Abstract
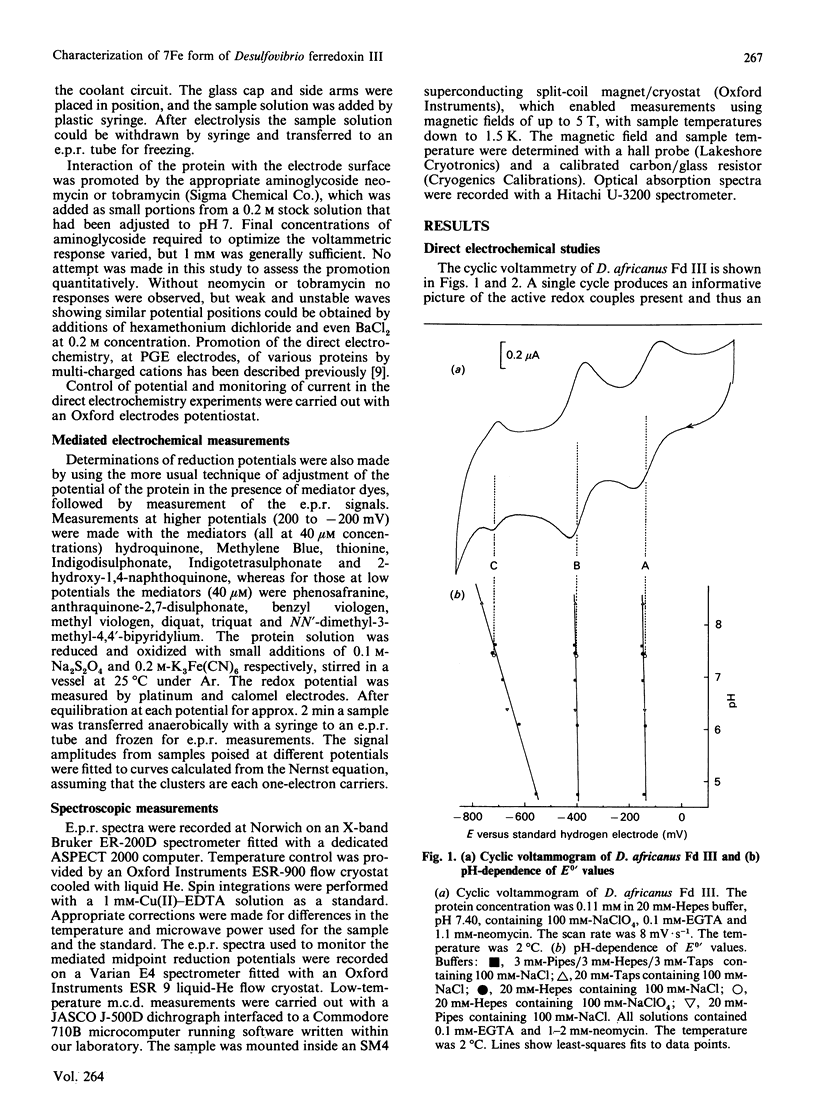
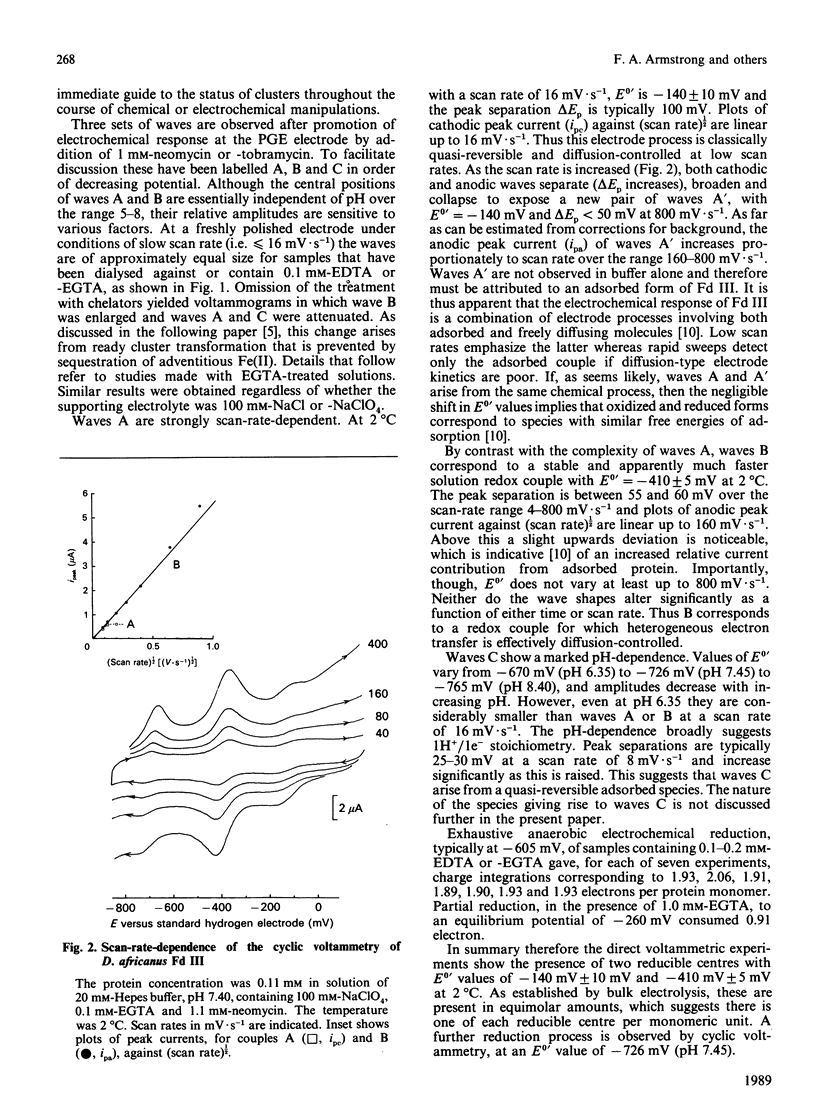
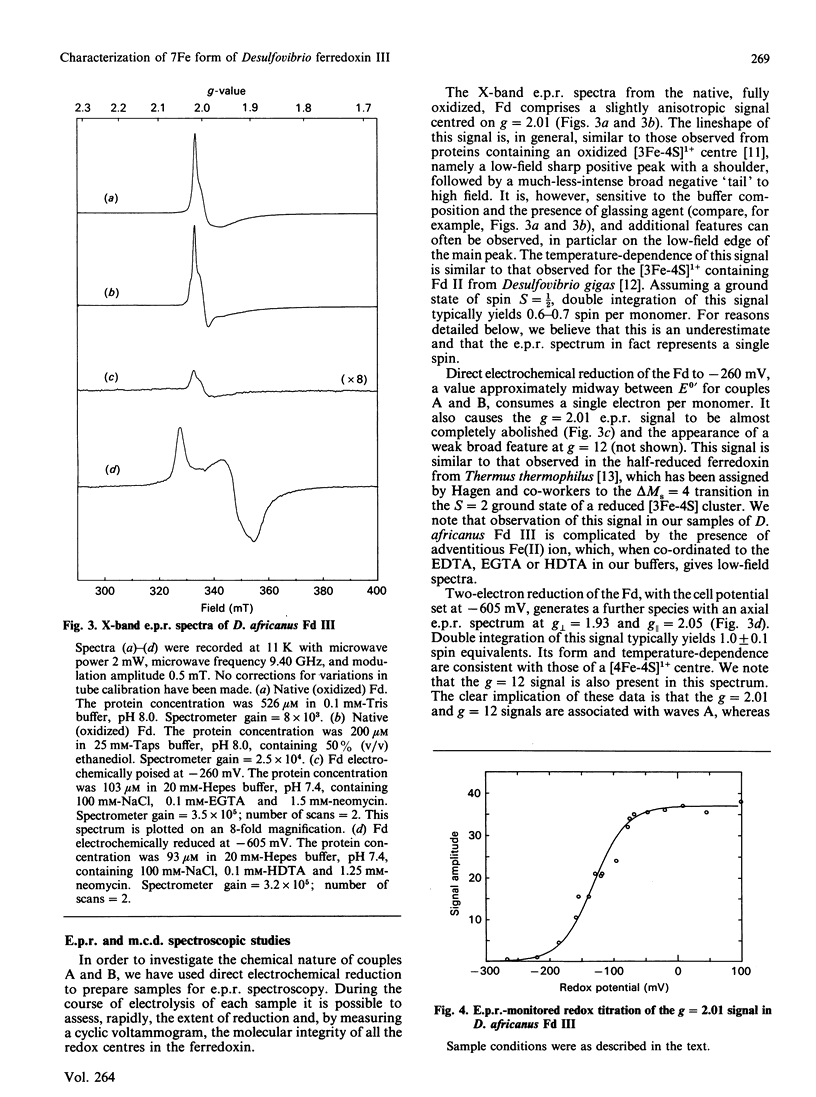
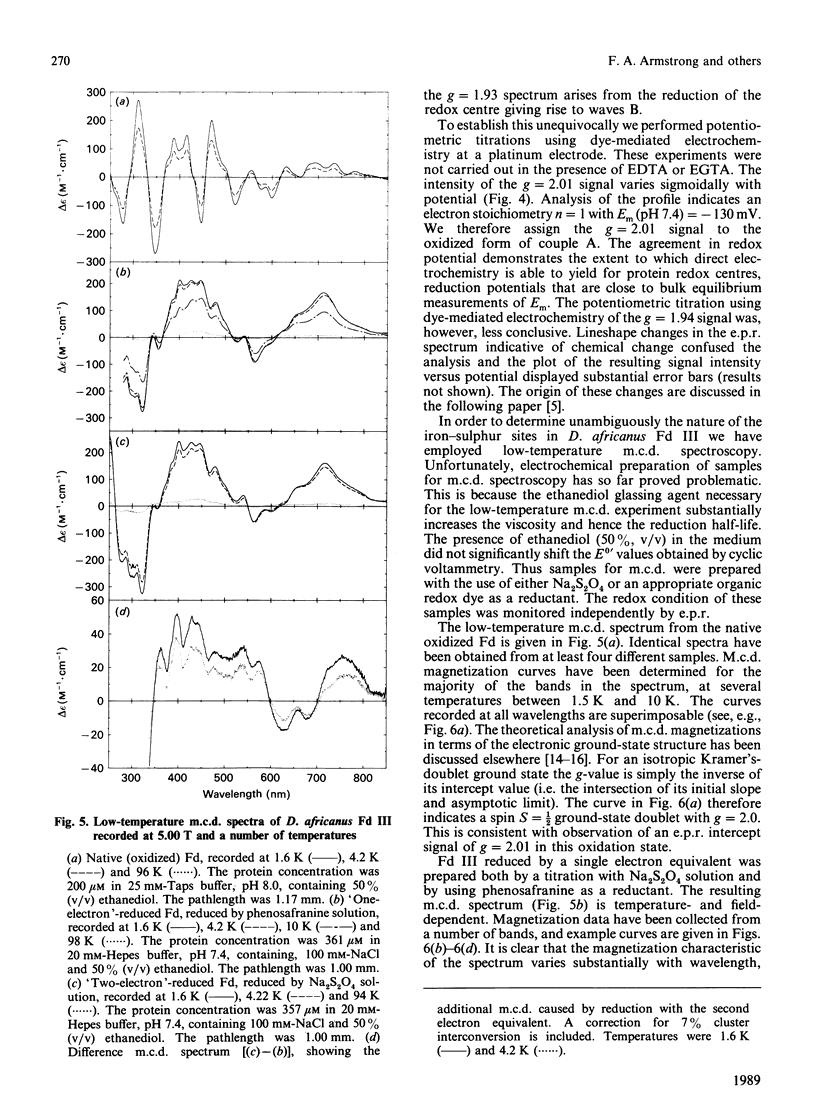
Desulfovibrio africanus ferredoxin III is a monomeric protein (Mr 6585) containing seven cysteine residues and 7-8 iron atoms and 6-8 atoms of acid-labile sulphur. It is shown that reversible unmediated electrochemistry of the two iron-sulphur clusters can be obtained by using a pyrolytic-graphite-'edge' carbon electrode in the presence of an appropriate aminoglycoside, neomycin or tobramycin, as promoter. Cyclic voltammetry reveals two well-defined reversible waves with E0' = -140 +/- 10 mV and -410 +/- 5 mV (standard hydrogen electrode) at 2 degrees C. Bulk reduction confirms that each of these corresponds to a one-electron process. Low-temperature e.p.r. and magnetic-c.d. spectroscopy identify the higher-potential redox couple with a cluster of core [3Fe-4S]1+.0 and the lower with a [4Fe-4S]2+.1+ centre. The low-temperature magnetic-c.d. spectra and magnetization properties of the three-iron cluster show that it is essentially identical with that in Desulfovibrio gigas ferredoxin II. We assign cysteine-11, -17 and -51 as ligands of the [3Fe-4S] core and cysteine-21, -41, -44 and -47 to the [4Fe-4S] centre.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Sieker L. C., Jensen L. H. Structure of a bacterial ferredoxin. J Biol Chem. 1973 Jun 10;248(11):3987–3996. [PubMed] [Google Scholar]
- Beinert H., Thomson A. J. Three-iron clusters in iron-sulfur proteins. Arch Biochem Biophys. 1983 Apr 15;222(2):333–361. doi: 10.1016/0003-9861(83)90531-3. [DOI] [PubMed] [Google Scholar]
- Fukuyama K., Nagahara Y., Tsukihara T., Katsube Y., Hase T., Matsubara H. Tertiary structure of Bacillus thermoproteolyticus [4Fe-4S] ferredoxin. Evolutionary implications for bacterial ferredoxins. J Mol Biol. 1988 Jan 5;199(1):183–193. doi: 10.1016/0022-2836(88)90388-9. [DOI] [PubMed] [Google Scholar]
- George S. J., Armstrong F. A., Hatchikian E. C., Thomson A. J. Electrochemical and spectroscopic characterization of the conversion of the 7Fe into the 8Fe form of ferredoxin III from Desulfovibrio africanus. Identification of a [4Fe-4S] cluster with one non-cysteine ligand. Biochem J. 1989 Nov 15;264(1):275–284. doi: 10.1042/bj2640275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. J., Richards A. J., Thomson A. J., Yates M. G. Azotobacter chroococcum 7Fe ferredoxin. Two pH-dependent forms of the reduced 3Fe clusters and its conversion to a 4Fe cluster. Biochem J. 1984 Nov 15;224(1):247–251. doi: 10.1042/bj2240247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen W. R., Dunham W. R., Johnson M. K., Fee J. A. Quarter field resonance and integer-spin/half-spin interaction in the EPR of Thermus thermophilus ferredoxin. Possible new fingerprints for three iron clusters. Biochim Biophys Acta. 1985 Apr 29;828(3):369–374. doi: 10.1016/0167-4838(85)90318-8. [DOI] [PubMed] [Google Scholar]
- Hatchikian C. E., Jones H. E., Bruschi M. Isolation and characterization of a rubredoxin and two ferredoxins from Desulfovibrio africanus. Biochim Biophys Acta. 1979 Dec 6;548(3):471–483. doi: 10.1016/0005-2728(79)90059-8. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C., Bruschi M. Characterization of a new type of ferredoxin from Desulfovibrio africanus. Biochim Biophys Acta. 1981 Jan 14;634(1):41–51. doi: 10.1016/0005-2728(81)90126-2. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Bennett D. E., Fee J. A., Sweeney W. V. Spectroscopic studies of the seven-iron-containing ferredoxins from Azotobacter vinelandii and Thermus thermophilus. Biochim Biophys Acta. 1987 Jan 5;911(1):81–94. doi: 10.1016/0167-4838(87)90273-1. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Thomson A. J., Richards A. J., Peterson J., Robinson A. E., Ramsay R. R., Singer T. P. Characterization of the Fe-S cluster in aconitase using low temperature magnetic circular dichroism spectroscopy. J Biol Chem. 1984 Feb 25;259(4):2274–2282. [PubMed] [Google Scholar]
- Johnson M. K., Thomson A. J., Robinson A. E., Rao K. K., Hall D. O. Low-temperature magnetic circular dichroism spectra and magnetisation curves of 4Fe clusters in iron-sulphur proteins from Chromatium and Clostridium pasteurianum. Biochim Biophys Acta. 1981 Feb 27;667(2):433–451. doi: 10.1016/0005-2795(81)90209-9. [DOI] [PubMed] [Google Scholar]
- Mullinger R. N., Cammack R., Rao K. K., Hall D. O., Dickson D. P., Johnson C. E., Rush J. D., Simopoulos A. Physicochemical characterization of the four-iron-four-sulphide ferredoxin from Bacillus stearothermophilus. Biochem J. 1975 Oct;151(1):75–83. doi: 10.1042/bj1510075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaka E., Ooi T. Examination of protein sequence homologies: IV. Twenty-seven bacterial ferredoxins. J Mol Evol. 1987;26(3):257–267. doi: 10.1007/BF02099857. [DOI] [PubMed] [Google Scholar]
- Stout C. D. 7-Iron ferredoxin revisited. J Biol Chem. 1988 Jul 5;263(19):9256–9260. doi: 10.2210/pdb3fd1/pdb. [DOI] [PubMed] [Google Scholar]
- Stout G. H., Turley S., Sieker L. C., Jensen L. H. Structure of ferredoxin I from Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1020–1022. doi: 10.1073/pnas.85.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. J., Johnson M. K. Magnetization curves of haemoproteins measured by low-temperature magnetic-circular-dichroism spectroscopy. Biochem J. 1980 Nov 1;191(2):411–420. doi: 10.1042/bj1910411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. J., Robinson A. E., Johnson M. K., Moura J. J., Moura I., Xavier A. V., Legall J. The three-iron cluster in a ferredoxin from Desulphovibrio gigas. A low-temperature magnetic circular dichroism study. Biochim Biophys Acta. 1981 Aug 28;670(1):93–100. doi: 10.1016/0005-2795(81)90053-2. [DOI] [PubMed] [Google Scholar]


