Abstract
Background
The aims of this narrative review were (i) to describe the current indications of SLKT, (ii) to report evolution of SLKT activity, (iii) to report the outcomes of SLKT, (iv) to explain the immune-protective effect of liver transplant on kidney transplant, (v) to explain the interest of delay kidney transplantation, using hypothermic machine perfusion (HMP), (vi) to report kidney after liver transplantation (KALT) indications and (vii) to describe the value of the increase in the use of extended criteria donors (ECD) and particular controlled donation after circulatory death (cDCD) transplant, thanks to the development of new organ preservation strategies.
Method
Electronic databases were screened using the keywords "Simultaneous", "Combined", "kidney transplantation" and "liver transplantation". The methodological and clinical heterogeneity of the included studies meant that meta-analysis was inappropriate.
Results
A total of 1,917 publications were identified in the literature search. Two reviewers screened all study abstracts independently and 1,107 of these were excluded. Thus, a total of 79 full text articles were assessed for eligibility. Of these, 21 were excluded. In total, 58 studies were included in this systematic review.
Conclusions
Simultaneous liver-kidney transplantation has made a significant contribution for patients with dual‐organ disease. The optimization of indication and selection of SLKT patients will reduce futile transplantation. Moreover, increasing the use of transplants from extended criteria donors, in particular cDCD, should be encouraged, thanks to the development of new modalities of organ preservation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00345-024-05174-z.
Keywords: Simultaneous liver-kidney transplantation, Liver transplantation, End-stage renal disease, Hypothermic machine perfusion
Introduction
Liver transplantation (LT) is an established treatment for patients with end-stage liver disease (ESLD) and acute liver failure. End-stage renal disease (ESRD) is common and increases mortality in wait-listed LT patients [1, 2]. ESRD in LT recipients is responsible of worse patient survival and outcomes [3–6]. Thus, ESRD has long been considered a contraindication to liver transplantation [2].
Simultaneous liver-kidney transplantations (SLKT), first reported in 1984 by Margreiter et al. [7] has been developed for patients with ESLD and ESRD, with optimal short-term and long-term outcomes [8]. In 2002, in the US, the model for end-stage liver disease (MELD) scoring system was introduced for organ allocation for wait-listed LT patients [9]. This scoring system includes the renal function of the LT candidates. Thus, since that time, the incidence of ESRD among LT recipients increased, resulting in an increase in SLKT activity since 2002 [5, 10, 11].
The aims of this narrative review were (i) to describe the current indications of SLKT, (ii) to report evolution of SLKT activity, (iii) to report the outcomes of SLKT, (iv) to explain the immune-protective effect of liver transplant on kidney transplant, (v) to explain the interest of delay kidney transplantation, using hypothermic machine perfusion (HMP), (vi) to report kidney after liver transplantation (KALT) indications and (vii) to describe the value of the increase in the use of extended criteria donors (ECD) and particular controlled donation after circulatory death (cDCD) transplant, thanks to the development of new organ preservation strategies.
Methods
Methodology
This article is a narrative review on simultaneous liver-kidney transplantation with the aim to describe the current indications of SLKT, to report evolution of SLKT activity, to report the outcomes of SLKT, to explain the immune-protective effect of liver transplant on kidney transplant, to explain the interest of delay kidney transplantation, using hypothermic machine perfusion (HMP), to report kidney after liver transplantation (KALT) indications and then to describe the value of the increase in the use of extended criteria donors (ECD) and particular controlled donation after circulatory death (cDCD) transplant, thanks to the development of new organ preservation strategies.
Search strategy and study selection
This narrative review has been conducted according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [12]. Studies (January 1, 1995 to April 31, 2023) were identified by highly sensitive searches of electronic databases (Embase, Medline, Cochrane Library databases, PubMed). The search was complimented by additional sources including the reference lists of included studies. Full strategy research is show in the Supplementary Table 1. All study designs were eligible for inclusion, except letter to the editor, case reports and studies published as conference abstracts only. Only studies published in 1995 and after were included to reflect current clinical practice. Language was restricted to English and French pragmatic reasons. Titles and abstracts of all identified studies were independently reviewed by two authors (T.P., B.M.) and discrepancies were resolved by a third reviewer (S.D.). The methodological and clinical heterogeneity of the included studies meant that meta-analysis was inappropriate. Therefore, a narrative synthesis of the data was performed.
A total of 1,917 publications were identified in the literature search. Two reviewers (T.P., B.M.) screened all study abstracts independently and 1,107 of these were excluded. Thus, a total of 79 full text articles were assessed for eligibility. Of these, 21 were excluded. In total, 58 studies were included in this systematic review (Supplementary Fig. 1).
Current indications of SLKT
The indications for SLKT can be divided into three categories [2, 13]:
-
(i)
ESLD with chronic kidney disease, including: glomerulonephritis, interstitial renal disease, polycystic disease, and calcineurin inhibitors (CNI) toxicity;
-
(ii)
ESLD with acute kidney injury, including: hepatorenal syndrome (HRS) and acute tubular necrosis;
-
(iii)
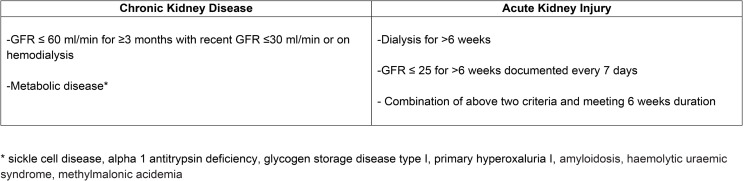
Metabolic disorders, including: primary hyperoxaluria I, alpha 1 antitrypsin deficiency, glycogen storage disease type I, sickle cell disease, amyloidosis, haemolytic uraemic syndrome, methylmalonic acidemia.
In 2017, an allocation policy for SLKT was created in the US, which defines eligibility criteria for SLKT [11, 14] (Fig. 1). Currently, SLKT indication is consensual for ESLD with chronic kidney disease or metabolic disorder but it remains a cause for debate for ESLD with acute renal failure, including hepatorenal syndrome (HRS). HRS is a functional acute kidney injury secondary to acute or chronic liver failure [13, 15, 16]. HRS is reversible as shown by the successful transplantation of affected kidneys from patients dying of liver failure with HRS to patients with normal liver function [17] and renal recovery following LT in patients with HRS [18]. However, patients with prolonged HRS may not recover renal function with LT alone and may require SLKT or kidney after LT (KALT) [19].
Fig. 1.
OPTN 2017 criterion for simultaneous liver-kidney transplantation
Evolution of SLKT activity
In 2002, the model for end-stage liver disease (MELD) scoring system was created in the US, (which prioritizes renal dysfunction) resulting in an over 400% increase of SLKT between 2001 (138 SLKT; 2.5% of LT) and 2016 (738 SLKT; 9.3% of LT) [9, 20]. Prior to introduction of the MELD system, SLKT accounted for only 1.7% of LT activity in 1990 in the US. This growing number of SLKT is not only a consequence of the MELD allocation system, but likely also because of changing practices in transplant centers [21]. In 2017, an UNOS allocation policy for SLKT was created in the US, which defines eligibility criteria for SLKT [11, 14]. Indeed, before this period, each transplant center decide the SLKT eligibility on a case-by‐case basis [2]. From the application of UNOS allocation policy, a progressive decrease of SLKT activity between 2016 and 2019 (738 SLKT in 2016 versus 704 SLKT in 2019) has been reported in the US [22].
In France, SLKT activity increased between 2010 (40 SLKT) and 2015 (74 SLKT) but in a lower proportion than in the US. Thus, in 2021, 58 (SLKT) were performed in France, accounted for 4.8% (58/1204) [23] of LT activity [23].
Indeed, in France, the allocation of LT is performed according to 3 main modalities: super-emergency, Liver score (since 2007) and “out of round”. Thus, Liver score considers the indication, the severity of the patient’s condition (MELD score in the case of cirrhosis, Alpha-fetoprotein score for hepatocarcinoma), the waiting time and the distance between the procurement and transplantation sites [24]. Therefore, ESRD has less impact on the LT indication.
In UK, this increase in SLKT activity has also been limited. Indeed, from January 2001 to December 2013, 5912 (98%) patients received a liver transplant alone and 123 (2%) a SLKT [25].
SLKT outcomes: patient and graft survival
National US transplant registry data and single center data analyses reported a 5-year patient survival rates after SLKT range from 64 to 76% [9, 26–29].
Patients with criteria for SLKT (Fig. 1) have better outcomes after receiving SLKT compared to LT alone recipients [28, 29]. Indeed, Hmoud et al. [28] reported patient survival, from the UNOS national database, comparing LT alone (with ESRD and wait-listed for KT) versus SLKT. They reported a significant higher 5-year patient survival in SLKT group (76% versus 55%). Similarly, Fong et al. [29] reported a significant higher 5-year patient survival in SLKT group, compared to LT alone (67.4% versus 62.9%).
In case of ESLD with acute renal failure, the etiology of acute renal failure impacts the LT outcomes. Indeed, Nadim et al. [30] compared LT outcomes, performed in acute renal failure patient, according to acute renal failure causes (HRS or acute tubular necrosis). They reported significant lower 5-year patient survival after LT in case of acute tubular necrosis (< 45% versus 75–80%).
Tinti et al. [25] reported the national UK experience on SLKT. Thus, from January 2001 to December 2013, 5912 (98%) patients received a LT and 123 (2%) a SLKT. They compared patient and graft survival between SLKT and LT, after stratifying on pretransplant estimated glomerular filtration rate (eGFR). They reported similar 5-year patient and graft survival between SLKT and LT, whatever pretransplant eGFR. However, they reported a significant higher patient survival, for patients on dialysis at time of transplantation, in SLKT group.
Immune-protection by the liver transplant in SLKT. Immunological aspects
In their study of the UNOS database, Fong et al. [31] and Simpson et al. [32] reported a lower incidence of kidney acute rejection and a higher kidney allograft survival in SLKT, as compared to KT alone. Indeed, the liver graft among SLKT recipients ensure an immune-protection to the kidney grafts, particularly in case of preformed donor-specific anti-HLA antibodies (DSA) [33–36].
Several mechanisms have been proposed, to explain the immunologic protection afforded by the liver to the kidney, which include adsorption of preformed DSA and removal by Kupffer cells and production by the liver allograft of soluble HLA class I antigens which can neutralize both pre-existing DSA and cytotoxic T lymphocytes [37].
Simultaneous or delayed KT, using hypothermic machine perfusion (HMP), after LT?
Studies from UNOS database reported a significant lower kidney transplant survival and higher kidney transplant primary non-function for SLKT in high risk medical patients (MELD scores > 30), compared to recipients with MELD scores < 30 [38].
The lower kidney transplant survival observed for SLKT recipients with MELD score > 30 is caused by the physiological disturbances generated by LT [39, 40]. Indeed, recipients of LT often require vasopressors to correct perioperative hypotension. This perioperative hypotension and vasopressors are harmful for kidney allograft [41], resulting in a lower kidney transplant survival.
In order to limit these detrimental effects on kidney allograft function, several teams reported their experience of delay of kidney transplantation [39, 40, 42, 43]. Thus, kidney transplants were preserved with hypothermic machine perfusion (HMP), to defer the KT until stabilization of the coagulopathy and hemodynamic status of the recipients.
Ekser et al. [39] compared the outcomes of simultaneous and delayed KT in SLKT. They included 69 simultaneous KT and 61 delayed KT. As expected, mean kidney cold ischemia time was longer in delayed KT group (10 and 50 h). They reported a significant higher delayed graft function (DGF), lower 1-year eGFR and higher 1-year and 5-year patient survival in delayed KT group. These results are confirmed by Lunsford et al. [40] in their multicentric retrospective study. From February 2004 to January 2017, they included 63 delayed KT and 161 simultaneous KT. They reported a significant higher 1-year, 3-year and 5-year patient and kidney transplant survival in delayed KT group.
Recently, Chang et al. [43] reported kidney outcomes of HMP versus static cold storage of kidney grafts in SLKT, from UNOS database. Kidney cold ischemia time was longer in the HMP group (12.8 h versus 10.0 h). They reported that HMP reduced DGF but not PNF.
Kidney after liver transplantation (KALT) or SLKT?
KALT was the historical indication of LT in recipients who develop ESRD due calcineurin inhibitor-induced nephrotoxicity. KALT, in an early stage after LT, has the advantage of a lower perioperative morbidity and greater supply of kidneys (deceased or living donor kidney grafts). Simpson et al. [32] compared outcomes of KALT and SLKT, from the UNOS national database. From 1996 to 2003, they included 352 KALT and 1,136 SLKT. They reported a significant shorter kidney transplant half-life and a lower rejection-free kidney transplant survival after KALT, compared to SLKT. Recently, Cullaro et al. [44] reported data on kidney fonction and survival comparing SLKT (according to MELD score) and KALT, also from the UNOS national database. They reported a higher risk of early kidney failure in SLKT recipients with an MELD score ≥ 25, as compared to KALT recipients. Thus, KALT should probably be considered in patients with high MELD score.
Extended criteria donor and donation after circulatory death (DCD) transplant. A way to increase the SLKT pool?
Main studies comparing donation after brain death (DBD) and cDCD SLKT are summarized in Tables 1, 2 and 3.
Table 1.
Studies evaluating cDCD SLKT and LT outcomes: studies, recipients and donors characteristics
| Author, date | Study type | Inclusion period | Arms (n° of recipients and donor type) |
Recipients age (years) | Recipient sex (% of male) | Preemptive KT (%) | Median biologic MELD | Retransplant rate (%) | Donor age (years) | Donor sex (% of male) |
|---|---|---|---|---|---|---|---|---|---|---|
| STUDIES EVALUATING cDCD VERSUS DBD IN SLKT | ||||||||||
| Nunez-Nateras et al., 2020 [45] | Retrospective cohort study | 2010–2018 | 30 cDCD SLKT | 57.1 | 53.3 | 36.7 | 24.2 |
Liver: 6.7 Kidney: 10 |
37.4 | 60.0 |
| 131 DBD SLKT | 54.8 | 59.5 | 26.0 | 28.9 |
Liver: 16 Kidney: 9.2 |
37.8 | 56.5 | |||
| Croome et al., 2020 [46] |
Retrospective UNOS cohort study |
2000–2010 (Era 1) |
94 cDCD SLKT | 53.7 | 66.0 | * | 27.6 | Liver: 9.6 | 32.3 | * |
|
2011–2018 (Era 2) |
208 cDCD SLKT | 56.7 | 63.0 | * | 27.8 | Liver: 2.4 | 33.0 | * | ||
| 624 DBD SLKT (propensity matched) | 56.1 | 63.8 | * | 27.7 | Liver: 2.2 | 34.8 | * | |||
| LaMattina et al., 2011 [47] | Retrospective cohort study | 1998–2008 | 5 cDCD SLKT | 47.0 | * | * | 33.0 | * | 35.0 | * |
| 32 DBD SLKT | 54.4 | * | * | 25.0 | * | 45.5 | * | |||
| STUDIES EVALUATING NRP VERSUS SRR IN cDCD LT | ||||||||||
| Hessheimer et al., 2019 [60] | Retrospective cohort study | 2012–2016 | 95 NRP cDCD LT | 54.8 | 77.9 | / | 15.1 | 2.1 | 53.8 | 66.3 |
| 117 SRR cDCD LT | 57.7 | 84.6 | / | 14.1 | 1.7 | 54.5 | 65.8 | |||
| Watson et al., 2019 [51] | Retrospective UK national database cohort study | 2011–2017 | 43 NRP cDCD LT | 60.0 | * | / | 15 | * | 41.0 | * |
| 187 SRR cDCD LT | 57.0 | * | / | 15 | * | 50.0 | * | |||
| Hessheimer et al., 2022 [50] | Retrospective cohort study | 2012–2019 | 545 NRP cDCD LT | 59.0 | 79.0 | / | 12.0 | 3.1 | 59.0 | 64.0 |
| 258 SRR cDCD LT | 58.0 | 83.0 | / | 12.0 | 1.2 | 58.0 | 65.0 | |||
| STUDIES EVALUATING NRP cDCD VERSUS DBD IN LT | ||||||||||
| Savier et al., 2020 [52] | Retrospective French national database cohort study | 2015–2019 | 50 NRP cDCD LT | 59.9 | * | / | 7 | * | 50.0 | 68.0 |
| 100 DBD LT | 58.4 | * | / | 10 | * | 50.0 | 61.0 | |||
| STUDIES EVALUATING NP VERSUS SCS IN LT | ||||||||||
| Nasralla et al., 2018 [56] | Prospective randomized controlled trial | 2014–2016 | 170 NP LT (107 DBD and 63 cDCD) | 55.0 | 71.1 | / | 13.0 | Liver: 9.9 | 56.0 | 59.1 |
| 164 SCS LT (104 DBD and 60 cDCD) | 55.0 | 73.3 | / | 14.0 | Liver: 7.9 | 56.0 | 57.1 | |||
| STUDIES EVALUATING HOPE VERSUS SCS IN LT | ||||||||||
| Schlegel et al., 2019 [61] | Retrospective cohort study | 2012–2017 | 50 HOPE cDCD LT | 58.0 | * | / | 11.0 | * | 57.0 | * |
| 50 SCS cDCD LT | 57.0 | * | / | 11.8 | * | 53.0 | * | |||
| 50 SCS DBD LT | 57.0 | * | / | 15.0 | * | 50.0 | * | |||
| Czigany et al., 2021 [62] | Prospective randomized controlled trial | 2017–2020 | 23 HOPE ECD DBD LT | 60.0 | 78.0 | / | 13.0 | * | 73.0 | 52 |
| 23 SCS ECD DBD LT | 63.0 | 87.0 | / | 17.0 | * | 71.0 | 56 | |||
| Schlegel et al., 2023 [57] | Prospective randomized controlled trial | 2015–2019 | 85 HOPE DBD LT | 60.0 | 64.7 | / | 20.0 | 5.9 | 59.0 | 52.9 |
| 85 SCS DBD LT | 57.0 | 78.8 | / | 19.0 | 2.4 | 62.0 | 50.0 | |||
| STUDIES EVALUATING NRP VERSUS HOPE IN cDCD LT | ||||||||||
| Muller et al., 2020 [58] | Retrospective cohort study | 2012–2019 | 132 NRP cDCD LT | 59.5 | * | / | 12.0 | * | 50.0 | * |
| 93 HOPE cDCD LT | 59.0 | * | / | 12.0 | * | 61.0 | * | |||
| STUDIES COMPARING NRP VERSUS NP IN cDCD LT | ||||||||||
| Mohkam et al., 2022 [63] | Retrospective cohort study | 2015–2019 | 34 NP cDCD LT | 56.0 | 52.9 | / | 12.0 | * | 48.0 | 58.8 |
| 68 NRP cDCD LT | 57.0 | 85.3 | / | 12.0 | * | 49.0 | 70.6 | |||
| Gaurav et al., 2022 [59] | Retrospective cohort study | 2013–2020 | 69 NRP cDCD LT | 56.0 | 70.0 | / | 14.0 | 12.0 | 51.0 | 65.0 |
| 67 NP cDCD LT | 59.0 | 67.0 | / | 14.0 | 5.0 | 52.0 | 58.0 | |||
| 97 SCS cDCD LT | 56.0 | 58.0 | / | 16.0 | 1.0 | 50.0 | 53.0 | |||
SLKT Simultaneous Liver-Kidney Transplantations; LT: Liver Transplantation; KT: Kidney Transplantation; DBD: Donation after Brain Death; cDCD: Controlled Donation after Circulatory Death; NP: Ex-Situ Normothermic Perfusion; NRP: Normothermic Regional Perfusion; SCS: Static Cold Storage; SRR: Super-Rapid Recovery; HOPE: Ex-Situ Hypothermic Oxygenated Perfusion; ECD: Extended Criteria Donors
Table 2.
Studies evaluating cDCD SLKT and LT outcomes: intra-operative and post-operative outcomes
| Author, date | Arms (Number of recipients and donor type) | NRP use (% and minutes) | WIT (minutes) | Liver CIT (hours) | Kidney CIT (hours) | Delayed graft function (%) | Primary non function (%) | 1-year creatinine (mg/dL) | 1-year eGFR (ml/min) | Dialysis at 1-year (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| STUDIES EVALUATING cDCD VERSUS DBD IN SLKT | ||||||||||
| Nunez-Nateras et al., 2020 [45] | 30 cDCD SLKT | 0% | 22.0 | 5.3 | 8.2 | 40 |
Liver: 3.3 Kidney: 0 |
1.36 | 57.7 | 3.3 |
| 131 DBD SLKT | / | / | 5.8 | 8.2 | 23.7 |
Liver: 0.8 Kidney: 3.8 |
1.37 | 56.3 | 1.5 | |
| Croome et al., 2020 [46] | 94 cDCD SLKT (Era 1) | 0% | 17.1 | 6.8 | 11.5 | * | * | * | * | * |
| 208 cDCD SLKT (Era 2) | 0% | 17.0 | 6.4 | 10.3 | * | * | * | * | * | |
| 624 DBD SLKT (propensity matched) (Era 2) | / | / | 6.4 | 11.4 | * | * | * | * | * | |
| LaMattina et al., 2011 [47] | 5 cDCD SLKT | 0% | 19.0 | 4.6 | 12.5 | 80.0 |
Liver: 0 Kidney: 0 |
* | * | * |
| 32 DBD SLKT | / | / | 8.0 | 12.5 | 31.0 |
Liver: 6.3 Kidney: 3.1 |
* | * | * | |
| STUDIES EVALUATING NRP VERSUS SRR IN cDCD LT | ||||||||||
| Hessheimer et al., 2019 [60] | 95 NRP cDCD LT |
100% 120 min |
19.2 | 5.6 | / | / | 2.0 | * | * | * |
| 117 SRR cDCD LT | 0% | 23.1 | 5.7 | / | / | 3.0 | * | * | * | |
| Watson et al., 2019 [51] | 43 NRP cDCD LT |
100% 123 min |
30.0 | 6.4 | / | / | 0 | * | 72 | * |
| 187 SRR cDCD LT | 0% | 27.0 | 7.4 | / | / | 7.0 | * | 70 | * | |
| Hessheimer et al., 2022 [50] | 545 NRP cDCD LT |
100% 111 min |
12.0 | 5.3 | / | / | 3.0 | * | * | * |
| 258 SRR cDCD LT | 0% | 14.0 | 5.6 | / | / | 6.0 | ** | * | ||
| STUDIES EVALUATING NRP cDCD VERSUS DBD IN LT | ||||||||||
| Savier et al., 2020 [52] | 50 NRP cDCD LT |
100% 190 min |
* | 5.8 | / | / | * | * | * | * |
| 100 DBD LT | / | / | 6.3 | / | / | * | * | * | * | |
| STUDIES EVALUATING NP VERSUS SCS IN LT | ||||||||||
| Nasralla et al., 2018 [56] | 170 NP LT (107 DBD and 63 cDCD) | 0% | 21.0 | 2.1 | / | / | 0.8 | * | * | * |
| 164 SCS LT (104 DBD and 60 cDCD) | 0% | 16.0 | 7.8 | / | / | 0 | * | * | * | |
| STUDIES EVALUATING HOPE VERSUS SCS IN LT | ||||||||||
| Schlegel et al., 2019 [61] | 50 HOPE cDCD LT | 0% | 31.0 | 4.4 | / | / | 0 | * | * | * |
| 50 SCS cDCD LT | 0% | 17.0 | 4.7 | / | / | 4.0 | * | * | * | |
| 50 SCS DBD LT | / | / | 5.0 | / | / | 2.0 | * | * | * | |
| Czigany et al., 2021 [62] | 23 HOPE ECD DBD LT | / | / | 8.3 | / | / | 4.0 | * | * | * |
| 23 SCS ECD DBD LT | / | / | 8.4 | / | / | 4.0 | * | * | * | |
| Schlegel et al., 2023 [57] | 85 HOPE DBD LT | / | / | 6.2 | / | / | 0 | * | * | * |
| 85 SCS DBD LT | / | / | 7.1 | / | / | 3.5 | * | * | * | |
| STUDIES EVALUATING NRP VERSUS HOPE IN cDCD LT | ||||||||||
| Muller et al., 2020 [58] | 132 NRP cDCD LT |
100% 184 min |
22.0 | 5.7 | / | / | 2.3 | * | * | * |
| 93 HOPE cDCD LT | 0% | 31.0 | 6.4 | / | / | 4.3 | * | * | * | |
| STUDIES COMPARING NRP VERSUS NP IN cDCD LT | ||||||||||
| Mohkam et al., 2022 [63] | 34 NP cDCD LT | 0% | 20.0 | 2.3 | / | / | 0 | * | * | * |
| 68 NRP cDCD LT |
100% 184 min |
21.0 | 5.8 | / | / | 1.5 | * | * | * | |
| Gaurav et al., 2022 [59] | 69 NRP cDCD LT |
100% 133 min |
19.0 | 6.7 | / | / | 0 | * | * | * |
| 67 NP cDCD LT | 0% | 15.0 | 6.6 | / | / | 1.5 | * | * | * | |
| 97 SCS cDCD LT | 0% | 15.0 | 7.2 | / | / | 5.2 | * | * | * | |
SLKT Simultaneous Liver-Kidney Transplantations; LT: Liver Transplantation; KT: Kidney Transplantation; DBD: Donation after Brain Death; cDCD: Controlled Donation after Circulatory Death; NP: Ex-Situ Normothermic Perfusion; NRP: Normothermic Regional Perfusion; SCS: Static Cold Storage; SRR: Super-Rapid Recovery; HOPE: Ex-Situ Hypothermic Oxygenated Perfusion; ECD: Extended Criteria Donors
Table 3.
Studies evaluating cDCD SLKT and LT outcomes: patient and graft survival
| Author, date | Arms (Number of recipients and donor type) | 1-year patient survival (%) | 1-year liver allograft survival (%) | 1-year kidney allograft survival (%) | 3-years patient survival (%) | 3-years liver allograft survival (%) | 3-years kidney allograft survival (%) |
|---|---|---|---|---|---|---|---|
| STUDIES EVALUATING cDCD VERSUS DBD IN SLKT | |||||||
| Nunez-Nateras et al., 2020 [45] | 30 cDCD SLKT | 96.7 | 93.3 | 93.3 | 93.3 | 90.0 | 90.0 |
| 131 DBD SLKT | 95.4 | 93.1 | 93.1 | 93.3 | 90.1 | 90.1 | |
| Croome et al., 2020 [46] | 94 cDCD SLKT (Era 1) | 72.9 | 69.2 | 67.4 | 64.8 | 59.5 | 58.4 |
| 208 cDCD SLKT (Era 2) | 87.6 | 83.1 | 86.5 | 82.4 | 76.6 | 79.4 | |
| 624 DBD SLKT (propensity matched) (Era 2) | 91.3 | 90.4 | 88.6 | 83.4 | 82.3 | 81.5 | |
| LaMattina et al., 2011 [47] | 5 cDCD SLKT | 100 | 100 | 100 | * | * | * |
| 32 DBD SLKT | 97 | 94 | 94 | * | * | * | |
| STUDIES EVALUATING NRP VERSUS SRR IN cDCD LT | |||||||
| Hessheimer et al., 2019 [60] | 95 NRP cDCD LT | 93 | 88 | / | 93 | 88 | / |
| 117 SRR cDCD LT | 88 | 83 | / | 84 | 76 | / | |
| Watson et al., 2019 [51] | 43 NRP cDCD LT | 97 | 98 | / | 94 | 93 | / |
| 187 SRR cDCD LT | 95 | 87 | / | 93 | 85 | / | |
| Hessheimer et al., 2022 [50] | 545 NRP cDCD LT | 92 | 90 | / | 89 | 87 | / |
| 258 SRR cDCD LT | 86 | 79 | / | 76 | 68 | / | |
| STUDIES EVALUATING NRP cDCD VERSUS DBD IN LT | |||||||
| Savier et al., 2020 [52] | 50 NRP cDCD LT | 97 | 95 | / | 83 | 80 | / |
| 100 DBD LT | 90 | 88 | / | 84 | 82 | / | |
| STUDIES EVALUATING NP VERSUS SCS IN LT | |||||||
| Nasralla et al., 2018 [56] | 170 NP LT (107 DBD and 63 cDCD) | 96 | 95 | / | * | * | / |
| 164 SCS LT (104 DBD and 60 cDCD) | 97 | 96 | / | * | * | / | |
| STUDIES EVALUATING HOPE VERSUS SCS IN LT | |||||||
| Schlegel et al., 2019 [61] | 50 HOPE cDCD LT | 98 | 92 | / | 94 | 92 | / |
| 50 SCS cDCD LT | 90 | 88 | / | 88 | 80 | / | |
| 50 SCS DBD LT | 92 | 88 | / | 92 | 88 | / | |
| Czigany et al., 2021 [62] | 23 HOPE ECD DBD LT | 91 | 91 | / | * | * | / |
| 23 SCS ECD DBD LT | 83 | 78 | / | * | * | / | |
| Schlegel et al., 2023 [57] | 85 HOPE DBD LT | 95.3 | 95.3 | / | * | * | / |
| 85 SCS DBD LT | 95.3 | 91.8 | / | * | * | / | |
| STUDIES EVALUATING NRP VERSUS HOPE IN cDCD LT | |||||||
| Muller et al., 2020 [58] | 132 NRP cDCD LT | 95 | 93 | / | 92 | 88 | / |
| 93 HOPE cDCD LT | 93 | 86 | / | 90 | 83 | / | |
| STUDIES COMPARING NRP VERSUS NP IN cDCD LT | |||||||
| Mohkam et al., 2022 [63] | 34 NP cDCD LT | 94.1 | 88.2 | / | * | * | / |
| 68 NRP cDCD LT | 98.5 | 93.9 | / | * | * | / | |
| Gaurav et al., 2022 [59] | 69 NRP cDCD LT | 94.0 | 93.0 | / | 94.0 | 90.0 | / |
| 67 NP cDCD LT | 94.0 | 88.0 | / | 90.0 | 76.0 | / | |
| 97 SCS cDCD LT | 94.0 | 84.0 | / | 88.0 | 76.0 | / | |
SLKT Simultaneous Liver-Kidney Transplantations; LT: Liver Transplantation; KT: Kidney Transplantation; DBD: Donation after Brain Death; cDCD: Controlled Donation after Circulatory Death; NP: Ex-Situ Normothermic Perfusion; NRP: Normothermic Regional Perfusion; SCS: Static Cold Storage; SRR: Super-Rapid Recovery; HOPE: Ex-Situ Hypothermic Oxygenated Perfusion; ECD: Extended Criteria Donors
Nunez-Nateras et al. [45] compared outcomes of DBD and cDCD SLKT. From January 2010 to December 2018, they included 30 cDCD SLKT and 131 DBD SLKT, from 2 US centers. Median warm ischemia time (WIT) was 24 min in the cDCD SLKT cohorts. They reported similar kidney DGF rates, similar 1-year patient survival (96.7% vs. 95.4% in cDCD and DBD), similar 1-year liver allograft survival (93.3% vs. 93.1%) and similar 1-year kidney allograft survival (93.3% vs. 93.1%). In their recent study, from UNOS database, Croome et al. [46] compared outcomes of cDCD SLKT performed between 2000 and 2010 and 2011–2018 and confirmed outcomes reported by Nunez-Nateras et al. [45]. Indeed, they reported better outcomes in 2011–2018 period: higher 3-years patient survival, higher 3-years liver allograft survival and higher 3-years kidney allograft survival. LaMattina et al. [47] reported the University of Wisconsin experience on cDCD SLKT and compared outcomes with DBD SLKT. From January 1998 to December 2008, they included 5 cDCD SLKT and 32 DBD SLKT. Median WIT was 19.0 min in cDCD SLKT group. They reported a non-significant higher kidney DGF rate in cDCD SLKT group and similar 1-year kidney and liver allograft survival (Kidney and Liver: 100% versus 94% in cDCD and DBD).
In-situ and ex-situ perfusion strategies to improve organ preservation in LT. Application for SLKT?
Main studies comparing in-situ and ex-situ perfusion strategies in LT are summarized in Tables 1, 2 and 3.
Normothermic regional perfusion (NRP), developed from extracorporeal membranous oxygenation (ECMO), was described by Johnson LB et al. in 1997 [48]. This in-situ normothermic perfusion allows to restore metabolic function by restoration of cellular energy substrates and allow to assess the suitability of the organs [49].
Recently, Hessheimer et al. [50] compared outcomes of Maastricht III cDCD liver transplantations performed with postmortem in-situ NRP versus super rapid recovery (SRR). They included 545 cDCD LT with in-situ NRP and 258 with SRR, from June 2012 and December 2019. They reported that NRP was a protective factor for ischaemic biliary complications and graft loss. Watson et al. [51] reported similar outcomes. They compared outcomes of Maastricht III cDCD liver transplantations performed with postmortem in-situ NRP versus SRR. From January 2011 to June 2017, they included 43 cDCD LT with in-situ NRP and 187 with SRR, in all UK centers. Median NRP duration was 123 min. They reported that NRP was associated with a reduction in 30-day graft loss, ischaemic biliary complications and anastomotic strictures.
In France, Savier et al. [52] compared outcomes of cDCD LT with in-situ NRP and DBD LT. They included 50 cDCD with in-situ NRP and 100 DBD. They reported similar arterial complications, biliary complications, 2-years graft survival and 2-years patient survival.
Moreover, ex-situ preservation is of paramount importance in the context of cDCD SLKT. Since the development of hypothermic machine perfusion by Alexis Carrel over a century ago [53], this preservation method has grown in its utility, initially in kidney transplantation. Indeed, many studies have demonstrated the benefit of HMP in kidney transplantation, particularly for its significant reduction of the incidence of delayed graft function for DCD and ECD [54].
In liver transplantation, in order to limit the negative effects of WIT, 2 main dynamic preservation strategies have been developed in clinical practice: ex-situ normothermic perfusion (NP) and ex-situ hypothermic oxygenated perfusion (HOPE) [55].
In 2018, Nasralla et al. [56] reported outcomes of the first randomized trial comparing conventional static cold storage (SCS) and ex-situ NP for liver transplantation. They included 170 NP liver transplantation and 164 SCS liver transplantation. The proportion of DBD were 62.9% and 63.4% in NP and SCS groups. They reported a significant decrease on peak AST (primary outcomes) of 49.4% and a significant decrease of early allograft dysfunction of 74% in NP group. They reported similar biliary complications, graft and patient survival between NP and SCS groups.
Recently, Schlegel et al. [57] compared outcomes of DBD LT with ex-situ HOPE and DBD LT with conventional SCS. From 2015 to 2019, they included 85 DBD LT with ex-situ HOPE and 85 DBD LT with conventional SCS. They reported similar major post-operative complications (Clavien ≥ III).
Currently, no study compared ex-situ normothermic perfusion (NP) and ex-situ hypothermic oxygenated perfusion (HOPE) for LT.
Muller et al. [58] compared outcomes of cDCD LT after in-situ NRP and ex-situ HOPE. They included 132 cDCD LT with in-situ NRP and 93 cDCD LT with ex-situ HOPE. They reported similar biliary complications rates, arterial thrombosis rates, primary non-function rates, 1-year graft and patient survival.
Recently, Gaurav et al. [59] reported outcomes of cDCD after in-situ NRP, cDCD after ex-situ NP and cDCD after conventional SCS. They reported a better early liver function using in-situ NRP and ex-situ NP, compared to conventional SCS.
Discussion
Some limitations of this narrative review should be mentioned. Firstly, the main limitation is the low level of included studies, the heterogeneity of evaluation between studies and the paucity of data. Indeed, several data were poorly reported, in particular mid-term patient survival and graft survival in studies evaluating DBD and DCD and in-situ and ex-situ preservation strategies.
Conclusion
Simultaneous liver-kidney transplantation has made a significant contribution for patients with dual-organ disease. The optimization of indication and selection of SLKT patients will reduce futile transplantation. Moreover, increasing the use of transplants from extended criteria donors, in particular cDCD, should be encouraged, thanks to the development of new modalities of organ preservation.
Author contributions
Thomas PRUDHOMME: Protocol development, Data collection, Data analysis, Manuscript writing Benoit MESNARD: Protocol development, Data collection, Data analysis, Manuscript writing Julien BRANCHEREAU: Protocol development, Manuscript writing Mathieu ROUMIGUIÉ: Data analysis, Manuscript editing Charlotte MAULAT: Data analysis, Manuscript editing Fabrice MUSCARI: Data analysis, Manuscript editing Nassim KAMAR: Data analysis, Manuscript editing Michel SOULIÉ: Data analysis, Manuscript editing Xavier GAMÉ: Data analysis, Manuscript editing Federico SALLUSTO: Data analysis, Manuscript editing Marc Olivier TIMSIT: Protocol development, Data collection, Data analysis, Manuscript editing Sarah DROUIN: Protocol development, Data collection, Data analysis, Manuscript writing and editing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- CNI
Calcineurin Inhibitors
- DBD
Donation after Brain Death
- DCD
Donation after Circulatory Death
- DGF
Delayed Graft Function
- DSA
Donor-Specific Anti-HLA Antibodies
- eGFR
Estimated Glomerular Filtration Rate
- ECD
Extended Criteria Donors
- ESLD
End-Stage Liver Disease
- ESRD
End-Stage Renal Disease
- HCV
Hepatitis C Virus
- HMP
Hypothermic Machine Perfusion
- HOPE
Ex-Situ Hypothermic Oxygenated Perfusion
- HRS
Hepatorenal Syndrome
- KALT
Kidney After Liver Transplantation
- KT
Kidney Transplantation
- LT
Liver Transplantation
- MELD
Model for End-Stage Liver Disease
- NP
Ex-Situ Normothermic Perfusion
- NRP
Normothermic Regional Perfusion
- OPTN
Organ Procurement and Transplantation Network
- PNF
Primary Non-Function
- SCS
Static Cold Storage
- SLKT
Simultaneous Liver-Kidney Transplantations
- SRR
Super-Rapid Recovery
- UNOS
United Network for Organ Sharing
- WIT
Warm Ischemia Time
Funding
Open access funding provided by Université Toulouse III - Paul Sabatier.
Declarations
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent
Patients have given prior consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saxena V, Lai JC (2015) Kidney failure and liver allocation: current practices and potential improvements. Adv Chronic Kidney Dis 22(5):391–398. 10.1053/j.ackd.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minnee RC, Darwish Murad S, Polak WG, Metselaar HJ (2019) Combined liver-kidney transplantation: two for the price of one? Transpl Int 32(9):913–915. 10.1111/tri.13438 [DOI] [PubMed] [Google Scholar]
- 3.Stepanova M, Wai H, Saab S, Mishra A, Venkatesan C, Younossi ZM (2015) The outcomes of adult liver transplants in the United States from 1987 to 2013. Liver Int 35(8):2036–2041. 10.1111/liv.12779 [DOI] [PubMed] [Google Scholar]
- 4.Bahirwani R, Reddy KR (2009) Outcomes after liver transplantation: chronic kidney disease. Liver Transpl 15(Suppl 2):S70–74. 10.1002/lt.21900 [DOI] [PubMed] [Google Scholar]
- 5.Nair S, Verma S, Thuluvath PJ (2002) Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology 35(5):1179–1185. 10.1053/jhep.2002.33160 [DOI] [PubMed] [Google Scholar]
- 6.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM (2003) Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 349(10):931–940. 10.1056/NEJMoa021744 [DOI] [PubMed] [Google Scholar]
- 7.Margreiter R, Kramar R, Huber C, Steiner E, Niederwieser D, Judmaier G, Vogel W (1984) Combined liver and kidney transplantation. Lancet 1(8385):1077–1078. 10.1016/s0140-6736(84)91486-7 [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Shu X, Schaubel DE, Sung RS, Magee JC (2016) Propensity score-based survival benefit of simultaneous liver-kidney transplant over liver transplant alone for recipients with pretransplant renal dysfunction. Liver Transpl 22(1):71–79. 10.1002/lt.24189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AK, Salameh H, Kuo YF, Wiesner RH (2014) Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation 98(2):216–221. 10.1097/TP.0000000000000048 [DOI] [PubMed] [Google Scholar]
- 10.Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N (2006) Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transpl 6(11):2651–2659. 10.1111/j.1600-6143.2006.01526.x [DOI] [PubMed] [Google Scholar]
- 11.Formica RN, Aeder M, Boyle G, Kucheryavaya A, Stewart D, Hirose R, Mulligan D (2016) Simultaneous liver-kidney allocation policy: a proposal to optimize appropriate utilization of Scarce resources. Am J Transpl 16(3):758–766. 10.1111/ajt.13631 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chava SP, Singh B, Zaman MB, Rela M, Heaton ND (2009) Current indications for combined liver and kidney transplantation in adults. Transpl Rev (Orlando) 23(2):111–119. 10.1016/j.trre.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 14.Asch WS, Bia MJ (2017) New Organ Allocation System for Combined liver-kidney transplants and the availability of kidneys for transplant to patients with stage 4–5 CKD. Clin J Am Soc Nephrol 12(5):848–852. 10.2215/CJN.08480816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singal AK, Ong S, Satapathy SK, Kamath PS, Wiesner RH (2019) Simultaneous liver kidney transplantation. Transpl Int 32(4):343–352. 10.1111/tri.13388 [DOI] [PubMed] [Google Scholar]
- 16.Buccheri S, Da BL (2022) Hepatorenal Syndrome: definitions, diagnosis, and management. Clin Liver Dis 26(2):181–201. 10.1016/j.cld.2022.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Koppel MH, Coburn JW, Mims MM, Goldstein H, Boyle JD, Rubini ME (1969) Transplantation of cadaveric kidneys from patients with hepatorenal syndrome. Evidence for the functionalnature of renal failure in advanced liver disease. N Engl J Med 280(25):1367–1371. 10.1056/NEJM196906192802501 [DOI] [PubMed] [Google Scholar]
- 18.Iwatsuki S, Popovtzer MM, Corman JL, Ishikawa M, Putnam CW, Katz FH, Starzl TE (1973) Recovery from hepatorenal syndrome after orthotopic liver transplantation. N Engl J Med 289(22):1155–1159. 10.1056/NEJM197311292892201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Leary JG, Levitsky J, Wong F, Nadim MK, Charlton M, Kim WR (2016) Protecting the kidney in liver transplant candidates: practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transpl 16(9):2516–2531. 10.1111/ajt.13790 [DOI] [PubMed] [Google Scholar]
- 20.Nair G, Nair V (2022) Simultaneous liver-kidney transplantation. Clin Liver Dis 26(2):313–322. 10.1016/j.cld.2022.01.011 [DOI] [PubMed] [Google Scholar]
- 21.Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, Feng S, Friedewald JJ, Hong JC, Kellum JA, Kim WR, Lake JR, Melton LB, Pomfret EA, Saab S, Genyk YS (2012) Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transpl 12(11):2901–2908. 10.1111/j.1600-6143.2012.04190.x [DOI] [PubMed] [Google Scholar]
- 22.OPTN/SRTR (2019) Annual Data Report: Liver. https://srtr.transplant.hrsa.gov/annual_reports/2019/Liver.aspx#LI_tx_slk_b64
- 23.Rapport d’activité (2019) de transplantation de l’Agence de Biomédecine https://rams.agence-biomedecine.fr/sites/default/files/pdf/2020-09/RAMS%202019%20ORGANES%20Rein.pdf
- 24.Guide du score Foie https://www.agence-biomedecine.fr/IMG/pdf/guide_score_foie_v3.pdf
- 25.Tinti F, Mitterhofer AP, Umbro I, Nightingale P, Inston N, Ghallab M, Ferguson J, Mirza DF, Ball S, Lipkin G, Muiesan P, Perera M (2019) Combined liver-kidney transplantation versus liver transplant alone based on KDIGO stratification of estimated glomerular filtration rate: data from the United Kingdom Transplant registry - a retrospective cohort study. Transpl Int 32(9):918–932. 10.1111/tri.13413 [DOI] [PubMed] [Google Scholar]
- 26.Aguilera V, Ferrer I, Berenguer M, Rivera J, Rubin A, Moya A, Pareja E, Sanchez J, Prieto M, Mir J (2013) Comparison of results of combined liver-kidney transplantation vs. isolated liver transplantation. Ann Hepatol 12(2):274–281 [PubMed] [Google Scholar]
- 27.Hibi T, Sageshima J, Molina E, Ciancio G, Nishida S, Chen L, Arosemena L, Mattiazzi A, Guerra G, Kupin W, Tekin A, Selvaggi G, Levi D, Ruiz P, Livingstone AS, Roth D, Martin P, Tzakis A, Burke GW (2012) Predisposing factors of diminished survival in simultaneous liver/kidney transplantation. Am J Transpl 12(11):2966–2973. 10.1111/j.1600-6143.2012.04121.x [DOI] [PubMed] [Google Scholar]
- 28.Hmoud B, Kuo YF, Wiesner RH, Singal AK (2015) Outcomes of liver transplantation alone after listing for simultaneous kidney: comparison to simultaneous liver kidney transplantation. Transplantation 99(4):823–828. 10.1097/TP.0000000000000438 [DOI] [PubMed] [Google Scholar]
- 29.Fong TL, Khemichian S, Shah T, Hutchinson IV, Cho YW (2012) Combined liver-kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplantation 94(4):411–416. 10.1097/TP.0b013e3182590d6b [DOI] [PubMed] [Google Scholar]
- 30.Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R (2012) Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl 18(5):539–548. 10.1002/lt.23384 [DOI] [PubMed] [Google Scholar]
- 31.Fong TL, Bunnapradist S, Jordan SC, Selby RR, Cho YW (2003) Analysis of the United Network for Organ Sharing database comparing renal allografts and patient survival in combined liver-kidney transplantation with the contralateral allografts in kidney alone or kidney-pancreas transplantation. Transplantation 76(2):348–353. 10.1097/01.TP.0000071204.03720.BB [DOI] [PubMed] [Google Scholar]
- 32.Simpson N, Cho YW, Cicciarelli JC, Selby RR, Fong TL (2006) Comparison of renal allograft outcomes in combined liver-kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS Database. Transplantation 82(10):1298–1303. 10.1097/01.tp.0000241104.58576.e6 [DOI] [PubMed] [Google Scholar]
- 33.Fung J, Makowka L, Tzakis A, Klintmalm G, Duquesnoy R, Gordon R, Todo S, Griffin M, Starzl T (1988) Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transpl Proc 20(1 Suppl 1):88–91 [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY (1995) Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation 59(6):919–921 [PubMed] [Google Scholar]
- 35.Zhang X, Wisel SA, Haas M, Kim I, Jordan S (2022) Both donor specific and non-donor specific HLA antibodies reduced in recipients post simultaneous liver/kidney transplant. Transpl Immunol 75:101744. 10.1016/j.trim.2022.101744 [DOI] [PubMed] [Google Scholar]
- 36.Cholbi E, Espi J, Ventura A, Ramos D, Ramos M, Luis M, Moreno E, Moreno M, Beneyto I, Hernandez J (2022) Combined liver-kidney transplantation in high immunologic risk recipients: kidney graft evolution. Transpl Proc 54(9):2475–2478. 10.1016/j.transproceed.2022.10.007 [DOI] [PubMed] [Google Scholar]
- 37.Sumimoto R, Kamada N (1990) Specific suppression of allograft rejection by soluble class I antigen and complexes with monoclonal antibody. Transplantation 50(4):678–682. 10.1097/00007890-199010000-00029 [DOI] [PubMed] [Google Scholar]
- 38.Habib S, Khan K, Hsu CH, Meister E, Rana A, Boyer T (2017) Differential Simultaneous liver and kidney transplant benefit based on severity of liver damage at the Time of Transplantation. Gastroenterol Res 10(2):106–115. 10.14740/gr803w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekser B, Mangus RS, Fridell W, Kubal CA, Nagai S, Kinsella SB, Bayt DR, Bell TM, Powelson JA, Goggins WC, Tector AJ (2017) A Novel Approach in Combined liver and kidney transplantation with long-term outcomes. Ann Surg 265(5):1000–1008. 10.1097/SLA.0000000000001752 [DOI] [PubMed] [Google Scholar]
- 40.Lunsford KE, Agopian VG, Yi SG, Nguyen DTM, Graviss EA, Harlander-Locke MP, Saharia A, Kaldas FM, Mobley CM, Zarrinpar A, Hobeika MJ, Veale JL, Podder H, Farmer DG, Knight RJ, Danovitch GM, Gritsch HA, Li XC, Ghobrial RM, Busuttil RW, Gaber AO (2020) Delayed implantation of pumped kidneys decreases renal allograft futility in combined liver-kidney transplantation. Transplantation 104(8):1591–1603. 10.1097/TP.0000000000003040 [DOI] [PubMed] [Google Scholar]
- 41.Mangus RS, Lutz AJ, Fridell JA, Kubal CA, Bush WJ, Tector AJ (2015) Minimal improvement in glomerular filtration rate in the First Year after Liver Transplantation. Transplantation 99(9):1855–1861. 10.1097/TP.0000000000000668 [DOI] [PubMed] [Google Scholar]
- 42.Ekser B, Mangus RS, Kubal CA, Powelson JA, Fridell JA, Goggins WC (2018) Excellent outcomes in combined liver-kidney transplantation: impact of kidney donor profile index and delayed kidney transplantation. Liver Transpl 24(2):222–232. 10.1002/lt.24946 [DOI] [PubMed] [Google Scholar]
- 43.Chang A, Schaubel DE, Chen M, Abt PL, Bittermann T (2022) Trends and Outcomes of Hypothermic Machine Perfusion Preservation of Kidney Allografts in simultaneous liver and kidney transplantation in the United States. Transpl Int 35:10345. 10.3389/ti.2022.10345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullaro G, Verna EC, Emond JC, Orandi BJ, Mohan S, Lai JC (2021) Early kidney allograft failure after simultaneous liver-kidney transplantation: evidence for utilization of the Safety Net? Transplantation 105(4):816–823. 10.1097/TP.0000000000003310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nunez-Nateras R, Reddy KS, Aqel BA, Heilman R, Morgan P, Mathur AK, Hewitt W, Heimbach J, Rosen C, Moss AA, Taner T, Jadlowiec CC (2020) Simultaneous liver-kidney transplantation from donation after cardiac death donors: an updated perspective. Am J Transpl 20(12):3582–3589. 10.1111/ajt.16191 [DOI] [PubMed] [Google Scholar]
- 46.Croome KP, Mao S, Yang L, Pungpapong S, Wadei HM, Taner CB (2020) Improved National results with simultaneous liver-kidney transplantation using donation after circulatory death donors. Liver Transpl 26(3):397–407. 10.1002/lt.25653 [DOI] [PubMed] [Google Scholar]
- 47.LaMattina JC, Mezrich JD, Fernandez LA, D’Alessandro AM, Bellingham JM, Musat AI, Foley DP (2011) Simultaneous liver and kidney transplantation using donation after cardiac death donors: a brief report. Liver Transpl 17(5):591–595. 10.1002/lt.22264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson LB, Plotkin JS, Howell CD, Njoku MJ, Kuo PC, Bartlett ST (1997) Successful emergency transplantation of a liver allograft from a donor maintained on extracorporeal membrane oxygenation. Transplantation 63(6):910–911 [DOI] [PubMed] [Google Scholar]
- 49.Sutherland AI, Oniscu GC (2016) Challenges and advances in optimizing liver allografts from donation after circulatory death donors. J Nat Sci Biol Med 7(1):10–15. 10.4103/0976-9668.175017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hessheimer AJ, de la Rosa G, Gastaca M, Ruiz P, Otero A, Gomez M, Alconchel F, Ramirez P, Bosca A, Lopez-Andujar R, Atutxa L, Royo-Villanova M, Sanchez B, Santoyo J, Marin LM, Gomez-Bravo MA, Mosteiro F, Villegas Herrera MT, Villar Del Moral, Gonzalez-Abos J, Vidal C, Lopez-Dominguez B, Llado J, Roldan L, Justo J, Jimenez I, Lopez-Monclus C, Sanchez-Turrion J, Rodriguez-Laiz V, Velasco Sanchez G, Lopez-Baena E, Caralt JA, Charco M, Tome R, Varo S, Marti-Cruchaga E, Rotellar P, Varona F, Barrera MA, Rodriguez-Sanjuan M, Briceno JC, Lopez J, Blanco D, Nuno G, Pacheco J, Coll D, Dominguez-Gil E, Fondevila B (2022) C Abdominal normothermic regional perfusion in controlled donation after circulatory determination of death liver transplantation: Outcomes and risk factors for graft loss. Am J Transplant 22 (4):1169–1181. 10.1111/ajt.16899 [DOI] [PubMed]
- 51.Watson CJE, Hunt F, Messer S, Currie I, Large S, Sutherland A, Crick K, Wigmore SJ, Fear C, Cornateanu S, Randle LV, Terrace JD, Upponi S, Taylor R, Allen E, Butler AJ, Oniscu GC (2019) In situ normothermic perfusion of livers in controlled circulatory death donation may prevent ischemic cholangiopathy and improve graft survival. Am J Transpl 19(6):1745–1758. 10.1111/ajt.15241 [DOI] [PubMed] [Google Scholar]
- 52.Savier E, Lim C, Rayar M, Orlando F, Boudjema K, Mohkam K, Lesurtel M, Mabrut JY, Pittau G, Begdadi N, Cherqui D, Adam R, Dondero F, Sepulveda A, Soubrane O, Bucur P, Barbier L, Salame E, Jasseron C, Antoine C, Riou B, Scatton O (2020) Favorable outcomes of liver transplantation from controlled circulatory death donors using Normothermic Regional Perfusion compared to Brain Death Donors. Transplantation 104(9):1943–1951. 10.1097/TP.0000000000003372 [DOI] [PubMed] [Google Scholar]
- 53.Sade RM (2005) Transplantation at 100 years: Alexis Carrel, pioneer surgeon. Ann Thorac Surg 80(6):2415–2418. 10.1016/j.athoracsur.2005.08.074 [DOI] [PubMed] [Google Scholar]
- 54.Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, van Kasterop-Kutz M, van der Heide JJ, Squifflet JP, van Heurn E, Kirste GR, Rahmel A, Leuvenink HG, Paul A, Pirenne J, Ploeg RJ (2009) Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 360(1):7–19. 10.1056/NEJMoa0802289 [DOI] [PubMed] [Google Scholar]
- 55.Monbaliu D, Pirenne J, Talbot D (2012) Liver transplantation using donation after Cardiac Death donors. J Hepatol 56(2):474–485. 10.1016/j.jhep.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 56.Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ, Garcia-Valdecasas JC, Heaton N, Imber C, Jassem W, Jochmans I, Karani J, Knight SR, Kocabayoglu P, Malago M, Mirza D, Morris PJ, Pallan A, Paul A, Pavel M, Perera M, Pirenne J, Ravikumar R, Russell L, Upponi S, Watson CJE, Weissenbacher A, Ploeg RJ, Friend PJ, Consortium for Organ Preservation in E (2018) A randomized trial of normothermic preservation in liver transplantation. Nature 557(7703):50–56. 10.1038/s41586-018-0047-9 [DOI] [PubMed] [Google Scholar]
- 57.Schlegel A, Mueller M, Muller X, Eden J, Panconesi R, von Felten S, Steigmiller K, Sousa Da Silva RX, de Rougemont O, Mabrut JY, Lesurtel M, Cerisuelo MC, Heaton ND, Allard MA, Adam R, Monbaliu D, Jochmans I, Haring MPD, Porte RJ, Parente A, Muiesan P, Kron P, Attia M, Kollmann D, Berlakovich G, Rogiers X, Petterson K, Kranich AL, Amberg S, Mullhaupt B, Clavien PA, Dutkowski P (2023) A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J Hepatol. 10.1016/j.jhep.2022.12.030 [DOI] [PubMed] [Google Scholar]
- 58.Muller X, Mohkam K, Mueller M, Schlegel A, Dondero F, Sepulveda A, Savier E, Scatton O, Bucur P, Salame E, Jeddou H, Sulpice L, Pittau G, Allard MA, Mabrut JY, Dutkowski P, Clavien PA, Lesurtel M (2020) Hypothermic oxygenated perfusion Versus Normothermic Regional Perfusion in Liver Transplantation from controlled donation after circulatory death: First International Comparative Study. Ann Surg 272(5):751–758. 10.1097/SLA.0000000000004268 [DOI] [PubMed] [Google Scholar]
- 59.Gaurav R, Butler AJ, Kosmoliaptsis V, Mumford L, Fear C, Swift L, Fedotovs A, Upponi S, Khwaja S, Richards J, Allison M, Watson CJE (2022) Liver transplantation outcomes from controlled circulatory death donors: SCS vs in situ NRP vs ex situ NMP. Ann Surg 275(6):1156–1164. 10.1097/SLA.0000000000005428 [DOI] [PubMed] [Google Scholar]
- 60.Hessheimer AJ, Coll E, Torres F, Ruiz P, Gastaca M, Rivas JI, Gomez M, Sanchez B, Santoyo J, Ramirez P, Parrilla P, Marin LM, Gomez-Bravo MA, Garcia-Valdecasas JC, Lopez-Monclus J, Bosca A, Lopez-Andujar R, Fundora-Suarez J, Villar J, Garcia-Sesma A, Jimenez C, Rodriguez-Laiz G, Llado L, Rodriguez JC, Barrera M, Charco R, Lopez-Baena JA, Briceno J, Pardo F, Blanco G, Pacheco D, Dominguez-Gil B, Sanchez Turrion V, Fondevila C (2019) Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol 70(4):658–665. 10.1016/j.jhep.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 61.Schlegel A, Muller X, Kalisvaart M, Muellhaupt B, Perera M, Isaac JR, Clavien PA, Muiesan P, Dutkowski P (2019) Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol 70(1):50–57. 10.1016/j.jhep.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 62.Czigany Z, Pratschke J, Fronek J, Guba M, Schoning W, Raptis DA, Andrassy J, Kramer M, Strnad P, Tolba RH, Liu W, Keller T, Miller H, Pavicevic S, Uluk D, Kocik M, Lurje I, Trautwein C, Mehrabi A, Popescu I, Vondran FWR, Ju C, Tacke F, Neumann UP, Lurje G (2021) Hypothermic oxygenated machine perfusion reduces early allograft Injury and improves post-transplant outcomes in Extended Criteria Donation Liver Transplantation from Donation after Brain Death: results from a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann Surg 274(5):705–712. 10.1097/SLA.0000000000005110 [DOI] [PubMed] [Google Scholar]
- 63.Mohkam K, Nasralla D, Mergental H, Muller X, Butler A, Jassem W, Imber C, Monbaliu D, Perera M, Laing RW, Garcia-Valdecasas JC, Paul A, Dondero F, Cauchy F, Savier E, Scatton O, Robin F, Sulpice L, Bucur P, Salame E, Pittau G, Allard MA, Pradat P, Rossignol G, Mabrut JY, Ploeg RJ, Friend PJ, Mirza DF, Lesurtel M Consortium for Organ Preservation in E (2022) in situ normothermic regional perfusion versus ex situ normothermic machine perfusion in liver transplantation from donation after circulatory death. Liver Transpl 28 (11):1716–1725. 10.1002/lt.26522 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.