Abstract
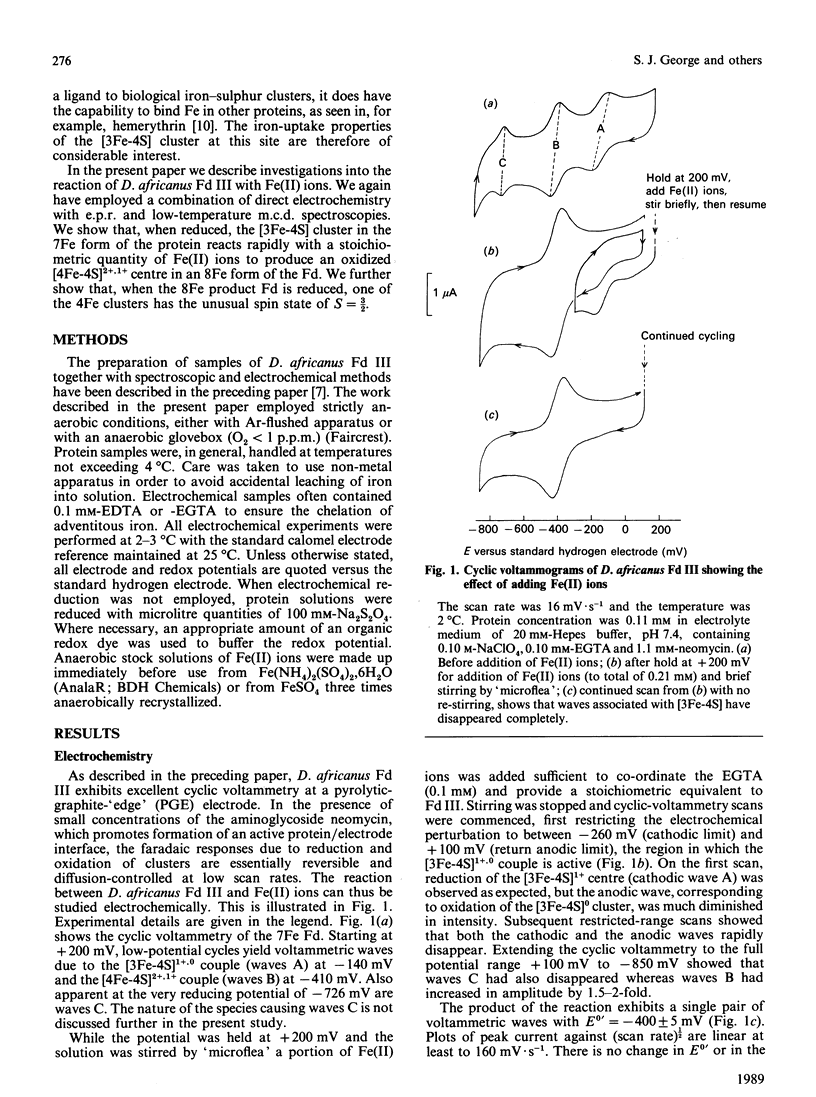
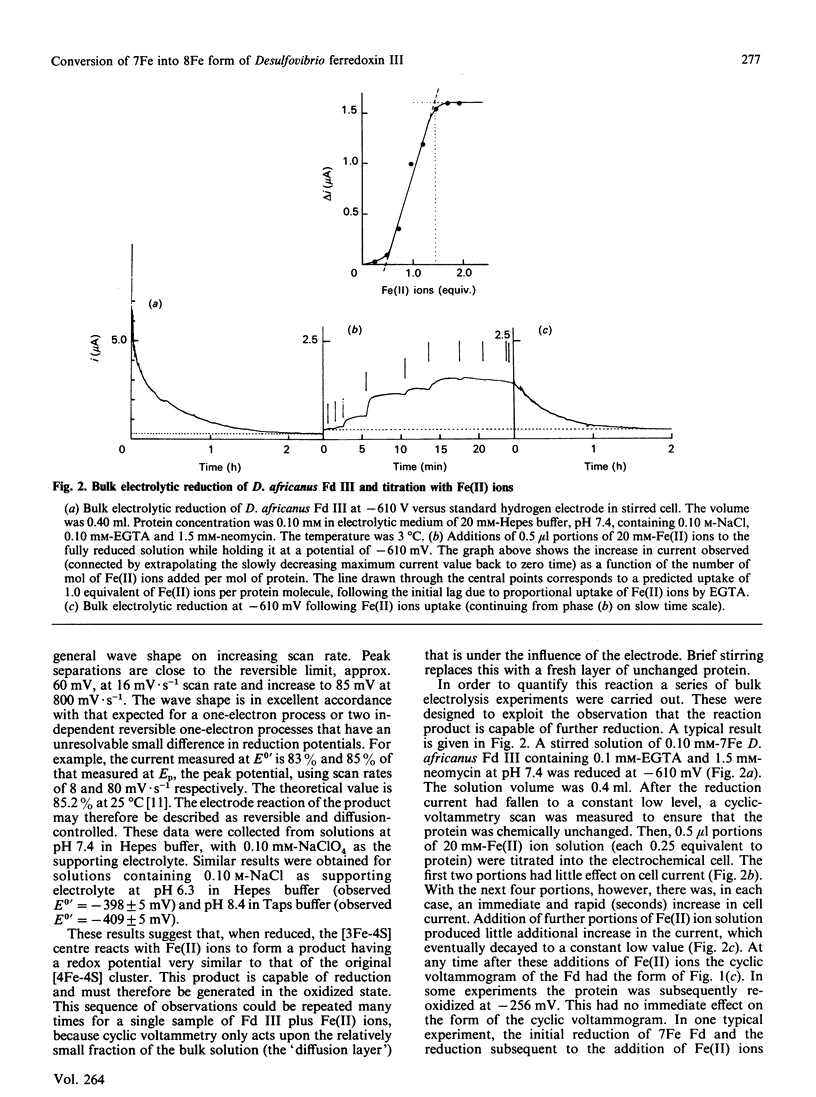
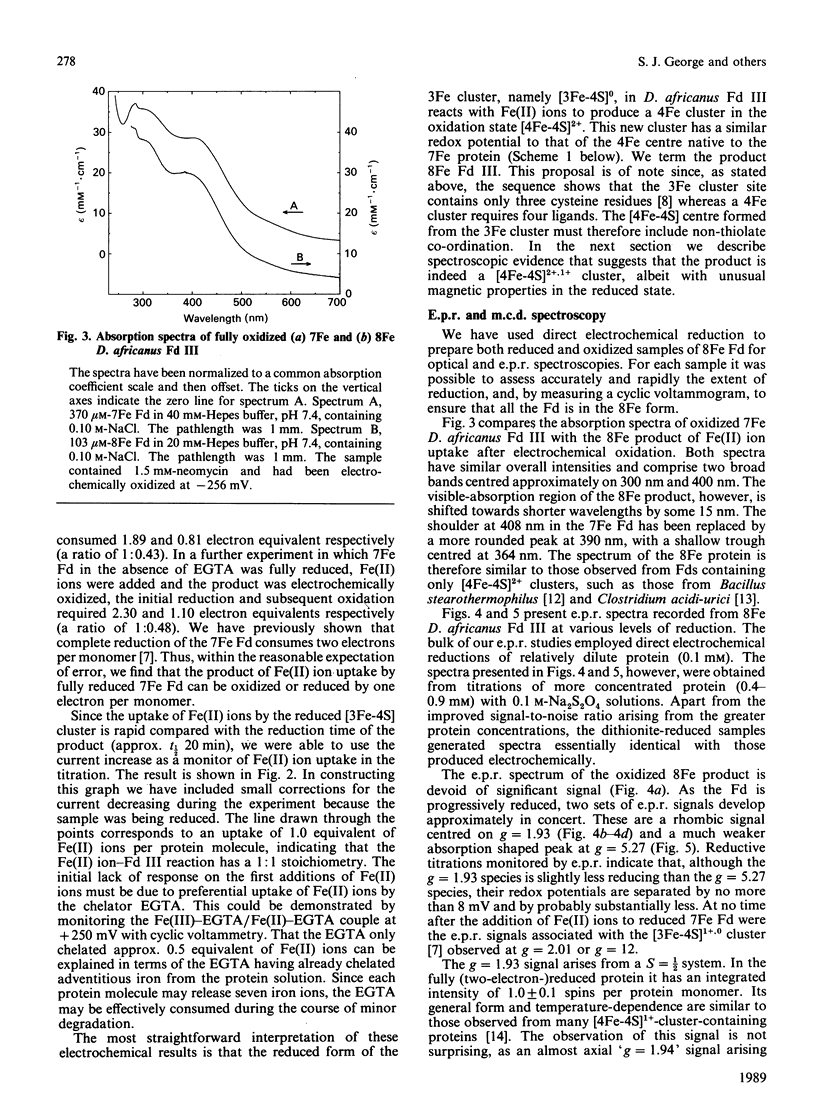
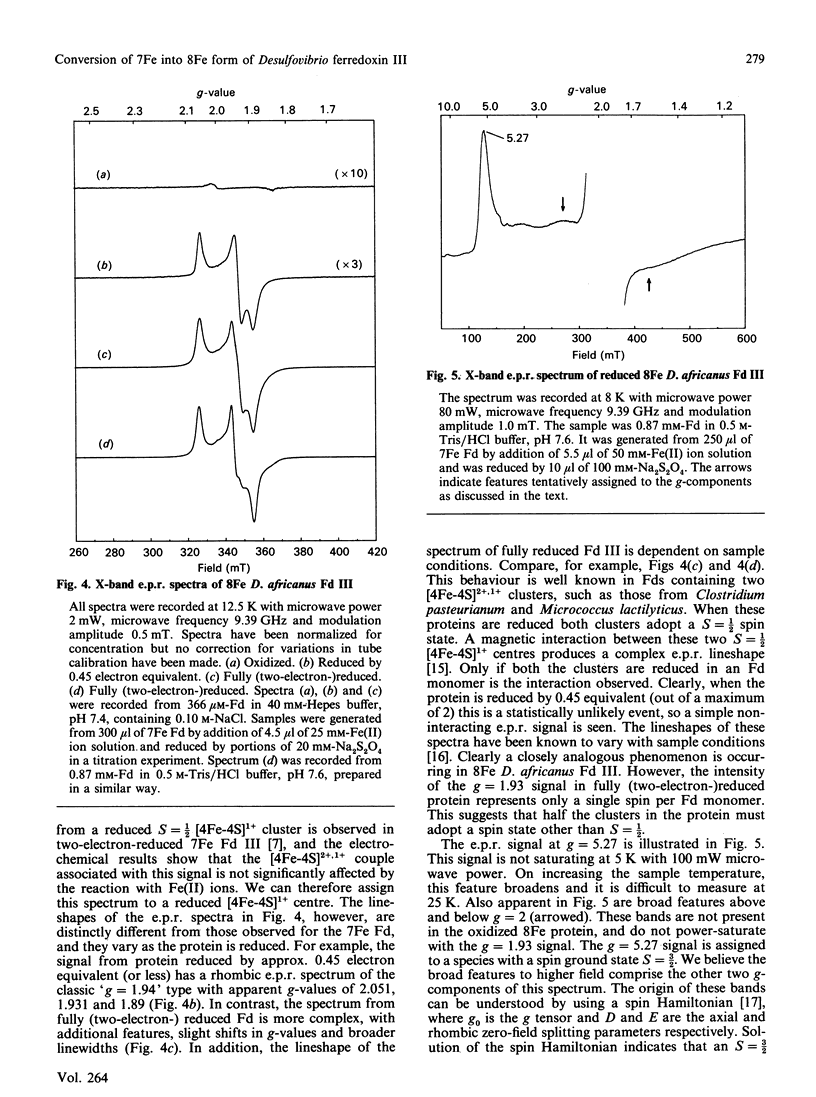
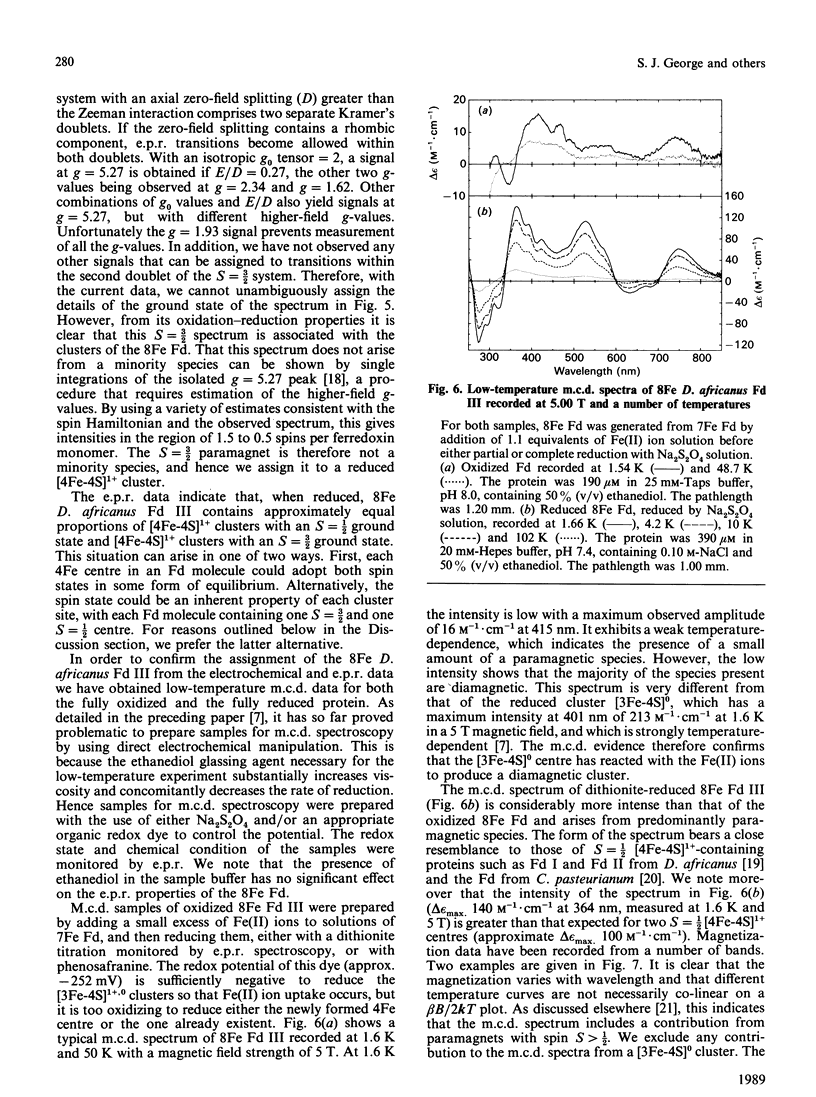
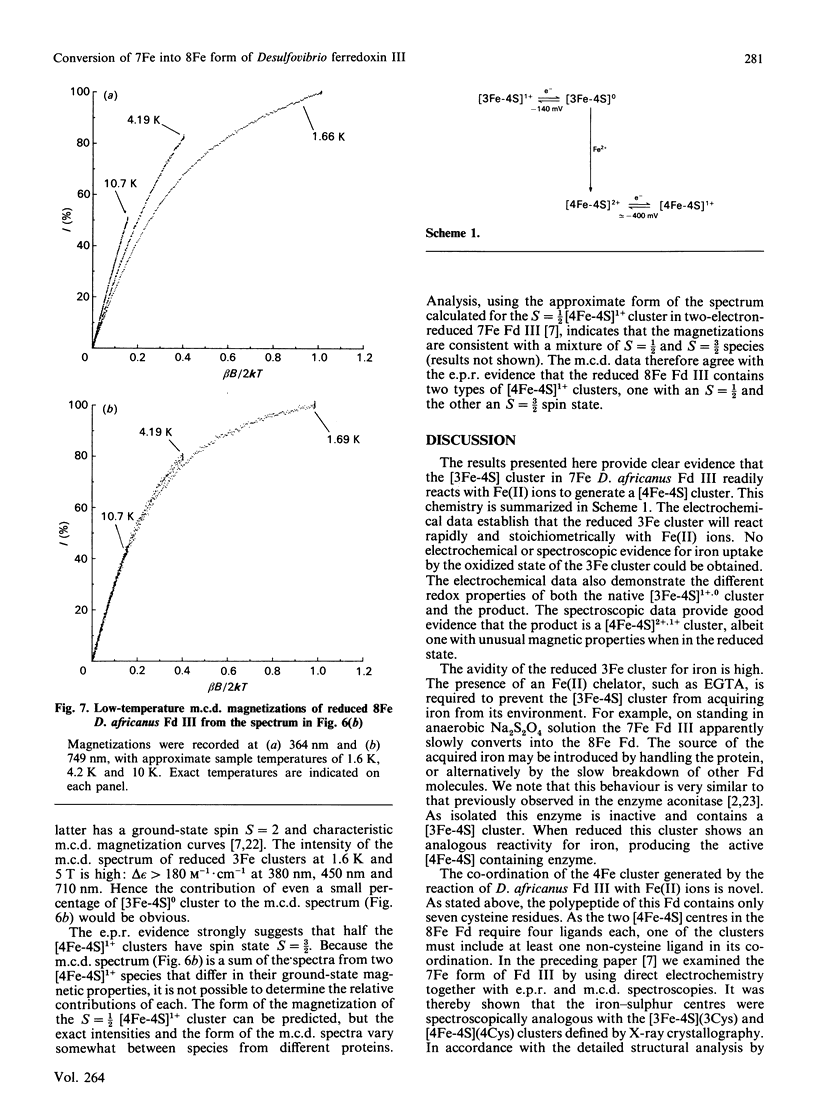
Desulfovibrio africanus ferredoxin III is a protein (Mr 6585) containing one [3Fe-4S]1+,0 and one [4Fe-4S]2+,1+ core cluster when aerobically isolated. The amino acid sequence contains only seven cysteine residues, the minimum required to ligand these two clusters. Cyclic voltammery by means of direct electrochemistry at a pyrolytic-graphite-'edge' electrode promoted by neomycin shows that, when reduced, the [3Fe-4S]0 centre reacts rapidly with Fe(II) ion to form a [4Fe-4S]2+ cluster. The latter, which can be reduced at a redox potential similar to that of the other [4Fe-4S] cluster, must include non-thiolate ligation. We propose that the carboxylate side chain of aspartic acid-14 is the most likely candidate, since this amino acid occupies the position of a cysteine residue in the sequence typical of an 8Fe ferredoxin. The magnetic properties at liquid-He temperature of this novel cluster, studied by low-temperature magnetic-c.d. and e.p.r. spectroscopy, are diamagnetic in the oxidized state and S = 3/2 in the one-electron-reduced state. This cluster provides a plausible model for the ligation states of the [4Fe-4S]1+ core in the S = 3/2 cluster of the iron protein of nitrogenase and in Bacillus subtilis glutamine:phosphoribosyl pyrophosphate amidotransferase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong F. A., George S. J., Cammack R., Hatchikian E. C., Thomson A. J. Electrochemical and spectroscopic characterization of the 7Fe form of ferredoxin III from Desulfovibrio africanus. Biochem J. 1989 Nov 15;264(1):265–273. doi: 10.1042/bj2640265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H., Emptage M. H., Dreyer J. L., Scott R. A., Hahn J. E., Hodgson K. O., Thomson A. J. Iron-sulfur stoichiometry and structure of iron-sulfur clusters in three-iron proteins: evidence for [3Fe-4S] clusters. Proc Natl Acad Sci U S A. 1983 Jan;80(2):393–396. doi: 10.1073/pnas.80.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H., Thomson A. J. Three-iron clusters in iron-sulfur proteins. Arch Biochem Biophys. 1983 Apr 15;222(2):333–361. doi: 10.1016/0003-9861(83)90531-3. [DOI] [PubMed] [Google Scholar]
- Bell S. H., Dickson D. P., Johnson C. E., Cammack R., Hall D. O., Rao K. K. Mössbauer spectroscopic evidence for the conversion of [4 Fe--4 S] clusters in Bacillus stearothermophilus ferredoxin into [3 Fe--3 S] clusters. FEBS Lett. 1982 Jun 1;142(1):143–146. doi: 10.1016/0014-5793(82)80238-x. [DOI] [PubMed] [Google Scholar]
- Cammack R. Effects of solvent on the properties of ferredoxins. Biochem Soc Trans. 1975;3(4):482–488. doi: 10.1042/bst0030482. [DOI] [PubMed] [Google Scholar]
- Fukuyama K., Nagahara Y., Tsukihara T., Katsube Y., Hase T., Matsubara H. Tertiary structure of Bacillus thermoproteolyticus [4Fe-4S] ferredoxin. Evolutionary implications for bacterial ferredoxins. J Mol Biol. 1988 Jan 5;199(1):183–193. doi: 10.1016/0022-2836(88)90388-9. [DOI] [PubMed] [Google Scholar]
- Gayda J. P., Bertrand P., More C., Le Gall J., Cammack R. C. Energy of the low-lying excited levels for some reduced [4Fe-4S] ferredoxins, from the relaxation broadening of the E.P.R. signals. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1265–1270. doi: 10.1016/0006-291x(81)90756-7. [DOI] [PubMed] [Google Scholar]
- Hagen W. R., Eady R. R., Dunham W. R., Haaker H. A novel S = 3/2 EPR signal associated with native Fe-proteins of nitrogenase. FEBS Lett. 1985 Sep 23;189(2):250–254. doi: 10.1016/0014-5793(85)81033-4. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Rabinowitz J. C. Molar extinction coefficient and iron and sulfide content of clostridial ferredoxin. J Biol Chem. 1970 Oct 10;245(19):4982–4987. [PubMed] [Google Scholar]
- Johnson M. K., Thomson A. J., Robinson A. E., Rao K. K., Hall D. O. Low-temperature magnetic circular dichroism spectra and magnetisation curves of 4Fe clusters in iron-sulphur proteins from Chromatium and Clostridium pasteurianum. Biochim Biophys Acta. 1981 Feb 27;667(2):433–451. doi: 10.1016/0005-2795(81)90209-9. [DOI] [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983 Sep 25;258(18):11098–11105. [PubMed] [Google Scholar]
- Lindahl P. A., Day E. P., Kent T. A., Orme-Johnson W. H., Münck E. Mössbauer, EPR, and magnetization studies of the Azotobacter vinelandii Fe protein. Evidence for a [4Fe-4S]1+ cluster with spin S = 3/2. J Biol Chem. 1985 Sep 15;260(20):11160–11173. [PubMed] [Google Scholar]
- Lindahl P. A., Gorelick N. J., Münck E., Orme-Johnson W. H. EPR and Mössbauer studies of nucleotide-bound nitrogenase iron protein from Azotobacter vinelandii. J Biol Chem. 1987 Nov 5;262(31):14945–14953. [PubMed] [Google Scholar]
- Mathews R., Charlton S., Sands R. H., Palmer G. On the nature of the spin coupling between the iron-sulfur clusters in the eight-iron ferredoxins. J Biol Chem. 1974 Jul 10;249(13):4326–4328. [PubMed] [Google Scholar]
- Moura J. J., Moura I., Kent T. A., Lipscomb J. D., Huynh B. H., LeGall J., Xavier A. V., Münck E. Interconversions of [3Fe-3S] and [4Fe-4S] clusters. Mössbauer and electron paramagnetic resonance studies of Desulfovibrio gigas ferredoxin II. J Biol Chem. 1982 Jun 10;257(11):6259–6267. [PubMed] [Google Scholar]
- Mullinger R. N., Cammack R., Rao K. K., Hall D. O., Dickson D. P., Johnson C. E., Rush J. D., Simopoulos A. Physicochemical characterization of the four-iron-four-sulphide ferredoxin from Bacillus stearothermophilus. Biochem J. 1975 Oct;151(1):75–83. doi: 10.1042/bj1510075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawara N., Ogata M., Yagi T., Wakabayashi S., Matsubara H. Amino acid sequence of ferredoxin I from Desulfovibrio vulgaris Miyazaki. J Biochem. 1988 Aug;104(2):196–199. doi: 10.1093/oxfordjournals.jbchem.a122441. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Adams M. W. Oxidation-reduction properties of the two Fe4S4 clusters in Clostridium pasteurianum ferredoxin. J Biol Chem. 1987 Apr 15;262(11):5125–5128. [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. Structure of activated aconitase: formation of the [4Fe-4S] cluster in the crystal. Proc Natl Acad Sci U S A. 1989 May;86(10):3639–3643. doi: 10.1073/pnas.86.10.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F. M., Adams M. W., Mortenson L. E., Münck E. Mössbauer study of Clostridium pasteurianum hydrogenase II. Evidence for a novel three-iron cluster. J Biol Chem. 1987 Jan 5;262(1):38–41. [PubMed] [Google Scholar]
- Thomson A. J., Robinson A. E., Johnson M. K., Moura J. J., Moura I., Xavier A. V., Legall J. The three-iron cluster in a ferredoxin from Desulphovibrio gigas. A low-temperature magnetic circular dichroism study. Biochim Biophys Acta. 1981 Aug 28;670(1):93–100. doi: 10.1016/0005-2795(81)90053-2. [DOI] [PubMed] [Google Scholar]
- Vollmer S. J., Switzer R. L., Debrunner P. G. Oxidation-reduction properties of the iron-sulfur cluster in Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1983 Dec 10;258(23):14284–14293. [PubMed] [Google Scholar]


