 2016;375(5):411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vercelli D. From Amish farm dust to bacterial lysates: the long and winding road to protection from allergic disease. Semin Immunol. 2023;68:101779. doi: 10.1016/j.smim.2023.101779. [DOI] [PMC free article] [PubMed] [Google Scholar]