Abstract
1. Spermine has previously been reported to be an activator of mitochondrial Ca2+ uptake [Nicchitta & Williamson (1984) J. Biol. Chem. 259, 12978-12983]. This is confirmed in the present studies on rat heart, liver and kidney mitochondria by using the activities of the Ca2+-sensitive intramitochondrial dehydrogenases (pyruvate, NAD+-isocitrate and 2-oxoglutarate dehydrogenases) as probes for matrix Ca2+, and also, for the heart mitochondria, by using entrapped fura-2. 2. As also found previously [Damuni, Humphreys & Reed (1984) Biochem. Biophys. Res. Commun. 124, 95-99], spermine activated extracted pyruvate dehydrogenase phosphate phosphatase. However, it was found to have no effects at all on the extracted NAD+-isocitrate or 2-oxoglutarate dehydrogenases. It also had no effects on activities of the enzymes in mitochondria incubated in the absence of Ca2+, or on the Ca2+-sensitivity of the enzymes in uncoupled mitochondria. 3. Spermine clearly activated 45Ca uptake by coupled mitochondria, but had no effect on Ca2+ egress from mitochondria previously loaded with 45Ca. 4. Spermine (with effective Km values of around 0.2-0.4 mM) caused an approx. 2-3-fold decrease in the effective ranges of extramitochondrial Ca2+ in the activation of the Ca2+-sensitive matrix enzymes in coupled mitochondria from all of the tissues. The effects of spermine appeared to be largely independent of the other effectors of mitochondrial Ca2+ transport, such as Mg2+ (inhibitor of uptake) and Na+ (promoter of egrees). 5. In the most physiological circumstance, coupled mitochondria incubated with Na+ and Mg2+, the presence of saturating spermine (2 mM) resulted in an effective extramitochondrial Ca2+ range for matrix enzyme activation of from about 30-50 nM up to about 800-1200 nM, with half-maximal effects around 250-400 nM-Ca2+. The implications of these findings for the regulation of matrix and extramitochondrial Ca2+ are discussed.
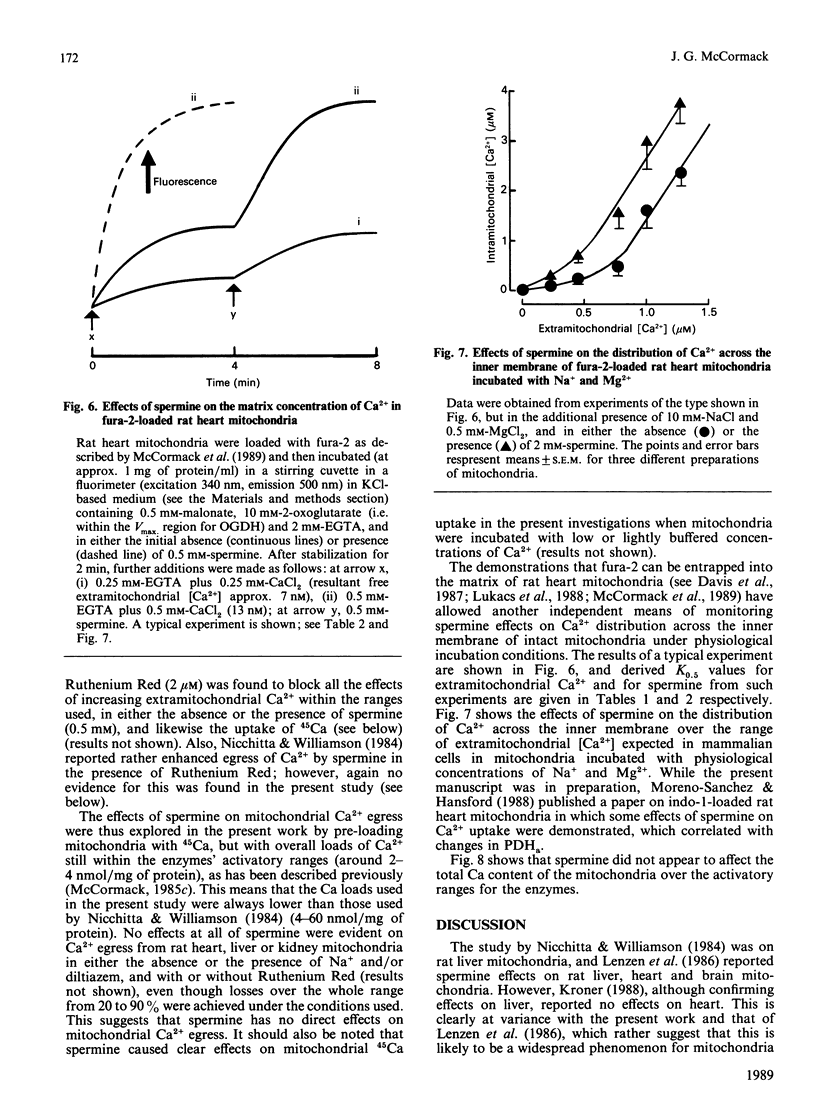
Full text
PDF
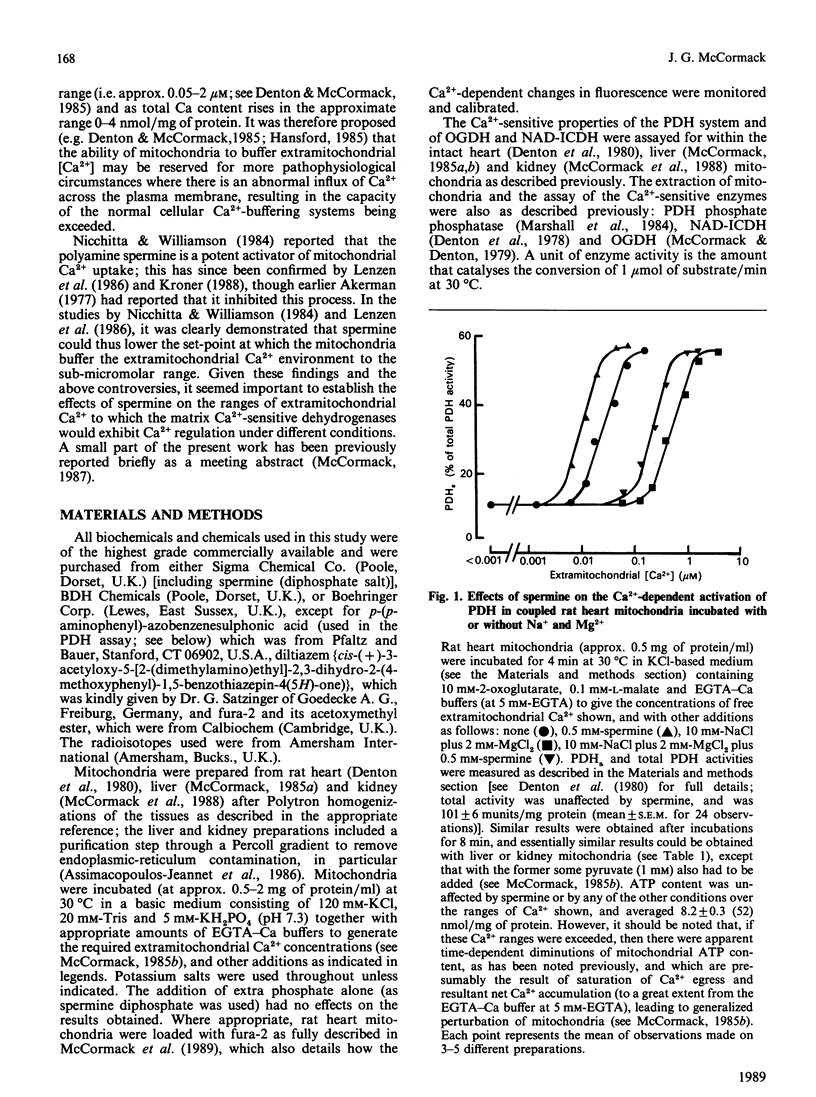
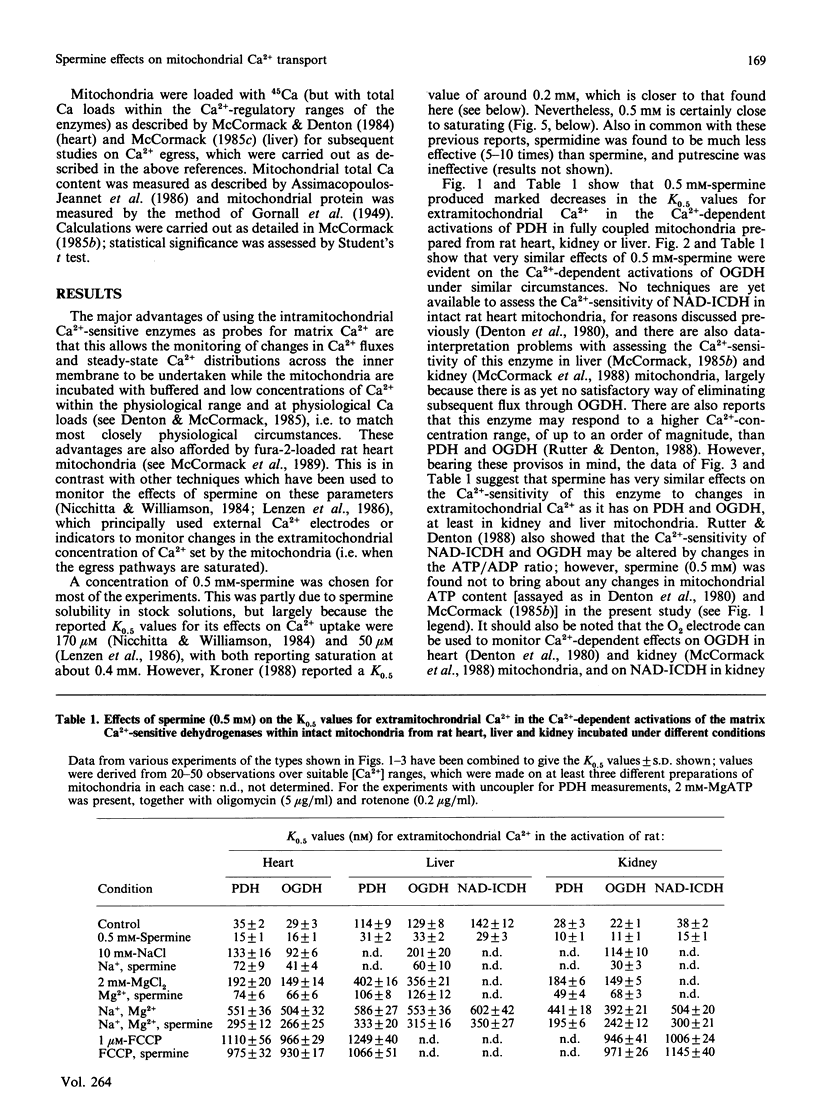
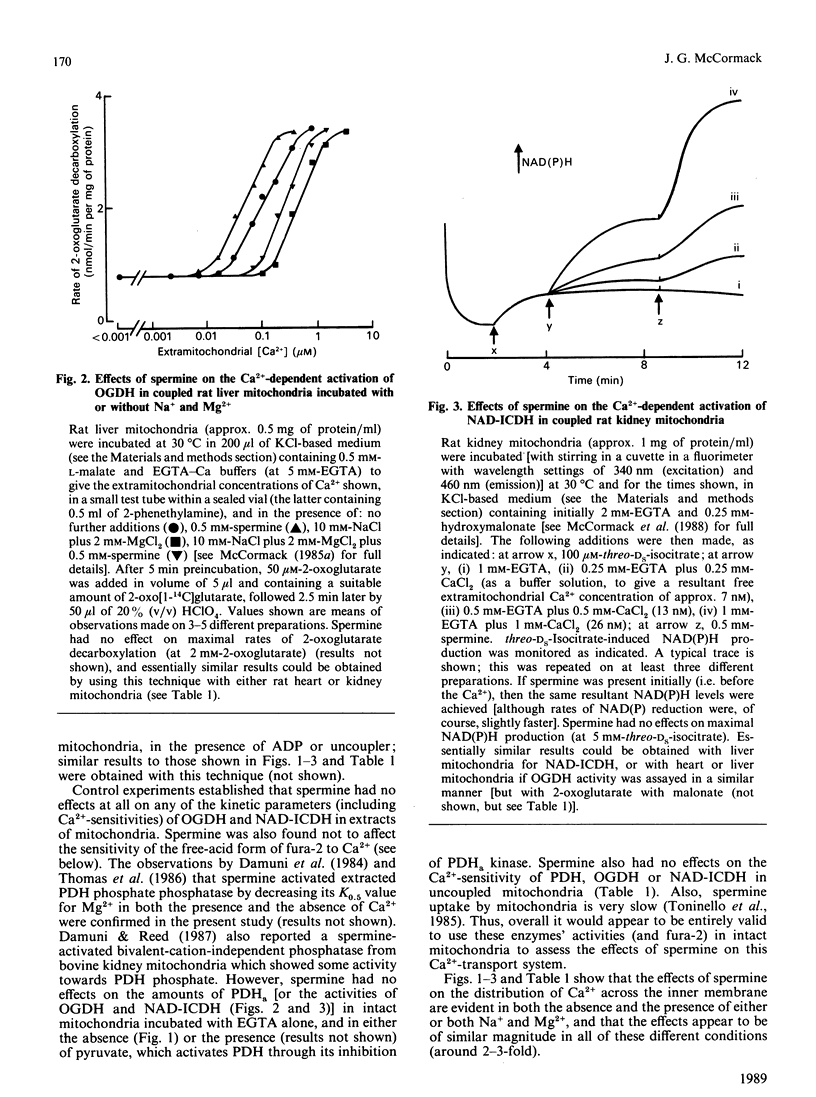
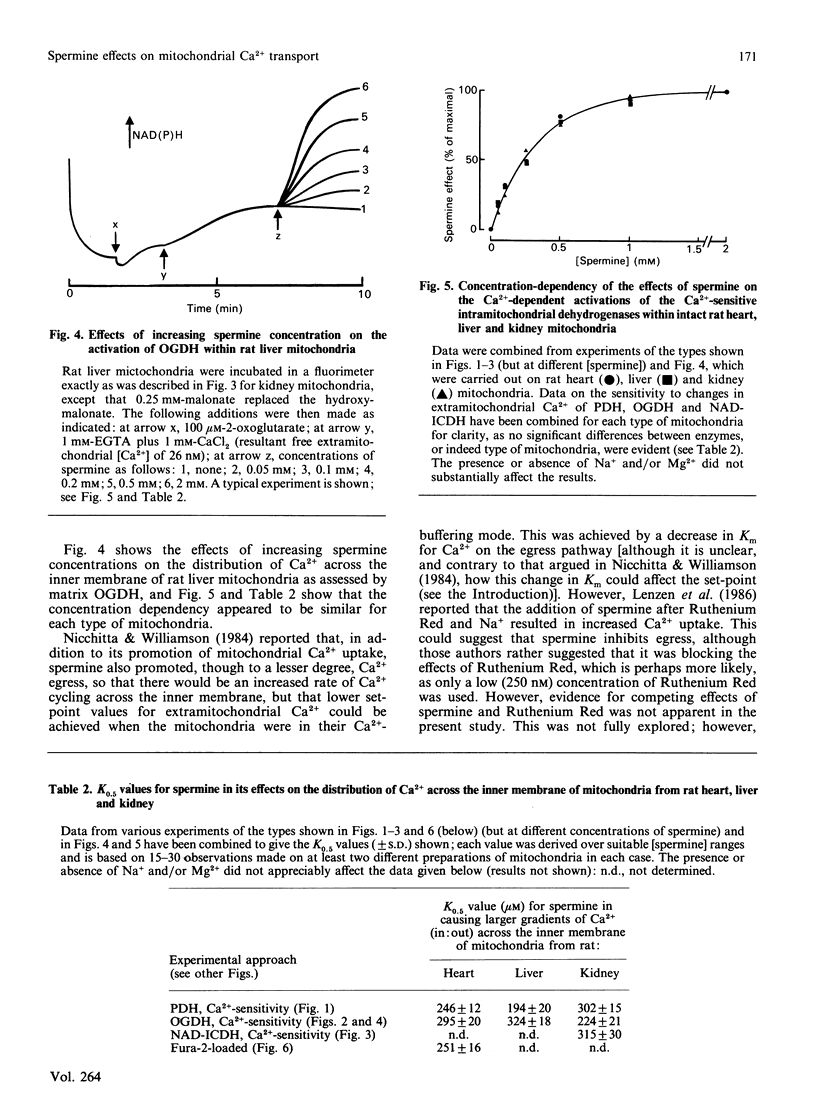


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E. Effect of Mg2+ and spermine on the kinetics of Ca2+ transport in rat-liver mitochondria. J Bioenerg Biomembr. 1977 Feb;9(1):65–72. doi: 10.1007/BF00745043. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F., McCormack J. G., Jeanrenaud B. Vasopressin and/or glucagon rapidly increases mitochondrial calcium and oxidative enzyme activities in the perfused rat liver. J Biol Chem. 1986 Jul 5;261(19):8799–8804. [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Chaffee R. R., Salganicoff L., Arine R. M., Rochelle R. H., Schultz E. L. Polyamine effects on succinate-linked and alphaketoglutarate- linked rat liver mitochondrial respiration. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1009–1016. doi: 10.1016/s0006-291x(77)80078-8. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Schwaller R., Eichenberger K. Structural dependency of the inhibitory action of benzodiazepines and related compounds on the mitochondrial Na+-Ca2+ exchanger. Biochem Pharmacol. 1988 Nov 15;37(22):4399–4403. doi: 10.1016/0006-2952(88)90623-5. [DOI] [PubMed] [Google Scholar]
- Damuni Z., Humphreys J. S., Reed L. J. Stimulation of pyruvate dehydrogenase phosphatase activity by polyamines. Biochem Biophys Res Commun. 1984 Oct 15;124(1):95–99. doi: 10.1016/0006-291x(84)90921-5. [DOI] [PubMed] [Google Scholar]
- Damuni Z., Reed L. J. Purification and characterization of a divalent cation-independent, spermine-stimulated protein phosphatase from bovine kidney mitochondria. J Biol Chem. 1987 Apr 15;262(11):5133–5138. [PubMed] [Google Scholar]
- Davis M. H., Altschuld R. A., Jung D. W., Brierley G. P. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem Biophys Res Commun. 1987 Nov 30;149(1):40–45. doi: 10.1016/0006-291x(87)91602-0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am J Physiol. 1985 Dec;249(6 Pt 1):E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G., Edgell N. J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J. 1980 Jul 15;190(1):107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., McCormack J. G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980 Sep 22;119(1):1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Richards D. A., Chin J. G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem J. 1978 Dec 15;176(3):899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. C., Koenig H. The role of polyamines in beta-adrenergic stimulation of calcium influx and membrane transport in rat heart. J Mol Cell Cardiol. 1988 Sep;20(9):789–799. doi: 10.1016/s0022-2828(88)80004-x. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Hayat L. H., Crompton M. Evidence for the existence of regulatory sites for Ca2+ on the Na+/Ca2+ carrier of cardiac mitochondria. Biochem J. 1982 Feb 15;202(2):509–518. doi: 10.1042/bj2020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig H., Goldstone A. D., Lu C. Y. Beta-adrenergic stimulation of Ca2+ fluxes, endocytosis, hexose transport, and amino acid transport in mouse kidney cortex is mediated by polyamine synthesis. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7210–7214. doi: 10.1073/pnas.80.23.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröner H. Spermine, another specific allosteric activator of calcium uptake in rat liver mitochondria. Arch Biochem Biophys. 1988 Nov 15;267(1):205–210. doi: 10.1016/0003-9861(88)90024-0. [DOI] [PubMed] [Google Scholar]
- LeFurgey A., Ingram P., Mandel L. J. Heterogeneity of calcium compartmentation: electron probe analysis of renal tubules. J Membr Biol. 1986;94(2):191–196. doi: 10.1007/BF01871198. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Hickethier R., Panten U. Interactions between spermine and Mg2+ on mitochondrial Ca2+ transport. J Biol Chem. 1986 Dec 15;261(35):16478–16483. [PubMed] [Google Scholar]
- Lukács G. L., Kapus A., Fonyó A. Parallel measurement of oxoglutarate dehydrogenase activity and matrix free Ca2+ in fura-2-loaded heart mitochondria. FEBS Lett. 1988 Feb 29;229(1):219–223. doi: 10.1016/0014-5793(88)80831-7. [DOI] [PubMed] [Google Scholar]
- Marshall S. E., McCormack J. G., Denton R. M. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat epididymal adipose tissue. Evidence against a role for Ca2+ in the activation of pyruvate dehydrogenase by insulin. Biochem J. 1984 Feb 15;218(1):249–260. doi: 10.1042/bj2180249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Bromidge E. S., Dawes N. J. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive dehydrogenases within intact rat-kidney mitochondria. Biochim Biophys Acta. 1988 Jul 27;934(3):282–292. doi: 10.1016/0005-2728(88)90088-6. [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Browne H. M., Dawes N. J. Studies on mitochondrial Ca2+-transport and matrix Ca2+ using fura-2-loaded rat heart mitochondria. Biochim Biophys Acta. 1989 Mar 23;973(3):420–427. doi: 10.1016/s0005-2728(89)80384-6. [DOI] [PubMed] [Google Scholar]
- McCormack J. G. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive enzymes from rat liver and within intact rat liver mitochondria. Biochem J. 1985 Nov 1;231(3):581–595. doi: 10.1042/bj2310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat heart. Evidence from studies with isolated mitochondria that adrenaline activates the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes by increasing the intramitochondrial concentration of Ca2+. Biochem J. 1984 Feb 15;218(1):235–247. doi: 10.1042/bj2180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J. 1979 Jun 15;180(3):533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G. Evidence that adrenaline activates key oxidative enzymes in rat liver by increasing intramitochondrial [Ca2+]. FEBS Lett. 1985 Jan 28;180(2):259–264. doi: 10.1016/0014-5793(85)81082-6. [DOI] [PubMed] [Google Scholar]
- McCormack J. G. Studies on the activation of rat liver pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase by adrenaline and glucagon. Role of increases in intramitochondrial Ca2+ concentration. Biochem J. 1985 Nov 1;231(3):597–608. doi: 10.1042/bj2310597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sánchez R., Hansford R. G. Dependence of cardiac mitochondrial pyruvate dehydrogenase activity on intramitochondrial free Ca2+ concentration. Biochem J. 1988 Dec 1;256(2):403–412. doi: 10.1042/bj2560403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta C. V., Williamson J. R. Spermine. A regulator of mitochondrial calcium cycling. J Biol Chem. 1984 Nov 10;259(21):12978–12983. [PubMed] [Google Scholar]
- Nicholls D., Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta. 1982 Sep 1;683(1):57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter G. A., Denton R. M. Regulation of NAD+-linked isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase by Ca2+ ions within toluene-permeabilized rat heart mitochondria. Interactions with regulation by adenine nucleotides and NADH/NAD+ ratios. Biochem J. 1988 May 15;252(1):181–189. doi: 10.1042/bj2520181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Thomas A. P., Diggle T. A., Denton R. M. Sensitivity of pyruvate dehydrogenase phosphate phosphatase to magnesium ions. Similar effects of spermine and insulin. Biochem J. 1986 Aug 15;238(1):83–91. doi: 10.1042/bj2380083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toninello A., Di Lisa F., Siliprandi D., Siliprandi N. Uptake of spermine by rat liver mitochondria and its influence on the transport of phosphate. Biochim Biophys Acta. 1985 May 28;815(3):399–404. doi: 10.1016/0005-2736(85)90366-9. [DOI] [PubMed] [Google Scholar]
- Wendt-Gallitelli M. F. Ca-pools involved in the regulation of cardiac contraction under positive inotropy. X-ray microanalysis on rapidly-frozen ventricular muscles of guinea-pig. Basic Res Cardiol. 1986;81 (Suppl 1):25–32. doi: 10.1007/978-3-662-11374-5_3. [DOI] [PubMed] [Google Scholar]


