Abstract
Background
Children treated in a pediatric intensive care unit (PICU) often receive several drugs together, among them drugs defined as high-alert medications (HAMs). Those drugs carry a high risk of causing patient harm, for example, due to a higher potential for interactions. HAMs should therefore be administered with caution, especially in a PICU.
Objectives
The objective of the current study was to identify drug–drug interactions involving HAMs that increase the risk of interaction-associated symptoms in pediatric intensive care.
Methods
In a retrospective study, we analyzed the electronic documentation of patients hospitalized for at least 48 h in a general PICU who received at least two different drugs within a 24-h interval. We assessed potential drug–drug interactions involving HAM on the basis of the two drug information databases UpToDate and drugs.com. Furthermore, we analyzed whether symptoms were observed after the administration of drug pairs that could lead to interaction-associated symptoms. For drug pairs involving HAM administered on at least 2% of patient days, and symptoms observed at least ten times after a respective drug pair, we calculated odds ratios, 95% confidence intervals, and p-values by using a univariate binary logistic regression.
Results
Among 315 analyzed patients, 81.3% (256/315) received drugs defined as high-alert medication for pediatric patients. Those high-alert medications were involved in 20,150 potential drug–drug interactions. In 14.0% (2830/20,150) of these, one or more symptoms were observed that could be a possible consequence of the interaction, resulting in 3203 observed symptoms affecting 56.3% (144/256) of patients receiving high-alert medication. The odds ratios for symptoms observed after a drug–drug interaction were increased for eight specific symptoms (each p ≤ 0.05), especially hemodynamic alterations and disturbances of electrolyte and fluid balance. The odds ratio was highest for decreased blood pressure observed after the administration of the drug pair fentanyl and furosemide (OR 5.06; 95% confidence interval 3.5–7.4; p < 0.001). Increased odds ratios for specific symptoms observed after drug–drug interactions resulted from eight combinations composed of eight different drugs: digoxin, fentanyl, midazolam, phenobarbital, potassium salts and vancomycin (high-alert medications), and the diuretics furosemide and hydrochlorothiazide (non-high-alert medications). The resulting drug pairs were: potassium salts–furosemide, fentanyl–furosemide, vancomycin–furosemide, digoxin–furosemide, digoxin–hydrochlorothiazide, fentanyl–phenobarbital, potassium salts–hydrochlorothiazide, and midazolam–hydrochlorothiazide.
Conclusions
In a cohort of PICU patients, this study identified eight specific drug pairs involving high-alert medications that may increase the risk of interaction-associated symptoms, mainly hemodynamic alterations and electrolyte/fluid balance disturbances. If the administration of those drug pairs is unavoidable, patients should be closely monitored.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40272-024-00641-x.
Key Points
| More than half of the patients receiving high-alert medications were affected by a total of 3203 symptoms observed after drug–drug interactions involving high-alert medications. More than one in four observed symptoms were associated with a drug–drug interaction at a significant odds ratio. |
| Specific drug pairs were identified that may increase the risk of interaction-associated symptoms, mainly categorized as hemodynamic alterations and fluid and electrolyte balance disturbances. Those drug pairs involved eight drugs frequently administered in a PICU. |
| Physicians should avoid the administration of these specific drug pairs, or if their administration is unavoidable, monitor patients closely for corresponding symptoms. |
Introduction
Children admitted to a pediatric intensive care unit (PICU) are often in a critical state of health and require complex drug treatment. Although administration of multiple drugs together leads to an increased risk of drug-related problems [1, 2], previous studies reported most patients in PICUs received a median number of ten different drugs per patient day [3, 4]. Especially in the PICU, so-called high-alert medications (HAMs) must be administered frequently. Due to various factors, such as a narrow therapeutic range or a high potential for drug–drug interactions (DDIs) [5, 6], these drugs bear a higher risk of causing patient harm compared with other drugs, according to the Institute for Safe Medication Practices (ISMP) [7]. Therefore, the administration of HAM should be given careful consideration. The ISMP developed its first list of HAMs for the acute care setting in 1995 [7]. Until now, few studies have identified specialized lists of HAM for children [6, 8–10]. Schilling et al. combined results from three previous studies to develop a list of 20 HAMs for pediatric patients in the German setting. They described DDI as a drug-related problem for half of those 20 [6].
There is scant literature about DDIs involving HAMs for pediatric patients or their implications for children admitted to a PICU. Therefore, we aimed to identify DDIs involving HAMs that may increase the risk of interaction-associated symptoms. We specifically targeted drug pairs that should be avoided in daily clinical practice or closely monitored if their administration is unavoidable. We did not distinguish between different severity grades for DDI and symptoms, as we aimed to assess the most common DDIs regardless of their classification according to the databases, and we endeavored not to overlook any relevant symptoms. Therefore, we also included drug–drug interactions with a low classification according to the databases, as these can also severely affect patients in a critical health state.
Material and Methods
Study Design
This retrospective study analyzed data from April 2018 to March 2019 obtained in a general PICU of a university hospital in Germany. Patients of all pediatric age groups were treated in the study unit, except neonates, who were treated in a separate neonatal intensive care unit. We assessed the electronic documentation for each patient in the hospital’s patient data management system to identify potential DDIs (pDDIs) involving at least one drug defined as a HAM. Furthermore, we analyzed symptoms observed after these pDDIs to detect interaction-associated symptoms.
We included patients hospitalized for at least 48 h in the study unit who received at least two different drugs within a 24-h interval during their stay. Patients on chemotherapy were excluded because they were mainly treated at the pediatric oncology unit of the university hospital and only transferred to the PICU for a short time if their health condition deteriorated severely.
The study titled “Adverse drug reactions in an interdisciplinary PICU” was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee at the Medical Faculty, Leipzig University, Germany (study number: 127/19-ek) on 2 May 2019. The ethics committee waived informed consent because this was a retrospective study, and patients’ treatment was not influenced.
Identification of HAM in the PICU and pDDIs Involving at Least One HAM in Clinical Care
On the basis of the electronic patient documentation, we first examined the administration of the 20 drugs that Schilling et al. [6] defined as HAM for hospitalized pediatric patients. We included 15 of those HAMs in our analysis because 5 of the 20 defined HAMs were not administered in our PICU during the study period: cyclosporine, phenytoin, amiodarone, vecuronium, and rocuronium. Second, we evaluated pDDIs that involved at least one HAM for each patient day. For this purpose, potentially interacting drug pairs were identified on the basis of two drug information databases: UpToDate (provided by Wolters Kluwer, Riverwoods, Illinois, USA) and drugs.com (provided by Drugsite Trust, Auckland, New Zealand). Each drug pair for which an interaction alert was reported in at least one of the databases was defined as a pDDI. To identify potentially interacting drug pairs, we considered a maximum time interval of 24 h between administering a HAM and another potentially interacting drug, regardless of whether the second drug was defined as a HAM. We considered a 24-h interval to be appropriate because estimating the correct half-lives of interacting drugs in individual patients in our cohort was hardly feasible due to the general developmental variability of pharmacokinetics and pharmacodynamics in children and the possible influence of the individual patient’s condition. Hence, if a potentially interacting drug was administered 24 h before or after a HAM, the event was categorized as a pDDI. If a pDDI occurred more than once within the defined time interval, it was counted only once. For continuous infusions, it was assessed for each drug administered concurrently whether a pDDI occurred due to the additional drug.
Identification of Interaction-Associated Symptoms
For each pDDI, we investigated whether symptoms that could be associated with it were observed after the administration. For this investigation, we examined the nurses’ and physicians’ daily documentation of the patient’s condition for symptoms that occurred within a 24-hour interval after administration of the second drug of the relevant drug pair. The documentation included automatically recorded vital parameters, laboratory parameters, and additional documentation, such as non-measurable symptoms as nausea or vomiting (Online Resource 1). For vital and laboratory parameters, age-dependent standard ranges for infants, children, and adolescents were determined by the treating physicians. For some patients, the attending physician adjusted the standard ranges to the patient’s health condition. In our analyses, we considered deviations from the determined patient–individual ranges. We focused on symptoms that were identified as possible consequences of a pDDI according to our database search in UpToDate and drugs.com. If at least one of these symptoms was associated with the relevant drug pair at a statistically significant odds ratio (OR), this was defined as a DDI. Since we took the underlying data on the symptoms from the documentation of physicians and nursing staff, it can be assumed that those symptoms were clinically relevant, as they would otherwise not have been documented.
To estimate the risk associated with the interaction of a particular drug pair for an observed symptom, we calculated the OR and 95% confidence interval. To ensure that the calculation was based on a sufficient occurrence of a particular drug pair and corresponding symptom, we set two criteria. First, we only considered potentially interacting drug pairs administered on at least 2% of patient days. Second, we focused only on corresponding symptoms observed at least ten times after a given drug pair. Combining potentially interacting drug pairs and symptoms that met these criteria, we created a contingency table that presents the frequency of the following combinations on each patient day: both the potentially interacting drug pair and corresponding symptom were observed; only the potentially interacting drug pair was observed; only the symptom was observed; and neither the potentially interacting drug pair nor the symptom was observed. On the basis of the contingency table, a univariate logistic regression was performed to obtain OR, 95% confidence interval, and p-value. The calculation was conducted using IBM SPSS Statistics Version 29 (IBM Corporation, Armonk, New York, USA). A p value ≤ 0.05 was considered to indicate significance.
Results
Characteristics of Patients and Administered Drugs
We examined 1263 patients admitted to the PICU during the study period for the inclusion criteria (Fig. 1). Of those, 315 (24.9%) patients fulfilled the inclusion criteria. Baseline patient characteristics are presented in Table 1. In total, 255 different drugs were administered to the patients. Of these drugs, 5.9% (15/255) were identified as HAM for hospitalized pediatric patients, according to the study by Schilling et al. [6] (Table 2). The most commonly administered sedative during the study period was midazolam [affected 173/315 (54.0%) patients on 1011/3788 (26.7%) patient days; Online Resource 2]. Potassium salts were the most frequently administered HAM, used on 39.0% of patient days (1477/3788), in 47.3% (149/315) of patients (Table 3).
Fig. 1.
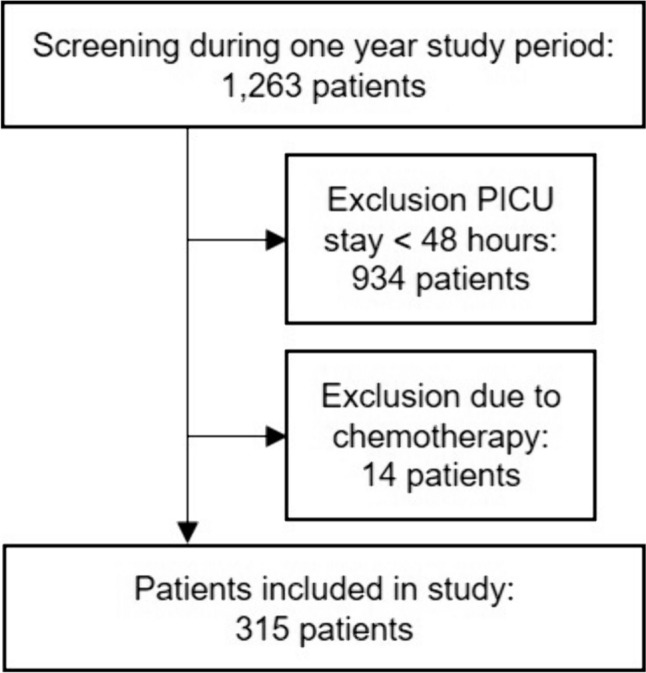
Flow chart of patient inclusion
Table 1.
Baseline patient characteristics
| Characteristics | Value |
|---|---|
| Number of patients, n (m/f) | 315 (183/132) |
| Median age, years (Q25/Q75; min/max) | 3.7 (0.8/11.3; 0.0/22.8) |
| Median weight, kg (Q25/Q75; min/max) | 13.0 (6.7/29.0; 2.3/156.0) |
| Median length of PICU stay, days (Q25/Q75; min/max) | 8 (4/14; 3/99) |
| Median simplified acute physiology score on PICU admission (Q25/Q75; min/max) | 13 (9/21; 2/50) |
| Status of ventilation at PICU admission, n (%) | |
| Not ventilated | 151 (47.9) |
| Non-invasive ventilation | 116 (36.8) |
| Invasive ventilation | 48 (15.3) |
| Death, n (%) | 6 (1.9) |
| Primary reason for PICU admission, n (%) | |
| Surgical | 167 (53.0) |
| Gastrointestinal | 53 (16.8) |
| Musculoskeletal | 40 (12.7) |
| Neurologic | 25 (7.9) |
| Oncologic | 23 (7.3) |
| Ears-nose-throat/maxillofacial | 13 (4.1) |
| Urologic | 7 (2.2) |
| Other | 6 (1.9) |
| Medical | 141 (44.8) |
| Respiratory | 60 (19.0) |
| Neurologic | 20 (6.3) |
| Sepsis | 15 (4.8) |
| Gastrointestinal | 13 (4.1) |
| Metabolic | 11 (3.5) |
| Cardiovascular | 5 (1.2) |
| Other | 17 (5.4) |
| Trauma | 7 (2.2) |
PICU pediatric intensive care unit
Table 2.
Characteristics of drug therapy
| Characteristics | Value |
|---|---|
| Total number of administered drugs, n | 43,200 |
| Number of different administered drugs, n | 255 |
| Median number of drugs per patient per day, n (Q25/Q75; min/max) | 10 (7/15;1/34) |
| Total number of administered HAM, n/N (%) | 5385/43,200 (12.5) |
| Number of different administered HAM, n/N (%) | 15/255 (5.9) |
| Median number of HAM per patient per day, n (Q25/Q75; min/max) | 1 (0/2; 0/8) |
HAM high-alert medication
Table 3.
Frequency of high-alert medications administered in the pediatric intensive care unit during the study period. In our analysis, we included 15 of 20 drugs defined as high-alert medications for hospitalized pediatric patients according to Schilling et al. [6]
| High-alert medication | Number of patients receiving the high-alert medication, n (%) (N = 315 patients) |
Number of patient days with the high-alert medication, n (%) (N = 3788 patient days) |
|---|---|---|
| Potassium salts | 149 (47.3) | 1477 (39.0) |
| Midazolam | 173 (54.9) | 1011 (26.7) |
| Vancomycin | 33 (10.5) | 449 (11.9) |
| Epinephrine | 74 (23.5) | 431 (11.4) |
| Clonidine | 30 (9.5) | 415 (11.0) |
| Phenobarbital | 65 (20.6) | 405 (10.7) |
| Fentanyl | 42 (13.3) | 389 (10.3) |
| Digoxin | 14 (4.4) | 302 (8.0) |
| Amphotericin B | 13 (4.1) | 131 (3.5) |
| Tacrolimus | 12 (3.8) | 127 (3.4) |
| Propofol | 40 (12.7) | 84 (2.2) |
| Dobutamine | 7 (2.2) | 69 (1.8) |
| Norepinephrine | 18 (5.7) | 35 (0.9) |
| Morphine | 12 (3.8) | 33 (0.9) |
| Dopamine | 3 (1.0) | 27 (0.7) |
The remaining five high-alert medications were not administered during the study period: cyclosporine, phenytoin, amiodarone, vecuronium, and rocuronium
pDDIs Involving at Least One HAM
Analyzing each patient’s electronic documentation, we identified 20,150 pDDIs involving at least one HAM on the basis of our database search in UpToDate and drugs.com. We calculated a rate of 78.7 pDDIs per patient that involved at least one HAM (20,150 pDDI involving at least one HAM/256 patients receiving HAM). The 20,150 pDDIs resulted from 469 different drug pairs. Of these potentially interacting drug pairs, 14.3% (67/469) were administered on at least 2% of patient days. The frequency of the potentially interacting drug pairs and their classifications according to the databases is presented in Online Resource 3.
Interaction-Associated Symptoms Identified in the PICU
We observed at least one symptom after 14.0% (2830/20,150) of pDDIs, resulting in a total of 3203 observed symptoms affecting 56.3% (144/256) of patients receiving HAM (Table 4). While we observed one symptom after the administration of 87.7% (2482/2830) of those pDDIs, more than one symptom was observed after 12.3% (348/2830) of pDDIs.
Table 4.
Frequency of symptoms observed after potential drug–drug interactions involving high-alert medications
| Symptom | Frequency of symptoms, n |
Frequency related to total of symptoms, % (N = 3203) |
Frequency of patients affected by the respective symptom after a pDDI involving HAM, n (%) (N = 256 patients receiving HAM) |
|---|---|---|---|
| Increased heart rate | 781 | 24.4 | 62 (24.2) |
| Hyponatremia | 390 | 12.2 | 52 (20.3) |
| Vomiting | 262 | 8.2 | 41 (16.0) |
| Hypokalemia | 243 | 7.6 | 18 (7.0) |
| Decreased blood pressure | 237 | 7.4 | 28 (10.9) |
| Respiratory depression | 164 | 5.1 | 24 (9.4) |
| Urinary retention | 137 | 4.3 | 29 (11.3) |
| Hyperkalemia | 131 | 4.1 | 43 (16.8) |
| Edema | 128 | 4.0 | 13 (5.1) |
| Nausea | 119 | 3.7 | 24 (9.4) |
| Agitation | 118 | 3.7 | 21 (8.2) |
| Decreased diuresis | 112 | 3.5 | 23 (9.0) |
| Decreased heart rate | 96 | 3.0 | 10 (3.9) |
| Hypomagnesemia | 57 | 1.8 | 14 (5.5) |
| Sweating | 46 | 1.4 | 9 (3.5) |
| Hypocalcemia | 43 | 1.3 | 12 (4.7) |
| Increased blood pressure | 43 | 1.3 | 12 (4.7) |
| Fever | 19 | 0.6 | 12 (4.7) |
| Dyspnea | 14 | 0.4 | 7 (2.7) |
| Seizures | 14 | 0.4 | 5 (2.0) |
| Constipation | 10 | 0.3 | 4 (1.6) |
| Diarrhea | 9 | 0.3 | 2 (0.8) |
| Dizziness | 8 | 0.2 | 3 (1.2) |
| Abdominal pain | 5 | 0.2 | 3 (1.2) |
| Sedation | 4 | 0.1 | 1 (0.4) |
| Excessive diuresis | 3 | 0.1 | 2 (0.8) |
| Hypercalcemia | 3 | 0.1 | 2 (0.8) |
| Increased PTH | 3 | 0.1 | 1 (0.4) |
| Exanthema | 2 | 0.1 | 2 (0.8) |
| Tachypnea | 2 | 0.1 | 2 (0.8) |
HAM high-alert medication, pDDI potential drug–drug interaction, PTH parathyroid hormone
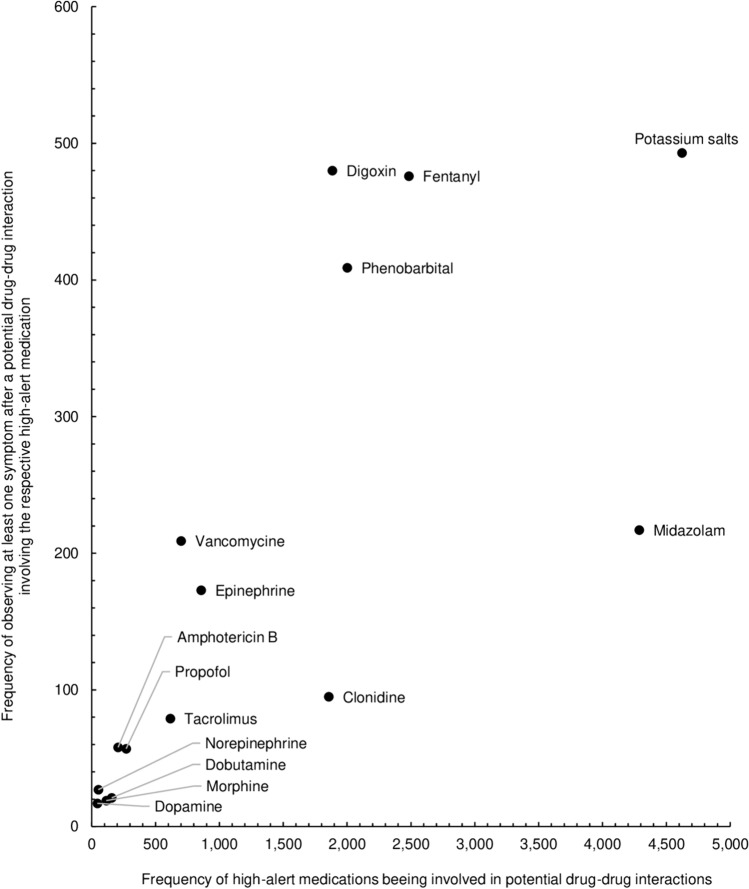
The most pDDIs after which we observed at least one symptom involved potassium salts (2.4%; 493/20,150), followed closely by digoxin (2.4%; 480/20,150) and fentanyl (2.4%; 476/20,150; Fig. 2).
Fig. 2.
For each high-alert medication, the number of potential drug–drug interactions (total interactions: N = 20,150) is plotted against how often at least one symptom was observed after a potential drug–drug interaction involving the respective high-alert medication (total interactions followed by symptoms: N = 2830)
For 33.1% (1061/3203) of observed symptoms, the preconditions for the calculation of the OR were fulfilled (Table 5). We found an increased OR for hyponatremia, hypokalemia, decreased blood pressure, increased heart rate, urinary retention, edema, sweating, and restlessness (each p ≤ 0.05; Table 5). Those eight specific symptoms accounted for 28.0% (897/3203) of all observed symptoms potentially related to DDI. These DDIs involved eight different drugs in eight different combinations. Of the eight drugs, 75% (6/8) were defined as HAM for pediatric patients: digoxin, fentanyl, midazolam, phenobarbital, potassium salts, and vancomycin. The remaining 25% (2/8) were diuretics not defined as HAM: furosemide and hydrochlorothiazide. The highest OR was found for decreased blood pressure observed after administration of the drug pair fentanyl and furosemide (OR 5.06; 95% CI 3.5–7.4; p < 0.001), followed by hypokalemia observed after administration of the drug pairs digoxin and furosemide (OR 4.16; 95% CI 3.1–5.6; p < 0.001) and digoxin and hydrochlorothiazide (OR 3.86; 95% CI 2.9–5.1; p < 0.001).
Table 5.
Drug–drug interactions involving high-alert medications and subsequent symptoms observed within 24 h after the administration of the respective drug–drug interaction
| pDDI | Classification | Associated symptom | Patient days with/without pDDI and symptom, N | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug 1 | Drug 2 | UpToDateb | drugs.comc | pDDI | Yes | Yes | No | No | Odds ratio [95% CI] | p value | |
| Symptom | Yes | No | Yes | No | |||||||
| Potassium saltsa | Furosemide | B | n/a | Hyponatremia | 163 | 667 | 341 | 2617 | 1.88 [1.5; 2.3] | < 0.001* | |
| Fentanyla | Furosemide | C | Moderate | Decreased blood pressure | 43 | 275 | 104 | 3366 | 5.06 [3.5; 7.4] | < 0.001* | |
| Urinary retention | 86 | 232 | 541 | 2929 | 2.01 [1.5; 2.6] | < 0.001* | |||||
| Increased heart rate | 76 | 242 | 521 | 2949 | 1.78 [1.3; 2.3] | < 0.001* | |||||
| Vancomycina | Furosemide | n/a | Moderate | Edema | 83 | 150 | 490 | 3065 | 3.46 [2.6; 4.6] | < 0.001* | |
| Decreased diuresis | 42 | 191 | 575 | 2980 | 1.14 [0.8; 1.6] | 0.459 | |||||
| Vomiting | 36 | 197 | 502 | 3053 | 1.11 [0.8; 1.6] | 0.573 | |||||
| Digoxina | Furosemide | n/a | Moderate | Hypokalemia | 89 | 134 | 523 | 3042 | 3.86 [2.9; 5.1] | < 0.001* | |
| Nausea | 10 | 213 | 177 | 3388 | 0.90 [0.5; 1.7] | 0.748 | |||||
| Increased heart rate | 35 | 188 | 562 | 3003 | 0.99 [0.7; 1.4] | 0.978 | |||||
| Hypomagnesemia | 12 | 211 | 238 | 3327 | 0.80 [0.4; 1.4] | 0.451 | |||||
| Digoxina | HCT | n/a | Moderate | Hypokalemia | 86 | 120 | 526 | 3056 | 4.16 [3.1; 5.6] | < 0.001* | |
| Increased heart rate | 29 | 177 | 568 | 3014 | 0.87 [0.6; 1.3] | 0.496 | |||||
| Fentanyla | Phenobarbitala | D | Major | Restlessness | 80 | 59 | 961 | 2688 | 3.79 [2.7; 5.4] | < 0.001* | |
| Sweating | 30 | 109 | 480 | 3169 | 1.82 [1.2; 2.8] | 0.005* | |||||
| Potassium saltsa | HCT | B | n/a | Hyponatremia | 85 | 229 | 419 | 3055 | 2.71 [2.1; 3.5] | < 0.001* | |
| Midazolama | HCT | n/a | Moderate | Decreased blood pressure | 20 | 168 | 127 | 3473 | 3.26 [2.0; 5.3] | < 0.001* | |
| Increased heart rate | 56 | 132 | 541 | 3059 | 2.40 [1.7; 3.3] | < 0.001* | |||||
For each drug combination and observed symptom, the frequencies of patient days on which the respective potential drug–drug interaction was or was not administered and whether the symptom was observed is shown. From those numbers, the odds ratios, 95% confidence intervals, and p-values were calculated using a univariate logistic regression
HCT hydrochlorothiazide, n/a not applicable (not listed in the respective database), pDDI potential drug–drug interaction
*Significant
aCategorized as high-alert medication for hospitalized pediatric patients according to Schilling et al. [6]
bClassification used in UpToDate: “D—Consider therapy modification; C—Monitor therapy; B—No action needed. Agents may interact with each other”
cClassification used in Drugs.com: “Major—Avoid combinations; Moderate—Usually avoid combination. Use it only under special circumstances; Minor—Take steps to circumvent the interaction risk and/or establish a monitoring plan”
Discussion
HAMs are Common Drugs Administered in the PICU
According to the ISMP, HAMs carry a higher risk of patient harm compared with ordinary drugs [7]. Even when used as prescribed, they significantly increase the risk of drug-related problems [11]. In our study, 81% of critically ill children received at least one drug defined as HAM for pediatric patients by Schilling et al. [6]. Potassium salts, midazolam, and vancomycin were the HAMs most frequently administered. This is in line with a previous study in a pediatric emergency setting reporting that 91% of patients were prescribed at least one HAM, with potassium salts being the most frequently administered [12].
More than 20,000 pDDIs with HAM During a 1-Year Study
It is widely known that pDDIs are highly prevalent in PICUs. They are associated with various factors, such as a high number of administered drugs, a complex chronic condition, or an increased length of hospitalization [4, 13, 14]. Although previous studies determined pDDI as a cause of drug-related problems with HAM for pediatric patients, there is only limited knowledge about the frequency of pDDIs in pediatric intensive care [6, 8, 10]. In our study, we found more than 20,000 pDDIs involving HAM in 256 pediatric patients over the 1-year study period. A previous Brazilian study of adult intensive care patients reported 846 HAM-related pDDIs in 60 patients [15]. Compared with our research, the Brazilian study reported a considerably lower rate of HAM-related pDDIs per patient (79 versus 14). Part of this difference may be explained by the fact that pediatric patients requiring intensive care are more susceptible to drug–drug interactions [16]. However, it may also be related to the fact that the Brazilian study was performed on the basis of the database Micromedex 2.0 only [15]. Several studies recommended using at least two databases to determine pDDIs in daily routine [17–19]. Thus, we used the two databases, UpToDate and drugs.com, to avoid underestimating any potential risks. However, since the concordance between different databases is limited, comparing various studies can be challenging [20, 21].
Physicians Should be Aware of Interaction-Associated Symptoms
For 2830 pDDIs, we observed 3203 symptoms occurring after the administration of the potentially interacting drug pairs. More than one in four detected symptoms were eventually associated with a DDI. Those interaction-associated symptoms comprised eight specific symptoms, mainly hemodynamic alterations or electrolyte and fluid balance disturbances. These symptoms were frequently reported in previous pediatric intensive care studies [3, 22–24]. The study presented here shows that DDI involving HAM should be considered a likely trigger for symptoms in addition to other factors, such as the underlying disease or non-drug treatments, such as surgeries. It can also be assumed that various factors contribute to the occurrence of a symptom. When identifying DDIs and following interaction-associated symptoms, we did not distinguish between different severity grades of DDI or symptoms, as the main aim of our study was to identify drug pairs that are frequently associated with symptoms that are considered clinically relevant by the responsible physicians and nurses. Physicians usually receive a considerable number of alerts when using a database-related interaction checker. This may quickly lead to over-alerting. Therefore, we aimed to provide physicians with a concise overview of clinically relevant DDIs that occur frequently in a PICU. Our findings could be implemented in commonly used database-related interaction checkers to draw physicians’ attention to drug pairs involving HAM that are potentially associated with an increased risk of adverse events.
We identified eight specific drug pairs composed of eight different drugs that may lead to an increased risk of interaction-associated symptoms. By calculating the OR for a DDI and a respective symptom, we took into account how often a symptom was observed on patient days when the interacting drug pair was administered compared with days when the respective drug pair was not administered. In particular, this should minimize the risk that certain combinations of DDI and symptoms are over- or underestimated. For the interaction of fentanyl and furosemide, we found the highest OR for the symptom of decreased blood pressure. Both drugs have been shown to belong to the top ten of the most frequently administered drugs and to be among the drugs most commonly involved in pDDIs in the pediatric intensive care setting [4]. In our study, DDI was associated with a potential fivefold increased risk of decreased blood pressure. The second highest OR, indicating a potential fourfold increased risk, was found for the interaction of digoxin with hydrochlorothiazide and the observed symptom of hypokalemia. Consequently, when the administration of drug pairs associated with a potentially increased risk of interaction-associated symptoms is unavoidable, patients should be closely monitored for potential symptoms.
Until now, few studies have dealt with interaction-associated symptoms in the pediatric intensive care setting [14, 25, 26]. One of those studies only focused on cytochrome P450-mediated drug–drug interactions [25]. Two other studies concentrated on symptoms on the basis of clinical monitoring and laboratory results, as we did in our research. Both studies also identified hemodynamic alterations and electrolyte and fluid balance disturbances as symptoms following DDIs. However, neither of those studies noted specific interactions that increased the risk of the detected symptoms [14, 26]. Our study went one step further by revealing eight interacting drug pairs that may increase the risk of the identified interaction-associated symptoms in clinical practice. We found symptoms that are widely known to follow the respective DDI, such as the association of hyponatremia with the DDI of potassium salts and furosemide, or the increased risk for hypokalemia associated with the DDI of digoxin and furosemide. However, we also observed symptoms after a DDI that we did not expect. For example, we unexpectedly found that the DDI of fentanyl and furosemide was associated with a potential risk increase for urinary retention, or that the DDI of vancomycin and furosemide was associated with edema. Especially for symptoms that unexpectedly are observed after a specific DDI, other factors, such as the state of illness or a surgery that could also lead to the symptom, should be critically evaluated.
Limitations
Some limitations have to be considered when interpreting our study results. First of all, the relevance of some drugs administered in our study can vary in different PICUs around the world. However, the 15 drugs defined as HAM that were in the focus of our study are used in many PICUs worldwide [4, 27–31].
As recommended by previous studies [17–19], we used two databases to prevent failure to detect interactions that could lead to interaction-associated symptoms. However, we could not identify a database specializing in DDI for pediatrics. Previous studies did not find an age-related trend in the magnitude of DDIs, although it should be noted that there are insufficient data for children under 2 years of age [32, 33]. In addition, extrapolating data from adults to children may over- or underestimate the severity of DDIs [34]. Additionally, as most databases are limited to the information on the interactions of two drugs, potential synergistic or antagonistic effects of combinations consisting of three or more drugs might be overlooked.
Furthermore, the allowed maximum time interval of 24 h between the administration of two drugs may be too long for an interaction for some drug pairs. According to a previous review by Bakker et al., the optimal time interval would consider the half-lives of interacting drugs [21]. However, due to the developmental variability of pharmacokinetics and pharmacodynamics in children, it is very challenging to determine standardized drug half-lives in the pediatric population [35]. In addition, the individual patients’ conditions, such as renal function, can also have significant influence on drugs’ half-lives [36]. In addition, a constant plasma concentration is aimed for with many drugs, which is why a longer-lasting interaction potential can be assumed, although the half-lives of the individual drugs are varying. To ensure a standardized approach for evaluating DDI, we established a 24-h time interval as described in the review by Bakker et al. if consideration of drug half-lives is not feasible [21]. This methodological approach might potentially increase the risk of overestimation.
The retrospective design is another limitation of this study, as using nurses’ and physicians’ daily documentation entails the risk of missing data. That could lead to information bias, as the documentation was not primarily compiled to answer research questions. Consequently, using the patient documentation as data basis may have an impact on the identification of symptoms themselves, and on the observed associations between interacting drugs pairs and subsequent symptoms. Furthermore, due to the retrospective design, we could not assess whether the physicians accepted certain expectable symptoms as an inevitable consequence of the chosen drug therapy because the patient’s state of health required the administration.
In addition, it should be kept in mind that the administration of a HAM alone and the underlying disease may also increase the risk of adverse events. However, we focused on acknowledged DDIs and interaction-associated symptoms reported in established databases. We endeavored to identify symptoms prone to being associated with a DDI by calculating ORs, as those interactions potentially contribute to evoking symptoms, or to prolonging or exacerbating existing symptoms. These drug combinations should therefore be given special consideration in the routine care of critically ill pediatric patients who are already at risk.
Conclusions
Our study sheds light on a topic about which knowledge is limited: symptoms associated with DDIs involving HAM. We showed that pDDIs involving HAM are very common in pediatric intensive care. More than one in four observed symptoms were associated with a DDI. These symptoms were mainly disturbances of electrolyte and fluid balance and hemodynamic alterations. Focusing on drug pairs with a potentially increased risk of triggering these symptoms, we identified eight specific drug pairs composed of eight different drugs. However, administration of these drug pairs may be unavoidable. In that case, patients should be carefully monitored for electrolyte and fluid balance disturbances and hemodynamic alterations, which were observed as the most frequent interaction-associated symptoms.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the physicians and nurses in the participating PICU for their helpful collaboration.
Declarations
Funding
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
A. Bertsche reports grants from UCB Pharma GmbH and honoraria for speaking engagements from Biogen GmbH, Desitin Arzneimittel GmbH, Eisai GmbH, GW Pharma GmbH, Neuraxpharm GmbH, Shire/Takeda GmbH, UCB Pharma GmbH, and ViroPharma GmbH. The other authors declare they have no conflicts of interests.
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to ethical and privacy considerations to protect the confidentiality of patients.
Ethics approval
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Medical Faculty, Leipzig University, Germany (127/19-ek). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
As this was a retrospective study and data were collected from patient records without any influence on patients’ treatment, the ethics committee waived informed consent.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
Conceptualization: Lisa Marie Kiesel and Martina Patrizia Neininger; methodology: Lisa Marie Kiesel, Martina Patrizia Neininger, Astrid Bertsche, Thilo Bertsche, Manuela Siekmeyer, and Wieland Kiess; formal analysis: Lisa Marie Kiesel; investigation: Lisa Marie Kiesel and Martina Patrizia Neininger; writing—original draft preparation: Lisa Marie Kiesel and Martina Patrizia Neininger; writing—review and editing: Astrid Bertsche, Thilo Bertsche, Manuela Siekmeyer, and Wieland Kiess; supervision: Martina Patrizia Neininger; project administration: Lisa Marie Kiesel and Martina Patrizia Neininger. All authors read and approved the final version.
References
- 1.Marquez C, Thompson R, Feinstein JA, et al. Identifying opportunities for pediatric medication therapy management in children with medical complexity. J Am Pharm Assoc. 2003;2022(62):1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinstein J, Dai D, Zhong W, et al. Potential drug-drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics. 2015;135:e99-108. 10.1542/peds.2014-2015 [DOI] [PubMed] [Google Scholar]
- 3.Kiesel LM, Bertsche A, Kiess W, et al. Intensive care drug therapy and its potential adverse effects on blood pressure and heart rate in critically ill children. World J Pediatr. 2023;19:902–11. 10.1007/s12519-023-00683-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai D, Feinstein JA, Morrison W, et al. Epidemiology of polypharmacy and potential drug–drug interactions among pediatric patients in ICUs of US children’s hospitals. Pediatr Crit Care Med. 2016;17:e218–28. 10.1097/PCC.0000000000000684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He M, Huang Q, Lu H, et al. Call for decision support for high-alert medication administration among pediatric nurses: findings from a large, multicenter, cross-sectional survey in China. Front Pharmacol. 2022;13: 860438. 10.3389/fphar.2022.860438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schilling S, Koeck JA, Kontny U, et al. High-alert medications for hospitalised paediatric patients - a two-step survey among paediatric clinical expert pharmacists in Germany. Pharmazie. 2022;77:207–15. [DOI] [PubMed] [Google Scholar]
- 7.Institute for Safe Medication Practices: High-alert medications in acute care settings. 2018. https://www.ismp.org/sites/default/files/attachments/2018-08/highAlert2018-Acute-Final.pdf. Accessed 08 Dec 2023.
- 8.Franke HA, Woods DM, Holl JL. High-alert medications in the pediatric intensive care unit. Pediatr Crit Care Med. 2019;10:85–90. 10.1097/PCC.0b013e3181936ff8 [DOI] [PubMed] [Google Scholar]
- 9.Maaskant JM, Eskes A, van Rijn-Bikker P, et al. High-alert medications for pediatric patients: an international modified Delphi study. Expert Opin Drug Saf. 2013;12:805–14. 10.1517/14740338.2013.825247 [DOI] [PubMed] [Google Scholar]
- 10.Bataille J, Prot-Labarthe S, Bourdon O, et al. Highalert medications in a French paediatric university hospital. J Eval Clin Pract. 2015;21:262–70. 10.1111/jep.12302 [DOI] [PubMed] [Google Scholar]
- 11.Loria G. Reduction of harm from high risk medications. Apollo Med. 2012;9:160–5. 10.1016/j.apme.2012.05.001 [DOI] [Google Scholar]
- 12.Melo VV, Costa MS, Soares AQ. Quality of prescription of high-alert medication and patient safety in pediatric emergency. Farm Hosp. 2014;38:9–17. [DOI] [PubMed] [Google Scholar]
- 13.Rao C, Shenoy V, Udaykumar P. Potential drug–drug interactions in the pediatric intensive care unit of a tertiary care hospital. J Pharmacol Pharmacother. 2019;10:63–8. 10.4103/jpp.JPP_27_19 [DOI] [Google Scholar]
- 14.Choi YH, Lee IH, Yang M, et al. Clinical significance of potential drug-drug interactions in a pediatric intensive care unit: a single-center retrospective study. PLoS ONE. 2021;16: e0246754. 10.1371/journal.pone.0246754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes ALB, Silvino ZR. Factors associated to potential drug interactions in one Intensive Care Unit: a cross-sectional study. Esc Anna Nery. 2019;23: e20180326. 10.1590/2177-9465-ean-2018-0326 [DOI] [Google Scholar]
- 16.Hassanzad M, Arenas-Lopez S, Baniasadi S. Potential drug-drug interactions among critically ill pediatric patients in a tertiary pulmonary center. J Clin Pharmacol. 2018;58:221–7. 10.1002/jcph.996 [DOI] [PubMed] [Google Scholar]
- 17.Suriyapakorn B, Chairat P, Boonyoprakarn S, et al. Comparison of potential drug-drug interactions with metabolic syndrome medications detected by two databases. PLoS ONE. 2019;14: e0225239. 10.1371/journal.pone.0225239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alkhalid ZN, Birand N. Determination and comparison of potential drug–drug interactions using three different databases in northern cyprus community pharmacies. Niger J Clin Pract. 2022;25:2005–9. 10.4103/njcp.njcp_448_22 [DOI] [PubMed] [Google Scholar]
- 19.Günay A, Demirpolat E, Ünal A, et al. A comparison of four drug-drug interaction databases for patients undergoing haematopoietic stem cell transplantation. J Clin Pharm Ther. 2022;47:1711–9. 10.1111/jcpt.13728 [DOI] [PubMed] [Google Scholar]
- 20.Vanham D, Spinewine A, Hantson P, et al. Drug-drug interactions in the intensive care unit: do they really matter? J Crit Care. 2017;38:97–103. 10.1016/j.jcrc.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 21.Bakker T, Dongelmans DA, Nabovati E, et al. Heterogeneity in the identification of potential drug-drug interactions in the intensive care unit: a systematic review, critical appraisal, and reporting recommendations. J Clin Pharmacol. 2022;62:706–20. 10.1002/jcph.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naseem F, Saleem A, Mahar IA, et al. Electrolyte imbalance in critically ill paediatric patients. Pak J Med Sci. 2019;35:1093–8. 10.12669/pjms.35.4.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wati DK. Measuring and managing fluid overload in pediatric intensive care unit. In: Erbay RH, editor. Current topics in intensive care medicine. 1st ed. London: IntechOpen; 2018. p. 3–12.
- 24.Buckley MS, Leblanc JM, Cawley MJ. Electrolyte disturbances associated with commonly prescribed medications in the intensive care unit. Crit Care Med. 2010;38:253–64. 10.1097/CCM.0b013e3181dda0be [DOI] [PubMed] [Google Scholar]
- 25.Li T, Hu B, Ye L, et al. Clinically significant cytochrome P450-mediated drug-drug interactions in children admitted to intensive care units. Int J Clin Pract. 2022;2022:2786914. 10.1155/2022/2786914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ismail M, Aziz S, Noor S, et al. Potential drug-drug interactions in pediatric patients admitted to intensive care unit of Khyber Teaching Hospital, Peshawar, Pakistan: a cross-sectional study. J Crit Care. 2017;40:243–50. 10.1016/j.jcrc.2017.04.028 [DOI] [PubMed] [Google Scholar]
- 27.Mlambo VC, Algaze CA, Mak K, et al. Impact of abnormal potassium on arrhythmia risk during pediatric digoxin therapy. Pediatr Cardiol. 2024;45:901–8. 10.1007/s00246-022-03051-3 [DOI] [PubMed] [Google Scholar]
- 28.Katz DT, Torres NS, Chatani B, et al. Care of pediatric solid organ transplant recipients: an overview for primary care providers. Pediatrics. 2020;146: e20200696. 10.1542/peds.2020-0696 [DOI] [PubMed] [Google Scholar]
- 29.Kleiber N, van Rosmalen J, Tibboel D, et al. Hemodynamic tolerance to IV clonidine infusion in the PICU. Pediatr Crit Care Med. 2018;19:e409–16. 10.1097/PCC.0000000000001602 [DOI] [PubMed] [Google Scholar]
- 30.Schindler E, Yamamoto T. New drugs for old problems: which inotropes for critically ill children? Pediatr Crit Care Med. 2018;19:674–5. 10.1097/PCC.0000000000001559 [DOI] [PubMed] [Google Scholar]
- 31.Groll AH, Tragiannidis A. Update on antifungal agents for paediatric patients. Clin Microbiol Infect. 2010;16:1343–53. 10.1111/j.1469-0691.2010.03334.x [DOI] [PubMed] [Google Scholar]
- 32.Salem F, Rostami-Hodjegan A, Johnson TN. Do children have the same vulnerability to metabolic drug–drug interactions as adults? A critical analysis of the literature. J Clin Pharmacol. 2013;53:559–66. 10.1002/jcph.13 [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez D, Sinha J. Pediatric drug-drug interaction evaluation: drug, patient population, and methodological considerations. J Clin Pharmacol. 2021;61:S175–87. 10.1002/jcph.1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salerno SN, Burckart GJ, Huang SM, et al. Pediatric drug-drug interaction studies: barriers and opportunities. Clin Pharmacol Ther. 2019;105:1067–70. 10.1002/cpt.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kearns GL. Impact of developmental pharmacology on pediatric study design: overcoming the challenges. J Allergy Clin Immunol. 2000;106:S128–38. 10.1067/mai.2000.109419 [DOI] [PubMed] [Google Scholar]
- 36.Levy G. Pharmacokinetics in renal disease. Am J Med. 1977;62:461–5. 10.1016/0002-9343(77)90397-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.