Graphical Abstract
Graphical Abstract.
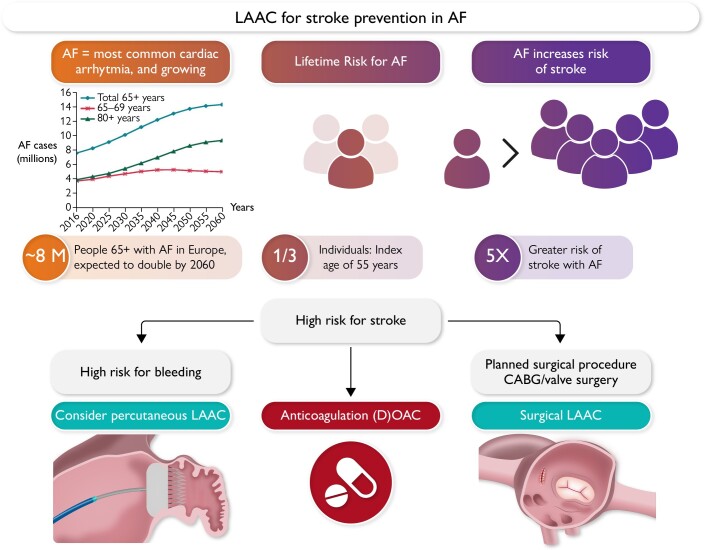
Rationale for left atrial appendage closure. Because of changing demographics, the number of atrial fibrillation patients is expected to double in industrialized countries within the next two decades. Patients with high risk for stroke have an indication for stroke preventive therapies consisting of either oral anticoagulation, percutaneous left atrial appendage closure in case of high bleeding risk, or surgical left atrial appendage closure in patients undergoing cardiac surgery. Several ongoing studies will more precisely define the optimal therapeutic approach for the individual patient. AF, atrial fibrillation, CABG, coronary artery bypass grafting; OAC, oral anticoagulation; LAAC, left atrial appendage closure.
Keywords: Atrial fibrillation, Left atrial appendage closure, Stroke prevention, Oral anticoagulation
Abstract
Atrial fibrillation (AF) is associated with an increased risk of stroke and systemic embolism, and the left atrial appendage (LAA) has been identified as a principal source of thromboembolism in these patients. While oral anticoagulation is the current standard of care, LAA closure (LAAC) emerges as an alternative or complementary treatment approach to reduce the risk of stroke or systemic embolism in patients with AF. Moderate-sized randomized clinical studies have provided data for the efficacy and safety of catheter-based LAAC, largely compared with vitamin K antagonists. LAA device iterations, advances in pre- and peri-procedural imaging, and implantation techniques continue to increase the efficacy and safety of LAAC. More data about efficacy and safety of LAAC have been collected, and several randomized clinical trials are currently underway to compare LAAC with best medical care (including non-vitamin K antagonist oral anticoagulants) in different clinical settings. Surgical LAAC in patients with AF undergoing cardiac surgery reduced the risk of stroke on background of anticoagulation therapy in the LAAOS III study. In this review, we describe the rapidly evolving field of LAAC and discuss recent clinical data, ongoing studies, open questions, and current limitations of LAAC.
Atrial fibrillation and stroke prevention: rationale for left atrial appendage closure
Atrial fibrillation (AF), the most prevalent cardiac arrhythmia, affects more than 50 million people worldwide.1 Current prevalence is estimated to be 2%–4%,2 but is expected to increase, in part due to increased life expectancy (Graphical Abstract).3
AF is a strong, independent risk factor for stroke, and thromboembolic strokes in AF patients are particularly large and severe contributing to the high medical, social, and economic burden.4,5
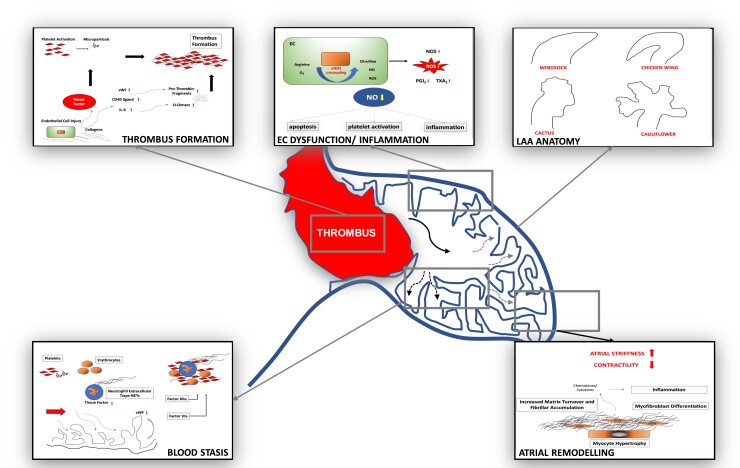
The left atrial appendage (LAA) has been identified as the principal source of intracardiac thrombi in AF patients, and thrombogenesis within the LAA has been related to local abnormalities in haemostasis, endothelial function, blood stasis, and LAA remodelling (Figure 1).6–9
Figure 1.
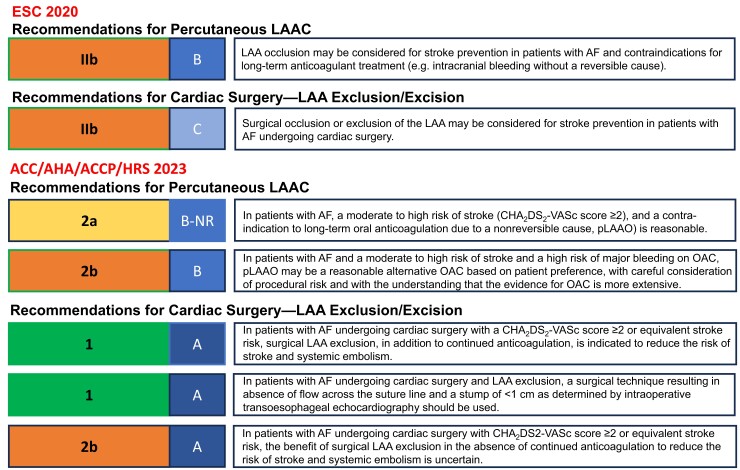
Present recommendations for left atrial appendage closure. Current guideline recommendations for left atrial appendage closure (ESC 2020, published before LAAOS III,1 and ACC/AHA/ACCP/HRS 202310). AF, atrial fibrillation; LAA, left atrial appendage; LAAC, LAA closure; OAC, oral anticoagulation; pLAAO, percutaneous left atrial appendage occlusion
Efforts to improve stroke prevention in patients with AF are therefore of paramount clinical importance.11 Risk assessments for stroke and bleeding in AF patients share several overlapping variables so that the highest risk of stroke is frequently associated with a particular high bleeding risk.12 Oral anticoagulation (OAC) is currently the mainstay of stroke preventive therapy in AF, but LAA closure (LAAC) is a rapidly developing field in this respect.
Oral vitamin K antagonists (VKA) have been shown to reduce the risk of stroke by approximately 60% compared with placebo.13 Non-vitamin K antagonist oral anticoagulants (NOACs) further reduce the risk of stroke or systemic embolism compared with VKA by 19% and are associated with a 10% relative risk reduction in mortality.14 While NOACs reduce the risk of intracranial haemorrhage (ICH) by 50% and major bleeding by 14% compared with VKA, gastrointestinal bleeding is more common, and major bleeding events still occur at a rate of 2%–3% per year, although patient populations at high bleeding risk and the elderly have been largely excluded in the Phase 3 clinical trials.14 Adherence rates for NOACs after 24 months have been reported in the range of 67%–79% in large-scale randomized clinical studies, i.e. >20% of patients may not continue chronic anticoagulation therapy under clinical trial conditions.15–18 Similar data were obtained in real-world registries.19,20 The main reasons for discontinuation of OAC include major bleeding, chronic renal failure, and a perceived high bleeding risk as well as reduced patient compliance.21–25
Exclusion of the LAA from the systemic circulation in patients with AF represents an alternative or complementary strategy to reduce the risk and severity of stroke.26 The US Food and Drug Administration (FDA) approval of catheter-based LAAC using the Watchman device and subsequently the Amulet device provided a therapeutic pathway as alternative treatment to OAC with warfarin. This catheter-based or surgical therapy may be especially attractive for prevention of thromboembolic events in AF patients at high bleeding risk. An EHRA/EAPCI expert consensus statement in 2020 discussed potential indications for catheter-based LAA occlusion in patients with AF and at high thromboembolic risk [i.e. CHA2DS2-VASc ≥ 2 (≥3 for females) - congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age 65–74 years, sex category] such as elevated bleeding risk under chronic OAC (i.e. status post-ICH, recurrent bleeds) and patients unwilling or unable to take OAC despite explanation or with a contraindication for OAC and suggested a careful individual risk–benefit analysis.27 Current guideline recommendations for LAAC are depicted in Figure 2.
Figure 2.
Proposed pathophysiology of left atrial appendage thrombus formation. Several anatomical, cellular, and physiological features contribute to thrombus formation within the left atrial appendage. Of note, different thromboembolic risk might be attributed to anatomic configurations of the left atrial appendage7 as well as flow and fluid dynamics.8,9 LAA, left atrial appendage; EC, endothelial cell; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; NOS, nitric oxide synthase; PGI2, prostaglandin I2; ROS, reactive oxygen species; TXA2, thromboxane A2; vWF, von Willebrandt factor; arrows indicate increase or decrease.
Left atrial appendage closure: current evidence
Surgical closure of the left atrial appendage
One in three patients undergoing cardiac surgery has a documented history of AF28 associated with increased risk of stroke and mortality. In approximately 80%–90% of these patients, OAC is indicated, but adherence to therapy is limited,29 and 10%–20% of patients are unsuitable for long-term anticoagulation therapy.30 Furthermore, some patients remain at high risk for stroke despite OAC.31 Surgical LAAC (S-LAAC) can be achieved endoscopically, e.g. by using the AtriClip (AtriCure) or Penditure (Medtronic) device, as a standalone procedure or in combination with endoscopic atrial ablation procedures. Endocardial suture ligation of the LAA is an invasive procedure (i.e. opened appendage) employing a cardiopulmonary bypass and is associated with high bleeding risk and incompleteness of occlusion in 10%–30%.32,33 Epicardial suture closure of the LAA is performed by either directly sewing the appendage or by tightening pre-tied suture loops around the base of the LAA (i.e. closed appendage) and can be performed without cardiopulmonary bypass with an operator- and technique-dependent success of complete closure between 23% and 100%.34,35 Surgical LAAC in patients with AF undergoing cardiac surgery may be a valuable option,36 further supported by the recent ATLAS37 and LAAOS III38 trials.
A large-scale retrospective registry from a US administrative database investigated the association between S-LAAC during concurrent cardiac surgery [coronary artery bypass grafting (CABG) and valve surgery] and long-term risk of stroke.28 After propensity matching, 4295 patients in each group were compared. Patients with S-LAAC showed significantly reduced risks of ischaemic stroke and embolism [hazard ratio (HR) .73, 95% confidence interval (CI) .56–.96; P = .03) as well as mortality (HR .71, 95% CI .60–.84; P < .001) after a mean follow-up of 2.1 years. In patients with documented AF, these differences were even more pronounced, while the event rate in patients without documented AF before surgery and in patients with AF taking OAC was not significantly different.
Another recently published registry39 determined the association of S-LAAC and readmission for thromboembolism [stroke, transient ischaemic attack (TIA), or systemic embolism] in >10 000 AF patients undergoing CABG or valve surgery. After adjustment, S-LAAO was associated with a significantly lower rate of thromboembolism [sub-distribution HR (sHR) .67, 95% CI .56–.81; P < .001] and all-cause mortality (HR .88, 95% CI .79–.97; P = .001). In this analysis, S-LAAC compared with no S-LAAC was associated with lower risk in patients discharged without anticoagulation but not in patients receiving OAC.
The recently published ATLAS feasibility trial37 randomized 562 cardiac surgery patients (CHA2DS2-VASc ≥ 2) with no pre-operative AF and in a 2:1 ratio to LAA exclusion (LAAE) with the AtriClip or no LAAE. The primary success rate of LAAE was 99% with low serious adverse events (<0.03%). At 1-year follow-up, 3.4% of LAAE patients compared with 5.6% without LAAE patients experienced a thromboembolic event. Oral anticoagulation was used by 32.5% of patients with post-operative AF that showed higher rates of bleeding (OAC vs. no OAC, 16.1% vs. 5.4%; P = .008).
The LAAOS III study randomized 4811 high-risk patients with documented AF or atrial flutter to S-LAAC or ‘standard of care’ among patients undergoing cardiac surgery.38 The study tested the combination of systemic and mechanical therapy for stroke prevention in AF. Oral anticoagulation was continued in a sizable proportion of patients in both treatment groups. The primary endpoint of stroke/systemic embolism after 4 years of follow-up was reduced by 33% in the S-LAAC group with up to 77% of patients taking OAC. Surgical LAAC was associated with minimal prolongation of procedure time and did not affect the risk of peri-operative mortality, myocardial infarction, hospitalization, or bleeding. In a landmark analysis, the effect of S-LAAC was apparent early (HR .82, 95% CI .57–1.18 during the first 30 days) and more pronounced over time (HR .58, 95% CI .43–.8 after 30 days). In an adjusted Cox proportional hazard analysis, the effect of LAAC was independent of use of anticoagulation confirming the concept of LAAE as stroke prevention therapy. In view of the observed benefit without increased risk, S-LAAC during cardiac surgery gained strong support in the 2023 ACC/AHA/ACCP/HRS guidelines on top of OAC for AF patients at high stroke risk undergoing cardiac surgery (Class I, level of evidence A).10 At the same time, these results generate interesting questions, i.e. whether percutaneous LAAC combined with OAC will provide similar or greater benefits in high-risk AF patients. In this regard, possible advantages of S-LAAC such as epicardial closure without a foreign body, more complete closure compared with percutaneous LAAC, and low risk for peri-device leaks with improved surgical techniques have to be considered.
Catheter-based left atrial appendage closure
Three moderate-sized randomized clinical trials (RCTs) compared catheter-based LAAC mainly with warfarin, and several large clinical registry studies and a number of smaller single and multicentre registries have been performed. The early RCTs focused on patients eligible for OAC, two compared LAAC with warfarin and one compared LAAC with NOACs. Large registries collected data of LAAC in patients non-eligible for long-term OAC. Compared with the large NOAC database, LAAC RCTs included substantially fewer patients, and more data from randomized studies are therefore clearly warranted.
Randomized clinical data
Catheter-based left atrial appendage closure vs. oral anticoagulation with vitamin K antagonists
Two studies examined the efficacy and safety of LAAC with the first-generation Watchman device (Boston Scientific, Marlborough, MA, USA) compared with OAC with warfarin (Table 1). The randomized controlled PROTECT-AF trial (NCT00129545)40 fulfilled the primary efficacy endpoint but failed the primary safety endpoint due to an excess in peri-procedural adverse events. Because of concern over peri-procedural complications, concerns about the patients’ risk factor profiles, poor adherence to mandated anticoagulation, and possible confounding by antiplatelet therapy, a second study was mandated by the FDA.41,42 The subsequent PREVAIL trial (NCT01182441)43 failed to reach the primary efficacy endpoint in view of low event rates but fulfilled late efficacy and safety. However, there was a signal of more ischaemic strokes in the LAAC group in PREVAIL that was more pronounced at later follow-up.42 A patient-level meta-analysis of both studies over an observation period of 5 years showed non-inferiority for the combined primary efficacy endpoint (stroke, systemic embolism, cardiovascular death).44 Rates of stroke and systemic embolism were comparable in both treatment groups (HR .96, 95% CI .60–1.54; P = .87). An interesting observation was a reduction in fatal and severe stroke post-LAAC (HR .45, 95% CI .21–.94; P = .034), although based on small event numbers. Cardiovascular (HR .59, 95% CI .37–.49; P = .027) and overall mortality (HR .73, 95% CI .54–.98; P = .035) were lower following catheter-based LAAC compared with VKA. In regard to safety, overall similar bleeding rates were observed (HR .91, 95% CI .64–1.29; P = .60), while significantly less bleeding complications following LAAC were reported after exclusion of procedure-related bleedings (HR .48, 95% CI .32–0.71; P = .0003).44 In aggregate, these randomized data support non-inferiority in efficacy following catheter-based LAAC after 5 years in high-risk AF patients eligible for long-term OAC in comparison to warfarin—the main limitation represents the sample size. While the NOACs were large scale, only 1114 patients were included in the randomized LAAC studies (PROTECT-AF and PREVAIL) diminishing the precision of treatment effects of low-frequency events. Additionally, the inclusion of cardiovascular/unexplained death as endpoint and wide non-/inferiority margins (PROTECT-AF 2.0, PREVAIL 1.75) have been noted as important limitations.27 Of note, the first-generation device and post-implant anti-thrombotic regimen differ from current clinical practice (Table 1).
Table 1.
Randomized left atrial appendage closure studies.
| Study | Indication | CHADS2*/CHA2DS2-VASc | HAS-BLED | Patients/randomization | Anti-thrombotic therapy | Primary endpoint | Main results |
|---|---|---|---|---|---|---|---|
| PROTECT-AF | Warfarin vs. LAAC (Watchman) OAC native patients | 2.2* | NA | 707 (2:1) | Warfarin + aspirin (81–325 mg) for 45 days, aspirin + clopidogrel 75 mg to 6 months post-LAAC, aspirin indefinitely | Efficacy: stroke, systemic TE, CV/non-explained death Safety: major bleeding |
Efficacy: non-inferiority of LAAC |
| PREVAIL | Warfarin vs. LAAC (Watchman) OAC native patients | 2.6* | NA | 407 (2:1) | Warfarin + aspirin (81 mg) for 45 days, aspirin + clopidogrel 75 mg to 6 months post-LAAC, aspirin indefinitely |
|
|
| PRAGUE-17 | NOAC vs. LAAC (Amulet 61.3%, Watchman 35.9%, Watchman FLX 2.8%) | 4.7 | 3.0 | 415 (1:1) | Aspirin 100 mg + clopidogrel 75 mg for 3 months, aspirin indefinitely NOAC: 95.5% apixaban |
Combined efficacy/safety: stroke/TIA, systemic TE, CV death, significant bleeding, significant procedure/device complications | Non-inferiority of LAAC Peri-procedural complications: 4.8% DRT 1.4% |
| AMULET IDE | Comparison of Amulet with Watchman device Non-valvular AF patients (CHA2DS2-VASc score ≥ 3), non-eligible for long-term oral anticoagulation |
4.6 | 3.2 | 1878 (1:1) | Amulet: DAPT or NOAC + aspirin 6 months, aspirin indefinitely Watchman: warfarin + aspirin 45 days, DAPT 45 days–6 months, aspirin indefinitely |
Primary safety endpoint: composite of procedure-related complications, or all-cause death, or major bleeding through 12 months Primary efficacy endpoint: composite of ischaemic stroke or systemic embolism through 18 months Primary mechanism of action endpoint: device closure at 45 days |
Amulet non-inferior for safety and efficacy Amulet superior for LAA occlusion Amulet more peri-procedural complications Amulet with higher complete sealing rate Similar rates for DRT (3.3% vs. 4.4%) Similar bleeding rates |
Abbreviations: CHADS2/CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke/transient ischemic attack, thromboembolic event, vascular disease, Age 65–74 years, sex category; CV, cardiovascular; DAPT, dual antiplatelet therapy; DRT, device related thrombus; HASBLED, hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol; LAA, left atrial appendage; LAAC, left atrial appendage closure; NOAC, non-vitamin K antagonist oral anticoagulants; OAC, oral anticoagulation; TE, thromboembolic event; TIA, transient ischemic attack.
Catheter-based left atrial appendage closure vs. non-vitamin K antagonist oral anticoagulant
The multicentre, prospective, randomized PRAGUE-17 study (NCT02426944) compared the efficacy and safety of LAAC vs. NOAC in high-risk AF patients eligible for long-term anticoagulation (n = 415).45,46 The composite primary endpoint consisting of stroke/TIA, systemic embolism, clinically significant bleeding (International Society on Thrombosis and Haemostasis definition), and cardiovascular mortality was observed in 8.6% in the LAAC and 11.9% in the NOAC group (sHR .81, 95% CI .56–1.18; P = .27; P = .006 for non-inferiority) after a median follow-up of 3.5 years.46 No differences were determined in the components of the combined endpoint: stroke/TIA (sHR 1.00, 95% CI .40–2.51), clinically significant bleeding (sHR .81, 95% CI .44–1.52), and cardiovascular death (sHR .75, 95% CI .34–1.62). The rate of peri-procedural complications was 4.5% following LAAC.45 Severe non-procedural bleedings were detected at a rate of 3.4% in the LAAC and 5.9% in the NOAC group (sHR .55, 95% CI .31–.97; P = .039). These event rates are comparable to those reported in ARISTOTLE (4.07%) and AVERROES (4.5%)17,47 studies, although patients undergoing LAAC were at higher bleeding risk. The composite endpoint in PRAGUE-17 has been criticized because thrombotic events and bleeding trend in different directions and no difference is a positive finding in non-inferiority trials.48 Accordingly, it has been argued that the results of the composite endpoint should be interpreted separately. Despite the limitation of small patient numbers, these initial results are encouraging in regard to treatment of a high-risk patient population, and larger studies are currently underway.
A meta-analysis of randomized trials comparing LAAC with OAC (i.e. PROTECT-AF, PREVAIL) and PRAGUE-1749 in 1516 randomized patients (LAAC = 933, OAC = 583, mean age 73.3 ± 7.7 years, CHA2DS2-VASc 4.1 ± 1.4, OAC group 65% warfarin, and 35% NOAC) showed no significant difference in stroke/thromboembolic risk [risk ratio (RR) .98, 95% CI .65–1.48; P = .92) after 3 years. As compared with OAC, the rate of ischaemic stroke was numerically higher post-LAAC (52/933 vs. 21/583 events, RR 1.48, 95% CI .89–2.46, P = .13; without peri-procedural stroke, 44/933 vs. 21/583 events, RR 1.27, 95% CI .76–2.14, P = .37). Left atrial appendage closure was associated with a lower risk of haemorrhagic strokes (5/933 vs. 14/583 events, RR .22, 95% CI .08–.58; P = .002) and cardiovascular (RR .65, 95% CI .44–.95; P = .03) and all-cause mortality (RR .78, 95% CI .62–.99; P = .04). The risk of severe bleeding was similar in both patient groups (RR .89, 95% CI .66–1.20; P = .46), while non-procedural bleeding was less frequent following LAAC (RR .53, 95% CI .38–.74; P = .0002). This meta-analysis is limited by possible patient selection bias in the LAAC group, open-label trial designs, and different OAC regimens (LAAC benefit mainly observed with warfarin). It is important to point out that the mechanism of mortality (cardiovascular and all-cause mortality) benefit not seen in LAAOS III remains unknown. Possible explanations comprise a decrease in haemorrhagic stroke, reduced severity of stroke, a reduction in non-procedure-related bleeding, or a play of chance.26,27 Interestingly, S-LAAC was not associated with a mortality benefit in LAAOS III, but this may be related to the fact that OAC was continued in both arms of this study.
Comparison of different devices for catheter-based left atrial appendage closure
The Amulet IDE study50 is the first large-scale randomized study to compare efficacy and safety of the Amulet double seal device (Abbott Vascular) and the first-generation Watchman 2.5 single seal device (Boston Scientific). A total of 1878 patients were enrolled, and the Amulet device was found non-inferior in regard to the primary safety endpoint (composite of procedure-related complications, all-cause death, or major bleeding at 12 months: 14.5% vs. 14.7%, P < .001 for non-inferiority), the primary effectiveness endpoint (composite of ischaemic stroke or systemic embolism at 18 months: 2.8% vs. 2.8%, P < .001 for non-inferiority), and the composite of stroke, systemic embolism, or cardiovascular/unexplained death (5.6% vs. 7.7%, P < .001 for non-inferiority; Table 1). Left atrial appendage occlusion at 45 days was higher for the Amulet compared with the Watchman 2.5 device (98.9% vs. 96.8%; difference = 2.03, 95% CI .41–3.66; P < .001 for non-inferiority; P = .003 for superiority). Three-year outcomes were recently published showing similar efficacy (stroke/systemic embolism/cardiovascular death 11.1% vs. 12.7%; P = .31) and safety (major bleeding: 16.1% vs. 14.7%; P = .46) of the Amulet vs. the Watchman 2.5 device, respectively.51
Registry data
EWOLUTION and global Amulet study
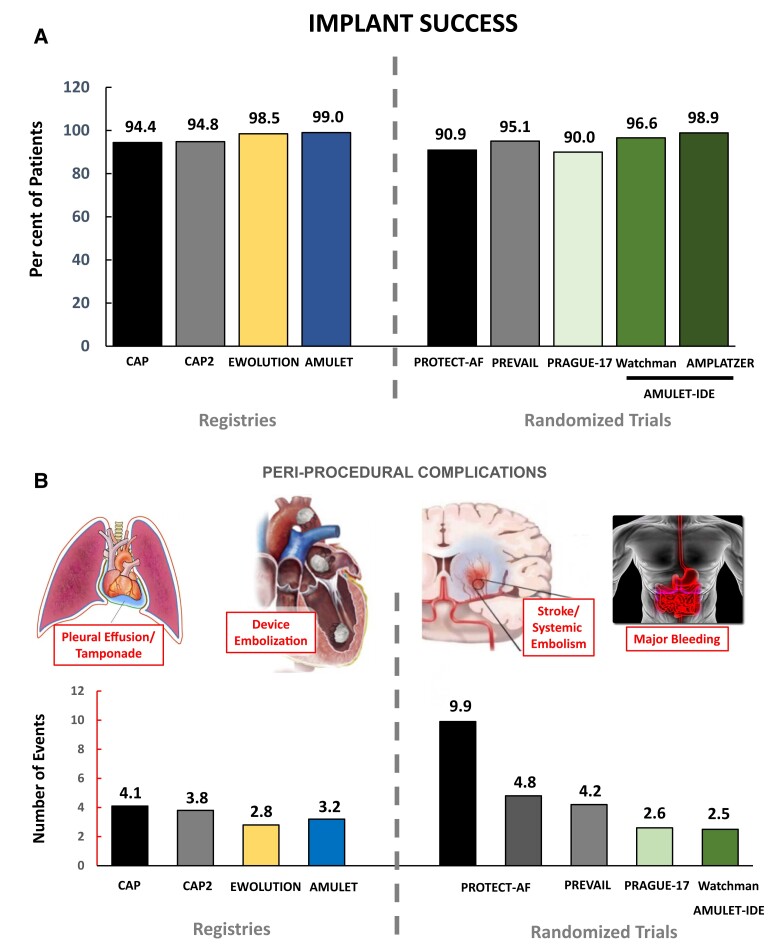
Additional data for catheter-based LAAC are derived from large-scale clinical registries, e.g. the EWOLUTION registry52 and global Amulet study,53 which report clinical routine use of LAAC in higher-risk AF patients. A summary of the results is depicted in Table 2. In aggregate, both registries show a higher rate of implantation success and lower rates of peri-procedural complications compared with initial studies (Figure 3), which can likely be explained at least in part by improved procedure planning, new device iterations with improved surface characteristics, as well as increased operator experience. However, inclusion into these prospective registries was voluntary. Therefore, selection bias should be taken into account when interpreting these data. Currently recruiting registries such as the French post-market study FLAAC-2 (NCT03434015) with 1020 patients will provide additional insights especially in regard to post-interventional anti-thrombotic therapy. Additional data are expected from long-term follow-up of the German LAARGE registry.54 Results from insurer databases confirm improved peri-procedural safety with significant sex and regional differences and higher overall complication rates of 4%–9%.55,56 In multivariate analyses, high- vs. low-volume hospitals showed a significantly reduced complication rate emphasizing the need for minimum institutional volumes for LAAC procedures to ascertain good quality of care and outcomes.57
Table 2.
Current prospective registries of left atrial appendage closure in high-risk atrial fibrillation patients with contraindications for long-term (direct) oral anticoagulants.
| Registry | Number of patients | Device | CHA2DS2-VASc score | HAS-BLED score | Results |
|---|---|---|---|---|---|
| EWOLUTION (NCT01972282) | 1025 Voluntary single-arm, prospective, non-randomized registry |
Watchman | 4.6 | 2.4 |
|
| Amplatzer Amulet (NCT02447081) | 1088 Voluntary single-arm, prospective, non-randomized registry Independent CEC |
Amulet | 4.2 | 3.3 |
|
| LAARGE (NCT02230748) | 643 Voluntary single-arm, prospective, non-randomized registry |
Watchman, Amplatzer cardiac plug Amulet |
4.7 | 4 |
|
| LISA (NCT03666780) | 500 Voluntary single-arm, prospective, non-randomized registry |
LAmbre | NA | NA | NA |
| FLAAC-2 (NCT03434015) | 1020 Voluntary single-arm, prospective, non-randomized registry |
Watchman, Amplatzer cardiac plug Amulet |
NA | NA | NA |
| PINNACLE FLX (NCT02702271) | 400 Voluntary single-arm, prospective, non-randomized registry, independent CEC |
Watchman FLX | 4.2 | 2.0 |
|
Abbreviations: CEC, clinical endpoint committee; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke/transient ischemic attack, thromboembolic event, vascular disease, Age 65–74 years, sex category; HASBLED, hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiac and cerebrovascular events; SAE, serious adverse event; TIA, transient ischemic attack.
Figure 3.
Implant success and peri-procedural safety of left atrial appendage closure. Over the last decade, implantation success and peri-procedural safety have improved due to developments in implantation technique, accumulating operator experience, and device development. (A) Implantation success is illustrated for multicentre registries and randomized trials over time. (B) Severe adverse events within 7 days post-implantation are shown consisting of vascular complications/bleeding, pericardial effusion/tamponade, stroke, and device embolization
Ongoing randomized trials
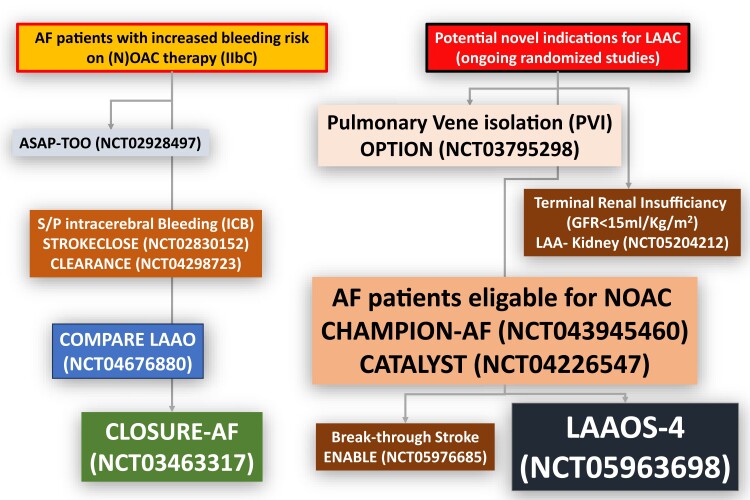
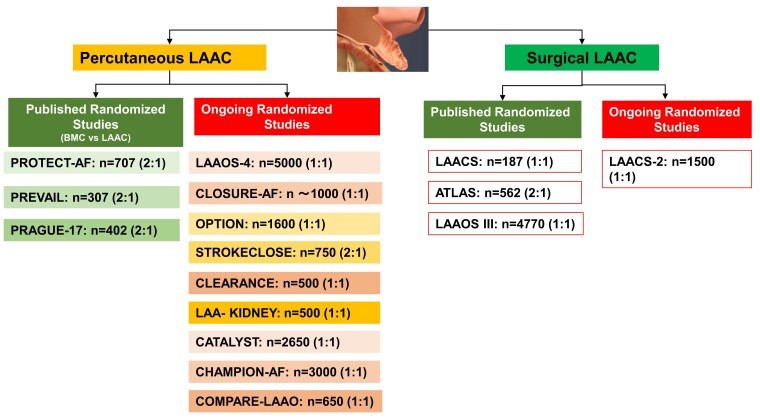
Ongoing randomized studies of catheter-based LAAC in different patient populations are summarized in Table 3 and Figure 4. Please refer to the Supplementary data for more information.
Table 3.
Ongoing randomized left atrial appendage closure studies
| Study | Patient number (randomization) | Main inclusion criteria | Main exclusion criteria | Primary endpoint |
|---|---|---|---|---|
| A) Patients with high bleeding risk (LAAC vs. best medical care) | ||||
| CLOSURE-AF (NCT03463317) | ∼1000 (1:1) | Non-valvular atrial fibrillation (CHA2DS2VASc ≥ 2) and high risk for bleeding:
|
|
Survival free of events (combined endpoint: stroke, systemic embolism, severe bleeding, cardiovascular or unexplained death > 2 years) |
| COMPARE LAAO (NCT04676880) | 609 (2:1) | Non-valvular atrial fibrillation (CHA2DS2VASc score ≥ 2), non-eligible for long-term oral anticoagulation |
|
1) Time to first occurrence of stroke 2) Time to combined endpoint: stroke, TIA, or systemic embolism 3) Peri-procedural complications |
| B) Patients without high bleeding risk (LAAC vs. NOAC) | ||||
| CHAMPION AF (NCT04394546) | 3000 (1:1) | Non-valvular atrial fibrillation (CHA2DS2VASc score ≥ 2), eligible for long-term oral anticoagulation |
|
|
| CATALYST (NCT04226547) | 2650 (1:1) | Non-valvular atrial fibrillation (CHA2DS2VASc score ≥ 3), eligible for long-term oral anticoagulation |
|
|
| C) Patients’ status post-intracerebral bleeding (LAAC vs. best medical care) | ||||
| CLEARANCE (NCT04298723) | 500 (1:1) | Non-valvular atrial fibrillation (CHA2DS2VASc score ≥ 2), status post-intracerebral bleeding > 6 weeks |
|
Combined endpoint: stroke, systemic embolism, severe bleeding, or CV/unexplained death (2-year follow-up) |
| STROKECLOSE (NCT02830152) | 750 (2:1) | Non-valvular atrial fibrillation (CHA2DS2VASc score ≥ 2), status post-intracerebral bleeding > 4 weeks but <6 months before randomization |
|
Combined endpoint: stroke, systemic embolism, severe bleeding, or death (5-year follow-up) |
| D) Patient status post-ischaemic stroke/TIA (LAAC vs. NOAC) | ||||
| OCCLUSION-AF (NCT03642509) | 750 (1:1) | Non-valvular atrial fibrillation (CHA2DS2VASc score ≥ 2), eligible for long-term oral anticoagulation. S/P stroke/TIA within 6 months before randomization |
|
Combined endpoint: stroke, systemic embolism, severe bleeding, or death (5-year follow-up) |
| E) LAAC and PVI (LAAC vs. DOAC) | ||||
| OPTION (NCT03795298) | 1600 (1:1) | Non-valvular atrial fibrillation (CHA2DS2VASc score ≥ 2), either S/P PVI 90–180 days ago or planned PVI < 10 days |
|
|
| F) LAAC and TAVR (LAAC vs. best medical care) | ||||
| Watch-TAVR (NCT03173534) | 350 (1:1) | Non-valvular atrial fibrillation (CHA2DS2VASc score ≥ 2) undergoing TAVR eligible for short-term warfarin |
|
First occurrence of all-cause mortality, stroke (ischaemic or haemorrhagic), or bleeding (life-threatening and major) through 1 year |
| G) LAAC and terminal renal insufficiency | ||||
| LAA-Kidney (NCT05204212) | 430 (1:1) | Non-valvular atrial fibrillation (CHA2DS2VASc score ≥ 2), end-stage chronic kidney disease (GFR < 15mL/min/1.73m2) |
|
Combined endpoint of stroke, systemic embolism, CV/unexplained death, and major bleeding (≥3 BARC) through 18 months of follow-up |
Abbreviations: CHA2DS2VASc ≥ 2, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or TIA or thromboembolism, vascular disease, age 65–74 years, sex category; BARC, Bleeding Academic Research Consortium; CV, cardiovascular; GFR, glomerular filtration rate; HAS-BLED, hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol; LAAC, left atrial appendage closure; LVEF, left ventricular ejection fraction; mRS, modified Rankin scale; NOAC, non-vitamin K antagonist oral anticoagulant; NYHA, New York Heart Association; PVI, pulmonary vein isolation; TIA, transient ischaemic attack.
Figure 4.
Present and potential future indications for left atrial appendage closure. Current guidelines1 recommend percutaneous left atrial appendage closure for atrial fibrillation patients with high risk of stroke not eligible for long-term oral anticoagulation. Because of an improved safety profile of left atrial appendage closure and encouraging data from prospective registries and randomized trials, current and additional indications for left atrial appendage closure are being extensively studied in randomized controlled trials. GFR, glomerular filtration rate.
New device developments for catheter-based left atrial appendage closure
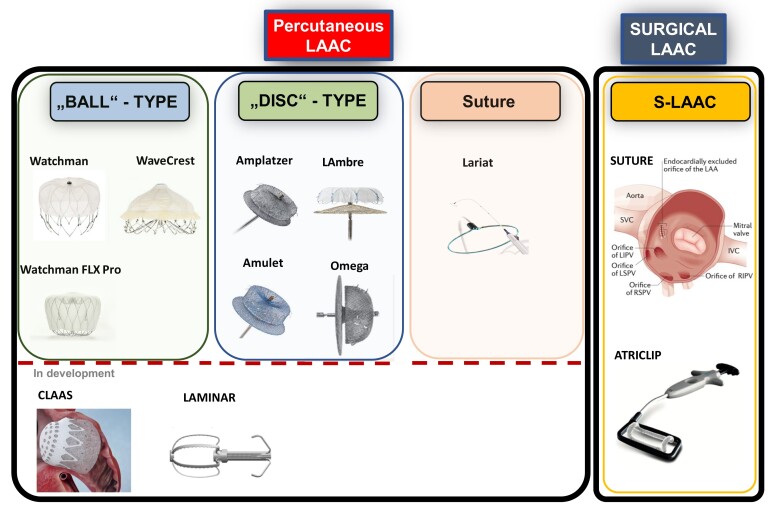
An ideal percutaneous LAAC device should be suitable for a large variety of different LAA anatomies, completely exclude the LAA from the circulation, and be associated with a very low rate of peri-procedural and long-term [i.e. device-related thrombus (DRT), peri-device leak (PDL)] complications. Currently used and novel devices for percutaneous LAAC are depicted in Figure 5.
Figure 5.
Percutaneous left atrial appendage closure devices and methods for surgical left atrial appendage closure. (A) Ball-type device (plug): endovascular delivery of a lobe or umbrella and closure by obstructing the neck of the left atrial appendage (Watchman, WAveCrest). (B) Disc-type device (pacifier): endovascular device of a lobe or umbrella (anchoring/obstructing the neck of the left atrial appendage) with an additional disc attached (sealing of the left atrial appendage ostium; Amplatzer Amulet, LAmbre). Left atrial appendage closure is dependent on sealing and endothelialization of the corpus/umbrella and/or disc (pacifier principle). (C) Ligation: endocardial and epicardial snaring and percutaneous suture ligation of the left atrial appendage (Lariat). (D) Surgical left atrial appendage closure: amputation by suture or Atriclip are depicted. Future devices: aims of future developments are better conformability (foam-based devices such as CLAAS), reducing the footprint of foreign material (left atrial appendage obliteration, i.e. Laminar device), and less thrombogenicity by anti-thrombotic covering. IVC, inferior vena cava; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; S-LAAC, surgical left atrial appendage closure; SVC, superior vena cava.
The recently introduced Watchman FLX (Boston Scientific) features a closed distal end, reduced metal exposure, and increased number of struts that enable better manoeuvrability and deliverability in order to further mitigate the risk of complications.58 This registry showed a comparatively high stroke and major bleeding rate (Table 2). The new iteration Watchman FLX Pro introduces a surface coating to promote endothelialization and additional markers for better visibility;59 clinical studies are required to assess whether these changes result in improved outcomes. The LAmbre (LifeTech Scientific) self-expanding nitinol device is composed of a distal flexible polyethylene terephthalate (PET) umbrella with atraumatic hooks that safely anchors the device and excludes the distal part of the LAA. The proximal semi-flexible disc is filled with sewn-in PET sealing the LAA ostium. This device can be custom ordered to close especially large LAA >32 mm27,60 and is available in 15 different sizes and 2 different designs suiting both single- and multi-lobed LAA morphologies. The Ultraseal device (CARDIA Inc., Eagan, MI, USA) is a novel LAAC device that conforms to LAA anatomy. It is composed of a distal soft bulb with hooks for anchoring and a proximal three-leaflet sail covered by a polyvinyl alcohol foam for LAA occlusion. A jointed technology allows adjustment to different ostium angles and LAA shapes.61
Novel device developments aim to improve implant as well as long-term safety and efficacy of LAAC and to overcome important limitations of current designs including perforation risk, narrow sizing requirements, the need for coaxial deployment, non-conformity (i.e. radial rigidity), the use of anti-thrombogenic coverings, as well as reduction in foreign material. First human data are now available for the Omega device (Eclipse Medical, Dublin, Ireland)62 characterized by increased stability due to the ‘self-retaining inverted cup’ feature, increased device malleability due to the independent inner and outer layers, and a highly flexible disc–cup articulation.62 The Sideris Transcatheter Patch (Custom Medical Devices) and its new iteration Prolipsis are frameless, bio-absorbable devices made from porous polyurethane that is delivered by a balloon and conforms to LAA anatomy. The Conformal Left Atrial Appendage Seal (CLAAS) features an expanded polytetrafluoroethylene-covered foam cup with an embedded nitinol endoskeleton and a tether release mechanism. The combination of a foam cup coupled with a compliant endoskeleton provides a less distortive, more conforming closure without requiring a strict coaxial angle. The Laminar device obliterates the LAA by rotation and holds the obliterated tissue into place by metallic retainers, leaving only a small footprint of foreign material in the left atrium. The Sierra ligation system (Aegis Medical Innovations, Inc.) allows electrocardiography-guided LAA ligation through an epicardial-only approach. An appendage grasper with jaws and mounted electrodes enables identification and capture of the LAA through electrographic navigation. A hollow suture loop is then advanced over the grasper and looped around the LAA for final cinching. A feasibility study was conducted in the USA and Canada (NCT02583178).
Uncertainties in left atrial appendage closure
Direct comparison of efficacy: left atrial appendage closure as an alternative or complementary strategy to oral anticoagulation
Non-vitamin K antagonist oral anticoagulant treatment is currently the standard of care for stroke prevention in AF patients. Limitations include dependence on patient adherence and a residual long-term risk for major bleeding. Patients at high risk of bleeding and elderly were under-represented in the available RCTs. Initial indirect comparisons and meta-analyses in particular from observational studies suggested improved efficacy and safety of LAAC compared with OAC.63–66 In a propensity-matched analysis (CHA2DS2-VASc and hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol scores) of LAAC patients of the Amulet registry (n = 1078) with medically treated patients (i.e. NOAC) from Denmark (n = 1184),67 a significant lower risk for the combined endpoint stroke/severe bleeding/death (HR .57, 95% CI .49–.67) was observed after 2 years. Risk for ischaemic stroke was similar in both groups (HR 1.11, 95% CI .71–1.75), and severe bleeding (HR .62, 95% CI .49–.79) and mortality (HR .53, 95% CI .43–.64) were significantly attenuated following LAAC. These registry data together with recent results from the randomized PRAGUE-17 study45 and an updated meta-analysis of randomized LAAC studies49 suggest a similar efficacy of LAAC with decreased post-interventional risk of severe bleeding compared with NOAC in a high-risk AF cohort. However, due to several limitations of these analyses such as retrospective nature or low patient numbers in the randomized LAAC trial, these data must be interpreted with caution. Thus, further comparative studies of percutaneous LAAC vs. NOAC are needed before interventional LAAC can be recommended as an alternative to anticoagulation in clinical routine, even in AF patients with high bleeding risk.
Patients with AF still have a residual risk for ischaemic stroke despite taking OACs.68 Several mechanisms including reduced compliance/adherence, reduced pharmacological efficacy of the anticoagulant in individual patients, or alternative stroke mechanisms may induce a stroke on OAC. Moreover, AF patients who have an ischaemic stroke despite previous OAC are at a higher risk for recurrent ischaemic stroke.69 In the LAAOS III trial, the number of patients taking OACs was not different between the surgical closure and best medical care group.38 Analogous to S-LAAC, two randomized studies will compare percutaneous LAAC plus NOAC vs. NOAC alone testing this new therapeutic paradigm in AF patients eligible for long-term anticoagulation. LAAOS-4 (NCT05963698) will employ the Watchman FLX device as a complementary therapy to best medical care in AF patients at high risk for stroke (CHA2DS2-VASc score ≥ 4) that are eligible for OAC therapy. This event-driven trial in 4000 patients with high residual stroke risk is powered to determine the primary efficacy endpoint of all-cause stroke/systemic thromboembolism. ENABLE will include AF patients who had a stroke despite anticoagulant therapy.
Bleeding risk and the fear of major bleeding are main factors for underuse of NOACs in eligible AF patients. The evidence from available Phase 2 trials suggests that unlike NOACs, drugs that target factor XI reduce thrombosis without a dose–response for bleeding. By uncoupling thrombosis from haemostasis, factor XI inhibitors have the potential for a more favourable benefit–risk profile.70 Asundexian, a small oral molecule inhibitor of factor XIa, was compared with apixaban in the PACIFIC-AF trial in regard to a composite of major or clinically relevant non-major bleeding in 753 AF patients. Although underpowered for efficacy, rates of bleeding severe enough to prompt medical care occurred one-third as often with asundexian than with apixaban.71 A Phase 3 outcome trial (LIBREXIA-AF, NCT05757869) will now evaluate the efficacy and safety of milvexian, a different factor XI/XIa inhibitor, for stroke prevention in patients with AF. If a favourable safety and efficacy profile of factor XI inhibitors can be observed, efficacy and safety of LAAC will likely have to be compared against these novel drugs in the future.
Post-implant anti-thrombotic treatment
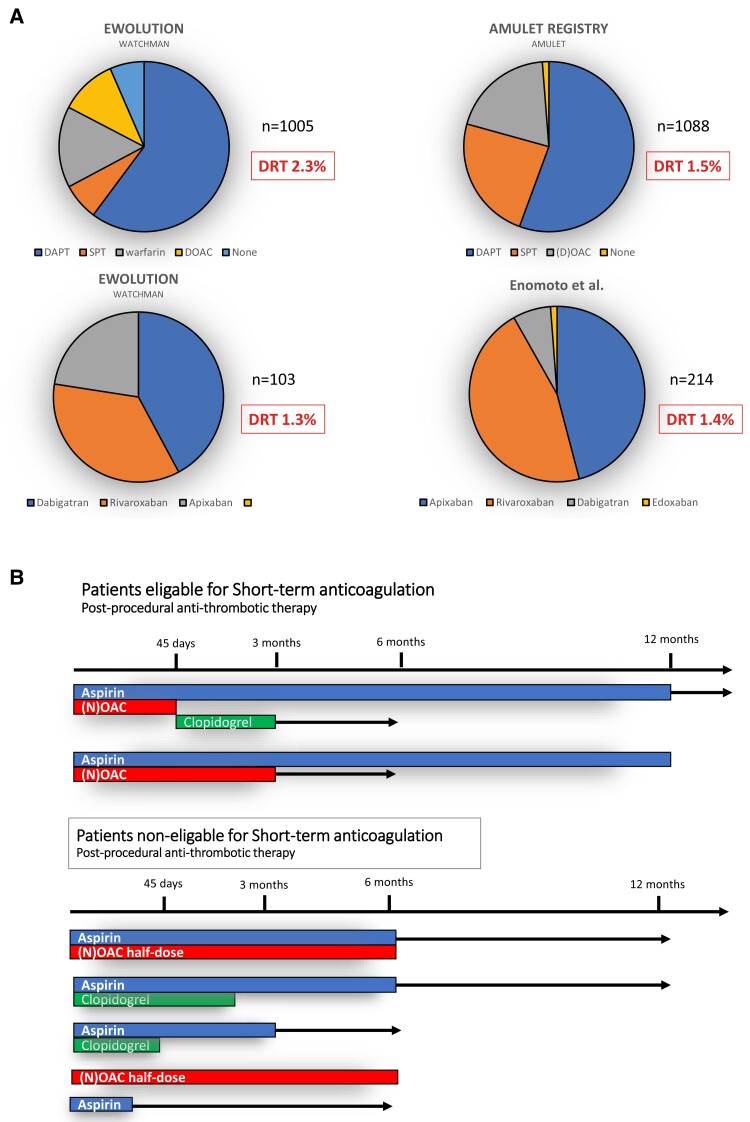
Post-procedural anti-thrombotic therapy is used to ensure endothelialization of the foreign device surface and to prevent device-associated thrombus formation but increases the risk for major bleedings in the high bleeding risk LAAC population, currently the most frequent adverse event after LAAC.50 There are no systematic human studies on the time frame of device endothelialization that can be variable or delayed in any individual patient and device.72 On the basis of experimental research in animals, endothelialization is presumed to last at least 90 days,73,74 but incomplete endothelialization has been described.72 Unfortunately, there is no current standard test to determine the degree of endothelialization of LAAC devices.75 Ideal short-term post-implant anti-thrombotic medication should prevent thrombus formation on the device without increase of bleeding. However, data on anti-thrombotic therapy after LAAC is scarce (Figure 6A). Results from the randomized Amulet IDE study showing comparative efficacy and safety of dual antiplatelet therapy (DAPT) vs. anticoagulation regimens are reassuring.50 Different anti-thrombotic regimens with variable durations are currently used (Figure 6B). Dependent on LAAC indications, the choice and duration of anti-thrombotic therapy should be individualized. Potential parameters to be considered are the indication for LAAC (i.e. major bleeding on OAC or aspirin, stroke on OAC without high bleeding risk), patient comorbidities (i.e. coronary artery disease, diabetes, heart failure with reduced ejection fraction), left atrial appearance on echocardiography (i.e. very large atria, spontaneous echo contrast), and quality of LAAC (i.e. presence of leaks, uncovered LAA lobes, device protrusion). Most European centres recommend DAPT regimens followed by antiplatelet monotherapy (APT) for a limited time or APT alone depending on the patient’s bleeding risk.52,76 Expert consensus recommends not to perform percutaneous LAAC if the patient is not eligible for a minimum of 4 weeks of APT.27 Termination of aspirin therapy should be strongly considered in regard to data about the randomized comparison of aspirin vs. NOAC in high-risk bleeding patients that determined significant bleeding rates in both groups. Ongoing randomized studies (CLOSURE-AF, STROKECLOSE, CHAMPION-AF, and CATALYST) together with the FADE-DRT trial (NCT04502017) will help to better define the optimal medical regimen post-LAAC.
Figure 6.
Post-implant anti-thrombotic therapy and rates of device-related thrombus. (A) Post-implant anti-thrombotic regimens used in multicentre clinical registries and device-related thrombus rates dependent on patient risk and physicians’ preference. (B) Different schemes of post-implant anti-thrombotic therapy according to patients’ risk profile. DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulants; DVT, device-related thrombus; SPT, single antiplatelet therapy.
Device-related thrombus
Device-related thrombus is observed with a frequency of 1.6%–16% of patients after LAAC,77–79 disparate numbers originating from lack of unified definitions for DRT and diversity of follow-up [i.e. transoesophageal echocardiography (TEE) or cardiac computed tomography angiography]. In PROTECT-AF, all device patients underwent TEE follow-up at 3, 6, and 12 months. Device-related thrombus was diagnosed in 5.7% of patients, with a primary efficacy event occurring at a rate of 3.4/100 patient-years.80 In an analysis of 1739 patients following Watchman 2.5 implantation, rates of DRT were affected by the time of imaging and type of concurrent anti-thrombotic medical therapy. Overall, DRT was diagnosed in 3.74% of patients.81 A history of stroke/TIA, peripheral artery or coronary artery disease, high CHA2DS2-VASc, decreased left ventricular ejection fraction, lower LAA emptying flow velocity, and larger LAA size were identified as predictors for DRT. Device-related thrombus was associated with a four-fold increased risk of ischaemic stroke or systemic embolism.81 In current prospective LAAC registries, DRT rates at 3-month TEE follow-up were 4.1% (EWOLUTION)82 and 2.2% (Amulet registry; Figure 6A).53 A French registry demonstrated an incidence of DRT of 7.2% with DRT being an independent predictor of ischaemic stroke or TIA during follow-up.78 Taken together, these data illustrate the importance of extended follow-up studies especially in high-risk patients. Given the association of decreased left atrial function, atrial size, and thromboembolic events, it is likely that left atrial haemodynamics, i.e. low-flow state due to fibrotic, immobile atrium, may constitute a risk factor for DRT. In this regard, permanent AF is associated with left atrial structural remodelling and fibrosis, a determinant of left atrial thrombosis83 and stroke even in patients treated with NOAC.84 Therefore, DRT could also be a marker for overall thromboembolic risk rather than being alone the source of thromboembolism. In accordance, the largest DRT registry identified hypercoagulability, pericardial effusion, renal insufficiency, implantation depth >10 mm from the pulmonary vein limbus, and non-paroxysmal AF as independent risk factors for DRT,85 while no signal was detected for different anti-thrombotic regimens. Besides clinical risk factors, the role of post-procedural anti-thrombotic therapy is disputed. A recent analysis reported an increased risk of DRT in patients without OAC86 that was confirmed in Amulet IDE,50 while the Euro-DRT registry detected DRT independent of device implantation and anti-thrombotic medication.87 In this regard, recent data determined a reduction of thrombin formation after LAAC by low-dose rivaroxaban compared with dual antiplatelet therapy (DAPT),88 and a recent study found a benefit of post-procedural low-dose NOAC vs. standard antiplatelet therapy.89 In addition to an international consensus on the diagnostic criteria and management of DRT, prospective studies on the optimal post-procedural treatment after LAAC are warranted. In any case, imaging follow-up after LAAC should be performed to exclude DRT. Risk of DRT needs to be particularly taken into account in the context of contraindications against OAC (i.e. recurrent major bleeding) with careful patient selection in regard to net clinical benefit.
Role of peri-device leaks
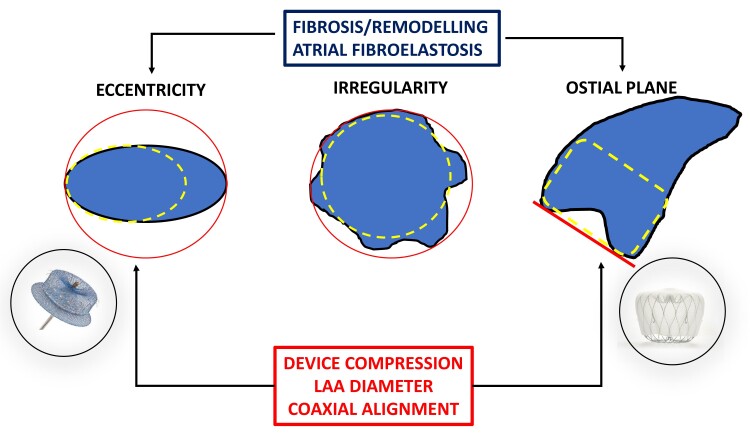
The efficacy of LAA closure is based on exclusion of the cul-de-sac structure of the LAA from the circulation, while persistent communication may not adequately prevent the passage of emboli from the LAA. Earlier studies in S-LAAC (using suture techniques) showed frequent incomplete closure.32 Residual PDLs also occur frequently post-percutaneous LAAC despite meticulous multimodality imaging.90 In PROTECT-AF, any PDL was classified by TEE in 40.9% after 45 days, while PDL >3 mm was shown in 13.3%.90 Similar numbers are documented in the Amulet IDE study (37.0% and 11.2% for Amulet and 53.9% and 25.9% for Watchman 2.5 at 45 days, respectively).91 In contemporary real-world registries, significant leaks were detected in 8.8% of patients with the Watchman 2.5 device,52 while the incidence of severe leaks >5 mm was observed only in 1% of cases. The global Amulet observational study reported PDL in 9.9% of patients at 45 days and significant PDL in 1.6%.53 For the newer Watchman FLX, a lower incidence of PDL was observed.58 However, also temporal changes in PDL incidence and size have been described.92 There is no consensus on the definition of PDL: the cut-off for a clinically significant PDL is arbitrary and varies (<3, >3, and >5 mm), and the optimal method of its detection (occurrence substantially higher with cardiac computed tomography compared with TEE75,93–95) as well as its mechanism and location is often not well defined. Anticoagulation has been advised for leaks >5 mm.27 Frequently, PDLs are associated with device malposition,96,97 insufficient device compression,98 larger LAA dimensions,97,98 or complex LAA anatomy.98 Of note, recent data implicate atrial remodelling in persistent AF associated with reduced deformation capacity due to reduced ostium elasticity and LAA compliance in leak generation (Figure 7). Two indexes of orifice morphology—ostium eccentricity and ostium irregularity—were identified that may assist pre-procedural prediction of residual leak formation.99 Most clinical studies investigating the clinical impact of PDL were underpowered due to low clinical event rates, varying clinical cut-offs, and treatment bias. Recent studies utilizing large databases determined an association of thromboembolic events and PDL. In the NCDR LAAO Registry,100 complete sealing was reported in 37 696 patients (73.4%) on 45-day follow-up imaging, while 13 258 patients (25.8%) had small leaks (>0–5 mm), and only 379 patients (0.7%) had large leaks (>5 mm). Small residual leaks were associated with higher odds for thromboembolic events, major bleeding, and major adverse events. Furthermore, a 5-year analysis of patients in the PROTECT-AF and PREVAIL randomized trials and their continuous access registries determined an association of small leaks (>0 and <5 mm) persisting at 1 year post-LAAO with a two-fold increase in stroke or systemic embolization due to higher odds of non-disabling stroke.101 In accordance with these data, a recent sub-analysis of the Amulet IDE trial showed that PDL >3 mm on 45-day TEE was associated with the composite endpoint of stroke, systemic embolism, or cardiovascular death.91 Therefore, all efforts should be made to achieve complete seal of the LAA whenever possible in order to mitigate the risk of future ischaemic or adverse events. This strategy requires a comprehensive approach that includes adequate pre- and intra-procedural imaging,102,103 an understanding of the underlying LAA anatomy, the mechanism of peri-device leaks,92 and an optimal device selection. Therapy of significant leaks (i.e. PDL > 5 mm) consists of continuation of OAC or transcatheter approaches effectively sealing leaks by coils,104 vascular plugs, nitinol-based devices,105 or radiofrequency energy application for stimulation of remodelling/tissue retraction,106 but long-term outcome or comparative data for these interventions are sparse.107 Progress in device development, closure techniques, and imaging (i.e. simulation software102) might lower the incidence of PDL in the future.
Figure 7.
Factors for leak generation following percutaneous left atrial appendage closure. Patient factors such as length of atrial fibrillation or ostial anatomy, device characteristics such as deformability, and implantation technique (coaxial device implantation, oversizing) play an important role in leak generation. Special care for device selection should be taken in complex anatomies, i.e. disc-type device for oval ostium in stiff left atrial appendage (red circle) or ball-type device for irregular ostial plane (red line).
Catheter-based left atrial appendage closure in multi-morbid and frail patients
Although LAAC might be appealing for older frail and comorbid patients who have a high bleeding risk, this patient group is particularly vulnerable to peri-procedural complications and major bleeding as mirrored in the HAS-BLED score, which includes age and comorbidities such as hypertension, chronic renal or liver disease, and history of bleeding.108–112 Presently, this cohort of patients is underrepresented in clinical trials of LAAC, and demographic development will lead to an increase of this population in the future. Several studies in a real-world cohort have demonstrated an association of age, female sex, body mass index, and comorbidity level with peri-procedural and long-term outcome.25 Therefore, adequate patient selection is important in real-world practice.25,61 However, patients undergoing LAAC in real-world practice represent a high-risk population, with a high comorbidity burden and a high rate of adverse events at follow-up, most of them not related to the LAAC device or procedure. However, only scarce data exist about treatment futility in these patients. In a recent multicentre registry including real-world (i.e. unselected) patients, early death after LAAC occurred in every sixth patient within 1 year after LAAC.113 Increased risk for early death was independently associated with older age, low body mass index, impaired renal function, diabetes, and heart failure. The risk of early death reached 50% in the presence of >3 of these risk factors. These results confirm that early death after LAAC is not related to procedural success or peri-procedural complications but to patients’ characteristics and estimated lifespan. Therefore, the precarious state of many patients undergoing LAAC highlights the urgent need for better patient stratification and selection as well as dedicated prospective studies in this field.
Summary and perspective
Catheter-based LAAC was initially developed as an alternative to anticoagulation to reduce the risk of stroke in AF patients with high risk of stroke and high bleeding risk. Initial moderate-sized clinical trials support non-inferiority in efficacy of device-based LAAC compared with OAC, especially with warfarin in high-risk AF patients. Recent early randomized data comparing LAAC with NOAC are encouraging, although conclusive evidence will need to await the results of ongoing larger-scale studies. Novel data suggest an important role for S-LAAC in concurrent cardiac surgery of AF patients to reduce the risk of subsequent stroke (Figure 8).
Figure 8.
Clinical evidence for left atrial appendage closure. Published and ongoing randomized studies in percutaneous and surgical left atrial appendage closure are depicted. BMC, best medical care; LAAC, left atrial appendage closure.
Improvement of LAA devices as well as current developments of pre-, peri-, and post-procedural imaging and planning will aid in further improvements of safety of percutaneous LAAC. The rapidly growing field of LAAC with expansion to lower-risk patient populations and novel indications in clinical studies warrants particular attention to unresolved issues in order to ensure long-term safety and efficacy. The benefit of LAAC in frail and multi-morbid patients non-eligible for OAC at increased peri- and post-procedural complications requires further study. In addition, controversy exists about the implications of intra-cardiac thrombus risk in operated patients, incidence of PDL, and appropriate therapies required in order to prevent post-procedural thromboembolic events.
Data of several currently ongoing RCTs comparing catheter-based LAAC with best medical care (including NOACs) in an AF population with high or lower risk for bleeding and for novel indications will define optimal patient selection and the future role of catheter-based LAAC.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
U.L. received research grants to the institution from Abbott and Bayer. C.S. received proctoring fees and speaker honoraria from Boston/LifeTech Scientific. A.T. has been a proctor/consultant for Abbott Vascular and Boston Scientific. V.F. reports relevant financial activities outside the submitted work from Medtronic, Biotronik, Abbott, Boston Scientific, Edwards Lifesciences, Zurich Heart, Berlin Heart, Novartis, Artivion, and LivaNova. V.Y.R. reports receiving grant support and consulting fees from Abbott and Boston Scientific and equity from Laminar Medical, all of whom manufacture LAA devices, and unrelated to this manuscript, he has consulted for and has equity in Ablacon, Acutus Medical, Affera, Medtronic, Apama Medical-Boston Scientific, Anumana, APN Health, Aquaheart, AtaCor, Autonomix, Axon Therapies, Backbeat, BioSig, CardiaCare, CardioFocus, CardioNXT/AFTx, Circa Scientific, CoRISMA, Corvia Medical, Dinova-Hangzhou DiNovA EP Technology, East End Medical, EPD-Philips, EP Frontiers, Epix Therapeutics-Medtronic, EpiEP, Eximo, Field Medical, Focused Therapeutics, HRT, InterShunt, Javelin, Kardium, Keystone Heart, LuxMed, MedLumics, Middlepeak, NeuTrace, Nuvera-Biosense Webster, Oracle Health, Pulse Biosciences, Restore Medical, Sirona Medical, SoundCath, and Valcare; unrelated to this work, has served as a consultant for Adagio Medical, AtriAN, Biosense Webster, BioTel Heart, Biotronik, Cairdac, Cardionomic, CoreMap, Fire1, Gore & Associates, Impulse Dynamics, Medtronic, Novartis, and Philips and has equity in DRS Vascular, Manual Surgical Sciences, Newpace, Nyra Medical, Surecor, and VizaraMed. S.W. has received research grants to the institution from Abbott, Biotronik, Boston Scientific, Medtronic, and Edwards Lifesciences.
Supplementary Material
Contributor Information
Ulf Landmesser, Department of Cardiology, Angiology and Intensive Care Medicine, Deutsches Herzzentrum der Charite (DHZC), Hindenburgdamm 30, 12203 Berlin, Germany; Berlin Institute of Health (BIH), Anna-Louisa-Karsch-Straße 2, 10178 Berlin, Germany; Friede Springer Cardiovascular Prevention Center@Charité, Hindenburgdamm 30, 12203 Berlin, Germany; DZHK Partner Site Berlin, Germany.
Carsten Skurk, Department of Cardiology, Angiology and Intensive Care Medicine, Deutsches Herzzentrum der Charite (DHZC), Hindenburgdamm 30, 12203 Berlin, Germany; DZHK Partner Site Berlin, Germany.
Apostolos Tzikas, Second Department of Cardiology, Hippocratic University Hospital, Aristotle University of Thessaloniki Department of Cardiology, Interbalkan Medical Center, Pylaia, Thessaloniki, Greece.
Volkmar Falk, Department of Cardiology, Angiology and Intensive Care Medicine, Deutsches Herzzentrum der Charite (DHZC), Hindenburgdamm 30, 12203 Berlin, Germany; Berlin Institute of Health (BIH), Anna-Louisa-Karsch-Straße 2, 10178 Berlin, Germany; Friede Springer Cardiovascular Prevention Center@Charité, Hindenburgdamm 30, 12203 Berlin, Germany; DZHK Partner Site Berlin, Germany; Department of Cardiothoracic Surgery, Deutsches Herzzentrum der Charite (DHZC), Berlin, Germany.
Vivek Y Reddy, Helmsley Electrophysiology Center, Mount Sinai Fuster Heart Hospital, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Stephan Windecker, Department of Cardiology, Bern University Hospital, Inselspital, University of Bern, Freiburgstrasse 18, 3010 Bern, Switzerland.
Data Availability
No data were generated or analysed for or in support of this paper.
Funding
All authors declare no funding for this contribution.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics—2019 update: a report from the American Heart Association. Circulation 2019;139:e56–528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 3. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 study. Circulation 2014;129:837–47. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holmes DR. Atrial fibrillation and stroke management: present and future. Semin Neurol 2010;30:528–36. 10.1055/s-0030-1268861 [DOI] [PubMed] [Google Scholar]
- 5. Lamassa M, Di Carlo A, Pracucci G, Basile AM, Trefoloni G, Vanni P, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (the European Community Stroke Project). Stroke 2001;32:392–8. 10.1161/01.STR.32.2.392 [DOI] [PubMed] [Google Scholar]
- 6. Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755–9. 10.1016/0003-4975(95)00887-X [DOI] [PubMed] [Google Scholar]
- 7. Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60:531–8. 10.1016/j.jacc.2012.04.032 [DOI] [PubMed] [Google Scholar]
- 8. Handke M, Harloff A, Hetzel A, Olschewski M, Bode C, Geibel A. Left atrial appendage flow velocity as a quantitative surrogate parameter for thromboembolic risk: determinants and relationship to spontaneous echocontrast and thrombus formation—a transesophageal echocardiographic study in 500 patients with cerebral ischemia. J Am Soc Echocardiogr 2005;18:1366–72. 10.1016/j.echo.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 9. Masci A, Barone L, Dede L, Fedele M, Tomasi C, Quarteroni A, et al. The impact of left atrium appendage morphology on stroke risk assessment in atrial fibrillation: a computational fluid dynamics study. Front Physiol 2018;9:1938. 10.3389/fphys.2018.01938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Writing Committee M, Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2024;83:109–279. 10.1016/j.jacc.2023.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery; Camm AJ, Kirchhof P, Lip GY, Schotten U, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–429. 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- 12. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J 2012;33:1500–10. 10.1093/eurheartj/ehr488 [DOI] [PubMed] [Google Scholar]
- 13. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 14. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. 10.1016/S0140-6736(13)62343-0 [DOI] [PubMed] [Google Scholar]
- 15. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 16. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 17. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 18. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. 10.1056/NEJMoa1310907 [DOI] [PubMed] [Google Scholar]
- 19. Johnson ME, Lefevre C, Collings SL, Evans D, Kloss S, Ridha E, et al. Early real-world evidence of persistence on oral anticoagulants for stroke prevention in non-valvular atrial fibrillation: a cohort study in UK primary care. BMJ Open 2016;6:e011471. 10.1136/bmjopen-2016-011471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paquette M, Riou Franca L, Teutsch C, Diener HC, Lu S, Dubner SJ, et al. Persistence with dabigatran therapy at 2 years in patients with atrial fibrillation. J Am Coll Cardiol 2017;70:1573–83. 10.1016/j.jacc.2017.07.793 [DOI] [PubMed] [Google Scholar]
- 21. Lip GY, Laroche C, Ioachim PM, Rasmussen LH, Vitali-Serdoz L, Petrescu L, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J 2014;35:3365–76. 10.1093/eurheartj/ehu374 [DOI] [PubMed] [Google Scholar]
- 22. Bassand JP, Accetta G, Camm AJ, Cools F, Fitzmaurice DA, Fox KA, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J 2016;37:2882–9. 10.1093/eurheartj/ehw233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meier B, Blaauw Y, Khattab AA, Lewalter T, Sievert H, Tondo C, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. EuroIntervention 2015;10:1109–25. 10.4244/EIJY14M09_18 [DOI] [PubMed] [Google Scholar]
- 24. Martinez C, Katholing A, Wallenhorst C, Freedman SB. Therapy persistence in newly diagnosed non-valvular atrial fibrillation treated with warfarin or NOAC. A cohort study. Thromb Haemost 2016;115:31–9. 10.1160/TH15-04-0350 [DOI] [PubMed] [Google Scholar]
- 25. Verheugt FW, Granger CB. Oral anticoagulants for stroke prevention in atrial fibrillation: current status, special situations, and unmet needs. Lancet 2015;386:303–10. 10.1016/S0140-6736(15)60245-8 [DOI] [PubMed] [Google Scholar]
- 26. Turagam MK, Kawamura I, Neuzil P, Nair D, Doshi S, Valderrabano M, et al. Severity of ischemic stroke after left atrial appendage closure vs nonwarfarin oral anticoagulants. JACC Clin Electrophysiol 2024;10:270–83. 10.1016/j.jacep.2023.10.012 [DOI] [PubMed] [Google Scholar]
- 27. Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYH, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—an update. EuroIntervention 2020;15:1133–80. 10.4244/EIJY19M08_01 [DOI] [PubMed] [Google Scholar]
- 28. Yao X, Gersh BJ, Holmes DR Jr, Melduni RM, Johnsrud DO, Sangaralingham LR, et al. Association of surgical left atrial appendage occlusion with subsequent stroke and mortality among patients undergoing cardiac surgery. JAMA 2018;319:2116–26. 10.1001/jama.2018.6024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc 2016;5:e003074. 10.1161/JAHA.115.003074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Brien EC, Holmes DN, Ansell JE, Allen LA, Hylek E, Kowey PR, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J 2014;167:601–9.e1. 10.1016/j.ahj.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 31. Piccini JP, Sievert H, Patel MR. Left atrial appendage occlusion: rationale, evidence, devices, and patient selection. Eur Heart J 2017;38:869–76. 10.1093/eurheartj/ehw330 [DOI] [PubMed] [Google Scholar]
- 32. Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiographic study. J Am Coll Cardiol 2000;36:468–71. 10.1016/S0735-1097(00)00765-8 [DOI] [PubMed] [Google Scholar]
- 33. Apostolakis E, Papakonstantinou NA, Baikoussis NG, Koniari I, Papadopoulos G. Surgical strategies and devices for surgical exclusion of the left atrial appendage: a word of caution. J Card Surg 2013;28:199–206. 10.1111/jocs.12055 [DOI] [PubMed] [Google Scholar]
- 34. Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol 2008;52:924–9. 10.1016/j.jacc.2008.03.067 [DOI] [PubMed] [Google Scholar]
- 35. Bakhtiary F, Kleine P, Martens S, Dzemali O, Dogan S, Keller H, et al. Simplified technique for surgical ligation of the left atrial appendage in high-risk patients. J Thorac Cardiovasc Surg 2008;135:430–1. 10.1016/j.jtcvs.2007.08.057 [DOI] [PubMed] [Google Scholar]
- 36. Melduni RM, Schaff HV, Lee HC, Gersh BJ, Noseworthy PA, Bailey KR, et al. Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality: a propensity score-matched analysis of 10 633 patients. Circulation 2017;135:366–78. 10.1161/CIRCULATIONAHA.116.021952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gerdisch MW, Garrett HE Jr, Mumtaz MA, Grehan JF, Castillo-Sang M, Miller JS, et al. Prophylactic left atrial appendage exclusion in cardiac surgery patients with elevated CHA(2)DS(2)-VASc score: results of the randomized ATLAS trial. Innovations (Phila) 2022;17:463–70. 10.1177/15569845221123796 [DOI] [PubMed] [Google Scholar]
- 38. Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. 10.1056/NEJMoa2101897 [DOI] [PubMed] [Google Scholar]
- 39. Friedman DJ, Piccini JP, Wang T, Zheng J, Malaisrie SC, Holmes DR, et al. Association between left atrial appendage occlusion and readmission for thromboembolism among patients with atrial fibrillation undergoing concomitant cardiac surgery. JAMA 2018;319:365–74. 10.1001/jama.2017.20125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534–42. 10.1016/S0140-6736(09)61343-X [DOI] [PubMed] [Google Scholar]
- 41. Waks JW, Manning WJ. Left atrial appendage closure to reduce the risk of thromboembolic complications in atrial fibrillation: pay now and possibly pay later? J Am Coll Cardiol 2015;65:2624–7. 10.1016/j.jacc.2015.03.593 [DOI] [PubMed] [Google Scholar]
- 42. Waksman R, Pendyala LK. Overview of the Food and Drug Administration circulatory system devices panel meetings on WATCHMAN left atrial appendage closure therapy. Am J Cardiol 2015;115:378–84. 10.1016/j.amjcard.2014.11.011 [DOI] [PubMed] [Google Scholar]
- 43. Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. 10.1016/j.jacc.2014.04.029 [DOI] [PubMed] [Google Scholar]
- 44. Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol 2017;70:2964–75. 10.1016/j.jacc.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 45. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–35. 10.1016/j.jacc.2020.04.067 [DOI] [PubMed] [Google Scholar]
- 46. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. Left atrial appendage closure versus non-warfarin oral anticoagulation in atrial fibrillation: 4-year outcomes of PRAGUE-17. J Am Coll Cardiol 2022;79:1–14. 10.1016/j.jacc.2021.10.023 [DOI] [PubMed] [Google Scholar]
- 47. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. 10.1056/NEJMoa1007432 [DOI] [PubMed] [Google Scholar]
- 48. Kim KS, Mandrola J, McIntyre WF, Whitlock RP, Belley-Cote EP. Primary composite outcome in PRAGUE-17: an unintended butterfly effect. J Am Coll Cardiol 2022;79:e497. 10.1016/j.jacc.2022.01.056 [DOI] [PubMed] [Google Scholar]
- 49. Turagam MK, Osmancik P, Neuzil P, Dukkipati SR, Reddy VY. Left atrial appendage closure versus oral anticoagulants in atrial fibrillation: a meta-analysis of randomized trials. J Am Coll Cardiol 2020;76:2795–7. 10.1016/j.jacc.2020.08.089 [DOI] [PubMed] [Google Scholar]
- 50. Lakkireddy D, Thaler D, Ellis CR, Swarup V, Sondergaard L, Carroll J, et al. Amplatzer Amulet left atrial appendage occluder versus Watchman device for stroke prophylaxis (Amulet IDE): a randomized controlled trial. Circulation 2021;144:1543–52. 10.1161/CIRCULATIONAHA.121.057063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lakkireddy D, Thaler D, Ellis CR, Swarup V, Gambhir A, Hermiller J, et al. 3-year outcomes from the Amplatzer Amulet left atrial appendage occluder randomized controlled trial (Amulet IDE). JACC Cardiovasc Interv 2023;16:1902–13. 10.1016/j.jcin.2023.06.022 [DOI] [PubMed] [Google Scholar]
- 52. Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm 2017;14:1302–8. 10.1016/j.hrthm.2017.05.038 [DOI] [PubMed] [Google Scholar]
- 53. Landmesser U, Tondo C, Camm J, Diener HC, Paul V, Schmidt B, et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: one-year follow-up from the prospective global Amulet observational registry. EuroIntervention 2018;14:e590–7. 10.4244/EIJ-D-18-00344 [DOI] [PubMed] [Google Scholar]
- 54. Ledwoch J, Franke J, Akin I, Geist V, Weiss C, Zeymer U, et al. WATCHMAN versus ACP or Amulet devices for left atrial appendage occlusion: a sub-analysis of the multicentre LAARGE registry. EuroIntervention 2020;16:e942–9. 10.4244/EIJ-D-19-01027 [DOI] [PubMed] [Google Scholar]
- 55. Thevathasan T, Degbeon S, Paul J, Wendelburg DK, Fureder L, Gaul AL, et al. Safety and healthcare resource utilization in patients undergoing left atrial appendage closure – a nationwide analysis. J Clin Med 2023;12:4573. 10.3390/jcm12144573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vuddanda VLK, Turagam MK, Umale NA, Shah Z, Lakkireddy DR, Bartus K, et al. Incidence and causes of in-hospital outcomes and 30-day readmissions after percutaneous left atrial appendage closure: a US nationwide retrospective cohort study using claims data. Heart Rhythm 2020;17:374–82. 10.1016/j.hrthm.2019.09.018 [DOI] [PubMed] [Google Scholar]
- 57. Nazir S, Ahuja KR, Kolte D, Isogai T, Michihata N, Saad AM, et al. Association of hospital procedural volume with outcomes of percutaneous left atrial appendage occlusion. JACC Cardiovasc Interv 2021;14:554–61. 10.1016/j.jcin.2020.11.029 [DOI] [PubMed] [Google Scholar]
- 58. Kar S, Doshi SK, Sadhu A, Horton R, Osorio J, Ellis C, et al. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX trial. Circulation 2021;143:1754–62. 10.1161/CIRCULATIONAHA.120.050117 [DOI] [PubMed] [Google Scholar]
- 59. Saliba WI, Kawai K, Sato Y, Kopesky E, Cheng Q, Ghosh SKB, et al. Enhanced thromboresistance and endothelialization of a novel fluoropolymer-coated left atrial appendage closure device. JACC Clin Electrophysiol 2023;9:1555–67. 10.1016/j.jacep.2023.04.013 [DOI] [PubMed] [Google Scholar]
- 60. Skurk C, Reinthaler M, Kasner M, Landmesser U. Large LAA—too big for closure?: LAA closure with the world’s biggest percutaneous closure device. JACC Cardiovasc Interv 2021;14:1846–7. 10.1016/j.jcin.2021.05.016 [DOI] [PubMed] [Google Scholar]
- 61. Asmarats L, Masson JB, Pagnotta PA, Cook S, Foresti M, Ibrahim R, et al. Percutaneous left atrial appendage closure with the Ultraseal device: insights from the initial multicenter experience. JACC Cardiovasc Interv 2018;11:1932–41. 10.1016/j.jcin.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 62. Wilkins B, Srimahachota S, De Backer O, Boonyartavej S, Lertsuwunseri V, Tumkosit M, et al. First-in-human results of the Omega left atrial appendage occluder for patients with non-valvular atrial fibrillation. EuroIntervention 2021;17:e376–9. 10.4244/EIJ-D-20-00552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yerasi C, Lazkani M, Kolluru P, Miryala V, Kim J, Moole H, et al. An updated systematic review and meta-analysis of early outcomes after left atrial appendage occlusion. J Interv Cardiol 2018;31:197–206. 10.1111/joic.12502 [DOI] [PubMed] [Google Scholar]
- 64. Li X, Wen SN, Li SN, Bai R, Liu N, Feng L, et al. Over 1-year efficacy and safety of left atrial appendage occlusion versus novel oral anticoagulants for stroke prevention in atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials and observational studies. Heart Rhythm 2016;13:1203–14. 10.1016/j.hrthm.2015.12.037 [DOI] [PubMed] [Google Scholar]
- 65. Bajaj NS, Parashar A, Agarwal S, Sodhi N, Poddar KL, Garg A, et al. Percutaneous left atrial appendage occlusion for stroke prophylaxis in nonvalvular atrial fibrillation: a systematic review and analysis of observational studies. JACC Cardiovasc Interv 2014;7:296–304. 10.1016/j.jcin.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 66. Sahay S, Nombela-Franco L, Rodes-Cabau J, Jimenez-Quevedo P, Salinas P, Biagioni C, et al. Efficacy and safety of left atrial appendage closure versus medical treatment in atrial fibrillation: a network meta-analysis from randomised trials. Heart 2017;103:139–47. 10.1136/heartjnl-2016-309782 [DOI] [PubMed] [Google Scholar]
- 67. Nielsen-Kudsk JE, Korsholm K, Damgaard D, Valentin JB, Diener HC, Camm AJ, et al. Clinical outcomes associated with left atrial appendage occlusion versus direct oral anticoagulation in atrial fibrillation. JACC Cardiovasc Interv 2021;14:69–78. 10.1016/j.jcin.2020.09.051 [DOI] [PubMed] [Google Scholar]
- 68. Xian Y, O'Brien EC, Liang L, Xu H, Schwamm LH, Fonarow GC, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes among patients with atrial fibrillation. JAMA 2017;317:1057–67. 10.1001/jama.2017.1371 [DOI] [PubMed] [Google Scholar]
- 69. Seiffge DJ, De Marchis GM, Koga M, Paciaroni M, Wilson D, Cappellari M, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol 2020;87:677–87. 10.1002/ana.25700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Weitz JI, Eikelboom JW. What is the future of factor XI inhibitors? Circulation 2022;146:1899–902. 10.1161/CIRCULATIONAHA.122.061132 [DOI] [PubMed] [Google Scholar]
- 71. Piccini JP, Caso V, Connolly SJ, Fox KAA, Oldgren J, Jones WS, et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet 2022;399:1383–90. 10.1016/S0140-6736(22)00456-1 [DOI] [PubMed] [Google Scholar]
- 72. Massarenti L, Yilmaz A. Incomplete endothelialization of left atrial appendage occlusion device 10 months after implantation. J Cardiovasc Electrophysiol 2012;23:1384–5. 10.1111/j.1540-8167.2012.02360.x [DOI] [PubMed] [Google Scholar]
- 73. Reinthaler M, Grosshauser J, Schmidt T, Unger J, Morgan R, Zimmermann F, et al. Preclinical assessment of a modified Occlutech left atrial appendage closure device in a porcine model. Sci Rep 2021;11:2988. 10.1038/s41598-021-82359-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kar S, Hou D, Jones R, Werner D, Swanson L, Tischler B, et al. Impact of Watchman and Amplatzer devices on left atrial appendage adjacent structures and healing response in a canine model. JACC Cardiovasc Interv 2014;7:801–9. 10.1016/j.jcin.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 75. Korsholm K, Jensen JM, Norgaard BL, Samaras A, Saw J, Berti S, et al. Peridevice leak following Amplatzer left atrial appendage occlusion: cardiac computed tomography classification and clinical outcomes. JACC Cardiovasc Interv 2021;14:83–93. 10.1016/j.jcin.2020.10.034 [DOI] [PubMed] [Google Scholar]
- 76. Korsholm K, Nielsen KM, Jensen JM, Jensen HK, Andersen G, Nielsen-Kudsk JE. Transcatheter left atrial appendage occlusion in patients with atrial fibrillation and a high bleeding risk using aspirin alone for post-implant antithrombotic therapy. EuroIntervention 2017;12:2075–82. 10.4244/EIJ-D-16-00726 [DOI] [PubMed] [Google Scholar]
- 77. Lempereur M, Aminian A, Freixa X, Gafoor S, Kefer J, Tzikas A, et al. Device-associated thrombus formation after left atrial appendage occlusion: a systematic review of events reported with the Watchman, the Amplatzer cardiac plug and the Amulet. Catheter Cardiovasc Interv 2017;90:E111–21. 10.1002/ccd.26903 [DOI] [PubMed] [Google Scholar]
- 78. Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Abbey S, et al. Device-Related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol 2018;71:1528–36. 10.1016/j.jacc.2018.01.076 [DOI] [PubMed] [Google Scholar]
- 79. Hildick-Smith D, Landmesser U, Camm AJ, Diener HC, Paul V, Schmidt B, et al. Left atrial appendage occlusion with the Amplatzer Amulet device: full results of the prospective global observational study. Eur Heart J 2020;41:2894–901. 10.1093/eurheartj/ehaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Main ML, Fan D, Reddy VY, Holmes DR, Gordon NT, Coggins TR, et al. Assessment of device-related thrombus and associated clinical outcomes with the WATCHMAN left atrial appendage closure device for embolic protection in patients with atrial fibrillation (from the PROTECT-AF trial). Am J Cardiol 2016;117:1127–34. 10.1016/j.amjcard.2016.01.039 [DOI] [PubMed] [Google Scholar]
- 81. Dukkipati SR, Kar S, Holmes DR, Doshi SK, Swarup V, Gibson DN, et al. Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation 2018;138:874–85. 10.1161/CIRCULATIONAHA.118.035090 [DOI] [PubMed] [Google Scholar]
- 82. Sedaghat A, Nickenig G, Schrickel JW, Ince H, Schmidt B, Protopopov AV, et al. Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device—insights from the EWOLUTION real world registry. Catheter Cardiovasc Interv 2021;97:E1019–24. 10.1002/ccd.29458 [DOI] [PubMed] [Google Scholar]
- 83. Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol 2011;57:831–8. 10.1016/j.jacc.2010.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJ, et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF trial. Eur Heart J 2015;36:288–96. 10.1093/eurheartj/ehu359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Simard T, Jung RG, Lehenbauer K, Piayda K, Pracon R, Jackson GG, et al. Predictors of device-related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol 2021;78:297–313. 10.1016/j.jacc.2021.04.098 [DOI] [PubMed] [Google Scholar]
- 86. Sondergaard L, Wong YH, Reddy VY, Boersma LVA, Bergmann MW, Doshi S, et al. Propensity-matched comparison of oral anticoagulation versus antiplatelet therapy after left atrial appendage closure with WATCHMAN. JACC Cardiovasc Interv 2019;12:1055–63. 10.1016/j.jcin.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 87. Sedaghat A, Vij V, Al-Kassou B, Gloekler S, Galea R, Furholz M, et al. Device-related thrombus after left atrial appendage closure: data on thrombus characteristics, treatment strategies, and clinical outcomes from the EUROC-DRT-registry. Circ Cardiovasc Interv 2021;14:e010195. 10.1161/CIRCINTERVENTIONS.120.010195 [DOI] [PubMed] [Google Scholar]
- 88. Duthoit G, Silvain J, Marijon E, Ducrocq G, Lepillier A, Frere C, et al. Reduced rivaroxaban dose versus dual antiplatelet therapy after left atrial appendage closure: ADRIFT a randomized pilot study. Circ Cardiovasc Interv 2020;13:e008481. 10.1161/CIRCINTERVENTIONS.119.008481 [DOI] [PubMed] [Google Scholar]
- 89. Della Rocca DG, Magnocavallo M, Di Biase L, Mohanty S, Trivedi C, Tarantino N, et al. Half-dose direct oral anticoagulation versus standard antithrombotic therapy after left atrial appendage occlusion. JACC Cardiovasc Interv 2021;14:2353–64. 10.1016/j.jcin.2021.07.031 [DOI] [PubMed] [Google Scholar]
- 90. Viles-Gonzalez JF, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage versus Warfarin Therapy for Prevention of Stroke in Patients with Atrial Fibrillation) substudy. J Am Coll Cardiol 2012;59:923–9. 10.1016/j.jacc.2011.11.028 [DOI] [PubMed] [Google Scholar]
- 91. Price MJ, Ellis CR, Nielsen-Kudsk JE, Thaler D, Gupta N, Koulogiannis K, et al. Peridevice leak after transcatheter left atrial appendage occlusion: an analysis of the Amulet IDE trial. JACC Cardiovasc Interv 2022;15:2127–38. 10.1016/j.jcin.2022.09.001 [DOI] [PubMed] [Google Scholar]
- 92. Alkhouli M, De Backer O, Ellis CR, Nielsen-Kudsk JE, Sievert H, Natale A, et al. Peridevice leak after left atrial appendage occlusion: incidence, mechanisms, clinical impact, and management. JACC Cardiovasc Interv 2023;16:627–42. 10.1016/j.jcin.2022.12.006 [DOI] [PubMed] [Google Scholar]
- 93. Holmes DR Jr, Alkhouli M. Comparison of cardiac computed tomography angiography and transoesophageal echocardiography for device surveillance after left atrial appendage closure. What we see depends on where we are looking from and what we are looking for. EuroIntervention 2019;15:650–1. 10.4244/EIJV15I8A119 [DOI] [PubMed] [Google Scholar]
- 94. Qamar SR, Jalal S, Nicolaou S, Tsang M, Gilhofer T, Saw J. Comparison of cardiac computed tomography angiography and transoesophageal echocardiography for device surveillance after left atrial appendage closure. EuroIntervention 2019;15:663–70. 10.4244/EIJ-D-18-01107 [DOI] [PubMed] [Google Scholar]
- 95. Samaras A, Papazoglou AS, Balomenakis C, Bekiaridou A, Moysidis DV, Patsiou V, et al. Residual leaks following percutaneous left atrial appendage occlusion and outcomes: a meta-analysis. Eur Heart J 2024;45:214–29. 10.1093/eurheartj/ehad828 [DOI] [PubMed] [Google Scholar]
- 96. Saw J, Fahmy P, DeJong P, Lempereur M, Spencer R, Tsang M, et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur Heart J Cardiovasc Imaging 2015;16:1198–206. 10.1093/ehjci/jev067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cochet H, Iriart X, Sridi S, Camaioni C, Corneloup O, Montaudon M, et al. Left atrial appendage patency and device-related thrombus after percutaneous left atrial appendage occlusion: a computed tomography study. Eur Heart J Cardiovasc Imaging 2018;19:1351–61. 10.1093/ehjci/jey010 [DOI] [PubMed] [Google Scholar]
- 98. Jaguszewski M, Manes C, Puippe G, Salzberg S, Muller M, Falk V, et al. Cardiac CT and echocardiographic evaluation of peri-device flow after percutaneous left atrial appendage closure using the AMPLATZER cardiac plug device. Catheter Cardiovasc Interv 2015;85:306–12. 10.1002/ccd.25667 [DOI] [PubMed] [Google Scholar]
- 99. Rajwani A, Shirazi MG, Disney PJS, Wong DTL, Teo KSL, Delacroix S, et al. Left atrial appendage eccentricity and irregularity are associated with residual leaks after percutaneous closure. JACC Clin Electrophysiol 2015;1:478–85. 10.1016/j.jacep.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 100. Alkhouli M, Du C, Killu A, Simard T, Noseworthy PA, Friedman PA, et al. Clinical impact of residual leaks following left atrial appendage occlusion: insights from the NCDR LAAO registry. JACC Clin Electrophysiol 2022;8:766–78. 10.1016/j.jacep.2022.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dukkipati SR, Holmes DR Jr, Doshi SK, Kar S, Singh SM, Gibson D, et al. Impact of peridevice leak on 5-year outcomes after left atrial appendage closure. J Am Coll Cardiol 2022;80:469–83. 10.1016/j.jacc.2022.04.062 [DOI] [PubMed] [Google Scholar]
- 102. De Backer O, Iriart X, Kefer J, Nielsen-Kudsk JE, Aminian A, Rosseel L, et al. Impact of computational modeling on transcatheter left atrial appendage closure efficiency and outcomes. JACC Cardiovasc Interv 2023;16:655–66. 10.1016/j.jcin.2023.01.008 [DOI] [PubMed] [Google Scholar]
- 103. Nielsen-Kudsk JE, Berti S, Caprioglio F, Ronco F, Arzamendi D, Betts T, et al. Intracardiac echocardiography to guide Watchman FLX implantation: the ICE LAA study. JACC Cardiovasc Interv 2023;16:643–51. 10.1016/j.jcin.2022.10.024 [DOI] [PubMed] [Google Scholar]
- 104. Della Rocca DG, Horton RP, Di Biase L, Bassiouny M, Al-Ahmad A, Mohanty S, et al. First experience of transcatheter leak occlusion with detachable coils following left atrial appendage closure. JACC Cardiovasc Interv 2020;13:306–19. 10.1016/j.jcin.2019.10.022 [DOI] [PubMed] [Google Scholar]
- 105. Piayda K, Sievert K, Della Rocca DG, Adeola OG, Alkhouli M, Yoo D, et al. Safety and feasibility of peri-device leakage closure after LAAO: an international, multi-center collaborative study. EuroIntervention 2021;17:e1033–40. 10.4244/EIJ-D-21-00286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Della Rocca DG, Murtaza G, Di Biase L, Akella K, Krishnan SC, Magnocavallo M, et al. Radiofrequency energy applications targeting significant residual leaks after Watchman implantation: a prospective, multicenter experience. JACC Clin Electrophysiol 2021;7:1573–84. 10.1016/j.jacep.2021.06.002 [DOI] [PubMed] [Google Scholar]
- 107. Charate R, Ahmed A, Della Rocca DG, Bloom S, Garg J, Pothineni NVK, et al. Evaluation of multimodality LAA leak closure methods following incomplete occlusion: the LAA leak study. JACC Cardiovasc Interv 2022;15:2158–70. 10.1016/j.jcin.2022.08.034 [DOI] [PubMed] [Google Scholar]
- 108. Wang A, Ferro EG, Song Y, Xu J, Sun T, Yeh RW, et al. Frailty in patients undergoing percutaneous left atrial appendage closure. Heart Rhythm 2022;19:814–21. 10.1016/j.hrthm.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang A, Ferro EG, Xu J, Song Y, Sun T, Strom JB, et al. Comparative performance of distinct frailty measures among patients undergoing percutaneous left atrial appendage closure. Pacing Clin Electrophysiol 2023;46:242–50. 10.1111/pace.14649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sanjoy S, Choi YH, Holmes D, Herrman H, Terre J, Alraies C, et al. Comorbidity burden in patients undergoing left atrial appendage closure. Heart 2021;107:1246–53. 10.1136/heartjnl-2020-317741 [DOI] [PubMed] [Google Scholar]
- 111. Sanjoy SS, Choi YH, Sparrow RT, Jneid H, Dawn Abbott J, Nombela-Franco L, et al. Outcomes of elderly patients undergoing left atrial appendage closure. J Am Heart Assoc 2021;10:e021973. 10.1161/JAHA.121.021973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nasasra AE, Brachmann J, Lewalter T, Akin I, Sievert H, Nienaber CA, et al. Comparison in patients <75 years of age-versus-those >75 years on one-year-events with atrial fibrillation and left atrial appendage occluder (from the prospective multicenter German LAARGE registry). Am J Cardiol 2020;136:81–6. 10.1016/j.amjcard.2020.09.017 [DOI] [PubMed] [Google Scholar]
- 113. Mesnier J, Cruz-Gonzalez I, Arzamendi D, Freixa X, Nombela-Franco L, Peral V, et al. Incidence and predictors of early death in patients undergoing percutaneous left atrial appendage closure. JACC Clin Electrophysiol 2022;8:1093–102. 10.1016/j.jacep.2022.06.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data were generated or analysed for or in support of this paper.