Abstract
Because hepatic stellate cells (HSCs) play a major role in fibrosis, we focused on HSCs as a potential target for the treatment of liver fibrosis. In this study, we attempted to identify drug candidates to inactivate HSCs and found that several proteasome inhibitors (PIs) reduced HSC viability. Our data showed that a second-generation PI, carfilzomib (CZM), suppressed the expression of fibrotic markers in primary murine HSCs at low concentrations of 5 or 10 nM. Since CZM was not toxic to HSCs up to a concentration of 12.5 nM, we examined its antifibrotic effects further. CZM achieved a clear reduction in liver fibrosis in the carbon tetrachloride (CCl4)-induced mouse model of liver fibrosis without worsening of liver injury. Mechanistically, RNA sequence analysis of primary HSCs revealed that CZM inhibits mitosis in HSCs. In the CCl4-injured liver, amphiregulin, which is known to activate mitogenic signaling pathways and fibrogenic activity and is upregulated in murine and human metabolic dysfunction-associated steatohepatitis (MASH), was downregulated by CZM administration, leading to inhibition of mitosis in HSCs. Thus, CZM and next-generation PIs in development could be potential therapeutic agents for the treatment of liver fibrosis via inactivation of HSCs without liver injury.
Keywords: Proteasome inhibitor, Carfilzomib, Hepatic stellate cell, Liver fibrosis
Subject terms: Drug discovery, Drug screening, Liver fibrosis
Liver fibrosis, a common feature of chronic liver diseases, including metabolic dysfunction-associated steatotic liver disease (MASLD; previously called non-alcoholic fatty liver disease [NAFLD]), results in sustained liver injury1. Advanced fibrosis eventually progresses to cirrhosis, which is directly related to a high incidence of mortality and hepatocellular carcinoma.
Despite the high prevalence of liver fibrosis, with the exception of resmetirom, no drugs are approved by the United States Food and Drug Administration (US FDA) for its treatment.
In March 2024, resmetirom, an oral thyroid hormone receptor beta (THB-β) agonist, became the first medication approved by the FDA for the treatment of MASH (metabolic dysfunction-associated steatohepatitis)2. Resmetirom demonstrated a significant reduction in hepatic fat and inflammation in patients with MASH, but its efficacy and adverse effects in clinical practice are unknown, and post-marketing trends need to be monitored. The most common adverse reactions reported in patients treated with resmetirom included diarrhea, nausea, pruritus, abdominal pain, vomiting, constipation, and dizziness. Therefore, an urgent need still exists for the development of effective drugs without significant adverse effects to treat liver fibrosis3.
Hepatic stellate cells (HSCs) are key cells responsible for liver fibrosis. HSCs have a quiescent phenotype in the normal liver; however, after liver injury, they transdifferentiate into a myofibroblast phenotype, become activated, and are responsible for extracellular matrix (ECM) protein synthesis and deposition during fibrosis. Activation of HSCs produces ECM proteins such as collagen, one of the most important contributors to fibrosis4.
Development of drugs to reduce the activity of HSCs is needed. In this study, we focused on HSCs as a therapeutic target of liver fibrosis with the aim of identifying drug candidates for liver fibrosis treatment.
Results
Drug screening assays revealed that proteasome inhibitors inactivate human stellate cells (LX-2 cells) at low concentrations
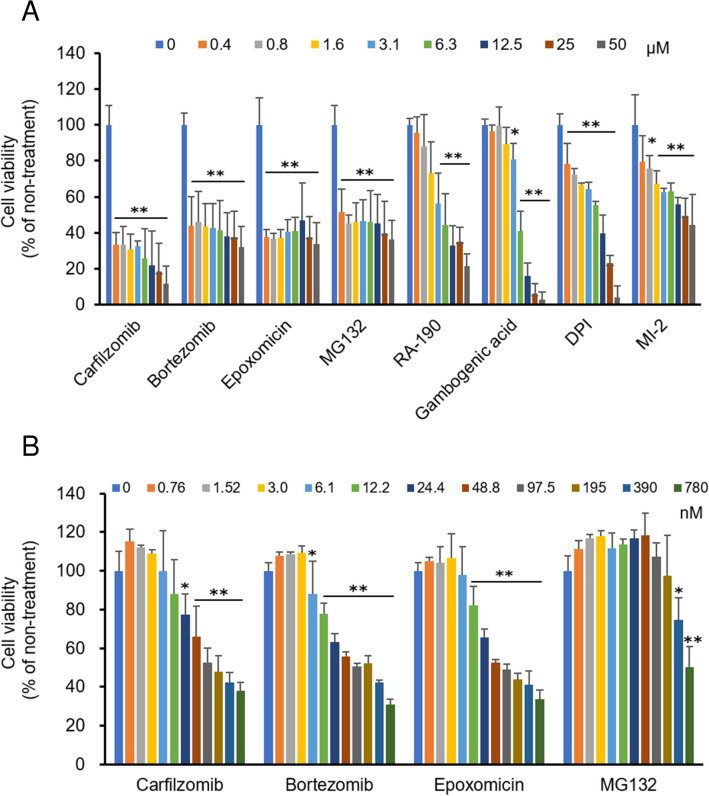
First, we searched for drug candidates to inactivate HSCs by assessing compounds in commercially available drug compound libraries for their effects on HSC viability. The inhibitory effects of 441 compounds (Supplementary Data 1) on 24-h viability were evaluated with LX-2 cells, which are human HSCs. We found that six drugs reduced HSC viability by more than 40% at a concentration of 12.5 μM. Since two of the six drugs, carfilzomib and epoxomicin, are proteasome inhibitors (PIs), two additional PIs commonly used in clinical and experimental settings, bortezomib (BZM) and MG132, respectively, were also examined (Fig. 1A). Among those eight drugs, carfilzomib (CZM), BZM, epoxomicin, and MG132 (all PIs) showed stronger inhibitory effects than the others (RA-190, gambogenic acid, diphenyleneiodonium (DPI), and Menin-MLL inhibitor 2 (MI-2)), at a low concentration of 0.4 μM. The PIs were further evaluated for their effects at lower concentrations than 0.4 μM because concentrations lower than those used for anti-tumor treatment are appropriate for anti-fibrotic treatment (Fig. 1B). CZM, BZM and epoxomicin showed stronger effects than MG132, and epoxomicin was used as a base chemical for the development of CZM. CZM and BZM, which are widely used in clinical practice as anti-tumor drugs for the treatment of multiple myeloma, strongly suppressed the viability of HSCs. Therefore, we decided to evaluate CZM and BZM further.
Figure 1.
Proteasome inhibitors suppress cell viability of LX-2 cells. (A) Cell viability of LX-2 cells treated with indicated drugs selected by first screening assay. (B) Cell viability of LX-2 cells treated with proteasome inhibitors. Mean ± SD data are displayed as fold changes relative to the non-treated group. Data were obtained from three independent experiments. *p < 0.05, **p < 0.01 (vs non-treated group).
Carfilzomib suppresses fibrogenic properties without cell injury in primary cultured mouse stellate cells at low concentrations
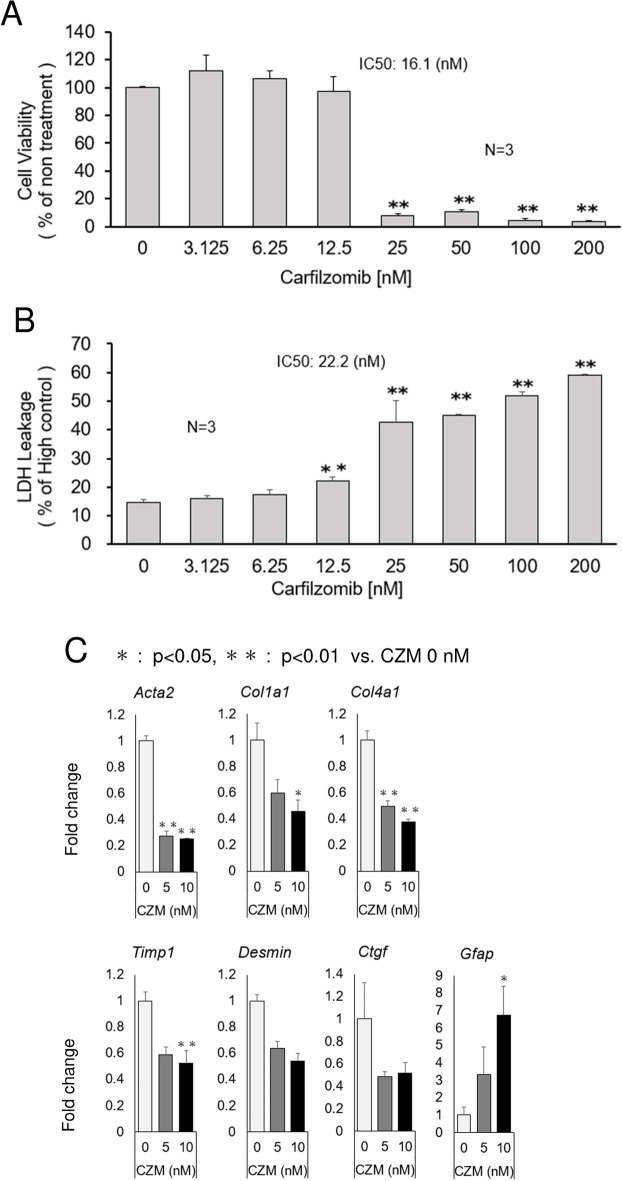
Due to their exceptional pathophysiological relevance, we used isolated HSCs from mouse livers for our experiments. Primary HSCs isolated from mouse livers were treated with BZM or CZM for 24 h to evaluate cell viability, cell toxicity and suppression of fibrogenic activity. Both drugs inhibited primary HSC viability at high concentrations, and the Half-maximal inhibitory concentration (IC50) values of CZM (16.1 nM, Fig. 2A) were two times higher than those of BZM (6.60 nM, Supplementary Fig. 1A). Importantly, CZM did not show toxicity, as assessed by lactate dehydrogenase (LDH) leakage, up to a concentration of 6.25 nM (Fig. 2B). In contrast, BZM induced LDH leakage at 6.25 nM (Supplementary Fig. 1B). Furthermore, CZM suppressed the expression of fibrotic markers at low concentrations (Fig. 2C). Alpha smooth muscle actin 2 (Acta2), Collagen type I alpha 1 (Col1a1), Collagen type IV alpha1 (Col4a1), Tissue inhibitor 1 of metalloproteinase 1 (Timp1), Desmin, and Connective tissue growth factor (Ctgf), hallmarks of HSC activation and hepatic fibrogenesis, were attenuated by CZM treatment at low concentrations of 5 or 10 nM, although attenuation of Desmin and Ctgf was not statistically significant. It is reported that Glial fibrillary acidic protein (GFAP) is expressed in quiescent or partially activated HSCs in liver tissue5–7. In rat liver, GFAP expression was detected in quiescent HSC during the acute phase response, whereas chronic liver injury led to a downregulation of GFAP which has therefore been suggested as an early marker of HSC activation8. In our study, (Gfap) expression was increased by CZM treatment (Fig. 2C). Although Gfap is not a well—established quiescent marker, decreased Acta2 and fibrotic markers expression by CZM treatment suggest that CZM inactivated HSCs, in other words, promoted the reversion of activated HSCs (Fig. 2C).
Figure 2.
Carfilzomib attenuates fibrogenic properties without cell injury in primary HSCs. (A) Cell viability after the treatment of murine primary HSCs with carfilzomib. (B) LDH leakage after the treatment of murine primary HSCs with carfilzomib. (C) mRNA levels of Acta2, Col1a1, Col4a1, Timp1, Desmin, Ctgf and Gfap determined by quantitative real time PCR analysis. Results were normalized to GAPDH expression. Mean ± SD data are displayed as fold changes relative to the non-treated group. Data were obtained from three independent experiments. *p < 0.05, **p < 0.01 (vs non-treated group).
Carfilzomib attenuates carbon tetrachloride-induced liver fibrosis without liver injury
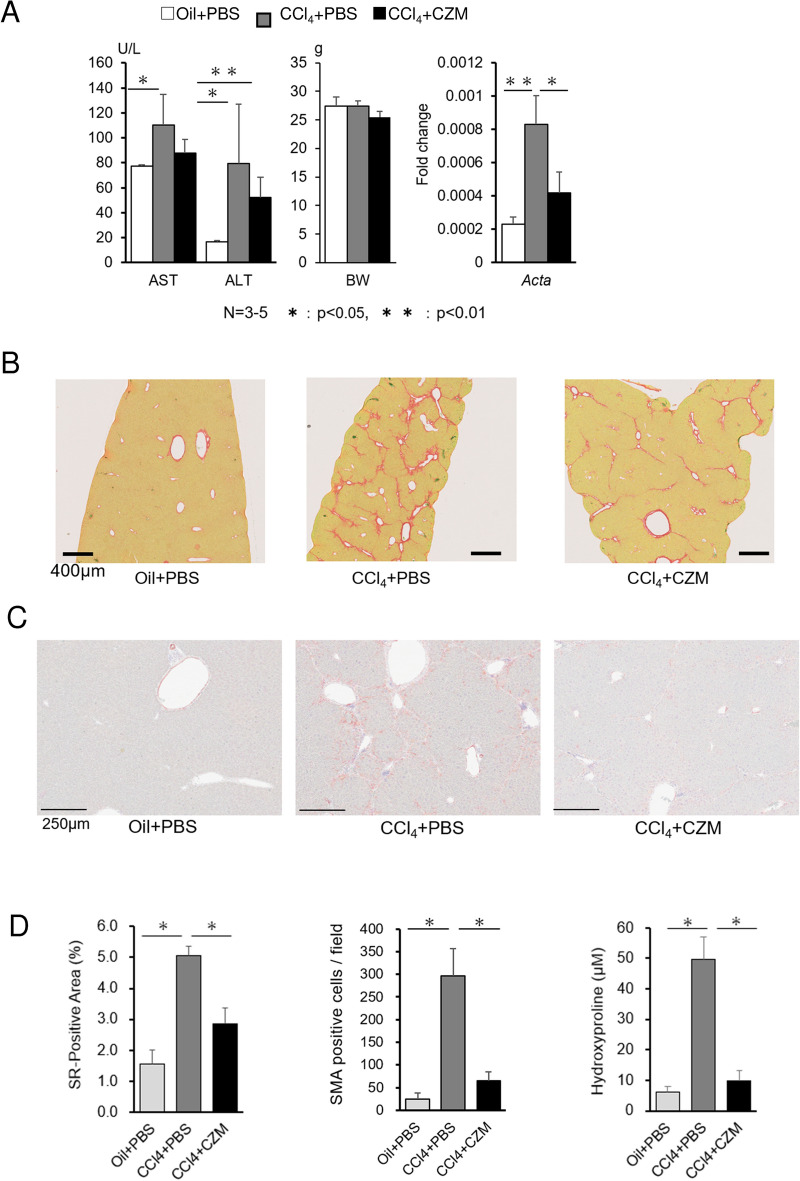
Mice were administered carbon tetrachloride (CCl4) or corn oil (Oil) for 10 weeks to induce fibrosis so that we could examine the in vivo effect of CZM administration. Then mice were injected intravenously with 5 mg/kg CZM or control phosphate-buffered saline (PBS) at 6 to 10 weeks of the CCl4 treatment period, and liver enzymes, body weight and liver fibrosis were evaluated. CZM treatment tended to improve the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) without a significant reduction in body weight (BW) (Fig. 3A). In addition, CZM reduced the area of positivity for picro-sirius red, which represents fibrotic tissue induced by CCl4 administration (Fig. 3B). Furthermore, in the CCl4 model, we performed a quantitative analysis of the area positive for picro-sirius red and determined the hepatic hydroxyproline content (Fig. 3D) to evaluate liver fibrosis and found that CZM significantly attenuated liver fibrosis progression. Since α-SMA is used as a reliable marker of activated and myofibroblastic HSCs, its immunoreactivity was confirmed. Strong α-SMA immunoreactivity was confirmed in the CCl4-injected mouse liver, and clearly suppressed by CZM treatment (Fig. 3C, D).
Figure 3.
Carfilzomib attenuates liver injury and fibrosis in CCl4-injected mice. (A) Serum AST, serum ALT, body weight (BW) values and Acta2 gene expression in the liver of treated mice. Mean ± SD data from each group are displayed. (B) Picro-sirius red staining of liver sections from representative mice treated with the indicated agents. (C) Alfa-smooth muscle actin (SMA) staining of liver sections from representative mice treated with the indicated agents. (D) Positive areas of SR and SMA staining. The area of red fiber, reflecting collagen deposition on sections from each mouse was calculated using Image J software. SMA-positive cells were counted. Hepatic hydroxyproline contents. Mean ± SD data from each group (5 mice per group) are displayed. *p < 0.05, **p < 0.01.
Carfilzomib reduces the expression of cytokines in the CCl4-injected mouse liver
Next, we performed a cytokine array analysis of livers of the CCl4 model mice to investigate the mechanism by which CZM suppresses liver fibrosis (Fig. 4A,B). CZM reduced the protein expression of several cytokines (PCSK9, Amphiregulin, CCL11/Eotaxin, Chemerin, Fetuin A/AHSG) in the CCl4-induced injured liver. Among them, Amphiregulin (Areg) is known to activate HSCs and induce fibrogenic activity via multiple mitogenic signaling pathways9. In addition, exogenous Areg stimulates proliferation of human HSC and it requires EGFR and the PI3K, MEK, p38 MAPK, PKC survival pathways9. It is also reported that Areg gene expression is induced in human and experimental liver injury10.
Figure 4.
Carfilzomib reduces cytokine expression in injured liver induced by CCl4. (A) Membranes of cytokine array incubated with liver lysate from CCl4-injected mouse liver. (B) Ratios, CCl4 + CZM vs CCl4 + PBS, of indicated cytokine expression calculated by densitometric analysis.
Carfilzomib suppresses mitotic gene expression of primary cultured mouse stellate cells
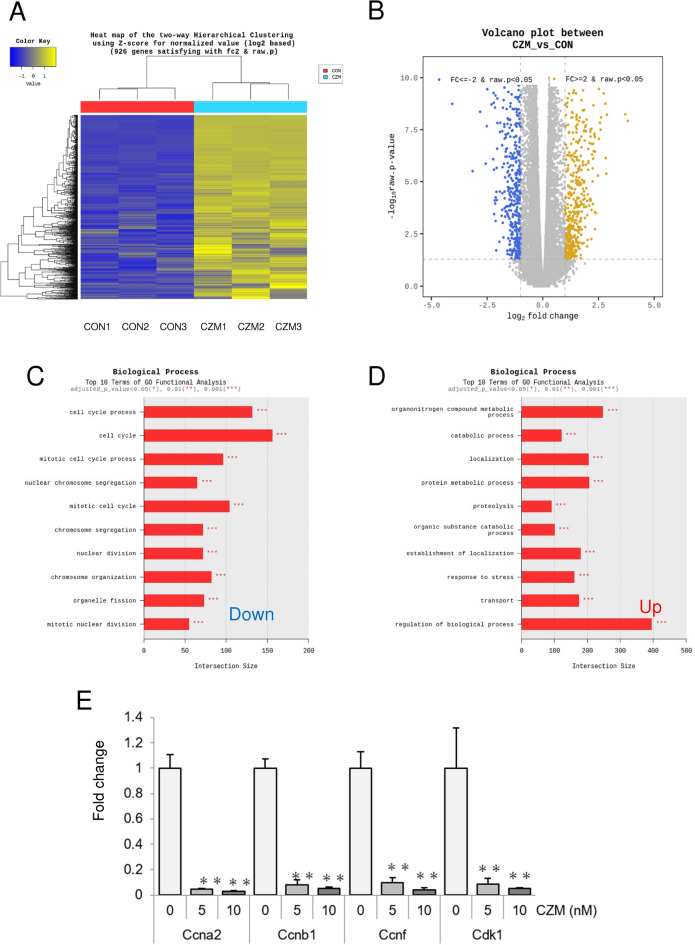
Finally, to explore the mechanism by which CZM inactivates HSCs and inhibits their fibrogenic properties, we analyzed CZM-treated primary HSCs by whole transcriptome sequencing and found that a total of 1,775 genes satisfied fold-change (FC) ≤ -2 (849 genes) or FC ≥ 2 (926 genes) (Fig. 5A,B). According to the results of GO functional analysis, most of the genes down-regulated by CZM are cell cycle-related genes (Fig. 5C), particularly mitosis-related genes. In contrast, the group of genes upregulated by CZM was diverse, including metabolic, catabolic and biological processes, localization, proteolysis, stress responses, transport, etc. (Fig. 5D). By realtime-qPCR analysis, we analyzed representative genes related to the mitosis and the cell cycle process including cyclin A2 (Ccna2), cyclin B1 (Ccnb1), cyclin F (Ccnf), and cyclin-dependent kinase 1 (Cdk1) (Fig. 5E). Consistent with the whole transcriptome sequencing results, their gene expressions were suppressed by CZM-treatment.
Figure 5.
Carfilzomib inhibits mitosis in primary HSCs. (A) Heat map of Hierarchical clustering of whole transcriptome sequencing. CZM treated primary HSCs (CZM) were analyzed and compared with non-treated control HSCs (CON). Three samples per group. (B) Volcano plot between CZM vs CON. (C) Top 10 terms downregulated by CZM treatment of GO functional analysis (biological process). (D) Top 10 terms upregulated by CZM treatment of GO functional analysis (biological process). (E) mRNA levels of Ccna2, Ccnb1, Ccnf and Cdk1 determined by quantitative real time PCR analysis. Results were normalized to GAPDH expression. Mean ± SD data are displayed as fold changes relative to the non-treated group. Data were obtained from three independent experiments. *p < 0.05, **p < 0.01 (vs non-treated group).
Discussion
Drug screening assays revealed that proteasome inhibitors inactivate human stellate cells (LX-2 cells) at low concentrations. The screening of drugs targeting HSCs was first conducted using reduction of cell viability as an indicator. We selected 441 compounds based on their potential for early clinical application from the perspective of drug repositioning, and the screening results showed that several PIs (CZM, BZM, epoxomicin, and MG132) inhibited the viability of human HSCs (LX-2 cells). MG132 is a PI commonly used in experimental studies, but is less effective than the other three, and epoxomicin was the base drug used as the starting point for the development of CZM, which led to its clinical application11. Since BZM and CZM are widely used in clinical practice as anti-tumor drugs for the treatment of multiple myeloma, and this study showed that both BZM and CZM inhibited the viability of LX-2 cells by approximately 20% at concentrations as low as around 10 nM, further studies were conducted on BZM and CZM.
Carfilzomib suppresses fibrogenic properties without toxicity in primary cultured mouse stellate cells at low concentrations. BZM and an inhibitor of I-kβα phosphorylation each induced apoptosis in primary rat HSCs12, but no detailed studies on the effect of BZM (besides cell death) on primary HSCs isolated from mouse livers or on the effect of CZM on HSCs have been reported. In this study, we used isolated HSCs from mouse livers for BZM and CZM experiments. BZM suppressed cell viability at low concentrations (IC50 values of 6.60 nM), but also showed toxicity, as assessed by LDH leakage at the same low concentrations (Supplementary Fig. 1A,B). In contrast, the IC50 values of CZM (16.1 nM) were twofold higher than those of BZM for suppressing cell viability (Fig. 2A), and importantly, CZM was not toxic up to a concentration of 6.25 nM, suggesting that CZM can exert anti-fibrotic effects without liver injury, making it safer than BZM (Fig. 2B).
PIs have been used clinically since BZM was approved by the US FDA in 200513, and drug development and clinical trials continued after the approval of the second-generation PI, CZM. Furthermore, BZM and CZM are used as anti-tumor drugs on the premise that they induce the death of tumor cells, but there is concern about liver toxicity due to hepatocyte death when they are administered to patients with liver fibrosis, the target of this treatment. A phase II clinical trial that evaluated BZM for the treatment of liver cancer did not show a clear benefit, and worsening liver function appeared to be a common occurrence, so the drug has not progressed beyond that point for that indication14. Of note, BZM degradation involves hepatic oxidative deboronation (the removal of a boronic group) by cytochrome P-450 (CYP) enzymes15, which may result in liver dysfunction and neurologic toxicity. In contrast, CZM is mainly metabolized and cleared by peptidase cleavage and epoxide hydrolysis, which is associated with rapid elimination and a favorable safety profile including a reduced incidence of neurologic toxicities16. Saeki et al. reported that in primary cultured rat hepatocytes and human hepatocytes, cytotoxicity was not confirmed with BZM 40 or 200 nM, respectively, higher doses than those we used for CZM in this study17. As a result of our experiments, PIs have emerged as promising drugs for the treatment of liver fibrosis, particularly because CZM showed a safety profile in agreement with clinical findings.
Next, we examined the inhibitory effect of CZM on the fibrogenic properties of HSCs. CZM suppressed the expression of fibrotic markers at concentrations much lower than the dose showing cell toxicity (Fig. 2C). Acta2, Col1a1, Col4a1, Timp1, Desmin, and Ctgf, hallmarks of HSC activation and liver fibrogenesis, were attenuated by CZM treatment at low concentrations of 5 or 10 nM, although the attenuation of Desmin and Ctgf was not statistically significant. Thus, CZM was found to reduce fibrosis-producing activity at low concentrations with no apparent cytotoxic effect on HSCs and possibly on hepatocytes. CZM may be applicable to fibrosis treatment via HSC suppression in a wide range of pathologies and liver diseases.
Carfilzomib attenuates carbon tetrachloride—induced liver fibrosis without liver injury. In preclinical, rodent liver fibrosis models, Saeki et al. reported that BZM inhibited liver fibrosis and hepatocarcinogenesis via anti-inflammatory effects in the rat diethylnitrosamine (DEN) administration plus choline-deficient L-amino acid refined (CDAA) diet model 10. They did not examine the effect of BZM on HSCs, and no reports were confirmed regarding CZM treatment on animal liver fibrosis models. BZM inhibited biliary fibrosis induced by bile duct ligation in mice18. The investigators examined the effect of BZM on HSCs and found that inhibition of nuclear factor-kappaB (NF-kB) induced apoptosis in LX-2 cells and primary rat HSCs. The CCl4-treated mouse, a representative animal model, was used to evaluate liver fibrosis because it develops liver fibrosis along with chronic liver injury. The fibrosis as indicated by Sirius Red staining and hydroxyproline was significantly improved in the CZM-treated group (Fig. 3B, D). In addition, there was no change in body weight after CZM administration, and the liver injury caused by CCl4 did not worsen, but slightly improved (Fig. 3A), indicating that CZM administration is safe for patients with a variety of chronic liver diseases. As far as we know, this is the first report that CZM inhibits liver fibrosis in rodent models. Previously, intraperitoneal injection of CZM was reported to improve liver injury caused by acetaminophen overdose in a mouse model of liver injury19. However, based on the pharmacokinetics of CZM, the effect of intraperitoneal administration is unclear, and CZM should be considered for intravenous injection in mice. The present study is the first report describing the effects of CZM on liver diseases.
Carfilzomib reduces the expression of cytokines in the CCl4-injected mouse liver. CZM reduced the protein expression of several cytokines (PCSK9, Amphiregulin, CCL11/Eotaxin, Chemerin, Fetuin A/AHSG) in the CCl4-induced injured liver (Fig. 4,B). Among those cytokines, PCSK9 and Areg were particularly decreased by CZM administration. Proprotein convertase subtilisin kexin type 9 (PCSK9) is a key regulator of low-density lipoprotein (LDL) cholesterol metabolism and the target of lipid-lowering drugs and is expressed mainly in hepatocytes. PCSK9 inhibitors reduce LDL leading to reduction in plaque volume and more importantly reduction in future cardiovascular events. PCSK9 is also highly expressed in undifferentiated human induced pluripotent stem cells (hiPSCs) and reported to have a new role as a potential cell cycle regulator involving to cellular proliferation and development in hiPSCs20. However, an association between PCSK9 and liver fibrosis or HSCs has not been reported.
Areg is most commonly identified as an epidermal growth factor receptor (EGFR) ligand and acts as a mitogen to induce mitosis in a variety of tissues, including an autocrine growth factor21–23. Importantly, Areg is known to be upregulated in a variety of liver diseases, including murine and human MASLD/MASH, to activate the mitogenic signaling pathway and fibrogenic activity in HSCs9. Specifically, the CCl4-induced liver injury model showed that Areg knockout attenuates liver fibrosis24. In the present study, Areg was suppressed by nearly 80% by CZM administration in the CCl4-induced mouse liver, suggesting that it may have been involved in the suppression of liver fibrosis.
Areg is expressed at the onset of liver injury either by partial hepatectomy or exposure to CCl425. The rise in Areg expression parallels an increase in α-smooth muscle actin (α-SMA), indicating an association between the activation of primary cultured HSC and Areg expression9. In accordance with these facts, Areg protein expression was suppressed by CZM treatment in the CCl4-injured liver and CZM inhibited mitosis in primary cultured HSCs in the present study, although mRNA expression of Areg was not decreased.
Carfilzomib suppresses mitotic gene expression of primary cultured mouse stellate cells. Whole transcriptome sequencing of CZM-treated primary HSCs demonstrated that most genes downregulated by CZM are cell cycle-related genes (Fig. 5C), especially mitosis-related genes. Areg is known as one of the EGFR ligands and stimulates the expression of cell proliferation–related early-response genes including Egr (early growth response), in an EGFR-dependent manner 10. In our RNA sequencing analysis, Egrs (Egr-1, -2, -3 and -4), especially Egr-4, were downregulated by CZM treatment (Supplementary Data 2, Representative genes 1).
Given that Areg is known to induce HSC fibrogenic activity via multiple mitogenic signaling pathways 9 and was suppressed by CZM in the CCl4-induced fibrotic liver in our study, CZM may antagonize Areg and could be a useful anti-fibrotic agent clinically in liver diseases such as MASLD/MASH.
Limitations
Association of drug concentrations between in vitro and in vivo experiments
At very low concentrations against LX-2 and primary HSC in vitro, CZM inhibited fibrogenic activity without toxicity. In contrast, the concentration used in vivo was based on a preclinical study of CZM (Demo, S. D. et al., 2007), a rapid intravenous (i.v.) dose of 5 mg/kg twice weekly used in a subcutaneous transplant model 26. In this study, 5 mg/kg dose of CZM i.v. administration induced enough suppressive activity in all rat tissues examined except the brain. In addition, the antitumor efficacy of CZM in mice bearing human tumor xenografts receiving biweekly administration of 5 mg/kg CZM was confirmed. In rats, CZM was rapidly cleared from the plasma compartment following i.v. administration. Noncompartmental analysis of CZM pharmacokinetics revealed an average terminal plasma half-life of ∼15 min. The conclusion is that CZM is cleared rapidly but promotes a widespread pharmacodynamic response and the compound does not readily cross the blood–brain barrier. Since prolonged exposure to CZM increases cytotoxicity, i.v. administration is better than the others. Although intraperitoneal injection was used in some rodent studies, its drug development process proved that CZM i.v. administration is superior and currently used in clinical practice. In summary, CZM i.v. administration was reported to inhibit tumor growth without seriously affecting the general condition and weight trends.
Mechanism of action of CZM
It was reported that PIs including MG-132 inhibit the degradation of I-kβ, thereby blocking the stimulation of NF-kB activity by tumor necrosis factor-α (TNF-α) and interleukin 1 beta (IL-1β) in activated HSCs. Thus, inhibition of I-kβα degradation is a potential target for anti-inflammatory therapy in the liver and might influence the activation process of HSCs following fibrotic stimuli27. However, the downregulation of cell cycle-related genes was more prominent as an effect of CZM treatment than an effect on NF-kB-responsive genes in this study.
As a mechanism that attenuates fibrosis, the anti-inflammatory effects of PI, especially via inhibition of NF-kB, may lead to cell cycle inhibition17. Since we found that CZM inhibited liver fibrosis via suppression of HSC activity, we sought to explore the mechanisms involved. However, we have not determined the mechanisms clearly, even though our results suggest that CZM directly inactivates HSC by suppression of mitosis leading to fibrosis suppression and Areg is possibly involved. Of note, the main aim in this study was not to induce cell death in HSCs, but to reduce their fibrogenic activity.
It was reported that proteasome inhibition induces hepatic stellate cell apoptosis by blockade of NF-kB activation18. In our study, the TOP 10 terms of GO functional analysis results of the whole transcriptome sequencing revealed that many of the genes related to cell cycle regulation and/or mitosis-related genes were repressed by CZM (Fig. 5C). On the other hand, when referring to the 428 representative target genes of NF-kB 28, only 17 genes among them were downregulated in primary mHSCs by CZM treatment suggesting that the blockade of NF-kB activation had limited involvement (Supplementary Data 2, Representative genes 2).
Apoptosis in LX-2 and primary rat HSCs was observed at a concentration of 10 μM which is 1,000 times higher than that required to suppress fibrotic properties in this study.
A recent report also focused on the usefulness of PI but analyzed the cytotoxicity of BZM and B cell lymphoma 2 (BCL2), and has not yet investigated the usefulness of CZM. BCL2 is known as an anti-apoptotic molecule and is upregulated in human cirrhotic livers and used for escape from cell death in HSC29. Recently, Qian et al. observed suppression of the apoptosis pathway along with induction of coregulated genes with BCL2 in HSC in transcriptome analysis of both human cirrhotic livers and mouse liver cells30. They concluded that BCL2 is an antifibrotic target that can be blocked by BZM and MG132, according to the CMap database. They also validated the effect of MG-132 at a high concentration of 20 μM in in vitro experiments. In that report, human liver cells and cell lines were examined, but primary cultured HSC and mouse fibrosis models were not. An analysis of the low concentrations we used is needed to assess an anti-fibrotic effect apart from apoptosis in HSC. Our findings that CZM is safer to use than BZM and that it can inhibit fibrotic potential at low concentrations that do not cause HSC cell death are important for the development of liver fibrosis therapy.
Limitations of cytokine array and RNA sequence analyses
The fact that CZM reduced Areg, which regulates mitosis in CCl4-induced liver fibrotic tissue, and that CZM administration to HSCs suppressed a group of genes related to the cell cycle, which is mainly responsible for mitosis, may be part of its mechanism. However, cytokines other than Areg, PCSK9, CCL11/Eotaxin, Chemerin, and Fetuin A/AHSG, which were extracted by antibody array, may also play a role. The whole transcriptome sequencing results also showed that a group of genes upregulated in HSCs by CZM treatment, including metabolic, catabolic and biological processes, localization, proteolysis, stress responses, transport, and others, were extensively affected by CZM. The involvement of these genes in HSCs is also an area for further investigation.
Effects on non-HSC cells
Anan et al. reported that bortezomib therapy was also associated with a reduction in hepatocyte apoptosis, a decrease in Kupffer cell/macrophage number and activation, and increased hepatocyte proliferation18. CZM may act similarly on hepatocytes and immune cells, such as Kupffer cell/macrophages, to inhibit inflammation and fibrosis.
Conclusion
To our knowledge, this is the first report to show that PIs, specifically CZM, attenuate liver fibrosis via suppression of HSC activity and expression of fibrosis markers. CZM and next-generation PIs in development have the potential to be therapeutic agents for the treatment of liver fibrosis via inactivation of HSCs.
Material and methods
Experimental procedures
All the experimental protocols were approved by the Institutional Research Committee of Kyoto Prefectural University of Medicine (KPUM). All the methods were performed in accordance with the relevant guidelines and regulations.
Cell culture
LX-2 cells were obtained from ATCC and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C with 5% CO2-in-air.
Primary cell culture
Primary cultures of HSCs in the liver were prepared as described previously31 with minor modifications. Livers were perfused and digested using pronase/collagenase and the gradient centrifugation method. Freshly isolated HSCs were cultured in DMEM + 10% FCS and + antibiotics. The second or third passage of these cells was used for cell viability, LDH leakage, and mRNA analyses.
Reagents
Bortezomib, carfilzomib, MG132 and chemical compound libraries (JAK/STAT compound library [L5400], TGF-beta/Smad compound library [L5600], Covalent Inhibitor Library [L5800], and Flavonoid Compound Library [L7700]) were purchased from Selleck Chemicals (Yokohama, Japan). TGF-β-SMAD, and Janus kinases (JAK)-signal transducer and activator of transcription protein (STAT) signaling pathways can act directly on HSCs, Covalent and Flavonoid provide an opportunity for drug repositioning (or repurposing). The detail of chemical compound libraries are described in Supplementary Data 1.
For analysis of cell viability, CellTiter-Glo Luminescent Cell Viability Assay (Promega, Tokyo, Japan), a homogeneous method to determine the number of viable cells in culture based on quantitation of the ATP present, which signals the presence of metabolically active cells, was used according to the manufacturer’s instructions.
To assess drug-induced cytotoxicity, LDH release was measured in cell culture supernatants using the LDH Cytotoxicity Assay kit (Nacalai tesque, Kyoto, Japan) according to the manufacturer's protocol. The absorbance at λ = 490 nm was measured using a microplate reader.
IC50 was calculated by Four Parameter Logistic (4PL) Curve Calculator (AAT Bioquest, Inc., California, USA).
Quantitative real-time PCR analysis
Real-time PCR was performed as described previously32, and the primers used for Real-time PCR analyses are listed in the Supplementary Table 1. Target gene levels were presented as a ratio of levels in the treated versus corresponding control groups.
Animals and treatment
A mouse model of CCl4 (FUJIFILM Wako, Osaka, Japan)—induced chronic fibrosis was used. CCl4 (0.5ml per kg of mouse, in a 10% solution of corn oil) or corn oil (Nacalai tesque, Kyoto, Japan) were injected intraperitoneally twice per week for up to 10 weeks. CZM (5mg per kg of mouse) or control PBS were administered intravenously twice per week at 6 to 10 weeks of CCl4 treatment period. CZM used for the treatment of mice were purchased from Ono pharmaceutical co. ltd (Osaka, Japan).
For the present experiments, 8 to 12-week-old C57/BL6 male mice were purchased from SHIMIZU Laboratory Supplies Co., Ltd (Kyoto, Japan)., maintained in a temperature- and light-controlled facility, and permitted consumption of water ad libitum. All animal experiments fulfilled the requirements for the ARRIVE guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) of KPUM (Approval no.: M2023-269 and M2023-270).
Analyses of liver specimens
To quantify the collagen content or the activated HSCs in the liver, liver sections were stained with picro-sirius red (Sigma-Aldrich, Japan) or anti-SMA antibody (ab5694, Abcam Limited., Cambridge, UK). The proportion of tissue stained with picro-sirius red was quantified by morphometric analysis with Image J software in five randomly selected fields per section. The number of SMA-positive cells as red spots was counted in five randomly selected fields per section.
Hydroxyproline content in whole-liver specimens was quantified with a Total Collagen Assay Kit (QuickZyme BioSciences B.V, Netherlands).
Plasma biochemical measurements
Serum AST, ALT, and LDH levels were measured by SRL, Inc (Tokyo, Japan).
Cytokine array analysis
Cytokine expression in liver tissue samples was analyzed with the Cytokine Array Kit (Mouse XL Cytokine Array Kit, R&D systems). Briefly, 20mg of liver tissues were lysed by bioMasher with 100 μl of lysis buffer, and 5 μl of tissue lysates were diluted with lysis buffer. Then, three samples for each group were mixed and incubated with array membranes.
After washing, membranes were incubated with Detection Antibody followed by Streptavidin-HRP. Cytokine blots were imaged with a ChemiDoc Imaging System (Bio-Rad, Tokyo, Japan) using ECL Prime Western blotting HRP substrate (Cytiva, Tokyo, Japan).
Whole transcriptome sequencing
We used an Illumina NovaSeq6000 (Novogene, Cambridge, UK) for whole transcriptome sequencing analysis of primary HSCs treated with CZM (CZM) were analyzed and compared with non-treated control HSCs (CON) (three samples per group). The results (Supplementary Data 2) were analyzed by Macrogen Japan, Hierarchical clustering, Pathway analysis and GO functional analysis were performed.
Statistical analysis
Results obtained in experiments are presented as the mean ± standard deviations. Comparisons were made by a two-tailed t test or Dunnett's multiple comparison test to calculate whether observed differences were statistically significant. Results with P values less than 0.05 were considered significant.
Supplementary Information
Acknowledgements
We would like to thank the members listed below for their support and guidance in completing our project. Dr. Hatsue Mizuhara; Department of Pharmacology, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Author contributions
A.F. (Data curation, Formal analysis), K.T. (Methodology, Formal analysis, Validation), A.T. (Data curation, Formal analysis), M.M. (Methodology, Formal analysis), M.K. (Supervision), T.O. (Funding acquisition), K.Y. (Supervision), Y.I. (Supervision), K.I. (Supervision, Validation) and K.A. (Supervision, Validation) and A.U. (Conceptualization, Data curation, Funding acquisition, Validation, Writing, Review & editing). Ayana Fujiwra and Keisuke Takemura. contributed equally to the work as co-first authors.
Funding
This study was supported by Japan Agency for Medical Research and Development (AMED) under Grant no. JP23fk0210115 and JP24fk0210115.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Supplementary Data 1 and Data 2 files include chemical libraries and the whole transcriptome sequencing data utilized in this manuscript, respectively.
Competing interests
Dr. Itoh Y received Speakers’ fee from ONO PHARMACEUTICAL CO., LTD. The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-70296-8.
References
- 1.Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol.79, 1542–1556. 10.1016/j.jhep.2023.06.003 (2023). 10.1016/j.jhep.2023.06.003 [DOI] [PubMed] [Google Scholar]
- 2.Harrison, S. A. et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med.390, 497–509. 10.1056/NEJMoa2309000 (2024). 10.1056/NEJMoa2309000 [DOI] [PubMed] [Google Scholar]
- 3.Umemura, A. et al. Potential therapeutic targets and promising agents for combating NAFLD. Biomedicines10, 901. 10.3390/biomedicines10040901 (2022). 10.3390/biomedicines10040901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang, S. & Friedman, S. L. Hepatic fibrosis: A convergent response to liver injury that is reversible. J. Hepatol.73, 210–211. 10.1016/j.jhep.2020.03.011 (2020). 10.1016/j.jhep.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buniatian, G., Hamprecht, B. & Gebhardt, R. Glial fibrillary acidic protein as a marker of perisinusoidal stellate cells that can distinguish between the normal and myofibroblast-like phenotypes. Biol. Cell87, 65–73 (1996). [PubMed] [Google Scholar]
- 6.Buniatian, G. H. Stages of activation of hepatic stellate cells: Effects of ellagic acid, an inhibiter of liver fibrosis, on their differentiation in culture. Cell Prolif.36, 307–319. 10.1046/j.1365-2184.2003.00287.x (2003). 10.1046/j.1365-2184.2003.00287.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neubauer, K., Knittel, T., Aurisch, S., Fellmer, P. & Ramadori, G. Glial fibrillary acidic protein: A cell type specific marker for Ito cells in vivo and in vitro. J. Hepatol.24, 719–730. 10.1016/s0168-8278(96)80269-8 (1996). 10.1016/s0168-8278(96)80269-8 [DOI] [PubMed] [Google Scholar]
- 8.Morini, S. et al. GFAP expression in the liver as an early marker of stellate cells activation. Ital. J. Anat. Embryol.110, 193–207 (2005). [PubMed] [Google Scholar]
- 9.McKee, C. et al. Amphiregulin activates human hepatic stellate cells and is upregulated in non alcoholic steatohepatitis. Sci. Rep.5, 8812. 10.1038/srep08812 (2015). 10.1038/srep08812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berasain, C. et al. Amphiregulin: An early trigger of liver regeneration in mice. Gastroenterology128, 424–432. 10.1053/j.gastro.2004.11.006 (2005). 10.1053/j.gastro.2004.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Kim, K. B. & Crews, C. M. From epoxomicin to carfilzomib: Chemistry, biology, and medical outcomes. Nat. Prod. Rep.30, 600–604. 10.1039/c3np20126k (2013). 10.1039/c3np20126k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anan, A. et al. Proteasome inhibition induces hepatic stellate cell apoptosis. Hepatology43, 335–344. 10.1002/hep.21036 (2006). 10.1002/hep.21036 [DOI] [PubMed] [Google Scholar]
- 13.Kane, R. C., Farrell, A. T., Sridhara, R. & Pazdur, R. United States Food and Drug Administration approval summary: Bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin. Cancer Res.12, 2955–2960. 10.1158/1078-0432.CCR-06-0170 (2006). 10.1158/1078-0432.CCR-06-0170 [DOI] [PubMed] [Google Scholar]
- 14.Kim, G. P. et al. An international, multicenter phase II trial of bortezomib in patients with hepatocellular carcinoma. Invest. New Drugs30, 387–394. 10.1007/s10637-010-9532-1 (2012). 10.1007/s10637-010-9532-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labutti, J. et al. Oxidative deboronation of the peptide boronic acid proteasome inhibitor bortezomib: Contributions from reactive oxygen species in this novel cytochrome P450 reaction. Chem. Res. Toxicol.19, 539–546. 10.1021/tx050313d (2006). 10.1021/tx050313d [DOI] [PubMed] [Google Scholar]
- 16.Yang, J. et al. Pharmacokinetics, pharmacodynamics, metabolism, distribution, and excretion of carfilzomib in rats. Drug Metab. Dispos.39, 1873–1882. 10.1124/dmd.111.039164 (2011). 10.1124/dmd.111.039164 [DOI] [PubMed] [Google Scholar]
- 17.Saeki, I. et al. Bortezomib induces tumor-specific cell death and growth inhibition in hepatocellular carcinoma and improves liver fibrosis. J. Gastroenterol.48, 738–750. 10.1007/s00535-012-0675-z (2013). 10.1007/s00535-012-0675-z [DOI] [PubMed] [Google Scholar]
- 18.Anan, A. et al. Proteasome inhibition attenuates hepatic injury in the bile duct-ligated mouse. Am. J. Physiol. Gastrointest. Liver Physiol.291, G709-716. 10.1152/ajpgi.00126.2006 (2006). 10.1152/ajpgi.00126.2006 [DOI] [PubMed] [Google Scholar]
- 19.Alanazi, A. et al. Therapeutic potential of carfilzomib, an irreversible proteasome inhibitor, against acetaminophen-induced hepatotoxicity in mice. J. Biochem. Mol. Toxicol.31, e21877. 10.1002/jbt.21877 (2017). 10.1002/jbt.21877 [DOI] [PubMed] [Google Scholar]
- 20.Roudaut, M. et al. PCSK9 regulates the NODAL signaling pathway and cellular proliferation in hiPSCs. Stem Cell Rep.16, 2958–2972. 10.1016/j.stemcr.2021.10.004 (2021). 10.1016/j.stemcr.2021.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoyab, M., Plowman, G. D., McDonald, V. L., Bradley, J. G. & Todaro, G. J. Structure and function of human amphiregulin: A member of the epidermal growth factor family. Science243, 1074–1076. 10.1126/science.2466334 (1989). 10.1126/science.2466334 [DOI] [PubMed] [Google Scholar]
- 22.Piepkorn, M., Lo, C. & Plowman, G. Amphiregulin-dependent proliferation of cultured human keratinocytes: Autocrine growth, the effects of exogenous recombinant cytokine, and apparent requirement for heparin-like glycosaminoglycans. J. Cell. Physiol.159, 114–120. 10.1002/jcp.1041590115 (1994). 10.1002/jcp.1041590115 [DOI] [PubMed] [Google Scholar]
- 23.Li, S., Plowman, G. D., Buckley, S. D. & Shipley, G. D. Heparin inhibition of autonomous growth implicates amphiregulin as an autocrine growth factor for normal human mammary epithelial cells. J. Cell. Physiol.153, 103–111. 10.1002/jcp.1041530114 (1992). 10.1002/jcp.1041530114 [DOI] [PubMed] [Google Scholar]
- 24.Perugorria, M. J. et al. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology48, 1251–1261. 10.1002/hep.22437 (2008). 10.1002/hep.22437 [DOI] [PubMed] [Google Scholar]
- 25.Berasain, C. et al. Epidermal growth factor receptor signaling in hepatocellular carcinoma: Inflammatory activation and a new intracellular regulatory mechanism. Dig. Dis.30, 524–531. 10.1159/000341705 (2012). 10.1159/000341705 [DOI] [PubMed] [Google Scholar]
- 26.Demo, S. D. et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res.67, 6383–6391. 10.1158/0008-5472.CAN-06-4086 (2007). 10.1158/0008-5472.CAN-06-4086 [DOI] [PubMed] [Google Scholar]
- 27.Hellerbrand, C. et al. Inhibition of NFkappaB in activated rat hepatic stellate cells by proteasome inhibitors and an IkappaB super-repressor. Hepatology27, 1285–1295. 10.1002/hep.510270514 (1998). 10.1002/hep.510270514 [DOI] [PubMed] [Google Scholar]
- 28.NF-kB Target Genes, <https://www.bu.edu/nf-kb/gene-resources/target-genes/>
- 29.Novo, E. et al. Overexpression of Bcl-2 by activated human hepatic stellate cells: Resistance to apoptosis as a mechanism of progressive hepatic fibrogenesis in humans. Gut55, 1174–1182. 10.1136/gut.2005.082701 (2006). 10.1136/gut.2005.082701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian, T. et al. Molecular signature predictive of long-term liver fibrosis progression to inform antifibrotic drug development. Gastroenterology162, 1210–1225. 10.1053/j.gastro.2021.12.250 (2022). 10.1053/j.gastro.2021.12.250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seki, E. et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med.13, 1324–1332. 10.1038/nm1663 (2007). 10.1038/nm1663 [DOI] [PubMed] [Google Scholar]
- 32.Kataoka, S. et al. Honokiol acts as a potent anti-fibrotic agent in the liver through inhibition of tgf-beta1/SMAD signaling and autophagy in hepatic stellate cells. Int. J. Mol. Sci.22, 13354. 10.3390/ijms222413354 (2021). 10.3390/ijms222413354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. Supplementary Data 1 and Data 2 files include chemical libraries and the whole transcriptome sequencing data utilized in this manuscript, respectively.